Abstract
Background
Circulating cell‐free mitochondrial DNA (ccf‐mtDNA) is a damage‐associated molecular pattern that reflects cell stress responses and tissue damage, but little is known about ccf‐mtDNA in preeclampsia. The main objectives of this study were to determine (1) absolute concentrations of ccf‐mtDNA in plasma and mitochondrial DNA content in peripheral blood mononuclear cells and (2) forms of ccf‐mtDNA transport in blood from women with preeclampsia and healthy controls. In addition, we sought to establish the association between aberrance in circulating DNA‐related metrics, including ccf‐mtDNA and DNA clearance mechanisms, and the clinical diagnosis of preeclampsia using bootstrapped penalized logistic regression.
Methods and Results
Absolute concentrations of ccf‐mtDNA were reduced in plasma from women with preeclampsia compared with healthy controls (P≤0.02), while mtDNA copy number in peripheral blood mononuclear cells did not differ between groups (P>0.05). While the pattern of reduced ccf‐mtDNA in patients with preeclampsia remained, DNA isolation from plasma using membrane lysis buffer resulted in 1000‐fold higher ccf‐mtDNA concentrations in the preeclampsia group (P=0.0014) and 430‐fold higher ccf‐mtDNA concentrations in the control group (P<0.0001). Plasma from women with preeclampsia did not induce greater Toll‐like receptor‐9–induced nuclear factor kappa‐light‐chain enhancer of activated B cells‐dependent responses in human embryonic kidney 293 cells overexpressing the human TLR‐9 gene (P>0.05). Penalized regression analysis showed that women with preeclampsia were more likely to have lower concentrations of ccf‐mtDNA as well as higher concentrations of nuclear DNA and DNase I compared with their matched controls.
Conclusions
Women with preeclampsia have aberrant circulating DNA dynamics, including reduced ccf‐mtDNA concentrations and DNA clearance mechanisms, compared with gestational age–matched healthy pregnant women.
Keywords: cell‐free DNA, DNase I, mitochondrial DNA, penalized regression analysis, preeclampsia
Subject Categories: Preeclampsia, Hypertension, Biomarkers, Inflammation, Physiology
Nonstandard Abbreviations and Acronyms
- ccf‐mtDNA
circulating cell‐free mitochondrial DNA
- mtDNA
mitochondrial DNA
- nDNA
nuclear DNA
- PBMCs
peripheral blood mononuclear cells
- TLR‐9
Toll‐like receptor‐9
Clinical Perspective
What Is New
Cell‐free circulating mitochondrial DNA concentrations are reduced in plasma from women with preeclampsia at the third trimester.
The majority of cell‐free circulating mitochondrial DNA is transported encapsulated in vesicles during pregnancy, and this phenomenon is more pronounced in preeclampsia.
Women with preeclampsia are more likely to have aberrant circulating DNA dynamics, including cell‐free circulating mitochondrial DNA concentrations and DNA clearance mechanisms, compared with gestational age–matched healthy pregnant women.
What Are the Clinical Implications?
Preeclampsia is associated with placental dysfunction, trophoblast cell death, and oxidative stress, which lead to release of proinflammatory factors into the maternal circulation.
Dysregulation of cell‐free circulating mitochondrial DNA concentrations and DNA clearance mechanisms during pregnancy may contribute to the development or establishment of the maternal cardiovascular syndrome in preeclampsia.
Preeclampsia is a prevalent, multifactorial complication of pregnancy diagnosed as de novo hypertension after the 20th week of gestation with end‐organ damage. 1 This pregnancy‐specific syndrome, which is seen in ≈5% to 8% of pregnancies worldwide, results in increased morbidity and mortality for fetus and mother alike. Currently, there is no cure for preeclampsia, and the most common approach to clinical management is delivery of the fetoplacental unit, 2 which often occurs preterm.
During healthy pregnancies, placental cell turnover results in release of cellular constituents and DNA into the maternal circulation in increasing amounts as pregnancy progresses. 3 , 4 , 5 Placentas from pregnancies with preeclampsia, however, exhibit signs of damage 6 and exaggerated rates of trophoblast cell death. 7 Subsequently, tissue damage and cell death results in increased release of antiangiogenic factors, proinflammatory cytokines, and DNA fragments 8 , 9 into the maternal circulation. Thus, concentrations of circulating factors are currently being studied as potential markers of early diagnosis and placental health and predictors of maternal and perinatal outcomes. 10 , 11 , 12
Circulating cell‐free mitochondrial DNA (ccf‐mtDNA) is considered a damage‐associated molecular pattern molecule, which elicits an immune response via activation of the pattern recognition receptor, Toll‐like receptor‐9 (TLR‐9). 9 We and others have previously reported that activation of TLR‐9 during pregnancy induced preeclampsia‐like signs in rats and mice. 13 , 14 In line with these observations, plasma from women with preeclampsia elicited greater TLR‐9–mediated inflammatory responses compared with plasma from healthy pregnant women. 15 Cross‐sectional and case‐control studies have reported increased mitochondrial DNA (mtDNA) copy number in serum and whole blood from women with preeclampsia in the third trimester 16 , 17 and at time of delivery. 18 On the other hand, ccf‐mtDNA was suppressed in the first trimester of women who later developed preeclampsia. 19 Experimental factors such as the choice of biological sample (ie, whole blood versus serum versus plasma 20 , 21 ), DNA extraction methods, and quantification protocols widely varied in these previous studies. This methodological variation raises questions about the biological specificity and interpretation of ccf‐mtDNA measurements in pregnant patients. 9 , 22 For example, quantification of mtDNA in whole blood does not distinguish between cellular and cell‐free forms of mtDNA, which correlate with distinct biological functions. 21 Furthermore, studies in maternal blood have not determined the biological forms of ccf‐mtDNA, such as quantities of mtDNA encapsulated in vesicular structures versus circulating non–membrane‐bound mtDNA. 23
The main objectives of this study were to determine (1) absolute concentrations of mtDNA in plasma (cell‐free fraction of blood, ccf‐mtDNA) and mtDNA content in peripheral blood mononuclear cells (cellular mtDNA) and (2) forms of ccf‐mtDNA transport (membrane bound versus non–membrane bound) in blood from women with preeclampsia and healthy controls. In addition, we sought to establish the association between aberrance in circulating DNA‐related metrics, including ccf‐mtDNA and DNA clearance mechanisms, and the clinical diagnosis of preeclampsia using bootstrapped penalized logistic regression. 24 , 25
Methods
Detailed methods are available in Data S1. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects
Deidentified subject information and blood samples were acquired from the Maternal Fetal Tissue Bank (IRB# 200910784) of the Women’s Health Tissue Repository at the University of Iowa who obtained informed consent from pregnant women to collect biological samples and clinical data. 26 Cases consisted of 19 pregnant women with preeclampsia and 19 healthy pregnant controls matched for gestational age (Table S1). As expected, the preeclampsia group exhibited higher mean, systolic, and diastolic blood pressures and a lower gestational age of delivery, neonatal weight, and APGAR scores (P<0.05) in comparison to the control pregnancies. Maternal venous blood was collected in the third trimester (mean=33.6 weeks) and separated into plasma and peripheral blood mononuclear cells (PBMCs). Plasma was stored at −80 °C, and PBMCs were stored in liquid N2 until further processing.
DNA Quantification
DNA was isolated and quantified as published previously, 27 with a few modifications. Previous studies have noted that ccf‐mtDNA can be membrane bound or non–membrane bound. 28 , 29 These data suggest vesicular and nonvesicular transport of ccf‐mtDNA within the circulation. To determine the contribution of membrane‐bound and ‐unbound ccf‐mtDNA in preeclampsia, DNA from plasma samples was isolated with lysis buffer and without lysis buffer. TaqMan chemistry‐based absolute quantification of nuclear DNA (nDNA) and ccf‐mtDNA was used as detailed elsewhere. 27 Isolated ccf‐mtDNA was quantified using a method modified from Kavlick et al 30 and detailed previously. 27 The target sequence for this analysis was the mitochondrial NADH:ubiquinone oxidoreductase core subunit 5 gene (mitochondrial NADH:ubiquinone oxidoreductase core subunit 5; GenBank Gene ID: 4540), spanning positions 13 288 to 12 392 of the mitochondrial genome. 31 The quantitative polymerase chain reaction primers, probes, and synthetic DNA standards used for ccf‐mtDNA quantification are detailed in Table S2. Concentrations of plasma DNA are expressed as pg/mL of plasma. Total DNA was calculated as the sum of ccf‐mtDNA and nDNA, in pg/mL plasma. Plasma ccf‐mtDNA is expressed in absolute concentrations as a percent of total DNA (%ccf‐mtDNA). The DNA content of PBMCs is presented as cell equivalent per µL DNA isolate for nDNA and as ccf‐mtDNA genome copies per cell equivalent.
Statistical Analysis
Normality tests, outlier removal and univariate analyses were performed using Prism (Version 8, GraphPad, San Diego, CA). Student’s t‐test and Mann‐Whitney U test were used for group comparisons. The effect of fetal sex on primary outcomes was assessed with a 2‐way ANOVA followed by Sidak’s correction to adjust for multiple comparisons. DNA outcomes are presented in violin plots showing medians and ranges. Subject characteristics are presented as mean with minimum and maximum unless otherwise indicated. The significance level was set at α=0.05 for all comparisons (before multiple testing comparisons).
Penalized Logistic Regression Model Selection
To identify the most important subject characteristics associated with the outcome of preeclampsia, bootstrapped penalized logistic regression was implemented (detailed description in Data S1). All elements of regression analyses were carried out in R software version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 Three penalized regression models (ie, least absolute shrinkage and selection operator, ridge regression, and elastic net regression) were fit, and their performances were compared to select the best model. To reduce the number of covariates and mitigate multicollinearity in regression models while not eliminating potentially important patient characteristics, related parameters were grouped a priori (Table S3), and one single characteristic was selected from each group to generate all possible combinations of independent characteristics (192 total data sets). Bootstrapped (R=500) 10‐fold cross‐validation least absolute shrinkage and selection operator, ridge, and elastic net were then performed on each data set, and best model fit was determined by lowest prediction error (ie, lowest median root mean squared error). 41 , 42
Application of Best Model to Explain the Relationship Between Maternal Plasma DNA Metrics and the Diagnosis of Preeclampsia
Predictive accuracy of the final model developed using real data was tested against a simulated naïve prediction data set using the bootstrap estimate (R=500) of the area under the receiver operator characteristic curve with 95% CI calculated from standard error. Characteristics selected by the final regression model at least 75% of the time (variable importance probability, 0.75) for all bootstrap samples were regarded as most important. 43 Adjusted odds ratios are reported.
Results
Maternal Plasma mtDNA and nDNA
Figure 1A illustrates the biological forms of mtDNA assessed in this investigation in maternal plasma from women with preeclampsia and gestational age–matched healthy pregnant women. Concentrations of ccf‐mtDNA were reduced in preeclampsia compared with healthy controls in both lysis buffer‐treated samples (Figure 1B) and in samples that were not treated with lysis buffer (Figure 1C). While the pattern of reduced ccf‐mtDNA in preeclampsia remained the same between methods of DNA extraction, the exact quantity of ccf‐mtDNA was different between extraction methods. Specifically, concentrations of ccf‐mtDNA were increased by 1000‐fold in samples that were treated with lysis buffer (3.294±0.828 versus 0.003±0.001 pg/mL, n=15; P=0.0014) in the preeclampsia group. On the other hand, concentrations of ccf‐mtDNA were increased by 430‐fold in samples treated with lysis buffer compared with samples not treated with lysis buffer (9.011±2.043 versus 0.021±0.004 pg/mL, n=18; P<0.0001) in the control group. Content of mtDNA in PBMCs did not differ between groups (Figure 1D).
Figure 1. Mitochondrial DNA in cell‐free and cellular forms in maternal plasma from pregnancies with preeclampsia and healthy controls.
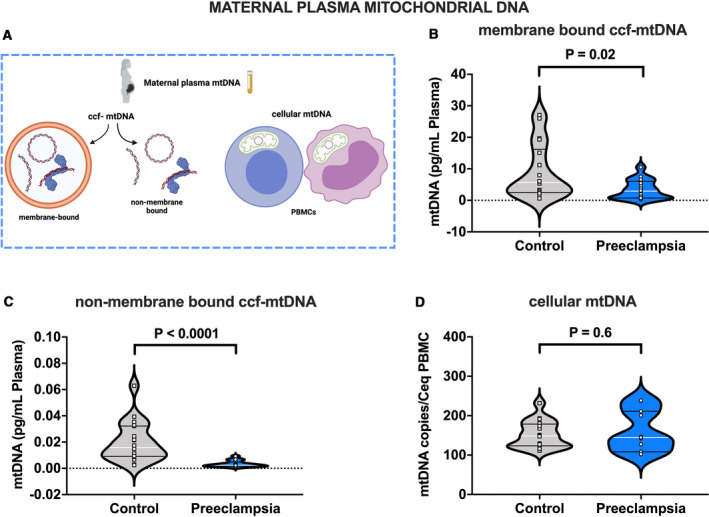
A, Biological forms of mtDNA assessed in this study, (B) ccf‐mtDNA concentrations (pg/mL) in plasma isolated using lysis buffer (membrane bound ccf‐mtDNA), (C) ccf‐mtDNA concentrations (pg/mL) in plasma isolated without lysis buffer (non‐membrane bound ccf‐mtDNA), (D) cellular mtDNA copies per estimated number of peripheral blood mononuclear cells (PBMCs), Student’s t‐test (B and D), Mann‐Whitney U test (C). B through C, n=18 controls, n=17 preeclampsia; (D) n=16 controls, n=11 preeclampsia. All values presented as median (white line), IQR (black lines). ccf‐mtDNA indicates circulating cell‐free mitochondrial DNA; Ceq, cell equivalent; and PBMC, peripheral blood mononuclear cell. Open squares=gestational age‐matched control; open circles=third trimester pregnancies with preeclampsia. A, was created with Biorender.com.
There were no group differences in plasma concentrations of cell‐free nDNA in samples treated with lysis buffer (Figure 2A), while plasma concentrations of cell‐free nDNA in samples treated without lysis buffer were increased in women with preeclampsia compared with healthy controls (Figure 2B). Content of cell‐free nDNA in PBMCs did not differ between groups (Figure 2C).
Figure 2. Nuclear DNA in cell‐free and cellular forms in maternal plasma from pregnancies with preeclampsia and healthy controls.
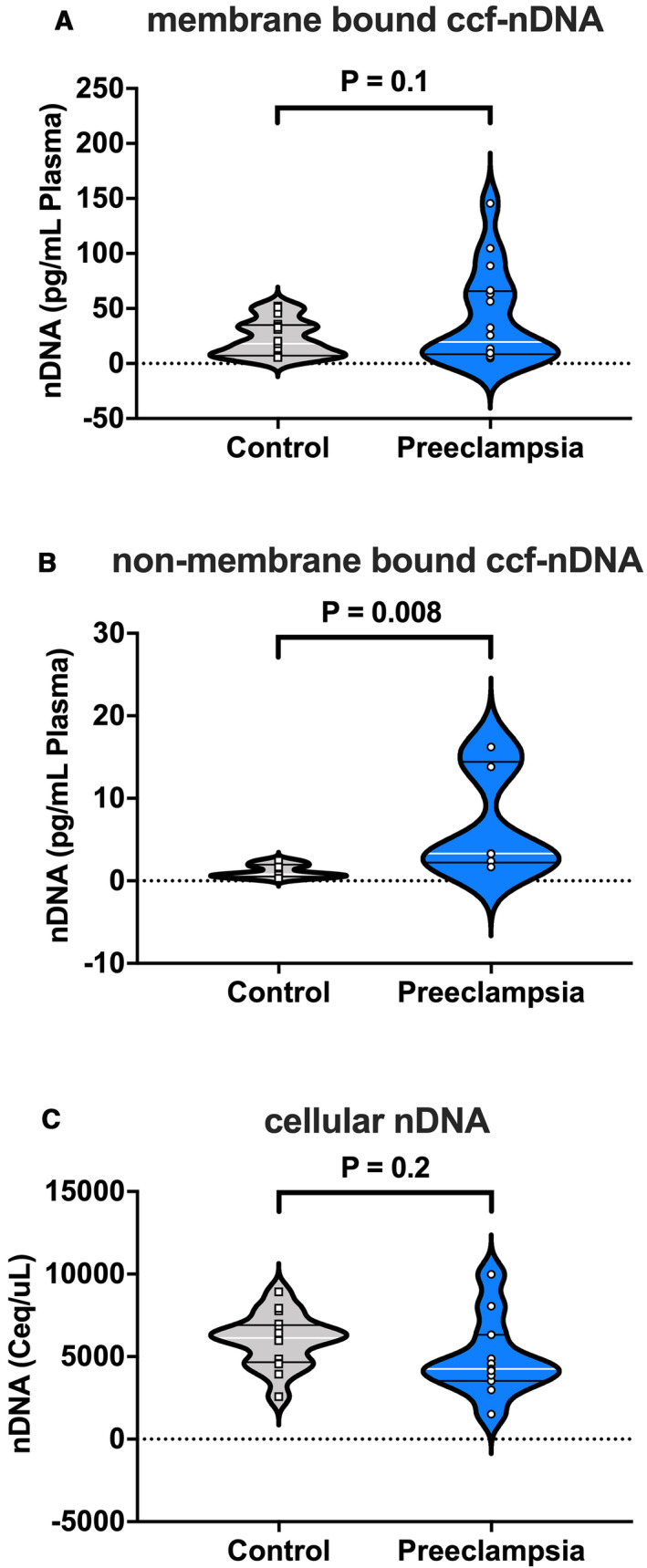
A, nDNA concentrations (pg/mL) in plasma isolated using lysis buffer (membrane bound ccf‐nDNA); and B, nDNA concentrations (pg/mL) in plasma isolated without lysis buffer (non–membrane‐bound ccf‐nDNA). C, nDNA concentration calculated as cell equivalents per microliter of peripheral blood mononuclear cell (PBMC) DNA isolate. Student’s t‐test (A and C), Mann‐Whitney U test (B). A, n=17 controls, n=16 preeclampsia; (B) n=8 controls, n=6 preeclampsia; (C) n=16 controls, n=11 preeclampsia. All values presented as median (white line), interquartile range (black lines). ccf‐nDNA indicates circulating cell‐free nuclear DNA; and Ceq, cell equivalent. Open squares=gestational age‐matched control; open circles=third trimester pregnancies with preeclampsia.
Plasma DNA concentrations were compared between pregnancies carrying male and female fetuses. There was neither a main effect for sex nor a group‐by‐sex interaction for plasma ccf‐mtDNA concentrations (Figure 3A); however, there was an increase (P=0.0003) in circulating nDNA in pregnancies with preeclampsia carrying female fetuses (Figure 3B).
Figure 3. Effect of fetal sex on maternal plasma concentrations of cell‐free DNA.
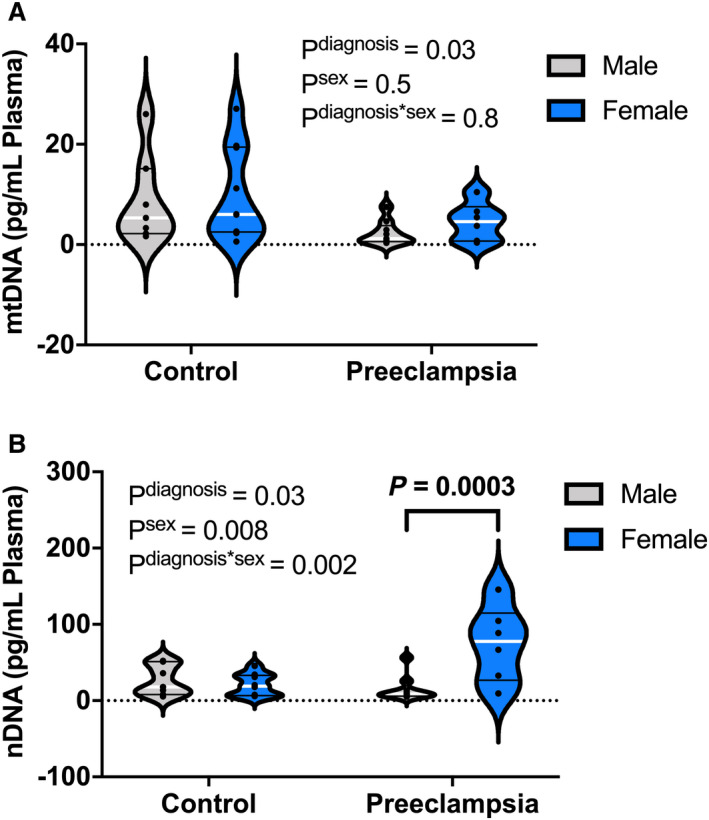
Membrane bound (A) ccf‐mtDNA (pg/mL) and (B) ccf‐nDNA (pg/mL) in plasma from pregnant women carrying female (control, n=9; preeclampsia, n=6) and male fetuses (control, n=7; preeclampsia, n=10). Data analyzed by 2‐way analysis of variance with Sidak’s post hoc analysis. All values presented as median (white line), interquartile range (black lines). nDNA, nuclear DNA; ccf‐mtDNA, circulating cell‐free mitochondrial DNA; ccf‐nDNA, circulating cell‐free nuclear DNA.
Collectively, these data demonstrate that the cell‐free form of circulating mtDNA and not the mtDNA content of PBMCs is reduced in plasma from women with preeclampsia compared with healthy gestational age–matched pregnant women and that the majority of ccf‐mtDNA is encapsulated in vesicular structures.
Effects of Maternal Plasma on TLR‐9–Dependent Inflammation
To assess the potential of maternal plasma to induce TLR‐9–mediated inflammatory responses, we treated human embryonic kidney 293 cells expressing a secreted embryonic alkaline phosphatase reporter gene (HEK‐Blue Null cells, control; Figure 4A) and human embryonic kidney 293 cells expressing a reporter gene and overexpressing the TLR‐9 gene (HEK‐Blue hTLR9 cells, Figure 4B) with plasma from women with preeclampsia and healthy controls (detailed methods in Data S1). Plasma from women with preeclampsia did not elicit greater nuclear factor kappa‐light‐chain enhancer of activated B cells –mediated proinflammatory responses in any of the cell lines (P≥0.096). To test whether inhibition of TLR‐9 in HEK‐Blue hTLR9 cells induced differential inflammatory responses when cells were treated with plasma from preeclamptic patients versus controls, we pretreated HEK‐Blue hTLR9 cells with chloroquine (an endolysosomal acidification inhibitor). Chloroquine treatment did not affect inflammatory responses of HEK‐Blue hTLR9 cells (Figure 4C) to either maternal plasma from preeclamptic patients (P=0.92) or controls (P=0.78). In summary, plasma from women with preeclampsia does not elicit greater TLR‐9–dependent inflammatory responses compared with plasma from healthy pregnant women.
Figure 4. TLR‐9–induced NF‐κB‐mediated inflammation in response to maternal plasma from women with preeclampsia and gestational age–matched healthy controls.
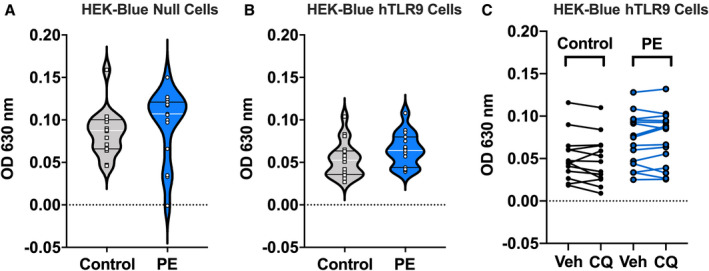
Nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB)‐mediated production of secreted embryonic alkaline phosphatase (SEAP) in (A) human embryonic kidney (HEK) 293 cells expressing an inducible SEAP reporter gene under the control of an NF‐κB inducible promoter (HEK‐Blue Null1, control cells; controls, n=16; preeclampsia, n‐15), (B) HEK293 cells overexpressing the human TLR‐9 gene and expressing an inducible SEAP reporter gene under the control of an NF‐κB inducible promoter (HEK‐Blue hTLR‐9; controls, n=18; preeclampsia, n=15), and (C) HEK‐Blue hTLR‐9 treated with chloroquine (CQ; controls, n=13; preeclampsia, n=15). All cells were exposed to 10% maternal plasma from women with preeclampsia or gestational age‐matched healthy pregnant women. Student’s t‐test. All values presented as median (white line), interquartile range (black lines). OD 630 nm, optical density at 630 nanometer wavelength light. Open squares=gestational age–matched control; open circles=third‐trimester pregnancies with preeclampsia.
DNA Clearance in Maternal Blood
Univariate analysis showed no differences (P=0.14) in plasma DNase I concentrations between control pregnancies and pregnancies with preeclampsia (Figure 5A). Moreover, there was no correlation (P=0.5) between DNase I concentrations and membrane‐bound ccf‐mtDNA in either group (Figure 5B). Plasma DNase I was not correlated to nDNA (P=0.2) or %ccf‐mtDNA (P=0.3) in control pregnancies (Figure 5C and 5D). In contrast, there were significant correlations (P≤0.04) between DNase I and nDNA and %ccf‐mtDNA in preeclampsia (Figure 5C and 5D). These data suggest that in preeclampsia, circulating DNase I concentrations are associated with cell‐free nDNA but not with membrane‐bound ccf‐mtDNA.
Figure 5. Maternal plasma concentrations of DNase I measured in healthy pregnancies and pregnancies with preeclampsia.
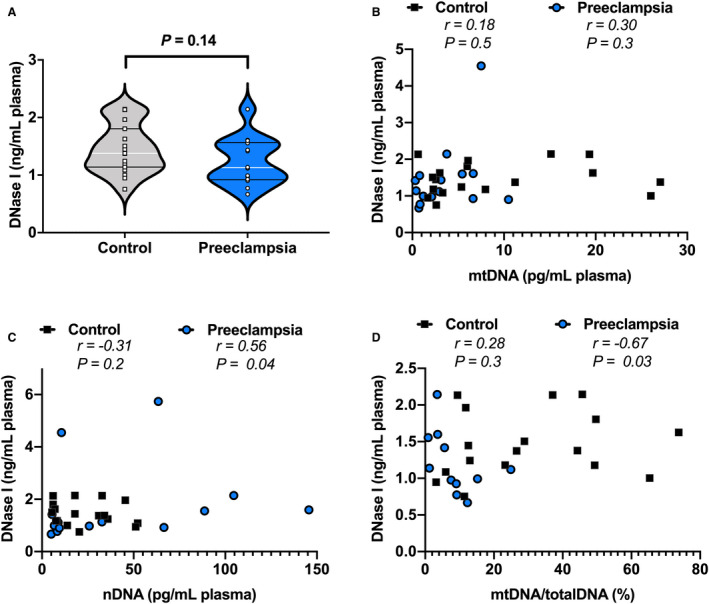
A, DNase I concentrations (ng/mL) in maternal plasma (controls, n=19; preeclampsia, n=14), and DNase I plotted as a concentration against (B) ccf‐mtDNA concentration (pg/mL) in plasma, (C) nDNA concentration (pg/mL), and (D) ccf‐mtDNA as a percent of total DNA. A, Student’s t‐test, values presented as median (white line), IQR (black lines). B through D, Spearman correlation with r and P values presented for each analysis. ccf‐mtDNA and nDNA used for Spearman correlations were derived from samples treated with lysis buffer to extract DNA. nDNA, nuclear DNA; ccf‐mtDNA, cell‐free circulating mitochondrial DNA; totalDNA, sum of ccf‐mtDNA and nDNA. Open squares=gestational age‐matched control; open circles=third trimester pregnancies with preeclampsia.
Contribution of Maternal Plasma DNA Metrics to the Clinical Diagnosis of Preeclampsia
A total of 192 data sets (all possible combinations of independent observations) were analyzed to determine the best combination of variables for modeling the relationships between clinical characteristics, circulating DNA metrics, and the diagnosis of preeclampsia. The best performing combination of characteristics and corresponding coefficients and odds ratios are listed in Table S4. After outlier removal via Mahalanobis distance, 44 the final data set used in model building included 35 patients (preeclampsia, n=17; control, n=18). Assessing model performance between ridge, least absolute shrinkage and selection operator, and elastic net penalized regression of the best‐performing data set over R=500 bootstraps showed that elastic net penalized regression produced the lowest prediction error (Table S5). 41 , 42 This model demonstrated an accuracy of ≈100% (95% CI, 0.9977–1.00; Table S6), and an area under the curve of the receiver operator characteristic value of ≈1 (95% CI, 0.9983–1.00; Table S6, Figure S1).
Application of the variable importance probability approach with 75% threshold (variable importance probability, 0.75) indicated that 9 of the subject characteristics were most important to the diagnosis of preeclampsia (Figure 6A). Characteristics with the largest effect (odds ratios) describing the diagnosis of preeclampsia were higher levels of nDNA and DNase I and having a history of preeclampsia (Figure 6B). Preeclampsia was also associated with lower concentrations of non–membrane‐bound ccf‐mtDNA, higher likelihood of having a cesarean delivery, higher body mass index at first obstetric visit, elevated mean arterial pressure, and higher maternal age. In summary, maternal circulating DNA metrics have made a significant contribution to the clinical diagnosis of preeclampsia.
Figure 6. Circulating DNA dynamics and patient characteristics most important to accurately determine preeclampsia diagnosis via elastic net penalized regression.
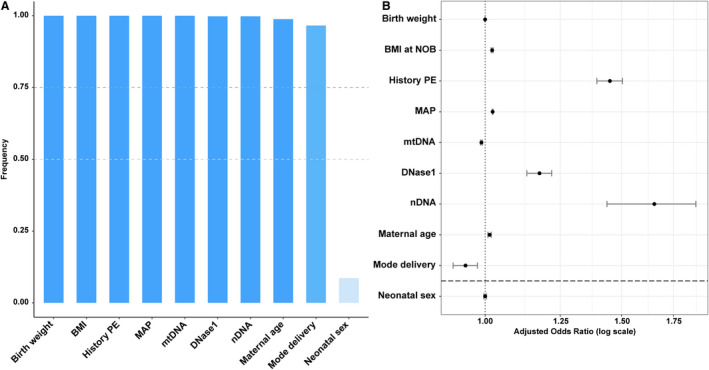
A, Subject characteristics whose variable importance probabilities (VIP) reached at least 75%. B, Adjusted odds ratios (OR) of selected subject characteristics. Vertical line represents OR=1 (no effect); horizontal line represents VIP 0.75 cutoff. BMI, body mass index; MAP, mean arterial pressure; mtDNA, mitochondrial DNA; nDNA, nuclear DNA; NOB, time of first (new) obstetric appointment.
Discussion
The main findings of this study are (1) plasma ccf‐mtDNA concentrations are reduced in women with preeclampsia compared with gestational age–matched healthy pregnant women, while there are no differences in cellular mtDNA copy number between groups; (2) the majority of ccf‐mtDNA is encapsulated in vesicles during pregnancy, and this phenomenon is more pronounced in preeclampsia; and (3) aberrance in circulating DNA dynamics, including reduced ccf‐mtDNA, is related to the clinical diagnosis of preeclampsia.
ccf‐mtDNA has been studied in various pathological conditions as a marker of disease‐associated changes in easily accessible body fluids such as blood samples. 45 , 46 The prognostic value of ccf‐mtDNA has been evaluated in patients with cancer, 47 trauma, 48 neurodegenerative diseases, 49 , 50 and multiorgan failure. 51 Furthermore, ccf‐mtDNA downstream signaling mediated by TLR‐9 activation has been implicated in cardiovascular disease, 52 diabetes, 53 and autoimmune disorders. 54 Here, we found reduced ccf‐mtDNA in plasma from women with preeclampsia compared with controls. These findings are in agreement with previous studies that also reported a reduction in ccf‐mtDNA in the first trimester of pregnant women who later developed preeclampsia. 19 We speculate that the amount of ccf‐mtDNA is correlated with the amount of placental mtDNA content. However, when preeclampsia was studied separately from intrauterine growth restriction, there was no consensus on the change in mtDNA content in placentas from these pregnancies. One study reported increased placental mtDNA content, 55 while another study showed no difference in placental mtDNA content 56 between pregnancies with preeclampsia and healthy controls. Interestingly, a reduction in mtDNA has also been observed with aging, 57 reflecting cellular metabolism and generalized catabolism of mitochondria in tissues. Although placental cell senescence increases with advancing gestational age as a function of normal placental growth in healthy pregnancies, greater levels of biomarkers of cell senescence have been reported in placentas from women with preeclampsia compared with gestational age–matched controls, suggesting accelerated aging. 58 , 59 , 60 While we speculate that the majority of ccf‐mtDNA is of placental origin in preeclampsia, maternal organ damage secondary to high blood pressure may also contribute to release and modification of mtDNA fragments into the maternal blood.
In this study, quantities of ccf‐mtDNA were determined in blood samples collected at the third trimester but before labor and delivery. In contrast, others noted an increase in ccf‐mtDNA in the serum of pregnant women (third trimester) suffering from preeclampsia when compared with controls. 16 Methodological differences in the protocol used to quantify ccf‐mtDNA may explain discrepancies between the results of our study and those from previous investigations. One of the major challenges of measuring ccf‐mtDNA is the specificity of current quantification protocols in determining absolute concentrations of ccf‐mtDNA in biological samples. 9 Most studies in pregnant patients have used relative polymerase chain reaction quantification approaches, 16 , 18 , 19 , 61 which rely on a nuclear housekeeping gene. However, we have recently reported that concentrations of nDNA increase with advancing gestational age during pregnancy, 27 making quantification of ccf‐mtDNA relative to nDNA liable to produce spurious results. In the present study, we overcome this challenge by using our previously published absolute polymerase chain reaction quantification protocol, 27 , 30 which uses a reference standard curve and is highly sensitive for detecting minute quantities of ccf‐mtDNA. 30 A second challenge is that a significant part of the mitochondrial genome is duplicated in the nuclear genome, 62 and therefore it can be coamplified by primers targeting ccf‐mtDNA. 9 Here, we address this challenge by amplifying a mitochondrial genome region (position 13,288‐12,392) that has few known mutations and is absent from the nuclear genome. 30 , 63 This approach protects against the effects of hypervariability and pseudogene contamination, respectively. To the best of our knowledge, this is the first study to use standardized conditions to isolate and quantify ccf‐mtDNA in plasma from women with preeclampsia.
While the pattern of reduced ccf‐mtDNA in preeclampsia was similar between methods of DNA extraction, DNA isolation with membrane lysis buffer resulted in 1000‐fold higher ccf‐mtDNA quantification in the preeclampsia group and 430‐fold higher ccf‐mtDNA quantification in the control group. Membrane‐bound and non–membrane‐bound cell‐free nDNA was also determined in these samples, and although the non‐membrane‐bound fraction was greater than the membrane‐bound nDNA, the magnitude of increase was much smaller than what we observed for ccf‐mtDNA (control, 27‐fold increase; preeclampsia, 15‐fold increase). Our observation that maternal blood contains ccf‐mtDNA packaged in vesicles as well as membrane‐unbound ccf‐mtDNA has been previously reported in conditions other than pregnancy or preeclampsia. 9 , 22 , 64 , 65 To the best of our knowledge, this is the first study to demonstrate that the vast majority of circulating cell‐free DNA, and particularly ccf‐mtDNA, is membrane bound in healthy pregnant women and more so in patients with preeclampsia. This observation can lead future studies to the identification of the source of ccf‐mtDNA in preeclampsia using cell‐surface markers and to the investigation of ccf‐mtDNA downstream signaling relative to its biological form and type of transport in the maternal circulation.
Extracellular mtDNA can elicit a proinflammatory response driven by activation of TLR‐9. 9 , 66 , 67 In this study, plasma from women with preeclampsia did not elicit greater TLR‐9–induced inflammation compared with plasma from healthy controls. Our findings contrast previous studies showing increased TLR‐9 activity in response to plasma from women with preeclampsia. 15 These previous studies monitored TLR‐9 activity using HEK‐Blue TLR‐9 reporter cells as we did in the present study. 15 Further, they used maternal blood from pregnant women at the third trimester, during which mtDNA in maternal plasma was increased in preeclampsia compared with healthy pregnancy. 15 However, control and preeclamptic patients were not matched for gestational age (control, 40±0.38 weeks versus preeclampsia, 37.29±0.83 weeks; P<0.01). ccf‐mtDNA increases with advancing gestational age, 27 and thus, assessing group differences between healthy pregnancies and pregnancy complications requires control of temporal variability by matching subjects for gestational age. In addition, circulating mtDNA was measured using a relative quantification protocol, and studies on TLR‐9 activity did not include control experiments (ie, HEK‐Blue Null cells, parental cell line of HEK‐Blue hTLR9).
Previous studies have suggested that plasma ccf‐mtDNA and cellular mtDNA copy number reflect different cellular processes. 68 Intracellular mtDNA copy number in PBMCs is thought to reflect variations in mitochondrial bioenergetics and biogenesis, 69 while ccf‐mtDNA is mostly associated with cellular stress and tissue damage. 9 , 21 In contrast to our findings in plasma samples, we detected no group differences in PBMC mtDNA copy number, suggesting that dysregulation occurred only in extracellular ccf‐mtDNA dynamics and not in cellular respiratory capacity of circulating cells in this cohort of women with preeclampsia.
We then examined the relationship between aberrance in circulating DNA dynamics and clinical diagnosis of preeclampsia using elastic net penalized regression. Our analysis demonstrated that dysregulation in circulating DNA dynamics, reflected by higher nDNA and DNase I concentrations, and lower non–membrane‐bound ccf‐mtDNA, were associated with the clinical diagnosis of preeclampsia. Well‐known relationships, such as a higher likelihood of experiencing preeclampsia if a subject has a history of preeclampsia, 70 , 71 and higher mean arterial blood pressure as part of the diagnostic criteria, 1 were also corroborated by the elastic net regression model.
DNase I is a significant component of cell‐free DNA degradation and DNase I activity has been shown to be increased in pregnant women with intrauterine growth restriction, 72 a common feature of pregnancies with preeclampsia. It has been speculated that ccf‐mtDNA can be degraded by circulating DNases when it is non–membrane bound but when it is encapsulated in vesicles may be protected from DNases. 73 , 74 Although this hypothesis has not been investigated in preeclampsia, it is noteworthy that in the present study concentrations of DNase I were correlated with cell‐free nDNA but not with ccf‐mtDNA, the majority of which was encapsulated in vesicular structures. Collectively, we have demonstrated the utility of penalized logistic regression in determining the association of circulating DNA‐related data with preeclampsia diagnosis.
Limitations of this study include a lack of diversity in subject race and ethnicity and small sample sizes, which may limit the generalizability of our results. Nonetheless, the demographics of the included subjects are representative of the Iowa population, where the samples were collected. Despite the small sample size, we were able to make well‐powered conclusions based on our statistical method. Furthermore, our assessment of DNA degradation mechanisms was limited to DNase I, while additional mechanisms may contribute to elimination of circulating cell‐free DNA, including filtration by the kidney or spleen, differential flux in DNA release and uptake by tissues, and degradation by other DNA hydrolases. 75 Despite these limitations, our study is the first to (1) provide a rigorous and accurate measurement of exact concentrations of ccf‐mtDNA; (2) determine ccf‐mtDNA forms of transport in healthy pregnancy and preeclampsia; and (3) identify an association between circulating cell‐free DNA dynamics, including ccf‐mtDNA and DNA clearance mechanisms, and clinical diagnosis of preeclampsia.
Conclusions
Previous studies have suggested that ccf‐mtDNA may be a potential pathogenic trigger or a contributor to the maintenance of the maternal syndrome in preeclampsia. 16 , 18 , 19 The present study demonstrates dysregulation of ccf‐mtDNA dynamics in preeclampsia. Findings from the present study set the foundation for future investigations to examine the role of biological forms of ccf‐mtDNA (ie, membrane bound versus non–membrane bound) in the pathogenesis of preeclampsia.
Sources of Funding
This research was supported in part by the American Heart Association (19TPA34850131 (Dr Goulopoulou), 18PRE33960162 (S. Cushen), 18SCG34350001 (Dr M. Santillan), and 19IPLOI34760288 (Dr M. Santillan)), the National Institutes of Health (HL0146562 (Dr Goulopoulou), T32 AG 020494 (S. Cushen), UL1TR002537 (Drs D. Santillan and M. Santillan), HD089940 (Dr M. Santillan), HD000849 (Dr M. Santillan), RR024980 (Dr M. Santillan), and 3UL1TR002537 (Dr M. Santillan)), and March of Dimes (#4‐FY18‐851 (Dr M. Santillan)).
Disclosures
Drs D. Santillan and M. Santillan hold patents related to the prediction and treatment of preeclampsia: US 293 #9 937 182 (April 10, 2018), EU #2 954 324, and PCT/US2018/027152. The remaining authors have no disclosures to report.
Supporting information
Acknowledgments
Computational and Analytics resources were provided by North Texas Scientific Computing, a division under the office of the Chief Information Officer for UNT and UNT System. We would like to acknowledge Drs Ravi Vadapalli and Richard Herrington (North Texas Scientific Computing) for reviewing R scripts and consulting on R code implementation.
Preprint posted on MedRxiv February 5, 2021. doi: https://doi.org/10.1101/2021.02.02.21250841
This manuscript was sent to Hossein Ardehali, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021726
For Sources of Funding and Disclosures, see page 10.
References
- 1. American College of O, Gynecologists and Task Force on Hypertension in P . Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' task force on hypertension in pregnancy. 2013;122:1122–1131. [DOI] [PubMed] [Google Scholar]
- 2. American College of Obstetrics and Gynecologists' Committee on Practice B‐O . Gestational Hypertension And Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135:E237–E260. [DOI] [PubMed] [Google Scholar]
- 3. Bischoff FZ, Lewis DE, Simpson JL. Cell‐free fetal DNA in maternal blood: kinetics, source and structure. Hum Reprod Update. 2005;11:59–67. doi: 10.1093/humupd/dmh053 [DOI] [PubMed] [Google Scholar]
- 4. Phillippe M. Cell‐free fetal DNA, telomeres, and the spontaneous onset of parturition. Reprod Sci. 2015;22:1186–1201. doi: 10.1177/1933719115592714 [DOI] [PubMed] [Google Scholar]
- 5. Sharp AN, Heazell AEP, Crocker IP, Mor G. Placental apoptosis in health and disease. Am J Reprod Immunol. 2010;64:159–169. doi: 10.1111/j.1600-0897.2010.00837.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falco Ml, Sivanathan J, Laoreti A, Thilaganathan B, Khalil A. Placental histopathology associated with pre‐eclampsia: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2017;50:295–301. doi: 10.1002/uog.17494 [DOI] [PubMed] [Google Scholar]
- 7. Travaglino A, Raffone A, Saccone G, Migliorini S, Maruotti GM, Esposito G, Mollo A, Martinelli P, Zullo F, D'Armiento M. Placental morphology, apoptosis, angiogenesis and epithelial mechanisms in early‐onset preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2019;234:200–206. doi: 10.1016/j.ejogrb.2018.12.039 [DOI] [PubMed] [Google Scholar]
- 8. Aucamp J, Bronkhorst AJ, Badenhorst CPS, Pretorius PJ. The diverse origins of circulating cell‐free DNA in the human body: a critical re‐evaluation of the literature. Biol Rev. 2018;93:1649–1683. doi: 10.1111/brv.12413 [DOI] [PubMed] [Google Scholar]
- 9. West AP, Shadel GS. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol. 2017;17:363–375. doi: 10.1038/nri.2017.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rana S, Salahuddin S, Mueller A, Berg AH, Thadhani RI, Karumanchi SA. Angiogenic biomarkers in triage and risk for preeclampsia with severe features. Pregnancy Hypertens. 2018;13:100–106. doi: 10.1016/j.preghy.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 11. Gil MM, Galeva S, Jani J, Konstantinidou L, Akolekar R, Plana MN, Nicolaides KH. Screening for trisomies by cfDNA testing of maternal blood in twin pregnancy: update of The Fetal Medicine Foundation results and meta‐analysis. Ultrasound Obstet Gynecol. 2019;53:734–742. doi: 10.1002/uog.20284 [DOI] [PubMed] [Google Scholar]
- 12. Kwak DW, Kim SY, Kim HJ, Lim JH, Kim Y‐H, Ryu HM. Maternal total cell‐free DNA in preeclampsia with and without intrauterine growth restriction. Sci Rep. 2020;10:11848. doi: 10.1038/s41598-020-68842-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goulopoulou S, Wenceslau CF, McCarthy CG, Matsumoto T, Webb RC. Exposure to stimulatory CpG oligonucleotides during gestation induces maternal hypertension and excess vasoconstriction in pregnant rats. Am J Physiol Heart Circ Physiol. 2016;310:H1015–H1025. doi: 10.1152/ajpheart.00834.2015 [DOI] [PubMed] [Google Scholar]
- 14. He B, Yang X, Li Y, Huang D, Xu X, Yang W, Dai Y, Zhang H, Chen Z, Cheng W. Tlr9 (Toll‐Like Receptor 9) agonist suppresses angiogenesis by differentially regulating Vegfa (Vascular Endothelial Growth Factor A) and Sflt1 (Soluble Vascular Endothelial Growth Factor Receptor 1) in preeclampsia. Hypertension. 2018;71:671–680. doi: 10.1161/HYPERTENSIONAHA.117.10510 [DOI] [PubMed] [Google Scholar]
- 15. Williamson RD, McCarthy FP, Kenny LC, McCarthy CM. Activation Of A Tlr9 Mediated innate immune response in preeclampsia. Sci Rep. 2019;9:5920. doi: 10.1038/s41598-019-42551-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marschalek J, Wohlrab P, Ott J, Wojta J, Speidl W, Klein KU, Kiss H, Pateisky P, Zeisler H, Kuessel L. Maternal serum mitochondrial DNA (mtDNA) levels are elevated in preeclampsia ‐ a matched case‐control study. Pregnancy Hypertens. 2018;14:195–199. doi: 10.1016/j.preghy.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 17. McCarthy C, Kenny LC. Therapeutically targeting mitochondrial redox signalling alleviates endothelial dysfunction in preeclampsia. Sci Rep. 2016;6:32683. doi: 10.1038/srep32683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qiu C, Hevner K, Enquobahrie DA, Williams MA. A case‐control study of maternal blood mitochondrial DNA copy number and preeclampsia risk. Int J Mol Epidemiol Genet. 2012;3:237–244. [PMC free article] [PubMed] [Google Scholar]
- 19. Busnelli A, Lattuada D, Ferrari S, Reschini M, Colciaghi B, Somigliana E, Fedele L, Ferrazzi E. Mitochondrial DNA copy number in peripheral blood in the first trimester of pregnancy and different preeclampsia clinical phenotypes development: a pilot study. Reprod Sci. 2019;26:1054–1061. doi: 10.1177/1933719118804410 [DOI] [PubMed] [Google Scholar]
- 20. Xia P, Radpour R, Zachariah R, Fan AXC, Kohler C, Hahn S, Holzgreve W, Zhong XY. Simultaneous quantitative assessment of circulating cell‐free mitochondrial and nuclear DNA by multiplex real‐time PCR. Genet Mol Biol. 2009;32:20–24. doi: 10.1590/S1415-47572009000100003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosa HS, Ajaz S, Gnudi L, Malik AN. A case for measuring both cellular and cell‐free mitochondrial DNA as a disease biomarker in human blood. FASEB J. 2020;34:12278–12288. doi: 10.1096/fj.202000959RR [DOI] [PubMed] [Google Scholar]
- 22. Trumpff C, Michelson J, Lagranha CJ, Taleon V, Karan KR, Sturm G, Lindqvist D, Fernström J, Moser D, Kaufman BA, et al. Stress and circulating cell‐free mitochondrial DNA: a systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion. 2021;59:225–245. doi: 10.1016/j.mito.2021.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miliotis S, Nicolalde B, Ortega M, Yepez J, Caicedo A. Forms of extracellular mitochondria and their impact in health. Mitochondrion. 2019;48:16–30. doi: 10.1016/j.mito.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 24. Makalic E, Schmidt FD. Review of modern logistic regression methods with application to small and medium sample size problems. In: Li J, ed. Ai 2010: advances in artificial intelligence Ai 2010 lecture notes in computer science. Berlin, Heidelberg: Springer; 2010. [Google Scholar]
- 25. Liao JG, Chin K‐V. Logistic regression for disease classification using microarray data: model selection in a large p and small n case. Bioinformatics. 2007;23:1945–1951. doi: 10.1093/bioinformatics/btm287 [DOI] [PubMed] [Google Scholar]
- 26. Santillan MK, Leslie KK, Hamilton WS, Boese BJ, Ahuja M, Hunter SK, Santillan DA. Collection of a lifetime: a practical approach to developing a longitudinal collection of women’s healthcare biological samples. Eur J Obst Gynecol Reprod Biol. 2014;179:94–99. doi: 10.1016/j.ejogrb.2014.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cushen SC, Sprouse ML, Blessing A, Sun J, Jarvis SS, Okada Y, Fu QI, Romero SA, Phillips NR, Goulopoulou S. Cell‐free mitochondrial DNA increases in maternal circulation during healthy pregnancy: a prospective, longitudinal study. Am J Physiol Regul Integr Comp Physiol. 2020;318:R445–R452. doi: 10.1152/ajpregu.00324.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keserű JS, Soltész B, Lukács J, Márton É, Szilágyi‐Bónizs M, Penyige A, Póka R, Nagy B. Detection of cell‐free, exosomal and whole blood mitochondrial DNA copy number in plasma or whole blood of patients with serous epithelial ovarian cancer. J Biotechnol. 2019;298:76–81. doi: 10.1016/j.jbiotec.2019.04.015 [DOI] [PubMed] [Google Scholar]
- 29. Ye W, Tang X, Liu C, Wen C, Li W, Lyu J. Accurate quantitation of circulating cell‐free mitochondrial DNA in plasma by droplet digital PCR. Anal Bioanal Chem. 2017;409:2727–2735. doi: 10.1007/s00216-017-0217-x [DOI] [PubMed] [Google Scholar]
- 30. Kavlick MF, Lawrence HS, Merritt RT, Fisher C, Isenberg A, Robertson JM, Budowle B. Quantification of human mitochondrial DNA using synthesized DNA standards. J Forensic Sci. 2011;56:1457–1463. doi: 10.1111/j.1556-4029.2011.01871.x [DOI] [PubMed] [Google Scholar]
- 31. Anderson S, Bankier AT, Barrell BG, De Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0 [DOI] [PubMed] [Google Scholar]
- 32. R Core Team . R: A Language and Environment for Statistical Computing. [Computer Program]. Vienna, Austria: R Foundation for Statistical Computing; 2020. Available at: https://www.R‐project.org/ [Google Scholar]
- 33. Fabricatr: imagine your data before you collect it. R Package Version 0.10.0 [Computer Program]. 2019.
- 34. Polanin JR, Snilstveit B. Converting between effect sizes. Campbell Syst Rev. 2016;12:1–13. [Google Scholar]
- 35. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J‐C, Müller M. pROC: an open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011;12:1–8. doi: 10.1186/1471-2105-12-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pcamixdata: multivariate analysis of mixed data. R Package Version 3.1 [Computer Program]. 2017.
- 37. Classdiscovery: classes and methods for "Class Discovery" with microarrays or proteomics. R Package Version 3.3.12. [Computer Program]. 2019.
- 38. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. doi: 10.18637/jss.v033.i01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Caret: Classification And Regression Training . R Package Version 6.0‐86. [Computer Program]. 2020.
- 40. Boot: Bootstrap R (S‐Plus) Functions. R Package Version 1.3‐25. [Computer Program]. 2020.
- 41. Price DT, McKenney DW, Nalder IA, Hutchinson MF, Kesteven JL. A comparison of two statistical methods for spatial interpolation of Canadian monthly mean climate data. Agric Forest Meteorol. 2000;101:81–94. doi: 10.1016/S0168-1923(99)00169-0 [DOI] [Google Scholar]
- 42. Willmott CJ. Some comments on the evaluation of model performance. Bull Am Meteorol Soc. 1982;63:1309–1313. doi: [DOI] [Google Scholar]
- 43. Mills PB, Holtz KA, Szefer E, Noonan VK, Kwon BK. Early predictors of developing problematic spasticity following traumatic spinal cord injury: a prospective cohort study. J Spinal Cord Med. 2020;43:315–330. doi: 10.1080/10790268.2018.1527082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leys C, Klein O, Dominicy Y, Ley C. Detecting multivariate outliers: use a robust variant of the Mahalanobis distance. J Exp Soc Psychol. 2018;74:150–156. doi: 10.1016/j.jesp.2017.09.011 [DOI] [Google Scholar]
- 45. Cao H, Wu J, Luo J, Chen X, Yang J, Fang L. Urinary mitochondrial DNA: a potential early biomarker of diabetic nephropathy. Diabetes Metab Res Rev. 2019;35:e3131. doi: 10.1002/dmrr.3131 [DOI] [PubMed] [Google Scholar]
- 46. Afrifa J, Zhao T, Yu J. Circulating mitochondria DNA, a non‐invasive cancer diagnostic biomarker candidate. Mitochondrion. 2019;47:238–243. doi: 10.1016/j.mito.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 47. Cicchillitti L, Corrado G, De Angeli M, Mancini E, Baiocco E, Patrizi L, Zampa A, Merola R, Martayan A, Conti L, et al. Circulating cell‐free DNA content as blood based biomarker in endometrial cancer. Oncotarget. 2017;8:115230–115243. doi: 10.18632/oncotarget.23247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Itagaki K, Kaczmarek E, Lee YT, Tang IT, Isal B, Adibnia Y, Sandler N, Grimm MJ, Segal BH, Otterbein LE, et al. Mitochondrial DNA released by trauma induces neutrophil extracellular traps. PLoS One. 2015;10:e0120549. doi: 10.1371/journal.pone.0120549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pyle A, Brennan R, Kurzawa‐Akanbi M, Yarnall A, Thouin A, Mollenhauer B, Burn D, Chinnery PF, Hudson G. Reduced cerebrospinal fluid mitochondrial DNA is a biomarker for early‐stage Parkinson’s disease. Ann Neurol. 2015;78:1000–1004. doi: 10.1002/ana.24515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Silzer T, Barber R, Sun J, Pathak G, Johnson L, O’Bryant S, Phillips N. Circulating mitochondrial DNA: new indices of type 2 diabetes‐related cognitive impairment in Mexican Americans. PLoS One. 2019;14:e0213527. doi: 10.1371/journal.pone.0213527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakahira K, Kyung S‐Y, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y, et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013;10:e1001577. doi: 10.1371/journal.pmed.1001577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McCarthy CG, Wenceslau CF, Goulopoulou S, Ogbi S, Baban B, Sullivan JC, Matsumoto T, Webb RC. Circulating mitochondrial DNA and toll‐like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats. Cardiovasc Res. 2015;107:119–130. doi: 10.1093/cvr/cvv137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Malik AN, Parsade CK, Ajaz S, Crosby‐Nwaobi R, Gnudi L, Czajka A, Sivaprasad S Altered circulating mitochondrial DNA and increased inflammation in patients with diabetic retinopathy. Diabetes Res Clin Pract. 2015;110:257–265. doi: 10.1016/j.diabres.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 54. Rykova E, Sizikov A, Roggenbuck D, Antonenko O, Bryzgalov L, Morozkin E, Skvortsova K, Vlassov V, Laktionov P, Kozlov V. Circulating DNA in rheumatoid arthritis: pathological changes and association with clinically used serological markers. Arthritis Res Ther. 2017;19:85. doi: 10.1186/s13075-017-1295-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pandey D, Yevale A, Naha R, Kuthethur R, Chakrabarty S, Satyamoorthy K. Mitochondrial DNA copy number variation ‐ a potential biomarker for early onset preeclampsia. Pregnancy Hypertens. 2021;23:1–4. doi: 10.1016/j.preghy.2020.10.002 [DOI] [PubMed] [Google Scholar]
- 56. Mandò C, De Palma C, Stampalija T, Anelli GM, Figus M, Novielli C, Parisi F, Clementi E, Ferrazzi E, Cetin I. Placental mitochondrial content and function in intrauterine growth restriction and preeclampsia. Am J Physiol Endocrinol Metab. 2014;306:E404–E413. doi: 10.1152/ajpendo.00426.2013 [DOI] [PubMed] [Google Scholar]
- 57. Mengel‐From J, Thinggaard M, Dalgård C, Kyvik KO, Christensen K, Christiansen L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum Genet. 2014;133:1149–1159. doi: 10.1007/s00439-014-1458-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Scaife PJ, Simpson A, Kurlak LO, Briggs LV, Gardner DS, Broughton Pipkin F, Jones CJP, Mistry HD. Increased placental cell senescence and oxidative stress in women with pre‐eclampsia and normotensive post‐term pregnancies. Int J Mol Sci. 2021;22:7295. doi: 10.3390/ijms22147295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sultana Z, Maiti K, Aitken J, Morris J, Dedman L, Smith R. Oxidative stress, placental ageing‐related pathologies and adverse pregnancy outcomes. Am J Reprod Immunol. 2017;77:1–10. [DOI] [PubMed] [Google Scholar]
- 60. Sultana Z, Maiti K, Dedman L, Smith R. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am J Obstet Gynecol. 2018;218:S762–S773. doi: 10.1016/j.ajog.2017.11.567 [DOI] [PubMed] [Google Scholar]
- 61. Colleoni F, Lattuada D, Garretto A, Massari M, Mandò C, Somigliana E, Cetin I. Maternal blood mitochondrial DNA content during normal and intrauterine growth restricted (IUGR) pregnancy. Am J Obstet Gynecol. 2010;203:E1–E6. doi: 10.1016/j.ajog.2010.05.027 [DOI] [PubMed] [Google Scholar]
- 62. Wang X, Weidling I, Koppel S, Menta B, Perez Ortiz J, Kalani A, Wilkins HM, Swerdlow RH. Detection of mitochondria‐pertinent components in exosomes. Mitochondrion. 2020;55:100–110. doi: 10.1016/j.mito.2020.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kavlick MF. Development of a triplex mtDNA qPCR assay to assess quantification, degradation, inhibition, and amplification target copy numbers. Mitochondrion. 2019;46:41–50. doi: 10.1016/j.mito.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 64. Al Amir Dache Z, Otandault A, Tanos R, Pastor B, Meddeb R, Sanchez C, Arena G, Lasorsa L, Bennett A, Grange T, et al. Blood contains circulating cell‐free respiratory competent mitochondria. FASEB J. 2020;34:3616–3630. doi: 10.1096/fj.201901917RR [DOI] [PubMed] [Google Scholar]
- 65. Cai J, Han YU, Ren H, Chen C, He D, Zhou L, Eisner GM, Asico LD, Jose PA, Zeng C. Extracellular vesicle‐mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J Mol Cell Biol. 2013;5:227–238. doi: 10.1093/jmcb/mjt011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hajizadeh S, Degroot J, Tekoppele JM, Tarkowski A, Collins LV. Extracellular mitochondrial DNA and oxidatively damaged DNA in synovial fluid of patients with rheumatoid arthritis. Arthritis Res Ther. 2003;5:R234–R240. doi: 10.1186/ar787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial damps cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lindqvist D, Wolkowitz OM, Picard M, Ohlsson L, Bersani FS, Fernström J, Westrin Å, Hough CM, Lin J, Reus VI, et al. Circulating cell‐free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacology. 2018;43:1557–1564. doi: 10.1038/s41386-017-0001-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Guitart‐Mampel M, Juarez‐Flores DL, Youssef L, Moren C, Garcia‐Otero L, Roca‐Agujetas V, Catalan‐Garcia M, Gonzalez‐Casacuberta I, Tobias E, Milisenda JC, et al. Mitochondrial implications in human pregnancies with intrauterine growth restriction and associated cardiac remodelling. J Cell Mol Med. 2019;23:3962–3973. doi: 10.1111/jcmm.14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bartsch E, Medcalf KE, Park AL, Ray JG; High Risk of Pre‐Eclampsia Identification Group . Clinical risk factors for pre‐eclampsia determined in early pregnancy: systematic review and meta‐analysis of large cohort studies. BMJ. 2016;353:I1753. doi: 10.1136/bmj.i1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shen M, Smith GN, Rodger M, White RR, Walker MC, Wen SW. Comparison of risk factors and outcomes of gestational hypertension and pre‐eclampsia. PLoS One. 2017;12:e0175914. doi: 10.1371/journal.pone.0175914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ershova E, Sergeeva V, Klimenko M, Avetisova K, Klimenko P, Kostyuk E, Veiko N, Veiko R, Izevskaya V, Kutsev S, et al. Circulating cell‐free DNA concentration and DNase I activity of peripheral blood plasma change in case of pregnancy with intrauterine growth restriction compared to normal pregnancy. Biomed Rep. 2017;7:319–324. doi: 10.3892/br.2017.968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lood C, Blanco LP, Purmalek MM, Carmona‐Rivera C, De Ravin SS, Smith CK, Hl M, Ledbetter JA, Elkon KB, Kaplan MJ . Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus‐like disease. Nat Med. 2016;22:146‐153. doi: 10.1038/nm.4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sisirak V, Sally B, D’Agati V, Martinez‐Ortiz W, Özçakar Z, David J, Rashidfarrokhi A, Yeste A, Panea C, Chida A, et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell. 2016;166:88–101. doi: 10.1016/j.cell.2016.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kustanovich A, Schwartz R, Peretz T, Grinshpun A. Life and death of circulating cell‐free DNA. Cancer Biol Ther. 2019;20:1057–1067. doi: 10.1080/15384047.2019.1598759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc: Series B. 1996;58:267–288. doi: 10.1111/j.2517-6161.1996.tb02080.x [DOI] [Google Scholar]
- 77. Hoerl AE, Kennard RW. Ridge regression: biased estimation for nonorthogonal problems. Technometrics. 2000;42:80–86. doi: 10.1080/00401706.2000.10485983 [DOI] [Google Scholar]
- 78. Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Series B. 2005;67:301–320. doi: 10.1111/j.1467-9868.2005.00503.x [DOI] [Google Scholar]
- 79. Fabricatr: Imagine Your Data Before You Collect It. [Computer Program]. Version R Package V. 0.14.0; 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


