Abstract
Background
The potential of phage therapy for the treatment of endovascular Staphylococcus aureus infections remains to be evaluated.
Methods and Results
The efficacy of a phage cocktail combining Herelleviridae phage vB_SauH_2002 and Podoviriae phage 66 was evaluated against a methicillin‐sensitive S. aureus strain in vitro and in vivo in a rodent model of experimental endocarditis. Six hours after bacterial challenge, animals were treated with (1) the phage cocktail. (2) subtherapeutic flucloxacillin dosage, (3) combination of the phage cocktail and flucloxacillin, or (4) saline. Bacterial loads in cardiac vegetations at 30 hours were the primary outcome. Secondary outcomes were phage loads at 30 hours in cardiac vegetations, blood, spleen, liver, and kidneys. We evaluated phage resistance 30 hours post infection in vegetations of rats under combination treatment. In vitro, phages synergized against S. aureus planktonic cells and the cocktail synergized with flucloxacillin to eradicated biofilms. In infected animals, the phage cocktail achieved bacteriostatic effect. The addition of low‐dose flucloxacillin elevated bacterial suppression (∆ of −5.25 log10 colony forming unit/g [CFU/g] versus treatment onset, P<0.0001) and synergism was confirmed (∆ of −2.15 log10 CFU/g versus low‐dose flucloxacillin alone, P<0.01). Importantly, 9/12 rats given the combination treatment had sterile vegetations at 30 hours. In vivo phage replication was partially suppressed by the antibiotic and selection of resistance to the Podoviridae component of the phage cocktail occurred. Plasma‐mediated inhibition of phage killing activity was observed in vitro.
Conclusions
Combining phages with a low‐dose standard of care antibiotic represents a promising strategy for the treatment of S. aureus infective endocarditis.
Keywords: endocarditis, phage antibiotic synergism, phage therapy, Staphylococcus aureus
Subject Categories: Animal Models of Human Disease, Infectious Endocarditis, Treatment
Nonstandard Abbreviations and Acronyms
- EE
experimental infective endocarditis
- IE
infective endocarditis
- MOI
multiplicity of infection
- PFU
plaque forming unit
Clinical Perspective
What Is New?
Therapy with Staphylococcus aureus bacteriophages synergizes with standard of care antibiotics for the treatment of experimental infective endocarditis.
What Are the Clinical Implications?
The addition of bacteriophages to standard‐of‐care antibiotic treatments at the beginning of the therapy increases bacterial load reduction within cardiac vegetations.
These findings suggest a reduction of the risk for typical S. aureus infective endocarditis‐related complications, such as septic embolism or acute valve damage and ultimately pave the way to shorter antibacterial treatment courses.
Staphylococcus aureus is one of the most common pathogens responsible for acute infective endocarditis (IE) on both native 1 and prosthetic valves. 2 Currently, S. aureus IE is managed primarily with a 4‐ to 6‐week course of intravenous antibiotic medication, and heart valve surgery may also be performed if indicated. 3 Even the most aggressive therapeutic plans are associated with substantial morbidity and mortality, with mortality rates reaching 50% in patients with prosthetic valve infection. 4 Thus, there remains a need for novel strategies that may improve outcomes in patients with IE.
Phage therapy, wherein bacterial viruses are used to treat bacterial infections, has been proposed as a salvage therapy, especially in the context of foreign body infections or multidrug‐resistant pathogens. 5 However, there is not yet sufficient evidence from randomized controlled trials to support widespread adoption of phage therapy. The available evidence suggests that phage therapy can be an effective alternative or complementary strategy to antibiotics for the treatment of S. aureus infections, including burn and chronic wound infections, 6 , 7 keratitis, 8 severe infections after cardiothoracic surgery, 9 prosthetic joint infections, 10 , 11 and ventricular‐assist device infections. 12
Recently, 2 Australian case series evaluated the safety and efficacy of a 3‐phage cocktail for the treatment of patients with S. aureus IE or S. aureus aortic graft infections. 13 , 14 Encouragingly, improved infection control and healing progress were documented with the adjunction of phages to antibiotic treatment. However, there were cases in which treatment failure and/or recurrence occurred, including some that were ultimately fatal. A reliable curative protocol for S. aureus endovascular infection treatment with phages has yet to be established.
Thus far, all patients who have received phage therapy for deep‐seated S. aureus infections have received the therapy in combination with antibiotic pharmacotherapy. Thus, it is still unknown whether phage therapy alone could clear such infections. Recently, using a model of experimental infective endocarditis (EE), we observed that phage therapy alone was as effective as ciprofloxacin alone for the treatment Pseudomonas aeruginosa EE and that combining phages and ciprofloxacin was highly synergistic and could even result in culture‐negative vegetations. 15 Thus, the aim of the current study was to evaluate the efficacy of phage therapy alone or in combination with the IE standard of care antibiotic flucloxacillin for the treatment of methicillin‐susceptible S. aureus (MSSA) EE in rats.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Bacterial Strains, Bacteriophages, Growth Conditions, and Evaluation of Phage Activity
A panel of 63 S. aureus strains isolated from humans and animals and representing a variety of sequence types was used (Table S1). Notably, among these was the MSSA strain Laus102, isolated from a healthy carrier. 16 Additionally, the P. aeruginosa strain ATCC 15442™ (LGC Standards, Molsheim, France) was used. The Podoviriae phage 66 was purchased from the National Collection of Type Cultures of Public Health England (#8289) and the Herelleviridae phage vB_SauH_2002 was isolated from sewage water previously. 17 Both phages were propagated in Laus102 cultures. The phage solutions were normalized to 1010 plaque‐forming units (PFU)/mL. Phage host range was determined using efficiency of plating assays on the aforementioned 63 S. aureus strains. The Myoviridae family phage vB_PaeM_4002, which infects P. aeruginosa, was isolated from a sewage water sample collected at the Vidy wastewater treatment plant, Lausanne, Switzerland (unpublished). Details on growth conditions and reagents are given in the Supplemental Material.
In Vitro Activity of Phages or Flucloxacillin Against Planktonic Cultures and Biofilms
Monophage and phage cocktail effects on 63 S. aureus strains were tested as described in the Supplemental Material and Table S1. Phage (only) or phage in combination with flucloxacillin was also tested against the MSSA isolate Laus102, using in vitro turbidity and time‐kill assays (described in the Supplemental Material).
S. aureus biofilms were produced, rinsed, and managed in 96‐well plates as previously described (Ref. [18] and Supplemental Material). Mature biofilms were treated for 24 hours at 37 °C with vB_SauH_2002, phage 66, or the phage cocktail at final multiplicity of infection (MOI) of 1, 10, and 100, in combination or not with flucloxacillin, at 1× and 10× minimum inhibitory concentration, in tryptic soy broth. Synergy was defined as a >2 log10 colony forming unit (CFU)/mL decrease in bacterial load compared with the decrease observed for the reference treatment alone. 19
Murine Infection Model
Female Wistar rats [Crl:WI(Han); Charles River, L’Abresle, France], weighing 180 to 200 g, were housed in specific pathogen‐free rooms (12‐hour light/dark conditions, 23±1 °C, water and food ad libitum). All animal experiments were carried out in accordance with Swiss Animal Protection Law guidelines and were approved by the Cantonal Committee on Animal Experiments of the State of Vaud (approval VD 879.10). For all manipulations, animals were anesthetized with a mixture of ketamine (Ketalar, 75 mg/kg) and xylazine (Xylasol, 0.5 mg/kg) given intraperitoneally. Buprenorphin (Temgesic, 0.15 mg/kg) was given intraperitoneally at the onset of surgery as an analgesic.
Induction of Infection
Catheter‐induced sterile aortic vegetations were produced in rats as previously described. 20 In parallel, an intravenous line was inserted via the jugular vein into the superior vena cava and connected to a programmable infusion pump (Pump 44; Harvard Apparatus, Inc., South Natick, MA) for delivery of antibacterial drugs according to a dosage regimen that mimics the kinetics of human intravenous antibiotic therapy. 21 Bacterial inocula were prepared from dilutions of fresh midexponential phase cultures (600 nm optical density (OD600nm)=0.6, ~108 CFU/mL). With the assistance of a programmable infusion pump, 1.30±0.35×105 CFU of bacteria in 500 µL (corresponding to 10 times the 90% infective dose 21 ) were inoculated to each animal 24 hours after catheterization. 22 The inoculum size was confirmed by colony counts on plates coated with tryptic soy agar (BD Difco, Becton Dickinson, Sparks, MD). Three uninfected animals were used for pharmacokinetic studies.
Treatment Protocol
We performed 4 sets of experiments with n=3 in each of the 5 groups. Six hours after the initiation of a bacterial challenge, animals were treated with either (1) a phage cocktail (vB_SauH_2002 and 66, 1010 PFU/mL each) injected as a 1‐mL bolus followed by continuous infusion at 0.3 mL/h for 24 hours (each rat received 8.2×1010 PFU over 24 hours, n=8); (2) a suboptimal IV dose of flucloxacillin mimicking human kinetic treatment (2 g every 12 hour for 24 hour instead of 2 g every 6 hours for 24 hours for an optimal treatment, n=11); (3) the phage cocktail plus flucloxacillin (dosages as previously) (n=12); or (4) mock therapy (saline, n=7). Ten animals were killed at the start of therapy (6 hours post infection) to assess infection severity at the onset of treatment. The remaining rats were killed 24 hours later (30 hours post infection).
Outcomes
The primary outcome was bacterial load in cardiac vegetations 30 hours after infection. Secondary outcomes were phage loads 30 hours post infection in cardiac vegetations, blood, spleen, liver, and kidneys. An additional outcome was the presence of phage‐resistant clones in the cardiac vegetations of rats given the phage cocktail/flucloxacillin combination treatment. Outcome assessment methods are described in the Supplemental Material.
Statistical Analysis
Differences between the groups were generally detected with 1‐way ANOVAs with Tukey correction for multiple comparisons. Phage loads in blood and organs were compared with unpaired t tests with Welch’s correction. All analyses were performed in Prism software (version 9, GraphPad, La Jolla, CA). Statistical test results were considered significant when P values <0.05 were obtained. Mean values are reported with SDs.
Results
Phage Cocktail of vB_SauH_2002 and 66 Had Synergistic Activity Against Planktonic S. aureus
The lytic activity of the Herelleviridae phage vB_SauH_2002 (Figure 1A) and the Podoviridae phage 66 (Figure 1B) against each of 63 S. aureus strains is summarized in Table S1. The anti‐S. aureus efficacy ranges of phage vB_SauH_2002 alone, phage 66 alone, or phage vB_SauH_2002 plus phage 66 (equimolar cocktail) covered ~83%, ~59%, and ~92% of the bacterial panel (Table S1). In diluted drop tests (Figure 1C), each of the 2 phages achieved very high titers against the MSSA strain Laus102 (ca. 1010 PFU/mL, Figure 1C). With respect to turbidity testing, the 2‐phage cocktail achieved more sustained S. aureus‐growth inhibition over 24 hours than either vB_SauH_2002 or phage 66 alone at the same MOI of 0.1 (P<0.0001) (Figure 1D through F).
Figure 1. In vitro activity against MSSA Laus102 of Myoviridae phage vB_HSa_2002, Podoviridae phage 66, or both in a 2‐phage cocktail. Electron micrograph of (A) vB_SauH_2002 and (B) phage 66.
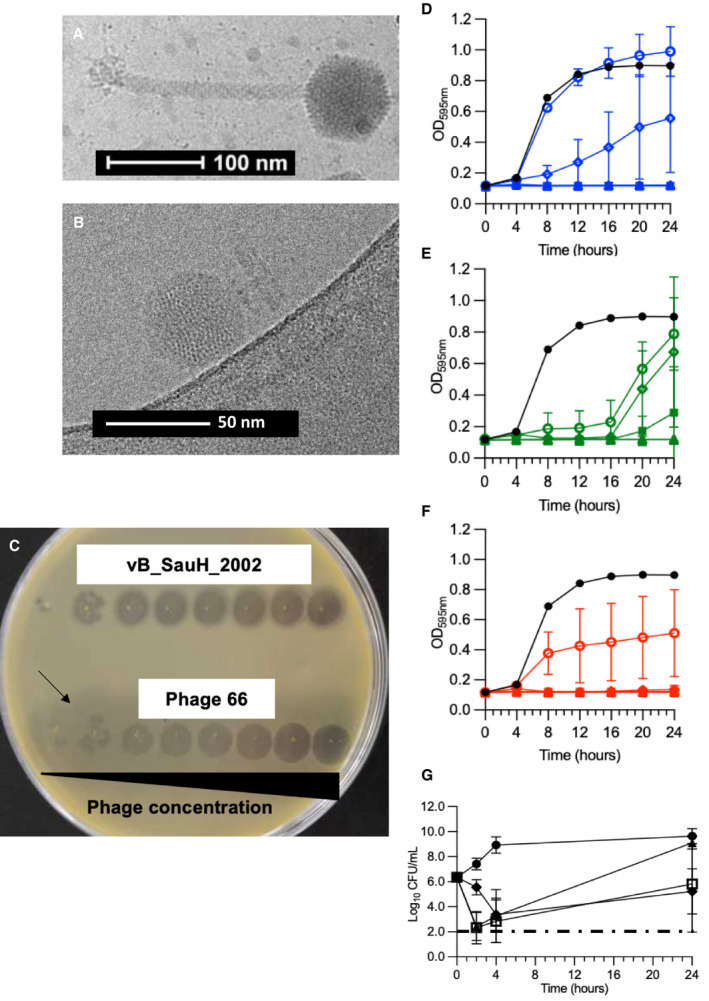
Antibacterial activity was tested through (C) diluted drop tests, (D through F) turbidity assays, and (G) time‐kill assays. C, The black arrow indicates the halo surrounding phage 66 lysis zones. Both phages were serially 10‐time diluted from right to left (starting concentration was 109 PFU/mL for both phages). D, Phage vB_SauH_2002 (blue). E, Phage 66 (green), and (F) phage cocktail (red). (D through F) Control without phages (closed black circles); phages at MOI=0.01 (open circles); phages at MOI=0.1 (open diamonds), MOI=1 (open triangles), MOI=10 (closed squares), and MOI=100 (closed triangles). G, Time‐kill assays were performed by challenging MSSA Laus102 with saline (closed circles); phage cocktail at MOI=1 (closed triangles), flucloxacillin at 1× MIC (closed diamonds) or a combination of both treatments (open squares). Means (±SDs) of 3 independent experiments performed in triplicate are shown in panels (D–G). One‐way ANOVA with Tukey correction for multiple comparison statistical tests was performed to compare either areas under the curves of curves obtained at MOI=0.1 (Figures 1D through 1F) or 24‐hour time points (Figure 1G). CFU indicates colony forming unit; MIC, minimum inhibitory concentration; MOI, multiplicity of infection; MSSA, methicillin‐susceptible Staphylococcus aureus; and PFU, plaque forming unit.
Time‐kill assay results are presented in Figure 1G. Notably, a significant loss of bacterial viability (ca. 4 log10 CFU/mL) was observed in time‐kill assays 2 hours after addition of the phage cocktail at an MOI of 1 (P<0.0001). Bacterial regrowth was observed 24 hours after the phage challenge but could be reduced by the addition of low‐dose (1× minimum inhibitory concentration, ie, 0.125 mg/mL) flucloxacillin (P<0.05). Interestingly, during the early hours of the time‐kill assay experiment, the phage cocktail achieved a greater magnitude of killing (~4 log10 CFU/mL at 2 hours) than flucloxacillin (~3 log10 CFU/mL at 4 hours) (P<0.0001). We did not observe evidence of synergism between the phages and the antibiotic in the time‐kill assay experiment (ie, nonsignificant difference between the phages+flucloxacillin and flucloxacillin alone at 24 hours, P=0.93).
Both Phages Synergized With Antibiotics to Clear Biofilms In Vitro
We further compared the efficacy of each single phage, the phage cocktail, flucloxacillin, and the combination of both for the treatment of MSSA biofilms in vitro (Figure 2). Surprisingly, although phage 66 was active against planktonic cells and exhibited exopolysaccharide depolymerase activity (evidenced by the formation of halos around PFUs 23 ) in the diluted drop test assay, phage 66 was ineffective against MSSA biofilms at all MOIs tested (1, 10, and 100) (Figure 2A). In contrast, phage vB_SauH_2002 achieved significant dose‐dependent biofilm clearance compared with the control treatment (∆3.22±0.55 log10 CFU/mL at MOI=1; ∆3.29±0.55 log10 CFU/mL at MOI=10), with particularly efficacious clearance being achieved at an MOI of 100 (∆4.51±0.55 log10 CFU/mL, P<0.0001) (Figure 2A). Moreover, phage vB_SauH_2002 synergized with phage 66 at an MOI of 10 (additional loss of viability of 2.26±0.55 log10 CFU/mL relative to vB_SauH_2002 alone; P<0.01) (Figure 2B). Substantial synergy between the phage cocktail (MOI=1) and low‐dose (1× minimum inhibitory concentration) flucloxacillin was observed (2.74±0.44 log10 CFU/mL additional clearance versus the phage cocktail at MOI=1 alone, P<0.0001) (Figure 2B).
Figure 2. Activity of bacteriophages against S. aureus biofilms with and without flucloxacillin.
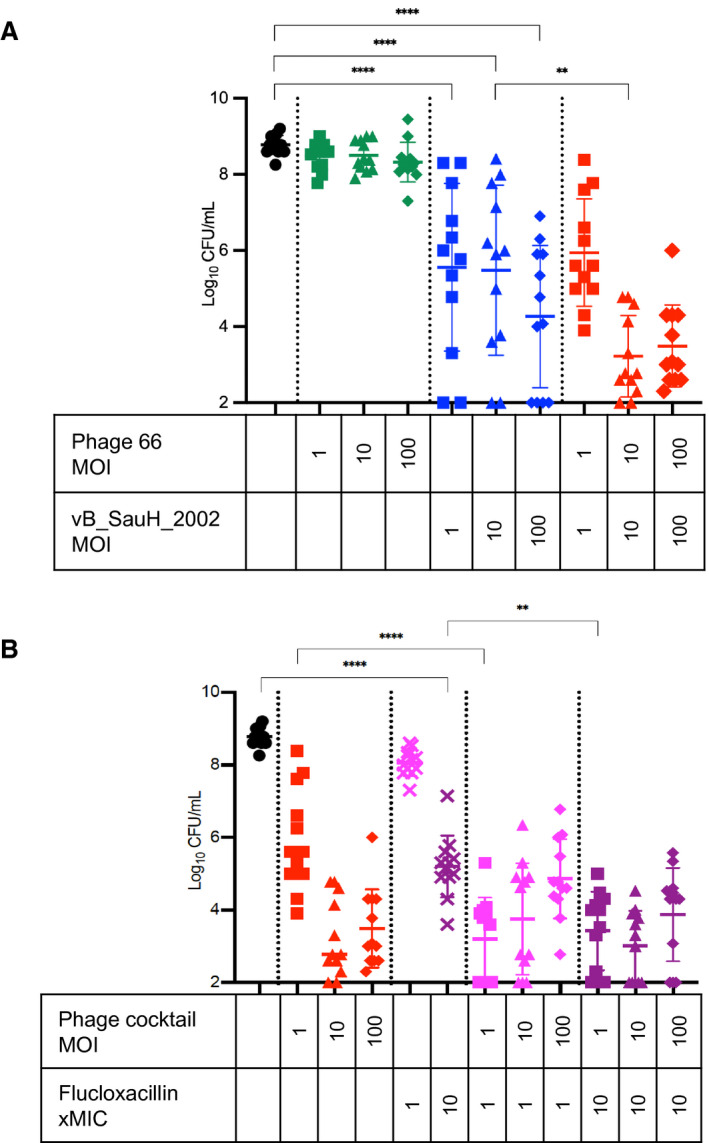
A, S. aureus Laus102 biofilms challenged for 24 hours with single phages or the phage cocktail. B, S. aureus Laus102 biofilms challenged for 24 hours with the phage cocktail (alone), flucloxacillin (alone), or the phage cocktail in combination with flucloxacillin. Means (±SDs) of 3 independent experiments performed in triplicate are depicted; **P<0.01; ****P<0.0001; 1‐way ANOVA with Tukey’s multiple comparisons test. CFU indicates colony forming unit; MIC, minimum inhibitory concentration; and MOI, multiplicity of infection.
Phages and Flucloxacillin Were Highly Synergistic Against S. aureus Experimental Endocarditis
At the onset of treatment, which occurred 6 hours after the bacterial challenge (Figure 3A), all rats harbored heavily infected vegetations (7.22±0.92 log10 CFU/g), and mock therapy (saline) allowed the bacterial load to increase to 9.20±1.05 log10 CFU/g 24 hours later (Figure 3B). Bacteriostasis (bacterial load similar to before phage application, ie, 6.57±1.03 log10 CFU/g versus 7.22±0.92 log10 CFU/g at the onset of treatment) was observed 24 hours after administration of the phage cocktail (1010 PFU in 1 mL saline followed by 8×1010 PFU over 24 hours via continuous intravenous infusion at 0.3 mL/h). Similarly, bacteriostasis was observed 24 hours after administration of a low dose of flucloxacillin every 12 hours (simulating antibacterial treatment in human patients) with 5.35±3.16 log10 CFU/g versus 7.22±0.92 log10 CFU/g at the onset of treatment. In sharp contrast, the combination of both treatments had a highly bactericidal effect (2.62±1.01 log10 CFU/g, ie, ∆ of −5.25 log10 CFU/g versus treatment onset, P<0.0001) owing to synergistic activity with the phage cocktail (∆ of −2.15 log10 CFU/g versus flucloxacillin alone, P<0.01). Importantly, the vegetations in 9 of 12 rats (75%) treated with the phage cocktail‐flucloxacillin combination were culture negative at 24 hours (Figure 3B).
Figure 3. Treatment of EE with a two‐phage cocktail in the presence or absence of low‐dose of flucloxacillin.
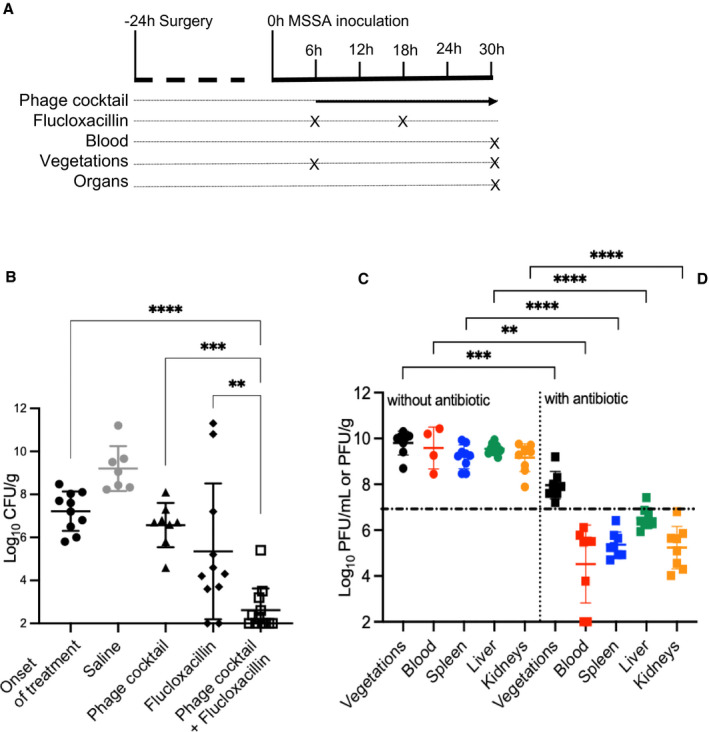
A, Study design with sampling time points (in hours). B, Bacterial loads in cardiac vegetations measured at 6 hours post infection (onset of treatment) in the control rats and 24 hours after the onset of treatment in rats given a mock therapy (saline), the phage cocktail (alone), a low dose of flucloxacillin (alone), or the phage cocktail in combination with flucloxacillin. Each symbol represents an animal (N=10, 7, 8, 11, and 12, respectively). C and D, In vivo phage pharmacokinetics. The phage concentrations observed in cardiac vegetations (black), blood (red), spleen (blue), liver (green), and kidneys (orange) from rats 24 hours after initiation of treatment with either (C) the phage cocktail (alone) or (D) the phage cocktail and flucloxacillin combination. Each dot represents an animal ([C] N=9, 4, 9, 9, and 8, respectively; and [D] n=12, 11, 11, 12, and 12, respectively). The black dotted‐dashed line represents the mean concentration of phages at 24 hours in the blood of healthy rats treated with the phage cocktail (alone) (N=3). Means (±SDs) are reported. **P<0.01; ***P<0.001, ****P<0.0001; 1‐way ANOVA with Tukey’s multiple comparisons test. CFU indicates colony forming unit; EE, experimental infective endocarditis; MSSA, methicillin‐susceptible Staphylococcus aureus; and PFU, plaque forming unit.
The Addition of a Subtherapeutic Dose of Flucloxacillin Affected Phage Titers In Vivo
As shown in Figure 3C, phage titers in blood samples collected from infected animals 24 hours after phage treatment initiation (9.59±0.91 log10 PFU/mL) were significantly higher (∆2.71±0.52 log10 PFU/mL, P<0.005) than those in noninfected animals (6.88±0.42 log10 PFU/mL). Similarly high phage titers were measured in cardiac vegetations (9.80±0.52 log10 PFU/g), spleen (9.20±0.52 log10 PFU/g), liver (9.54±0.23 log10 PFU/g), and kidneys (9.16±0.61 log10 PFU/g) in EE animals. The addition of low‐dose flucloxacillin decreased phage titers drastically in all body compartments (cardiac vegetation ∆1.84±0.28 log10 PFU/mL, P<0.001; blood ∆5.06±0.75 log10 PFU/g, P<0.01; spleen ∆3.84±0.27 log10 PFU/g, P<0.0001; liver ∆3.02±0.18 log10 PFU/g, P<0.0001; and kidneys ∆3.92±0.39 log10 PFU/g, P<0.0001 versus phage cocktail alone). After rats were on a subtherapeutic dose of flucloxacillin for 24 hours, phage levels in their blood were even lower than those of noninfected animals (∆2.35±0.65 log10 PFU/mL, P<0.01, Figure 3D).
Bacterial Resistance Occurred In Vivo for Phage 66 But Not for vB_SauH_2002
Screening for phage/antibiotic‐resistant clones in bacteria recovered from cardiac vegetations treated with the phage and antibiotic in combination resulted in the recovery of S. aureus colonies in 2/12 treated rats (Table S2). Of 36 clones that were recovered, 23 (63%) were susceptible to each single phage and to the phage cocktail (Susceptible, Susceptible, and Susceptible to the phage vB_SauH_2002, phage 66, and the phage cocktail, respectively [SSS] pattern of resistance), whereas 13 were resistant to phage 66 while retaining sensitivity to phage vB_SauH_2002 and the phage cocktail (Susceptible, Resistant, and Susceptible to the phage vB_SauH_2002, phage 66, and the phage cocktail, respectively [SRS] pattern of resistance). No clones harboring resistance to phage vB_SauH_2002 or to the phage cocktail were recovered from any of the animals (Table S2).
Sequencing of the genomes of 6 phage 66‐resistant clones showed that all 6 clones harbored single nucleotide polymorphisms in several genes coding for transposases and that 1 clone, namely clone 16C8, harbored 1 additional point mutation in tarS leading to a frameshift (bold and underlined in Table S3). Interestingly, all 13 phage 66‐resistant clones were as virulent as the parent strain in a Galleria mellonella infection model (data not shown).
Rat Plasma Impaired the Lytic Activity of S. aureus But Not of P. aeruginosa Phages In Vitro
As shown in Figure 4A, the addition of 10% rat plasma to S. aureus Laus102 cultures 30 min before administration of the phage cocktail at an MOI of 100 inhibited the in vitro bactericidal activity of phages dramatically (7.58±1.36 CFU/mL with plasma versus 2.00±0.00 CFU/mL without plasma, 4 hours after phage treatment, P<0.0001). In sharp contrast, phage‐induced killing of P. aeruginosa 4 hours after administration of phage vB_PaeM_4002 was not altered significantly by the addition of 10% plasma (2.02±0.07 CFU/mL with plasma and 1.69±0.86 CFU/mL without plasma; Figure 4B, P=0.33). Of note, no significant effect on phage killing activity was observed when phages, instead of bacteria, were preincubated for 30 minutes with 10% plasma and washed twice in saline before being added to bacterial cultures in the absence of plasma (data not shown).
Figure 4. In vitro time‐kill assays after 30‐minute preincubation of the bacterial cells in 10% rat plasma.
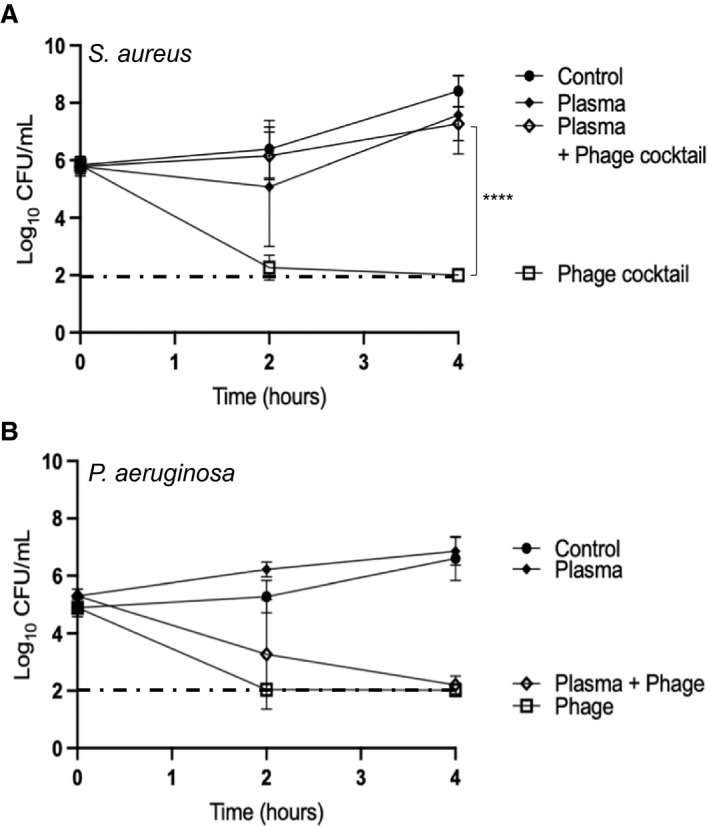
A, Phage cocktail at MOI=100 on S. aureus Laus102. B, Phage vB_PaeM_4002 at MOI=100 on P. aeruginosa strain ATCC® 15442™. The mean±SD of 3 independent experiments performed in triplicates are shown. The black dotted‐dashed line represents the limit of detection (100 CFU/mL). ****P<0.0001; 1‐way ANOVA with Tukey’s multiple comparisons test. CFU indicates colony forming unit; EE, experimental infective endocarditis; IE, infective endocarditis; MOI, multiplicity of infection; and PFU, plaque forming unit.
Discussion
It is unclear whether phage susceptibility assays performed in vitro predict phage behavior in vivo reliably and to what extent combining phages with standard of care antibiotics represents a potentially promising strategy. 24 In the current study, we designed a 2‐phage cocktail for the treatment of EE due to MSSA. We selected the recently isolated and highly lytic Herelleviridae phage vB_SauH_2002 and the Podoviridae phage 66, based on the supposition that its exopolysaccharide depolymerase activity might facilitate antimicrobial activity against bacteria that are embedded in biofilms. 25 Our standard in vitro phage susceptibility testing results (drop tests, turbidity, and time‐kill assays) were encouraging, and further suggested that the 2 phages indeed produced synergized activity against S. aureus biofilms. Extending these promising in vitro results, we observed a 2.6 log10 CFU/g decrease in cardiac vegetations from living rats treated with the phages for 24 hours, compared with mock‐therapy controls. However, the phage cocktail alone achieved only a bacteriostatic effect, failing to clear S. aureus EE completely despite effective in vivo phage amplification (also known as. phage auto‐dosing), 26 which produced a local MOI of ~103 in cardiac vegetations at the time of necropsy.
The therapeutic failure of phages alone in our S. aureus EE model contrasts with the efficacy of phage therapy alone observed in an S. aureus experimental ventilator‐associated pneumonia model. 17 , 27 , 28 This discrepancy could be related to differing mechanisms of disease. In the pneumonia model, bacterial toxins play a major role in lung tissue destruction and plasma proteins are not expected to interfere much with phage‐induced bacterial killing. 29 , 30 In contrast, plasma proteins in general and coagulation factors in particular play a major role in S. aureus endovascular infections, wherein plasma fibrinogen and fibronectin attach to the surfaces of circulating S. aureus cells, thereby promoting valve infection indirectly. 31 Hence, inhibition of S. aureus phage activity by blood proteins 32 might limit phage efficacy in the EE model employed in this study. Indeed, we confirmed that preincubating bacterial cells, but not phages, with rat plasma inhibited the bactericidal activity of the phages against S. aureus but not P. aeruginosa. Strong inhibition of S. aureus phage lytic activity by rabbit and human serum was demonstrated in the 1930s, leading researchers at that time to hypothesize that bacteria might be protected by what they called “a colloidal coating of serum,” preventing phage penetration into the bacterial cell surface. 33 , 34 , 35 Consistent with these almost century‐old observations, it was shown recently that S. aureus phage K propagation was impaired in whole blood, plasma, and serum 36 compared with propagation in growth media devoid of blood proteins. The molecular mechanism mediating plasma/serum‐mediated phage resistance has yet to be elucidated.
Evidence in support of the therapeutic potential of phage‐antibiotic synergisms has been growing. 37 A major potential drawback of the addition of antibiotics is their potential impact on phage pharmacokinetics given that inhibition of bacterial growth also limits the ability of phages to replicate within target bacteria. 26 When a subtherapeutic dosage of flucloxacillin was given concomitantly with our phage cocktail, we detected an altered phage pharmacokinetic profile evidenced by markedly reduced levels of circulating phages compared with levels seen when the phage treatment was administered alone. Notwithstanding, synergism of the 2 treatments emerged in vivo, with 75% of vegetations being found to be culture‐negative after only 24 hours of combined treatment.
Regarding resistance selection, all clones recovered from cardiac‐vegetation homogenates of rats treated with the phage cocktail/antibiotic combination remained susceptible to flucloxacillin (not shown), the Herelleviridae vB_SauH_2002, and the phage cocktail. Resistance to the Podoviridae phage 66 was observed in one third of the clones. Interestingly, a single phage 66‐resistant clone had a mutation leading to a frameshift likely deleterious in tarS. TarM and TarS are involved in α‐ and β‐O‐glycosylation of N‐acetyl‐D‐glucosamine residues of the wall teichoic acids, a main phage receptor in S. aureus. TarS‐mediated β‐O‐glycosylation has been shown to be required for S. aureus susceptibility to Podoviridae. 38 Whereas point mutations in tarM have been shown to alter susceptibility to Podoviridae, 38 the present work identified a tarS mutation that may underlie a Podoviridae‐resistance mechanism. Of note, the absence of genetic mutations in the remaining phage 66‐resistant clones, with the exception of transposase genes likely unrelated to the phage 66‐resistance phenotype, suggested the selection in our EE model of an adaptive mechanism of Podoviridae resistance mediated through the differential expression of α‐ and β‐N‐acetyl‐D‐glucosamine, as previously reported in other models of infectious diseases. 39 Finally, resistance to phage 66 did not affect virulence in a Galleria mellonella model of S. aureus infectious disease (not shown), affirming the notion that development of phage resistance is not always associated with an in vivo fitness cost. 40
Conclusions
Taken together, the present encouraging results are informative for phage therapy development, particularly for the treatment of S. aureus endovascular infections. For IE applications, S. aureus phages would not be given as monotherapy but rather in combination with antibiotics. 41 , 42 Indeed, phages may accelerate bacterial load reduction at infection sites at the start of therapy. This type of intervention may improve infection‐related cardiac dysfunction in general, 43 potentially truncating the period of systemic embolization risk, and thus, ultimately, shortening the duration of antibiotic therapy needed. Each of these considerations should be addressed in future translational and clinical trials.
To the best of our knowledge, this study is among very few investigations of synergism between phages and antibiotics in vivo, and the very first to report efficacy of phage‐antibiotic combinations for the treatment of S. aureus EE. In a previous study, we investigated P. aeruginosa IE, a relatively uncommon but difficult‐to‐treat infection, 44 based on the availability of the PhagoBurn phage cocktail. 45 The present model has far greater clinical relevance because S. aureus is the predominant IE pathogen. 46 Combination therapy outperformed either phage or antibiotic treatment alone, consistent with recent publications on this topic. 15 , 47 , 48 Deciphering the mechanisms behind the observed synergy however would require a thorough investigation (eg, transcriptomic analysis 49 ), something that is out of the scope of the present study.
Caution in relation to the use of phage therapy for IE treatment remains warranted. Synergism cannot be assumed and there are potential risks of adverse effects of combining phages with antibiotics. 50 Moreover, positive results in phage susceptibility testing may or may not translate into positive outcomes in vivo. A systemic understanding of in vivo interactions among bacteria, phages, antibiotics, and host defense mechanisms is needed to better define the role of phage therapy and its modality of prescription for the treatment of endovascular infections in humans.
Sources of Funding
This work was supported by the Swiss National Foundation (grants #320030_176216 and #CR31I3_166124 to YAQ and GR), and by an unrestricted grant from the Novartis Foundation (to YAQ).
Disclosures
GR worked as a consultant for Phagomed GmbH and is a former employee of the University of Lausanne, which licensed phage vB_SauH_2002 to PhagoMed GmBH.
Supporting information
Data S1
Tables S1–S3
Figure S1
Acknowledgments
We thank Aurélie Marchet and Jean Daraspe for providing outstanding technical assistance and Philippe Moreillon for critical advice and fruitful discussions. We thank the University of Lausanne for having provided us with the phage vB_SauH_2002. The bacterial strains noted with an asterisk in Table S1 were provided by NARSA (the Network on Antimicrobial Resistance in S. aureus) for distribution by BEI Resources, National Institute of Allergy and Infectious Disease, and National Institutes of Health.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023080
For Sources of Funding and Disclosures, see page 9.
References
- 1. Fowler VG, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293:3012–3021. doi: 10.1001/jama.293.24.3012 [DOI] [PubMed] [Google Scholar]
- 2. Wang A, Athan E, Pappas PA, Fowler VG Jr, Olaison L, Pare C, Almirante B, Munoz P, Rizzi M, Naber C, et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA. 2007;297:1354–1361. doi: 10.1001/jama.297.12.1354 [DOI] [PubMed] [Google Scholar]
- 3. Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435–1486. doi: 10.1161/CIR.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 4. Chirouze C, Alla F, Fowler VG, Sexton DJ, Corey GR, Chu VH, Wang A, Erpelding M‐L, Durante‐Mangoni E, Fernández‐Hidalgo N, et al. Impact of early valve surgery on outcome of Staphylococcus aureus prosthetic valve infective endocarditis: analysis in the International Collaboration of Endocarditis‐Prospective Cohort Study. Clin Infect Dis. 2015;60:741–749. doi: 10.1093/cid/ciu871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pirnay JP, Ferry T, Resch G. Recent progress towards the implementation of phage therapy in Western medicine. FEMS Microbiol Rev. 2021;fuab040:1–17. doi: 10.1093/femsre/fuab040 [DOI] [PubMed] [Google Scholar]
- 6. Rose T, Verbeken G, Vos DD, Merabishvili M, Vaneechoutte M, Lavigne R, Jennes S, Zizi M, Pirnay JP. Experimental phage therapy of burn wound infection: difficult first steps. Int J Burns Trauma. 2014;4:66–73. [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta P, Singh HS, Shukla VK, Nath G, Bhartiya SK. Bacteriophage therapy of chronic nonhealing wound: clinical study. Int J Low Extrem Wounds. 2019;18:171–175. doi: 10.1177/1534734619835115 [DOI] [PubMed] [Google Scholar]
- 8. Fadlallah A, Chelala E, Legeais JM. Corneal infection therapy with topical bacteriophage administration. Open Ophthalmol J. 2015;9:167–168. doi: 10.2174/1874364101509010167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rubalskii E, Ruemke S, Salmoukas C, Boyle EC, Warnecke G, Tudorache I, Shrestha M, Schmitto JD, Martens A, Rojas SV, et al. Bacteriophage therapy for critical infections related to cardiothoracic surgery. Antibiotics (Basel). 2020;9:232. doi: 10.3390/antibiotics9050232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doub JB, Ng VY, Johnson AJ, Slomka M, Fackler J, Horne B, Brownstein MJ, Henry M, Malagon F, Salvage BB. Bacteriophage therapy for a chronic MRSA prosthetic joint infection. Antibiotics. 2020;9:241. doi: 10.3390/antibiotics9050241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferry T, Kolenda C, Batailler C, Gustave C‐A, Lustig S, Malatray M, Fevre C, Josse J, Petitjean C, Chidiac C, et al. Phage therapy as adjuvant to conservative surgery and antibiotics to salvage patients with relapsing S. aureus prosthetic knee infection. Front Med (Lausanne). 2020;7:570572. doi: 10.3389/fmed.2020.570572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aslam S, Lampley E, Wooten D, Karris M, Benson C, Strathdee S, Schooley RT. Lessons learned from the first 10 consecutive cases of intravenous bacteriophage therapy to treat multidrug‐resistant bacterial infections at a single center in the United States. Open Forum Infect Dis. 2020;7:ofaa389. doi: 10.1093/ofid/ofaa389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petrovic Fabijan A, Lin RCY, Ho J, Maddocks S, Ben Zakour NL, Iredell JR, Khalid A, Venturini C, Chard R, Morales S, et al. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat Microbiol. 2020;5:465–472. doi: 10.1038/s41564-019-0634-z [DOI] [PubMed] [Google Scholar]
- 14. Gilbey T, Ho J, Cooley LA, Petrovic Fabijan A, Iredell JR. Adjunctive bacteriophage therapy for prosthetic valve endocarditis due to Staphylococcus aureus . Med J Aust. 2019;211:142–143 e1. doi: 10.5694/mja2.50274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oechslin F, Piccardi P, Mancini S, Gabard J, Moreillon P, Entenza JM, Resch G, Que YA. Synergistic interaction between phage therapy and antibiotics clears Pseudomonas Aeruginosa infection in endocarditis and reduces virulence. J Infect Dis. 2017;215:703–712. doi: 10.1093/infdis/jiw632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Resch G, Francois P, Morisset D, Stojanov M, Bonetti EJ, Schrenzel J, Sakwinska O, Moreillon P. Human‐to‐bovine jump of Staphylococcus aureus CC8 is associated with the loss of a beta‐hemolysin converting prophage and the acquisition of a new staphylococcal cassette chromosome. PLoS One. 2013;8:e58187. doi: 10.1371/journal.pone.0058187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prazak J, Iten M, Cameron DR, Save J, Grandgirard D, Resch G, Goepfert C, Leib SL, Takala J, Jakob SM, et al. Bacteriophages improve outcomes in experimental Staphylococcus aureus ventilator‐associated pneumonia. Am J Respir Crit Care Med. 2019;200:1126–1133. doi: 10.1164/rccm.201812-2372OC [DOI] [PubMed] [Google Scholar]
- 18. Tasse J, Cara A, Saglio M, Villet R, Laurent F. A steam‐based method to investigate biofilm. Sci Rep. 2018;8:13040. doi: 10.1038/s41598-018-31437-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chin JN, Jones RN, Sader HS, Savage PB, Rybak MJ. Potential synergy activity of the novel ceragenin, CSA‐13, against clinical isolates of Pseudomonas aeruginosa, including multidrug‐resistant P. aeruginosa . J Antimicrob Chemother. 2008;61:365–370. doi: 10.1093/jac/dkm457 [DOI] [PubMed] [Google Scholar]
- 20. Heraief E, Glauser MP, Freedman LR. Natural history of aortic valve endocarditis in rats. Infect Immun. 1982;37:127–131. doi: 10.1128/iai.37.1.127-131.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Que YA, Entenza JM, Francioli P, Moreillon P. The impact of penicillinase on cefamandole treatment and prophylaxis of experimental endocarditis due to methicillin‐resistant Staphylococcus aureus . J Infect Dis. 1998;177:146–154. [DOI] [PubMed] [Google Scholar]
- 22. Veloso TR, Chaouch A, Roger T, Giddey M, Vouillamoz J, Majcherczyk P, Que YA, Rousson V, Moreillon P, Entenza JM. Use of a human‐like low‐grade bacteremia model of experimental endocarditis to study the role of Staphylococcus aureus adhesins and platelet aggregation in early endocarditis. Infect Immun. 2013;81:697–703. doi: 10.1128/IAI.01030-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hernandez‐Morales AC, Lessor LL, Wood TL, Migl D, Mijalis EM, Cahill J, Russell WK, Young RF, Gill JJ. Genomic and biochemical characterization of acinetobacter podophage petty reveals a novel lysis mechanism and tail‐associated depolymerase activity. J Virol. 2018;92:1–18. doi: 10.1128/JVI.01064-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Segall AM, Roach DR, Strathdee SA. Stronger together? Perspectives on phage‐antibiotic synergy in clinical applications of phage therapy. Curr Opin Microbiol. 2019;51:46–50. doi: 10.1016/j.mib.2019.03.005 [DOI] [PubMed] [Google Scholar]
- 25. Hughes KA, Sutherland IW, Jones MV. Biofilm susceptibility to bacteriophage attack: the role of phage‐borne polysaccharide depolymerase. Microbiology. 1998;144(Pt 11):3039–3047. doi: 10.1099/00221287-144-11-3039 [DOI] [PubMed] [Google Scholar]
- 26. Danis‐Wlodarczyk K, Dabrowska K, Abedon ST. Phage therapy: the pharmacology of antibacterial viruses. Curr Issues Mol Biol. 2020;40:81–164. doi: 10.21775/cimb.040.081 [DOI] [PubMed] [Google Scholar]
- 27. Prazak J, Valente L, Iten M, Grandgirard D, Leib SL, Jakob SM, Haenggi M, Que YA, Cameron DR. Nebulized bacteriophages for prophylaxis of experimental ventilator‐associated pneumonia due to methicillin‐resistant Staphylococcus aureus . Crit Care Med. 2020;48:1042–1046. doi: 10.1097/CCM.0000000000004352 [DOI] [PubMed] [Google Scholar]
- 28. Prazak J, Valente L, Iten M, Federer L, Grandgirard D, Soto S, Resch G, Leib SL, Jakob SM, Haenggi M, et al. Benefits of aerosolized phages for the treatment of pneumonia due to methicillin‐resistant Staphylococcus aureus (MRSA): an experimental study in rats. J Infect Dis. 2021;jiab112:1–8. doi: 10.1093/infdis/jiab112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stulik L, Malafa S, Hudcova J, Rouha H, Henics BZ, Craven DE, Sonnevend AM, Nagy E. α‐Hemolysin activity of methicillin‐susceptible Staphylococcus aureus predicts ventilator‐associated pneumonia. Am J Respir Crit Care Med. 2014;190:1139–1148. doi: 10.1164/rccm.201406-1012OC [DOI] [PubMed] [Google Scholar]
- 30. Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. Poring over pores: α‐hemolysin and Panton‐Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–1406. doi: 10.1038/nm1207-1405 [DOI] [PubMed] [Google Scholar]
- 31. Que YA, Moreillon P. Infective endocarditis. Nat Rev Cardiol. 2011;8:322–336. doi: 10.1038/nrcardio.2011.43 [DOI] [PubMed] [Google Scholar]
- 32. Rakieten ML, Zalkan G, Rakieten TL. Bacteriophage inhibition by serum. Yale J Biol Med. 1935;7:541–554. [PMC free article] [PubMed] [Google Scholar]
- 33. Gratia A, Jaumain D. Dualité du principe lytique du colibacille et du staphylocoque. C R Des Séances Soc Biol Fil. 1921;85:882–884. [Google Scholar]
- 34. Gratia A, Mutsaars W. L’action inhibitrice du sérum normal sur la lyse du staphylocoque doré par les bactériophages staphylococciques polyvalents. C R Des Séances Soc Biol Fil. 1931;106:943–945. [Google Scholar]
- 35. Mutsaars W. De l’action protectrice exercée par le sérum normal sur les staphylocoques dorés, contre la fixation du bactériophage. C R Des Séances Soc Biol Fil. 1931;108:235–237. [Google Scholar]
- 36. Frati K, Malagon F, Henry M, Velazquez Delgado E, Hamilton T, Stockelman MG, Duplessis C, Biswas B. Propagation of S. aureus Phage K in presence of human blood. Biomed J Sci Tech Res. 2019;18:13815–13819. [Google Scholar]
- 37. Tagliaferri TL, Jansen M, Horz HP. Fighting pathogenic bacteria on two fronts: phages and antibiotics as combined strategy. Front Cell Infect Microbiol. 2019;9:22. doi: 10.3389/fcimb.2019.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li X, Gerlach D, Du X, Larsen J, Stegger M, Kuhner P, Peschel A, Xia G, Winstel V. An accessory wall teichoic acid glycosyltransferase protects Staphylococcus aureus from the lytic activity of Podoviridae. Sci Rep. 2015;5:17219. doi: 10.1038/srep17219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mistretta N, Brossaud M, Telles F, Sanchez V, Talaga P, Rokbi B. Glycosylation of Staphylococcus aureus cell wall teichoic acid is influenced by environmental conditions. Sci Rep. 2019;9:3212. doi: 10.1038/s41598-019-39929-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mangalea MR, Duerkop BA. Fitness trade‐offs resulting from bacteriophage resistance potentiate synergistic antibacterial strategies. Infect Immun. 2020;88:1–15. doi: 10.1128/IAI.00926-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kebriaei R, Lev KL, Stamper KC, Lehman SM, Morales S, Rybak MJ. Bacteriophage AB‐SA01 cocktail in combination with antibiotics against MRSA‐VISA strain in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2020;65:1–5. doi: 10.1128/AAC.01863-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lerche CJ, Schwartz F, Theut M, Fosbol EL, Iversen K, Bundgaard H, Hoiby N, Moser C. Anti‐biofilm approach in infective endocarditis exposes new treatment strategies for improved outcome. Front Cell Dev Biol. 2021;9:643335. doi: 10.3389/fcell.2021.643335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xiong YQ, Kupferwasser LI, Zack PM, Bayer AS. Comparative efficacies of liposomal amikacin (MiKasome) plus oxacillin versus conventional amikacin plus oxacillin in experimental endocarditis induced by Staphylococcus aureus: microbiological and echocardiographic analyses. Antimicrob Agents Chemother. 1999;43:1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramireddy S, Gudipati S, Zervos M. Expect the unexpected: a rare case of Pseudomonas aeruginosa endocarditis. Idcases. 2020;21:e00787. doi: 10.1016/j.idcr.2020.e00787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jault P, Leclerc T, Jennes S, Pirnay JP, Que Y‐A, Resch G, Rousseau AF, Ravat F, Carsin H, Le Floch R, et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double‐blind phase 1/2 trial. Lancet Infect Dis. 2019;19:35–45. doi: 10.1016/S1473-3099(18)30482-1 [DOI] [PubMed] [Google Scholar]
- 46. Vogkou CT, Vlachogiannis NI, Palaiodimos L, Kousoulis AA. The causative agents in infective endocarditis: a systematic review comprising 33,214 cases. Eur J Clin Microbiol Infect Dis. 2016;35:1227–1245. doi: 10.1007/s10096-016-2660-6 [DOI] [PubMed] [Google Scholar]
- 47. Morrisette T, Kebriaei R, Lev KL, Morales S, Rybak MJ. Bacteriophage therapeutics: a primer for clinicians on phage‐antibiotic combinations. Pharmacotherapy. 2020;40:153–168. doi: 10.1002/phar.2358 [DOI] [PubMed] [Google Scholar]
- 48. Rodriguez‐Gonzalez RA, Leung CY, Chan BK, Turner PE, Weitz JS. Quantitative models of phage‐antibiotic combination therapy. mSystems. 2020;5:1–13. doi: 10.1128/mSystems.00756-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Simon K, Pier W, Kruttgen A, Horz HP. Synergy between phage Sb‐1 and oxacillin against methicillin‐resistant Staphylococcus aureus . Antibiotics. 2021;10:849. doi: 10.3390/antibiotics10070849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Burmeister AR, Fortier A, Roush C, Lessing AJ, Bender RG, Barahman R, Grant R, Chan BK, Turner PE. Pleiotropy complicates a trade‐off between phage resistance and antibiotic resistance. Proc Natl Acad Sci USA. 2020;117:11207–11216. doi: 10.1073/pnas.1919888117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. CLSI: methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. In: CLSI standards M07. 11th, Wayne, PA: Clinical and Laboratory Institute; 2018. [Google Scholar]
- 52. Acs N, Gambino M, Brondsted L. Bacteriophage enumeration and detection methods. Front Microbiol. 2020;11:594868. doi: 10.3389/fmicb.2020.594868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007 [DOI] [PubMed] [Google Scholar]
- 54. Kutter E. Phage host range and efficiency of plating. Methods Mol Biol. 2009;501:141–149. [DOI] [PubMed] [Google Scholar]
- 55. Dell RB, Holleran S, Ramakrishnan R. Sample size determination. ILAR J. 2002;43:207–213. doi: 10.1093/ilar.43.4.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sakwinska O, Giddey M, Moreillon M, Morisset D, Waldvogel A, Moreillon P. Staphylococcus aureus host range and human‐bovine host shift. Appl Environ Microbiol. 2011;77:5908–5915. doi: 10.1128/AEM.00238-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kos VN, Desjardins CA, Griggs A, Cerqueira G, Van Tonder A, Holden MT, Godfrey P, Palmer KL, Bodi K, Mongodin EF, et al. Comparative genomics of vancomycin‐resistant Staphylococcus aureus strains and their positions within the clade most commonly associated with Methicillin‐resistant S. aureus hospital‐acquired infection in the United States. MBio. 2012;3:1–9. doi: 10.1128/mBio.00112-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed‐field gel electrophoresis typing of oxacillin‐resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113–5120. doi: 10.1128/JCM.41.11.5113-5120.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cameron DR, Ramette A, Prazak J, Entenza J, Haenggi M, Que YA, Resch G. Draft genome sequence of methicillin‐resistant Staphylococcus aureus strain AW7, isolated from a patient with bacteremia. Microbiol Resour Announc. 2019;8:1–2. doi: 10.1128/MRA.00806-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin‐resistant Staphylococcus aureus strain and a biofilm‐producing methicillin‐resistant Staphylococcus epidermidis strain. J Bacteriol. 2005;187:2426–2438. doi: 10.1128/JB.187.7.2426-2438.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Leuenberger A, Sartori C, Boss R, Resch G, Oechslin F, Steiner A, Moreillon P, Graber HU. Genotypes of Staphylococcus aureus: on‐farm epidemiology and the consequences for prevention of intramammary infections. J Dairy Sci. 2019;102:3295–3309. doi: 10.3168/jds.2018-15181 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S3
Figure S1


