Abstract
Background
Guidelines promote shared decision‐making (SDM) for anticoagulation in patients with atrial fibrillation. We recently showed that adding a within‐encounter SDM tool to usual care (UC) increases patient involvement in decision‐making and clinician satisfaction, without affecting encounter length. We aimed to estimate the extent to which use of an SDM tool changed adherence to the decided care plan and clinical safety end points.
Methods and Results
We conducted a multicenter, encounter‐level, randomized trial assessing the efficacy of UC with versus without an SDM conversation tool for use during the clinical encounter (Anticoagulation Choice) in patients with nonvalvular atrial fibrillation considering starting or reviewing anticoagulation treatment. We conducted a chart and pharmacy review, blinded to randomization status, at 10 months after enrollment to assess primary adherence (proportion of patients who were prescribed an anticoagulant who filled their first prescription) and secondary adherence (estimated using the proportion of days for which treatment was supplied and filled for direct oral anticoagulant, and as time in therapeutic range for warfarin). We also noted any strokes, transient ischemic attacks, major bleeding, or deaths as safety end points. We enrolled 922 evaluable patient encounters (Anticoagulation Choice=463, and UC=459), of which 814 (88%) had pharmacy and clinical follow‐up. We found no differences between arms in either primary adherence (78% of patients in the SDM arm filled their first prescription versus 81% in UC arm) or secondary adherence to anticoagulation (percentage days covered of the direct oral anticoagulant was 74.1% in SDM versus 71.6% in UC; time in therapeutic range for warfarin was 66.6% in SDM versus 64.4% in UC). Safety outcomes, mostly bleeds, occurred in 13% of participants in the SDM arm and 14% in the UC arm.
Conclusions
In this large, randomized trial comparing UC with a tool to promote SDM against UC alone, we found no significant differences between arms in primary or secondary adherence to anticoagulation or in clinical safety outcomes.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: clinicaltrials.gov. Identifier: NCT02905032.
Keywords: adherence, anticoagulation, atrial fibrillation, communication, conversation aid, decision aid, shared decision‐making
Subject Categories: Health Services, Quality and Outcomes, Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- DOAC
direct oral anticoagulant
- PDC
percentage days covered
- SDM
shared decision‐making
- TTR
time in therapeutic range
- UC
usual care
Clinical Perspective
What Is New?
In a large randomized multicenter trial, the use of a tool, effective and efficient at promoting shared decision‐making about whether and how to use anticoagulants to prevent strokes in patients with atrial fibrillation, did not significantly improve adherence to anticoagulants or have any discernible effect on safety end points.
What Are the Clinical Implications?
These results should inform the rationale and perhaps affect the strength of recommendation for shared decision‐making in clinical practice guidelines and of policies to promote the adoption of shared decision‐making in practice when the main rationale for each is to increase the uptake and adherence to effective care, such as anticoagulation, rather than the promotion of patient‐centered care.
Atrial fibrillation (AF) is a highly prevalent condition 1 , 2 and contributor to stroke risk and increased morbidity and mortality. 1 Although anticoagulation can reduce the stroke risk by two thirds, 3 patients often opt not to take these medications, exposing many to the risk of potentially preventable strokes. 4 , 5 , 6 , 7 In part to address this treatment gap, the American cardiovascular societies have all endorsed shared decision‐making (SDM), patients and clinicians working together in making decisions about treatment, to individualize anticoagulation in patients with AF at risk of stroke. 8 Many tools have been developed to promote SDM in this setting, 9 , 10 , 11 , 12 , 13 but, until recently, most had not been prospectively evaluated.
Our group recently completed and reported a randomized clinical trial that evaluated Anticoagulation Choice, 14 , 15 an SDM conversation aid for use within the encounter on anticoagulation use for stroke prevention in patients with AF. 16 We demonstrated that the SDM tool was used properly and contributed to patient involvement in decision‐making and to clinician satisfaction, without affecting treatment decisions or encounter length in comparison to usual care (UC). In addition, we had hypothesized that successful SDM would result in decisions that are more consistent with a patient’s goals and preferences and that this would translate to improved adherence to a chosen treatment and, ultimately, better clinical outcomes. To this end, we performed a prespecified 10‐month follow‐up analysis of our trial data to estimate the extent to which the addition of an SDM tool to UC, versus UC alone, changed primary or secondary adherence to the decided care plan or the rate of clinical safety end points.
Methods
Trial Design
This is a prespecified, 10‐month follow‐up analysis of a previously reported multicenter encounter‐randomized controlled trial (SDM4Afib [Shared Decision‐Making for Atrial Fibrillation] Trial) comparing clinical outcomes in UC with and without the use of the Anticoagulation Choice tool. 16 The Institutional Review Boards at the coordinating center (Institutional Review Board No. 16‐005409) and all participating sites approved study procedures. The trial was registered at ClinicalTrials.gov (NCT02905032), and the study protocol was published. 14 The SDM tool is freely available online (http://anticoagulationdecisionaid.mayoclinic.org). 17 Trial data, except for encounter video recordings that cannot be deidentified, will be made publicly available through National Heart, Lung, and Blood Institute data repository, as per their policy (https://www.nhlbi.nih.gov/grants‐and‐training/policies‐and‐guidelines/nhlbi‐policy‐for‐data‐sharing‐from‐clinical‐trials‐and‐epidemiological‐studies).
This study evaluated pharmacy and clinical data collected during the 12 months before and 10 months after enrollment to assess the following: (1) anticoagulation start and continuation rates (primary and secondary adherence) and (2) clinical safety outcomes. A data and safety monitoring board met before study initiation, approved its charter, and met biannually thereafter. They monitored study conduct, data quality, and safety signals. On review of the results, the board released the data for publication.
Setting and Participants
The SDM4AFib Trial enrolled patients cared for in emergency departments, outpatient safety net, primary care and cardiology clinics, and inpatient hospital services at academic medical centers, a suburban group practice, and an urban safety‐net health system. Clinicians (physicians, pharmacists, advance practice providers, and nurses) were eligible for participation if they had a planned conversation with patients with AF to discuss anticoagulation for stroke prevention. Adult patients (aged ≥18 years) were eligible if they had a diagnosis of nonvalvular AF, were at high risk of a thromboembolic event (ie, had a CHA2DS2‐VASc [congestive heart failure, hypertension, age ≥75 years, diabetes, stroke or transient ischemic attack, vascular disease, age 65 to 74 years, sex category] score of ≥1 in men or ≥2 in women), and were able to read and understand the informed consent document. The population consisted of 2 cohorts: the start and the review cohort. The start cohort had never used anticoagulation or used anticoagulation and discontinued >6 months before enrollment, and had started an anticoagulant during the randomization visit, in the emergency department, or in an inpatient stay within 10 days of the enrolled encounter. Patients in the review cohort were already taking an anticoagulant at the time of the enrolled encounter. Patients with mechanical heart valves, prior left atrial appendage occlusion device implantation, or moderate or severe rheumatic mitral stenosis were excluded.
Data Collection
The patient‐ and clinician‐reported data collection process has been described previously. 14 Blinded to study arm, each site’s study coordinator reviewed the patient’s electronic health record at baseline and 10 months after enrollment, and entered these data into Research Electronic Data Capture. Study data were collected and managed using Research Electronic Data Capture electronic data capture tools hosted at Mayo Clinic. 18 , 19 Research Electronic Data Capture is a secure, web‐based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources. If a patient had no documented contact with the health care system between 9 and 12 months after the index visit, the patient was contacted by telephone (up to 3 attempts) to obtain information about any safety outcomes. We contacted the patient’s pharmacy 10 months after patient enrollment, requesting information for all prescriptions filled in the previous 22 months (12 months before enrollment and 10 months after enrollment). If a patient reported using >1 pharmacy, then all listed pharmacies were contacted.
Outcomes
The primary outcomes have been previously published. 14 This study included 2 sets of prespecified secondary outcomes: (1) primary and secondary medication adherence and (2) clinical safety outcomes.
Primary and Secondary Adherence
The initial choice of anticoagulation agent, warfarin or a direct oral anticoagulant (DOAC), and any changes, and the reasons for these changes documented in clinical notes, were extracted from the medical record along with all international normalized ratio (INR) values.
Primary adherence was defined as the proportion of patients with pharmacy records who were prescribed an anticoagulant and who went on to fill the first prescription within 30 days.
To calculate the secondary adherence to DOACs, we calculated the percentage days covered (PDC) as the number of days supplied to the patient in each prescription fill over the 300 days (10 months) of observation. If the patient had a note in the medical record that indicated the patient stopped the medication or if the patient died before the 300 days, we censored the follow‐up at that date. Some patients switched between DOAC and warfarin during the follow‐up period. For these patients, the follow‐up (1) began at the first prescription of DOAC after warfarin or (2) ended at the first prescription of warfarin if a DOAC was prescribed first. We required that a patient had at least 30 evaluable days to calculate and report the PDC. We assessed prescriptions during the 12 months before enrollment to use all previous prescriptions to accurately count total days supplied. The 12‐month period before study enrollment was also used to compare adherence rates before and after enrollment. A subanalysis was also performed, limited to patients with complete pharmacy records. Complete pharmacy coverage was defined as having data from all pharmacies listed in the patients’ medical record.
To estimate the secondary adherence to warfarin (PDC could be imprecise because the days covered may vary as the dose is adjusted over time), we used the Rosendaal method 20 to calculate the time in therapeutic range (TTR) for patients who were on warfarin, based on number of days a patient was in therapeutic range (INR between 2.0 and 3.0) over the number of days within the evaluation period. Patients had to have at least 2 INR values within 30 days to calculate the TTR. Patients were censored at the time of the last INR result.
Safety Outcomes
The statistical team periodically compiled a list of potential safety events extracted from the medical record and submitted them to the site’s principal investigator for review and confirmation. If the site principal investigator recruited the patient, then another participating clinician at the site conducted the review. The investigator’s decision on the event, which was made blind to allocation, was used for analysis.
Events extracted were major bleeding, stroke or transient ischemic attack, and death, classified as cardiovascular, bleeding related, cancer, infection/sepsis, or unknown. A major bleed was defined using the International Society on Thrombosis and Haemostasis guidelines. 21 For bleeds, we calculated the count and percentage of patients with ≥1 bleeds in each study arm along with the median and interquartile range of bleeds. Stroke included both hemorrhagic and ischemic stroke as ascertained from diagnoses documented in the medical record. Death related to any underlying cause was included.
Sample Size
As previously reported, the recruitment goal was 1000 patient encounters (500 per arm). 14 We anticipated that 90% of recruited patients would start or continue using an anticoagulant. We requested pharmacy records of all enrolled patients regardless of their treatment decision. On the basis of our own experience, we expected to receive >85% of those records. Thus, we expected ≈765 patient records would be available for assessment of secondary adherence at 10 months. In our review of the Optum database, 40% of patients were adherent to anticoagulation (>80% PDC, the threshold used by Center for Medicare and Medicaid Services) at 12 months. 6 Assuming an expected 60% PDC in the UC cohort, the study should have at most (considering the reduction in power with any potential clustering) 80% power to detect a 9% difference (69% PDC in the intervention arm), with a 2‐sided test and an α of 0.05.
Statistical Analysis
We conducted this trial and analyzed its results according to the intention‐to‐treat principle, including all encounters in the arm to which they were randomly assigned. Per the statistical analysis plan, no multiple imputation was conducted for missing pharmacy data because we accounted for missing data in the power calculation. We analyzed outcomes with mixed‐effects models adjusting by fixed effects of arm, cohort (start versus review cohort), and stroke risk (CHA2DS2‐VASc score of <2 versus ≥2 for men or <3 versus ≥3 for women) 22 with random effects for clinic and clinician. For binary outcomes, we conducted multivariable logistic regression, reporting odds ratios (ORs) and 95% CIs. For continuous outcomes, we conducted multivariable generalized linear regression, reporting adjusted mean differences with 95% CIs.
Kaplan‐Meier curves for each study arm were constructed to represent the time to anticoagulation start after the initial prescription and the time on anticoagulation. All patients with an initial prescription of DOAC or warfarin were evaluable. The time represents the date of the first fill (0) until the end of the days supplied or end of follow‐up (300 days). If a patient received his/her first fill 90 days after enrollment, then we considered this day 0; if he/she still had medication coverage past day 300, he/she was censored on that day. To further describe the population, alluvial plots were used to show the relationship between trial arm, preenrollment adherence, and subsequent primary and secondary adherence. Adherence to anticoagulants in the 12 months before enrollment and the interaction with arm within the review cohort was tested using the χ2 test statistic of the differences in the log likelihood. All tests are 2 sided, and analyses were conducted in Stata. 23 As planned, there were few clinical safety events so we report the rates for all safety events, without a statistical assessment of difference.
Results
Participant Characteristics and Treatment Choices
Figure 1 describes the flow of participants and the accrual of follow‐up data. Recruitment began in January 2017 and was completed in June 2019. Patients and clinicians both consented to participate in 942 (52%) of the 1827 eligible encounters; 922 (98%) were enrolled and available for analyses: 463 randomly allocated to intervention, and 459 allocated to UC. All patient factors were balanced across arms (Table 1). Table 2 describes the treatment decisions. Approximately 45% of patients and clinicians chose warfarin. Of the 55% choosing a DOAC, most (≈60%) selected apixaban. Approximately one third of patients stopped anticoagulation during follow‐up, and one fifth decided to change to another agent during follow‐up. The results are presented both with the “start” and “continuation” cohorts pooled and separately in Table 2 and Table 3.
Figure 1. Flow diagram, demonstrating patient enrollment and available follow‐up data.
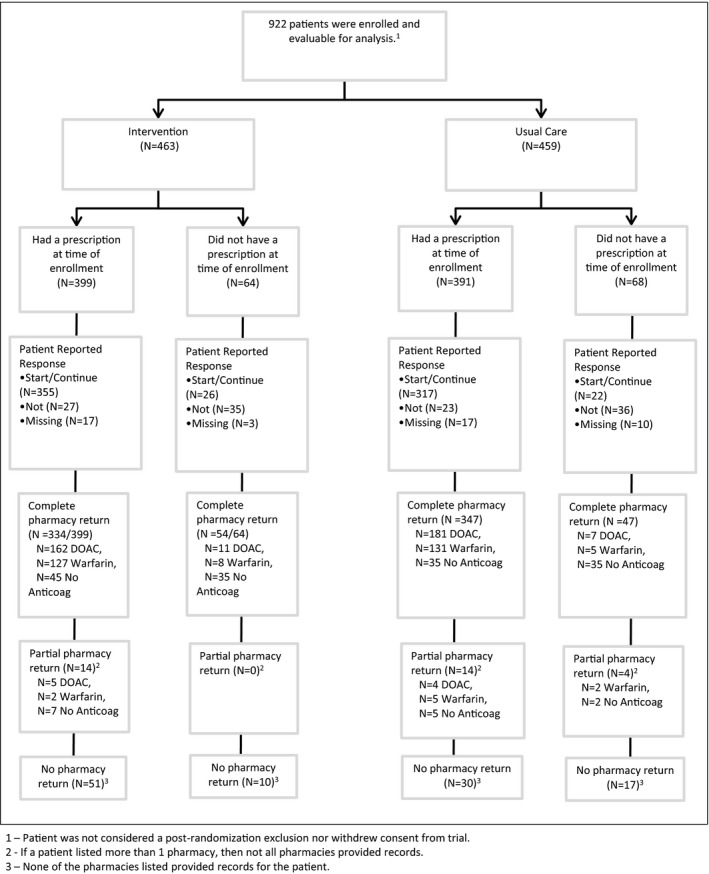
Anticoag indicates anticoagulation; and DOAC, direct oral anticoagulant.
Table 1.
Characteristics of Study Participants Who Had Documentation in the Medical Record
| Characteristics |
Intervention (n=463) |
Usual care (n=459) |
|---|---|---|
| Age, mean (SD), y | 71 (11) | 71 (10) |
| Women, n (%) | 172 (37) | 191 (42) |
| White race, n (%)* | 387 (85) | 380 (84) |
| CHA2DS2‐VASc score, mean (SD) | 3.5 (1.5) | 3.5 (1.5) |
| HAS‐BLED score, mean (SD) | 2.1 (1.1) | 2.1 (1.0) |
| Serum creatinine, N | 331 | 327 |
| Mean (SD) | 1.1 (0.6) | 1.2 (0.8) |
| Cohort, n (%) | ||
| Start (treatment naïve) | 98 (21) | 99 (22) |
| Review | 365 (79) | 360 (78) |
| General health, n (%) † , ‡ | 28 | 31 |
| Excellent/very good | 153 (35) | 138 (32) |
| Good | 188 (43) | 184 (43) |
| Fair/poor | 84 (22) | 106 (25) |
| Total medicines, mean (SD) † , § | 8.1 (4.7) | 7.6 (4.2) |
| Taking aspirin/NSAIDS and/or antiplatelet agents, n (%) † , || | 172 (40) | 151 (36) |
CHA2DS2‐VASc indicates congestive heart failure, hypertension, age ≥75 years, diabetes, stroke or transient ischemic attack, vascular disease, age 65 to 74 years, sex category.
Missing (n=16; 7 in intervention arm).
Patient reported.
Missing (n=59; 28 in intervention arm).
Prescription and over the counter per day, missing (n=65; 32 in intervention arm).
Missing (n=69; 31 in intervention arm).
Table 2.
Treatment Decisions Documented in the Medical Record
| Variable | Intervention (N=463) |
Usual care (N=459) |
Adjusted odds ratio (95% CI)|| , * | ICC (site) | ICC (clinician) |
|---|---|---|---|---|---|
| Start/continue anticoagulant | 399 (86) | 391 (85) | 1.11 (0.71–1.73) | 0.03 | 0.21 |
| Warfarin | 174 (44) | 177 (45) | 1.10 (0.79–1.53) | 0.23 | 0.41 |
| DOAC | 225 (56) | 214 (55) | |||
| Apixaban | 132 (59) | 125 (58) | |||
| Rivaroxaban | 84 (37) | 80 (37) | |||
| Dabigatran | 8 (4) | 8 (4) | |||
| Edoxaban | 1 (0.4) | 1 (0.5) | |||
| Start/continue anticoagulant | |||||
| Start cohort | 59/98 (60) | 54/99 (55) | 3.04 (0.94–9.87) | 0.17 | 0.46 |
| Warfarin | 9 (15) | 18 (33) | |||
| DOAC | 50 (85) | 36 (67) | |||
| Review cohort | 340/365 (93) | 337/360 (94) | 0.99 (0.70–1.40) | 0.22 | 0.39 |
| Warfarin | 165 (49) | 159 (47) | |||
| DOAC | 175 (51) | 178 (53) | |||
| Medication change † | 72 (18) | 86 (22) | 0.79 (0.55–1.14) | 0.05 | 0.20 |
| Chose to stop | 25 (35) | 31 (36) | |||
| Chose to change | 47 (65) | 55 (64) | |||
Data are given as number (percentage) or number/total (percentage). DOAC indicates direct oral anticoagulant; and ICC, intraclass correlation.
Multivariable logistic regression, adjusted by intervention, start vs review cohort, cluster effect of health care site and clinician.
Adjusted odds ratio is for the between‐arm comparison of intervention vs usual care.
First documented change (one DOAC to another, warfarin to DOAC, or DOAC to warfarin) after index encounter in the medical record.
Table 3.
Adherence to Anticoagulation Based on Pharmacy Fill Records and INR Data
| Variable |
Intervention (N=402) |
Usual care (N=412) |
Adjusted odds ratio (95% CI)‡ , * |
|---|---|---|---|
| Patients with complete records, n (%) | 388 (97) | 394 (96) | |
| Patients with partial records, n (%) | 14 (3) | 18 (4) | |
| No anticoagulants on record, n (%) | 87 (22) | 77 (19) | |
| Prescriptions filled, n (%) † | 315 (78) | 335 (81) | 0.83 (0.57 to 1.19) |
| Warfarin | 138 (44) | 143 (43) | |
| DOAC | 177 (56) | 192 (57) | |
| Low‐risk cohort, n prescription filled/N (%) ‡ | 67/97 (70) | 71/90 (79) | 0.96 (0.50 to 1.85) |
| Warfarin | 23 | 23 | |
| DOAC | 44 | 48 | |
| High‐risk cohort, n prescription filled/N (%) ‡ | 248/308 (81) | 264/322 (82) | 0.73 (0.44 to 1.21) |
| Warfarin | 115 | 120 | |
| DOAC | 133 | 144 | |
| Secondary adherence: DOAC | N=183 | N=191 | |
| PDC, mean (95% CI) § | 74.1 (69.7 to 78.5) | 71.6 (67.6 to 75.7) | 2.4 (−3.5 to 8.3) || |
| PDC ≥80%, n (%) | 113 (62) | 102 (53) | 1.42 (0.96 to 2.11) |
| Start cohort | N=41 | N=38 | |
| PDC ≥80%, n (%) | 21 (51) | 18 (47) | 1.16 (0.51 to 2.62) |
| Review cohort | N=142 | N=153 | |
| PDC, mean (95% CI) | 74.8 (69.8 to 79.8) | 73.3 (68.9 to 77.7) | |
| PDC ≥80%, n (%) | 92 (65) | 84 (55) | 1.49 (1.00 to 2.22) |
| Secondary adherence: warfarin ¶ | N=154 | N=161 | |
| Missing INR, n (%)# | 21 (14) | 13 (8) | |
| No. of INR tests, median (IQR) | 18 (11 to 20) | 15 (8.5 to 20) | |
| INR results in therapeutic range (2.0–3.0), median (IQR) | 10 (6 to 12) | 9 (4 to 12) | |
| TTR, mean (95% CI), % ** | 66.6 (61.9 to 71.4) | 64.4 (42.8 to 54.1) | |
| TTR ≥80%, n/N (%) ¶ | 50/122 (41) | 57/131 (44) | 0.96 (0.55 to 1.67) |
| Start cohort | N=8 | N=13 | |
| No. of INR tests, median (IQR) | 18 (4 to 20) | 17 (8 to 20) | |
| INR results in therapeutic range (2.0–3.0), median (IQR) | 9 (1 to 12) | 7 (2 to 12) | |
| TTR, mean (95% CI), % ** | 61.1 (40.2 to 82.0) | 50.0 (30.6 to 69.5) | |
| TTR ≥80%, n (%) ¶ | 2 (25) | 2 (15) | … |
| Review cohort | N=114 | N=118 | |
| No. of INR tests, median (IQR) | 18 (12 to 20) | 15 (9 to 20) | |
| INR results in therapeutic range (2.0–3.0), median (IQR) | 10 (6 to 12) | 9 (4 to 12) | |
| TTR, mean (95% CI), % ** | 70.9 (66.7 to 75.1) | 70.3 (65.5 to 75.2) | |
| TTR ≥80%, n (%) ¶ | 48 (42) | 55 (47) | 0.85 (0.48 to 1.50) |
DOAC indicates direct oral anticoagulant; INR, international normalized ratio; IQR, interquartile range; PDC, percentage days covered; and TTR, time in therapeutic range.
Multivariable logistic regression, adjusted by intervention, start vs review cohort, cluster effect of health care site and clinician.
Adjusted odds ratio is for the between‐arm comparison of intervention vs usual care.
Initial prescription after index encounter (N=34 patients had prescriptions for DOAC first and then warfarin after, and N=16 for intervention).
CHA2DS2‐VASc (congestive heart failure, hypertension, age ≥75 years, diabetes, stroke or transient ischemic attack, vascular disease, age 65 to 74 years, sex category) score of <2 vs ≥2 for men or <3 vs ≥3 for women.
Adherence to DOACs includes patients with documentation of a prescription for a DOAC who did not fill them (for those with available fill information, N=183 Anticoagulation Choice and N=191 usual care).
The adjusted mean difference was calculated from a multivariable generalized linear regression model, adjusted by intervention, start vs review cohort, cluster effect of health care site and clinician.
Patients who started on warfarin or who chose to switch to warfarin during follow‐up.
No INR test results reported in the medical record (ie, test could have been completed and reported at a different health care system).
Patients with ≥2 INR results and with test results covering ≥30 days.
Primary and Secondary Adherence
Approximately 80% of patients filled their first prescription, and this proportion was not significantly different across trial arms, either in the overall population with pharmacy records or after stratification by CHA2DS2‐VASc score (low versus high risk) (Table 3). The time to filling of the initial prescription (Figure 2A) and the rate of subsequent adherence (Figure 2B) were also similar across arms. Among patients who chose treatment with a DOAC, the PDC was similar between groups (74.1% versus 71.6% in the intervention and UC arms, respectively). Among patients who chose treatment with warfarin, the TTR was similar between groups (66.6% versus 64.4% in the intervention and UC arms, respectively), with 41% and 44% of patients achieving a TTR ≥80% in the intervention and UC arms, respectively. Of the 725 patients in the review cohort, 424 had adherence data (PDC for DOAC and/or TTR for warfarin) before enrollment and after enrollment. Among patients taking DOAC in the review cohort, PDCDOAC was better (65%) in SDM than in UC (55%) (OR, 1.49; 95% CI, 1.00–2.22) (Table 2). Among patients in the review cohort, there was modest interaction between preenrollment adherence and the impact of SDM on subsequent adherence (OR, 1.75; 95% CI, 1.05–2.93; P=0.03; Table S1). Figure 3 (and Figures S1 through S3) demonstrates adherence to anticoagulation before enrollment and after exposure by trial arm and across relevant subgroups.
Figure 2. Kaplan‐Meier curves, demonstrating the time to start anticoagulation after the initial prescription by arm (A) and the time on anticoagulation (secondary adherence) by arm (B).
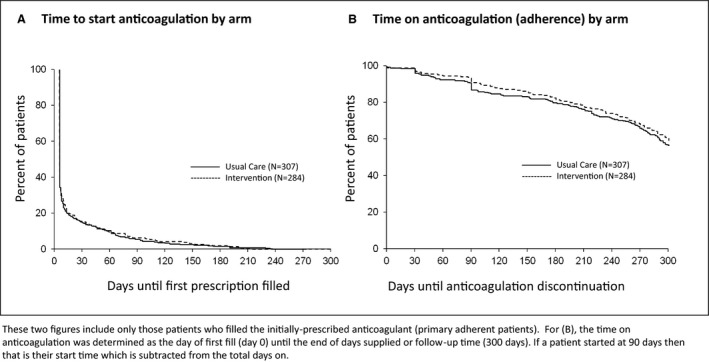
Figure 3. Alluvial plot, demonstrating the evolution of anticoagulation fills and adherence before enrollment and after exposure, by trial arm.
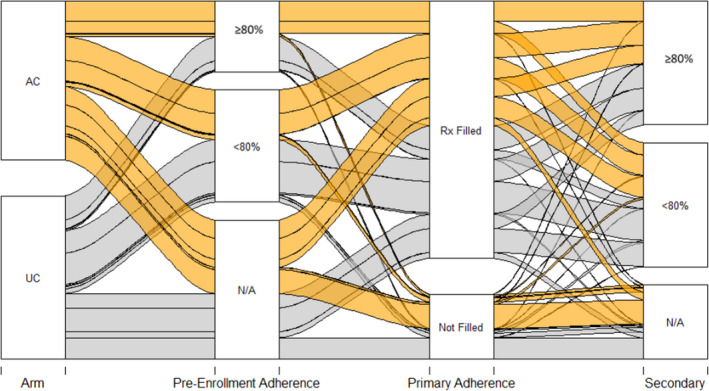
Primary adherence reflects a prescription fill after the index visit, whereas secondary adherence reflects the percentage days covered (for patients on a direct oral anticoagulant) and time in therapeutic range (for patients on warfarin). Patients fell in the not applicable (N/A) category for secondary adherence if they did not have ≥30 days of coverage by medication fills or international normalized ratio values. AC indicates Anticoagulation Choice (shared decision‐making tool); Rx, prescription; and UC, usual care.
Clinical Safety Outcomes
The rates of clinical safety outcomes were similar between groups, with 13% and 14% of patients in the intervention and UC arms, respectively, having a clinical safety outcome (Table 4). Major bleeding was the most frequent outcome, with 47 (10%) and 48 (11%) patients having an event during follow‐up in the intervention and UC arms, respectively. Approximately the same proportion of patients experienced a stroke or died in both treatment arms during the 10‐month follow‐up, and the rates of both stroke and death were similar in the 2 arms.
Table 4.
Safety Outcomes
| Outcome |
Intervention (N=459) |
Usual care (N=456) |
|---|---|---|
| Any major bleeding, cerebrovascular event, or death from any cause, n (%) | 59 (13) | 64 (14) |
| Major bleeding, n (%) | 47 (10) | 48 (11) |
| Cerebrovascular event, n (%) | 7 (2) | 6 (1) |
| Transient ischemic attack | 1 | 0 |
| Ischemic stroke | 5 | 5 |
| Stroke, type unknown | 1 | 1 |
| Death, n (%) | 15 (3) | 19 (4) |
| Cardiovascular* | 4 | 6 |
| Bleeding | 2 | 1 |
| Cancer | 3 | 3 |
| Infection or sepsis | 1 | 1 |
| Cause unknown | 5 | 8 |
Myocardial infarction, stroke, heart failure, or pulmonary embolism.
Discussion
In this study, the largest randomized trial of SDM to date in the care of patients with atrial fibrillation to date, 10 the use of a within‐encounter SDM tool did not result in increased rates of anticoagulation initiation or adherence. This lack of demonstrable benefit is despite the fact that the intervention appeared to have been properly implemented. As presented in the initial report of this trial, manual review of their video‐recorded encounters revealed that clinicians used the intervention correctly in most cases and that there was no substantial contamination between trial arms. 14 These results leave us to assess critically the value of using SDM tools in practice, particularly as a patient‐centered intervention to improve adherence to therapy via SDM.
Most of the existing literature has focused on evaluating the ability of SDM tools to promote behaviors consistent with SDM and to improve psychological outcomes of SDM (eg, knowledge, decisional conflict, or regret) rather than the downstream clinical impact of collaborative clinician‐patient interactions. The results of trials evaluating the impact of SDM on medication adherence and clinical outcomes have been heterogeneous. For instance, a recent cluster randomized clinical trial of an SDM program for patients with type 2 diabetes in primary care demonstrated higher levels of risk knowledge with the intervention, but no impact on the primary end point of medication adherence. 24 In contrast, one randomized clinical trial in patients with poorly controlled asthma demonstrated improvement in treatment adherence with an SDM intervention. 25 There are other ongoing studies evaluating the impact of SDM on treatment adherence in a broad range of conditions, ranging from bipolar disorder 26 to hormonal contraception. 27 To our knowledge, there are no other trials of SDM interventions assessing their impact on primary and secondary medication adherence and clinical outcomes.
It seems almost self‐evident that an intervention designed to ensure high‐quality SDM would result in more patient‐centered decisions and higher probability of long‐term medication adherence and favorable clinical outcomes, but this and other studies suggest that this is not easy to demonstrate. In the current era, more and more clinicians are aware of the value and underlying assumptions of SDM and, as such, the magnitude of the effect of these interventions could be attenuated by high‐quality interactions that are part of UC. In addition, many patients in the study were already taking anticoagulants (the continuation cohort), and it may be unlikely that a single intervention, as studied herein, would affect their medication adherence or their clinical trajectory. The latest Cochrane review of interventions to promote adherence to medications (which included several studies about warfarin but none about DOACs) also reported disappointing results. 28 The authors noted that most trials enrolled patients who do not have documented nonadherence at baseline, limiting the opportunity for the intervention to improve adherence. It is also worth considering the extent to which participating in SDM helps a patient overcome the barriers that patients face in maintaining adherence (ie, the issue of adherence may not be an issue of choice but rather the result of the ongoing work of implementation after the decision). 29 , 30 It is possible that an intervention targeting patients with nonadherence at baseline and patients with direct experience of the consequences of AF or anticoagulation (for instance, patients with prior stroke or those with concerns about recurrent bleeding, medication cost, polypharmacy, or labile INRs) could have yielded different results, but the current data appear to be broadly applicable to the patients typically engaging in anticoagulation conversations.
Further complicating the analysis of the link between SDM and clinical and behavioral outcomes is the question of the quality of the decision itself. Certainly, some “good” decisions can sometimes have bad outcomes, yet we hope for a high‐quality decision to usually contribute to favorable medical outcomes. Currently, the field of SDM is limited in its ability to evaluate if a decision, at the time it was made, made optimal intellectual, practical, and emotional sense for the patient and his/her situation. 31
These results should inform the rationale and perhaps affect the strength of recommendation for SDM in clinical practice guidelines and of policies to promote the adoption of SDM in practice when the main rationale for it is to increase the uptake and adherence to effective care, such as anticoagulation, rather than the promotion of patient‐centered care.
We acknowledge several limitations of this trial. To precisely exclude meaningful differences in safety outcomes, we would have needed a much larger trial, as large or larger than the trials used to demonstrate the efficacy of warfarin and of DOACs. Larger trials would be particularly necessary to parse any differences in treatment pattern, adherence, or outcomes between the “start” and “continuation” cohorts. The trial enrolled a wide range of patients, including patients who were adherent to a stable anticoagulation regimen, a population unlikely to benefit from the intervention’s effect on adherence, if any were present. We note, however, that we enrolled fewer women and minorities relative to the prevalence of atrial fibrillation in these populations. Although we were able to obtain fairly complete pharmacy and laboratory data, there was some missing data on prescription fills and we lacked INR data in >10% of the warfarin‐treated population. These missing data could have affected our estimates of medication adherence. In addition, we acknowledge that the rate of anticoagulation was higher in both trial arms than might be typically observed among unselected patients in routine clinical practice. This high level of anticoagulation could attenuate the ability to detect a difference in anticoagulation rate between arms and may indicate a difference between trial participants and nonparticipants. Last, our results may not apply to patients who would have opted to not take part in our trial, such as patients with reduced capacity to adhere to complex or expensive treatments.
Conclusions
In conclusion, in a large, randomized trial, a within‐encounter SDM tool for use in conversations on anticoagulation for stroke prevention in patients with atrial fibrillation had no significant effect on treatment adherence or clinical safety outcomes.
Appendix
SDM4AFib (Shared Decision‐Making for Atrial Fibrillation) Trial Investigators
Steering Committee
Principal investigator: Victor M. Montori; Study statistician: Megan E. Branda; Coinvestigators: Juan P. Brito, Marleen Kunneman, Ian Hargraves; Study coordinator: Angela L. Sivly; Study manager: Kirsten Fleming; Site principal investigators: Bruce Burnett (Park Nicollet‐Health Partners, Minneapolis, MN), Mark Linzer and Haeshik Gorr (Hennepin Healthcare, Minneapolis, MN), Elizabeth Jackson and Erik Hess (University of Alabama at Birmingham), Takeki Suzuki and James Hamilton IV (University of Mississippi Medical Center, Jackson, MS), and Peter A. Noseworthy (Mayo Clinic, Rochester, MN).
Site Teams (alphabetical order)
Hennepin Healthcare: Haeshik Gorr, Alexander Haffke, Mark Linzer, Jule Muegge, Sara Poplau, Benjamin Simpson, Miamoua Vang, and Mike Wambua. Mayo Clinic: Joel Anderson, Emma Behnken, Fernanda Bellolio, Juan P. Brito, Renee Cabalka, Michael Ferrara, Kirsten Fleming, Rachel Giblon, Ian Hargraves, Jonathan Inselman, Marleen Kunneman, Annie LeBlanc, Alexander Lee, Victor Montori, Peter Noseworthy, Marc Olive, Paige Organick, Nilay Shah, Angela Sivly, Gabriela Spencer‐Bonilla, Amy Stier, Anjali Thota, Henry Ting, Derek Vanmeter, and Claudia Zeballos‐Palacios. Park Nicollet‐ Health Partners: Carol Abullarade, Bruce Burnett, Lisa Harvey, and Shelly Keune. University of Alabama at Birmingham: Elizabeth Jackson, Erik Hess, Timothy Smith, Shannon Stephens. University of Mississippi Medical Center: Bryan Barksdale, James Hamilton IV, Theresa Hickey, Roma Peters, Memrie Price, Takeki Suzuki, Connie Watson, and Douglas Wolfe.
Data Safety and Monitoring Board
Gordon Guyatt (chair), Brian Haynes, and George Tomlinson.
Expert Advisory Panel
Paul Daniels, Bernard Gersh, Erik Hess, Thomas Jaeger, Robert McBane, and Peter Noseworthy (chair).
Sources of Funding
The trial was funded by and conducted independently of the National Heart, Lung, and Blood Institute of the US National Institutes of Health (RO1 HL131535‐01 and RO1 HL131535‐03S1). The funding body had no influence on the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
None.
Supporting information
Table S1
Figures S1–S3
Acknowledgments
The investigators thank all the patients, caregivers, clinicians, study coordinators, patient and expert advisors, and members of the data monitoring board who kindly and enthusiastically made this trial possible.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023048
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Victor M. Montori, Email: Montori.Victor@mayo.edu.
the SDM4AFib (Shared Decision‐Making for Atrial Fibrillation) Trial Investigators:
Victor M. Montori, Megan E. Branda, Juan P. Brito, Marleen Kunneman, Ian Hargraves, Angela L. Sivly, Kirsten Fleming, Bruce Burnett, Mark Linzer, Haeshik Gorr, Elizabeth Jackson, Erik Hess, Takeki Suzuki, James Hamilton, Peter A. Noseworthy, Haeshik Gorr, Alexander Haffke, Mark Linzer, Jule Muegge, Sara Poplau, Benjamin Simpson, Miamoua Vang, Mike Wambua, Joel Anderson, Emma Behnken, Fernanda Bellolio, Juan P. Brito, Renee Cabalka, Michael Ferrara, Kirsten Fleming, Rachel Giblon, Ian Hargraves, Jonathan Inselman, Marleen Kunneman, Annie LeBlanc, Alexander Lee, Victor Montori, Peter Noseworthy, Marc Olive, Paige Organick, Nilay Shah, Angela Sivly, Gabriela Spencer‐Bonilla, Amy Stier, Anjali Thota, Henry Ting, Derek Vanmeter, Claudia Zeballos‐Palacios, Park Nicollet, Carol Abullarade, Bruce Burnett, Lisa Harvey, Shelly Keune, Elizabeth Jackson, Erik Hess, Timothy Smith, Shannon Stephens, Bryan Barksdale, James Hamilton, Theresa Hickey, Roma Peters, Memrie Price, Takeki Suzuki, Connie Watson, Douglas Wolfe, Gordon Guyatt, Brian Haynes, George Tomlinson, Paul Daniels, Bernard Gersh, Erik Hess, Thomas Jaeger, Robert McBane, and Peter Noseworthy
References
- 1. Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14:195–203. doi: 10.11909/j.issn.1671-5411.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063 [DOI] [PubMed] [Google Scholar]
- 3. Tawfik A, Bielecki JM, Krahn M, Dorian P, Hoch JS, Boon H, Husereau D, Pechlivanoglou P. Systematic review and network meta‐analysis of stroke prevention treatments in patients with atrial fibrillation. Clin Pharmacol. 2016;8:93–107. doi: 10.2147/CPAA.S105165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gallagher AM, Rietbrock S, Plumb J, van Staa TP. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis? J Thromb Haemost. 2008;6:1500–1506. doi: 10.1111/j.1538-7836.2008.03059.x [DOI] [PubMed] [Google Scholar]
- 5. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645.e4. doi: 10.1016/j.amjmed.2009.11.025 [DOI] [PubMed] [Google Scholar]
- 6. Yao X, Abraham NS, Alexander GC, Crown W, Montori VM, Sangaralingham LR, Gersh BJ, Shah ND, Noseworthy PA. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc. 2016;5:e003074. doi: 10.1161/JAHA.115.003074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hernandez I, He M, Chen N, Brooks MM, Saba S, Gellad WF. Trajectories of oral anticoagulation adherence among Medicare beneficiaries newly diagnosed with atrial fibrillation. J Am Heart Assoc. 2019;8:e011427. doi: 10.1161/JAHA.118.011427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. doi: 10.1161/CIR.0000000000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Neill ES, Grande SW, Sherman A, Elwyn G, Coylewright M. Availability of patient decision aids for stroke prevention in atrial fibrillation: a systematic review. Am Heart J. 2017;191:1–11. doi: 10.1016/j.ahj.2017.05.014 [DOI] [PubMed] [Google Scholar]
- 10. Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, Holmes‐Rovner M, Llewellyn‐Thomas H, Lyddiatt A, Thomson R, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi: 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clarkesmith DE, Lip GYH, Lane DA. Patients' experiences of atrial fibrillation and non‐vitamin K antagonist oral anticoagulants (NOACs), and their educational needs: a qualitative study. Thromb Res. 2017;153:19–27. doi: 10.1016/j.thromres.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 12. Thomson RG, Eccles MP, Steen IN, Greenaway J, Stobbart L, Murtagh MJ, May CR. A patient decision aid to support shared decision‐making on anti‐thrombotic treatment of patients with atrial fibrillation: randomised controlled trial. Qual Saf Health Care. 2007;16:216–223. doi: 10.1136/qshc.2006.018481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eckman MH, Costea A, Attari M, Munjal J, Wise RE, Knochelmann C, Flaherty ML, Baker P, Ireton R, Harnett BM, et al. Shared decision‐making tool for thromboprophylaxis in atrial fibrillation – a feasibility study. Am Heart J. 2018;199:13–21. doi: 10.1016/j.ahj.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 14. Kunneman M, Branda ME, Noseworthy PA, Linzer M, Burnett B, Dick S, Spencer‐Bonilla G, Fernandez CA, Gorr H, Wambua M, et al. Shared decision‐making for stroke prevention in atrial fibrillation: study protocol for a randomized controlled trial. Trials. 2017;18:443. doi: 10.1186/s13063-017-2178-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeballos‐Palacios CL, Hargraves IG, Noseworthy PA, Branda ME, Kunneman M, Burnett B, Gionfriddo MR, McLeod CJ, Gorr H, Brito JP, et al. Developing a conversation aid to support shared decision‐making: reflections on designing anticoagulation choice. Mayo Clin Proc. 2019;94:686–696. doi: 10.1016/j.mayocp.2018.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kunneman M, Branda ME, Hargraves IG, Sivly AL, Lee AT, Gorr H, Burnett B, Suzuki T, Jackson EA, Hess E, et al. Assessment of shared decision‐making for stroke prevention in patients with atrial fibrillation: a randomized clinical trial. JAMA Intern Med. 2020;180:1215–1224. doi: 10.1001/jamainternmed.2020.2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montori VM, Kunneman M, Brito JP. Shared decision‐making and improving health care: the answer is not in. JAMA. 2017;318:617–618. doi: 10.1001/jama.2017.10168 [DOI] [PubMed] [Google Scholar]
- 18. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. doi: 10.1055/s-0038-1651587 [DOI] [PubMed] [Google Scholar]
- 21. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 22. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 23. StataCorp LLC . Stata Statistical Software: Release 15. 2017. Stata Press. [Google Scholar]
- 24. Buhse S, Heller T, Kasper J, Mühlhauser I, Müller UA, Lehmann T, Lenz M. An evidence‐based shared decision‐making programme on the prevention of myocardial infarction in type 2 diabetes: protocol of a randomised‐controlled trial. BMC Fam Pract. 2013;14:155. doi: 10.1186/1471-2296-14-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, Vollmer WM, Better Outcomes of Asthma Treatment Study Group . Shared treatment decision‐making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181:566–577. doi: 10.1164/rccm.200906-0907OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Samalin L, Honciuc M, Boyer L, de Chazeron I, Blanc O, Abbar M, Llorca PM. Efficacy of shared decision‐making on treatment adherence of patients with bipolar disorder: a cluster randomized trial (ShareD‐BD). BMC Psychiatry. 2018;18:103. doi: 10.1186/s12888-018-1686-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Molina‐Férnandez MI, Raigal‐Aran L, de la Flor‐Lopez M, Prata P, Font‐Jimenez I, Valls‐Fonayet F, March‐Jardi G, Escuriet‐Peiro R, Rubio‐Rico L. The effectiveness of a digital shared decision‐making tool in hormonal contraception during clinical assessment: study protocol of a randomized controlled trial in Spain. BMC Public Health. 2019;19:1224. doi: 10.1186/s12889-019-7572-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, Agoritsas T, Mistry N, Iorio A, Jack S, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;11:CD000011. doi: 10.1002/14651858.CD000011.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hargraves IG, Montori VM, Brito JP, Kunneman M, Shaw K, LaVecchia C, Wilson M, Walker L, Thorsteinsdottir B. Purposeful SDM: a problem‐based approach to caring for patients with shared decision‐making. Patient Educ Couns. 2019;102:1786–1792. doi: 10.1016/j.pec.2019.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brand‐McCarthy SR, Delaney RK, Noseworthy PA. Can shared decision‐making improve stroke prevention in atrial fibrillation? Implications of the updated guidelines. Circ Cardiovasc Qual Outcomes. 2020;13:e006080. doi: 10.1161/CIRCOUTCOMES.119.006080 [DOI] [PubMed] [Google Scholar]
- 31. Kunneman M, LaVecchia CM, Singh Ospina N, Abu Dabrh AM, Behnken EM, Wilson P, Branda ME, Hargraves IG, Yost KJ, Frankel RM, et al. Reflecting on shared decision‐making: a reflection‐quantification study. Health Expect. 2019;22:1165–1172. doi: 10.1111/hex.12953 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figures S1–S3


