Abstract
Background
Suboptimal cardiovascular health (CVH) and social determinants of health (SDOH) have a significant impact on maternal morbidity and mortality. We aimed to evaluate the association of SDOH with suboptimal CVH among pregnant women in the United States.
Methods and Results
We examined cross‐sectional data of pregnant women aged 18 to 49 years from the National Health Interview Survey (2013–2017). We ascertained optimal and suboptimal CVH based on the presence of 0 to 1 and ≥2 risk factors (hypertension, diabetes, hyperlipidemia, current smoking, obesity, and insufficient physical activity), respectively. We calculated an aggregate SDOH score representing 38 variables from 6 domains (economic stability; neighborhood, physical environment, and social cohesion; community and social context; food; education; and healthcare system) and divided into quartiles. We used Poisson regression model to evaluate the association of SDOH with suboptimal CVH and risk factors. Our study included 1433 pregnant women (28.8±5.5 years, 13% non‐Hispanic Black). Overall, 38.4% (95% CI, 33.9–43.0) had suboptimal CVH versus 51.7% (95% CI, 47.0–56.3) among those in the fourth SDOH quartile. Risk ratios of suboptimal CVH, smoking, obesity, and insufficient physical activity were 2.05 (95% CI, 1.46–2.88), 8.37 (95% CI, 3.00–23.43), 1.54 (95% CI, 1.17–2.03), and 1.19 (95% CI, 1.01–1.42), respectively among those in the fourth SDOH quartile compared with the first quartile.
Conclusions
Over 50% of pregnant women with the highest SDOH burden had suboptimal CVH, highlighting the public health urgency for interventions in socially disadvantaged pregnant women with renewed strategies toward improving modifiable risk factors, especially smoking and insufficient physical activity.
Keywords: cardiovascular disease, cardiovascular health, cardiovascular risk factors, maternal health, pregnancy, social determinants of health
Subject Categories: Cardiovascular Disease, Pregnancy
Nonstandard Abbreviations and Acronyms
- APO
adverse pregnancy outcomes
- CVH
cardiovascular health
- SDOH
social determinants of health
Clinical Perspective
What Is New?
Adverse social determinants of health (SDOH) are associated with maternal mortality and morbidity. The association of cumulative SDOH with cardiovascular risk factors such as hypertension, smoking, obesity, dyslipidemia, and physical inactivity during pregnancy is not well understood.
We examined the association of aggregate SDOH risk across 6 domains with cardiovascular health during pregnancy in a large nationally representative sample and found that >50% of women with the most adverse SDOH profile had suboptimal cardiovascular health (≥2 risk factors) during pregnancy.
What Are the Clinical Implications?
Adoption of a polysocial risk assessment tool can identify women during pregnancy with suboptimal cardiovascular health.
Health policy and public health interventions need urgent implementation of strategies to mitigate the impact of SDOH on cardiovascular health of young women. This is critical for sustainable preventive efforts toward reducing cardiovascular risk factors burden in pregnant women.
Maternal mortality in the United States has increased steadily from 7 per 100 000 live births in 1987 to nearly 17 per 100 000 live births in 2016. 1 Cardiovascular disease (CVD) accounts for about one third of all pregnancy‐related deaths and remains the leading cause of death during pregnancy. 1 , 2 Additionally, over the past 2 decades, maternal cardiovascular risk factors such as hypertension, 3 diabetes, 4 , 5 , 6 and obesity 7 have also increased, suggesting a higher at‐risk birthing population. Concurrently, there has been a proportional increase in adverse pregnancy outcomes (APOs) such as preeclampsia, 8 gestational diabetes, 9 preterm delivery, 10 and small‐for‐gestational age infants 11 with growing evidence suggesting their strong association with severe maternal morbidity and premature coronary artery disease, heart failure, and stroke. 12 , 13 , 14 , 15 , 16 , 17
There are well‐recognized racial and ethnic disparities in maternal outcomes, with non‐Hispanic Black women being 3 to 4 times more likely to die from pregnancy‐related causes as compared with White women. 18 Sociodemographic disadvantage—measured collectively as social determinants of heath (SDOH) burden—is strongly associated with poor maternal health and is a major driver of disparities in maternal outcomes. 17 , 19 , 20 , 21 SDOH encompass income, education, occupational status, neighborhood environment, food insecurity, and a variety of health system factors that are tied to poor maternal outcomes; their inclusion into existing care delivery paradigms has provided unique opportunities for personalized medical care, improved the spectrum of patient care, and advanced health equity. 22 , 23 , 24
Socioeconomic factors are potent determinants of cardiovascular risk and contribute to the poor outcomes in patients with existing CVD. 25 , 26 The American Heart Association and the American College of Obstetricians and Gynecologists have recognized the importance of preserving women’s cardiovascular health (CVH) across the life course. 27 The state of disparities in CVH has been studied extensively in the general US population. 28 , 29 However, few studies have examined the link between SDOH and CVH during pregnancy. 30
To date, studies conducted in pregnant women on the association of CVD risk factors focus on only 1 determinant or 1 class of determinant such as lower socioeconomic status, rural location, or immigrant status and/or their influence on pregnancy outcome (when considered, the other determinants are treated as potential confounding variables). 31 , 32 So, the distribution of SDOH and how they aggregate and how they potentially accumulate in the population of pregnant women is still largely unknown. Considering simultaneously all of SDOH could help define a descriptive approach was never conducted in a large population‐based study of pregnant women. Based on prior studies on CVD risk factors in pregnant women with lower socioeconomic status, we expect to find higher prepregnancy hypertension, obesity, and smoking. 30 To date, the association of cumulative SDOH burden on CVH has not been evaluated in pregnant women. Accordingly, we sought to examine the association between SDOH and suboptimal CVH in a nationally representative sample of pregnant women in the United States.
Methods
The data that support the findings of this study are available from the corresponding author upon request.
Data Source
We used the National Health Interview Survey (NHIS), a cross‐sectional database that is updated annually by the National Center for Health Statistics/Centers for Disease Control and Prevention and is the principal source of information on the health of noninstitutionalized US population. 33 The NHIS operationalizes complex, multistage probability sampling, incorporating stratification, clustering, and oversampling, to provide representative estimates for the noninstitutionalized US population. 34 The NHIS questionnaire is composed of 4 core sections: Household Composition, Family Core, Sample Child Core, and Adult Sample Core. The Household Composition file collects information on individuals living under the same household and the Family Core collects sociodemographic characteristics per family, such as general health indicators, physical limitations, injuries, and insurance coverage.
From each family, 1 child and 1 adult (Sample Child and Adult Core files, respectively) are selected randomly for a more in‐depth questionnaire to gain insights about the health behaviors and specific disease‐related information, healthcare barriers, and associated financial constraints. We used the Sample Adult Core questionnaire as its base, further supplemented with information from the Household Composition and Family Core files. This study was exempt from the institutional review board given the de‐identified and public availability nature of data. 35
Study Design and Population
We performed a cross‐sectional analysis of pooled data from the NHIS between 2013 and 2017. Our study population included pregnant women aged 18 to 49 years (Figure 1).
Figure 1. Flow diagram indicating total population of individuals eligible for study inclusion from the National Health Interview Survey between 2013 and 2017 and eligibility criteria for final sample of pregnant women 18 to 49 years of age.
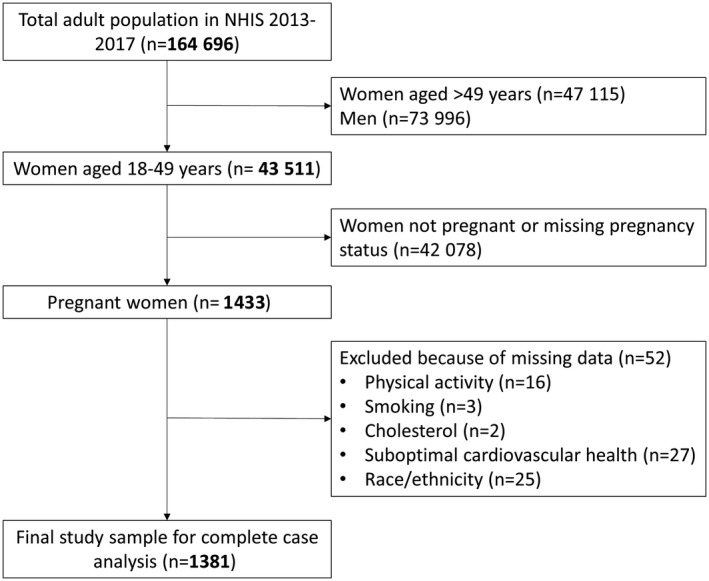
NHIS indicates National Health Interview Survey.
Ascertainment of Pregnancy Status
Consistent with prior studies, pregnancy status was ascertained using self‐report of pregnancy at the time of survey. 36
Ascertainment of SDOH Aggregate Score
We designed a comprehensive measure of SDOH using a list of 38 individual subcomponents across 6 domains based on Kaiser Family Foundation’s model and other variables published in literature that have been demonstrated to affect CVH (Table S1). 37 Final SDOH score included the following domains and variables: economic stability: employment, income, and financial burden (inability to adhere with treatment and delaying and/or foregoing health care because of cost); neighborhood, physical environment, and social cohesion: house tenure and neighborhood quality; community and social context: psychological distress (feeling sad, restless/fidgety, nervous, hopeless, worthless, and everything as an effort); education: English language proficiency, highest education attained, and health literacy (use of health information technology); food: food insecurity; and health care: insurance status, usual source of care, delayed/forgone care in accessing health care, and quality of health care. For factors with binary response, we assigned a value of 0 if the response to a factor was favorable (eg, presence of health insurance) and 1 if otherwise (eg, absence of health insurance). For factors with multiple response and those assessed using multiple items (eg, 10‐item questionnaire for food insecurity), we categorized the responses as favorable (factor value “0”) or unfavorable (factor value “1”) using cutoffs per previous studies. 38
For each participant, an aggregate SDOH score was calculated, representing the total number of unfavorable SDOH across the 6 domains with a minimum of 0 and a maximum of 38 (Table S1). To assess the distribution of our SDOH scores we constructed a histogram that is included as Figure S1. Owing to the largely positive skew of our data as shown in the histogram and the fact that a very small proportion of observations were found below SDOH score of 3 (2.24%) and above SDOH score of 20 (3.78%), we treated individuals below SDOH score of 3 as having an SDOH score of 3 and those above 20 as having a score of 20. To assess for a linear relationship between SDOH score and our outcomes of interest, we constructed b‐splines (Figure S2). For outcomes whose relationship with SDOH scores was not clear (high cholesterol) or were linear (suboptimal CVH, obesity, and insufficient physical activity), we modeled SDOH using quartiles for easy interpretation and better comparison. Conversely, for outcomes whose relationship with SDOH score was not linear (hypertension and smoking) we used the inflection points of our splines as cutoffs for the purposes of our regression models. Quartiles were created as follows: first (score 3 to 6); second (score 7 to 9); third (score 10 to 13); and fourth (score ≥14). The first quartile was defined as the most favorable SDOH profile, whereas the fourth quartile was defined as the most unfavorable SDOH profile.
Ascertainment of CVH Status
CVH was determined using self‐reported traditional cardiovascular risk factors except diet. 39 Diabetes, hypertension, and hypercholesterolemia were ascertained based on self‐report. Current smoking status was self‐reported and obesity was assessed based on body mass index ≥30 kg/m2 during pregnancy. Women were determined to have insufficient physical activity during pregnancy if not engaging in ≥75 min/week of vigorous‐intensity activity, ≥150 min/week moderate‐intensity activity or combination, or a total combination of ≥150 minutes per week of moderate/vigorous‐intensity aerobic physical activity. Diet was not included to determine CVH status because the NHIS survey did not collect dietary information. Individuals were stratified into 2 mutually exclusive groups of CVH: suboptimal (≥2 cardiovascular risk factors) and optimal CVH (0–1 cardiovascular risk factors).
Statistical Analysis
We used survey‐specific descriptive statistics to obtain weighted national estimates for SDOH, CVH, and other demographic/clinical participant characteristics. We reported categorical variables as numbers and proportions and continuous variables as means and SDs. We estimated age‐adjusted prevalence of suboptimal CVH and cardiovascular risk factors (except diabetes owing to small sample size), overall and across the SDOH aggregate score quartiles. We used direct age‐adjustment using the standard US Census 2000 population. 40 We performed Bayesian regression with b‐splines to assess linearity between SDOH scores and (1) cardiovascular risk factors and (2) CVH. We performed multivariable Poisson regression analyses, adjusting for age and race and ethnicity, to test the association between SDOH and (1) cardiovascular risk factors and (2) CVH. We modeled the association between SDOH and individual cardiovascular risk factors or CVH using quartiles (for obesity, insufficient physical activity, high cholesterol, and suboptimal cardiovascular health) or cutoffs based on the inflection points from our b‐splines (for hypertension and smoking).
All statistical analyses were survey‐specific using person weights and variance estimation to account for complex survey design of NHIS. Variance estimation for the entire pooled cohort was obtained from the Integrated Public Use Microdata Series (http://www.ipums.org). 41 All analyses were performed using Stata®, version 16 (StataCorp, LP, College Station, TX, USA).
Results
Our study included 1433 pregnant women (mean age, 28.8±5.5 years), representing 2.2 million pregnant women in the United States annually. Characteristics of the study participants by pregnancy status are described in Table S2. The unadjusted prevalence of suboptimal CVH was 33.6% among pregnant women, corresponding to 752 289 women in the United States annually. Overall, the unadjusted prevalence of insufficient physical activity (59.8% versus 46.7%) and obesity (38.4% versus 31%) was higher in pregnant versus nonpregnant women. In contrast, prevalence of hypertension (12.9% versus 8.7%), diabetes (3.5% versus 1.6%), high cholesterol (10.7% versus 4.4%), and smoking (15.4% versus 8.6%) were higher in nonpregnant women versus pregnant women. Pregnant women were more likely to be unemployed, have low family income, rent or make other arrangements for housing, and believe that people in the neighborhood did not help each other and cannot be trusted (Table S3).
The age‐adjusted prevalence of suboptimal CVH and cardiovascular risk factors by SDOH score quartiles among pregnant women are demonstrated in Figure 2, respectively. Age‐adjusted prevalence of suboptimal CVH was 38.4% (95% CI, 33.9–43.0) in pregnant women. Suboptimal CVH was present in 51.7% (95% CI, 47.0–56.3) of the pregnant women in the fourth SDOH quartile when compared with 27.4% (95% CI, 23.1–31.8) among those in the first SDOH quartile. Insufficient physical activity was the most common risk factor at 63.1% (95% CI, 58.5–67.6) followed by obesity at 39% (95% CI, 34.1–43.9), hypertension at 16.4% (95% CI, 13.4–19.4), smoking at 11.8% (95% CI, 8.6–15), and hyperlipidemia at 9.9% (95% CI, 5.6–14.3). The prevalence of hyperlipidemia, smoking, obesity, and insufficient physical activity was the highest among pregnant women in the fourth SDOH quartile when compared with those in the first SDOH quartile.
Figure 2. Age‐adjusted prevalence of cardiovascular risk factors and suboptimal CVH among pregnant women in the United States, overall and by SDOH quartiles.
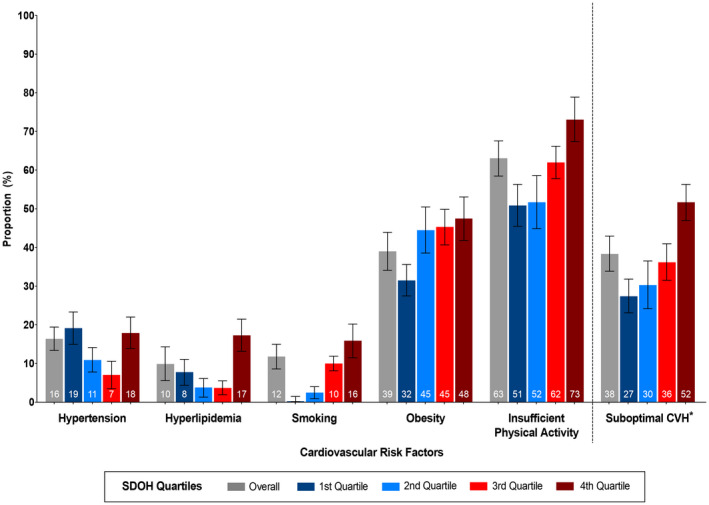
*Suboptimal CVH defined as ≥2 cardiovascular risk factors (hypertension, diabetes, hyperlipidemia, smoking, obesity, and insufficient physical activity). CVH indicates cardiovascular health; and SDOH, social determinants of health.
Figure 3 demonstrates the adjusted risk ratios (RRs) of cardiovascular risk factors and suboptimal CVH across SDOH quartiles. In adjusted Poisson regression models, there was a stepwise increase in the RRs of suboptimal CVH with increasing quartiles of SDOH score. Pregnant women in the third SDOH quartile had RR 1.82 (95% CI, 1.28–2.60) and fourth SDOH quartile had RR 2.05 (95% CI, 1.46–2.88) compared with those in the first SDOH quartile. A similar stepwise increase in the RR of smoking was noted with increasing quartiles of SDOH scores; pregnant women in the third SDOH quartile had RR 5.37 (95% CI, 1.84–15.62) and fourth SDOH quartile had RR 8.37 (95% CI, 3.00–23.43) compared with those in the first SDOH quartile. Pregnant women in the fourth quartile also had higher RR of obesity (RR, 1.54; 95% CI, 1.17–2.03) and insufficient physical activity (RR, 1.19; 95% CI, 1.01–1.42), compared with those in the first quartile.
Figure 3. Associations of SDOH with cardiovascular risk factors and suboptimal CVH among pregnant women in the United States.
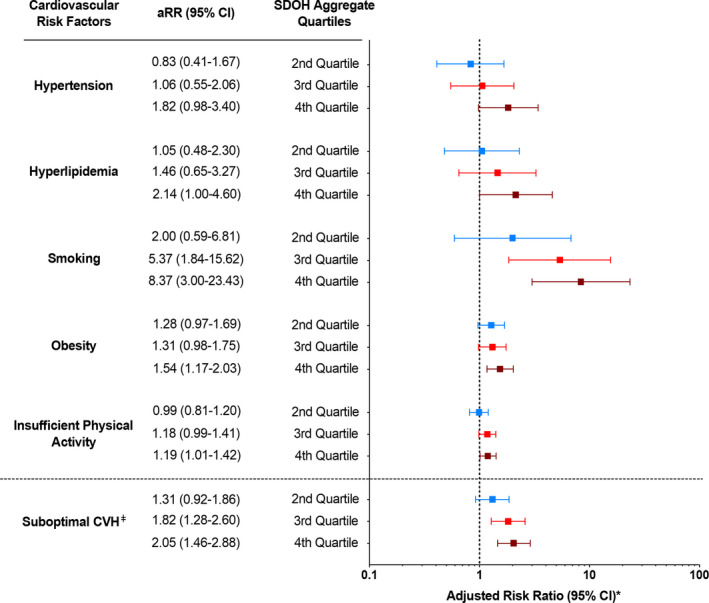
*Adjusted for age and race and ethnicity with first SDOH quartile as the reference; ǂSuboptimal CVH defined as ≥2 cardiovascular risk factors (hypertension, diabetes, hyperlipidemia, smoking, obesity, and insufficient physical activity). aRR indicates adjusted risk ratio; CVH, cardiovascular health; and SDOH, social determinants of health.
Discussion
We demonstrated that one third of pregnant women had suboptimal CVH. Among pregnant women with the most unfavorable SDOH score quartile, more than half had suboptimal CVH and 2.1‐fold higher relative risk of suboptimal CVH compared with those in the most favorable SDOH quartiles. Those in the most unfavorable SDOH score quartile had 8.4‐fold, 1.5‐fold, and 1.2‐fold higher relative risk of smoking, obesity, and insufficient physical activity, respectively, compared with those in the most favorable SDOH quartile.
To our knowledge, this is the first study to examine the association between SDOH, traditional cardiovascular risk factors, and overall CVH in a large, nationally representative sample of pregnant women in the United States. These data show an increasing prevalence of cardiovascular risk factors and suboptimal CVH with worsening SDOH burden. Given the rise in maternal morbidity and mortality in the United States, 1 these findings are relevant, underscoring the burden of adverse risk factor profile during pregnancy and the impact of SDOH on overall CVH.
Underlying CVH is an important predictor of maternal and fetal outcome. 30 Traditional measures of socioeconomic status, such as education, income, and occupation, have been explored extensively with regard to their relationship to CVH. Lower socioeconomic status is associated with a greater prevalence of cardiovascular risk factors and a higher mortality resulting from CVD among pregnant women. 26 SDOH, including education, occupation, income, wealth, social class, ethnicity, family structure, or living arrangements are fundamental “upstream” determinants of health and disease. These characteristics create conditions or circumstances that shape “downstream” risk factors (eg, smoking, alcohol and drug use, unhealthy diet, lack of physical activity, obesity, hypertension, and diabetes), which can predispose to CVD morbidity and mortality.
We examined the association between SDOH and suboptimal CVH in a vulnerable population. Significant demographic and socioeconomic disparities continue to exist in maternal care and impact outcomes. 21 , 42 , 43 , 44 Previous studies have established the association between unfavorable socioeconomic position or individual factors as such as minority race and ethnicity, physical circumstances such as segregated neighborhood environment, public or no insurance coverage, and lower education levels and increased incidence of maternal death and maternal morbidity. 21 Nationally, the pregnancy‐related mortality among non‐Hispanic Black women is 42.8 deaths per 100 000 live births and is higher in rural areas as compared with urban areas. 43 , 44
Maternal cardiovascular risk factors studied here such as hypertension, obesity, physical inactivity, and smoking are well‐recognized risk factors for APOs. 8 , 11 , 14 Although we did not specifically examine racial or ethnic and place‐based disparities as well as the impact of suboptimal CVH on APOs herein, prior studies have demonstrated a higher prevalence of gestational hypertension, preeclampsia, and eclampsia 1 in non‐Hispanic Black women, compared with White women (odds ratio [OR], 2.85; 95% CI, 1.38–5.53). 43 Future studies establishing the impact of SDOH, maternal CVH, and APOs are needed.
Previous studies have explored the impact of SDOH on CVH and health behaviors in pregnancy. 45 , 46 , 47 , 48 , 49 , 50 Self‐reported birth certificate data have indicated that 7.2% of mothers smoked at one point during pregnancy, with the prevalence of smoking decreasing with increasing education among women with a completed high school education or higher education. 46 Some studies have linked 3 indicators of unfavorable SDOH such as low income, education, and unemployment with increased prenatal smoking behavior. 45 Our study also demonstrates a higher prevalence of smoking (16.2%) in those with the most unfavorable (fourth quartile) of SDOH. Interventions on smoking cessation are multipronged and should incorporate recommendations by American College of Obstetricians and Gynecologists. 51 Clinicians should inquire about all types of tobacco or nicotine use, including cigarette smoking, use of e‐cigarettes or vaping products, hookahs, snus, lozenges, patches, and gum, during the prepregnancy, pregnancy, and postpartum periods. They should also individualize care by offering psychosocial, behavioral, and pharmacotherapy interventions, with the use of cessation‐aid services and resources, including digital resources, aimed at education of women regarding the perinatal risks associated with tobacco use, including orofacial clefts, fetal growth restriction, placenta previa, preterm prelabor rupture of membranes, small for gestation age infant, increased perinatal mortality, ectopic pregnancy, and decreased maternal thyroid function. 51
Physical activity during pregnancy has been reported to be suboptimal in previous studies with a significant decline in later trimesters. 47 One study showed that higher educational level during pregnancy was associated with higher exercise (OR, 1.82; 95% CI, 1.28–2.60) 48 . Our study demonstrates that insufficient physical activity in pregnancy was a common risk factor (63.1%) in the overall population, with the highest prevalence (74%) among participants with the most unfavorable SDOH risk profile.
Obesity—in the form of prepregnancy body mass index and gestational weight gain is associated with poor pregnancy outcomes such as cesarean sections and preeclampsia. 7 Prepregnancy obesity is associated with lower maternal socioeconomic status. 49 Recent data show that one half of women who gave birth in the United States were significantly overweight or had obesity before becoming pregnant. 50 Mothers who were older; had less education; were non‐Hispanic Black, non‐Hispanic American Indian, or Alaska Native; and had Medicaid as the principal source of payment for the delivery were more likely to have obesity before pregnancy. 7 Our study found that approximately 2 in every 5 pregnant women were obese. Our findings are especially important considering the growing prevalence of obesity and its impact on pregnancy outcomes.
Implications for Patient Care and Public Policy
Detailed discussion on the health policy changes is outside the purview of this article but we would like to briefly address the domains of the SDOH and hope that they are recognized at clinician level with improved educational curricula aimed at medical school level. Addressing the various domains of SDOH requires a broad range of actions that involve collaboration of multiple sectors (eg, education, justice, and employment) and local, provincial, and federal levels of government, physicians and other allied healthcare workers.
We examined the burden of SDOH on individual cardiovascular risk factors and suboptimal CVH, providing insights into their potential impact on CVH of young mothers. The impact of poor maternal CVH lasts beyond pregnancy. A study of 2302 multinational mother–child dyads showed that poorer maternal CVH at a mean of 28 weeks’ gestation was significantly associated with higher risks for poorer offspring CVH at ages 10 to 14 years. 52 Although the approach to addressing these inequities on maternal and child health is complex and multipronged, it is important to recognize the broad pillars of interventions at the patient, provider, health system, and public health policy levels.
First, regionalization of health care is critical toward large institutions expanding their outreach and providing high‐value and high‐quality health services to underserved and high‐risk populations, including maternal and child health services for vulnerable pregnant women. 19 , 20 , 21 Second, there is a need for expansion of prepregnancy assessment of cardiovascular risk factors, such as implementing cholesterol screening during the first trimester before expected changes in lipid metabolism and their management during pregnancy. 36 , 52 , 53 Additionally, there needs to be improved education of clinicians about racial and ethnic disparities and impact of SDOH on maternal outcomes, cultural competency, and better reporting of the outcomes and quality of care in those with unfavorable SDOH through electronic health records. 17 , 54 , 55
Third, state policy efforts should target opportunities for improved access to preventive services in the postpartum period, so all women and especially those with unfavorable SDOH continue to have follow‐up for interpregnancy care. 19 , 20 , 21 , 26 , 56 Lastly, nationwide efforts should address the challenge of maternal mortality and morbidity in the context of SDOH, which are interconnected and have cascading downstream effects that require multisector interventions to improve health. Prioritizing improving healthcare access and quality, education, and adverse social and community contexts such as discrimination and economic stability can lead to big structural changes.
Study Strengths
Our study has several strengths. First, the NHIS is the nation’s largest in‐person household survey and presents a unique opportunity to study SDOH extensively in a nationally representative sample of non‐institutionalized US adults. Second, NHIS data allow for examining the association among various SDOH and a variety of clinical and nonclinical outcomes. Third, this is the first population‐based study to examine the intersection of SDOH and CVH among pregnant women, which is an important area of study in the field of CVH. 17 , 18 , 19 , 20 , 21
Study Limitations
Our study has a few limitations. First, the NHIS is based on self‐reported information; hence, there is a potential for recall bias of unfavorable SDOH. Further, although we adjusted for all possible confounders, the risk for residual confounding is ever present. The cross‐sectional nature of our study and lack of APOs data precludes us from establishing causality and examine the impact of SDOH on APOs directly. Causality may be bidirectional (ie, individuals with cardiovascular risk factors have worse SDOH or given an unfavorable SDOH profile, there is an increased risk for developing cardiovascular risk factors). Second, the SDOH domains studied are reported as aggregate sums and divided into quartiles. Although this approach maybe statistically adequate, we acknowledge that it may lack granularity. However, the components of SDOH are interconnected and considering social conditions in isolation would generate limited results. 57 Third, we included a number of self‐reported components of American Heart Association‐designed Life Simple’s 7 construct except diet (not a part of NHIS survey questionnaire) to ascertain suboptimal CVH; however, age‐adjusted prevalence of diabetes by SDOH quartiles was not performed because the numbers for diabetes were too low to be statistically relevant while performing individual analysis. Future analyses should include information on diet and diabetes and their association with SDOH. Fourth, although the association of worsening SDOH burden with suboptimal CVH may differ across race and ethnicity, we did not perform analysis stratified by race and ethnicity because of limited sample size. However, our formal interaction testing did not suggest differential impact across different racial or ethnic groups. Future larger studies are needed to confirm these findings across specific racial/ethnic groups. Lastly, because we included only pregnant women aged 18 to 45 years, the study findings may not be generalizable to pregnant women under the age of 18 years.
Conclusions
Aggregate unfavorable SDOH risk score is associated with suboptimal CVH in pregnant women. Our findings suggest that women with the most unfavorable SDOH profile have 2.1‐fold higher relative risk of suboptimal CVH, 8.4‐fold higher relative risk of smoking, 1.5‐fold greater relative risk of obesity, and 1.2‐fold higher relative risk of insufficient physical activity. Suboptimal CVH is common and more than half of pregnant women with the highest risk of SDOH inequities have suboptimal CVH, highlighting the public health urgency for intervention in high‐risk women. Knowledge of SDOH must inform clinical decision making and policy‐making process to enhance quality of care, mitigate cardiovascular risk factors especially smoking and insufficient physical activity, and improve health outcomes in this vulnerable population.
Sources of Funding
None.
Disclosures
Dr Sharma is supported by Blumenthal Scholar’s Fund. Dr Nasir is supported by the Katz Academy for Translational Research and serves as a consultant for Amgen, Novartis, and Novo Nordisk. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Figures S1–S3
Acknowledgments
Author contributions: G. Sharma and G.R. Grandhi analyzed and drafted the article and are co‐first authors. G. Sharma, G.R. Grandhi, and K. Nasir were involved in the study conception and design. I. Acquah checked the accuracy of the data and performed data analysis. G. Sharma, G.R. Grandhi, R. Mszar, I. Acquah, S. Mahajan, S.U. Khan, Z. Javed, L.S. Mehta, M. Gulati, M Cainzos‐Achirica, R.S. Blumenthal, and K. Nasir participated in the interpretation of the results and critical revision of the article.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022837
For Sources of Funding and Disclosures, see page 8.
REFERENCES
- 1. Petersen EE, Davis NL, Goodman D, Cox S, Mayes N, Johnston E, Syverson C, Seed K, Shapiro‐Mendoza CK, Callaghan WM, et al. Vital Signs: pregnancy‐related deaths, United States, 2011–2015, and strategies for prevention, 13 States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy‐related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366–373. doi: 10.1097/AOG.0000000000002114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina EV. Hypertensive disorders and pregnancy‐related stroke: frequency, trends, risk factors, and outcomes. Obstet Gynecol. 2015;125:124–131. doi: 10.1097/AOG.0000000000000590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Admon LK, Winkelman TNA, Moniz MH, Davis MM, Heisler M, Dalton VK. Disparities in chronic conditions among women hospitalized for delivery in the United States, 2005–2014. Obstet Gynecol. 2017;130:1319–1326. doi: 10.1097/AOG.0000000000002357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Correa A, Bardenheier B, Elixhauser A, Geiss LS, Gregg E. Trends in prevalence of diabetes among delivery hospitalizations, United States, 1993–2009. Matern Child Health J. 2015;19:635–642. doi: 10.1007/s10995-014-1553-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deputy NP, Kim SY, Conrey EJ, Bullard KM. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth — United States, 2012–2016. MMWR Morb Mortal Wkly Rep. 2018;67:1201–1207. doi: 10.15585/mmwr.mm6743a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Branum AM, Kirmeyer SE, Gregory ECW. Prepregnancy body mass index by maternal characteristics and state: data from the birth certificate, 2014. Natl Vital Stat Rep. 2016;65:1–11. [PubMed] [Google Scholar]
- 8. ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:1. [DOI] [PubMed] [Google Scholar]
- 9. Hypertension in Pregnancy . Report of the American College of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–1131. [DOI] [PubMed] [Google Scholar]
- 10. Richards JL, Kramer MS, Deb‐Rinker P, Rouleau J, Mortensen L, Gissler M, Morken N‐H, Skjærven R, Cnattingius S, Johansson S, et al. Temporal trends in late preterm and early term birth rates in 6 high‐income countries in North America and Europe and association with clinician‐initiated obstetric interventions. JAMA. 2016;316:410–419. doi: 10.1001/jama.2016.9635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lane‐Cordova AD, Khan SS, Grobman WA, Greenland P, Shah SJ. Long‐term cardiovascular risks associated with adverse pregnancy outcomes: JACC review topic of the week. J Am Coll Cardiol. 2019;73:2106–2116. doi: 10.1016/j.jacc.2018.12.092 [DOI] [PubMed] [Google Scholar]
- 12. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew‐Graham CA, et al. Preeclampsia and future cardiovascular health. A systematic review and meta‐analysis. Circ Cardiovasc Qual Outcomes. 2017;10:e003497. doi: 10.1161/CIRCOUTCOMES.116.003497 [DOI] [PubMed] [Google Scholar]
- 13. Honigberg MC, Zekavat SM, Aragam K, Klarin D, Bhatt DL, Scott NS, Peloso GM, Natarajan P. Long‐term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol. 2019;74:2743–2754. doi: 10.1016/j.jacc.2019.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, Smith GN, Gore GC, Ray JG, Nerenberg K, et al. Cardiovascular disease‐related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019;139:1069–1079. doi: 10.1161/CIRCULATIONAHA.118.036748 [DOI] [PubMed] [Google Scholar]
- 15. Wu P, Gulati M, Kwok CS, Wong CW, Narain A, O'Brien S, Chew‐Graham CA, Verma G, Kadam UT, Mamas MA. Preterm delivery and future risk of maternal cardiovascular disease: a systematic review and meta‐analysis. J Am Heart Assoc. 2018;7:e007809. doi: 10.1161/JAHA.117.007809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shields LE, Wiesner S, Klein C, Pelletreau B, Hedriana HL. Use of maternal early warning trigger tool reduces maternal morbidity. Am J Obstet Gynecol. 2016;214:527.e1–527.e6. doi: 10.1016/j.ajog.2016.01.154 [DOI] [PubMed] [Google Scholar]
- 17. Howell EA. Reducing disparities in severe maternal morbidity and mortality. Clin Obstet Gynecol. 2018;61:387–399. doi: 10.1097/GRF.0000000000000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Race, ethnicity, and nativity differentials in pregnancy‐related mortality in the United States: 1993–2006. Obstet Gynecol. 2012;120:261–268. doi: 10.1097/AOG.0b013e31825cb87a [DOI] [PubMed] [Google Scholar]
- 19. Harrington RA, Califf RM, Balamurugan A, Brown N, Benjamin RM, Braund WE, Hipp J, Konig M, Sanchez E, Joynt Maddox KE. Call to action: rural health: a presidential advisory from the American Heart Association and American Stroke Association. Circulation. 2020;141:e615–e644. doi: 10.1161/CIR.0000000000000753 [DOI] [PubMed] [Google Scholar]
- 20. ACOG Committee Opinion No. 586: health disparities in rural women. Obstet Gynecol. 2014;123:384–388. [DOI] [PubMed] [Google Scholar]
- 21. Wang E, Glazer KB, Howell EA, Janevic TM. Social determinants of pregnancy‐related mortality and morbidity in the United States: a systematic review. Obstet Gynecol. 2020;135:896–915. doi: 10.1097/AOG.0000000000003762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gadson A, Akpovi E, Mehta PK. Exploring the social determinants of racial/ethnic disparities in prenatal care utilization and maternal outcome. Semin Perinatol. 2017;41:308–317. doi: 10.1053/j.semperi.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 23. Andermann A. Screening for social determinants of health in clinical care: moving from the margins to the mainstream. Public Health Rev. 2018;39:19. doi: 10.1186/s40985-018-0094-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Daniel H, Bornstein SS, Kane GC; Health and Public Policy Committee of the American College of P . Addressing social determinants to improve patient care and promote health equity: an American College of Physicians Position Paper. Ann Intern Med. 2018;168:577–578. doi: 10.7326/M17-2441 [DOI] [PubMed] [Google Scholar]
- 25. Lang T, Lepage B, Schieber A‐C, Lamy S, Kelly‐Irving M. Social determinants of cardiovascular diseases. Public Health Rev. 2011;33:601–622. doi: 10.1007/BF03391652 [DOI] [Google Scholar]
- 26. Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz‐Flores S, Davey‐Smith G, Dennison‐Himmelfarb CR, Lauer MS, Lockwood DW, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:873–898. doi: 10.1161/CIR.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 27. Brown HL, Warner JJ, Gianos E, Gulati M, Hill AJ, Hollier LM, Rosen SE, Rosser ML, Wenger NK. Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation. 2018;137:e843–e852. doi: 10.1161/CIR.0000000000000582 [DOI] [PubMed] [Google Scholar]
- 28. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 29. Ning H, Labarthe DR, Shay CM, Daniels SR, Hou L, Van Horn L, Lloyd‐Jones DM. Status of cardiovascular health in US children up to 11 years of age: the National Health and Nutrition Examination Surveys 2003–2010. Circ Cardiovasc Qual Outcomes. 2015;8:164–171. doi: 10.1161/CIRCOUTCOMES.114.001274 [DOI] [PubMed] [Google Scholar]
- 30. Perak AM, Ning H, Khan SS, Van Horn LV, Grobman WA, Lloyd‐Jones DM. Cardiovascular health among pregnant women, aged 20 to 44 years, in the United States. J Am Heart Assoc. 2020;9:e015123. doi: 10.1161/JAHA.119.015123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boakye E, Sharma G, Ogunwole SM, Zakaria S, Vaught AJ, Kwapong YA, Hong X, Ji Y, Mehta L, Creanga AA, et al. Relationship of preeclampsia with maternal place of birth and duration of residence among non‐hispanic black women in the United States. Circ Cardiovasc Qual Outcomes. 2021;14:e007546. doi: 10.1161/CIRCOUTCOMES.120.007546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cameron NA, Molsberry R, Pierce JB, Perak AM, Grobman WA, Allen NB, Greenland P, Lloyd‐Jones DM, Khan SS. Pre‐pregnancy hypertension among women in rural and urban areas of the United States. J Am Coll Cardiol. 2020;76:2611–2619. doi: 10.1016/j.jacc.2020.09.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. National Center for Health Statistics . Available at: https://www.cdc.gov/nchs/nhis/about_nhis.htm. Accessed November 1st, 2020.
- 34. NHIS data, questionnaires and related documentation. Available at: https://www.cdc.gov/nchs/nhis/data‐questionnaires‐documentation.htm. Accessed November 1, 2020.
- 35. IRB Exemption . Available at: http://www.hhs.gov/ohrp/regulations‐and‐policy/decision‐trees/. Accessed November 1, 2020.
- 36. Mszar R, Mahajan S, Valero‐Elizondo J, Grandhi GR, Caraballo C, Gopal DJ, Nemiroff RL, Soffer DE, Cainzos‐Achirica M, Sharma G, et al. Disparities in cholesterol screening among a nationally representative sample of pregnant women in the United States. Eur J Prev Cardiol. 2020. doi: 10.1093/eurjpc/zwaa100 [DOI] [PubMed] [Google Scholar]
- 37. Artiga S, Hinton E. Beyond health care: the role of social determinants in promoting health and health equity. Available at: https://www.kff.org/racial‐equity‐and‐health‐policy/issue‐brief/beyond‐health‐care‐the‐role‐of‐social‐determinants‐in‐promoting‐health‐and‐health‐equity/. Accessed October 1, 2021.
- 38. USDA Economic Research Service . Available at: https://www.ers.usda.gov/topics/food‐nutrition‐assistance/food‐security‐in‐the‐us/measurement/#survey. Accessed June 7, 2021.
- 39. Singh J, Valero‐Elizondo J, Salami JA, Warraich HJ, Ogunmoroti O, Spatz ES, Desai N, Rana JS, Virani SS, Blankstein R, et al. Favorable modifiable cardiovascular risk profile is associated with lower healthcare costs among cancer patients: the 2012–2013 medical expenditure panel survey. J Am Heart Assoc. 2018;7:e007874. doi: 10.1161/JAHA.117.007874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Age standardization and population estimates. Available at: https://wwwn.cdc.gov/nchs/nhanes/tutorials/module8.aspx. Accessed November 1, 2020.
- 41. Blewett L, Rivera Drew JA, Griffin R, King M, Williams K. IPUMS health surveys: national health interview survey, version 6.2.
- 42. Vilda D, Wallace M, Dyer L, Harville E, Theall K. Income inequality and racial disparities in pregnancy‐related mortality in the US. SSM Popul Health. 2019;9:100477. doi: 10.1016/j.ssmph.2019.100477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shahul S, Tung A, Minhaj M, Nizamuddin J, Wenger J, Mahmood E, Mueller A, Shaefi S, Scavone B, Kociol RD, et al. Racial disparities in comorbidities, complications, and maternal and fetal outcomes in women with preeclampsia/eclampsia. Hypertens Pregnancy. 2015;34:506–515. doi: 10.3109/10641955.2015.1090581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kozhimannil KB, Interrante JD, Henning‐Smith C, Admon LK. Rural‐urban differences in severe maternal morbidity and mortality in the US, 2007–15. Health Aff. 2019;38:2077–2085. doi: 10.1377/hlthaff.2019.00805 [DOI] [PubMed] [Google Scholar]
- 45. Yang I, Hall LA, Ashford K, Paul S, Polivka B, Ridner SL. Pathways from socioeconomic status to prenatal smoking: a test of the reserve capacity model. Nurs Res. 2017;66:2–11. doi: 10.1097/NNR.0000000000000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Drake P, Driscoll AK, Mathews TJ. Cigarette Smoking During Pregnancy: United States, 2016. NCHS Data Brief, no 305. Hyattsville, MD: National Center for Health Statistics; 2018. [PubMed] [Google Scholar]
- 47. Hesketh KR, Evenson KR. Prevalence of U.S. pregnant women meeting 2015 ACOG physical activity guidelines. Am J Prev Med. 2015;2016:e87–e89. doi: 10.1016/j.amepre.2016.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nascimento SL, Surita FG, Godoy AC, Kasawara KT, Morais SS. Physical activity patterns and factors related to exercise during pregnancy: a cross sectional study. PLoS One. 2015;10:e0128953. doi: 10.1371/journal.pone.0128953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Singh GK, DiBari JN. Marked disparities in pre‐pregnancy obesity and overweight prevalence among US Women by race/ethnicity, nativity/immigrant status, and sociodemographic characteristics, 2012–2014. J Obes. 2019;2019:2419263. doi: 10.1155/2019/2419263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Martin J, Hamilton B, Osterman M, Driscoll A, Drake P. Births: final data for 2016. Natl Vital Stat Rep. 2018;67:1–54. [PubMed] [Google Scholar]
- 51. Tobacco and nicotine cessation during pregnancy: ACOG Committee opinion, number 807. Obstet Gynecol. 2020;135:e221–e229. [DOI] [PubMed] [Google Scholar]
- 52. Perak AM, Lancki N, Kuang A, Labarthe DR, Allen NB, Shah SH, Lowe LP, Grobman WA, Lawrence JM, Lloyd‐Jones DM, et al. Associations of maternal cardiovascular health in pregnancy with offspring cardiovascular health in early adolescence. JAMA. 2021;325:658–668. doi: 10.1001/jama.2021.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mszar R, Gopal DJ, Chowdary R, Smith CL, Dolin CD, Irwin ML, Soffer D, Nemiroff R, Lewey J. Racial/ethnic disparities in screening for and awareness of high cholesterol among pregnant women receiving prenatal care. J Am Heart Assoc. 2021;10:e017415. doi: 10.1161/JAHA.120.017415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sharma G, Zakaria S, Michos ED, Bhatt AB, Lundberg GP, Florio KL, Vaught AJ, Ouyang P, Mehta L. Improving cardiovascular workforce competencies in cardio‐obstetrics: current challenges and future directions. J Am Heart Assoc. 2020;9:e015569. doi: 10.1161/JAHA.119.015569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moscrop A, Ziebland S, Bloch G, Iraola JR. If social determinants of health are so important, shouldn't we ask patients about them? BMJ. 2020;371:m4150. doi: 10.1136/bmj.m4150 [DOI] [PubMed] [Google Scholar]
- 56. ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140–e150. [DOI] [PubMed] [Google Scholar]
- 57. Figueroa JF, Frakt AB, Jha AK. Addressing social determinants of health: time for a polysocial risk score. JAMA. 2020;323:1553–1554. doi: 10.1001/jama.2020.2436 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S3


