Abstract
Background
Insufficient evidence is available for patients with acute ischemic stroke with atrial fibrillation (AF) to determine the efficacy and safety of different dosages of intravenous thrombolysis treatment. This study examined clinical outcomes in Chinese patients with stroke with and without AF after intravenous thrombolysis treatment with different intravenous thrombolysis doses.
Methods and Results
This multicenter, prospective cohort study recruited 2351 patients with acute ischemic stroke (1371 with AF and 980 without AF) treated with intravenous thrombolysis using alteplase. The Totaled Health Risks in Vascular Events score is a validated risk‐scoring tool used for assessing patients with acute ischemic stroke with and without AF. We evaluated favorable functional outcome at day 90 and symptomatic intracranial hemorrhage within 24 to 36 hours and outcomes of the patients receiving different doses of alteplase. Compared with the non‐AF group, the AF group exhibited a 2‐ to 3‐fold increased risk of symptomatic intracranial hemorrhage according to the National Institute of Neurological Disorders and Stroke standard (relative risk [RR], 2.10 [95% CI, 1.35–3.26]). Favorable functional outcome at 90 days and symptomatic intracranial hemorrhage rates according to the European Cooperative Acute Stroke Study II and the Safe Implementation of Thrombolysis in Stroke‐Monitoring Study standards did not significantly differ between the AF and non‐AF groups. In addition, the low‐dose alteplase subgroup exhibited an increased risk of symptomatic intracranial hemorrhage according to the National Institute of Neurological Disorders and Stroke standard (RR, 2.84 [95% CI, 1.63–4.96]). A validation study confirmed these findings after adjustment for scores determined using different stroke risk‐scoring tools.
Conclusions
Different alteplase dosages did not affect functional status at 90 days in the AF and non‐AF groups. Thus, the adoption of low‐dose alteplase simply because of AF is not recommended.
Keywords: acute stroke, atrial fibrillation, functional outcome, intravenous thrombolysis, symptomatic intracranial hemorrhage
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke, Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- FFO
favorable functional outcome
- FI
functional independence
- IPTW
inverse probability of treatment weighting
- IVT
intravenous thrombolysis
- SICH
symptomatic intracranial hemorrhage
Clinical Perspective
What Is New
After adjustment for the Totaled Health Risks in Vascular Events and other prognostic risk scores, clinical outcomes were comparable between stroke patients with and without atrial fibrillation treated with intravenous thrombolysis.
Increased symptomatic intracranial hemorrhage was only observed in patients treated with a lower dose of alteplase according to the National Institute of Neurological Disorders and Stroke standard (any point decline in National Institutes of Health Stroke Scale score) but not for ECASS II (European Cooperative Acute Stroke Study II) and SITS‐MOST (Safe Implementation of Thrombolysis in Stroke‐Monitoring Study) standards (National Institutes of Health Stroke Scale of ≥4 points).
What Are the Clinical Implications?
This study determined 90‐day clinical outcomes between the AF and non‐AF groups.
In our cohort of Asian patients, AF was not the reason indicated for prescribing low‐dose alteplase.
The prevalence of atrial fibrillation (AF) increases with age. 1 , 2 , 3 AF causes one‐fourth of acute ischemic stroke cases worldwide. 4 , 5 , 6 In Taiwan, AF is the most common arrhythmia and accounts for 20% to 25% of acute ischemic stroke. 7 , 8 Studies have reported that AF‐related stroke causes more disabilities compared with stroke not related to AF. 9 , 10 Despite receiving intravenous thrombolysis (IVT) treatment, patients with AF‐related acute ischemic stroke had poorer functional outcomes and higher hemorrhagic transformation risk. 11 , 12 In addition, compared with White patients with AF, Asian patients with AF had a higher incidence of stroke 13 , 14 and up to 2‐ to 4‐fold higher risk of intracranial bleeding. 15 , 16 , 17 , 18
Few risk‐scoring tools are available to predict clinical outcomes in acute ischemic stroke with AF. The THRIVE (Totaled Health Risks in Vascular Events) score 19 assesses age, and the National Institutes of Health Stroke Scale (NIHSS) score assesses at the time of hospital admission and evaluates the presence of hypertension, diabetes, and AF. Studies have reported the broad utility of the THRIVE score 19 to predict clinical outcomes, namely functional status, mortality, and hemorrhagic transformation after IVT. 20 , 21 In addition, the applicability of the THRIVE score in Chinese patients with acute ischemic stroke treated with IVT was validated. 22 , 23
Since the earlier J‐ACT (Japan Alteplase Clinical Trial) 24 in 2006 advocated using a lower dose of alteplase (0.6 mg/kg) to achieve equivalent efficacy and ensure safety of IVT in patients with acute ischemic stroke, the debate on the different dosages of IVT for Asian patients with stroke has been ongoing. Our previous studies, the TTT‐AIS (Taiwan Thrombolytic Therapy for Acute Ischemic Stroke) study I and II, 25 , 26 and a 2019 octogenarian study, 27 have reported an association of a lower dose with decreased mortality and increased favorable functional outcomes (FFOs) for mild stroke. This study evaluated clinical outcomes after IVT treatment between stroke patients with AF and those without AF by adjusting the THRIVE score and determined outcomes in patients with AF‐related stroke treated with different IVT doses.
METHODS
The data that support the findings of this study are available from the corresponding author (A.‐C.C. [achch@cc.kmu.edu.tw]) upon reasonable request.
Study Design and Participants
The TTT‐AIS study included a nationwide Chinese stroke cohort with longitudinal follow‐up data for 90 days. 25 , 26 This multicenter, prospective cohort study included patients from 30 hospitals throughout all regions in Taiwan from December 1, 2004 to December 31, 2016. On arrival at the hospital within 3 hours of stroke onset, patients with acute ischemic stroke were treated with IVT, with alteplase as the thrombolytic agent. The inclusion criteria were the following: (1) receiving IVT treatment adhering to the National Institute of Neurological Disorders and Stroke (NINDS) criteria, 28 (2) undergoing brain computed tomography (CT) before IVT and another CT scan within 24 to 36 hours after IVT, and (3) having complete data of the IVT dose using alteplase. According to the ECASS (European Cooperative Acute Stroke Study), early ischemic changes on brain CT were defined as subtle gray matter hypodensity, subtle cortical hypodensity, loss of the insular ribbon, sulcal effacement attributable to early edema, and the hyperdense middle cerebral artery sign. 29 , 30 , 31 A dose of 0.9 mg/kg (0.86–0.95 mg/kg) of alteplase for IVT was defined as the standard‐dose subgroup, whereas 0.6 mg/kg (0.55–0.65 mg/kg), 0.7 mg/kg (0.66–0.75 mg/kg), and 0.8 mg/kg (0.76–0.85 mg/kg) were defined as low‐dose subgroups. 25 , 32 , 33 , 34 The exclusion criteria for IVT were based on the SITS‐MOST (Safe Implementation of Thrombolysis in Stroke‐Monitoring Study) criteria. 35 The following data for each enrolled patient with stroke were prospectively retrieved and registered: age; sex; history of hypertension, diabetes, and coronary artery disease; alcohol use; presence of AF; the baseline NIHSS score; laboratory testing data; and antithrombotic medications. Written informed consent was obtained from patients who were mentally competent or from patients’ legal surrogates when they could not provide the consent. This study was approved by the institutional review board of Kaohsiung Medical University Hospital (reference number: KMUH‐IRB‐20140305).
Measurement of Risk Scores in Patients With Stroke
To evaluate each patient’s THRIVE score, 20 1 point each was assigned for the age of 60 to 79 years, AF, hypertension, and diabetes; 2 points each for the age ≥80 years and an NIHSS score of 11 to 20 at baseline, and 4 points for an NIHSS score of ≥21 at baseline. In addition, we used additional validated risk‐scoring tools for Chinese patients with stroke treated after IVT 36 despite no inclusion of AF in these scoring tools to estimate hemorrhagic transformation risk and clinical response. The following risk‐scoring tools were used to examine the baseline severity of the enrolled patients with stroke: the HAT (Hemorrhage After Thrombolysis) score 37 ; the SITS‐SICH (Safe Implementation of Thrombolysis in Stroke‐Symptomatic Intracranial Hemorrhage) score 38 ; the Cucchiara score 39 ; SEDAN (Blood Sugar, Early Infarct Signs and Hyperdense Cerebral Artery Sign, Age, and NIHSS) score 40 ; SPAN‐100 Index (Stroke Prognostication Using Age and National Institutes of Health Stroke Scale‐100 Index) 41 ; and GRASPS (Glucose, Race, Age, Sex, Pressure, Stroke Severity) score 42 (see Table 1 for details).
Table 1.
Risk‐Scoring Models Used in the Validation Study
| Stroke risk‐scoring tools | Variables required | Cutoff values (points obtained for each item) |
|---|---|---|
| THRIVE score 20 | Age, y | 60–79 (1), ≥80 (2) |
| NIHSS | 11–20 (2), ≥21 (4) | |
| Hypertension | Yes (1) | |
| Diabetes | Yes (1) | |
| Atrial fibrillation | Yes (1) | |
| HAT score 37 | NIHSS | 15–20 (1), >20 (2) |
| Glucose >200 mg/dL or diabetes | Yes (1) | |
| Hypodensity on CT | <1/3 of the MCA territory (1), ≥1/3 of the MCA territory (2) | |
| SITS‐SICH score 38 | Age, y | ≥72 (1) |
| NIHSS | 7–12 (1), ≥13 (2) | |
| Glucose | ≥180 mg/dL (2) | |
| Systolic blood pressure | ≥146 mm Hg (1) | |
| Weight | ≥95 kg (1) | |
| Onset to thrombolytic time | ≥180 min (1) | |
| Aspirin monotherapy | Yes (2) | |
| Aspirin+clopidogrel | Yes (3) | |
| Hypertension | Yes (1) | |
| Cucchiara score 39 | Age | >60 (1) |
| NIHSS | >10 (1) | |
| Glucose | >150 mg/dL (1) | |
| Platelet count | <150 000/mm3 (1) | |
| SEDAN score 40 | Glucose | 145–216 mg/dL (1), >216 mg/dL (2) |
| Early infarct on CT | Yes (1) | |
| Dense cerebral artery sign on CT | Yes (1) | |
| Age, y | >75 (1) | |
| NIHSS | ≥10 (1) | |
| SPAN‐100 Index 41 | Age+NIHSS | ≥100 (1) |
| GRASPS score 42 | Age, y | ≤60 (8), 61–70 (11), 71–80 (15), >80 (17) |
| NIHSS | 0–5 (20), 6–10 (27), 11–15 (34), 16–20 (40), >20 (42) | |
| Glucose | <100 (2), 100–149 (6), ≥150 (8) | |
| Systolic blood pressure | <120 (10), 120–149 (14), 150–179 (18), ≥180 (21) | |
| Race | Asian (9), non‐Asian (0) | |
| Sex | Male (4), female (0) |
CT indicates computed tomography; GRASPS, Glucose, Race, Age, Sex, Pressure, Stroke Severity; HAT, Hemorrhage After Thrombolysis; MCA, middle cerebral artery; NIHSS, National Institutes of Health Stroke Scale; SEDAN, Blood Sugar, Early Infarct Signs, and Hyperdense Cerebral Artery Sign, Age, and National Institutes of Health Stroke Scale; SITS‐SICH, Safe Implementation of Treatment in Stroke‐Symptomatic Intracerebral Hemorrhage; SPAN‐100, Stroke Prognostication Using Age and National Institutes of Health Stroke Scale‐100 Index; and THRIVE, the Totaled Health Risks in Vascular Events.
Measurement of Clinical Outcomes
The primary objective was to determine differences in FFO rates at 90 days and symptomatic intracranial hemorrhage (SICH) after IVT within 24 to 36 hours between the AF and non‐AF groups. Two standards of favorable functional status 43 were applied: (1) FFO was defined as a modified Rankin Scale (mRS) score of 0 to 1, and (2) functional independence (FI) was defined as an mRS score of 0 to 2. Three standards of SICH within 24 to 36 hours were used: (1) the NINDS standard, 28 in which any intracranial hemorrhage occurred with the deterioration of the NIHSS score to ≥1 or death; (2) the ECASS II standard, 44 in which any intracranial hemorrhage occurred with the deterioration of the NIHSS score to ≥4 or death; and (3) the SITS‐MOST standard, 35 in which a type 2 parenchymal hemorrhage occurred (a local or remote parenchymal intracranial hemorrhage exceeding 30% of the infarct) with the deterioration of the NIHSS score to ≥4 or death within 36 hours. The secondary objective was to examine clinical outcomes in the subgroups of patients with stroke treated with IVT using low‐ and standard‐dose alteplase.
Statistical Analysis
The Student t test and Pearson χ2 test were performed to examine differences in continuous and categorical variables, respectively, between the AF and non‐AF groups. Univariate multiple Poisson regression models were used to determine the relative risks (RRs) and their 95% CIs. In these models, the study outcomes of interest were considered as dependent variables. AF versus non‐AF was included as the independent variable, and the THRIVE score 20 or other stroke risk‐scoring tools, prestroke mRS, early ischemic changes on brain CT, and use of antithrombotic medications were included as fixed‐effects covariates. Thirty hospitals were included as a random‐effects factor. The heterogeneity of the association between the different doses of IVT was estimated by adding an interaction term to the model. We performed a separate validation study to confirm the primary and secondary objectives by using stroke risk‐scoring tools, namely the HAT score, 37 SITS‐SICH score, 38 the Cucchiara score, 39 the SEDAN score, 40 the SPAN‐100 Index, 41 and the GRASPS score. 42 Statistical significance was defined as P<0.05. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Sensitivity Analysis
We performed data imputation for the baseline and outcome variables with loss to follow‐up at 90 days and used the inverse probability of treatment weighting (IPTW) to reduce selection bias in observational studies. 45 , 46 For data imputation, we used fully conditional specification multiple imputation that is performed on a variable‐by‐variable basis by using a set of conditional densities (with linear regression for continuous variables and logistic regression for categorical variables). The number of multiple imputations was determined using the quadratic rule with the formula M=1+df×γ2, 47 , 48 where M was the number of imputations, df was the desired degrees of freedom, and γ was the fraction of missing information. The standardized mean difference was determined to investigate whether characteristics were balanced between AF and non‐AF groups, and an absolute difference of a standardized mean difference of <0.1 was defined as a balanced status. 45 , 46 Three sensitivity analyses were conducted: (1) primary objectives were determined between the AF and non‐AF groups, (2) a subgroup analysis of different alteplase doses (0.6 versus 0.9 mg/kg) was performed between the AF and non‐AF groups, and (3) a subgroup analysis for different alteplase doses (0.6 versus 0.9 mg/kg) was performed after adjustment for scores determined using different risk‐scoring tools. Patients who received alteplase doses of 0.7 and 0.8 mg/kg were excluded from the subgroup analysis. Multiple imputation and IPTW were performed using SAS 9.4 (SAS Institute).
RESULTS
Baseline Demographic Characteristics
From December 1, 2004 to December 31, 2016, there were 2351 patients with acute ischemic stroke (1371 without AF and 980 with AF) who completed IVT treatment and were enrolled in this study (Table 2). The study flowchart is presented in Figure 1. The mean ages, proportions of female patients, and mean dosages of alteplase were 71.7±11.9 and 66.4±13.0 years (P<0.0001), 41.8% and 33.2% (P<0.0001), and 0.78±0.14 and 0.81±0.13 mg/kg (P<0.0001) in the AF and non‐AF groups, respectively. No significant difference in the mean arterial pressure was observed between the AF and non‐AF groups. The proportions of prestroke mRS of 0 to 2 was similar in the AF and non‐AF groups (56.8% and 55.8%, P=0.6285), and a higher proportion of the patients in the non‐AF group than in the AF group had mild stroke (NIHSS score ≤10). The AF group had higher THRIVE scores than the non‐AF group (4.1±1.8 versus 3.3±1.8, P<0.0001). Moreover, a higher proportion of the patients in the AF group than in the non‐AF group exhibited early ischemic changes (15.3% versus 11.5%, P=0.0062), and received warfarin before stroke (4.4% versus 0.8%, P<0.0001). Furthermore, 682 (29.0%), 738 (31.4%), 550 (23.3%), and 381 (16.2%) patients received 0.9, 0.8, 0.7, and 0.6 mg/kg of alteplase, respectively.
Table 2.
Baseline Characteristics of Patients With and Without AF (N=2351)
| Characteristics | Non‐AF, n=1371 | AF, n=980 | P value |
|---|---|---|---|
| Age, y | 66.4±13.0 | 71.7±11.9 | <0.0001 |
| Age groups, % | <0.0001 | ||
| <60 y | 29.7% (407/1371) | 17.0% (167/980) | |
| 60–79 y | 54.9% (752/1371) | 54.8% (537/980) | |
| ≥80 y | 15.5% (212/1371) | 28.2% (276/980) | |
| Female sex, % | 33.2% (456/1371) | 41.8% (410/980) | <0.0001† |
| Medical history, % | |||
| Hypertension | 69.3% (946/1365) | 74.8% (733/980) | 0.0036 |
| Diabetes | 33.5% (457/1365) | 30.4% (298/980) | 0.1164 |
| Hyperlipidemia | 37.9% (520/1371) | 31.5% (309/980) | 0.0014† |
| Coronary artery disease | 11.4% (156/1365) | 17.0% (167/980) | 0.0001† |
| Alcoholism | 8.1% (110/1365) | 6.9% (68/980) | 0.3126 |
| Prestroke mRS | 0.6285 | ||
| mRS of 0–2 | 56.8% (779/1371) | 55.8% (547/980) | |
| mRS of 3–5 | 43.2% (592/1371) | 44.2% (433/980) | |
| CT scan of brain at baseline | 0.0062† | ||
| Normal | 88.5% (1214/1371) | 84.7% (830/980) | |
| Early ischemic changes | 11.5% (157/1371) | 15.3% (150/980) | |
| Stroke severity, % * | <0.0001† | ||
| Mild, NIHSS of ≤10 | 45.2% (620/1371) | 28.8% (282/980) | |
| Moderate, NIHSS of 11–20 | 39.5% (542/1371) | 45.8% (449/980) | |
| High, NIHSS of ≥21 | 15.2% (209/1371) | 25.4% (249/980) | |
| Alteplase dose, mg/kg | 0.81±0.13 | 0.78±0.14 | <0.0001† |
| Groups of alteplase dosage, % | <0.0001† | ||
| Standard dose, 0.9 mg/kg | 32.7% (448/1371) | 23.9% (234/980) | |
| Low dose, 0.6–0.8 mg/kg | 67.3% (923/1371) | 76.1% (746/980) | |
| MAP on arrival, mm Hg | 114.0±21.2 | 113.6±20.6 | 0.5843 |
| Time to treatment, min | 116.8±60.0 | 135.1±45.0 | <0.0001 |
| Fasting glucose, mg/dL | 155.0±81.5 | 145.4±86.3 | 0.0038† |
| THRIVE score | 3.3±1.8 | 4.1±1.8 | <0.0001† |
| Groups of THRIVE score, % | <0.0001† | ||
| 0–2 | 38.0% (521/1371) | 20.8% (204/980) | |
| 3–5 | 48.4% (663/1371) | 55.9% (548/980) | |
| 6–9 | 13.6% (187/1371) | 23.3% (228/980) | |
| Pre‐stroke antithrombotic medications | |||
| Aspirin | 10.9% (149/1371) | 11.0% (108/980) | 0.9070 |
| P2Y12 inhibitors | 1.8% (25/1371) | 1.9% (19/980) | 0.8388 |
| Dual antiplatelet | 1.3% (18/1371) | 1.8% (17/980) | 0.4051 |
| Warfarin | 0.8% (11/1371) | 4.4% (43/980) | <0.0001† |
Continuous variables are expressed as mean±standard deviation. AF indicates atrial fibrillation; CT, computed tomography; mRS, modified Rankin scale; and NIHSS, National Institutes of Health Stroke Scale.
Statistically significant at P<0.05.
The cutoff interval for the baseline NIHSS score was in accordance with the THRIVE (Totaled Health Risks in Vascular Events) score classification.
Figure 1. Flow diagram of the study.
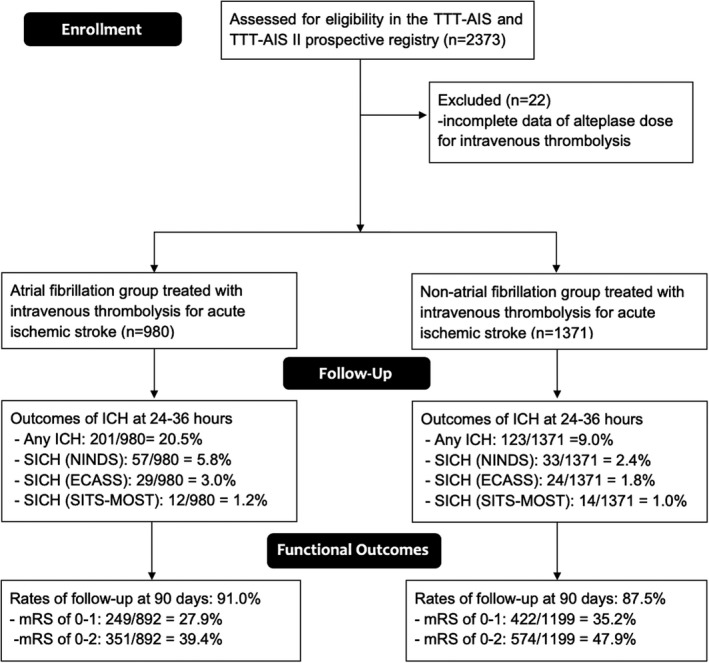
ECASS indicates European Cooperative Acute Stroke Study; ICH, intracranial hemorrhage; mRS, modified Rankin Scale; NINDS, National Institute of Neurological Disorders and Stroke; SICH, symptomatic intracranial hemorrhage; SITS‐MOST, Safe Implementation of Thrombolysis in Stroke‐Monitoring Study; and TTT‐AIS, Taiwan Thrombolytic Therapy for Acute Ischemic Stroke.
Primary Objectives (AF Versus Non‐AF Group)
The primary objectives are listed in Table 3, and the functional outcome distribution in terms the mRS score at 90 days is illustrated in Figure 2. The AF group exhibited decreased FFO (RR, 0.90 [95% CI, 0.81–0.99]; P=0.0458) and FI (RR, 0.86 [95% CI, 0.77–0.96]; P=0.0099). After adjustment for the THRIVE score, no significant differences in both FFO (adjusted RR, 0.99 [95% CI, 0.89–1.11]; P=0.8645) and FI (adjusted RR, 1.01 [95% CI, 0.89–1.13]; P=0.9164) were observed between the AF and non‐AF groups. The 90‐day mortality rate did not significantly differ between the 2 groups. In addition, the AF group had a 2‐fold increased SICH rate determined according to the NINDS standard than did the non‐AF group (RR, 2.41 [95% CI, 1.57–3.71]; P<0.0001; adjusted RR, 2.14 [95% CI, 1.36–3.37]; P=0.0010). However, SICH rates determined according to ECASS II and SITS‐MOST standards did not significantly differ between the 2 groups. The relationship between the THRIVE score and clinical outcomes is presented in Figure 3.
Table 3.
Analysis of the Risk of Unfavorable Functional Outcomes and SICH With AF
| Characteristics | AF | Non‐AF | RR (95% CI) | P value | Adjusted RR (95% CI)† | P value |
|---|---|---|---|---|---|---|
| Functional outcome of mRS on day 90 | ||||||
| 0: No symptoms | 13.3% (119/892) | 17.9% (215/1199) | ||||
| 1: No substantive disability | 14.6% (130/892) | 17.3% (207/1199) | ||||
| 2: Slight disability | 11.4% (102/892) | 12.7% (152/1199) | ||||
| 3: Moderate disability | 11.8% (105/892) | 15.5% (186/1199) | ||||
| 4: Moderate to severe disability | 18.4% (164/892) | 16.4% (196/1199) | ||||
| 5: Severe disability | 20.1% (179/892) | 11.4% (137/1199) | ||||
| 6: Death | 10.4% (93/892) | 8.8% (106/1199) | 1.18 (0.89–1.56) | 0.2457 | 0.99 (0.73–1.33) | 0.9258 |
| Favorable outcomes at 90 d | ||||||
| mRS of 0–1, FFO | 27.9% (249/892) | 35.2% (422/1199) | 0.90 (0.81–0.99) | 0.0458 * | 0.99 (0.89–1.11) | 0.8645 |
| mRS of 0–2, FI | 39.4% (351/892) | 47.9% (574/1199) | 0.86 (0.77–0.96) | 0.0099 * | 1.01 (0.89–1.13) | 0.9164 |
| SICH at 24–36 h | ||||||
| By NINDS standard | 5.8% (57/980) | 2.4% (33/1371) | 2.41 (1.57–3.71) | <0.0001 * | 2.14 (1.36–3.37) | 0.0010 * |
| By ECASS II standard | 3.0% (29/980) | 1.8% (24/1371) | 1.69 (0.98–2.90) | 0.0571 | 1.43 (0.81–2.54) | 0.2145 |
| By SITS‐MOST standard | 1.2% (12/980) | 1.0% (14/1371) | 1.19 (0.55–2.59) | 0.6444 | 0.99 (0.44–2.20) | 0.9726 |
AF indicates atrial fibrillation; ECASS II, European Cooperative Acute Stroke Study II; FFO, favorable functional outcome; FI, functional independence; mRS, modified Rankin Scale; NINDS, National Institute of Neurological Disorders and Stroke; RR, relative risk; SICH, symptomatic intracranial hemorrhage; and SITS‐MOST, Safe Implementation of Thrombolysis in Stroke‐Monitoring Study.
Multivariable Poisson regression was adjusted for fixed effects of the THRIVE (Totaled Health Risks in Vascular Events) score, prestroke mRS, early ischemic changes, and prestroke antithrombotic medications, and random effects for 30 hospitals.
Statistically significant at P<0.05.
Figure 2. Distribution of functional outcomes at 90 days.
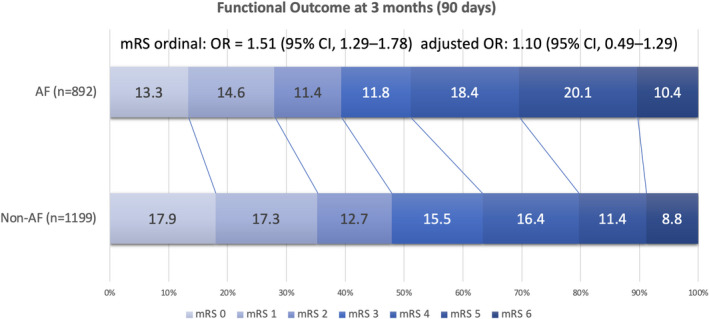
The data set shows no differences in functional outcomes between the atrial fibrillation (AF) and non‐AF groups after adjustment for the THRIVE (Totaled Health Risks in Vascular Events) score (adjusted odds ratio [OR], 1.10 [95% CI, 0.49–1.29]). mRS indicates modified Rankin Scale.
Figure 3. The relationship between the THRIVE (Totaled Health Risks in Vascular Events) score and clinical outcomes.
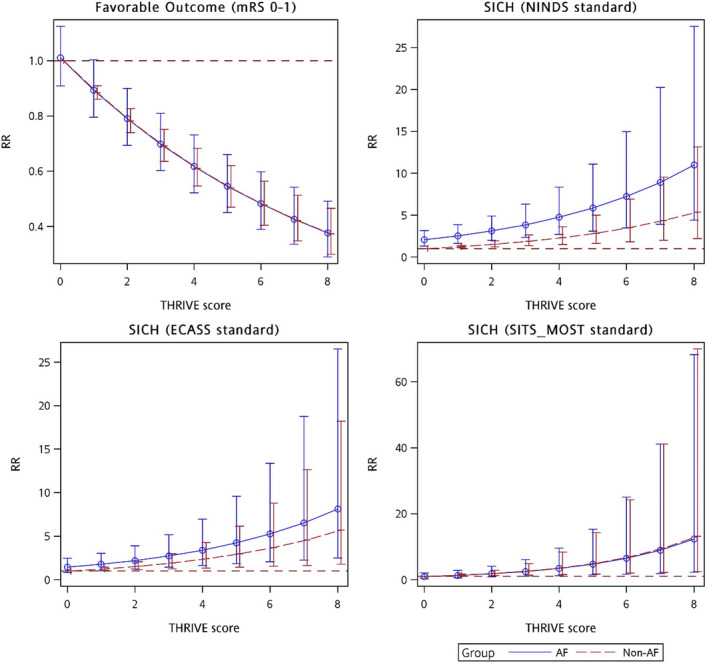
AF indicates atrial fibrillation; ECASS, European Cooperative Acute Stroke Study; mRS, modified Rankin Scale; NINDS, National Institute of Neurological Disorders and Stroke; SICH, symptomatic intracranial hemorrhage; RR, relative risk; and SITS‐MOST, Safe Implementation of Thrombolysis in Stroke‐Monitoring Study.
Secondary Objectives (AF Versus Non‐AF Group With Different Doses of IVT)
Among the enrolled patients, 1669 and 682 patients with acute ischemic stroke were treated with low‐ and standard‐dose alteplase, respectively (Table 4). The functional outcome distribution at 90 days for the different doses of alteplase subgroups is presented in Figure 4. For the patients receiving the standard dose, the good outcome status at 90 days and SICH rates did not significantly differ between the AF and non‐AF groups. For the patients receiving a low dose, the AF group exhibited a >2‐fold increased risk of SICH according to the NINDS standard (adjusted RR, 2.87 [95% CI, 1.63–5.04]; P=0.0003) compared with the non‐AF group. The interaction terms between different doses of alteplase were significant for SICH according to the NINDS standard (P=0.0047). However, no significant differences in the good outcome status at 90 days and SICH rates according to ECASS II and SITS‐MOST standards were observed between the AF and non‐AF groups. The relationship between the THRIVE score and clinical outcomes for the AF group receiving different doses of alteplase is presented in Figure 5.
Table 4.
Subgroup Analysis by Different Doses of Tissue Plasminogen Activator
| Characteristics | AF | Non‐AF | RR (95% CI) | P value | Adjusted RR (95% CI)† | P value | P value (interaction) ‡ |
|---|---|---|---|---|---|---|---|
| Low‐dose subgroup, n=1669 | |||||||
| Favorable outcomes at 90 d | |||||||
| mRS of 0–1 | 26.0% (177/680) | 34.1% (274/804) | 0.89 (0.79–1.01) | 0.0642 | 0.97 (0.86–1.10) | 0.6436 | 0.3445 |
| mRS of 0–2 | 37.1% (252/680) | 47.6% (383/804) | 0.83 (0.73–0.95) | 0.0074 * | 0.95 (0.83–1.09) | 0.4827 | 0.7323 |
| SICH at 24–36 h | |||||||
| By NINDS standard | 6.2% (46/746) | 2.0% (18/923) | 3.17 (1.83–5.45) | <0.0001 | 2.87 (1.63–5.04) | 0.0003 * | 0.0047 * |
| By ECASS II standard | 3.1% (23/746) | 1.7% (16/923) | 1.78 (0.94–3.37) | 0.0769 | 1.61 (0.83–3.12) | 0.1618 | 0.3625 |
| By SITS‐MOST standard | 1.2% (9/746) | 0.9% (9/923) | 1.24 (0.49–3.12) | 0.6515 | 1.05 (0.40–2.75) | 0.9140 | 0.6415 |
| Standard‐dose subgroup, n=682 | |||||||
| Favorable outcomes at 90 d | |||||||
| mRS of 0–1 | 34.0% (72/212) | 37.4% (148/395) | 0.95 (0.77–1.17) | 0.6061 | 1.05 (0.85–1.30) | 0.6467 | |
| mRS of 0–2 | 46.7% (99/212) | 48.4% (191/395) | 0.97 (0.77–1.22) | 0.7878 | 1.15 (0.91–1.46) | 0.2469 | |
| SICH at 24–36 h | |||||||
| By NINDS standard | 4.7% (11/234) | 3.3% (15/448) | 1.40 (0.64–3.06) | 0.3927 | 1.26 (0.54–2.93) | 0.5956 | |
| By ECASS II standard | 2.6% (6/234) | 1.8% (8/448) | 1.44 (0.50–4.13) | 0.5029 | 1.16 (0.37–3.65) | 0.8005 | |
| By SITS‐MOST standard | 1.3% (3/234) | 1.1% (5/448) | 1.15 (0.27–4.81) | 0.8494 | 0.90 (0.19–4.32) | 0.8938 | |
AF indicates atrial fibrillation; ECASS II, European Cooperative Acute Stroke Study II; mRS, modified Rankin Scale; NINDS, National Institute of Neurological Disorders and Stroke; RR, relative risk; SICH, symptomatic intracranial hemorrhage; and SITS‐MOST, Safe Implementation of Thrombolysis in Stroke‐Monitoring Study.
Multivariable Poisson regression was adjusted for fixed effects of the Totaled Health Risks in Vascular Events score, prestroke mRS, early ischemic changes, and prestroke antithrombotic medications, and random effects for 30 hospitals.
The interaction term was between AF status and alteplase dose.
P<0.05.
Figure 4. The distribution of functional outcomes at 90 days for subgroups receiving different doses of alteplase subgroups.
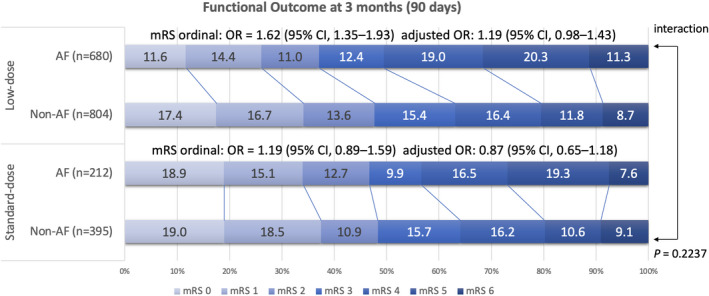
The data set shows no significant differences in functional outcomes between the atrial fibrillation (AF) and non‐AF groups after adjustment for the THRIVE (Totaled Health Risks in Vascular Events) score in the low‐dose subgroup (adjusted odds ratio [OR], 1.19 [95% CI, 0.98–1.43]) and standard‐dose subgroup (adjusted OR, 0.87 [95% CI, 0.65–1.18]), and no interaction was observed between different doses of alteplase (P=0.2237). mRS indicates modified Rankin Scale.
Figure 5. The relationship between the THRIVEs (Totaled Health Risks in Vascular Event) score and clinical outcomes for the atrial fibrillation group treated with different doses of alteplase.
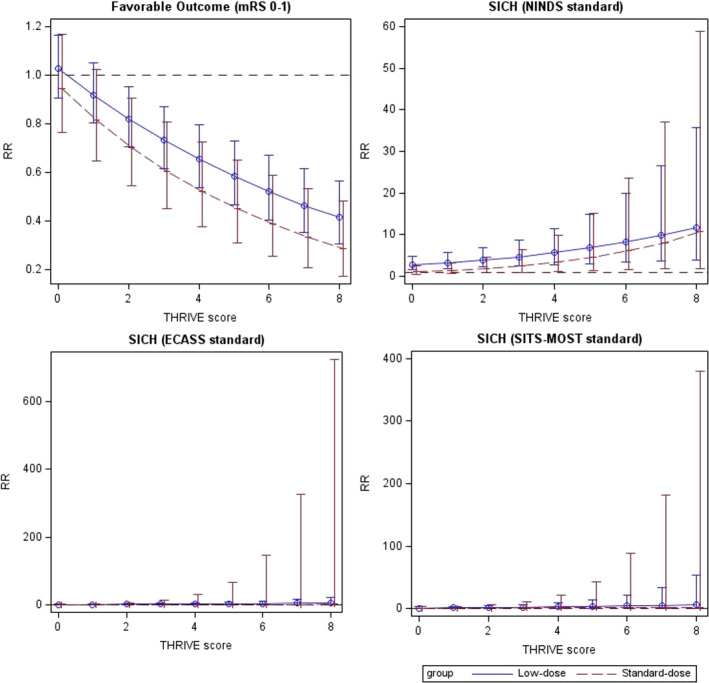
The zero‐inflated Poisson regression models were used to plot the relationship between the THRIVE score and symptomatic intracranial hemorrhage (SICH) by the ECASS II (European Cooperative Acute Stroke Study II) and the SITS‐MOST (Safe Implementation of Thrombolysis in Stroke‐Monitoring Study) standards. mRS indicates modified Rankin Scale; NINDS, National Institute of Neurological Disorders and Stroke; and RR, relative risk.
Validation Study (Adjustment for Different Risk‐Scoring Tools)
To substantiate our findings, we verified the primary and secondary objectives by using different stroke risk‐scoring tools (Table 5). Similar to the findings based on the THRIVE score, the low‐dose subgroup exhibited an increased risk of SICH according to the NINDS standard after adjustment for the HAT score (adjusted RR, 2.92 [95% CI, 1.66–5.13]; P=0.0001), SITS‐SICH score (adjusted RR, 2.83 [95% CI, 1.62–4.95]; P=0.0002), Cucchiara score (adjusted RR, 2.86 [95% CI, 1.63–5.04]; P=0.0001), SEDAN score (adjusted RR, 2.94 [95% CI, 1.68–5.16]; P=0.0001), SPAN‐100 Index (adjusted RR, 2.90 [95% CI, 1.64–5.11]; P=0.0001), and GRASPS score (adjusted RR, 2.56 [95% CI, 1.46–4.49]; P=0.0010). However, the standard‐dose subgroup demonstrated no difference in all clinical outcomes after adjustment for scores determined using any risk‐scoring tools. In addition, characteristics between the low‐dose and standard‐dose subgroups were analyzed for age (69.1±12.6 versus 67.4±13.2 years, P=0.0030), proportion of the female patients (37.3% versus 35.8%, P=0.4964), and baseline NIHSS scores (13.9±6.7 versus 13.8±7.9, P=0.6721), and indicated high similarity between the low‐dose and standard‐dose subgroups. The distribution of each risk‐scoring system for the AF and non‐AF groups is presented in Figure 6.
Table 5.
Validation Study With the Different Risk‐Scoring Models
| Adjustment model | Adjusted RR ‡ (95% CI) for AF vs non‐AF Groups | |||||
|---|---|---|---|---|---|---|
| HAT score | SITS‐SICH score | Cucchiara score | SEDAN score | SPAN‐100 | GRASPS score | |
| Low‐dose subgroup, n=1669 | ||||||
| Favorable outcomes at 90 d | ||||||
| mRS of 0–1 | 0.94 (0.83–1.07) | 0.95 (0.84–1.07) | 0.95 (0.84–1.08) | 0.94 (0.83–1.07) | 0.96 (0.84–1.08) | 0.99 (0.87–1.12) |
| mRS of 0–2 | 0.91 (0.79–1.04) | 0.91 (0.80–1.05) | 0.93 (0.81–1.07) | 0.90 (0.79–1.04) | 0.92 (0.80–1.06) | 0.98 (0.86–1.13) |
| SICH at 24–36 h | ||||||
| By NINDS standard | 2.92 (1.66–5.13)* | 2.83 (1.62–4.95)* | 2.86 (1.63–5.04)* | 2.94 (1.68–5.16)* | 2.90 (1.64–5.11)* | 2.56 (1.46–4.49) † |
| By ECASS II standard | 1.55 (0.80–3.00) | 1.49 (0.77–2.86) | 1.50 (0.77–2.91) | 1.54 (0.80–2.96) | 1.54 (0.79–3.01) | 1.35 (0.70–2.61) |
| By SITS‐MOST standard | 1.08 (0.41–2.80) | 1.08 (0.42–2.79) | 1.08 (0.41–2.81) | 1.13 (0.44–2.93) | 1.05 (0.40–2.74) | 0.91 (0.35–2.34) |
| Standard‐dose subgroup, n=682 | ||||||
| Favorable outcomes at 90 d | ||||||
| mRS of 0–1 | 0.98 (0.79–1.21) | 1.00 (0.81–1.23) | 0.99 (0.80–1.23) | 0.99 (0.80–1.22) | 0.98 (0.79–1.21) | 1.04 (0.84–1.29) |
| mRS of 0–2 | 1.04 (0.82–1.32) | 1.06 (0.84–1.35) | 1.06 (0.84–1.34) | 1.05 (0.83–1.32) | 1.03 (0.81–1.30) | 1.14 (0.90–1.44) |
| SICH at 24–36 h | ||||||
| By NINDS standard | 1.38 (0.61–3.16) | 1.42 (0.62–3.22) | 1.37 (0.59–3.16) | 1.46 (0.63–3.34) | 1.49 (0.65–3.41) | 1.23 (0.55–2.75) |
| By ECASS II standard | 1.32 (0.43–4.01) | 1.33 (0.44–4.03) | 1.39 (0.45–4.29) | 1.28 (0.42–3.91) | 1.52 (0.50–4.58) | 1.18 (0.39–3.51) |
| By SITS‐MOST standard | 1.04 (0.22–4.88) | 1.07 (0.23–5.01) | 0.96 (0.20–4.64) | 1.06 (0.23–4.93) | 1.33 (0.30–5.86) | 0.89 (0.21–3.87) |
AF indicates atrial fibrillation; ECASS II, European Cooperative Acute Stroke Study II; GRASPS, Glucose, Race, Age, Sex, Pressure, Stroke Severity; HAT, Hemorrhage After Thrombolysis; mRS, modified Rankin Scale; NINDS, National Institute of Neurological Disorders and Stroke; RR, relative risk; SEDAN, Blood Sugar, Early Infarct Signs and Hyperdense Cerebral Artery Sign, Age, and the National Institutes of Health Stroke Scale; SICH, symptomatic intracranial hemorrhage; SITS‐MOST, Safe Implementation of Thrombolysis in Stroke‐Monitoring Study; SITS‐SICH, Safe Implementation of Treatment in Stroke‐Symptomatic Intracerebral Hemorrhage; and SPAN‐100, Stroke Prognostication Using Age and National Institutes of Health Stroke Scale‐100 Index.
Statistically significant at P<0.001.
Statistically significant at P<0.05.
Models were adjusted for fixed effects of each scoring tools, prestroke mRS, early ischemic changes, and prestroke antithrombotic medications, and random effects for 30 hospitals.
Figure 6. The distribution of each risk‐scoring system for atrial fibrillation (AF) and non‐AF groups.
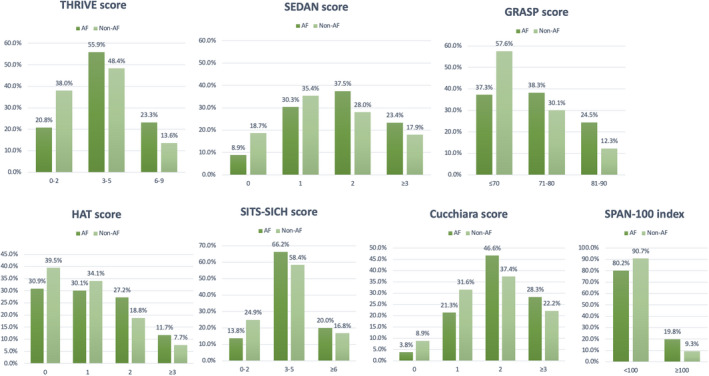
ECASS II indicates European Cooperative Acute Stroke Study II; GRASP, Glucose, Race, Age, Sex, Pressure, Stroke Severity; HAT, Hemorrhage After Thrombolysis; SEDAN, the Blood Sugar, Early Infarct Signs and Hyperdense Cerebral Artery Sign, Age, and National Institutes of Health Stroke Scale; SITS‐SICH, the Safe Implementation of Treatment in Stroke‐Symptomatic Intracerebral Hemorrhage; SPAN‐100, Stroke Prognostication Using Age and National Institutes of Health Stroke Scale‐100 Index; and THRIVE, Totaled Health Risks in Vascular Events.
Sensitivity Analysis
The number of imputations was 30 after applying the quadratic rule. Before imputation and IPTW, the characteristics at baseline between the AF and non‐AF groups were nearly the same as those of the original data set (Table 6). After IPTW, age, sex, medical comorbidities (ie, hypertension, diabetes, hyperlipidemia, coronary artery disease, and alcoholism), prestroke mRS score, brain CT findings at baseline, NIHSS score on arrival, alteplase dose, mean arterial pressure, time to treatment, THRIVE score, and use of antithrombotic medications were balanced between the AF and non‐AF groups (standardized mean difference <0.1). After imputation and IPTW (Table 7), the primary objectives were comparable between the AF and non‐AF groups except for SICH according to the NINDS standard, which exhibited twice the risk in the AF group. In subgroup analysis, the patients with AF who received 0.6 mg/kg of alteplase exhibited a 2‐fold increased risk of SICH according to the NINDS standard after adjustment for the THRIVE score (Table 8) and scores obtained using other stroke risk‐scoring tools (Table 9). However, in both AF and non‐AF groups, comparable outcomes were observed between the low‐dose (0.6 mg/kg) and standard‐dose (0.9 mg/kg) subgroups.
Table 6.
Characteristics of the Data Set After Data Imputation and IPTW (N=2351)
| Characteristics | Before IPTW | SMD | After IPTW | SMD | ||
|---|---|---|---|---|---|---|
| Non‐AF, N=1371 | AF, N=980 | Non‐AF, N=1371 | AF, N=980 | |||
| Age, y | 66.4±13.0 | 71.7±11.9 | 0.4263 | 68.7±12.9 | 68.8±12.4 | 0.0067 |
| Female sex, % | 33.2% (456/1371) | 41.8% (410/980) | 0.1778 | 36.4% (499/1371) | 35.9% (352/980) | −0.0100 |
| Medical history, % | ||||||
| Hypertension | 69.3% (950/1371) | 74.8% (733/980) | −0.1229 | 71.8% (984/1371) | 73.0% (715/980) | −0.0270 |
| Diabetes | 33.5% (459/1371) | 30.4% (298/980) | 0.0659 | 31.9% (437/1371) | 32.3% (317/980) | −0.0075 |
| Hyperlipidemia | 37.9% (520/1371) | 31.5% (309/980) | 0.1347 | 35.0% (480/1371) | 36.0% (353/980) | −0.0209 |
| Coronary artery disease | 11.5% (157/1365) | 17.0% (167/980) | −0.1582 | 14.1% (193/1371) | 14.0% (137/980) | 0.0012 |
| Alcoholism | 8.1% (111/1371) | 6.9% (68/980) | 0.0439 | 7.9% (108/1371) | 7.8% (76/980) | −0.0035 |
| Prestroke mRS | 0.0202 | −0.0251 | ||||
| mRS of 0–2 | 56.6% (776/1371) | 55.4% (543/980) | 51.7% (709/1371) | 52.9% (518/980) | ||
| mRS of 3–5 | 43.2% (592/1371) | 44.2% (433/980) | 48.3% (662/1371) | 47.1% (462/980) | ||
| CT scan of brain at baseline | 0.1134 | −0.0002 | ||||
| Normal | 88.5% (1214/1371) | 84.7% (830/980) | 86.3% (1183/1371) | 86.4% (847/980) | ||
| Early ischemic changes | 11.5% (157/1371) | 15.3% (150/980) | 13.7% (188/1371) | 13.6% (133/980) | ||
| Stroke severity, % | 0.3468 | 0.0622 | ||||
| Mild, NIHSS of ≤10 | 45.2% (620/1371) | 28.8% (282/980) | 26.7% (366/1371) | 25.2% (247/980) | ||
| Moderate, NIHSS of 11–20 | 39.5% (542/1371) | 45.8% (449/980) | 29.2% (400/1371) | 27.8% (272/980) | ||
| High, NIHSS of ≥21 | 15.2% (209/1371) | 25.4% (249/980) | 44.1% (605/1371) | 47.0% (461/980) | ||
| Alteplase dose, mg/kg | 0.81±0.13 | 0.78±0.14 | −0.2295 | 0.80±0.14 | 0.80±0.14 | −0.0003 |
| Groups of alteplase dosage, % | 0.1963 | 0.0018 | ||||
| Standard dose, 0.9 mg/kg | 32.7% (448/1371) | 23.9% (234/980) | 29.0% (398/1371) | 28.9% (283/980) | ||
| Low dose, 0.6–0.8 mg/kg | 67.3% (923/1371) | 76.1% (746/980) | 71.0% (973/1371) | 71.1% (697/980) | ||
| MAP on arrival, mm Hg | 113.4±21.4 | 113.7±20.7 | 0.0167 | 112.9±21.5 | 113.6±20.5 | 0.0346 |
| Time to treatment, min | 116.8±60.0 | 135.1±45.0 | 0.3439 | 125.5±59.2 | 126.4±47.0 | 0.0177 |
| Fasting glucose, mg/dL | 150.9±81.2 | 144.2±67.6 | −0.0908 | 148.2±75.3 | 151.5±95.2 | 0.0440 |
| THRIVE score | 3.3±1.8 | 4.1±1.8 | 0.4446 | 3.6±1.9 | 3.6±1.8 | 0.0001 |
| Prestroke antithrombotic medications | ||||||
| Aspirin | 10.9% (149/1371) | 11.0% (108/980) | −0.0049 | 10.9% (149/1371) | 10.4% (102/980) | 0.0155 |
| P2Y12 inhibitors | 1.8% (25/1371) | 1.9% (19/980) | −0.0085 | 1.8% (25/1371) | 1.9% (19/980) | 0.0032 |
| Dual antiplatelet | 1.3% (18/1371) | 1.8% (17/980) | −0.0344 | 1.4% (19/1371) | 1.4% (14/980) | −0.0002 |
| Warfarin | 0.8% (11/1371) | 4.4% (43/980) | −0.2270 | 2.5% (34/1371) | 2.3% (23/980) | 0.0110 |
Continuous variables are expressed as mean±standard deviation. AF indicates atrial fibrillation; CT, computed tomography; IPTW, inverse probability of treatment weighting; MAP, mean arterial pressure; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SMD, standardized mean difference; and THRIVE, Totaled Health Risks in Vascular Events.
The number of imputations was 30.
Table 7.
Sensitivity Analysis I: The Risk of Unfavorable Functional Outcomes and SICH With AF
| Characteristics | AF | Non‐AF | After IPTW | |||
|---|---|---|---|---|---|---|
| RR (95% CI) | P value | Adjusted RR (95% CI)* | P value | |||
| Functional outcome of mRS at 90 d | ||||||
| 0: No symptoms | 13.1% (128/980) | 17.9% (245/1371) | ||||
| 1: No substantive disability | 14.0% (137/980) | 16.8% (230/1371) | ||||
| 2: Slight disability | 11.5% (113/980) | 12.0% (165/1371) | ||||
| 3: Moderate disability | 11.4% (112/980) | 15.7% (215/1371) | ||||
| 4: Moderate to severe disability | 19.5% (191/980) | 17.7% (243/1371) | ||||
| 5: Severe disability | 20.7% (203/980) | 11.9% (163/1371) | ||||
| 6: Death | 9.8% (96/980) | 8.0% (110/1371) | 1.12 (0.92–1.37) | 0.2618 | 0.99 (0.73–1.33) | 0.9258 |
| Favorable outcomes at 90 d | ||||||
| mRS of 0–1, FFO | 27.4% (268/980) | 34.9% (479/1371) | 1.03 (0.96–1.10) | 0.4244 | 0.97 (0.87–1.07) | 0.5077 |
| mRS of 0–2, FI | 38.5% (377/980) | 47.0% (644/1371) | 1.04 (0.96–1.12) | 0.3046 | 0.97 (0.87–1.09) | 0.6430 |
| SICH at 24–36 h | ||||||
| By NINDS standard | 5.8% (57/980) | 2.4% (33/1371) | 2.00 (1.47–2.74) | <0.0001 * | 2.14 (1.36–3.37) | 0.0010 * |
| By ECASS II standard | 3.0% (29/980) | 1.8% (24/1371) | 1.32 (0.89–1.95) | 0.1680 | 1.43 (0.81–2.54) | 0.2145 |
| By SITS‐MOST standard | 1.2% (12/980) | 1.0% (14/1371) | 0.90 (0.51–1.61) | 0.7331 | 0.99 (0.44–2.20) | 0.9726 |
AF indicates atrial fibrillation; ECASS II, European Cooperative Acute Stroke Study II; FFO, favorable functional outcome; FI, functional independence; IPTW, inverse probability of treatment weighting; mRS, modified Rankin Scale; NINDS, National Institute of Neurological Disorders and Stroke; RR, relative risk; SICH, symptomatic intracranial hemorrhage; and SITS‐MOST, Safe Implementation of Thrombolysis in Stroke‐Monitoring Study.
Multivariable Poisson regression was adjusted for fixed effects of the THRIVE (Totaled Health Risks in Vascular Events) score, prestroke mRS, early ischemic changes, and use of antithrombotic medications, and random effects for 30 hospitals.
Statistically significant at P<0.05.
Table 8.
Sensitivity Analysis II: Subgroup Analysis by Different Doses of Alteplase
| Characteristics | AF | Non‐AF | After IPTW | Interaction† | |||
|---|---|---|---|---|---|---|---|
| RR (95% CI) | P value | Adjusted RR ‡ (95% CI) | P value | ||||
| Low dose of 0.6 mg/kg subgroup, N=381 | |||||||
| mRS of 0–1 | 23.4% (44/188) | 34.2% (66/193) | 0.98 (0.83–1.16) | 0.8194 | 0.96 (0.81–1.14) | 0.6473 | 0.1829 |
| mRS of 0–2 | 33.5% (63/188) | 47.7% (92/193) | 0.91 (0.75–1.09) | 0.2954 | 0.91 (0.75–1.10) | 0.3359 | 0.1620 |
| SICH at 24–36 h | |||||||
| By NINDS standard | 5.9% (11/188) | 2.1% (4/193) | 2.36 (1.04–5.34) | 0.0400 * | 2.63 (1.10–6.32) | 0.0302 * | 0.0009 * |
| By ECASS II standard | 3.7% (7/188) | 1.6% (3/193) | 2.04 (0.76–5.47) | 0.1560 | 2.52 (0.86–7.43) | 0.0927 | 0.3505 |
| By SITS‐MOST standard | 1.6% (3/188) | 0.5% (1/193) | 4.37 (0.64–29.84) | 0.1323 | 4.49 (0.64–31.58) | 0.1313 | 0.8338 |
| Standard dose of 0.9 mg/kg subgroup, N=682 | |||||||
| mRS of 0–1 | 33.8% (79/234) | 37.1% (166/448) | 0.91 (0.79–1.04) | 0.1619 | 1.05 (0.91–1.20) | 0.5198 | |
| mRS of 0–2 | 46.2% (108/234) | 52.5% (213/448) | 0.85 (0.73–0.96) | 0.0315 * | 1.09 (0.94–1.27) | 0.2353 | |
| SICH at 24–36 h | |||||||
| By NINDS standard | 4.7% (11/234) | 3.3% (15/448) | 1.23 (0.71–2.11) | 0.4587 | 1.66 (0.92–3.01) | 0.0917 | |
| By ECASS II standard | 2.6% (6/234) | 1.8% (8/448) | 1.21 (0.57–2.58) | 0.6190 | 1.32 (0.57–3.05) | 0.5149 | |
| By SITS‐MOST standard | 1.3% (3/234) | 1.1% (5/448) | 1.07 (0.39–2.96) | 0.8892 | 1.42 (0.47–4.34) | 0.5359 | |
AF indicates atrial fibrillation; ECASS II, European Cooperative Acute Stroke Study II; IPTW, inverse probability of treatment weighting; mRS, modified Rankin Scale; NINDS, National Institute of Neurological Disorders and Stroke; RR, relative risk; SICH, symptomatic intracranial hemorrhage; and SITS‐MOST, Safe Implementation of Thrombolysis in Stroke‐Monitoring Study.
The interaction term was between AF status and alteplase dose.
Statistically significant at P<0.05.
Multivariable Poisson regression was adjusted for fixed effects of the THRIVE (Totaled Health Risks in Vascular Events) score, prestroke mRS, early ischemic changes, and use of antithrombotic medications, and random effects for 30 hospitals.
Table 9.
Sensitivity Analysis III: Different Risk‐Scoring Models
| Adjustment model | Adjusted RR (95% CI) for AF vs non‐AF groups after IPTW | |||||
|---|---|---|---|---|---|---|
| HAT score | SITS‐SICH score | Cucchiara score | SEDAN score | SPAN‐100 | GRASPS score | |
| Low dose of 0.6 mg/kg subgroup, N=381 | ||||||
| Favorable outcomes at 90 d | ||||||
| mRS of 0–1 | 0.96 (0.81–1.15) | 0.94 (0.79–1.11) | 0.94 (0.79–1.11) | 0.94 (0.80–1.12) | 0.93 (0.79–1.11) | 0.95 (0.80–1.13) |
| mRS of 0–2 | 0.93 (0.77–1.12) | 0.88 (0.72–1.06) | 0.88 (0.72–1.06) | 0.89 (0.73–1.07) | 0.88 (0.72–1.06) | 0.90 (0.74–1.08) |
| SICH at 24–36 h | ||||||
| By NINDS standard | 2.48 (1.03–5.95)* | 2.42 (1.01–5.79)* | 2.82 (1.15–6.90)* | 2.60 (1.08–6.25)* | 2.52 (1.06–5.98)* | 2.33 (0.97–5.63) |
| By ECASS II standard | 2.31 (0.78–6.83) | 2.10 (0.71–6.24) | 2.60 (0.86–7.84) | 2.35 (0.80–6.94) | 2.51 (0.86–7.33) | 2.18 (0.73–6.55) |
| By SITS‐MOST standard | 3.44 (0.46–25.90) | 4.04 (0.56–29.16) | 4.04 (0.56–29.16) | 4.27 (0.61–29.70) | 5.13 (0.74–35.72) | 4.13 (0.58–29.48) |
| Standard dose of 0.9 mg/kg subgroup, N=682 | ||||||
| Favorable outcomes at 90 d | ||||||
| mRS of 0–1 | 1.03 (0.90–1.18) | 1.06 (0.92–1.21) | 1.06 (0.92–1.21) | 1.06 (0.93–1.21) | 1.03 (0.90–1.18) | 1.06 (0.92–1.21) |
| mRS of 0–2 | 1.07 (0.93–1.25) | 1.15 (0.99–1.34) | 1.15 (0.99–1.34) | 1.12 (0.96–1.30) | 1.12 (0.95–1.30) | 1.15 (0.98–1.34) |
| SICH at 24–36 h | ||||||
| By NINDS standard | 1.25 (0.73–2.16) | 1.70 (0.94–3.07) | 1.65 (0.91–2.99) | 1.63 (0.90–2.95) | 1.78 (0.98–3.22) | 1.68 (0.93–3.01) |
| By ECASS II standard | 1.24 (0.58–2.64) | 1.24 (0.54–2.85) | 1.34 (0.58–3.10) | 1.17 (0.51–2.68) | 1.42 (0.62–3.28) | 1.38 (0.61–3.16) |
| By SITS‐MOST standard | 1.11 (0.40–3.09) | 1.13 (0.38–3.38) | 1.22 (0.39–3.84) | 1.15 (0.38–3.48) | 1.39 (0.46–4.20) | 1.47 (0.50–4.37) |
Models were adjusted for fixed effects of each scoring tools, prestroke mRS, early ischemic changes, and prestroke antithrombotic medications, and random effects for 30 hospitals. AF indicates atrial fibrillation; ECASS II, European Cooperative Acute Stroke Study II; GRASPS, Glucose, Race, Age, Sex, Pressure, Stroke Severity; HAT, Hemorrhage After Thrombolysis; IPTW, inverse probability of treatment weighting; mRS, modified Rankin Scale; NINDS, National Institute of Neurological Disorders and Stroke; RR, relative risk; SEDAN, the Blood Sugar, Early Infarct Signs and Hyperdense Cerebral Artery Sign, Age, and National Institutes of Health Stroke Scale; SICH, symptomatic intracranial hemorrhage; SITS‐MOST, Safe Implementation of Thrombolysis in Stroke‐Monitoring Study; SITS‐SICH, the Safe Implementation of Treatment in Stroke‐Symptomatic Intracerebral Hemorrhage; and SPAN‐100, Stroke Prognostication Using Age and National Institutes of Health Stroke Scale‐100 Index.
P<0.05.
DISCUSSION
To our knowledge, this is the first study to analyze clinical outcomes in patients with acute ischemic stroke with and without AF by using different SICH definitions, and evaluate the effects of different dosages of alteplase with respect to FFO at 90 days and SICH within 24 to 36 hours after IVT. The results revealed that the AF group had a higher risk of intracranial hemorrhage. However, clinical outcomes, FFO at 90 days, FI, and mortality were unaffected by AF. Compared with the non‐AF group, the AF group exhibited increased SICH rates only according to the NINDS standard 28 (with the deterioration of the NIHSS score to ≥1) and not ECASS II and SITS‐MOST standards (with the deterioration of NIHSS scores to ≥4). We observed an increased risk of SICH in the patients with stroke treated with low‐dose alteplase.
Our measurements for functional status are consistent with most studies, 49 , 50 , 51 , 52 , 53 , 54 , 55 reporting that patients with AF tended to have unfavorable functional outcomes at 90 days before adjustment for any covariates. Consistent with previous studies, 51 , 52 , 53 , 54 our results revealed that the trend of unfavorable outcomes in patients with AF was eliminated after adjustment for the baseline NIHSS scores and other demographic characteristics. By contrast, a recent meta‐analysis in 2021 12 reported that patients with AF had poorer functional outcomes at 90 days and higher SICH rates than patients without AF. However, this meta‐analysis 12 was limited by the statistical methods because the pooled odds ratios of each study in this meta‐analysis were obtained simply by calculating the binary outcome number between patients with and without AF (the event and nonevent number in a 2×2 table). Hence, the pooled odds ratios were unadjusted for baseline stroke severity and other characteristic differences between AF and non‐AF groups.
AF‐related thrombi are larger and more resistant to IVT as reported in previous studies, 56 , 57 and more than half of the patients with AF had failed recanalization. In the current study, only the low‐dose subgroup exhibited higher SICH rates according to the NINDS standard. Low‐dose alteplase might lead to the incomplete resolution of AF‐related thrombi, thus resulting in secondary hemorrhagic transformation; however, the secondary hemorrhagic transformation was not too severe to affect the FFO and FI at 90 days. SICH rates determined according to ECASS II and SITS‐MOST standards did not significantly differ between the AF and non‐AF groups. These data highlight the rationality of using standard‐dose alteplase for patients with AF and support the safety of standard‐dose alteplase in Asian patients with AF.
This study has many strengths. First, this study included a larger prospective cohort of patients with and without AF ever treated with IVT than that in previous studies. 49 , 50 , 51 , 52 , 53 , 54 , 55 Second, a solid definition for hemorrhagic transformation was adopted based on NINDS, ECASS II, and SITS‐MOST standards to differentiate between varying severity levels of SICH. Third, by replacing the THRIVE score with the HAT score, SITS‐SICH score, SEDAN score, Cucchiara score, SPAN‐100 Index, and GRASPS score, our study validated the robustness of the primary and secondary objectives. Fourth, we used a Poisson regression model instead of a logistic regression model because our primary analysis fulfilled the assumption of Poisson distribution 58 , 59 (counting of the rare event of SICH in a large cohort). Finally, this study provides real‐world data for patients with stroke treated with IVT using different dosages of alteplase. In Taiwan, most physicians prefer a low or standard dose of alteplase, and the ratio is ≈2.4:1 (sample size of low dose/standard dose=1669/682). Because we included enough patients in both the low‐ and standard‐dose subgroups, our results were stable and can be generalized to other Asian populations.
In the sensitivity analysis, we performed data imputation and IPTW to determine the robustness of our findings. The rates of follow‐up at 90 days were 91.0% and 87.5% in the AF and non‐AF groups, respectively. The statistical findings suggested that ≈10% of missing at random can be replaced with substituted values. 60 , 61 In our study, the probability of a missing value was supposed to be dependent on observed quantities (such as age, medical comorbidities, and stroke severity) but to be independent of unobserved data, which fulfilled the condition for missing at random. In general, the results of our sensitivity analysis were the same as those obtained before multiple imputation and IPTW. The patients in the AF group who received 0.6 mg/kg (N=381) exhibited a 2‐fold of significantly increased risk of SICH according to the NINDS standard after adjustment for the THRIVE score, SITS‐SICH score, Cucchiara score, SEDAN score, and SPAN‐100 Index but at borderline significance for the HAT and GRASPS scores. This borderline significance can be attributed to the inclusion of an insufficient sample size in the subgroup receiving 0.6 mg/kg of alteplase (N=381) compared with the low‐dose subgroup receiving 0.6 to 0.8 mg/kg of alteplase (N=1669).
Our study has some limitations. Because our study ended in 2016, additional analysis of clinical outcomes before treatment and after the addition of new oral anticoagulants could not be performed. In Taiwan, new oral anticoagulants (dabigatran, rivaroxaban, apixaban, and edoxaban) were approved between 2013 and 2016 62 ; therefore, most enrolled patients were not yet being treated with them. In addition, before 2016, second‐generation thrombectomy technique was not a standard treatment in Taiwan and was not covered by the national insurance programs. Finally, our results did not include the factor of thrombectomy. The ASPECTS (Alberta Stroke Programme Early CT Score) was not determined in our TTT‐AIS study; therefore, we did not adjust for it. However, our results were determined to be robust after adjustment for subtle early ischemic changes; this finding is consistent with that of an earlier study indicating the lack of clinical significance of subtle early ischemic changes for patients with stroke treated with alteplase. 31
In conclusion, this study examined the 90‐day clinical outcomes in patients having acute ischemic stroke with and without AF. Although the patients with AF receiving low‐dose alteplase had higher SICH rates according to the NINDS standard, the different dosages of alteplase exerted no overt effect on the functional status at 90 days in the AF and non‐AF groups. Thus, the adoption of a low‐dose alteplase simply because of AF is not recommended.
APPENDIX
The TTT‐AIS Study Group Investigators: Sheng‐Feng Lin, Chien‐Fu Chen, Han‐Hwa Hu, Bo‐Lin Ho, Chih‐Hung Chen, Lung Chan, Huey‐Juan Lin, Yu Sun, Yung‐Yang Lin, Po‐Lin Chen, Shinn‐Kuang Lin, Cheng‐Yu Wei, Yu‐Te Lin, Jiunn‐Tay Lee, A‐Ching Chao.
Sources of Funding
This study was supported by grants from Kaohsiung Medical University Hospital, Kaohsiung, Taiwan, reference number KMUH109‐9R72 and the Ministry of Science and Technology, Taiwan, reference number MOST‐108‐2314‐B‐037 ‐038 ‐MY3.
Disclosures
None.
Acknowledgments
Author Contributions: Each author contributed to the conception and design of the study, acquisition, analysis, and interpretation of data. S.‐F.L. wrote the first draft of the article.
For Sources of Funding and Disclosures, see page 16.
Contributor Information
Han‐Hwa Hu, Email: hanhwa@hotmail.com.
A‐Ching Chao, Email: achch@cc.kmu.edu.tw.
Taiwan Thrombolytic Therapy for Acute Ischemic Stroke Study Group:
Sheng‐Feng Lin, Chien‐Fu Chen, Han‐Hwa Hu, Bo‐Lin Ho, Chih‐Hung Chen, Lung Chan, Huey‐Juan Lin, Yu Sun, Yung‐Yang Lin, Po‐Lin Chen, Shinn‐Kuang Lin, Cheng‐Yu Wei, Yu‐Te Lin, Jiunn‐Tay Lee, and A‐Ching Chao
REFERENCES
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.STR.22.8.983 [DOI] [PubMed] [Google Scholar]
- 2. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. doi: 10.1093/eurheartj/eht280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chien KL, Su TC, Hsu HC, Chang WT, Chen PC, Chen MF, Lee YT. Atrial fibrillation prevalence, incidence and risk of stroke and all‐cause death among Chinese. Int J Cardiol. 2010;139:173–180. doi: 10.1016/j.ijcard.2008.10.045 [DOI] [PubMed] [Google Scholar]
- 4. Sposato LA, Cipriano LE, Saposnik G, Ruíz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta‐analysis. Lancet Neurol. 2015;14:377–387. doi: 10.1016/S1474-4422(15)70027-X [DOI] [PubMed] [Google Scholar]
- 5. Brachmann J, Morillo CA, Sanna T, Di Lazzaro V, Diener HC, Bernstein RA, Rymer M, Ziegler PD, Liu S, Passman RS. Uncovering atrial fibrillation beyond short‐term monitoring in cryptogenic stroke patients: three‐year results from the cryptogenic stroke and underlying atrial fibrillation trial. Circ Arrhythm Electrophysiol. 2016;9:e003333. doi: 10.1161/CIRCEP.115.003333 [DOI] [PubMed] [Google Scholar]
- 6. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 7. Wang KL, Wu CH, Huang CC, Wu TC, Naditch‐Brûlé L, Steg PG, Lin SJ, Chiang CE. Complexity of atrial fibrillation patients and management in Chinese ethnicity in routine daily practice: insights from the RealiseAF Taiwanese cohort. J Cardiol. 2014;64:211–217. doi: 10.1016/j.jjcc.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 8. Tsai L‐K, Lee I‐H, Chen Y‐L, Chao T‐F, Chen Y‐W, Po HL, Lien L‐M, Chu P‐H, Huang W‐C, Lin T‐H, et al. Diagnosis and treatment for embolic stroke of undetermined source: consensus statement from the Taiwan Stroke Society and Taiwan Society of Cardiology. J Formos Med Assoc. 2021;120:93–106. doi: 10.1016/j.jfma.2020.05.026 [DOI] [PubMed] [Google Scholar]
- 9. Tu HTH, Campbell BCV, Christensen S, Desmond PM, De Silva DA, Parsons MW, Churilov L, Lansberg MG, Mlynash M, Olivot J‐M, et al. Worse stroke outcome in atrial fibrillation is explained by more severe hypoperfusion, infarct growth, and hemorrhagic transformation. Int J Stroke. 2015;10:534–540. doi: 10.1111/ijs.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tu HTH, Campbell BCV, Christensen S, Collins M, De Silva DA, Butcher KS, Parsons MW, Desmond PM, Barber PA, Levi CR, et al. Pathophysiological determinants of worse stroke outcome in atrial fibrillation. Cerebrovasc Dis. 2010;30:389–395. doi: 10.1159/000316886 [DOI] [PubMed] [Google Scholar]
- 11. Yue R, Li D, Yu J, Li S, Ma Y, Huang S, Zeng Z, Zeng R, Sun X. Atrial fibrillation is associated with poor outcomes in thrombolyzed patients with acute ischemic stroke: a systematic review and meta‐analysis. Medicine (Baltimore). 2016;95:e3054. doi: 10.1097/MD.0000000000003054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu Y, Ji C. Efficacy and safety of thrombolysis for acute ischemic stroke with atrial fibrillation: a meta‐analysis. BMC Neurol. 2021;21:66. doi: 10.1186/s12883-021-02095-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chao TF, Liu CJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chen TJ, Lip GY, et al. Using the CHA2DS2‐VASc score for refining stroke risk stratification in 'low‐risk' Asian patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:1658–1665. doi: 10.1016/j.jacc.2014.06.1203 [DOI] [PubMed] [Google Scholar]
- 14. Nielsen PB, Chao TF. The risks of risk scores for stroke risk assessment in atrial fibrillation. Thromb Haemost. 2015;113:1170–1173. doi: 10.1160/TH15-03-0210 [DOI] [PubMed] [Google Scholar]
- 15. Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–315. doi: 10.1016/j.jacc.2007.01.098 [DOI] [PubMed] [Google Scholar]
- 16. Goto S, Zhu J, Liu L, Oh B‐H, Wojdyla DM, Aylward P, Bahit MC, Gersh BJ, Hanna M, Horowitz J, et al. Efficacy and safety of apixaban compared with warfarin for stroke prevention in patients with atrial fibrillation from East Asia: a subanalysis of the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Am Heart J. 2014;168:303–309. doi: 10.1016/j.ahj.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 17. Hori M, Connolly SJ, Zhu J, Liu LS, Lau C‐P, Pais P, Xavier D, Kim SS, Omar R, Dans AL, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non‐Asians with atrial fibrillation. Stroke. 2013;44:1891–1896. doi: 10.1161/STROKEAHA.113.000990 [DOI] [PubMed] [Google Scholar]
- 18. Wong KSL, Hu DY, Oomman A, Tan R‐S, Patel MR, Singer DE, Breithardt G, Mahaffey KW, Becker RC, Califf R, et al. Rivaroxaban for stroke prevention in East Asian patients from the ROCKET AF trial. Stroke. 2014;45:1739–1747. doi: 10.1161/STROKEAHA.113.002968 [DOI] [PubMed] [Google Scholar]
- 19. Flint AC, Cullen SP, Faigeles BS, Rao VA. Predicting long‐term outcome after endovascular stroke treatment: the totaled health risks in vascular events score. AJNR Am J Neuroradiol. 2010;31:1192–1196. doi: 10.3174/ajnr.A2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flint AC, Faigeles BS, Cullen SP, Kamel H, Rao VA, Gupta R, Smith WS, Bath PM, Donnan GA, Lees KR, et al. THRIVE score predicts ischemic stroke outcomes and thrombolytic hemorrhage risk in VISTA. Stroke. 2013;44:3365–3369. doi: 10.1161/STROKEAHA.113.002794 [DOI] [PubMed] [Google Scholar]
- 21. Kamel H, Patel N, Rao VA, Cullen SP, Faigeles BS, Smith WS, Flint AC. The totaled health risks in vascular events (THRIVE) score predicts ischemic stroke outcomes independent of thrombolytic therapy in the NINDS tPA trial. J Stroke Cerebrovasc Dis. 2013;22:1111–1116. doi: 10.1016/j.jstrokecerebrovasdis.2012.08.017 [DOI] [PubMed] [Google Scholar]
- 22. Chen W, Liu G, Fang J, Wang Y, Song Y, Pan Y, Li H, Liu L, Wang C, Wang DZ, et al. External validation of the totaled health risks in vascular events score to predict functional outcome and mortality in patients entered into the China National Stroke Registry. J Stroke Cerebrovasc Dis. 2016;25:2331–2337. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.021 [DOI] [PubMed] [Google Scholar]
- 23. Pan Y, Peng Y, Chen W, Wang Y, Lin YI, He Y, Wang N, Wang Y. THRIVE‐c score predicts clinical outcomes in Chinese patients after thrombolysis. Brain Behav. 2018;8:e00927. doi: 10.1002/brb3.927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamaguchi T, Mori E, Minematsu K, Nakagawara J, Hashi K, Saito I, Shinohara Y; Group JACTJ‐A . Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J‐ACT). Stroke. 2006;37:1810–1815. doi: 10.1161/01.STR.0000227191.01792.e3 [DOI] [PubMed] [Google Scholar]
- 25. Chao A‐C, Liu C‐K, Chen C‐H, Lin H‐J, Liu C‐H, Jeng J‐S, Hu C‐J, Chung C‐P, Hsu H‐Y, Sheng W‐Y, et al. Different doses of recombinant tissue‐type plasminogen activator for acute stroke in Chinese patients. Stroke. 2014;45:2359–2365. doi: 10.1161/STROKEAHA.114.005245 [DOI] [PubMed] [Google Scholar]
- 26. Chao AC, Hsu HY, Chung CP, Liu CH, Chen CH, Teng MM, Peng GS, Sheng WY, Hu HH; Group TTT‐AIS . Outcomes of thrombolytic therapy for acute ischemic stroke in Chinese patients: the Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT‐AIS) study. Stroke. 2010;41:885–890. doi: 10.1161/STROKEAHA.109.575605 [DOI] [PubMed] [Google Scholar]
- 27. Chao A‐C, Han KE, Lin S‐F, Lin R‐T, Chen C‐H, Chan L, Lin H‐J, Sun YU, Lin Y‐Y, Chen P‐L, et al. Low‐dose versus standard‐dose intravenous alteplase for octogenerian acute ischemic stroke patients: a multicenter prospective cohort study. J Neurol Sci. 2019;399:76–81. doi: 10.1016/j.jns.2019.01.047 [DOI] [PubMed] [Google Scholar]
- 28. Group NIoNDaSr‐PSS . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 29. von Kummer R, Allen KL, Holle R, Bozzao L, Bastianello S, Manelfe C, Bluhmki E, Ringleb P, Meier DH, Hacke W. Acute stroke: usefulness of early CT findings before thrombolytic therapy. Radiology. 1997;205:327–333. doi: 10.1148/radiology.205.2.9356611 [DOI] [PubMed] [Google Scholar]
- 30. Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, Boysen G, Bluhmki E, Höxter G, Mahagne MH. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017–1025. doi: 10.1001/jama.1995.03530130023023 [DOI] [PubMed] [Google Scholar]
- 31. Patel SC, Levine SR, Tilley BC, Grotta JC, Lu M, Frankel M, Haley EC, Brott TG, Broderick JP, Horowitz S, et al. Lack of clinical significance of early ischemic changes on computed tomography in acute stroke. JAMA. 2001;286:2830–2838. doi: 10.1001/jama.286.22.2830 [DOI] [PubMed] [Google Scholar]
- 32. Ong CT, Wong YS, Wu CS, Su YH. Outcome of stroke patients receiving different doses of recombinant tissue plasminogen activator. Drug Des Devel Ther. 2017;11:1559–1566. doi: 10.2147/DDDT.S133759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou XY, Wang SS, Collins ML, Davis SM, Yan B. Efficacy and safety of different doses of intravenous tissue plasminogen activator in Chinese patients with ischemic stroke. J Clin Neurosci. 2010;17:988–992. doi: 10.1016/j.jocn.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 34. Chen CH, Hsieh CY, Lai TB, Chuang MT, Chen WL, Sun MC. Optimal dose for stroke thrombolysis in Asians: low dose may have similar safety and efficacy as standard dose. J Thromb Haemost. 2012;10:1270–1275. doi: 10.1111/j.1538-7836.2012.04761.x [DOI] [PubMed] [Google Scholar]
- 35. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke‐Monitoring Study (SITS‐MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 36. Sung SF, Chen SC, Lin HJ, Chen YW, Tseng MC, Chen CH. Comparison of risk‐scoring systems in predicting symptomatic intracerebral hemorrhage after intravenous thrombolysis. Stroke. 2013;44:1561–1566. doi: 10.1161/STROKEAHA.111.000651 [DOI] [PubMed] [Google Scholar]
- 37. Lou M, Safdar A, Mehdiratta M, Kumar S, Schlaug G, Caplan L, Searls D, Selim M. The HAT Score: a simple grading scale for predicting hemorrhage after thrombolysis. Neurology. 2008;71:1417–1423. doi: 10.1212/01.wnl.0000330297.58334.dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mazya M, Egido JA, Ford GA, Lees KR, Mikulik R, Toni D, Wahlgren N, Ahmed N; Investigators S . Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: Safe Implementation of Treatments in Stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke. 2012;43:1524–1531. doi: 10.1161/STROKEAHA.111.644815 [DOI] [PubMed] [Google Scholar]
- 39. Cucchiara B, Tanne D, Levine SR, Demchuk AM, Kasner S. A risk score to predict intracranial hemorrhage after recombinant tissue plasminogen activator for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2008;17:331–333. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 40. Strbian D, Engelter S, Michel P, Meretoja A, Sekoranja L, Ahlhelm FJ, Mustanoja S, Kuzmanovic I, Sairanen T, Forss N, et al. Symptomatic intracranial hemorrhage after stroke thrombolysis: the SEDAN score. Ann Neurol. 2012;71:634–641. doi: 10.1002/ana.23546 [DOI] [PubMed] [Google Scholar]
- 41. Saposnik G, Guzik AK, Reeves M, Ovbiagele B, Johnston SC. Stroke prognostication using age and NIH Stroke Scale: SPAN‐100. Neurology. 2013;80:21–28. doi: 10.1212/WNL.0b013e31827b1ace [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Menon BK, Saver JL, Prabhakaran S, Reeves M, Liang LI, Olson DM, Peterson ED, Hernandez AF, Fonarow GC, Schwamm LH, et al. Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue‐type plasminogen activator. Stroke. 2012;43:2293–2299. doi: 10.1161/STROKEAHA.112.660415 [DOI] [PubMed] [Google Scholar]
- 43. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6 [DOI] [PubMed] [Google Scholar]
- 44. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, et al. Randomised double‐blind placebo‐controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European‐Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/S0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 45. Hade EM, Lu B. Bias associated with using the estimated propensity score as a regression covariate. Stat Med. 2014;33:74–87. doi: 10.1002/sim.5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8:206–213. doi: 10.1007/s11121-007-0070-9 [DOI] [PubMed] [Google Scholar]
- 48. von Hippel PT. How many imputations do you need? A two‐stage calculation using a quadratic rule. Sociol Methods Res. 2020;49:699–718. doi: 10.1177/0049124117747303 [DOI] [Google Scholar]
- 49. Bluhmki E, Chamorro A, Dávalos A, Machnig T, Sauce C, Wahlgren N, Wardlaw J, Hacke W. Stroke treatment with alteplase given 3.0‐4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009;8:1095–1102. doi: 10.1016/S1474-4422(09)70264-9 [DOI] [PubMed] [Google Scholar]
- 50. Kimura K, Iguchi Y, Shibazaki K, Iwanaga T, Yamashita S, Aoki J. IV t‐PA therapy in acute stroke patients with atrial fibrillation. J Neurol Sci. 2009;276:6–8. doi: 10.1016/j.jns.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 51. Frank B, Fulton R, Weimar C, Shuaib A, Lees KR; Collaborators V . Impact of atrial fibrillation on outcome in thrombolyzed patients with stroke: evidence from the Virtual International Stroke Trials Archive (VISTA). Stroke. 2012;43:1872–1877. doi: 10.1161/STROKEAHA.112.650838 [DOI] [PubMed] [Google Scholar]
- 52. Padjen V, Bodenant M, Jovanovic DR, Ponchelle‐Dequatre N, Novakovic N, Cordonnier C, Beslac‐Bumbasirevic L, Leys D. Outcome of patients with atrial fibrillation after intravenous thrombolysis for cerebral ischaemia. J Neurol. 2013;260:3049–3054. doi: 10.1007/s00415-013-7119-4 [DOI] [PubMed] [Google Scholar]
- 53. Saposnik G, Gladstone D, Raptis R, Zhou L, Hart RG; Group IotRotCSNRatSORCSW . Atrial fibrillation in ischemic stroke: predicting response to thrombolysis and clinical outcomes. Stroke. 2013;44:99–104. doi: 10.1161/STROKEAHA.112.676551 [DOI] [PubMed] [Google Scholar]
- 54. Šaňák D, Herzig R, Král M, Bártková A, Zapletalová J, Hutyra M, Školoudík D, Vlachová I, Veverka T, Horák D, et al. Is atrial fibrillation associated with poor outcome after thrombolysis? J Neurol. 2010;257:999–1003. doi: 10.1007/s00415-010-5452-4 [DOI] [PubMed] [Google Scholar]
- 55. Mehrpour M, Afrakhte M, Shojaei SF, Sohrabi A, Ashayeri R, Esmaeili S, Bahadori M. Factors predicting the outcome of intravenous thrombolysis in stroke patients before rt‐PA administration. Caspian J Intern Med. 2019;10:424–430. doi: 10.22088/cjim.10.4.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shi ZS, Loh Y, Walker G, Duckwiler GR; Investigators MaMM . Endovascular thrombectomy for acute ischemic stroke in failed intravenous tissue plasminogen activator versus non‐intravenous tissue plasminogen activator patients: revascularization and outcomes stratified by the site of arterial occlusions. Stroke. 2010;41:1185–1192. doi: 10.1161/STROKEAHA.109.568451 [DOI] [PubMed] [Google Scholar]
- 57. Kimura K, Iguchi Y, Yamashita S, Shibazaki K, Kobayashi K, Inoue T. Atrial fibrillation as an independent predictor for no early recanalization after IV‐t‐PA in acute ischemic stroke. J Neurol Sci. 2008;267:57–61. doi: 10.1016/j.jns.2007.09.036 [DOI] [PubMed] [Google Scholar]
- 58. Re KI. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2014;179:1034–1035. doi: 10.1093/aje/kwt435 [DOI] [PubMed] [Google Scholar]
- 59. McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074 [DOI] [PubMed] [Google Scholar]
- 60. Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—a practical guide with flowcharts. BMC Med Res Methodol. 2017;17:162. doi: 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Khan SI, Hoque ASML. SICE: an improved missing data imputation technique. J Big Data. 2020;7:37. doi: 10.1186/s40537-020-00313-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lin SF, Lu YH, Bai CH. Risk of recurrent stroke for Asian stroke patients treated with non‐vitamin K antagonist oral anticoagulant and warfarin. Ther Adv Chronic Dis. 2020;11:2040622320974853. doi: 10.1177/2040622320974853 [DOI] [PMC free article] [PubMed] [Google Scholar]


