Heart failure with preserved ejection fraction (HFpEF) accounts for up to 50% of patients with heart failure and has high rates of adverse clinical outcomes, similar to heart failure with reduced ejection fraction (HFrEF). 1 Although HFpEF was once thought to be diastolic heart failure, it is now understood to be a heterogeneous clinical syndrome, with several underlying pathophysiologic mechanisms. It is likely that different clinical HFpEF phenotypes might not derive similar benefits from the same therapeutic interventions. 2 , 3 Inflammation is highly prevalent in heart failure and is recognized as one of the factors in the pathogenesis of HFpEF. 4 , 5 Potential benefits of immunomodulatory therapies were demonstrated in animal models. 4 , 6 Nonetheless, the evidence in humans is limited to small pilot studies with conflicting results, and the clinical implications remain to be determined. 7 , 8
The vagus nerve plays a critical role in the inflammatory reflex, where it serves as an afferent and efferent pathway to communicate between brain and peripheral organs, including the heart. 9 Sensory vagus nerve afferents are activated by the presence of proinflammatory cytokines in peripheral tissues and convey the signal to the brain. This signal leads to the release of acetylcholine from vagus nerve efferents into the reticuloendothelial system, which consequently suppresses inflammation by inhibiting proinflammatory cytokine synthesis and release. Therefore, it is understandable that vagus nerve stimulation (VNS) could attenuate the proinflammatory state known to be a key pathological mechanism in HF, especially HFpEF, and improve cardiac remodeling. After multiple experimental animal models showed promising benefits of VNS, 10 the feasibility of long‐term VNS in patients with heart failure was first reported by Schwartz and colleagues, which demonstrated improvement in functional status, quality of life, and left ventricular volume in HFrEF following implantation of a vagus nerve stimulator. 11 However, the benefits were not consistently reproduced in larger clinical trials to date. 12 A large pivotal VNS study in HFrEF (ANTHEM‐HFrEF) is ongoing and is going to deliver the definitive answer on whether VNS via an implantable impulse generator can improve clinical outcomes. 13 .
Alternatively, VNS can be performed in a less invasive manner by transcutaneous stimulation of the auricular branch of the vagus nerve using tragus of the ear as an anatomical landmark. 14 Low‐level transcutaneous vagus nerve stimulation (LLTS) has been shown to decrease inflammatory cytokines in animal models as well as in patients with atrial fibrillation. 15 , 16 , 17 Its application in HFpEF has also been reported. Long‐term intermittent LLTS in a rat model with HFpEF led to decrease in inflammatory markers and improvement in diastolic function compared with the sham group. In patients with HFpEF, the improvement in left ventricular global longitudinal strain (GLS) was observed during LLTS. 18 The long‐term use of LLTS in ambulatory patients with HFpEF has not yet been described.
In this issue of the Journal of the American Heart Association (JAHA), Stavrakis and colleagues presented a single‐center, randomized, double‐blind, sham‐controlled study to examine the effect of long‐term LLTS in patients with HFpEF with a predominantly inflammatory‐metabolic phenotype (defined as having at least 2 of the 4 following comorbidities: aged ≥65 years, diabetes, hypertension, and body mass index ≥30 kg/m2). The treatment group was assigned to have self‐performed stimulation via the ear clip electrode at the tragus (20 Hz, 1 mA below the discomfort threshold) for 1 hour daily for 3 months. Patients in the sham group received the same instruction except for the location of the electrode at the ear lobe. The study sample size was reduced from the initial calculation of 72 patients to 52 patients because of a slower than expected enrollment rate during the COVID‐19 pandemic. A total of 48 patients completed the study and were available for the analysis. Changes in echocardiographic measurements of both diastolic function (the ratio of early mitral inflow Doppler velocity/early diastolic mitral annulus velocity) and systolic function (left ventricular GLS) were used as coprimary outcomes. LLTS was only shown to improve GLS (−18.6±2.5% versus −16.0±2.4%; P=0.02) but not the ratio of early mitral inflow Doppler velocity/early diastolic mitral annulus velocity (9.9±1.5 versus 10.5±1.5; P=0.53). LLTS also resulted in larger improvement in the Minnesota Living With Heart Failure Questionnaire score, but no difference was observed in 6‐minute walk distance.
Although the study showed lower inflammatory markers, including tumor necrosis factor‐α and interleukin‐8, following LLTS, it cannot be simply assumed that amelioration of inflammation had led to improved outcomes. The authors, therefore, performed an exploratory analysis that demonstrates the inverse association between the change in tumor necrosis factor‐α levels and the change in GLS. This key finding could suggest the causality between these 2 outcomes; however, it is uncertain whether LLTS improves both inflammation and cardiac function independently, reduces inflammation, which in turn improves GLS, or vice versa.
LLTS is an emerging concept in patients with HFpEF and associated comorbidities, such as atrial fibrillation, for which effective treatments are currently limited. Because of the noninvasive and simplistic nature of LLTS, the adherence to self‐administered therapy was reasonably high (>90% in both arms) with no device‐related adverse effects. Nonetheless, the effects of LLTS are ideally reproduced and expanded to clinically meaningful end points. Although GLS is a strong predictor of adverse clinical outcomes in HFpEF, 20 it is plausible yet unknown whether interventions to improve GLS, including LLTS, will affect clinical outcomes.
In addition to larger‐scale randomized clinical trials to assess the effect of LLTS, there are some factors to consider to optimize the benefits of LLTS in patients with HFpEF. As the basis of LLTS is inflammatory attenuation, patients with a proinflammatory state might hypothetically derive more benefits from LLTS, which is in keeping with the authors′ intention to only enroll patients with a predominantly inflammatory‐metabolic phenotype. This notion is supported by the result of this study that patients with greater changes in inflammatory markers also had greater improvement in GLS. Therefore, careful selection of candidates with a proinflammatory state should be a prerequisite. This can be challenging in HFpEF, which consists of multiple and overlapping phenotypes. Assessment of the degree of inflammation incorporating comorbidity risk score or inflammatory cytokine levels might be helpful to predict treatment responses. Furthermore, optimization of stimulation parameters involving intensity, duration, and frequency could potentiate effect of LLTS and needs to be further investigated.
It is still too early to conclude the use of LLTS in patients with HFpEF, albeit the study demonstrates improvement in GLS, inflammatory markers, and quality of life. With further refinement in candidate selection and optimization of stimulation program, we are hopeful to see the abandoned VNS revive and transform into a more feasible and effective therapy back in the heart failure arena (Figure).
Figure 1. Effects of low‐level transcutaneous vagus nerve stimulation.
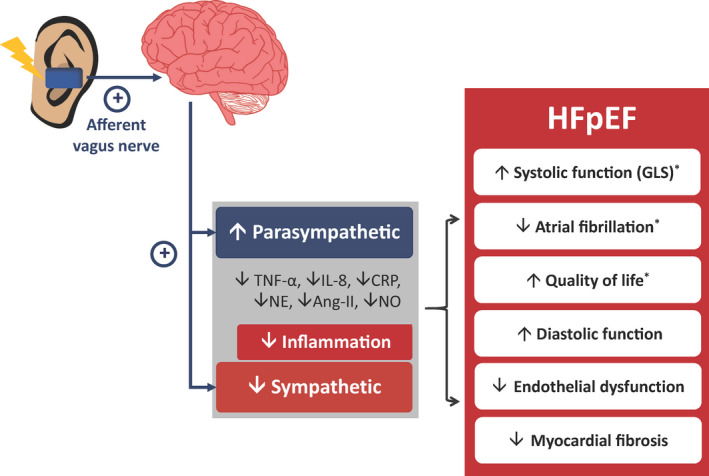
Patients with heart failure have a low vagal tone and high sympathetic activity. Low‐level transcutaneous vagus nerve stimulation via the auricular branch leads to an increase in efferent vagal activity and resultant increased parasympathetic tone with a decreased sympathetic tone. Parasympathetic activation results in attenuation of inflammation, which is one of the pathophysiologic mechanisms involved in heart failure with preserved ejection fraction (HFpEF). Low‐level transcutaneous vagus nerve stimulation has been shown to improve outcomes in both animal models and patients with HFpEF. (Note: The asterisk indicates the results from randomized‐controlled studies in humans). Ang‐II indicates angiotensin‐II; CRP, C‐reactive protein; GLS, global longitudinal strain; IL‐8, interleukin‐8; NE, norepinephrine; and TNF‐α, tumor necrosis factor‐α.
Disclosures
None.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Disclosures, see page 3.
See Article by Stavrakis et al.
References
- 1. Lam CS, Donal E, Kraigher‐Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shah SJ, Katz DH, Deo RC. Phenotypic spectrum of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:407–418. doi: 10.1016/j.hfc.2014.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, Yarde M, Wang Z, Bhattacharya PT, Chirinos DA, et al. Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail. 2020;8:172–184. doi: 10.1016/j.jchf.2019.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Empel V, Brunner‐La Rocca HP. Inflammation in HFpEF: key or circumstantial? Int J Cardiol. 2015;189:259–263. doi: 10.1016/j.ijcard.2015.04.110 [DOI] [PubMed] [Google Scholar]
- 5. Tromp J, Westenbrink BD, Ouwerkerk W, van Veldhuisen DJ, Samani NJ, Ponikowski P, Metra M, Anker SD, Cleland JG, Dickstein K, et al. Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2018;72:1081–1090. doi: 10.1016/j.jacc.2018.06.050 [DOI] [PubMed] [Google Scholar]
- 6. Adamo L, Rocha‐Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17:269–285. doi: 10.1038/s41569-019-0315-x [DOI] [PubMed] [Google Scholar]
- 7. Van Tassell BW, Arena R, Biondi‐Zoccai G, McNair Canada J, Oddi C, Abouzaki NA, Jahangiri A, Falcao RA, Kontos MC, Shah KB, et al. Effects of interleukin‐1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D‐HART pilot study). Am J Cardiol. 2014;113:321–327. doi: 10.1016/j.amjcard.2013.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, et al. Effect of phosphodiesterase‐5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex–linking immunity and metabolism. Nat Rev Endocrinol. 2012;8:743–754. doi: 10.1038/nrendo.2012.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Ferrari GM. Vagal stimulation in heart failure. Cardiovasc Transl Res. 2014;7:310–320. doi: 10.1007/s12265-014-9540-1 [DOI] [PubMed] [Google Scholar]
- 11. Schwartz PJ, De Ferrari GM, Sanzo A, Landolina M, Rordorf R, Raineri C, Campana C, Revera M, Ajmone‐Marsan N, Tavazzi L, et al. Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Fail. 2008;10:884–891. doi: 10.1016/j.ejheart.2008.07.016 [DOI] [PubMed] [Google Scholar]
- 12. Byku M, Mann DL. Neuromodulation of the failing heart: lost in translation? JACC Basic Transl Sci. 2016;1:95–106. doi: 10.1016/j.jacbts.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fudim M, Abraham WT, von Bardeleben RS, Lindenfeld J, Ponikowski PP, Salah HM, Khan MS, Sievert H, Stone GW, Anker SD, et al. Device therapy in chronic heart failure: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2021;78:931–956. doi: 10.1016/j.jacc.2021.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fallgatter AJ, Neuhauser B, Herrmann MJ, Ehlis AC, Wagener A, Scheuerpflug P, Reiners K, Riederer P. Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. J Neural Transm (Vienna). 2003;110:1437–1443. doi: 10.1007/s00702-003-0087-6 [DOI] [PubMed] [Google Scholar]
- 15. Wang Z, Yu L, Wang S, Huang B, Liao K, Saren G, Tan T, Jiang H. Chronic intermittent low‐level transcutaneous electrical stimulation of auricular branch of vagus nerve improves left ventricular remodeling in conscious dogs with healed myocardial infarction. Circ Heart Fail. 2014;7:1014–1021. doi: 10.1161/CIRCHEARTFAILURE.114.001564 [DOI] [PubMed] [Google Scholar]
- 16. Zhou L, Filiberti A, Humphrey MB, Fleming CD, Scherlag BJ, Po SS, Stavrakis S. Low‐level transcutaneous vagus nerve stimulation attenuates cardiac remodelling in a rat model of heart failure with preserved ejection fraction. Exp Physiol. 2019;104:28–38. doi: 10.1113/EP087351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R, Po SS. Low‐level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol. 2015;65:867–875. doi: 10.1016/j.jacc.2014.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tran N, Asad Z, Elkholey K, Scherlag BJ, Po SS, Stavrakis S. Autonomic neuromodulation acutely ameliorates left ventricular strain in humans. J Cardiovasc Transl Res. 2019;12:221–230. doi: 10.1007/s12265-018-9853-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stavrakis S, Elkholey K, Morris L, Niewiadomska M, Ul Abideen Asad Z, Humphrey MB. Neuromodulation of inflammation to treat heart failure with preserved ejection fraction: a pilot randomized clinical trial. J Am Heart Assoc. 2021;10:e023582. doi: 10.1161/JAHA.121.023582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132:402–414. doi: 10.1161/CIRCULATIONAHA.115.015884 [DOI] [PMC free article] [PubMed] [Google Scholar]


