Abstract
Background
Saphenous vein grafts (SVGs) are broadly used in coronary artery bypass grafting despite their inferior patency compared with arterial grafts. Recently, the no‐touch technique (NT), in which an SVG is harvested with a pedicle of perivascular adipose tissue (PVAT) without conduit distension, was shown to improve long‐term patency compared with conventional preparation (CV), wherein outer tissue is removed with distension. The NT was also reportedly associated with reduced atherosclerosis. Although endothelial damage provoked by conventional distension may underlie poor patency when CV is performed, the precise mechanisms underlying the salutary effects of the NT have been unclear.
Methods and Results
Residual SVGs prepared with CV (CV‐SVGs) or NT (NT‐SVGs) were obtained during coronary artery bypass grafting. Nitric oxide (NO2 −/NO3 − (NOx)) levels after 24 hours of tissue culture were quantified. The protein expression and localization were analyzed. The isometric force of SVG strips was measured. NT‐SVGs showed superior NOx production to CV‐SVGs. PVAT generated the majority of NOx in NT‐SVGs. PVAT highly expressed arginosuccinate synthase 1, a rate‐limiting enzyme in the molecular circuit for NO synthesis, thereby continuously providing the substrate for NO. A substantial level of endothelial NO synthase was also expressed in PVAT. Pharmacological inhibition of arginosuccinate synthase 1 or endothelial NO synthase significantly suppressed the NOx production in NT‐SVGs. PVAT induced vasorelaxation through NO production, even in the endothelium‐denuded SVG strips.
Conclusions
Preserving PVAT was predominantly involved in the superior NOx production in NT‐SVGs. Since NO plays crucial roles in suppressing atherosclerosis, this mechanism may greatly contribute to the excellent patency in NT‐SVGs.
Keywords: conventional preparation, coronary artery bypass grafting, nitric oxide, no‐touch technique, saphenous vein graft
Subject Categories: Cardiovascular Surgery, Vascular Biology
Coronary artery bypass grafting (CABG) and percutaneous coronary intervention are performed as primary therapy for ischemic heart disease. CABG has been shown to be superior to percutaneous coronary intervention for reducing morbidity and mortality in patients with multivessel disease or diabetes. Arterial revascularization has been advocated because of its superior patency; however, saphenous vein grafts (SVGs) are still broadly used because of their ease of manipulation. 1 Previously, SVGs were usually prepared with the conventional method (CV), which involves the removal of outer tissue and conduit distension against spastic reaction. However, their long‐term patency rate was significantly inferior to that of the internal thoracic artery (ITA). 2 Vein graft adaptation to the arterial circulation may accelerate atherosclerosis in SVGs.
In the mid‐1990s, the no‐touch technique (NT), which harvests the saphenous vein (SV) with its pedicle of perivascular adipose tissue (PVAT), was introduced. 3 , 4 SVGs prepared via the NT (NT‐SVGs), which preserve the outer tissue intact without conduit distension, showed reduced atherosclerosis compared with those prepared via the CV (CV‐SVGs) at 8.5 years after CABG. 3 A randomized controlled trial reported the superior patency of NT‐SVGs to CV‐SVGs, which was comparable to that of the left ITA, at 16 years after surgery. 4 Subsequently, the 2018 European Society of Cardiology/European Association for Cardio‐Thoracic Surgery Guidelines on myocardial revascularization recommended the NT for preparing SVGs. 5
Although the routine use of the NT has spread worldwide, the predominant mechanism through which NT provides its salutary effect has remained elusive. Evidence suggests that CV‐mediated distension damages the endothelium and induces inflammation, a potential cause of atherosclerosis and thrombosis. 6 Endothelial denudation was reproducibly observed after CV. 7 It is conceivable that endothelial damage caused by conventional distension underlies the poor patency of CV‐SVGs; however, atherosclerosis and thrombosis are attributable to many factors, so factors other than the endothelium may be involved in the mechanism underlying the increased patency rate in the NT group. For instance, the vasa vasorum, which is preserved in NT‐SVGs, was also suggested to suppress atherosclerosis. In addition, we actually found that the endothelial morphology in CV‐SVGs was preserved to a comparable degree to that in NT‐SVGs when distending pressure was reduced (<300 mm Hg). 8 Given that the distending pressure varies among surgeons, and only a higher pressure (>700 mm Hg) induces endothelial damage, a substantial portion of patients with CV‐SVGs may have a preserved endothelium.
In the present study, we examined the functionality of the endothelium using residual CV‐SVGs, NT‐SVGs, and ITAs obtained during CABG before anastomosis. Because the production of nitric oxide (NO) is a major function of endothelium, we quantified and compared it among CV‐SVGs, NT‐SVGs and ITAs ex vivo. We found that NT‐SVGs showed superior NO2 −/NO3 − (NOx) production to CV‐SVGs. Unexpectedly, our result suggested that PVAT generates the majority of NOx in NT‐SVGs. PVAT highly expresses arginosuccinate synthase 1 (ASS1), a rate‐limiting enzyme in the citrulline‐arginosuccinate‐arginine cycle, thereby continuously providing the substrate for NO synthesis. A substantial level of endothelial NO synthase (eNOS) is also expressed in PVAT. Thus, this study suggests that preserving PVAT is predominantly involved in the superior production of NOx in NT‐SVGs in comparison to CV‐SVGs. Given that NO regulates vascular homeostasis through multiple factors (eg, inhibition of atherosclerosis and thrombosis, regulation of vascular tone, etc.), this mechanism may greatly contribute to the excellent patency of NT‐SVGs.
Although previous studies with animal models have described the role of PVAT in vascular homeostasis, 9 it is difficult to simply translate the results from animals to human atherosclerosis. Although studies with human SVs have clarified the significance of PVAT, whether or how PVAT contributes to NO production is unclear, because no study has compared NO production between the endothelium and PVAT. 10 Accordingly, this is the first study to delineate the crucial role of PVAT in the production of NOx in human NT‐SVGs. This finding may provide significant insight into vascular biology and surgical methodologies, offering clues to the development of novel strategies for the regulation of vascular homeostasis.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files. A Supplemental Methods section is available in Data S1.
Ethics Approval and Consent to Participate
This study was approved by Institutional Review Boards of Yamaguchi University Hospital (H2019‐092) and followed the Declaration of Helsinki and the ethical standards of the responsible committee on human experimentation. All participants granted their informed consent.
Surgical Aspects
The details were as described previously. 8 The patient characteristics are summarized in Tables S1 and S2.
SV Harvesting Techniques
The vein was removed from the leg after dissection, connected to the cannula inserted into the femoral artery, and dilated with arterial pressure for 10 minutes before sewing of anastomoses (Figure S1). More information is described in Data S1.
NOx Measurement
The SV was cut into 4‐mm ring segments, placed in 24‐well plates, and tissue‐cultured at 37°C for 24 hours. 11 Serum‐free medium supplemented with antibiotics was used in the presence or absence of acetylcholine (10−6 M), N omega‐Nitro‐L‐arginine methyl ester hydrochloride (10−4 M), and α‐methyl‐DL‐aspartic acid (10−3 M). 12 The NOx content in the conditioned medium was measured with the improved Griess method (QuantiChrom Nitric Oxide Assay Kit; BioAssay Systems, Hayward, CA, USA), which allows for the reliable quantitation of NO2 − (nitrite)/NO3 − (nitrate) (Table S3). 11 More information is described in Data S1.
Immunoblot and Immunohistochemical Analyses
The details were as described previously. 13
Measurements of Isometric force in SVG
Measurements of isometric force of vascular strips (1.0 × 4.0 mm) were carried out as described previously. 14
Results
The residual SVG harvested via the CV or NT was obtained (Figure 1A). After 24 hours of tissue culture, the NOx produced by each SVG method ex vivo was measured (Figure 1B). Because the endothelium is exposed to blood flow and sensitized by shear stress and vasoactive mediators in vivo, we tissue cultured SVGs in the absence or presence of acetylcholine in order to evaluate the endothelial function at baseline or during stimulation, respectively. A low level of NOx was produced by the CV‐SVG at baseline (Figure 1B). There was not obvious change in the level of NOx in response to acetylcholine stimulation in the CV group. In contrast, a modest level of NOx was produced by the NT‐SVG at baseline (Figure 1B). The level of NOx was significantly upregulated in response to acetylcholine stimulation and this significance was abrogated in the presence of N omega‐Nitro‐L‐arginine methyl ester hydrochloride, an inhibitor of NOS, in the NT group. This result suggests that the NT‐SVG preserves NOx production more markedly than the CV‐SVG. When the value of NOx was normalized by dry weight of SV, the NT‐SVG significantly displayed a high production of NOx during acetylcholine treatment (Table S4), so the same conclusion was obtained. Because the NT‐SVG contains SV and outer tissue, we also normalized the value with the dry weight of total tissue (Table S5). Although statistical significance was abrogated by this normalization, we believe that this is due to the surgical characteristics of NT‐SVG and does not deny the superior productivity of NOx in NT‐SVG.
Figure 1. PVAT plays a major role in the production of NOx in NT‐SVGs.

The residual SVG harvested via the CV, the residual SVG harvested via the NT, and PVAT were tissue cultured in the presence or absence of acetylcholine (10−6 M) and L‐NAME (10−4 M) for 24 h. The levels of NOx in the conditioned medium were measured. Box plots show the center line as the median, box limits as the upper and lower quartiles, whiskers as the minimum and maximum values, dots as outlier values, and “X” as the average value. (A) Representative images of the CV, NT, and ITA are shown. Scale bar, 1 cm. (B) NT‐SVGs produced more NOx than CV‐SVGs; this occurred in response to acetylcholine in an eNOS‐dependent manner (n=9–11, Steel‐Dwass test, # P<0.05 vs CV control, † P<0.05 vs CV acetylcholine (+), ‡ P<0.05 vs CV acetylcholine(+) L‐NAME(+)). (C) PVAT produced a substantial level of NO. When a CV‐SVG specimen was incubated with PVAT, the NOx level was restored to a similar degree to that observed in NT‐SVG specimens (n=5‐10, Steel test, # P<0.05 vs CV control). (D) The NOx levels were compared between vessels harvested via the NT without manual distension (Distension (−)) and those harvested via the NT with manual distension (Distension (+)). There was no significant difference between the groups (n=5, N.S., not significant). (E) The ITA produced a relatively high level of NOx (n=5, Student’s t‐test, # P<0.05 vs control). CV indicates conventional preparation; eNOS, endothelial NO synthase; ITA, internal thoracic artery; L‐NAME, N omega‐Nitro‐L‐arginine methyl ester hydrochloride; NO, nitric oxide; NOx, NO2 −/NO3 −; NT, no‐touch technique; PVAT, perivascular adipose tissue; and SVG, saphenous vein graft.
Although endothelial damage provoked by conventional distension might impair the NOx production in the CV‐SVG, the perivascular tissue preserved in the NT‐SVG might play a role in producing NOx. These 2 factors may account for the difference in the NOx production between SVGs obtained using the 2 techniques. Because PVAT is a major component of perivascular tissues, we first quantified the NOx produced by PVAT. Interestingly, a substantial level of NOx was produced by PVAT both at baseline and during acetylcholine stimulation (Figure 1C). When CV‐SVG was incubated with PVAT, the level of NOx was significantly upregulated and restored to a level similar to that observed in the NT group (Figure 1B and 1C). This result suggests that PVAT is a major source of NOx in the NT‐SVG.
Next, we examined whether or not NOx production was impaired by conventional distension. We measured the NOx generated by the NT‐SVG in the absence or presence of manual distension. As a result, the NOx production was not significantly affected by conduit distension (Figure 1D). Because we used a relatively low pressure (<300 mm Hg) for manual distension, endothelial damage might not have been significantly induced there. Thus, PVAT, rather than endothelium, is suggested to be associated with the superior production of NOx in the NT‐SVG.
As a reference, we examined the NOx production in the ITA. The level of NOx produced by the ITA was comparable or superior to that obtained by the NT‐SVG both at baseline and during acetylcholine stimulation (Figure 1E). The deviations in the data obtained by the ITA were smaller than those obtained by SVGs (Figure 1B through 1E). Given that the ITA clinically displays the highest patency among all grafts, our results regarding NOx production appeared to be positively correlated with their patency rates.
It would have been ideal to evaluate the NOx production in the CV‐SVG, NT‐SVG, CV‐SVG with PVAT, and NT‐SVG without PVAT using the same SVG specimen, both at baseline and during acetylcholine stimulation. However, this experiment was technically difficult because of the limited length of the residual SVG. Instead, we examined these 4 groups derived from the same SVG specimen only at baseline. As shown, the levels of NOx from the NT‐SVG, CV‐SVG with PVAT, and NT‐SVG without PVAT were significantly higher than that from the CV‐SVG (Figure S2). Interestingly, the level of NOx from the NT‐SVG was significantly higher than that from the NT‐SVG without PVAT (Figure S2). This result further supports the pivotal role of PVAT in NOx production.
We subsequently investigated the molecular mechanisms by which PVAT produces high levels of NOx. NOS catalyzes the conversion of L‐arginine to L‐citrulline and Nω ‐hydroxy‐L‐arginine, an intermediate that subsequently forms NO. 15 The by‐product of citrulline is converted to arginosuccinate by ASS1, followed by de novo synthesis of arginine by arginosuccinate lyase (Figure 2A). In this molecular circuit, ASS1 is considered a rate‐limiting enzyme. 15 We examined the protein levels of ASS1 and eNOS in CV‐SVG, PVAT, and ITA specimens. Interestingly, the level of ASS1 in PVAT was significantly higher than that in the CV‐SVG and ITA specimens (Figure 2B). A substantial level of eNOS was also expressed in PVAT (Figure 2B). α‐methyl‐DL‐aspartic acid, a pharmacological inhibitor of ASS1, 12 significantly ameliorated the NOx production in response to acetylcholine in NT‐SVG (Figure 2C). These results suggest that ASS1 and eNOS in PVAT play an important role in producing NOx in the NT‐SVG. The same conclusion was obtained when the value of NOx was normalized by the dry weight of the SV (Table S6) or total tissue (Table S7).
Figure 2. PVAT expresses a high level of ASS1, a rate limiting enzyme for the synthesis of NO, in NT‐SVGs.

(A) A schematic illustration depicting the molecular circuit of NO synthesis. (B) The ASS1 and eNOS levels in the CV‐SVG, PVAT, and ITA were analyzed by immunoblotting. Perilipin is an adipocyte marker. GAPDH is a loading control (n=7–10, Steel test, # P<0.05 vs CV). Representative images and summaries are shown. Error bars represent the SD. (C) NOx levels were measured in conditioned medium of NT‐SVG in the presence or absence of acetylcholine (10−6 M) or MDLA (10−3 M), a specific inhibitor of ASS1, after 24 h of tissue culture. (n=6, Dunnett test, # P<0.05 vs control). Error bars represent the SD. (D, E) The localization of ASS1 in CV‐SVGs (D) and NT‐SVGs (E) was assessed by immunohistochemistry. vWF is an endothelial marker. Representative images are shown. The demarcated area shows a higher magnification view. Scale bar, 200 µm. ASS1 indicates arginosuccinate synthase 1; CV, conventional preparation; eNOS, endothelial NO synthase; EVG, Elastica Van Gieson staining; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; ITA, internal thoracic artery; L‐NAME, N omega‐Nitro‐L‐arginine methyl ester hydrochloride; MDLA, α‐methyl‐DL‐aspartic acid; NO, nitric oxide; NOx, NO2 −/NO3 −; NT, no‐touch technique; PVAT, perivascular adipose tissue; SVG, saphenous vein graft; and vWF, Von Willebrand Factor.
Although countless studies have investigated eNOS in vasculature, the characteristics of ASS1 have been poorly understood. To clarify the localization of these molecules, we conducted an immunohistochemical staining of CV‐SVG and NT‐SVG specimens. The signal of von Willebrand Factor, a representative marker of the endothelium, 8 was equally detected throughout the vascular wall in both types of SVG, suggesting that the endothelium was morphologically preserved in both (Figure 2D and 2E). The signal of ASS1 was predominantly found in the endothelium of CV‐SVG specimens, suggesting a significant role of the endothelium in producing arginosuccinate (Figure 2D). In addition to the endothelium, the strong expression of ASS1 was detected in PVAT in NT‐SVG specimens (Figure 2E). The signal of ASS1 appeared in adipocytes, encircling lipid droplets. This result further supports that preservation of PVAT is a crucial factor in the high level of ASS1 expressed in NT‐SVGs. As expected, the signal of eNOS was predominantly observed in the endothelium in both types of SVG (Figure S3). The signal of eNOS was weakly and fragmentally observed in adipocytes in NT‐SVG specimens (Figure S3). Taken together with the results of the immunoblot analyses (Figure 2B), we concluded that PVAT significantly expresses eNOS and that the protein level in PVAT is not less than that in the CV‐SVG.
To determine whether or not NO derived from PVAT functionally protects SVGs, we measured the vasorelaxation in the CV‐SVG with or without PVAT. Acetylcholine induced weak relaxation during 40 mM K+‐induced precontraction in the CV‐SVG (Figure 3A left and 3C). In the presence of PVAT, the CV‐SVG displayed more relaxation than in its absence in response to acetylcholine (Figure 3A middle and 3C), and this significance was abrogated by pretreatment with N omega‐Nitro‐L‐arginine methyl ester hydrochloride (Figure 3A right and 3C). This result suggests that PVAT induces vasorelaxation through NO. Next, we removed the endothelium in CV‐SVGs and conducted the same experiment. In the CV‐SVG without endothelium, acetylcholine induced contraction during 40 mM K+‐induced precontraction (Figure 3B left and 3D). In the presence of PVAT, CV‐SVG displayed the significant relaxation in response to acetylcholine even without endothelium (Figure 3B middle and 3D), and this significance was abrogated by pretreatment with N omega‐Nitro‐L‐arginine methyl ester hydrochloride (Figure 3B right and 3D). This result suggests that NO derived from PVAT diffuses to vascular smooth muscle and functionally induces vasorelaxation, even in the endothelium‐denuded SVG.
Figure 3. NO derived from PVAT functionally induces vasorelaxation in SVGs.
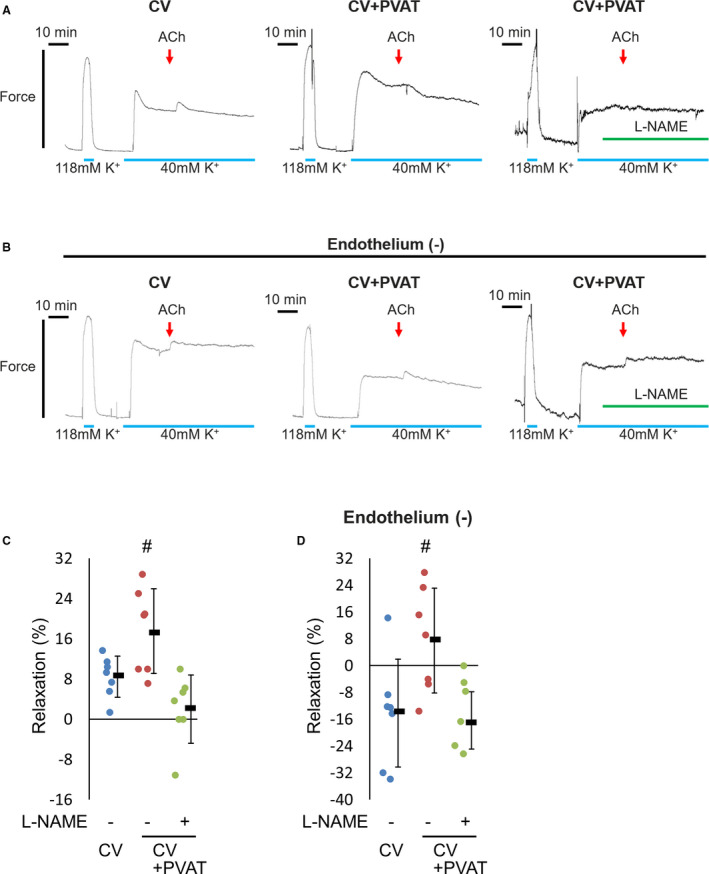
The vascular tone was examined in the residual SVG harvested via the CV in the presence or absence of PVAT. The effects of acetylcholine (10−4 M) and L‐NAME (10−4 M) were tested. The relaxation was described as a percentage of the response to the steady‐state tension induced by 40 mM K+. Error bars represent the SD. (A) Intact strips were used. Representative recordings of the CV (left), CV with PVAT (middle) and CV with PVAT pretreated with L‐NAME (right) are shown. (B) Endothelium‐denuded strips were used. Representative recordings of the CV (left), CV with PVAT (middle), and CV with PVAT pretreated with L‐NAME (right) are shown. (C) Summary of intact strips of SVGs (n=7, Dunnett test, # P<0.05 vs CV). (D) Summary of endothelium‐denuded strips of SVGs (n=7, Dunnett test, # P<0.05 vs CV). CV indicates conventional preparation; L‐NAME, N omega‐Nitro‐L‐arginine methyl ester hydrochloride; NO, nitric oxide; PVAT, perivascular adipose tissue; and SVG, saphenous vein graft.
Discussion
In the present study, we clarified for the first time that PVAT is the predominant source of NOx in the NT‐SVG by analyzing residual SVGs obtained during CABG before grafting. PVAT expresses high levels of ASS1 and eNOS, which is advantageous in the continuous cycling of the molecular circuit for NO synthesis. This mechanism may be deeply associated with the superior patency of NT‐SVGs in comparison to CV‐SVGs.
Previous studies with animal models have suggested that PVAT plays a role in NO production. 9 Studies with high‐fat diet and/or genetic manipulation models also reported the pathophysiological significance of PVAT in mediating inflammation, eNOS uncoupling, and other processes. 9 However, few studies have compared the NO production among vascular components, such as the endothelium and PVAT. To our knowledge, the present study is the first to identify PVAT as a predominant source of NO in human vasculature.
In the last experiment, it was demonstrated that PVAT functionally relaxes SVG specimens through NO production. In the intact CV‐SVG, PVAT significantly induced vasorelaxation (17.52%±8.43%, Figure 3C). In the endothelium‐denuded CV‐SVG, the PVAT‐mediated vasorelaxation (7.48%±15.64%, Figure 3D) was less marked than that seen in the intact CV‐SVG; nonetheless, this result suggests that PVAT plays an important role in relaxing the SVG as well as the endothelium.
Conclusions
In conclusion, the present study clarified—for the first time—that PVAT is a predominant source of NOx in the NT‐SVG, which may be significantly associated with its superior patency. This finding has shed light on a long‐standing enigma and will contribute to the future development of innovative strategies for the regulation of vascular homeostasis.
Limitations
Several limitations associated with the present study warrant mention. More information is described in Data S2.
Sources of Funding
This work was supported in part by Grants‐in‐Aids for Scientific Research (B) (19H03739 to K.H.) and Young Scientists (19K17601 to T.S.) from the Japan Society for the Promotion of Science; and grants from MSD Life Science Foundation; Cardiovascular Research Fund; The Ichiro Kanehara Foundation; and The Mochida Memorial Foundation.
Disclosures
None.
Supporting information
Data S1. Supplemental Methods
Data S2. Limitations
Tables S1–S7
Figures S1–S3
Acknowledgments
We thank Yukari Hironaka for technical support and Dr. Brian Quinn for his linguistic comments and help with the article.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020637
For Sources of Funding and Disclosures, see page 7.
Contributor Information
Toshiro Saito, Email: saitot@yamaguchi-u.ac.jp.
Kimikazu Hamano, Email: kimikazu@yamaguchi-u.ac.jp.
References
- 1. Gaudino M, Lorusso R, Rahouma M, Abouarab A, Tam DY, Spadaccio C, Saint‐Hilary G, Leonard J, Iannaccone M, D'Ascenzo F, et al. Radial artery versus right internal thoracic artery versus saphenous vein as the second conduit for coronary artery bypass surgery: a network meta‐analysis of clinical outcomes. J Am Heart Assoc. 2019;8:e010839. doi: 10.1161/JAHA.118.010839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sabik JF 3rd, Lytle BW, Blackstone EH, Houghtaling PL, Cosgrove DM. Comparison of saphenous vein and internal thoracic artery graft patency by coronary system. Ann Thorac Surg. 2005;79:544–551. doi: 10.1016/j.athoracsur.2004.07.047 [DOI] [PubMed] [Google Scholar]
- 3. Johansson BL, Souza DS, Bodin L, Filbey D, Loesch A, Geijer H, Bojo L. Slower progression of atherosclerosis in vein grafts harvested with 'no touch' technique compared with conventional harvesting technique in coronary artery bypass grafting: an angiographic and intravascular ultrasound study. Eur J Cardiothorac Surg. 2010;38:414–419. doi: 10.1016/j.ejcts.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 4. Samano N, Geijer H, Liden M, Fremes S, Bodin L, Souza D. The no‐touch saphenous vein for coronary artery bypass grafting maintains a patency, after 16 years, comparable to the left internal thoracic artery: a randomized trial. J Thorac Cardiovasc Surg. 2015;150:880–888. doi: 10.1016/j.jtcvs.2015.07.027 [DOI] [PubMed] [Google Scholar]
- 5. Sousa‐Uva M, Neumann F‐J, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J‐P, Falk V, Head SJ, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg. 2019;55:4–90. doi: 10.1093/ejcts/ezy289 [DOI] [PubMed] [Google Scholar]
- 6. Thatte HS, Khuri SF. The coronary artery bypass conduit: I. Intraoperative endothelial injury and its implication on graft patency. Ann Thorac Surg. 2001;72:S2245–S2252. doi: 10.1016/S0003-4975(01)03272-6 [DOI] [PubMed] [Google Scholar]
- 7. Dashwood MR, Loesch A. Surgical damage of the saphenous vein and graft patency. J Thorac Cardiovasc Surg. 2007;133:274–275. doi: 10.1016/j.jtcvs.2006.09.029 [DOI] [PubMed] [Google Scholar]
- 8. Saito T, Kurazumi H, Suzuki R, Matsuno Y, Mikamo A, Hamano K. Preserving the endothelium in saphenous vein graft with both conventional and no‐touch preparation. J Cardiothorac Surg. 2020;15:317. doi: 10.1186/s13019-020-01352-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang L, Garcia‐Barrio MT, Chen YE. Perivascular adipose tissue regulates vascular function by targeting vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2020;40:1094–1109. doi: 10.1161/ATVBAHA.120.312464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, Shirodaria C, Sayeed R, Petrou M, De Silva R, et al. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation. 2013;127:2209–2221. doi: 10.1161/CIRCULATIONAHA.112.001133 [DOI] [PubMed] [Google Scholar]
- 11. Ying WZ, Aaron K, Sanders PW. Dietary salt activates an endothelial proline‐rich tyrosine kinase 2/c‐Src/phosphatidylinositol 3‐kinase complex to promote endothelial nitric oxide synthase phosphorylation. Hypertension. 2008;52:1134–1141. doi: 10.1161/HYPERTENSIONAHA.108.121582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao J, Suyama A, Chung H, Fukuda T, Tanaka M, Matsui T. Ferulic acid enhances nitric oxide production through up‐regulation of argininosuccinate synthase in inflammatory human endothelial cells. Life Sci. 2016;145:224–232. doi: 10.1016/j.lfs.2015.12.044 [DOI] [PubMed] [Google Scholar]
- 13. Saito T, Uchiumi T, Yagi M, Amamoto R, Setoyama D, Matsushima Y, Kang D. Cardiomyocyte‐specific loss of mitochondrial p32/C1qbp causes cardiomyopathy and activates stress responses. Cardiovasc Res. 2017;113:1173–1185. doi: 10.1093/cvr/cvx095 [DOI] [PubMed] [Google Scholar]
- 14. Morikage N, Kishi H, Sato M, Guo F, Shirao S, Yano T, Soma M, Hamano K, Esato K, Kobayashi S. Cholesterol primes vascular smooth muscle to induce Ca2 sensitization mediated by a sphingosylphosphorylcholine‐Rho‐kinase pathway: possible role for membrane raft. Circ Res. 2006;99:299–306. doi: 10.1161/01.RES.0000235877.33682.e9 [DOI] [PubMed] [Google Scholar]
- 15. Goodwin BL, Solomonson LP, Eichler DC. Argininosuccinate synthase expression is required to maintain nitric oxide production and cell viability in aortic endothelial cells. J Biol Chem. 2004;279:18353–18360. doi: 10.1074/jbc.M308160200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods
Data S2. Limitations
Tables S1–S7
Figures S1–S3


