Abstract
Background
Individuals of the same chronological age may exhibit diverse susceptibilities to death. However, few studies have investigated the associations between blood pressure and the accelerated aging.
Methods and Results
A cross‐sectional study was conducted in 288 adults aged ≥50 years. We assessed the DNA methylation‐based measures of biological age using CpG sites on the Illumina HumanMethylationEPIC BeadChip. Epigenetic age acceleration metrics were derived by regressing residuals (ΔAge) and ratios (aging rate) of DNA methylation age on chronological age. Dose‐response relationships between blood pressure and epigenetic age acceleration were quantified using multiple linear regression and restricted cubic regression models. We found that each 10–mm Hg increase in systolic blood pressure was associated with 0.608 (95% CI, 0.231–0.984) years increase in ΔAge and 0.007 (95% CI, 0.002–0.012) increase in aging rate; meanwhile, for pulse pressure, the increase was 1.12 (95% CI, 0.625–1.61) years for ΔAge and 0.013 (95% CI, 0.007–0.020) for aging rate. Subgroup analysis showed that the significant associations of systolic blood pressure and pulse pressure with epigenetic age acceleration appeared to be limited to women, although interactions between blood pressure and sex were not significant (P values for interaction >0.05). The combination of women and hypertension was associated with a much higher increase in ΔAge (β [95% CI], 4.05 [1.07–7.02]) and aging rate (β [95% CI], 0.047 [0.008–0.087]), compared with male participants without hypertension.
Conclusions
Our findings suggested that high systolic blood pressure and pulse pressure were associated with the epigenetic age acceleration, providing important clues for relationships between blood pressure and epigenetic aging.
Keywords: blood pressure, DNA methylation age, epigenetic age acceleration
Subject Categories: Aging, Epidemiology, Cardiovascular Disease, Epigenetics
Nonstandard Abbreviations and Acronyms
- DBP
diastolic blood pressure
- DMR
differentially methylated region
- DNAm age
DNA methylation age
- PP
pulse pressure
- SBP
systolic blood pressure
Clinical Perspective
What Is New?
In the study, we assessed epigenetic age acceleration by DNA methylation‐based measures and found that high systolic blood pressure and pulse pressure are significantly associated with increased epigenetic age acceleration with a dose‐response trend.
The associations of blood pressure with epigenetic age acceleration were influenced by sexual difference, which appeared to be limited to women, and the combination of women and hypertension was associated with a much higher increase in accelerated aging than the combined men and nonhypertension.
What Are the Clinical Implications?
Our findings indicate significant relationships between blood pressure and DNA methylation‐derived measures of epigenetic age acceleration in older adults, providing important clues for identifying effective antiaging interventions in controlling blood pressure.
The aging population is increasing in both developed and developing parts of the world. With remarkable improvements in life expectancy, aging has become a critical public health priority. 1 Accumulating evidence indicated that aging is characterized by accumulation of chronic diseases, among which hypertension or hypotension represents one of the most prevalent and potentially modifiable risk factors. 2 , 3 Most observational studies have found that changes in systolic blood pressure (SBP) or diastolic blood pressure (DBP) were associated with a shortened life span. 4 , 5 , 6 However, whether blood pressure contributes significantly to the accelerated aging remains unclear.
It is well known that aging is an inevitable biological process, but individual decline in physical function is associated with accelerated aging, resulting in different lifespans between people. 7 Individuals of the same chronological age may exhibit different predisposition to age‐related conditions or death, which is likely reflective of the discrepancy in biological age. 7 Because chronological age is an imperfect proxy of the aging process, mounting biomarkers have been reported as predictors of biological age, such as telomere length, mitochondrial damage, and epigenetic alterations. 8 , 9 Recent evidence indicated that DNA methylation‐derived biomarkers exhibit statistically age‐associated physiological decline. 10 , 11 Studies have observed that age‐dependent changes in DNA methylation have been reported to be involved in millions of CpG methylations in the human genome. 12 , 13 With the availability of oligonucleotide arrays to vast CpG sites in the human genome, sets of CpGs coupled with a mathematical algorithm were imputed to assess DNA methylation age (DNAm age) referring to epigenetic clocks. 14 , 15 Pivotal aspects of age‐related conditions are demonstrated to be captured by DNAm age, which has been reported to well predict lifespan of individuals. 13 , 16 The application of DNAm age can invariably reveal differences in physiological status among individuals with the same chronological age, where DNAm age exceeding chronological age is described as the epigenetic age acceleration. 13
Because of the increasing mortality of cardiovascular events, it is crucial to explore the effects of blood pressure on the epigenetic age acceleration to emphasize the importance of blood pressure management. In the present study, we measured DNA methylation at ≈850 000 CpGs in a cross‐sectional study and quantified the associations of blood pressure with epigenetic age acceleration by DNA methylation‐based measures.
METHODS
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population
This was a cross‐sectional study (n=288), and participants originated from rural towns of Hongshuihe region, located in southern China, with a relatively high proportion of long‐lived people. 17 Three towns (Wuzhuan, Sanshi, and Donglan) were selected using a stratified and cluster sampling approach. Individuals aged ≥50 years with residence in selected towns for >10 years were recruited to participate in the survey in appointed clinics with the assistance of local medical staff. Individuals were excluded if (1) they were aphasic, deaf, or blind; (2) they had psychiatric disturbances; or (3) they experienced severe organic diseases, such as stroke, cachexia, myocardial infarction, or cancers. Recruited participants received detailed standardized questionnaire; and information, such as age, sex, education, history of disease, and lifestyles, was collected by face‐to face interview. Anthropometric metrics, such as height and weight, were conducted by qualified medical practitioners. With an exclusion of 8 individuals who refused to provide blood samples or failed to complete investigation survey, a total of 280 participants were included in the current study for subsequent analysis. The study was approved by the ethics committee of Guangxi Medical University. Each participant enrolled was informed of why and what the survey was performed and gave written informed consent before investigation.
Measurement of Blood Pressure
All the participants were asked not to exercise, smoke, and have stimulants for at least half an hour before the measurement. After sitting for at least 5 minutes, blood pressure was measured for most subjects in a sitting position with an electronic sphygmomanometer by placing the center of the cuff's rubber bag on the brachial artery and keeping the lower edge of the cuff 2 to 3 cm away from the elbow line. For centenarians unable to sit upright, blood pressure was measured in a recumbent position. SBP and DBP were obtained on the basis of readings of the machine, and 2 or 3 measurements were taken to ensure the reading was accurate. Pulse pressure (PP) and mean arterial pressure (MAP) were calculated as follows: PP=SBP–DBP; MAP=DBP+PP÷3. Participants with measured SBP ≥140 mm Hg or DBP ≥90 mm Hg, or receipt of antihypertensive medications, were defined as having hypertension.
Assessment of DNA Methylation Age Acceleration
Peripheral venous blood samples were collected from each participant, and DNA from white blood cells was isolated as previously described. 18 Infinium HuamnMethylationEPIC BeadChip arrays (Illumina Inc) were used to measure DNA methylation at a single‐nucleotide resolution at >850 000 CpG sites, according to manufacturer’s instructions. Data of BeadChip were filtered if samples with 10% of CpG probes with detection P>0.01; sites of single‐nucleotide polymorphisms rather than CpG probes; sites with bead counts <3 in 5% of samples or detection P>0.01; sites extended on single‐nucleotide polymorphisms with mirror allele frequency >0.05; or sites blasted to multiple different chromosomal regions. After filtering quality control, normalized β values (proportions of methylated DNA) of selected CpGs were imputed for calculating DNAm age through the Horvath method (https://dnamage.genetics.ucla.edu/). The calculation of Horvath’s DNAm age was derived from an elastic net penalized regression of 353 CpGs integrated with blood cell proportions. 15 The collective effects of 353 CpGs produce a composite multivariate biomarker, and the high accuracy of Horvath’s DNAm age has been validated using 8000 publicly available microarray samples, including the whole blood. 19 , 20 , 21 Scatter plot for the correlation between chronological age and Horvath’s DNAm age was presented in Figure S1. The correlation between chronological age and estimated methylation age was 0.88, with an root mean square error of 6.11 years, which showed a relatively higher accuracy and less error in our population. Epigenetic age acceleration was assessed by the measurement of ΔAge and aging rate. 14 , 15 ΔAge was derived by regressing DNAm age on chronological age and predicting the residuals from a linear model. Aging rate was assessed by ratios of DNAm age to chronological age.
Statistics Analysis
Associations of SBP, DBP, PP, and MAP with epigenetic age acceleration were tested by multiple linear regression models and quantified using estimated changes and 95% CIs of epigenetic age acceleration (ΔAge and aging rate) by each 10–mm Hg increase in blood pressure as continuous variables. Adjusting covariates were chosen on the basis of a priori knowledge and included sex (men/women), body mass index (BMI; kg/m2), socioeconomic status (low/middle/high), cigarette smoking (yes/no), alcohol drinking (yes/no), physical activity (yes/no), sleep duration (hours), hyperlipidemia (yes/no), and diabetes (yes/no). 22 , 23 Restricted cubic spline models with 3 knots at 5th, 50th, and 95th percentiles were fitted to graphically visualize the dose‐response trajectory between blood pressures metrics (SBP, DBP, PP, and MAP) and epigenetic age acceleration (ΔAge and aging rate). In addition, the epigenome‐wide analysis for the differentially methylated region (DMR) associated with hypertension was conducted using Bumphunter function with adjustment for confounding factors and estimated cell type proportions by Houseman et al. 24 , 25 As a known risk factor for hypertension, BMI can be considered acceptable to discriminate individuals with hypertension from healthy subjects. Receiver‐operating characteristic analyses were conducted to determine the area under the curves of ΔAge, aging rate, and BMI for hypertension.
Several additional sensitivity analyses were conducted in the present study to evaluate the robustness of our results. To assess whether associations of blood pressure with epigenetic age acceleration were affected by inclusion of individuals with receipt of antihypertensive medication, sensitivity analyses were conducted in participants without taking antihypertensive drugs. Other measures of DNAm age were also calculated on the basis of 71 CpGs using Hannum’s clock to assess the epigenetic age acceleration. 14 To further explore differences in estimated changes varied by sexual difference, we assessed associations of epigenetic age acceleration with a 10–mm Hg increase in blood pressure among subgroups using multiple linear regression models with adjustment for potential confounders. We included a product term between each blood pressure measure and sex as a continuous variable in the multiple linear regression model to test the interaction between blood pressure and sex in association with epigenetic age acceleration. Statistic powers for the sample size (n=280) with multiple linear regression models were calculated using “pwr” packages. Assuming a significance level of 0.05 and r 2 of predictors varying from 0.16 to 0.23, the statistic power of the study could reach at least 90%. We performed all analyses using R software (version 4.0.2; R Core Team), and 2‐tailed P<0.05 was defined as statistical significance in the present study.
RESULTS
Characteristics of the Study Population
As summarized in Table 1, the overall population had an average chronological age of 78.5 years. The mean (SD) values of estimated DNAm age, ΔAge, and aging rate were 77.2 (11.4), −3.18 (0.43), and 0.952 (0.11), respectively. Most participants were women (57.15%), and they were less likely to consume cigarettes (12.9%) or alcohol (23.9%). The prevalence rates of diabetes, hyperlipidemia, and hypertension were 8.2%, 36.1%, and 52.5%, respectively. Individuals with hypertension were more likely to be chronologically and biologically older and have a higher proportion of hyperlipidemia (shown in Table S1). The proportions of women and alcohol drinking in hypertensive participants were higher than those in nonhypertensive participants, although significant difference was not observed (P>0.05). The mean SBP, DBP, PP, and MAP were 143, 80.3, 62.7, and 101 mm Hg, relatively.
Table 1.
General Characteristics of the Study Population (n=280)
| Characteristics | Values |
|---|---|
| Sex, women, n (%) | 160 (57.1) |
| Chronological age, mean±SD, y | 78.5±16.1 |
| DNAm age, mean±SD, y | 77.2±11.4 |
| ΔAge, mean±SD, y* | −3.18±0.43 |
| Aging rate, mean±SD † | 0.952±0.11 |
| BMI, mean±SD, kg/m2 | 21.9±3.48 |
| Socioeconomic status, n (%) | |
| Lower | 110 (39.3) |
| Middle | 87 (31.1) |
| Higher | 83 (29.6) |
| Cigarette smoking, n (%) | 36 (12.9) |
| Alcohol drinking, n (%) | 67 (23.9) |
| Sleep, mean±SD, h | 9.63±1.58 |
| Physical activity, n (%) | 130 (46.4) |
| Diabetes, n (%) | 23 (8.2) |
| Hyperlipidemia, n (%) | 101 (36.1) |
| Hypertension, n (%) | 147 (52.5) |
| SBP, mean±SD, mm Hg | 143±24.6 |
| DBP, mean±SD, mm Hg | 80.3±13.3 |
| PP, mean±SD, mm Hg | 62.7±18.5 |
| MAP, mean±SD, mm Hg | 101±15.6 |
Data are presented as number (percentage) for categorical variables and mean±SD for continuous variables. BMI indicates body mass index; DBP, diastolic blood pressure; DNAm age, DNA methylation age; MAP, mean arterial pressure; PP, pulse pressure; and SBP, systolic blood pressure.
ΔAge was calculated as residual from regressing DNAm age on chronological age.
Aging rate was calculated as the methylation age divided by chronological age.
Associations of Blood Pressure With Epigenetic Age Acceleration
The univariate analysis showed that confounding factors, including sex, BMI, socioeconomic status, cigarette smoking, sleep duration, and physical activity, were significantly associated with epigenetic age acceleration (P<0.05; shown in Table S2). Both SBP and PP were found to be positively related to the accelerated DNA methylation aging, even with graded adjustments for related risk factors (shown in Table 2). After fully adjusted related covariates, each 10–mm Hg increment of SBP was associated with 0.608 (95% CI, 0.231–0.984) years increase in ΔAge and 0.007 (95% CI, 0.002–0.012) increase in aging rate; meanwhile, for PP, the increase was 1.120 (95% CI, 0.625–1.61) years in ΔAge and 0.013 (95% CI, 0.007–0.020) in aging rate. The results of restricted cubic splines in Figures 1 and 2 visually revealed significantly linear relationships of SBP and PP with ΔAge as well as of SBP and PP with aging rate (P overall <0.05 and P nonlinear >0.05). Increasing SBP and PP were significantly related to elevated ΔAge and aging rate in a likely linear dose‐dependent manner.
Table 2.
Relationships Between Blood Pressure and Epigenetic Age Acceleration (n=280)
| Variable | Estimated changes of ΔAge per 10–mm Hg increase in blood pressure | Estimated changes of aging rate per 10–mm Hg increase in blood pressure | ||
|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | |
| SBP | ||||
| Model 1 | 0.640 (0.265 to 1.015) | <0.001 | 0.008 (0.003 to 0.013) | 0.003 |
| Model 2 | 0.623 (0.239 to 1.007) | 0.002 | 0.008 (0.003 to 0.013) | 0.003 |
| Model 3 | 0.612 (0.222 to 1.001) | 0.002 | 0.008 (0.002 to 0.013) | 0.005 |
| Model 4 | 0.608 (0.231 to 0.984) | 0.002 | 0.007 (0.002 to 0.012) | 0.004 |
| DBP | ||||
| Model 1 | −0.191 (−0.924 to 0.543) | 0.611 | −0.002 (−0.011 to 0.008) | 0.727 |
| Model 2 | −0.122 (−0.859 to 0.615) | 0.746 | −0.001 (−0.01 to 0.009) | 0.873 |
| Model 3 | −0.096 (−0.814 to 0.622) | 0.794 | −0.001 (−0.011 to 0.009) | 0.843 |
| Model 4 | −0.087 (−0.809 to 0.635) | 0.813 | −0.001 (−0.010 to 0.009) | 0.862 |
| PP | ||||
| Model 1 | 1.186 (0.696 to 1.676) | <0.001 | 0.014 (0.007 to 0.021) | <0.001 |
| Model 2 | 1.182 (0.674 to 1.69) | <0.001 | 0.014 (0.008 to 0.021) | <0.001 |
| Model 3 | 1.154 (0.654 to 1.654) | <0.001 | 0.014 (0.007 to 0.021) | <0.001 |
| Model 4 | 1.120 (0.625 to 1.61) | <0.001 | 0.013 (0.007 to 0.02) | <0.001 |
| MAP | ||||
| Model 1 | 0.491 (−0.113 to 1.095) | 0.112 | 0.007 (−0.002 to 0.015) | 0.119 |
| Model 2 | 0.468 (−0.152 to 1.088) | 0.140 | 0.006 (−0.002 to 0.014) | 0.142 |
| Model 3 | 0.415 (−0.207 to 1.038) | 0.192 | 0.005 (−0.003 to 0.014) | 0.199 |
| Model 4 | 0.470 (−0.137 to 1.08) | 0.130 | 0.006 (−0.002 to 0.014) | 0.163 |
Model 1 is the crude model, which did not adjust any covariates. Model 2 is the basically adjusted model, including sex and body mass index. Model 3 is the further adjusted model, including factors of model 2 plus socioeconomic status, smoking status, drinking status, physical activity, and sleep duration. Model 4 is the fully multivariate model, including factors of model 3 plus history of hyperlipidemia and diabetes. β indicates regression coefficient; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; and SBP, systolic blood pressure.
Figure 1. The restricted cubic splines for the associations between blood pressure and ΔAge (n=280).
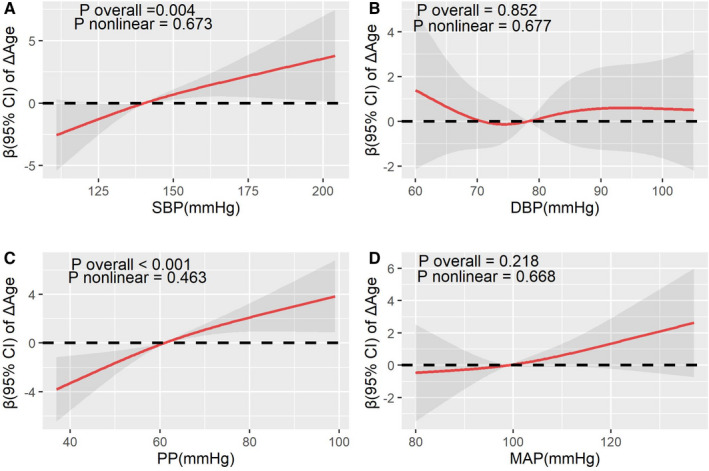
The lines represent adjusted regression coefficient (β) (95% CI) based on restricted cubic spline for SBP (A), DBP (B), PP (C), and MAP (D) with knots at 5th, 50th, and 95th percentiles. The β was estimated with adjustment for sex, body mass index, socioeconomic status, smoking status, drinking status, physical activity, sleep duration, hyperlipidemia, and diabetes. ΔAge was calculated as residual from regressing DNA methylation age on chronological age. DBP indicates diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; and SBP, systolic blood pressure.
Figure 2. The restricted cubic splines for the associations between blood pressure and aging rate (n=280).
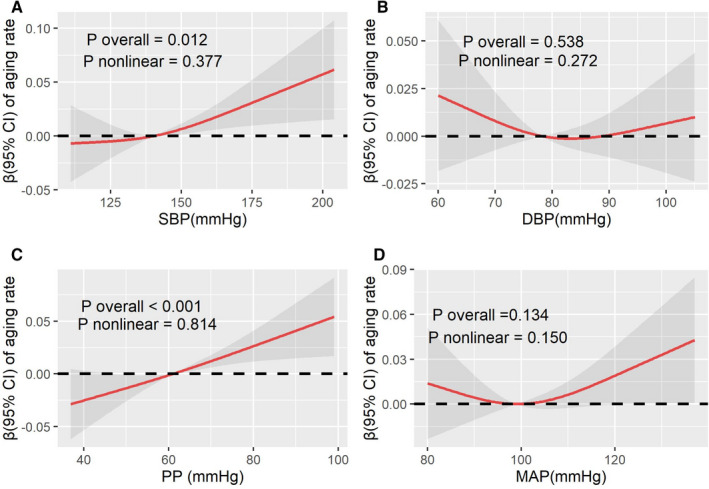
The lines represent adjusted regression coefficient (β) (95% CI) based on restricted cubic spline for SBP (A), DBP (B), PP (C), and MAP (D) with knots at 5th, 50th, and 95th percentiles. The β was estimated with adjustment for sex, body mass index, socioeconomic status, smoking status, drinking status, physical activity, sleep duration, hyperlipidemia, and diabetes. Aging rate was calculated as DNA methylation age divided by chronological age. DBP indicates diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; and SBP, systolic blood pressure.
In addition, we also performed a sensitivity analysis for relationships between blood pressure (SBP, DBP, PP, and MAP) and the epigenetic age acceleration using the DNA methylation age estimated with the Hannum’s clock and found the results were consistent with primary analyses (shown in Table S3). The relations of blood pressure with ΔAge and aging rate in participants without taking antihypertensive medications were also consistent with those in the total population. Each 10–mm Hg increment of SBP was significantly related to 0.613 years increase in ΔAge and 0.009 increase in aging rate (P<0.05). For PP, each 10–mm Hg increment of PP was significantly related to 0.973 years increase in ΔAge and 0.012 increase in aging rate (P<0.05) (shown in Table S4).
Subgroup Analysis
Table 3 showed the relations of blood pressure with epigenetic age acceleration stratified by sex. We found that significant relations of blood pressure with epigenetic age acceleration were only observed in women (P<0.05). For female participants, estimated change of ΔAge and aging rate per 10–mm Hg increment of SBP were 0.810 (95% CI, 0.282–1.338) and 0.010 (95% CI, 0.003–0.017), relatively; meanwhile, estimated change of ΔAge and aging rate per 10–mm Hg increment of PP were 1.492 (95% CI, 0.751–2.233) and 0.018 (95% CI, 0.008–0.028), respectively. The interactions between blood pressure and sex in association with epigenetic age acceleration were not significant (all P values for interaction >0.05).
Table 3.
Associations of Blood Pressure With Epigenetic Age Acceleration Stratified by Sex (n=280)
| Variables | Blood pressure, mean±SD, mm Hg | Estimated changes of ΔAge per 10–mm Hg increase in blood pressure | Estimated changes of aging rate per 10–mm Hg increase in blood pressure | ||||
|---|---|---|---|---|---|---|---|
| β (95% CI) | P value* | P for interaction † | β (95% CI) | P value* | P for interaction † | ||
| SBP | |||||||
| Men | 135±15.6 | 0.277 (−0.214 to 0.768) | 0.272 | 0.376 | 0.003 (−0.004 to 0.010) | 0.403 | 0.507 |
| Women | 154±22.2 | 0.810 (0.282 to 1.338) | 0.003 | 0.010 (0.003 to 0.017) | 0.005 | ||
| DBP | |||||||
| Men | 73.1±7.59 | −0.495 (−1.500 to 0.510) | 0.336 | 0.223 | −0.007 (−0.021 to 0.007) | 0.353 | 0.229 |
| Women | 89.7±13.9 | 0.021 (−0.079 to 0.12) | 0.682 | 0.003 (−0.010 to 0.016) | 0.635 | ||
| PP | |||||||
| Men | 51.6±15.9 | 0.591 (−0.003 to 1.185) | 0.054 | 0.297 | 0.007 (−0.002 to 0.015) | 0.113 | 0.424 |
| Women | 77.2±18.3 | 1.492 (0.751 to 2.233) | <0.001 | 0.018 (0.008 to 0.028) | <0.001 | ||
| MAP | |||||||
| Men | 90.3±6.87 | 0.038 (−0.803 to 0.880) | 0.929 | 0.331 | 0 (−0.012 to 0.012) | 0.968 | 0.404 |
| Women | 115±14.7 | 0.074 (−0.009 to 0.158) | 0.084 | 0.009 (−0.001 to 0.020) | 0.092 | ||
ΔAge was calculated as residual from regressing DNA methylation age on chronological age. Aging rate was calculated as DNA methylation age divided by chronological age. β indicates regression coefficient; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; and SBP, systolic blood pressure.
P values were estimated using multiple linear regression models, adjusted for sex, body mass index, socioeconomic status, smoking status, drinking status, physical activity, sleep duration, hyperlipidemia, and diabetes.
P values for interaction were estimated by including a product term of each blood pressure measure and sex in multiple linear regression models.
We observed that hypertension was significantly associated with epigenetic age acceleration (P<0.05; shown in Table S5). The adjusted estimated changes of ΔAge and aging rate were 3.10 (95% CI, 1.07–5.13) and 0.04 (95% CI, 0.015–0.065), respectively. Figure S2 shows that the area under the curves of ΔAge, aging rate, and BMI for hypertension were 0.687, 0.690, and 0.687, respectively, indicating that measures of epigenetic age acceleration (ΔAge and aging rate) can also be considered as predictors of hypertension. Epigenome‐wide analysis for DMR associated with hypertension showed that 11 independent DMRs were identified associated with hypertension at a false discovery rate <0.05. Most DMRs contained CpGs annotated to genes, spanning first exon, the body, TSS200, TSS1500, and 5' untranslated region (shown in Table S6). We further examined the joint effects of hypertension and sex on epigenetic age acceleration (shown in Table 4). Compared with men without hypertension, estimated changes of ΔAge and aging rate in women with hypertension were 4.045 (95% CI, 1.074–7.017) and 0.047 (95% CI, 0.008–0.087), respectively.
Table 4.
Joint Associations of Hypertension and Sex in Relation to Epigenetic Age Acceleration (n=280)
| Groups | Proportions of hypertension, n (%) |
Estimated changes of ΔAge β (95% CI) |
P value |
Estimated changes of aging rate β (95% CI) |
P value |
|---|---|---|---|---|---|
| Nonhypertension and men | … | 0 (Reference) | … | 0 (Reference) | … |
| Nonhypertension and women | … | −0.288 (−3.09 to 2.51) | 0.322 | −0.006 (−0.041 to 0.029) | 0.423 |
| Hypertension and men | 58 (48.3) | 0.132 (−2.61 to 2.88) | 0.295 | 0.001 (−0.036 to 0.038) | 0.254 |
| Hypertension and women | 89 (55.6) | 4.05 (1.07 to 7.02) | <0.001 | 0.047 (0.008 to 0.087) | <0.001 |
ΔAge was calculated as residual from regressing DNA methylation age on chronological age. Aging rate was calculated as DNA methylation age divided by chronological age. The β was estimated with adjustment for sex, body mass index, socioeconomic status, cigarette smoking, alcohol drinking, physical activity, sleep duration, hyperlipidemia, and diabetes. β indicates regression coefficient.
DISCUSSION
In the present study, we found that high SBP and PP are significantly associated with increased epigenetic age acceleration with a dose‐response trend. The significant associations of SBP and PP with epigenetic age acceleration appeared to be limited to women, although interactions between blood pressure and sex were not significant. The combination of women and hypertension was associated with a much higher increase in accelerated aging than the combined men and nonhypertension.
Individuals of the same chronological age may exhibit different predisposition to age‐related conditions or death, which is likely reflective of the discrepancy in biological age (ie, DNAm age). In the present study, the DNAm age of individuals was lower than chronological age. The possible reason may be that our study population originated from Hongshuihe region, which has been noted as a high‐longevity area because it has leading centenarian ratios among the counties in China. 17 The study participants tended to have a prolonged lifespan with a relatively lower biological age.
Variation of interindividual difference exists in age‐associated cardiometabolic dysregulation, especially for older adults. Higher risks of obesity and dyslipidemia were also found in participants with increasing epigenetic age acceleration. 26 , 27 Pottinger and his colleagues observed that poor cardiovascular health was associated with the epigenetic age acceleration in a cross‐sectional analysis of 2170 menopausal women. 28 In the present study, an increase in epigenetic age acceleration per 10–mm Hg of blood pressure was found to be <1 year. Several cardiometabolic risk factors pose threats to the premature decline of physical functioning attributable to accelerated aging, and blood pressure may play a small part in such declines. 29 , 30 , 31 Ammous and his colleagues found that epigenetic age acceleration may be attributed to a worse cardiometabolic file, such as higher SBP, PP, triglycerides, and fasting insulin, and lower low‐density cholesterol. 29 A longitudinal study from the WHI (Women's Health Initiative) revealed that accelerated epigenetic aging has been associated with lifestyle factors, such as diet, physical activity, and alcohol consumption. 26 Ammous et al reported that a 10–mm Hg increase in epigenetic age acceleration was associated with an ≈1‐year increase in SBP and PP.
A cross‐sectional study conducted among 42 middle‐aged adults reported that higher SBP and DBP is associated with shortened telomere length, but the sample size of the study limited the validity of their results. 32 Epigenetic age acceleration exhibits statistically age‐associated physiological decline than telomere length. In the present study, SBP and PP were found to be positively related to the accelerated DNA methylation aging. Consistent with our study, increased epigenetic age acceleration was found to be associated with higher SBP and PP in a Black cohort with a high prevalence of hypertension. 29 The reason why significant associations were observed for SBP and PP may be that the 2 measures have better implicative power than DBP and MAP for coronary artery disease. Increased arterial stiffness promotes an increase in SBP and a decrease in DBP, leading to an increase in PP, but not MAP, which acts as an offsetting effect. 33 , 34 Kong et al found that SBP and PP had stronger relationships with coronary artery disease than DBP and MAP. 35 In a study of 1390 Black individuals, Smith and his colleagues found that epigenetic age acceleration may act as a subclinical biomarker for damage to peripheral vascular and heart, which may help to better characterize the functional mechanisms underlying organ damage from aging. 36
Methylation of DNA plays an important role in regulation of the expression of individual genes. It is reported that 5‐methylcytosine DNA levels have been reported to be lower in patients with essential hypertension compared with healthy controls. 37 Our findings of epigenome‐wide analysis showed that hypermethylation or hypomethylation of DMRs was found independently associated with hypertension. Similarly, some studies reported that blood pressure was associated with hypermethylation or hypomethylation of several single genes. 38 , 39 Alexeeff and his colleagues found that SBP and DBP were negatively associated with methylation of interferon‐γ gene and positively associated with methylation of inducible NO synthase and toll‐like receptor 2 genes. 38 The results of Manuel and his colleagues showed that decreases in SBP and DBP were significantly associated with hypomethylation of Nuclear Factor Kappa B Subunit 1 gene. 39 However, comparing with the studies focusing on several genes, we used DNAm age with the status of multiple CpG sites to explore the associations between blood pressure and DNA methylation. The epigenetic clock of Horvath was established with 353 CpG sites, of which 160 CpGs were negatively related to aging while the remaining 193 CpGs had a negative association. 15 Of note, NADPH Oxidase 4 gene, a reactive oxygen species generating enzyme expressed in endothelium, was reported to exert potentially beneficial effects on vasodilator function and blood pressure in transgenic rats treated as overexpression of Nox4. 40 Intriguingly, CpG located in Nox4 was used in methylation age estimation of Horvath epigenetic clock, which was negatively associated with the age predictor.
Because of different hereditary and habitual diets, it has been shown that sex is associated with differences in epigenetic response. 41 In the present study, we found that significant associations of blood pressure with epigenetic aging were only observed in women. As has been previously reported, the blood pressure of women is typically higher than that of men. 42 Most observational studies showed that more women than men die of cardiovascular disease, indicating that the sex difference was associated with interindividual variability in the regulation of blood pressure. 43 , 44 , 45 In addition, hundreds of genes differentially expressed between sexes in an age‐dependent manner result in sexual difference in blood pressure. For example, genes encoding angiotensin II type 2 receptor and angiotensin‐converting enzyme 2 are located on the X chromosome and are associated with relative cardiovascular discrepancy for men and women. 46 Epigenetic age may take a role in sexual dimorphism relating to the regulation of blood pressure and cardiovascular function, and further studies with larger sample size were required to verify our findings.
Our research has the following strengths. First, SBP, DBP, PP, and MAP were adopted as the metrics of blood pressure, which provided a comprehensive reflection of cardiovascular function than just using SBP and DBP. It was reported that PP and MAP were more sensitive to aortic regurgitation or aortic stiffness, but the same level of attention to SBP and DBP has not given to PP and MAP in previous studies. 47 , 48 Second, we assessed epigenetic age acceleration (ΔAge and aging rate) of individuals to further explore the relationships between blood pressure and aging pace, which provides important clues for relationships between blood pressure and epigenetic aging in an Asian population. Finally, linear and nonlinear models were performed in the study with adjustment for potential confounding variables, making our findings more convincing. However, there are some limitations worth noting. First, the cross‐sectional nature of the study cannot allow us to infer the causality between blood pressure and epigenetic age acceleration. Therefore, our findings should be confirmed in further prospective cohort studies. Second, our study was restricted in the population aged ≥50 years, which might limit the generalizability of our findings to younger populations. On the other hand, we minimize the confounding effects by age variation. Finally, adjusted covariates, such as cigarette smoking and alcohol drinking, were self‐reported, which may result in potential recall bias. However, we conducted questionnaire investigation through face‐to‐face interviews, and completed questionnaires were logically checked by another trained investigator.
CONCLUSIONS
We found that both SBP and PP were positively related to the elevated ΔAge and aging rate, which were influenced by sexual difference. Our findings suggested that high SBP and PP were associated with the epigenetic age acceleration, providing important clues for relationships between blood pressure and epigenetic aging.
Sources of Funding
This work was supported by the Innovation Research Team of Guangxi Natural Science Foundation (2017GXNSFGA198003).
Disclosures
None.
Supporting information
Tables S1–S6
Figures S1–S2
Acknowledgments
We gratefully thank all the colleagues and the study participants in the study.
Author contributions: Dr Xiao contributed to the analysis and interpretation of data and drafting of the manuscript; Drs Zan, Liu, Xu, Li, and Chen were involved in the acquisition of data and critical revision of the manuscript. Drs Yang and Zhang were the guarantors of this work and take responsibility for the study concept and design and revision of the manuscript for important intellectual content.
Supplementary Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022257
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Zhiyong Zhang, Email: rpazz@163.com.
Xiaobo Yang, Email: yangx@gxmu.edu.cn.
References
- 1. Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018;561:45–56. doi: 10.1038/s41586-018-0457-8 [DOI] [PubMed] [Google Scholar]
- 2. Rapsomaniki E, Timmis A, George J, Pujades‐Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1·25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wahidi N, Lerner AJ. Blood pressure control and protection of the aging brain. Neurotherapeutics. 2019;16:569–579. doi: 10.1007/s13311-019-00747-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rastas S, Pirttilä T, Viramo P, Verkkoniemi A, Halonen P, Juva K, Niinistö L, Mattila K, Länsimies E, Sulkava R. Association between blood pressure and survival over 9 years in a general population aged 85 and older. J Am Geriatr Soc. 2006;54:912–918. doi: 10.1111/j.1532-5415.2006.00742.x [DOI] [PubMed] [Google Scholar]
- 5. Lv Y‐B, Gao X, Yin Z‐X, Chen H‐S, Luo J‐S, Brasher MS, Kraus VB, Li T‐T, Zeng Y, Shi X‐M. Revisiting the association of blood pressure with mortality in oldest old people in China: community based, longitudinal prospective study. BMJ. 2018;361:k2158. doi: 10.1136/bmj.k2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kagiyama S, Fukuhara M, Ansai T, Matsumura K, Soh I, Takata Y, Sonoki K, Awano S, Takehara T, Iida M. Association between blood pressure and mortality in 80‐year‐old subjects from a population‐based prospective study in Japan. Hypertens Res. 2008;31:265–270. doi: 10.1291/hypres.31.265 [DOI] [PubMed] [Google Scholar]
- 7. Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. 2018;10:573–591. doi: 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. López‐Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, Gibson J, Henders AK, Redmond P, Cox SR, et al. DNA methylation age of blood predicts all‐cause mortality in later life. Genome Biol. 2015;16:25. doi: 10.1186/s13059-015-0584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Unnikrishnan A, Freeman WM, Jackson J, Wren JD, Porter H, Richardson A. The role of DNA methylation in epigenetics of aging. Pharmacol Ther. 2019;195:172–185. doi: 10.1016/j.pharmthera.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones MJ, Goodman SJ, Kobor MS. DNA methylation and healthy human aging. Aging Cell. 2015;14:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiao F‐H, Kong Q‐P, Perry B, He Y‐H. Progress on the role of DNA methylation in aging and longevity. Brief Funct Genomics. 2016;15:454–459. [DOI] [PubMed] [Google Scholar]
- 13. Horvath S, Raj K. DNA methylation‐based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19:371–384. [DOI] [PubMed] [Google Scholar]
- 14. Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan J‐B, Gao Y, et al. Genome‐wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng Y, Joyce BT, Colicino E, Liu L, Zhang W, Dai QI, Shrubsole MJ, Kibbe WA, Gao T, Zhang Z, et al. Blood epigenetic age may predict cancer incidence and mortality. EBioMedicine. 2016;5:68–73. doi: 10.1016/j.ebiom.2016.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deng Q, Wei Y, Zhao Y, Han X, Yin J. Understanding the natural and socioeconomic factors behind regional longevity in Guangxi, China: is the centenarian ratio a good enough indicator for assessing the longevity phenomenon? Int J Environ Res Public Health. 2018;15:938. doi: 10.3390/ijerph15050938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xiao L, Zan G, Feng X, Bao Y, Huang S, Luo X, Xu X, Zhang Z, Yang X. The associations of multiple metals mixture with accelerated DNA methylation aging. Environ Pollut. 2021;269:116230. doi: 10.1016/j.envpol.2020.116230 [DOI] [PubMed] [Google Scholar]
- 19. El Khoury LY, Gorrie‐Stone T, Smart M, Hughes A, Bao Y, Andrayas A, Burrage J, Hannon E, Kumari M, Mill J, et al. Systematic underestimation of the epigenetic clock and age acceleration in older subjects. Genome Biol. 2019;20:283. doi: 10.1186/s13059-019-1810-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horvath S, Erhart W, Brosch M, Ammerpohl O, von Schönfels W, Ahrens M, Heits N, Bell JT, Tsai P‐C, Spector TD, et al. Obesity accelerates epigenetic aging of human liver. Proc Natl Acad Sci USA. 2014;111:15538–15543. doi: 10.1073/pnas.1412759111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grant CD, Jafari N, Hou L, Li Y, Stewart JD, Zhang G, Lamichhane A, Manson JE, Baccarelli AA, Whitsel EA, et al. A longitudinal study of DNA methylation as a potential mediator of age‐related diabetes risk. Geroscience. 2017;39:475–489. doi: 10.1007/s11357-017-0001-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fiorito G, McCrory C, Robinson O, Carmeli C, Rosales CO, Zhang Y, Colicino E, Dugué P‐A, Artaud F, McKay GJ, et al. Socioeconomic position, lifestyle habits and biomarkers of epigenetic aging: a multi‐cohort analysis. Aging. 2019;11:2045–2070. doi: 10.18632/aging.101900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carey RM, Muntner P, Bosworth HB, Whelton PK. Reprint of: prevention and control of hypertension: JACC health promotion series. J Am Coll Cardiol. 2018;72:2996–3011. [DOI] [PubMed] [Google Scholar]
- 24. Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, Wiencke JK, Kelsey KT. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13:86. doi: 10.1186/1471-2105-13-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jaffe AE, Murakami P, Lee H, Leek JT, Fallin MD, Feinberg AP, Irizarry RA. Bump hunting to identify differentially methylated regions in epigenetic epidemiology studies. Int J Epidemiol. 2012;41:200–209. doi: 10.1093/ije/dyr238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quach A, Levine ME, Tanaka T, Lu AT, Chen BH, Ferrucci L, Ritz B, Bandinelli S, Neuhouser ML, Beasley JM, et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging. 2017;9:419–446. doi: 10.18632/aging.101168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nannini DR, Joyce BT, Zheng Y, Gao T, Liu L, Yoon G, Huan T, Ma J, Jacobs DR Jr, Wilkins JT, et al. Epigenetic age acceleration and metabolic syndrome in the coronary artery risk development in young adults study. Clin Epigenetics. 2019;11:160. doi: 10.1186/s13148-019-0767-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pottinger TD, Khan SS, Zheng Y, Zhang W, Tindle HA, Allison M, Wells G, Shadyab AH, Nassir R, Martin LW, et al. Association of cardiovascular health and epigenetic age acceleration. Clin Epigenetics. 2021;13:42. doi: 10.1186/s13148-021-01028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ammous F, Zhao W, Ratliff SM, Mosley TH, Bielak LF, Zhou X, Peyser PA, Kardia SLR, Smith JA. Epigenetic age acceleration is associated with cardiometabolic risk factors and clinical cardiovascular disease risk scores in African Americans. Clin Epigenetics. 2021;13:55. doi: 10.1186/s13148-021-01035-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang RC, Lillycrop KA, Beilin LJ, Godfrey KM, Anderson D, Mori TA, Rauschert S, Craig JM, Oddy WH, Ayonrinde OT, et al. Epigenetic age acceleration in adolescence associates with BMI, inflammation, and risk score for middle age cardiovascular disease. J Clin Endocrinol Metab. 2019;104:3012–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Irvin MR, Aslibekyan S, Do A, Zhi D, Hidalgo B, Claas SA, Srinivasasainagendra V, Horvath S, Tiwari HK, Absher DM, et al. Metabolic and inflammatory biomarkers are associated with epigenetic aging acceleration estimates in the GOLDN study. Clin Epigenetics. 2018;10:56. doi: 10.1186/s13148-018-0481-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Insel KC, Merkle CJ, Hsiao C‐P, Vidrine AN, Montgomery DW. Biomarkers for cognitive aging part I: telomere length, blood pressure and cognition among individuals with hypertension. Biol Res Nurs. 2012;14:124–132. doi: 10.1177/1099800411406433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abhayaratna WP, Srikusalanukul W, Budge MM. Aortic stiffness for the detection of preclinical left ventricular diastolic dysfunction: pulse wave velocity versus pulse pressure. J Hypertens. 2008;26:758–764. doi: 10.1097/HJH.0b013e3282f55038 [DOI] [PubMed] [Google Scholar]
- 34. Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: systematic review and meta‐analysis. BMJ. 2016;354:i4098. doi: 10.1136/bmj.i4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kong MG, Kim HL, Kim MA, Kim M, Park SM, Yoon HJ, Shin MS, Hong KS, Shin GJ, Shim WJ. Relationships between blood pressure measurements and target organ damage: data from the Korea women's chest pain registry. J Clin Hypertens (Greenwich). 2018;20:1724–1730. doi: 10.1111/jch.13417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith JA, Raisky J, Ratliff SM, Liu J, Kardia SLR, Turner ST, Mosley TH, Zhao W. Intrinsic and extrinsic epigenetic age acceleration are associated with hypertensive target organ damage in older African Americans. BMC Med Genomics. 2019;12:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smolarek I, Wyszko E, Barciszewska AM, Nowak S, Gawronska I, Jablecka A, Barciszewska MZ. Global DNA methylation changes in blood of patients with essential hypertension. Med Sci Monit. 2010;16:Cr149–Cr155. [PubMed] [Google Scholar]
- 38. Alexeeff SE, Baccarelli AA, Halonen J, Coull BA, Wright RO, Tarantini L, Bollati V, Sparrow D, Vokonas P, Schwartz J. Association between blood pressure and DNA methylation of retrotransposons and pro‐inflammatory genes. Int J Epidemiol. 2013;42:270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Macías‐González M, Martín‐Núñez GM, Garrido‐Sánchez L, García‐Fuentes E, Tinahones FJ, Morcillo S. Decreased blood pressure is related to changes in NF‐kB promoter methylation levels after bariatric surgery. Surg Obes Relat Dis. 2018;14:1327–1334. doi: 10.1016/j.soard.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 40. Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom‐Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, et al. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol. 2011;31:1368–1376. doi: 10.1161/ATVBAHA.110.219238 [DOI] [PubMed] [Google Scholar]
- 41. Gunes SO, Metin Mahmutoglu A, Agarwal A. Genetic and epigenetic effects in sex determination. Birth Defects Res C Embryo Today. 2016;108:321–336. [DOI] [PubMed] [Google Scholar]
- 42. Joyner MJ, Wallin BG, Charkoudian N. Sex differences and blood pressure regulation in humans. Exp Physiol. 2016;101:349–355. doi: 10.1113/EP085146 [DOI] [PubMed] [Google Scholar]
- 43. Colafella KMM, Denton KM. Sex‐specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol. 2018;14:185–201. doi: 10.1038/nrneph.2017.189 [DOI] [PubMed] [Google Scholar]
- 44. Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133:916–947. doi: 10.1161/CIR.0000000000000351 [DOI] [PubMed] [Google Scholar]
- 45. Ventura‐Clapier R, Dworatzek E, Seeland U, Kararigas G, Arnal J‐F, Brunelleschi S, Carpenter TC, Erdmann J, Franconi F, Giannetta E, et al. Sex in basic research: concepts in the cardiovascular field. Cardiovasc Res. 2017;113:711–724. doi: 10.1093/cvr/cvx066 [DOI] [PubMed] [Google Scholar]
- 46. Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, Arnold AP, Sandberg K. Sex chromosome effects unmasked in angiotensin II‐induced hypertension. Hypertension. 2010;55:1275–1282. doi: 10.1161/HYPERTENSIONAHA.109.144949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Avanzini F, Alli C, Boccanelli A, Chieffo C, Franzosi MG, Geraci E, Maggioni AP, Marfisi RM, Nicolosi GL, Schweiger C, et al. High pulse pressure and low mean arterial pressure: two predictors of death after a myocardial infarction. J Hypertens. 2006;24:2377–2385. doi: 10.1097/01.hjh.0000251897.40002.bf [DOI] [PubMed] [Google Scholar]
- 48. Pastor‐Barriuso R, Banegas JR, Damián J, Appel LJ, Guallar E. Systolic blood pressure, diastolic blood pressure, and pulse pressure: an evaluation of their joint effect on mortality. Ann Intern Med. 2003;139:731–739. doi: 10.7326/0003-4819-139-9-200311040-00007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figures S1–S2


