Abstract
Background
The use of large‐bore (LB) arterial access and guiding catheters has been advocated for complex percutaneous coronary intervention. However, the impact of LB transradial access (TRA) and transfemoral access (TFA) on extremity dysfunction is currently unknown.
Methods and Results
The predefined substudy of the COLOR (Complex Large‐Bore Radial PCI) trial aimed to assess upper and lower‐extremity dysfunction after LB radial and femoral access. Upper‐extremity function was assessed in LB TRA‐treated patients by the Quick Disabilities of the Arm, Shoulder, and Hand questionnaire and lower‐extremity function in LB TFA‐treated patients by the Lower Extremity Functional Scale questionnaire. Extremity pain and effect of access site complications and risk factors on extremity dysfunction was also analyzed. There were 343 patients who completed analyzable questionnaires. Overall, upper and lower‐extremity function did not decrease over time when LB TRA and TFA were used for complex percutaneous coronary intervention, as represented by the median Quick Disabilities of the Arm, Shoulder, and Hand score (6.8 at baseline and 2.1 at follow‐up, higher is worse) and Lower Extremity Functional Scale score (56 at baseline and 58 at follow‐up, lower is worse). Clinically relevant extremity dysfunction occurred in 6% after TRA and 9% after TFA. A trend for more pronounced upper‐limb dysfunction was present in female patients after LB TRA (P=0.05). Lower‐extremity pain at discharge was significantly higher in patients with femoral access site complications (P=0.02).
Conclusions
Following LB TRA and TFA, self‐reported upper and lower‐limb function did not decrease over time in the majority of patients. Clinically relevant limb dysfunction occurs in a small minority of patients regardless of radial or femoral access.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03846752.
Keywords: complex PCI, chronic total occlusion, large‐bore arterial access, extremity dysfunction
Subject Categories: Percutaneous Coronary Intervention, Catheter-Based Coronary and Valvular Interventions
Nonstandard Abbreviations and Acronyms
- COLOR
Complex Large‐Bore Radial PCI
- LEFS
Lower Extremity Functional Scale
- MCID
minimal clinically important difference
- QuickDASH
Quick Disabilities of the Arm, Shoulder, and Hand
- RAS
radial artery spasm
- TFA
transfemoral access
- TRA
transradial access
Clinical Perspective
What Is New?
Following large‐bore arterial access for complex percutaneous coronary intervention, self‐reported upper and lower‐extremity function does not decrease over time in the majority of patients.
What Are the Clinical Implications?
Informing patients about the small possibility for extremity dysfunction should be considered when obtaining informed consent before complex percutaneous coronary intervention, irrespective of which access site will be used.
The radial artery has become the standard access site for percutaneous coronary intervention (PCI). For PCI of complex coronary lesions, the femoral artery is still used in a considerable proportion of patients because of the need for large‐bore access (≥7F), and is associated with higher success rates for chronic total occlusions. 1 Recent efforts to miniaturize transradial equipment have led to increased adoption of transradial access (TRA) for complex PCI. As shown recently by the COLOR (Complex Large‐Bore Radial PCI) trial, large‐bore TRA with the Glidesheath Slender 7F (Terumo, Tokyo, Japan) sheath leads to similar procedural success rates for PCI of complex coronary lesions, while significantly reducing clinically relevant access site–related complications compared with transfemoral access (TFA). 2 Nevertheless, TRA might lead to disabling upper‐extremity dysfunction, especially in patients with large‐bore access because of sheath‐to‐artery mismatch and subsequent vascular injury. 3 , 4 , 5 , 6 However, upper‐extremity dysfunction after large‐bore TRA has never been studied up to now. Similarly, little is known about the incidence of lower‐extremity dysfunction after TFA, especially for large‐bore TFA. 7 , 8 , 9 The aim of our study was to assess upper and lower‐extremity dysfunction after large‐bore access for complex PCI.
Methods
Study Design
Study data are available on request from the authors (see the Data Sharing Plan provided in Data S1 for details). This study was a predefined substudy of the COLOR trial. The COLOR trial was an investigator‐initiated international multicenter study with a prospective, open‐label, randomized controlled superiority design. Full study rationale and protocol have been published previously. 10 The primary end point of the COLOR trial was the occurrence of clinically significant bleeding or vascular complications at discharge related to the randomized access site. The main results of the COLOR trial have been published previously. 2
Patient Population
All patients aged 18 years or older presenting with chronic coronary syndrome, unstable angina, or non–ST‐segment–elevation myocardial infarction and planned for PCI of complex coronary lesions including chronic total occlusion, left main stem, heavily calcified lesions, and complex bifurcations in whom the operator anticipated a 7F guiding catheter was indicated, were screened for inclusion in the COLOR trial. Patients with ST‐segment–elevation myocardial infarction or cardiogenic shock were excluded. Patients with contraindications for femoral or radial access, such as occlusive peripheral artery disease, known severe radial artery spasm (RAS), or known anatomical variants prohibiting radial or femoral access on both sides were also excluded. Enrolling centers were the Isala Heart Center (Zwolle, the Netherlands; coordinating center), Radboud University Medical Center (Nijmegen, the Netherlands), Catharina Hospital (Eindhoven, the Netherlands), Elisabeth‐Krankenhaus (Essen, Germany), NorthWest Clinics (Alkmaar, the Netherlands), Frimley National Health Service Foundation Trust (Surrey, United Kingdom), Onze Lieve Vrouwe Gasthuis Hospital (Amsterdam, the Netherlands), Centre Hospitalier Universitaire de Charleroi (Charleroi, Belgium), VU University Medical Center (Amsterdam, the Netherlands), Hospital Oost‐Limburg (Genk, Belgium), Geneva University Hospital (Geneva, Switzerland), and ZNA Middelheim (Antwerpen, Belgium).
Trial Organization
The trial was approved by the appropriate ethics review board at each site. Written informed consent was obtained from all patients before enrollment. The trial was designed in accordance with the Declaration of Helsinki. All data were collected in an electronic data‐capturing system, the eDREAM (electronic case record form Diagnostic Research and Management). Diagram BV (Zwolle, the Netherlands), was responsible for overall trial and data management, as well as monitoring of the study. Evaluation of serious adverse events was performed by an independent data safety monitoring board. A clinical events committee reviewed and adjudicated all end point–related adverse events. The COLOR trial has been registered in the ClinicalTrials.gov database (URL: https://www.clinicaltrials.gov; Unique identifier: NCT03846752).
Objectives
The primary objective of this study was to evaluate the impact of large‐bore TRA and TFA on upper and lower‐extremity dysfunction.
Secondary objectives were:
The association between extremity dysfunction and access site–related bleeding or vascular complications
To identify risk factors for upper and lower‐extremity dysfunction after large‐bore radial and femoral access.
Questionnaires
Extremity dysfunction was assessed using 2 validated questionnaires. All patients were asked to answer both questionnaires right before the PCI procedure and at the 30‐day follow‐up. For upper‐extremity dysfunction, the Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) questionnaire was used (Data S2). The QuickDASH questionnaire contains 11 items and is an abbreviated version of the 30‐item Disabilities of the Arm, Shoulder, and Hand outcome measure, with similar reliability and responsiveness. 11 Ten of the 11 items need to be completed for the scores to be valid. Each item is graded on a 5‐point Likert scale. Each item has 5 response options from 1 (no difficulty to perform, no symptom, or no impact) to 5 (unable to do, severe symptom, or high impact). The assigned values for all completed responses are averaged and then transformed to a score of 0 to 100 by subtracting 1 from the average score and multiplying this by 25. A higher QuickDASH score indicates worse upper‐limb function or symptoms. The minimal clinically important difference (MCID) score for the QuickDASH that corresponds to a change in clinical status appreciated by the patient varies between 8 and 14 points. 12 , 13 Lower‐extremity dysfunction was measured with the Lower Extremity Functional Scale (LEFS) (Data S3), a reliable and responsive 20‐item questionnaire used for assessing lower‐extremity functional impairment in a wide array of patient groups with lower‐extremity conditions. 14 , 15 Seventeen out of the 20 items need to be completed for the scores to be valid. Each item has 5 response options from 0 (extreme difficulty or unable to perform activity) to 4 (no difficulty). The maximum possible score is 80 points, indicating high function, and the minimum possible score is 0 points, indicating low function. The MCID score for the LEFS that corresponds to a change in clinical status varies between 9 and 12 points. 14 , 16
Besides extremity dysfunction, pain related to the access site was measured using the visual analog scale (VAS). The VAS pain scale was a 10‐point pain scale, where 0=no pain, 1=slight pain, through 10, which equates to the patient’s worst imaginable pain. VAS scores were collected directly after the procedure, at discharge, and at the 30‐day follow‐up.
End Points
Upper‐extremity function was assessed as follows:
QuickDASH score at baseline and follow‐up for all patients treated with large‐ bore radial access
Proportion of patients exceeding 1 or both QuickDASH MCID thresholds
Subgroup analyses based on access site distribution
Access site pain (VAS) after procedure, at discharge, and at follow‐up
Influence of TRA‐related bleeding and vascular complications on upper‐extremity dysfunction
Risk factors for extremity dysfunction and pain on upper‐extremity dysfunction
Lower‐extremity function was assessed as follows:
LEFS score at baseline and follow‐up for all patients treated with large‐bore femoral access
Proportion of patients exceeding 1 or both LEFS MCID thresholds
Subgroup analyses based on access site distribution
Access site pain (VAS) after procedure, at discharge, and at follow‐up
Influence of TFA‐related bleeding and vascular complications on lower‐extremity dysfunction
Risk factors for extremity dysfunction and pain on lower‐extremity dysfunction
Statistical Analysis
Primary analysis assessed upper‐extremity function decrease in all patients treated with large‐bore TRA and lower‐extremity function decrease in all patients treated with large‐bore TFA (per protocol analysis). Secondary analyses consisted of upper and lower‐extremity function decrease in predefined subgroups (single radial, biradial, single femoral, and bifemoral access). Influence of bleeding and vascular complications end points and prespecified known or potential modifiers for extremity dysfunction (female sex, hypertension, diabetes, RAS, and ultrasound‐guided puncture) were analyzed as well and tested in uni‐ and multivariate analyses for prediction of clinically relevant extremity dysfuntion. 17 , 18 , 19 Depending on the distribution of the data, between‐group tests were performed by using t tests or Mann‐Whitney U tests. Within‐subject tests were performed by using the paired t test or Wilcoxon signed rank test. A proportion of subjects were tested by using the Pearson χ2 test or Fisher exact test. Normally distributed data are expressed as mean±standard deviation and nonnormally distributed data as median with interquartile range.
Results
Patient and Access Site Characteristics
The mean age of the total population was 69 years, and 81% were men. The primary indication for complex PCI was chronic coronary syndrome (85%). Fifty‐nine percent of patients were treated with single large‐bore transradial or transfemoral access, and 41% were treated with an additional access site (ie, in case of chronic total occlusion PCI). Twelve percent of patients with dual arterial access had biradial access, 22% bifemoral access, and 66% had a combination of radial and femoral access. Forty‐four percent of secondary access sheath size was 7F. Baseline characteristics of the entire cohort as well as for both single‐access and dual‐access groups stratified by randomized access strategy were highly comparable (Table S1 through S3). Figure 1 provides a complete graphic overview of access site distribution and analyzable questionnaires.
Figure 1. Flowchart representing access site distribution and questionnaire response.
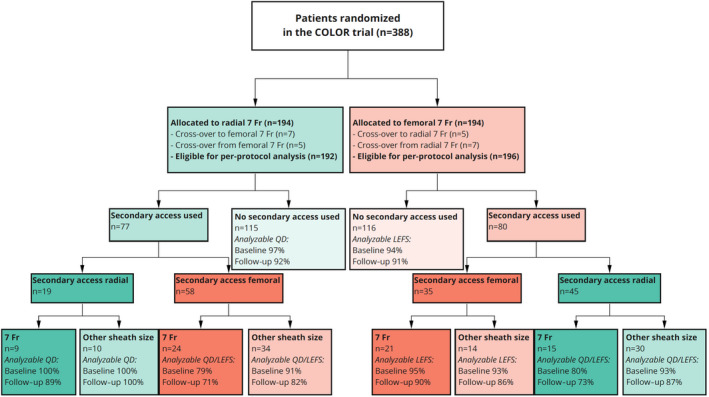
The percentages shown reflect the proportion of patients with analyzable questionnaires. Fr indicates French; LEFS, Lower Extremity Functional Scale; and QD, QuickDASH, Quick Disabilities of Arm, Shoulder, and Hand. COLOR indicates Complex Large‐Bore Radial PCI trial.
Questionnaires
Three hundred eighty‐two patients (98%) had completed 1 or more analyzable questionnaires. Three hundred forty‐three patients (88%) had completed analyzable questionnaires appropriate for their corresponding access site(s) at baseline as well as follow‐up (181 and 169 patients for large‐bore TRA, respectively, and 182 and 174 patients for large‐bore TFA, respectively [Figure 1]). Baseline characteristics were highly comparable between the complete COLOR trial cohort and the cohort analyzed in the current substudy (Table S1).
Upper Extremity Function
Median QuickDASH score at baseline was 6.8 (interquartile range, 0–27). At the 30‐day follow‐up, the median QuickDASH score was 2.5 (interquartile range, 0–16), indicating no decreased limb function at follow‐up compared with baseline for the complete cohort (Figure 2). A total of 9% of patients experienced a decrease of upper‐limb function expressed by an MCID ≥8. When an MCID of ≥14 was applied, 6% of patients experienced clinically relevant upper‐limb dysfunction. All QuickDASH outcomes for patients treated with primary large‐bore TRA are displayed in Table 1. Patients with single 7F radial access had a lower QuickDASH score at follow‐up compared with baseline as well (4.5 compared with 6.8). The same applies for biradial‐treated patients (1.1 versus 4.5). For patients treated with secondary radial access in case of primary femoral access (n=45), 9.3% experienced clinically relevant decrease of upper‐extremity function. Details about radial‐treated subgroups are provided in Table 2.
Figure 2. Quick Disabilities of Arm, Shoulder, and Hand (QuickDASH) score for primary transradial‐treated patients and Lower Extremity Functional Scale (LEFS) score for primary transfemoral‐treated patients at baseline and at the 30‐day follow‐up.
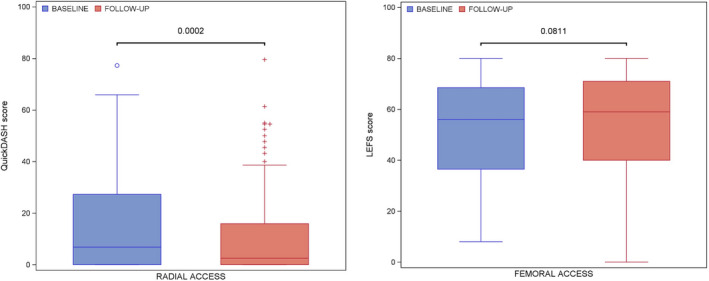
Table 1.
QuickDASH and LEFS Scores at Baseline and Follow‐Up (Per Protocol Analysis)
| Baseline QuickDASH score, median (IQR) | 6.8 (0–27) |
| Follow‐up QuickDASH score, median (IQR) | 2.5 (0–16) |
| P value for QuickDASH difference | 0.0002 |
| Patients with worsening QuickDASH score | 37 (22%) |
| MCID ≥8, n (%) | 16 (9%) |
| MCID ≥14, n (%) | 10 (6%) |
| Baseline LEFS score, median (IQR) | 56 (37–69) |
| Follow‐up LEFS score, median (IQR) | 59 (40–71) |
| P value for LEFS difference | 0.08 |
| Patients with worsening LEFS score | 47 (27%) |
| MCID ≥9, n (%) | 18 (11%) |
| MCID ≥12, n (%) | 15 (9%) |
IQR indicates interquartile range; LEFS, Lower Extremity Functional Scale; MCID, minimal clinically important difference; and QuickDASH, Quick Disabilities of Arm, Shoulder, and Hand.
Table 2.
QuickDASH Scores for Single Radial and Biradial Access Subgroups
| Single radial, n=115 | Value |
|---|---|
| QuickDASH baseline, median (IQR) | 6.8 (0–27) |
| QuickDASH follow‐up, median (IQR) | 4.5 (0–16) |
| P value for QuickDASH difference | 0.01 |
| MCID ≥8, n (%) | 11 (11%) |
| MCID ≥14, n (%) | 6 (6%) |
| Biradial, n=19 | Value |
|---|---|
| QuickDASH baseline, median (IQR) | 4.5 (0–20) |
| QuickDASH follow‐up, median (IQR) | 1.1 (0–6.8) |
| P value for QuickDASH difference | 0.006 |
| MCID ≥8, n (%) | 0 (0%) |
| MCID ≥14, n (%) | 0 (0%) |
IQR indicates interquartile range; MCID, minimal clinically important difference; and QuickDASH, Quick Disabilities of Arm, Shoulder, and Hand.
Lower Extremity Function
Median LEFS score at follow‐up was 59 (interquartile range, 40–71), which was comparable to median LEFS at baseline (56 [interquartile range, 37–69]; P=0.08) (Figure 2). Twenty‐seven percent of large‐bore TFA‐treated patients experienced a decrease in LEFS score at follow‐up compared with baseline, reflecting worse lower‐limb function. Eleven percent of primary large‐bore TFA‐treated patients experienced clinically significant worsening of lower‐limb function when MCID ≥9 was applied (9% with MCID ≥12). For patients treated with secondary femoral access in case of primary radial access (n=58), 10.6% experienced clinically relevant decrease of lower‐extremity function. LEFS outcomes for primary large‐bore TFA‐treated patients are displayed in Table 2. Median baseline and follow‐up LEFS scores were comparable for single large‐bore femoral access and bifemoral access as well (Table 3).
Table 3.
LEFS Scores for Single and Double Femoral Access Subgroups
| Single femoral, n=114 | Value |
|---|---|
| LEFS baseline, median (IQR) | 60 (39–67) |
| LEFS follow‐up, median (IQR) | 60 (40–69) |
| P value for LEFS difference | 0.06 |
| MCID ≥9, n (%) | 8 (8%) |
| MCID ≥12, n (%) | 6 (6%) |
| Bifemoral, n=35 | Value |
|---|---|
| LEFS baseline, median (IQR) | 43 (28–66) |
| LEFS follow‐up, median (IQR) | 49 (26–66) |
| P value for LEFS difference | 0.16 |
| MCID ≥9, n (%) | 5 (16%) |
| MCID ≥12, n (%) | 5 (16%) |
IQR indicates interquartile range; LEFS, Lower Extremity Functional Scale; and MCID, minimal clinically important difference.
Access Site Pain
Postprocedural access site pain was present in 36% for primary TRA (median VAS, 3.2) and 37% for primary TFA‐treated patients (median VAS, 3.3) (P=0.96). At discharge and follow‐up, pain was present in 25% and 18% of patients, respectively, with no significant difference between both randomized strategies. The same applied for secondary access–related VAS scores. All VAS data are displayed in Table 4.
Table 4.
Extremity Pain for Primary and Secondary Access Sites
| Randomized access site | Primary access radial | Primary access femoral | P value |
|---|---|---|---|
| Postprocedural pain present, n (%) | 70 (36) | 71 (37) | 0.92 |
| VAS, median±SD | 3.2±2.1 | 3.3±2.2 | 0.96 |
| Discharge pain present, n (%) | 45 (23) | 52 (27) | 0.41 |
| VAS, median±SD | 2.7±1.9 | 3.5±2.0 | 0.22 |
| Follow‐up pain present, n (%) | 35 (18) | 35 (18) | 0.94 |
| VAS, median±SD | 2.9±1.4 | 2.8±1.5 | 0.90 |
| Secondary access site | Secondary access radial | Secondary access femoral | |
|---|---|---|---|
| Postprocedural pain present, n (%) | 28 (44) | 37 (40) | 0.62 |
| VAS, median±SD | 2.3±1.5 | 3.4±2.5 | 0.12 |
| Discharge pain present, n (%) | 16 (25) | 32 (35) | 0.21 |
| VAS, median±SD | 2.2±1.6 | 2.9±2.0 | 0.65 |
| Follow‐up pain present, n (%) | 11 (18) | 19 (21) | 0.58 |
| VAS, median±SD | N/A | 2.0±1.0 | N/A |
N/A indicates not available (not enough data); and VAS, visual analog scale.
Access Site Complications
Clinically significant decrease in upper and lower‐limb function was compared for patients with and without clinically relevant access site complications, as defined as the primary end point of the COLOR trial. For patients with Bleeding Academic Research Consortium scores 2, 3, or 5 bleeding or vascular complications requiring intervention of the randomized radial access site, 0% experienced clinically significant decrease of upper‐extremity function (MCID ≥8) compared with 6% of patients without clinically significant radial access site complications (P=1.0). For femoral‐treated patients, percentage of patients with clinically significant decrease of lower‐limb function was both 10% with and without clinically relevant access site complications. Median VAS at discharge was significantly higher for femoral‐treated patients with access site complications compared with patients without (6 versus 3, P=0.02). Table 5 displays extremity dysfunction in relation with important bleeding or vascular access site complications.
Table 5.
Effect of Access Site Bleeding on Extremity Dysfunction (Per Protocol Analysis)
| 7F primary radial access | Important bleeding or vascular complication | No important bleeding or vascular complication | P value |
|---|---|---|---|
| QD MCID ≥8, n (%) | 0/8 (0%) | 10/162 (6%) | 1.0 |
| QD MCID ≥14, n (%) | 0/8 (0%) | 16/162 (10%) | 1.0 |
| VAS discharge, median (IQR) | 5 (5–5) | 2 (1–3) | 0.28 |
| VAS follow‐up, median (IQR) | N/A | 2.5 (2–4) | N/A |
| Primary and/or secondary radial access | Any bleeding, BARC 0–5 | No bleeding, BARC 0 | |
|---|---|---|---|
| QD MCID ≥8, n (%) | 3/36 (8%) | 17/174 (10%) | 1.0 |
| QD MCID ≥14, n (%) | 2/36 (6%) | 9/174 (5%) | 1.0 |
| VAS discharge, median (IQR) | 2 (2–5) | 2 (1–3) | 0.72 |
| VAS follow‐up, median (IQR) | 3 (3–3) | 2 (2–4) | 1.0 |
| 7F primary femoral access | Important bleeding or vascular complication | No important bleeding or vascular complication | |
|---|---|---|---|
| LEFS MCID ≥9, n (%) | 3 (10%) | 15 (10%) | 1.0 |
| LEFS MCID ≥12, n (%) | 2 (6%) | 13 (9%) | 1.0 |
| VAS discharge, median (IQR) | 6 (5–7) | 3 (2–4) | 0.02 |
| VAS follow‐up, median (IQR) | 3.5 (2–5) | 2 (2–3) | 0.38 |
| Primary and/or secondary femoral access | Any bleeding, BARC 0–5 | No bleeding, BARC 0 | |
|---|---|---|---|
| LEFS MCID ≥9, n (%) | 11 (11%) | 12 (10%) | 0.67 |
| LEFS MCID ≥12, n (%) | 8 (8%) | 10 (8%) | 0.93 |
| VAS discharge, median (IQR) | 4 (2.5–5.5) | 2.5 (1–5) | 0.15 |
| VAS follow‐up, median (IQR) | 3 (1.5–4.0) | 2 (1–2) | 0.18 |
BARC indicates Bleeding Academic Research Consortium; IQR, interquartile range; LEFS, Lower Extremity Functional Scale; MCID, minimal clinically important difference; N/A, not available (not enough data); QD, Quick Disabilities of Arm, Shoulder, and Hand; and VAS, visual analog scale.
Risk Factors
Table 6 displays extremity dysfunction in relation to sex, RAS, and ultrasound‐guided puncture. Female patients treated with large‐bore TRA did show a trend toward increased occurrence of clinically relevant upper‐limb dysfunction (MCID ≥8) compared with male patients (20% versus 7%, P=0.05). Incidence of RAS was 6.7% for TRA‐treated patients, and did not lead to increased extremity dysfunction. In uni‐ and multivariate analyses, female sex was the only independent predictor of MCID ≥8 (Table S4), whereas for LEFS ≥9, no independent risk factors could be identified (Table S5). Ultrasound‐guided puncture in TFA‐treated patients resulted in a lower median VAS at discharge compared with patients without ultrasound‐guided puncture (4 versus 2, P=0.008), but VAS at follow‐up was comparable.
Table 6.
Effect of Risk Factors on Extremity Dysfunction (Per Protocol Analysis)
| Primary and/or secondary radial access | Male sex | Female sex | P value |
|---|---|---|---|
| QD MCID ≥14, n (%) | 8 (5%) | 3 (9%) | 0.40 |
| QD MCID ≥8, n (%) | 13 (7%) | 7 (20%) | 0.05 |
| VAS discharge, median (IQR) | 2 (1–3) | 3 (2–5) | N/A |
| VAS follow‐up, median (IQR) | 2 (2–3) | 4.5 (4–5) | N/A |
| Primary and/or secondary femoral access | Male sex | Female sex | |
|---|---|---|---|
| LEFS MCID ≥9, n (%) | 17 (10%) | 6 (13%) | 0.41 |
| LEFS MCID ≥12, n (%) | 12 (7%) | 6 (13%) | 0.22 |
| VAS discharge, median (IQR) | 3.5 (2–5) | 3.5 (2–6) | 0.67 |
| VAS follow‐up, median (IQR) | 2 (2–3) | 2 (1–4) | 0.95 |
| Primary and/or secondary radial access | RAS | No RAS | |
|---|---|---|---|
| QD MCID ≥14, n (%) | 0 (0%) | 11 (6%) | 1.0 |
| QD MCID ≥8, n (%) | 2 (15%) | 18 (9%) | 0.36 |
| VAS discharge, median (IQR) | 3 (1–3) | 2 (1–5) | 1.0 |
| VAS follow‐up, median (IQR) | 4.5 (4–5) | 2 (2–3) | 0.09 |
| Primary and/or secondary radial access | Ultrasound guided | Not ultrasound guided | |
|---|---|---|---|
| QD MCID ≥14, n (%) | 0 (0%) | 11 (6%) | 1.0 |
| QD MCID ≥8, n (%) | 1 (7%) | 19 (10%) | 1.0 |
| VAS discharge, median (IQR) | 1.5 (1–5) | 2.5 (1.5–3) | 0.53 |
| VAS follow‐up, median (IQR) | N/A | 2.5 (2–4) | N/A |
| Primary and/or secondary femoral access | Ultrasound guided | Not ultrasound guided | |
|---|---|---|---|
| LEFS MCID ≥9, n (%) | 9 (10%) | 14 (11%) | 0.90 |
| LEFS MCID ≥12, n (%) | 6 (7%) | 12 (9%) | 0.53 |
| VAS discharge, median (IQR) | 2 (1–3) | 4 (4–6) | 0.008 |
| VAS follow‐up, median (IQR) | 2 (2–3) | 2.5 (1–3.5) | 1.0 |
IQR indicates interquartile range; LEFS, Lower Extremity Functional Scale; MCID, minimal clinically important difference; N/A, not available (not enough data); QD, Quick Disabilities of Arm, Shoulder, and Hand; RAS, radial artery spasm; and VAS, visual analog scale.
Discussion
This study is the first to assess the impact of large‐bore radial and femoral access on extremity dysfunction and pain. In the complete study cohort, upper‐extremity function does not decrease over time after large‐bore TRA. The proportion of patients with clinically relevant decrease in upper‐extremity function at the 30‐day follow‐up is limited (6% or 9% depending on the applied MCID cutoff value). These outcomes are observed in the entire large‐bore TRA‐treated group as well as patients treated with biradial access and a radial/femoral hybrid approach in case of dual arterial access for chronic total occlusion PCI. Patients treated with large‐bore TFA had a lower LEFS score at follow‐up, reflecting decrease in lower‐extremity function, but this difference is not statistically significant.
Pain at discharge and follow‐up was comparable for large‐bore TRA‐ and TFA‐treated patients and was usually mild. Clinically relevant access site–related complications did not lead to a significant decrease in extremity dysfunction, although the occurrence of this primary end point was low in TRA‐treated patients. Of note, incidence of radial artery occlusion was low (<1%) but not routinely assessed or confirmed with ultrasound; therefore, a relation between radial artery occlusion and upper‐extremity dysfunction could not be assessed. TFA‐treated patients who had clinically relevant access site complications had a significantly higher VAS score at discharge, which can probably be explained by femoral nerve compression caused by prolonged hemostasis therapy and/or large hematoma. This difference in lower‐extremity pain was not observed at the 30‐day follow‐up. In radial access, no difference in pain scores was observed comparing patients with or without access site complications, although limited by the low complication rate.
For TRA‐treated patients, overall incidence of RAS was 6.7%. Previous studies have described a higher incidence of RAS, varying between 10% and 16%. 18 , 20 However, this is influenced by whether a clinical or angiographic definition of spasm is used, the former being dependent on operator as well as patient. In addition, selection bias may have caused a lower incidence of RAS, because patients with known severe RAS were excluded in this trial or could have refused informed consent because of previous painful transradial procedures. Sheath‐to‐artery mismatch is more likely to occur in female patients, which may explain the trend toward a higher incidence of clinically relevant upper‐limb dysfunction in female patients compared with male patients in this trial.
Ultrasound‐guided femoral puncture has been shown to reduce postprocedural pain in previous studies. 21 This is confirmed in the present trial, showing significantly lower VAS score at discharge for ultrasound‐guided femoral puncture. This difference could not be observed at the 30‐day follow‐up. The proportion of patients with clinically relevant lower‐limb dysfunction was comparable for TFA with and without ultrasound guidance. There is a possible selection bias involved, because ultrasound may be used if first or second puncture failed or in patients with known difficult femoral access, for example because of peripheral arterial disease. Consistent use of ultrasound‐guided femoral artery puncture by well‐trained and experienced operators may have a beneficial influence on both vascular complications and extremity dysfunction.
Previous studies assessing upper‐extremity dysfunction by using the QuickDASH questionnaire scores before and after transradial access are limited. The ACRA (Assessment of Disability After Coronary Procedures Using Radial Access) anatomy and perfusion studies examined hand function in relation to palmar arch completeness and digital hand perfusion, respectively, and used the QuickDASH questionnaire as an outcome measure. 22 , 23 Similar to the present study (although not statistically significant), no significant decrease in upper‐limb function was observed in the total population at short‐term follow‐up in the ACRA study. However, the proportion of patients with clinically significant decrease in hand function, using an MCID threshold of 14, was <5%, whereas in the present study this percentage is slightly higher (5%–6%). Nonetheless, caution must be used to compare both trials, because not only sheath diameter but also study populations are not similar. Mean age and presence of vascular risk factors are higher in the current study population, and the same applies for procedural time and lesion complexity. These factors possibly influence extremity dysfunction, although no studies have evaluated such predictors. 24 To our knowledge, no previous studies have been performed evaluating lower‐extremity dysfunction using the LEFS questionnaire; therefore, the current results on lower‐extremity function cannot be compared with historical data.
Several tests and questionnaires are available to assess upper‐extremity dysfunction. The Cold Intolerance Severity Scale can be used to assess pathological cold intolerance, which commonly occurs after a variety of upper‐extremity injuries, but is limited to detecting sensibility disorders. 25 The Michigan Hand Outcomes questionnaire is a more extensive questionnaire but focuses exclusively on hand function, and has not previously been described in a TRA population. 26 The QuickDASH score has proven to be a validated outcome measure with good test–rest reliability to monitor upper‐extremity disability for both clinical and research purposes. No serious alternative questionnaires for measuring lower‐extremity function are available to our knowledge.
The present study has some limitations. First, because of missing or incomplete questionnaires, not all patients could be analyzed for both baseline and follow‐up, introducing a chance for response bias. Proportion of missing or unanalyzable questionnaires was low, though, and baseline characteristics of both the complete cohort and analyzable cohort were similar. Second, because patients might report less upper‐extremity symptoms at follow‐up when their complex coronary artery lesions have been successfully treated by PCI, questionnaire bias may have affected the outcome by the design of the questions and how the questionnaires are administered or completed. 27 As a result, the questionnaire scores at follow‐up can show improvement, although limb function itself is not expected to improve after large‐bore arterial access. Third, the use of secondary access may have influenced primary and secondary outcome parameters, although the results were consistent among single‐ and double‐access subgroups. Fourth, assessment of pain after PCI and at discharge may have been influenced by periprocedural medical treatment such as use of analgesics.
Conclusions
Following large‐bore TRA and TFA, self‐reported upper‐limb and lower‐limb function did not decrease over time in the vast majority of patients. Clinically relevant limb dysfunction occurs in a small minority of patients regardless of radial or femoral large‐bore access. Of note, previous studies have shown that upper‐extremity dysfunction resolves in the majority of patients at 1 year follow‐up. 28
Sources of Funding
Terumo EMEA (Leuven, Belgium) supported the investigator‐initiated COLOR study by an unrestricted grant.
Disclosures
Drs van Leeuwen, Aminian, Dens, and Iglesias are consultants for Terumo. Drs Iglesias and Schmitz have received honoraria/speakers fees from Terumo. The remaining authors have no disclosures to report.
Supporting information
Data S1–S3
Tables S1–S5
Acknowledgments
The authors thank the members of the Clinical Event Committee Eugene P. McFadden (Chair), J. J. Wykrzykowska (member) and J. Daemen (member).
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023691
For Sources of Funding and Disclosures, see page 9.
References
- 1. Tanaka Y, Moriyama N, Ochiai T, Takada T, Tobita K, Shishido K, Sugitatsu K, Yamanaka F, Mizuno S, Murakami M, et al. Transradial coronary interventions for complex chronic total occlusions. JACC Cardiovasc Interv. 2017;10:235–243. doi: 10.1016/j.jcin.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 2. Meijers TA, Aminian A, van Wely M, Teeuwen K, Schmitz T, Dirksen MT, Rathore S, van der Schaaf RJ, Knaapen P, Dens J, et al. Randomized comparison between radial and femoral large‐bore access for complex percutaneous coronary intervention. JACC Cardiovasc Interv. 2021;14:1293–1303. doi: 10.1016/j.jcin.2021.03.041 [DOI] [PubMed] [Google Scholar]
- 3. van der Heijden D, van Leeuwen M, Janssens G, Hermie J, Lenzen M, Ritt M, van de Ven P, Kiemeneij F, van Royen N. Endothelial dysfunction and the occurrence of radial artery spasm during transradial coronary procedures: the ACRA‐Spasm study. EuroIntervention. 2016;12:1263–1270. doi: 10.4244/EIJV12I10A207 [DOI] [PubMed] [Google Scholar]
- 4. Di Serafino L, Pyxaras SA, Mangiacapra F, Dierickx K, Toth G, Bartunek J, De Bruyne B, Van Mieghem C, Wijns W, Barbato E. Influence of transradial versus transfemoral diagnostic heart catheterisation on peripheral vascular endothelial function. EuroIntervention. 2013;8:1252–1258. doi: 10.4244/EIJV8I11A193 [DOI] [PubMed] [Google Scholar]
- 5. Costa F, van Leeuwen MAH, Daemen J, Diletti R, Kauer F, van Geuns R‐J, Ligthart J, Witberg K, Zijlstra F, Valgimigli M, et al. The Rotterdam radial access research: ultrasound‐based radial artery evaluation for diagnostic and therapeutic coronary procedures. Circ Cardiovasc Interv. 2016;9:e003129. doi: 10.1161/CIRCINTERVENTIONS.115.003129 [DOI] [PubMed] [Google Scholar]
- 6. Yonetsu T, Kakuta T, Lee T, Takayama K, Kakita K, Iwamoto T, Kawaguchi N, Takahashi K, Yamamoto G, Iesaka Y, et al. Assessment of acute injuries and chronic intimal thickening of the radial artery after transradial coronary intervention by optical coherence tomography. Eur Heart J. 2010;31:1608–1615. doi: 10.1093/eurheartj/ehq102 [DOI] [PubMed] [Google Scholar]
- 7. Kinnaird TD, Stabile E, Mintz GS, Lee CW, Canos DA, Gevorkian N, Pinnow EE, Kent KM, Pichard AD, Satler LF, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930–935. doi: 10.1016/S0002-9149(03)00972-X [DOI] [PubMed] [Google Scholar]
- 8. Kent KC, Moscucci M, Gallagher SG, DiMattia ST, Skillman JJ. Neuropathy after cardiac catheterization: incidence, clinical patterns, and long‐term outcome. J Vasc Surg. 1994;19:1008–1013;discussion 1013–4. doi: 10.1016/S0741-5214(94)70212-8 [DOI] [PubMed] [Google Scholar]
- 9. El‐Ghanem M, Malik AA, Azzam A, Yacoub HA, Qureshi AI, Souayah N. Occurrence of femoral nerve injury among patients undergoing transfemoral percutaneous catheterization procedures in the United States. J Vasc Interv Neurol. 2017;9:54–58. [PMC free article] [PubMed] [Google Scholar]
- 10. Meijers TA, Aminian A, Teeuwen K, van Wely M, Schmitz T, Dirksen MT, van der Schaaf RJ, Iglesias JF, Agostoni P, Dens J, et al. Complex Large‐Bore Radial percutaneous coronary intervention: rationale of the COLOR trial study protocol. BMJ Open. 2020;10:e038042. doi: 10.1136/bmjopen-2020-038042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beaton DE, Wright JG, Katz JN, Amadio P, Bombardier C, Cole D, Davis A, Hudak P, Marx R, Hawker G, et al. Development of the QuickDASH: COmparison of three item‐reduction approaches. J Bone Jt Surg ‐ Ser A. 2005;87:1038–1046. doi: 10.2106/JBJS.D.02060 [DOI] [PubMed] [Google Scholar]
- 12. Mintken PE, Glynn P, Cleland JA. Psychometric properties of the shortened disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) and Numeric Pain Rating Scale in patients with shoulder pain. J Shoulder Elb Surg. 2009;18:920–926. doi: 10.1016/j.jse.2008.12.015 [DOI] [PubMed] [Google Scholar]
- 13. Sorensen AA, Howard D, Tan WH, Ketchersid J, Calfee RP. Minimal clinically important differences of 3 patient‐rated outcomes instruments. J Hand Surg Am. 2013;38:641–649. doi: 10.1016/j.jhsa.2012.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Binkley J, Stratford P, Lott S, Riddle D. The lower extremity functional scale. Phys Ther. 1999;79:371–383. [PubMed] [Google Scholar]
- 15. Mehta SP, Fulton A, Quach C, Thistle M, Toledo C, Evans NA. Measurement properties of the lower extremity functional scale: a systematic review. J Orthop Sports Phys Ther. 2016;46:200–216. doi: 10.2519/jospt.2016.6165 [DOI] [PubMed] [Google Scholar]
- 16. McCormack J, Underwood F, Slaven E, Cappaert T. The Minimum clinically important difference on the visa‐a and lefs for patients with insertional achilles tendinopathy. Int J Sports Phys Ther. 2015;10:639–644. [PMC free article] [PubMed] [Google Scholar]
- 17. Ruhnau J. Prevalence of and risk factors for radial artery complications after transradial cardiac catheterization. Circulation. 2013. [Google Scholar]
- 18. Gorgulu S, Norgaz T, Karaahmet T, Dagdelen S. Incidence and predictors of radial artery spasm at the beginning of a transradial coronary procedure. J Interv Cardiol. 2013;26:208–213. doi: 10.1111/joic.12000 [DOI] [PubMed] [Google Scholar]
- 19. Ercan S, Unal A, Altunbas G, Kaya H, Davutoglu V, Yuce M, Ozer O. Anxiety score as a risk factor for radial artery vasospasm during radial interventions: a pilot study. Angiology. 2014;65:67–70. doi: 10.1177/0003319713488931 [DOI] [PubMed] [Google Scholar]
- 20. Van Der Heijden DJ, Van Leeuwen MAH, Janssens GN, Hermie J, Lenzen MJ, Ritt MJPF, Van De Ven PM, Kiemeneij F, Van Royen N. Endothelial dysfunction and the occurrence of radial artery spasm during transradial coronary procedures: the ACRA‐Spasm study. EuroIntervention. 2016;12:1263–1270. doi: 10.4244/EIJV12I10A207 [DOI] [PubMed] [Google Scholar]
- 21. Katircibasi MT, Günes H, Aykan AÇ, Aksu E, Özgül S. Comparison of ultrasound guidance and conventional method for common femoral artery cannulation: a prospective study of 939 patients. Acta Cardiol Sin. 2018;34:394. doi: 10.6515/ACS.201809_34(5).20180524A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Leeuwen MAH, Hollander MR, van der Heijden DJ, van de Ven PM, Opmeer KHM, Taverne YJHJ, Ritt MJPF, Kiemeneij F, van Mieghem NM, van Royen N. The ACRA Anatomy Study (Assessment of Disability After Coronary Procedures Using Radial Access). Circ Cardiovasc Interv. 2017;10. doi: 10.1161/circinterventions.117.005753 [DOI] [PubMed] [Google Scholar]
- 23. van Leeuwen MAH, van der Heijden DJ, Hollander MR, Mulder MJ, van de Ven PM, Ritt MJPF, Kiemeneij F, van Mieghem NM, van Royen N. ACRA Perfusion Study. Circ Cardiovasc Interv. 2019;12. doi: 10.1161/CIRCINTERVENTIONS.118.007641 [DOI] [PubMed] [Google Scholar]
- 24. Ul Haq MA, Rashid M, Kwok CS, Wong CW, Nolan J, Mamas MA. Hand dysfunction after transradial artery catheterization for coronary procedures. World J Cardiol. 2017;9:609. doi: 10.4330/wjc.v9.i7.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruijs ACJ, Jaquet JB, Daanen HAM, Hovius SER. Cold Intolerance of the hand measured by the CISS questionnaire in a normative study population. J Hand Surg Am. 2006;31:533–536. doi: 10.1016/j.jhsb.2006.04.013 [DOI] [PubMed] [Google Scholar]
- 26. Chung KC, Pillsbury MS, Walters MR, Hayward RA. Reliability and validity testing of the Michigan Hand Outcomes Questionnaire. J Hand Surg Am. 1998;23:575–587. doi: 10.1016/S0363-5023(98)80042-7 [DOI] [PubMed] [Google Scholar]
- 27. Choi BCK, Pak AWP. A catalog of biases in questionnaires. Prev Chronic Dis. 2005;2:A13. [PMC free article] [PubMed] [Google Scholar]
- 28. van Leeuwen MAH, Hollander MR, van der Heijden DJ, van de Ven PM, Opmeer KHM, Taverne YJHJ, Ritt MJPF, Kiemeneij F, van Mieghem NM, van Royen N. The ACRA Anatomy Study (Assessment of Disability After Coronary Procedures Using Radial Access): a comprehensive anatomic and functional assessment of the vasculature of the hand and relation to outcome after transradial catheterization. Circ Cardiovasc Interv. 2017;10. doi: 10.1161/CIRCINTERVENTIONS.117.005753 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1–S3
Tables S1–S5


