Abstract
Background
Human aging is associated with increased risk of thrombosis, but the mechanisms are poorly defined. We hypothesized that aging induces peroxide‐dependent release of neutrophil extracellular traps that contribute to thrombin generation and thrombosis.
Methods and Results
We studied C57BL6J mice and littermates of glutathione peroxidase‐1 transgenic and wild‐type mice at young (4 month) and old (20 month) ages and a healthy cohort of young (18–39 years) or middle‐aged/older (50–72 years) humans. In plasma, we measured thrombin generation potential and components of neutrophil extracellular traps (cell‐free DNA and citrullinated histone). Aged wild‐type mice displayed a significant increase in thrombin generation that was decreased in aged glutathione peroxidase‐1 transgenic mice. Both aged wild‐type and aged glutathione peroxidase‐1 transgenic mice demonstrated similar elevation of plasma cell‐free DNA compared with young mice. In contrast, plasma levels of citrullinated histone were not altered with age or genotype. Release of neutrophil extracellular traps from neutrophils in vitro was also similar between young and aged wild‐type or glutathione peroxidase‐1 transgenic mice. Treatment of plasma or mice with DNase 1 decreased age‐associated increases in thrombin generation, and DNase 1 treatment blocked the development of experimental venous thrombi in aged C57BL6J mice. Similarly, thrombin generation potential and plasma cell‐free DNA, but not citrullinated histone, were higher in middle‐aged/older humans, and treatment of plasma with DNase 1 reversed the increase in thrombin generation.
Conclusions
We conclude that DNase 1 limits thrombin generation and protects from venous thrombosis during aging, likely by hydrolyzing cell‐free DNA.
Keywords: age, aging, extracellular traps, oxidative stress, thrombosis
Subject Categories: Thrombosis
Nonstandard Abbreviations and Acronyms
- cfDNA
cell‐free DNA
- ETP
endogenous thrombin potential
- Gpx1 Tg
glutathione peroxidase‐1 transgenic
- H3Cit
citrullinated histone
- NETs
neutrophil extracellular traps
- PMA
phorbol myristate acetate
- PPP
platelet poor plasma
- WT
wild type
Clinical Perspective
What Is New?
Aging increases circulating cell‐free DNA in a NETosis‐independent pathway.
DNase 1 protects from age‐induced increase in endogenous thrombin potential and venous thrombosis.
What Are the Clinical Implications?
Cell‐free DNA and endogenous thrombin potential may serve as the biomarkers for a prothrombotic state during aging.
Therapeutic potential of DNase 1 for the treatment of age‐associated thrombotic events should be considered.
Human aging is associated with an increased risk of thrombotic complications, 1 , 2 , 3 but the mechanisms of thrombus development are not fully elucidated. Emerging data suggest that plasma from patients with thrombotic events such as myocardial infarction, 4 acute ischemic stroke, 5 or deep vein thrombosis 6 exhibits increased potential for thrombin generation, which can be quantitated as endogenous thrombin potential (ETP), when exposed to an extrinsic trigger such as tissue factor. Increased ETP has also been reported in conditions such as diabetes, 7 sepsis, 8 , 9 and cancer‐associated thrombosis. 10 , 11 Studies have suggested that human aging is associated with an increase in markers of thrombin generation even in the absence of any known thrombotic risk factors or a clinical thrombotic event. 12 , 13 , 14 It is likely that the subset of older healthy subjects exhibiting increased potential for thrombin generation is more vulnerable to a future thrombotic event. Therefore, better understanding of the early mechanisms leading to increased potential for thrombin generation in aging may lead to the development of diagnostics for identifying an underlying subclinical prothrombotic state.
Using experimental models of thrombosis, we 15 and others 16 , 17 have demonstrated that aged mice display increased susceptibility to arterial and venous thrombosis. We have further observed that aged mice overexpressing the antioxidant glutathione peroxidase‐1 (Gpx1; an enzyme that converts peroxides to water) are protected from increased thrombotic susceptibility. 15 However, it is not known whether, like humans, aged mice also display increased potential for thrombin generation and whether it is modulated by peroxide. It has been demonstrated that hydrogen peroxide (H2O2) mediates activation of neutrophils in vitro, and subsequent release of neutrophil extracellular traps (NETs), in a process called NETosis. 18 NETosis involves chromatin decondensation that occurs through peptidylarginine deiminase 4–mediated deimination of nuclear histones. 19 NETs contain cell‐free DNA (cfDNA), histones, citrullinated histone H3 (H3Cit), elastase, and myeloperoxidase. The cfDNA and histones are pro‐thrombotic in nature. 20 Extracellular traps are classically defined to be released from neutrophils, 21 but recently other cells such as eosinophils, 22 mast cells, 23 and basophils 24 also have been documented to release extracellular traps. Components of NETs have been identified in plasma and tissues during aging. For example, tissues of elderly patients with severe vasculitis exhibit dense infiltration of H3Cit‐positive neutrophils, 25 , 26 and elevated plasma cfDNA has been observed with human aging in the absence of any vascular risk factors. 27 One study also observed NETosis in conjunction with cardiac and lung fibrosis in murine aging. 28 Furthermore, levels of reactive oxygen species are known to increase with human aging in several tissues, including neutrophils, 29 and reactive oxygen species such as H2O2 are known to release NETs in vitro. 18 It is not known, however, whether increased NETosis in aging leads to increased thrombin generation potential in a peroxide‐dependent manner or whether it contributes to age‐associated thrombosis.
Components of NETs such as cfDNA and histones can contribute to increased thrombin generation, 8 and NETs have been proposed to promote the development of venous thrombosis in mice. 30 We hypothesized that aging induces peroxide‐dependent release of NETs that contribute to thrombin generation and venous thrombosis. Herein, using mice overexpressing Gpx1 and their wild‐type (WT) littermates, and plasma samples from healthy young and middle‐aged/older human subjects, we demonstrate that both murine and human aging is associated with increased potential for thrombin generation, which is likely mediated through cfDNA. Surprisingly, the aging‐related prothrombotic effects of cfDNA were independent of peroxide and NETosis, suggesting an alternative mechanism for generation of cfDNA during aging. Finally, our data in mice demonstrated that treatment with DNase 1 protects aged C57BL6J mice from increased susceptibility to venous thrombosis.
METHODS
The data that support the findings are available from the corresponding author upon reasonable request.
Mice
C57BL6J mice and mice overexpressing Gpx1 (Gpx1 transgenic [Gpx1 Tg] mice) 31 and their WT littermates were included in the study design. Genotyping for the Gpx1 transgene was performed using real‐time polymerase chain reaction. 32 All animal protocols were approved by the University of Iowa Animal Care and Use Committee. Male and female mice were examined at 4 and 20 months of age. Blood samples were collected in 3.2% Na‐citrate through heart puncture. For treatment of mice with DNase 1 followed by thrombin generation assay, mice were infused with either 1 µg/g of DNase 1 or heat‐inactivated DNase 1 (free of other proteases, LS006344 DPRFS; Worthington Biochemicals, NJ) retro‐orbitally, 60 minutes before blood collection.
Human Subjects
Stored plasma samples utilized in this study were available from healthy human subjects recruited under 2 Institutional Review Board–approved protocols: a randomized clinical study (IRB# 201201739, NCT01775865), 33 and a study of platelets in aging (IRB#201309851). From NCT01775865, only the baseline samples were used before any intervention. Samples from a total of 27 young (age 18–39 years) and 28 middle aged/older subjects (age 50–72 years) were analyzed. The proportion of men and women was similar between groups, and all subjects were non‐Hispanic White individuals. Healthy subjects were recruited from the Iowa City community through flyers and email advertisements to participate in a single visit, cross‐sectional study. Subjects were free of cardiovascular and metabolic disease, were not taking any medications for a known chronic condition, and were ambulatory and independent. All blood samples were collected in the morning after an overnight fast from an antecubital intravenous catheter 15 minutes after catheterization or via butterfly needle into tubes containing 3.2% Na‐citrate. All participants gave written informed consent. Recruitment and blood collection protocol was approved by Institutional Review Board at the University of Iowa.
Thrombin Generation
We measured thrombin generation using platelet poor plasma (PPP) or platelet rich plasma in a Calibrated Automated Thrombogram (CAT, Diagnostica Stago, Inc, Parsippany, NJ). The details of methods are provided in Data S1.
Prothrombin, DNase 1, and Components of NETs
Plasma levels of prothrombin were measured using murine and human specific enzyme‐linked immunosorbent assay kits (Molecular Innovations, MI). Plasma levels of DNase 1 were quantified using a commercially available kit (from LifeSpan BioSciences, Inc. for mouse and Cloud Clone Corp. for human). Quantification of circulating cfDNA in plasma samples was performed using the Qubit dsDNA HS Assay kit (Invitrogen, Life Technologies, Carlsbad, CA) according to manufacturer’s instructions. The levels of H3Cit were measured using a commercially available kit (Caymen Chemical, MI).
Experimental Venous Thrombosis
Mice anesthetized with isoflurane underwent a stasis model of experimental deep vein thrombosis, 15 where inferior vena cava and all other visible side and back branches were ligated. Mice received retro‐orbitally either 2.5 µg/g DNase 1 or heat‐inactivated DNase 1 (Pulmozyme, Genentech Inc., CA), 30 minutes before experimental thrombosis. Thereafter, the same amount was injected intraperitoneally every 12 hours. This protocol was adapted from previous reports 30 , 34 with minor modifications. After 48 hours of ligation, mice were euthanized, and thrombus developed in inferior vena cava was harvested for measurement of the length and weight.
Statistical Analysis
All data were analyzed using GraphPad Prism software. Normality testing was performed using D’agostino‐Pearson omnibus. A 2‐way ANOVA with the Tukey test for multiple comparisons was performed in mice for measures of thrombin generation, cfDNA, and H3Cit and a 3‐way ANOVA with Šídák's multiple comparisons test was used in studies using DNase 1. Kruskal–Wallis with the Dunn’s test for multiple comparisons was used to analyze data for experimental venous thrombosis. In human plasma samples, mean and SD were reported for continuous measures stratified by the young versus middle‐aged/older groups; count and percentage were reported for categorical measures stratified by the young versus middle‐aged/older groups. Continuous measures between the young versus middle‐aged/older groups were compared using unpaired t test. Linear regression analysis was performed to determine effects of age and cfDNA on thrombin generation. Statistical significance was defined as a value of P<0.05.
RESULTS
Murine Aging Is Associated With Increased Thrombin Generation Potential That Is Moderately Reversed With Gpx1 Overexpression
To determine whether thrombin generation potential is increased during aging and whether this effect is modulated through peroxide, we first performed a fluorometric‐based thrombin generation assay in PPP collected from young and aged WT and Gpx1 Tg mice. For ETP, which is measured as area under the thrombin generation curve, 2‐way ANOVA indicated a significant main effect for age (P<0.01) but not for genotype (P=0.68). However, there was a significant interaction between age and genotype (P<0.05), reflecting a loss of age‐related increase in ETP in Gpx1 Tg mice (Figure 1A, Table S1). Accordingly, the ETP was significantly increased in aged WT mice compared with young WT mice (P<0.001), whereas ETP was not significantly different in aged Gpx1 Tg mice compared with either young WT mice (P=0.069) or young Gpx1 Tg mice (P=0.066). These findings suggest that peroxide contributes to aging‐associated increases in thrombin generation potential. A similar pattern was observed for thrombin peak, with a significant main effect for age (P<0.01) but not for genotype (P=0.69) or interaction between age and genotype (P=0.07) (Figure 1B, Table S1). Aged WT mice displayed a significantly higher thrombin peak than young WT mice (P<0.05), but the thrombin peak in aged Gpx1 Tg mice was not significantly different from young Gpx1 Tg mice (P=0.8) or aged WT mice (P=0.7). The lag time, which represents the time to onset of thrombin generation, did not differ significantly among the groups (Figure 1C, Table S1). Plasma prothrombin levels were also not significantly different among the groups (Figure S1, Table S1).
Figure 1. Murine aging is associated with increased thrombin generation, which is moderately reversed with Gpx1 overexpression.
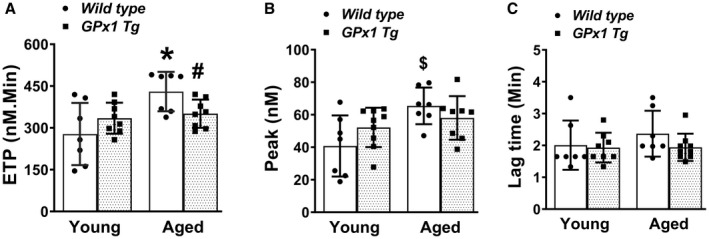
Thrombin generation was measured using calibrated automated thrombogram in platelet poor plasma in young and aged wild‐type mice as well as littermates overexpressing Gpx1 (glutathione peroxidase‐1 transgenic [Gpx1 Tg]). A, Endogenous thrombin potential (ETP). B, Peak thrombin generation. C, Lag time. Data expressed as mean±SD. *P<0.001 vs young wild type, # P<0.05 vs aged wild type, and $ P<0.05 vs young wild type (2‐way ANOVA with Tukey test). N=7 to 8 in each group.
Aged Mice Display Elevated Levels of cfDNA Regardless of Gpx1 Overexpression
Since NETs are known to contribute to thrombin generation, we next tested whether there was an increase in components of NETs in the plasma of aged mice and whether they are modulated by peroxide. We measured levels of cfDNA and H3Cit in plasma samples from WT and Gpx1 Tg mice in both age groups. Two‐way ANOVA indicated a significant main effect for age on cfDNA (P<0.0001, Figure 2A, Table S1), but the effects for genotype (P=0.16) or the interaction of age and genotype (P=0.6) on cfDNA were not significant. A significant increase in the levels of cfDNA in both aged WT mice (P<0.0001 versus young WT mice) and aged Gpx1 Tg mice (P<0.0001 versus young Gpx1 Tg mice) was observed and the levels of cfDNA in aged WT mice were similar to those in aged Gpx 1 Tg mice (P=0.5). These findings suggest that age‐dependent elevation in cfDNA is not modulated by peroxide. In contrast to cfDNA, H3Cit levels did not differ significantly among the groups (main effects of age, P=0.7, genotype, P=0.7, and interaction, P=0.6, Figure 2B, Table S1). Regression analysis revealed a positive linear relationship between cfDNA and both ETP and thrombin peak (P<0.05 for both, Figure 2C and 2D). The relationship between cfDNA and H3Cit was not significant (data not shown). The absence of an increase in H3Cit in aged WT mice suggests that age‐associated changes in cfDNA and ETP may not be mediated via NETosis.
Figure 2. Aged mice display elevated levels of cfDNA regardless of Gpx1 overexpression and cfDNA has a positive effect on thrombin generation.
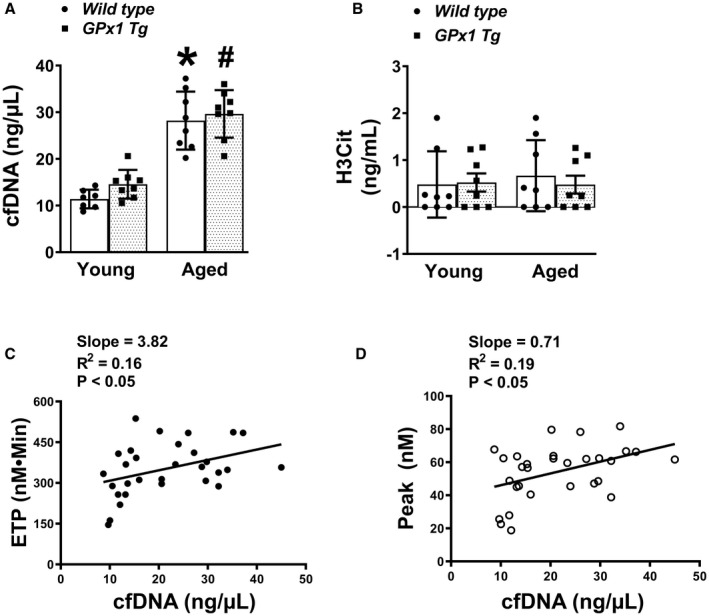
Components of neutrophil extracellular traps were measured in plasma from young and aged wild‐type mice and littermates overexpressing Gpx1 (glutathione peroxidase‐1 transgenic [Gpx1 Tg]). A, Plasma cell‐free DNA (cfDNA). B, Plasma citrullinated histone (H3Cit). Data for (A and B) are expressed as mean±SD. C, Linear regression analysis between cfDNA and endogenous thrombin potential (ETP). D, Linear regression analysis between cfDNA and thrombin peak. *P<0.0001 vs young wild type and # P<0.001 vs young GPx1 Tg mice (2‐way ANOVA with Tukey test). N=7 to 8 in each group.
We next utilized in vitro approaches to directly test the susceptibility of neutrophils from young or aged mice to undergo NETosis and whether this response is modulated by peroxide. We isolated neutrophils from mice and seeded on coverslips. Neutrophils were isolated from young or aged WT or Gpx1 Tg mice, and release of NETs with phorbol myristate acetate (PMA) stimulation was monitored using fluorescent microscopy. The percentage of neutrophils releasing NETs in response to PMA were comparable between the groups (Figure S2); neither the main effect for age (P=0.58) nor genotype (P>0.99) nor the interaction of age and genotype (P=0.52) were significant. Next, neutrophils from young WT, aged WT, or aged Gpx1 Tg mice were suspended in pooled mouse plasma and incubated with either the vehicle buffer (control) or PMA. Compared with vehicle, stimulation with PMA increased levels of cfDNA, H3Cit, and ETP (the main effect for PMA was significant, with P<0.05, P<0.001, and P<0.001, respectively, Figure S3). Within the PMA‐treated groups, however, no further elevations in cfDNA, H3Cit, or ETP were observed in aged WT or aged Gpx1 Tg mice compared with young WT mice. These findings suggest that (1) neutrophils from young and aged mice release similar levels of NETs and produce similar increases in ETP after PMA activation, and (2) peroxide does not modulate the in vitro release of NETs from neutrophils upon PMA stimulation, even in aged mice.
Platelet‐Dependent Thrombin Generation Is Not Altered by Murine Aging
Since histones can promote thrombin generation through platelet activation, 35 we next tested whether platelet‐dependent thrombin generation is increased with age and whether mice overexpressing Gpx1 are protected. Platelet‐dependent thrombin generation was measured in platelet rich plasma. In contrast to findings with PPP, no significant main effects of age, genotype, or the interaction of age and genotype on ETP or peak thrombin generation were observed in platelet rich plasma (Figure S4, Table S1); these findings suggest that platelet‐dependent thrombin generation does not change with age in this model.
Incubation of Plasma Samples With DNase 1 Decreases Thrombin Generation Potential in Mice
First, to determine whether levels of endogenous DNase 1 are affected by age, we measured DNase 1 antigen levels in plasma samples (Figure S5, Table S1). The DNase 1 levels were not found to be altered because of age (P=0.9) or genotype (P=0.6). Next, to assess the potential mechanistic role of cfDNA in driving thrombin generation during aging, we treated PPP samples from young or aged mice with either DNase 1 or heat‐inactivated DNase 1 (as a control) before measurement of thrombin generation. By 3‐way ANOVA, the main effect of treatment was significant (P<0.001, Figure 3, Table S1). However, the main effect of age or genotype was not significant. Also, there were no significant interactions between age, genotype, or treatment. We observed 31% and 26% reductions in ETP in young WT and young Gpx1 Tg mice, respectively, and 43% and 39% reductions in aged WT and aged Gpx1 Tg mice, respectively, with DNase 1 treatment. Also, within the DNase 1–treated groups, we did not observe any differences in ETP between young or aged mice or mice with Gpx 1 overexpression. These findings indicate that DNase 1 treatment normalized the differences in ETP between young and aged mice.
Figure 3. In vitro treatment of plasma with DNase 1 lowered age‐associated increase in thrombin generation potential in mice regardless of genotype.
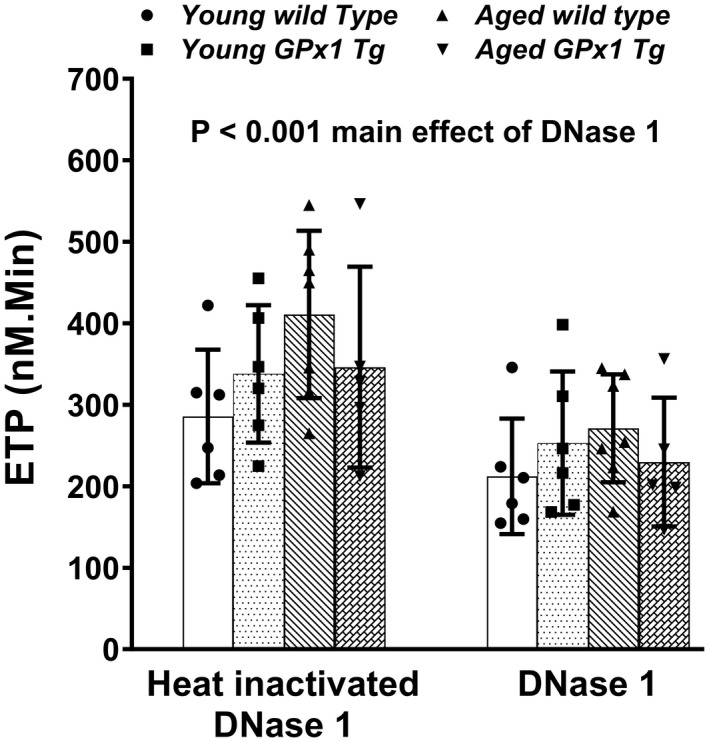
Platelet poor plasma from young and aged wild‐type mice or glutathione peroxidase‐1 transgenic (Gpx1 Tg) littermates were treated with either 20 µg/mL of DNase 1 or same dose of heat‐inactivated DNase 1 as control for 60 minutes, and thrombin generation (endogenous thrombin potential [ETP]) was measured. Data are expressed as mean±SD. Three‐way ANOVA with Šídák's multiple comparisons. N=5 to 7 in each group.
Treatment of Aged Mice With DNase 1 Lowers Plasma cfDNA Burden and Protects From Increased Thrombin Generation Potential Ex Vivo and Susceptibility to Venous Thrombosis In Vivo
To determine whether lowering of cfDNA in vivo would decrease thrombin generation potential and protect aged mice from increased susceptibility to thrombosis, we infused mice retro‐orbitally with DNase 1 or heat‐inactivated DNase 1. First, we performed a dose–response study in young C57BL6J mice and observed that 1 mg/kg DNase 1 significantly decreased levels of circulating cfDNA 60 minutes postinfusion (Figure S6). Compared with mice infused with heat‐inactivated DNase 1, infusion of DNase 1 significantly lowered cfDNA and ETP (main effect for treatment was P<0.0001 and P<0.01, respectively, by 3‐way ANOVA, Figure 4A and 4B, Table S1). We also observed a significant main effect of age (P<0.0001 for cfDNA, and P<0.01 for ETP), but not of genotype and there were no significant interactions between age, genotype, or treatment. Post DNase 1 infusion, the levels of cfDNA or ETP were similar in all 4 groups of mice, suggesting that infusion of DNase 1 normalized differences between young and aged mice regardless of genotype. Accordingly, while the linear regression analysis within the heat‐inactivated DNase 1 infusion group revealed a positive relation between cfDNA and ETP (slope=10, R 2=0.25 and P<0.05, Figure 4C), this relationship was lost after DNase 1 treatment (slope=−6.8, R 2=0.11 and P=0.11, Figure 4D).
Figure 4. In vivo treatment with DNase 1 lowered potential of plasma to generate thrombin ex vivo in aged mice regardless of genotype.
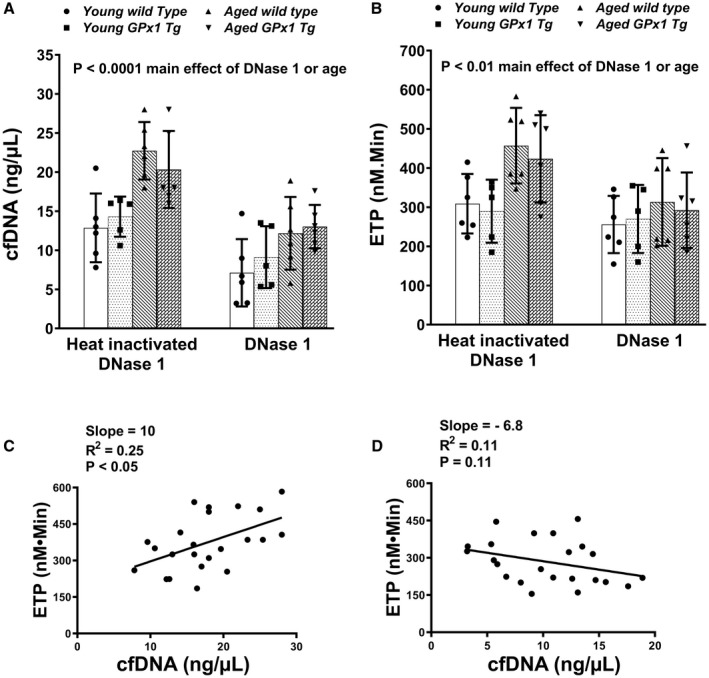
Young and aged wild‐type mice or glutathione peroxidase‐1 transgenic (Gpx1 Tg) littermates were treated retro‐orbitally with either 1 mg/kg of DNase 1 or heat‐inactivated DNase 1. Blood was collected 60 minutes postinfusion, and plasma cell‐free DNA (cfDNA) (A) and endogenous thrombotic potential (ETP) (B) were measured. Data are expressed as mean±SD and analyzed with 3‐way ANOVA with Šídák's multiple comparisons. N=5 to 6 in each group. Linear regression analysis for cfDNA and ETP was performed in mice infused with heat‐inactivated DNase 1 (C) or DNase 1 (D).
Finally, in a murine experimental deep vein thrombosis model, we tested the effect of elevated cfDNA on venous thrombosis in aged mice. Since overexpression of Gpx1 did not alter NETosis, cfDNA levels, or the effect of DNase 1 treatment on ETP, we chose to study the role of cfDNA in venous thrombosis by infusing DNase 1 in young and aged WT mice rather than Gpx1 Tg mice. We infused young and aged C57BL6J mice before and every 12 hours during the 48 hours of inferior vena cava ligation with either DNase 1 or heat‐inactivated DNase 1. We compared the length and weight of thrombi that developed at 48 hours after ligation. The main effect of treatment for both length and weight of thrombi was significant (P<0.01), and there was also a significant interaction between age and treatment for the length (P<0.01), but not weight (P=0.1) of the thrombus. Aged control mice developed longer‐length thrombi than young mice (P<0.05, Figure 5A), and treatment with DNase 1 significantly reduced the thrombus length and weight in aged mice (P<0.01 and P<0.001, respectively, Figure 5A and 5B).
Figure 5. Treatment of aged C57BL6J mice with DNase 1 blocked development of venous thrombi in vivo.
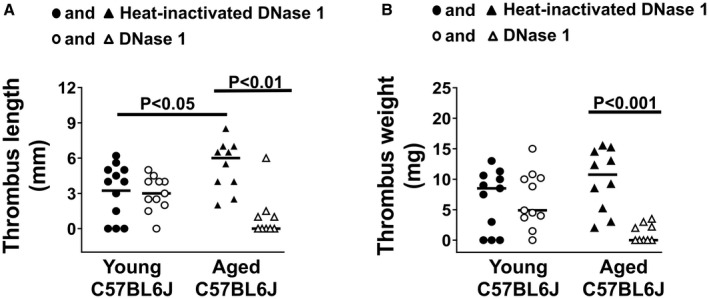
Young and aged C57BL6J mice were treated with either DNase 1 or heat‐inactivated DNase 1 control, during experimental venous thrombosis. Inferior vena cava was ligated in a stasis model and 48 hours later venous thrombus was harvested. A, Thrombus length. B, Thrombus weight. Kruskal–Wallis with Dunn’s test for multiple comparison. N=9 to 12 in each group.
Together, these findings suggest that DNase 1 protects from age‐associated increased thrombin generation potential and susceptibility to venous thrombosis in mice.
Human Aging Is Associated With Increased Thrombin Generation Potential and Elevated cfDNA
To examine how the aforementioned findings in aged mice are translated into human aging, we examined PPP samples from a healthy cohort of young and middle‐aged/older humans. The baseline characteristics of the human subjects are described in Table S2; body mass index and blood pressure were within the clinically normal range for all the subjects. The ETP was higher in middle aged/older subjects compared with young subjects (P<0.0001, Figure 6A, Table S3). Regression analysis also showed a positive relationship between age and ETP (slope=13.8, R 2=0.23, P<0.001, Figure 6B). Likewise, peak thrombin generation was higher in middle aged/older subjects compared with young subjects (P<0.01, Figure 6C, Table S3), and was positively associated with age (slope=2.36, R 2=0.15, P<0.01, Figure 6D). The lag time was not different between the 2 age groups (P=0.3, Figure 6E, Table S3), and regression analysis also did not show significant association with age (Figure 6F). Plasma levels of prothrombin were similar in both age groups (Figure S7).
Figure 6. Human aging is associated with increased thrombin generation.
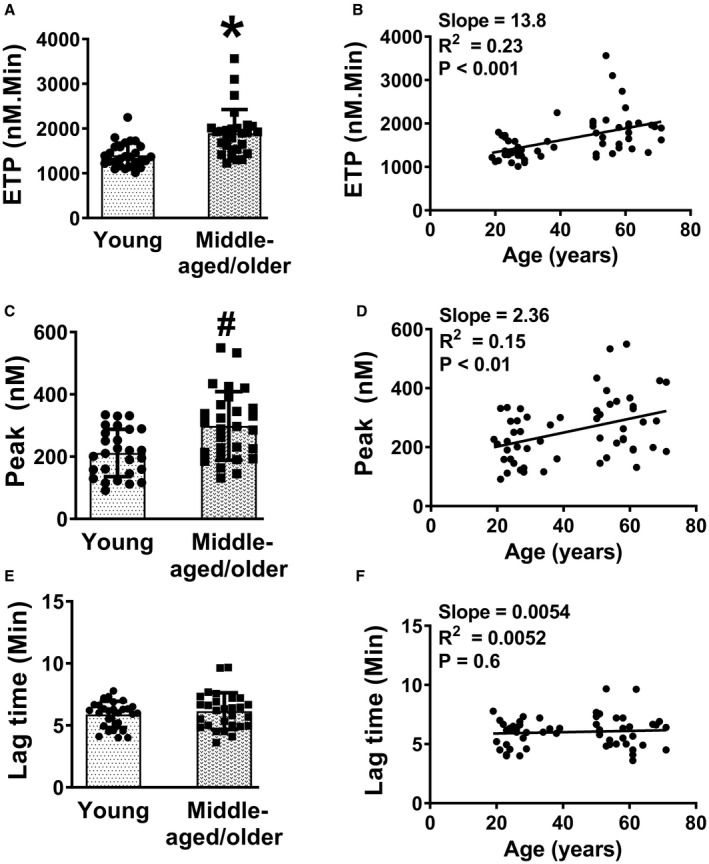
Thrombin generation was measured using platelet poor plasma from young and middle‐aged/older subjects. A, Endogenous thrombin potential (ETP). B, Linear regression analysis of ETP with age. C, Peak thrombin generation. D, Linear regression analysis of peak thrombin generation with age. E, Lag time. F, Linear regression analysis of lag time with age. Data for (A, C, and E) are expressed as mean±SD *P<0.0001 and # P<0.01 vs young subjects. Unpaired t test for (A, C, and E) and linear regression for (B, D, and F). N=27 to 28 in each group.
Similar to our findings in aged mice, we observed an elevation in plasma levels of cfDNA in middle‐aged/older human subjects compared with young subjects (P<0.001, Figure 7A, Table S3) and linear regression analysis revealed a positive relationship between cfDNA and age (slope=0.036, R 2=0.47, P<0.001, Figure 7B). Plasma levels of endogenous DNase 1 did not differ with age (P=0.9, Figure S8, Table S3).
Figure 7. Human aging is associated with elevated levels of cfDNA.
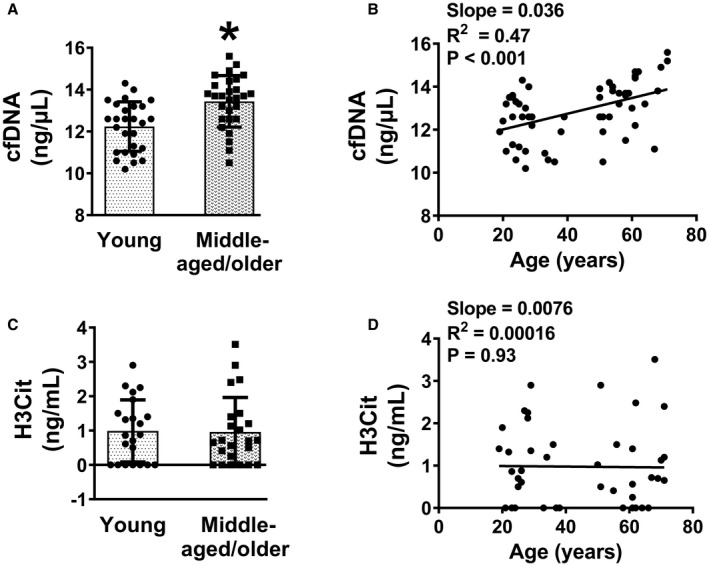
Components of neutrophil extracellular traps were measured in plasma from young and middle‐aged/older subjects. A, Plasma cell‐free DNA (cfDNA). B, Linear regression analysis of cfDNA with age. C, Plasma citrullinated histone (H3Cit). D, Linear regression analysis of H3Cit with age. Data for (A and C) are presented as mean±SD. *P<0.001 vs young subjects. Unpaired t test was used for (A and C) and linear regression analysis for (B and D). N=27 to 28 for (A), 22 to 23 for (C), 55 for (B), and 45 for (D).
In contrast to cfDNA levels, plasma levels of H3Cit were not found to be altered with age in humans (Figure 7C and 7D). In regression analysis, we observed that the elevation in plasma cfDNA with age was also positively associated with ETP (P<0.05, Figure S9). We next utilized an in vitro approach to directly test the susceptibility of neutrophils from young and middle‐aged/older subjects to undergo NETosis. Neutrophils from each group were added to pooled human plasma and incubated with either the vehicle buffer (control) or PMA at 37 °C for 60 minutes to activate neutrophils to release NETs. Similar to findings in mice, we observed PMA‐dependent increases in levels of cfDNA, H3Cit, and ETP in both age groups (main effect of PMA treatment was P<0.05 for H3Cit and P<0.01 for cfDNA and ETP, Figure S10). No age‐dependent increase was observed for any of these parameters upon PMA treatment (main effect of age was P=0.4 for cfDNA, P=0.9 for H3Cit, and P=0.3 for ETP).
Incubation of Human Plasma Samples With DNase 1 Decreased Thrombin Generation Potential
Finally, to determine the role of cfDNA in driving thrombin generation potential ex vivo during human aging, we treated PPP samples with DNase 1 or control (heat‐inactivated DNase 1) before measurement of thrombin generation (Figure 8, Table S3). The comparison for main effect of treatment, age, and the interaction of age and treatment was significant (P<0.0001, P<0.01, and P<0.05 respectively). Furthermore, we observed that treatment with DNase 1 led to 43% reduction in ETP compared with heat‐inactivated DNase 1 for young subjects (P<0.01) and 52% reduction for middle‐aged/older subjects (P<0.0001). These findings suggest that the DNase 1 treatment normalized the differences in ETP between young and middle/aged older mice.
Figure 8. Increased thrombin generation potential in aged humans is likely mediated through cfDNA.
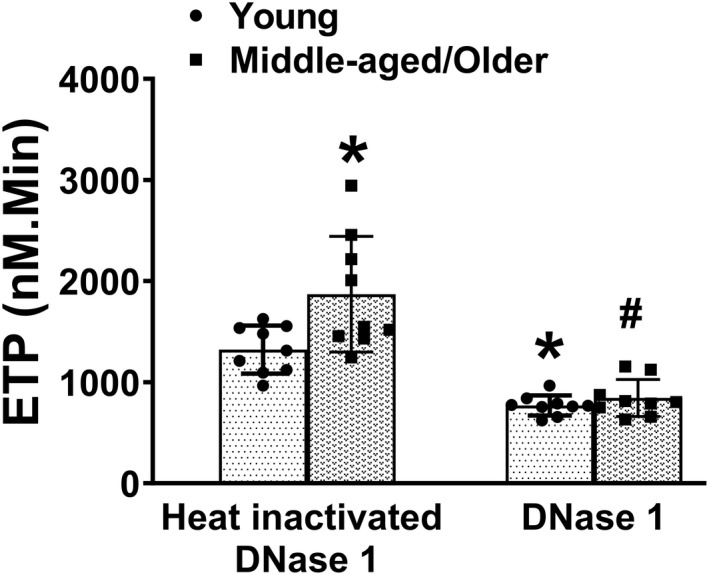
Platelet poor plasma from young and middle aged/older subjects was treated with either 20 µg/mL of DNase 1 or same dose of heat‐inactivated DNase 1 as control for 60 minutes and endogenous thrombin potential (ETP) was measured. Data are presented as mean±SD. *P<0.01 vs young subjects treated with heat‐inactivated DNase 1, # P<0.0001 vs middle aged/older subjects treated with heat‐inactivated DNase 1 (2‐way ANOVA, with Tukey test). N=9 in each group. cfDNA indicates cell‐free DNA.
DISCUSSION
In the present study, we addressed whether NETs contribute to age‐associated increased potential for thrombin generation and thrombosis. Our study reveals 3 major findings. First, we observed that both human and murine aging are associated with an increase in thrombin generation potential as well as elevation in cfDNA, but not in H3Cit, a specific marker of NETosis. Second, we demonstrated that overexpression of Gpx1 provided only modest protection of aged mice from developing increased thrombin generation potential, and the protective effect of Gpx1 was independent of cfDNA. Finally, we observed that treatment with DNase 1 reduced thrombin generation potential in aged mice and in human plasma and blocked the development of venous thrombi in aged mice. Taken together, these findings suggest that aging leads to elevation in cfDNA in a NETosis‐ and peroxide‐independent manner, and treatment with DNase 1 is sufficient to decrease ETP and thrombosis burden.
Aging is a major risk factor for clinical thrombotic events. 1 , 2 , 3 It is associated with altered levels of several procoagulant factors 36 , 37 , 38 that may potentially contribute to a prothrombotic state. In the present study, we measured thrombin generation potential in plasma samples from aged mice and humans. The thrombin generation assay measures the potential of plasma to generate thrombin in response to an extrinsic trigger (tissue factor); the area under the thrombin generation curve over its entire time course is quantitated as the ETP by means of a fluorescently labeled synthetic substrate. In our study, we observed an increase in thrombin generation potential with both human and murine aging, consistent with prior reports in aged humans. 13 , 14 One limitation of our human samples is that the sample size was inadequate to assess for potential differences in ETP that may occur over a broad age range. This will be an important question for future study given that the risk of thrombosis increases with every decade past 50 years of age. 39 , 40 Another limitation is that we could not determine effects of race/ethnicity on thrombin generation in our aged cohort.
One interesting observation in our murine studies was that age‐induced increases in thrombin generation potential were moderately decreased by overexpression of Gpx1. This suggests that the age‐associated increase in thrombin generation is mediated, at least in part, by peroxides. Peroxide levels are known to increase with aging, which is mainly attributable to a decline in anti‐oxidant systems. 41 , 42 These findings are in accordance with our previous work demonstrating that aging is accompanied by peroxide‐dependent increases in experimental thrombosis. 15 The effect of Gpx1 overexpression on thrombin generation was modest, however, and it is likely that peroxides contribute to aging‐related thrombosis primarily via effects on platelet activation rather than thrombin generation. 15
Recently, NETs have emerged as major mediators of increased thrombotic susceptibility in animal models, 30 , 34 and increased markers of NETs have been reported in human patients with thrombosis. 43 , 44 , 45 , 46 Histones and cfDNA have been proposed to enhance thrombin generation through platelet‐dependent and ‐independent mechanisms, respectively. 8 , 35 While histones contribute to thrombin generation by activating platelets through TLR2 and TLR4 leading to surface phosphatidyl serine exposure, cfDNA is known to activate coagulation through the contact activation pathway. In vivo, we observed a low level of plasma H3Cit, a specific marker of NETosis, in young mice or humans, and it did not increase with age. In contrast, we observed age‐dependent increases in plasma cfDNA in both mice and humans. The lack of effect of aging on plasma H3Cit may explain the absence of platelet‐dependent increase in thrombin generation potential with age in mice. In contrast, the profound increase in ETP in PPP in aged mice or humans is consistent with the elevation in cfDNA.
The lack of change in plasma levels of H3Cit with age in our study was unexpected, since recently a group has reported increased NETosis with age in mice 28 or in aged individuals with severe vasculitis. 25 , 26 We considered the possibility that the assay we used may have limited sensitivity to detect small changes in plasma H3Cit. Therefore, since neutrophils are the source of NETs, we used direct in vitro approaches to determine whether neutrophils from aged mice have higher potential to undergo NETosis and whether overexpression of Gpx1 protects from this effect of aging. Upon PMA activation of neutrophils isolated from young and aged WT or Gpx1 Tg mice and seeded on coverslips in the presence of HBSS media, we observed a similar extent of NETosis when visualized by fluorescent microscopy. Furthermore, when neutrophils were suspended in plasma and activated with PMA, we observed a similar elevation in cfDNA, H3Cit, and ETP regardless of age or genotype. Neutrophils from young or aged humans suspended in plasma also showed similar increases in cfDNA, H3Cit, and ETP in both age groups upon PMA activation. These findings suggest that age in the absence of an underlying disease condition is not a major mediator of NETosis in our model. The observed differences in findings may have resulted from the use of somewhat older C57BL6J mice (24–27 months old) in the previous report 28 compared with our use of slightly younger mice (up to 20 months). We have observed that C57BL6J mice beyond 20 months of age often develop inflammatory health conditions, including dermatitis and spontaneous neoplasms that have also been reported by others, 47 so we excluded mice >20 months of age. Recent studies have reported alternative mechanisms of release of extracellular traps, other than reactive oxygen species or histone citrullination, some of which could potentially be important in aging and need to be explored in future study design. 48 , 49 , 50
A previous report has suggested that neutrophils are activated by hydrogen peroxide and release NETs in vitro. 18 However, similar release of NETs from neutrophils in WT or mice overexpressing Gpx1 in our study may suggest that endogenous peroxides do not have a major role in release of NETs during aging. Overall, our findings of elevations in plasma cfDNA with age in the absence of any alterations in plasma H3Cit or PMA‐driven in vitro NETosis suggest that increases in plasma cfDNA in aging may have occurred because of NETosis‐independent mechanisms. We considered the possibility that the changes in endogenous DNase 1 levels with age may mediate the changes in the levels of circulating cfDNA. However, our findings did not suggest any alterations in DNase 1 levels in either mice or humans with age. It may be that the cellular stress mechanisms such as senescence, necrosis, apoptosis, or increased cellular turnover that occur with age 51 may contribute to elevated circulating cfDNA in our model. It is also important to note that the Qubit cfDNA assay we utilized detects both histone‐free and histone‐bound cfDNA. It may be that the elevation in histone‐free cfDNA could be partly mitochondrial in origin 52 and mitochondrial and nuclear DNA may exert differential procoagulant activities. A limitation of our study therefore is that we could not identify the source of cfDNA, and future studies focusing on distinguishing the origin of cfDNA may aid in developing targeted therapies.
cfDNA contributes to thrombin generation by activating FXI and FXII. 8 In vitro treatment with DNase 1 lowers the ETP in plasma samples from patients with sepsis. 8 Similarly, in vivo treatment of mice with DNase 1 lowers thrombin generation potential 53 and protects from thrombotic consequences such as stroke, 54 atherosclerosis, 55 and venous thrombosis. 30 , 34 Therefore, we sought to determine whether the elevated plasma cfDNA observed in aged mice and humans modulates increases in ETP and thrombosis. We observed that treatment of plasma with DNase 1 in vitro significantly decreased ETP in both young and middle aged/older humans, and that either in vitro treatment of plasma or in vivo treatment of mice with DNase 1 decreased ETP in all groups of mice. In these assays, thrombin generation was initiated via a low concentration of tissue factor (1 pmol/L), which triggers the extrinsic pathway of coagulation. Under these conditions, continued thrombin generation is dependent on thrombin‐mediated FXI activation through a feed‐forward mechanism. 56 We speculate, therefore, that elevated plasma cfDNA in aging leads to enhanced thrombin generation by promoting thrombin‐dependent activation of FXI, similar to other negatively charged endogenous surfaces such as polyphosphate. 57 The observation that DNase 1 treatment lowered thrombin generation and normalized the differences between the young and aged cohorts suggests that cfDNA is a likely mediator of increased thrombin generation in aging. A complementary approach in future to consider would be to study mouse model lacking and/or overexpressing DNase 1 under conditions of aging.
Finally, we observed that treatment with DNase 1 also decreased the increased susceptibility to venous thrombosis in aged mice. These findings corroborate the clinical findings where patients with FXI deficiency have lower incidence of deep vein thrombosis and cardiovascular events, 58 , 59 , 60 and FXI is currently a target for novel anticoagulant therapies in development. 61 The fact that after DNase 1 treatment aged mice developed substantially smaller‐sized thrombi than younger mice treated with DNase 1 suggests that venous thrombosis is not only exacerbated by cfDNA during aging but also rather is dependent on it. This differential requirement for cfDNA in young versus aged mice in mediating venous thrombosis in our model is interesting and should be explored in future studies. Some of these effects can also be driven by a potential role of cfDNA in modulating clot structure and delaying fibrinolysis, since in the setting of elevated cfDNA in a cell‐free system, DNase 1 is shown to protect from formation of dense clot structure and in potentiating fibrinolysis. 9 Alternatively, DNase 1 may also exert a protective effect independent of cfDNA in aged mice. 62
With regard to our in vivo findings with DNase 1 treatment, it is important to mention that a study reported that NETs itself is not prothrombotic, 20 but its components such as cfDNA and histones are. This study also showed that degradation of NETs by DNase 1 would release cfDNA and histones to augment thrombin generation. Based on these findings, in our aged mice, if NETosis was indeed occurring in vivo and expelling NETs actively, then treatment with DNase 1 would have worsened the effects on venous thrombosis by releasing procoagulant histones. In fact, we observed that treatment with DNase 1 instead protected from developing venous thrombi in aged mice and is consistent with elevation in circulating cfDNA but not H3Cit in our aging models. Together these findings support the idea that in our model, NETosis is not the mediator of venous thrombosis. Our findings are also consistent with a report where Jiménez‐Alcázar and colleagues, 63 in a prospective cohort study, observed that cfDNA but not NETs was associated with the extent of venous thromboembolism in humans aged >65 years.
In summary, these findings suggest that aging leads to cfDNA‐associated increases in thrombin generation and venous thrombosis. Our findings also suggest that plasma cfDNA and ETP may be considered early markers of a prothrombotic state during aging. Given the emerging evidence that use of DNase 1 is beneficial in experimental thrombotic and inflammatory conditions in mice, 30 , 34 , 64 and the recent use of DNase 1 in patients with Alzheimer disease, 65 cystic fibrosis, 66 or in an ongoing trial to treat patients with SARS‐CoV‐2, 67 our findings underscore the clinical potential of this treatment to target thrombotic consequences in aging.
Sources of Funding
This work was possible through funding from National Institutes of Health AG049784 to Dayal, AG043722 to Pierce, and U54TR001356 to the University of Iowa, Veteran Affairs I01 CX001932‐01 to Dayal, American Heart Association 18IPA34180014 to Dayal and 19POST34380100 to Kumar and University of Iowa pilot Dance Marathon award to Sharathkumar and Dayal.
Disclosures
None.
Supporting information
Acknowledgments
The authors thank Lauren Points, Amy K. Stroud, and Lyndsey E. DuBose for technical assistance with the human samples and Dr Chaorong Wu for statistical assistance. Also, we acknowledge the Diagnostica Stago Inc. (Parsippany NJ) for lending Fluoroskan Ascent instrument and partial supply of reagents for thrombin generation assay.
Author contributions: Kumar designed and conducted the experiments, interpreted the results, and co‐wrote the manuscript. Sonkar assisted with DNase 1 infusion and conducted in vivo thrombosis studies. Swamy also conducted experiments. Sharathkumar aided with thrombin generation assay. Pierce collected, processed, and stored the human samples. Dayal directed the project, designed the experiments, interpreted the results, and wrote the manuscript. All authors assisted with the preparation and editing of the manuscript.
This manuscript was sent to Francis Miller, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021188
For Sources of Funding and Disclosures, see page 12.
REFERENCES
- 1. Howard G, Manolio TA, Burke GL, Wolfson SK, O'Leary DH. Does the association of risk factors and atherosclerosis change with age? An analysis of the combined ARIC and CHS cohorts. The Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health Study (CHS) Investigators. Stroke. 1997;28:1693–1701. doi: 10.1161/01.STR.28.9.1693 [DOI] [PubMed] [Google Scholar]
- 2. Roger VL, Jacobsen SJ, Weston SA, Goraya TY, Killian J, Reeder GS, Kottke TE, Yawn BP, Frye RL. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136:341–348. doi: 10.7326/0003-4819-136-5-200203050-00005 [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al.; American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S . Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 4. Smid M, Dielis AW, Spronk HM, Rumley A, van Oerle R, Woodward M, ten Cate H, Lowe G. Thrombin generation in the Glasgow Myocardial Infarction Study. PLoS One. 2013;8:e66977. doi: 10.1371/journal.pone.0066977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carcaillon L, Alhenc‐Gelas M, Bejot Y, Spaft C, Ducimetiere P, Ritchie K, Dartigues JF, Scarabin PY. Increased thrombin generation is associated with acute ischemic stroke but not with coronary heart disease in the elderly: the Three‐City cohort study. Arterioscler Thromb Vasc Biol. 2011;31:1445–1451. doi: 10.1161/ATVBAHA.111.223453 [DOI] [PubMed] [Google Scholar]
- 6. van Hylckama VA, Baglin CA, Luddington R, MacDonald S, Rosendaal FR, Baglin TP. The risk of a first and a recurrent venous thrombosis associated with an elevated D‐dimer level and an elevated thrombin potential: results of the THE‐VTE study. J Thromb Haemost. 2015;13:1642–1652. doi: 10.1111/jth.13043 [DOI] [PubMed] [Google Scholar]
- 7. Gajos G, Konieczynska M, Zalewski J, Undas A. Low fasting glucose is associated with enhanced thrombin generation and unfavorable fibrin clot properties in type 2 diabetic patients with high cardiovascular risk. Cardiovasc Diabetol. 2015;14:44. doi: 10.1186/s12933-015-0207-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, Liaw PC. Neutrophil extracellular traps promote thrombin generation through platelet‐dependent and platelet‐independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34:1977–1984. doi: 10.1161/ATVBAHA.114.304114 [DOI] [PubMed] [Google Scholar]
- 9. Gould TJ, Vu TT, Stafford AR, Dwivedi DJ, Kim PY, Fox‐Robichaud AE, Weitz JI, Liaw PC. Cell‐free DNA modulates clot structure and impairs fibrinolysis in sepsis. Arterioscler Thromb Vasc Biol. 2015;35:2544–2553. doi: 10.1161/ATVBAHA.115.306035 [DOI] [PubMed] [Google Scholar]
- 10. Leiba M, Malkiel S, Budnik I, Rozic G, Avigdor A, Duek A, Nagler A, Kenet G, Livnat T. Thrombin generation as a predictor of thromboembolic events in multiple myeloma patients. Blood Cells Mol Dis. 2017;65:1–7. doi: 10.1016/j.bcmd.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 11. Ay C, Dunkler D, Simanek R, Thaler J, Koder S, Marosi C, Zielinski C, Pabinger I. Prediction of venous thromboembolism in patients with cancer by measuring thrombin generation: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2011;29:2099–2103. doi: 10.1200/JCO.2010.32.8294 [DOI] [PubMed] [Google Scholar]
- 12. Cushman M, Psaty BM, Macy E, Bovill EG, Cornell ES, Kuller LH, Tracy RP. Correlates of thrombin markers in an elderly cohort free of clinical cardiovascular disease. Arterioscler Thromb Vasc Biol. 1996;16:1163–1169. doi: 10.1161/01.ATV.16.9.1163 [DOI] [PubMed] [Google Scholar]
- 13. Haidl H, Cimenti C, Leschnik B, Zach D, Muntean W. Age‐dependency of thrombin generation measured by means of calibrated automated thrombography (CAT). Thromb Haemost. 2006;95:772–775. doi: 10.1160/TH05-10-0685 [DOI] [PubMed] [Google Scholar]
- 14. Wu J, Zhao HR, Zhang HY, Ge YL, Qiu S, Zhao J, Song Y, Zhao JZ, Lu SS. Thrombin generation increasing with age and decreasing with use of heparin indicated by calibrated automated thrombogram conducted in Chinese. Biomed Environ Sci. 2014;27:378–384. [DOI] [PubMed] [Google Scholar]
- 15. Dayal S, Wilson KM, Motto DG, Miller FJ Jr, Chauhan AK, Lentz SR. Hydrogen peroxide promotes aging‐related platelet hyperactivation and thrombosis. Circulation. 2013;127:1308–1316. doi: 10.1161/CIRCULATIONAHA.112.000966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang J, Zhou X, Fan X, Xiao M, Yang D, Liang BO, Dai M, Shan L, Lu J, Lin Z, et al. mTORC1 promotes aging‐related venous thrombosis in mice via elevation of platelet volume and activation. Blood. 2016;128:615–624. doi: 10.1182/blood-2015-10-672964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McDonald AP, Meier TR, Hawley AE, Thibert JN, Farris DM, Wrobleski SK, Henke PK, Wakefield TW, Myers DD Jr. Aging is associated with impaired thrombus resolution in a mouse model of stasis induced thrombosis. Thromb Res. 2010;125:72–78. doi: 10.1016/j.thromres.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 18. Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noubouossie DF, Whelihan MF, Yu YB, Sparkenbaugh E, Pawlinski R, Monroe DM, Key NS. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood. 2017;129:1021–1029. doi: 10.1182/blood-2016-06-722298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 22. Ueki S, Melo RC, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion‐competent eosinophil granules in humans. Blood. 2013;121:2074–2083. doi: 10.1182/blood-2012-05-432088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. von Kockritz‐Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby‐Teglund A, Rohde M, Medina E. Phagocytosis‐independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018 [DOI] [PubMed] [Google Scholar]
- 24. Morshed M, Hlushchuk R, Simon D, Walls AF, Obata‐Ninomiya K, Karasuyama H, Djonov V, Eggel A, Kaufmann T, Simon H‐U, et al. NADPH oxidase‐independent formation of extracellular DNA traps by basophils. J Immunol. 2014;192:5314–5323. doi: 10.4049/jimmunol.1303418 [DOI] [PubMed] [Google Scholar]
- 25. Matsuda Y, Itabashi M, Tachibana Y, Sugihara T, Sakashita Y, Matsubara T, Murayama S, Yumura W, Shimizu A, Takei T, et al. Citrullinated histone H3 expression in anti‐neutrophil cytoplasmic antibody‐associated vasculitis in older Japanese autopsy patients. Geriatr Gerontol Int. 2019;19:259–264. doi: 10.1111/ggi.13596 [DOI] [PubMed] [Google Scholar]
- 26. Matsuda Y, Hamayasu H, Seki A, Nonaka K, Wang T, Matsumoto T, Hamano Y, Sumikura H, Kumasaka T, Murayama S, et al. Presence of citrullinated histone H3‐positive neutrophils in microscopic polyangiitis from the early phase: an autopsy proven case. Pathol Int. 2016;66:466–471. doi: 10.1111/pin.12434 [DOI] [PubMed] [Google Scholar]
- 27. Jylhava J, Nevalainen T, Marttila S, Jylha M, Hervonen A, Hurme M. Characterization of the role of distinct plasma cell‐free DNA species in age‐associated inflammation and frailty. Aging Cell. 2013;12:388–397. [DOI] [PubMed] [Google Scholar]
- 28. Martinod K, Witsch T, Erpenbeck L, Savchenko A, Hayashi H, Cherpokova D, Gallant M, Mauler M, Cifuni SM, Wagner DD. Peptidylarginine deiminase 4 promotes age‐related organ fibrosis. J Exp Med. 2017;214:439–458. doi: 10.1084/jem.20160530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ogawa K, Suzuki K, Okutsu M, Yamazaki K, Shinkai S. The association of elevated reactive oxygen species levels from neutrophils with low‐grade inflammation in the elderly. Immun Ageing. 2008;5:13. doi: 10.1186/1742-4933-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheng WH, Ho YS, Ross DA, Han Y, Combs GF Jr, Lei XG. Overexpression of cellular glutathione peroxidase does not affect expression of plasma glutathione peroxidase or phospholipid hydroperoxide glutathione peroxidase in mice offered diets adequate or deficient in selenium. J Nutr. 1997;127:675–680. doi: 10.1093/jn/127.5.675 [DOI] [PubMed] [Google Scholar]
- 32. Chrissobolis S, Didion SP, Kinzenbaw DA, Schrader LI, Dayal S, Lentz SR, Faraci FM. Glutathione peroxidase‐1 plays a major role in protecting against angiotensin II‐induced vascular dysfunction. Hypertension. 2008;51:872–877. doi: 10.1161/HYPERTENSIONAHA.107.103572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DuBose LE, Voss MW, Weng TB, Kent JD, Dubishar KM, Lane‐Cordova A, Sigurdsson G, Schmid P, Barlow PB, Pierce GL. Carotid beta‐stiffness index is associated with slower processing speed but not working memory or white matter integrity in healthy middle‐aged/older adults. J Appl Physiol (1985). 2017;122:868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hisada Y, Grover SP, Maqsood A, Houston R, Ay C, Noubouossie DF, Cooley BC, Wallén H, Key NS, Thålin C, et al. Neutrophils and neutrophil extracellular traps enhance venous thrombosis in mice bearing human pancreatic tumors. Haematologica. 2020;105:218–225. doi: 10.3324/haematol.2019.217083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT. Extracellular histones promote thrombin generation through platelet‐dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118:1952–1961. doi: 10.1182/blood-2011-03-343061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tracy RP, Bovill EG, Fried LP, Heiss G, Lee MH, Polak JF, Psaty BM, Savage PJ. The distribution of coagulation factors VII and VIII and fibrinogen in adults over 65 years. Results from the Cardiovascular Health Study. Ann Epidemiol. 1992;2:509–519. doi: 10.1016/1047-2797(92)90100-5 [DOI] [PubMed] [Google Scholar]
- 37. Tofler GH, Massaro J, Levy D, Mittleman M, Sutherland P, Lipinska I, Muller JE, D'Agostino RB. Relation of the prothrombotic state to increasing age (from the Framingham Offspring Study). Am J Cardiol. 2005;96:1280–1283. doi: 10.1016/j.amjcard.2005.06.072 [DOI] [PubMed] [Google Scholar]
- 38. Mari D, Coppola R, Provenzano R. Hemostasis factors and aging. Exp Gerontol. 2008;43:66–73. doi: 10.1016/j.exger.2007.06.014 [DOI] [PubMed] [Google Scholar]
- 39. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ III. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study. Arch Intern Med. 1998;158:585–593. doi: 10.1001/archinte.158.6.585 [DOI] [PubMed] [Google Scholar]
- 40. Wolf PA, Lewis A, Lecture C. Contributions of epidemiology to the prevention of stroke. Circulation. 1993;88:2471–2478. doi: 10.1161/01.CIR.88.5.2471 [DOI] [PubMed] [Google Scholar]
- 41. Rey C, Vericel E, Nemoz G, Chen W, Chapuy P, Lagarde M. Purification and characterization of glutathione peroxidase from human blood platelets. Age‐related changes in the enzyme. Biochim Biophys Acta. 1994;1226:219–224. doi: 10.1016/0925-4439(94)90032-9 [DOI] [PubMed] [Google Scholar]
- 42. Kishido T, Unno K, Yoshida H, Choba D, Fukutomi R, Asahina S, Iguchi K, Oku N, Hoshino M. Decline in glutathione peroxidase activity is a reason for brain senescence: consumption of green tea catechin prevents the decline in its activity and protein oxidative damage in ageing mouse brain. Biogerontology. 2007;8:423–430. doi: 10.1007/s10522-007-9085-7 [DOI] [PubMed] [Google Scholar]
- 43. Lee KH, Cavanaugh L, Leung H, Yan F, Ahmadi Z, Chong BH, Passam F. Quantification of NETs‐associated markers by flow cytometry and serum assays in patients with thrombosis and sepsis. Int J Lab Hematol. 2018;40:392–399. doi: 10.1111/ijlh.12800 [DOI] [PubMed] [Google Scholar]
- 44. Laridan E, Denorme F, Desender L, Francois O, Andersson T, Deckmyn H, Vanhoorelbeke K, De Meyer SF. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;82:223–232. doi: 10.1002/ana.24993 [DOI] [PubMed] [Google Scholar]
- 45. Hofbauer TM, Mangold A, Scherz T, Seidl V, Panzenböck A, Ondracek AS, Müller J, Schneider M, Binder T, Hell L, et al. Neutrophil extracellular traps and fibrocytes in ST‐segment elevation myocardial infarction. Basic Res Cardiol. 2019;114:33. doi: 10.1007/s00395-019-0740-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Langseth MS, Opstad TB, Bratseth V, Solheim S, Arnesen H, Pettersen AA, Seljeflot I, Helseth R. Markers of neutrophil extracellular traps are associated with adverse clinical outcome in stable coronary artery disease. Eur J Prev Cardiol. 2018;25:762–769. doi: 10.1177/2047487318760618 [DOI] [PubMed] [Google Scholar]
- 47. Kohnken R, Porcu P, Mishra A. Overview of the use of murine models in leukemia and lymphoma research. Front Oncol. 2017;7:22. doi: 10.3389/fonc.2017.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, Menninger S, Eickhoff J, Nussbaumer P, Klebl B, et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol. 2018;3:eaar6689. doi: 10.1126/sciimmunol.aar6689 [DOI] [PubMed] [Google Scholar]
- 49. Kenny EF, Herzig A, Kruger R, Muth A, Mondal S, Thompson PR, Brinkmann V, Bernuth HV, Zychlinsky A. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife. 2017;6:e24437. doi: 10.7554/eLife.24437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Claushuis TAM, van der Donk LEH, Luitse AL, van Veen HA, van der Wel NN, van Vught LA, Roelofs J, de Boer OJ, Lankelma JM, Boon L, et al. Role of peptidylarginine deiminase 4 in neutrophil extracellular trap formation and host defense during Klebsiella pneumoniae‐induced pneumonia‐derived sepsis. J Immunol. 2018;201:1241–1252. [DOI] [PubMed] [Google Scholar]
- 51. Teo YV, Capri M, Morsiani C, Pizza G, Faria AMC, Franceschi C, Neretti N. Cell‐free DNA as a biomarker of aging. Aging Cell. 2019;18:e12890. doi: 10.1111/acel.12890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, Benatti S, Gibellini L, Cotichini R, Stazi MA, et al. Circulating mitochondrial DNA increases with age and is a familiar trait: implications for "inflamm‐aging". Eur J Immunol. 2014;44:1552–1562. doi: 10.1002/eji.201343921 [DOI] [PubMed] [Google Scholar]
- 53. Wang Y, Luo L, Braun OO, Westman J, Madhi R, Herwald H, Morgelin M, Thorlacius H. Neutrophil extracellular trap‐microparticle complexes enhance thrombin generation via the intrinsic pathway of coagulation in mice. Sci Rep. 2018;8:4020. doi: 10.1038/s41598-018-22156-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. De Meyer SF, Suidan GL, Fuchs TA, Monestier M, Wagner DD. Extracellular chromatin is an important mediator of ischemic stroke in mice. Arterioscler Thromb Vasc Biol. 2012;32:1884–1891. doi: 10.1161/ATVBAHA.112.250993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yamamoto K, Yamada H, Wakana N, Kikai M, Terada K, Wada N, Motoyama S, Saburi M, Sugimoto T, Kami D, et al. Augmented neutrophil extracellular traps formation promotes atherosclerosis development in socially defeated apoE(‐/‐) mice. Biochem Biophys Res Commun. 2018;500:490–496. doi: 10.1016/j.bbrc.2018.04.115 [DOI] [PubMed] [Google Scholar]
- 56. Matafonov A, Sarilla S, Sun MF, Sheehan JP, Serebrov V, Verhamme IM, Gailani D. Activation of factor XI by products of prothrombin activation. Blood. 2011;118:437–445. doi: 10.1182/blood-2010-10-312983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Choi SH, Smith SA, Morrissey JH. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood. 2011;118:6963–6970. doi: 10.1182/blood-2011-07-368811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Preis M, Hirsch J, Kotler A, Zoabi A, Stein N, Rennert G, Saliba W. Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood. 2017;129:1210–1215. doi: 10.1182/blood-2016-09-742262 [DOI] [PubMed] [Google Scholar]
- 59. Salomon O, Steinberg DM, Koren‐Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111:4113–4117. doi: 10.1182/blood-2007-10-120139 [DOI] [PubMed] [Google Scholar]
- 60. Salomon O, Steinberg DM, Zucker M, Varon D, Zivelin A, Seligsohn U. Patients with severe factor XI deficiency have a reduced incidence of deep‐vein thrombosis. Thromb Haemost. 2011;105:269–273. doi: 10.1160/TH10-05-0307 [DOI] [PubMed] [Google Scholar]
- 61. Buller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE, Segers A, Verhamme P, Weitz JI; Investigators F‐AT . Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372:232–240. doi: 10.1056/NEJMoa1405760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Carminita E, Crescence L, Brouilly N, Altie A, Panicot‐Dubois L, Dubois C. DNAse‐dependent, NET‐independent pathway of thrombus formation in vivo. Proc Natl Acad Sci USA. 2021;118:e2100561118. doi: 10.1073/pnas.2100561118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jiménez‐Alcázar M, Limacher A, Panda R, Méan M, Bitterling J, Peine S, Renné T, Beer JH, Aujesky D, Lämmle B, et al. Circulating extracellular DNA is an independent predictor of mortality in elderly patients with venous thromboembolism. PLoS One. 2018;13:e0191150. doi: 10.1371/journal.pone.0191150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang S, Xie T, Sun S, Wang K, Liu B, Wu X, Ding W. DNase‐1 treatment exerts protective effects in a rat model of intestinal ischemia‐reperfusion injury. Sci Rep. 2018;8:17788. doi: 10.1038/s41598-018-36198-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tetz V, Tetz G. Effect of deoxyribonuclease I treatment for dementia in end‐stage Alzheimer's disease: a case report. J Med Case Rep. 2016;10:131. doi: 10.1186/s13256-016-0931-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liao TH. Deoxyribonuclease I and its clinical applications. J Formos Med Assoc. 1997;96:481–487. [PubMed] [Google Scholar]
- 67. Medicine. NUSNLo . ClinicalTrials.gov: Dornase Alfa for ARDS in patients with SARS‐CoV‐2 (DORNASESARS2). Available at: https://clinicaltrials.gov/ct2/show/NCT04402970. Accessed May 27, 2020.
- 68. Swamydas M, Luo Y, Dorf ME, Lionakis MS. Isolation of mouse neutrophils. Curr Protoc Immunol. 2015;110:3.20.1–3.20.15. doi: 10.1002/0471142735.im0320s110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Clark RA, Nauseef WM. Isolation and functional analysis of neutrophils. Curr Protoc Immunol. 2001;Chapter 7:Unit 7.23. [DOI] [PubMed] [Google Scholar]
- 70. Hemker HC. Calibrated automated thrombinography (CAT). Thromb Res. 2005;115:255. doi: 10.1016/j.thromres.2004.06.042 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


