Abstract
Background
Hypertensive disorders of pregnancy are growing public health problems that contribute to maternal morbidity, mortality, and future risk of cardiovascular disease. Given established rural‐urban differences in maternal cardiovascular health, we described contemporary trends in new‐onset hypertensive disorders of pregnancy in the United States.
Methods and Results
We conducted a serial, cross‐sectional analysis of 51 685 525 live births to individuals aged 15 to 44 years from 2007 to 2019 using the Centers for Disease Control and Prevention Natality Database. We included gestational hypertension and preeclampsia/eclampsia in individuals without chronic hypertension and calculated the age‐adjusted incidence (95% CI) per 1000 live births overall and by urbanization status (rural or urban). We used Joinpoint software to identify inflection points and calculate rate of change. We quantified rate ratios to compare the relative incidence in rural compared with urban areas. Incidence (95% CI) of new‐onset hypertensive disorders of pregnancy increased from 2007 to 2019 in both rural (48.6 [48.0–49.2] to 83.9 [83.1–84.7]) and urban (37.0 [36.8–37.2] to 77.2 [76.8–77.6]) areas. The rate of annual increase in new‐onset hypertensive disorders of pregnancy was more rapid after 2014 with greater acceleration in urban compared with rural areas. Rate ratios (95% CI) comparing incidence of new‐onset hypertensive disorders of pregnancy in rural and urban areas decreased from 1.31 (1.30–1.33) in 2007 to 1.09 (1.08–1.10) in 2019.
Conclusions
Incidence of new‐onset hypertensive disorders of pregnancy doubled from 2007 to 2019 with persistent rural‐urban differences highlighting the need for targeted interventions to improve the health of pregnant individuals and their offspring.
Keywords: gestational hypertension, hypertensive disorders of pregnancy, preeclampsia, rural, urban
Subject Categories: Pregnancy, Preeclampsia
Nonstandard Abbreviations and Acronyms
- CDC
Centers for Disease Control and Prevention
- WONDER
Wide‐ranging Online Data for Epidemiologic Research
Clinical Perspective
What Is New?
The incidence of new‐onset hypertensive disorders of pregnancy (gestational hypertension and preeclampsia/eclampsia) have nearly doubled in both rural and urban areas in the United States from 2007 to 2019 with accelerating rates since 2014.
The rural‐urban gap in new‐onset hypertensive disorders of pregnancy decreased from 2007 to 2019 as a result of increasing rates in urban areas, rather than improvements in rural areas.
Differences in the incidence of new‐onset hypertensive disorders of pregnancy by race, ethnicity, and United States region persisted throughout the study period in both rural and urban areas.
What Are the Clinical Implications?
Future research is needed to identify the individual and environmental factors that contribute to increasing rates of new‐onset hypertensive disorders of pregnancy in both rural and urban areas in the United States.
Public health policy changes are necessary to equitably reverse the accelerating rates of new‐onset hypertensive disorders of pregnancy in the United States.
Pregnancy is a crucial time that uniquely influences the cardiovascular health of both the pregnant individual and child. Hypertensive disorders of pregnancy in particular have major implications for cardiovascular health. 1 Specifically new‐onset hypertensive disorders of pregnancy, defined as new‐onset hypertension arising during pregnancy (gestational hypertension and preeclampsia/eclampsia), is associated with more than double the risk of coronary heart disease, heart failure, stroke and cardiovascular‐related mortality, 2 , 3 and account for up to 44% of deaths within the first 6 days postpartum. 4 Additionally, the cardiovascular associations of hypertensive disorders of pregnancy extend to offspring, who have increased risks of hypertension, obesity, and lifetime cardiovascular disease into young adulthood. 5
Despite advances in cardiovascular and obstetric care, hypertensive disorders of pregnancy and pregnancy‐related mortality remain major public health problems in the United States. From 1987 to 2004, the incidence of gestational hypertension almost tripled from 10.7 to 30.6 per 1000 deliveries, and the incidence of preeclampsia increased from 23.6 to 29.4 per 1000 deliveries. 6 Furthermore, US maternal mortality rates are the highest among developed countries, reaching 20.1 per 100 000 live births in 2019. 7 , 8 Given the scope of the problem, the American Heart Association and American College of Obstetricians and Gynecologists released a joint statement recognizing pregnancy as an important milestone that shapes cardiovascular health. 9
Identifying the social determinants of health that contribute to hypertension during pregnancy is an important step towards improving maternal cardiovascular health. Individuals in rural areas are confronted with distinct challenges that may contribute to a rural‐urban gap in adverse pregnancy outcomes. 10 , 11 For example, from 2007 to 2015, rates of severe maternal morbidity and mortality were 9% higher in rural compared with urban areas, and this rural‐urban gap is predicted to continue to grow. 12 Recent work has demonstrated that individuals with live births in rural areas are up to 20% more likely to have chronic hypertension than those in urban areas; this gap was amplified in non‐Hispanic Black individuals reinforcing known racial and ethnic differences in maternal health. 13
Therefore, we aimed to describe trends in hypertensive disorders of pregnancy from 2007 to 2019 among individuals in rural and urban areas in the United States, overall and stratified by age, self‐identified racial and ethnic groups, and US region.
Methods
We performed a serial, cross‐sectional analysis of maternal data from all live births in the United States to individuals aged 15 to 44 years from 2007 to 2019 (N=51 685 525) using data obtained from the Centers for Disease Control and Prevention (CDC) Wide‐Ranging Online Data for Epidemiologic Research (WONDER) and Natality Database. 14 The data set reports information on maternal demographics, medical history, and complications of pregnancies recorded on infant birth certificates documented by the professional attendant at birth using data obtained from the individual and their health records. Births were included regardless of gestational age or plurality. The “pregnancy associated hypertension” checkbox (referred to as hypertensive disorders of pregnancy in this analysis) was listed under “maternal risk factors” and defined as either gestational hypertension or preeclampsia (with or without a diagnosis of eclampsia or severe features). Data on hemolysis, elevated liver enzymes and low platelet count was not available in the CDC WONDER. All data are publicly available and can be accessed at https://wonder.cdc.gov/natality.html.
We calculated the incidence of hypertensive disorders of pregnancy per 1000 live births by urbanization status (rural or urban), as well as within each rural (micropolitan and noncore area) and urban subgroup (large metropolitan, large fringe/suburban, medium metropolitan, small metropolitan area) based on the pregnant individual’s residence. Rates were age‐standardized with the first year of the study period as the reference population (2007) to allow for comparisons over time, by subgroups and to existing literature. 6 Rural/urban designations were based on the 2013 National Center for Health Statistics urban‐rural classification scheme for counties. 15 A rural area was designated as a county in a metropolitan statistical area with a population <50 000. We additionally performed subgroup analyses to calculate the age‐standardized incidence of hypertensive disorders of pregnancy by self‐identified racial and ethnic groups (American Indian/Alaskan Native, Asian American/Pacific Islander, Hispanic/Latina, non‐Hispanic Black, non‐Hispanic White) and US census region (Midwest, Northeast, West, South), as well as in 5‐year standardized age categories (15–19, 20–24, 25–29, 30–34, 35–39, and 40–44 years) in rural and urban areas. Because this analysis included individuals who had >1 pregnancy resulting in a live birth during the study period, we conducted a sensitivity analysis restricted to nulliparous individuals from 2016 to 2019 when data were available for this subgroup.
To analyze trends in the incidence of hypertensive disorders of pregnancy from 2007 to 2019, we used Joinpoint statistical software which models consecutive linear segments on a log scale to identify points in time (joinpoints) at which there are statistically significant changes in trends as previously described for analysis of surveillance statistics over time. 16 , 17 Using this approach, we identified a single inflection point in 2014 in the overall incidence, and then calculated the slope (average annual percent change) of the overall study period and the slopes of the 2 periods defined by the inflection point both by rural‐urban status and within each age group and racial and ethnic group, and US census region.
To compare the incidence of hypertensive disorders of pregnancy between rural and urban areas, we calculated rate ratios overall and within each age group, racial and ethnic group, and US census region at the inflection point in 2014 as well as in 2007 and 2019. Rate ratios and their corresponding 95% CIs were calculated using Stata v14.2 (College Station, TX). 18 This study was exempt from Institutional Review Board approval because of the deidentified nature of the data.
Results
Study Population Demographics
Of the 51 685 525 live births to individuals aged 15 to 44 years in the United States from 2007 to 2019, (Table 1) the majority of births were to individuals from urban areas (86.3%). Compared with urban areas, in rural areas a greater proportion of births occurred among younger (aged <30 years) and non‐Hispanic White individuals living in the Midwest and South. A similar proportion of births occurred in individuals with a high school education or greater and in individuals who received prenatal care in rural and urban areas.
Table 1.
Descriptive Statistics for All Women in the United States Aged 15 to 44 Years With Live Births Between 2007 and 2019, Stratified by Rural or Urban Status
| Demographics | Urban | Rural |
|---|---|---|
| n | 44 616 094 | 7 069 431 |
| Age, % | ||
| 15–19 y | 6.91 | 10.12 |
| 20–24 y | 21.19 | 29.85 |
| 25–29 y | 28.38 | 30.62 |
| 30–34 y | 26.98 | 19.74 |
| 35–39 y | 13.58 | 8.04 |
| 40–44 y | 2.97 | 1.64 |
| Race or ethnicity, % | ||
| American Indian/Alaskan Native | 0.58 | 3.31 |
| Asian American/Pacific Islander | 7.18 | 1.39 |
| Hispanic/Latina | 25.35 | 12.13 |
| Non‐Hispanic Black | 15.72 | 9.35 |
| Non‐Hispanic White | 50.35 | 73.31 |
| Region (%) | ||
| Midwest | 19.19 | 32.73 |
| Northeast | 17.23 | 8.20 |
| South | 37.66 | 44.07 |
| West | 25.92 | 15.01 |
| Education, % | ||
| Less than high school | 13.47 | 14.89 |
| High school/GED | 20.82 | 25.88 |
| Some college | 17.06 | 18.73 |
| Bachelor's/associate's degree | 22.92 | 18.91 |
| Master's or doctorate degree | 9.61 | 4.39 |
| Initiation of prenatal care, % | ||
| Never | 1.39 | 1.11 |
| During months 1–5 | 74.28 | 73.34 |
Overall Trends in Hypertensive Disorders of Pregnancy
The age‐adjusted incidence of hypertensive disorders of pregnancy increased among individuals in both rural and urban areas from 2007 to 2019 (Figure 1, Table S1). From 2007 to 2019, the incidence increased from 48.6 (95% CI, 48.0–49.2) to 83.9 (95% CI, 83.1–84.7) in rural areas and from 37.0 (95% CI, 36.8–37.2) to 77.2 (95% CI, 76.8–77.6) in urban areas. These increases represented an overall average annual percent change during the whole study period of 6.5% per year (95% CI, 6.0–6.9) in urban areas and 4.7% per year (95% CI, 4.2–5.2) in rural areas (Table 2). When evaluating average annual percent change in the 2 time periods, increases occurred in both rural and urban areas, with greater average annual percent change in the 2014 to 2019 epoch, reaching as high as 7.7% per year (95% CI, 6.7–8.8) in rural areas and 9.4% per year (95% CI, 8.5–10.3) in urban areas (Table 2). Similar patterns of change were observed in each rural and urban subgroup (Table 2, Table S1). Although both rural and urban areas had increasing rates over time, rate ratios comparing the incidence of hypertensive disorders of pregnancy in rural and urban areas decreased from 1.31 (95% CI, 1.30–1.33) in 2007 to 1.09 (95% CI, 1.08–1.10) in 2019 (Table 3) given the high rate of increase in urban areas.
Figure 1. Trends in the age‐adjusted incidence of new‐onset hypertensive disorders of pregnancy per 1000 live births and average annual percent change in rural and urban areas in the United States, 2007 to 2019.
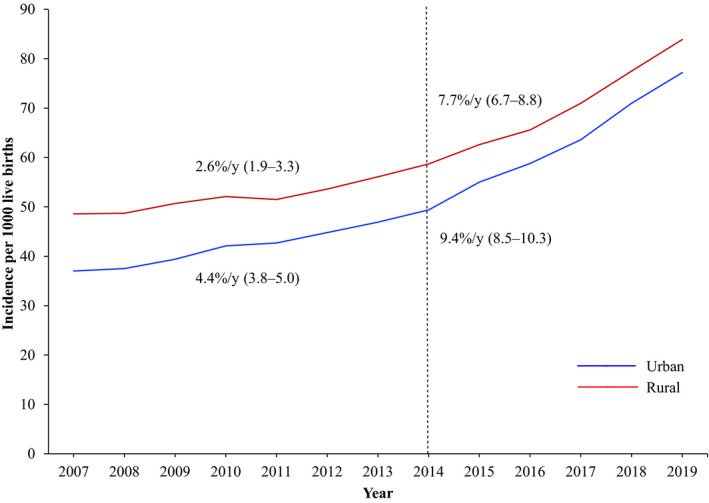
The incidence of hypertensive disorders of pregnancy approximately doubled in both rural and urban areas from 2007 to 2019 with accelerating rates since 2014. The rural‐urban gap decreased over the study period because of more rapid increases in the rates of hypertensive disorders of pregnancy among individuals with live births in urban compared with rural areas.
Table 2.
Average Annual Percent Change (95% CI) in the Incidence of New‐Onset Hypertensive Disorders of Pregnancy Across Two Periods Based on Identified Inflection Points (2007–2014, 2014–2019) Stratified by Rural‐Urban Status
| Rural/Urban Category | Average Annual Percent Change (95% CI) | ||
|---|---|---|---|
| 2007–2019 | 2007–2014 | 2014–2019 | |
| Rural | 4.7 (4.2–5.2) | 2.6 (1.9–3.3) | 7.7 (6.7–8.8) |
| Micropolitan | 4.8 (4.3–5.2) | 2.6 (1.9–3.2) | 7.9 (6.9–8.9) |
| Noncore | 4.4 (3.9–5.0) | 2.7 (2.0–3.4) | 6.9 (6.0–7.7) |
| Urban | 6.5 (6.0–6.9) | 4.4 (3.8–5.0) | 9.4 (8.5–10.3) |
| Large Central Metropolitan | 7.0 (6.5–7.6) | 5.1 (4.4–5.9) | 9.8 (8.6–10.9) |
| Large Fringe/Suburban | 6.2 (5.7–6.7) | 4.0 (3.4–4.7) | 9.4 (8.4–10.4) |
| Medium Metropolitan | 6.0 (5.6–6.5) | 4.2 (3.6–4.8) | 8.7 (7.8–9.6) |
| Small Metropolitan | 5.7 (5.1–6.3) | 3.0 (2.2–3.8) | 9.6 (8.4–10.8) |
Table 3.
Rate Ratios (95% CI) of New‐Onset Hypertensive Disorders of Pregnancy in Rural Compared With Urban Areas in the United States Stratified by Age, Race, Ethnicity, and Region in 2007, 2014, and 2019
| Demographic Characteristics | Year | ||
|---|---|---|---|
| 2007 | 2014 | 2019 | |
| Overall | 1.31 (1.30–1.33) | 1.19 (1.17–1.20) | 1.09 (1.08–1.10) |
| Age group, y | |||
| Age 15–19 | 1.23 (1.19–1.27) | 1.14 (1.09–1.18) | 0.99 (0.95–1.03) |
| Age 20–24 | 1.26 (1.23–1.29) | 1.15 (1.13–1.18) | 1.06 (1.04–1.08) |
| Age 25–29 | 1.29 (1.26–1.32) | 1.16 (1.14–1.19) | 1.1 (1.08–1.12) |
| Age 30–34 | 1.39 (1.35–1.43) | 1.25 (1.22–1.28) | 1.12 (1.09–1.14) |
| Age 35–39 | 1.43 (1.37–1.49) | 1.25 (1.21–1.3) | 1.15 (1.12–1.19) |
| Age 40–44 | 1.26 (1.16–1.38) | 1.17 (1.08–1.26) | 1.08 (1.01–1.15) |
| Race or Ethnicity | |||
| American Indian/Alaskan Native | 1.21 (1.11–1.33) | 1.17 (1.08–1.13) | 1.03 (0.96–1.11) |
| Asian American/Pacific Islander | 1.29 (1.11–1.50) | 0.98 (0.84–1.14) | 1.12 (0.99–1.26) |
| Hispanic/Latina | 1.33 (1.27–1.40) | 1.08 (1.04–1.13) | 0.97 (0.93–1.00) |
| Non‐Hispanic Black | 1.14 (1.09–1.19) | 1.09 (1.05–1.13) | 0.99 (0.95–1.02) |
| Non‐Hispanic White | 1.20 (1.18–1.21) | 1.13 (1.11–1.15) | 1.04 (1.03–1.05) |
| Region | |||
| Midwest | 1.22 (1.20–1.25) | 1.01 (0.99–1.03) | 0.90 (0.88–0.92) |
| Northeast | 1.16 (1.11–1.21) | 1.20 (1.14–1.26) | 1.12 (1.08–1.16) |
| South | 1.19 (1.17–1.21) | 1.16 (1.14–1.18) | 1.11 (1.10–1.13) |
| Midwest | 1.67 (1.61–1.72) | 1.28 (1.24–1.32) | 1.16 (1.12–1.19) |
Our sensitivity analysis restricted to nulliparas revealed similar trends in the incidence of hypertensive disorders of pregnancy from 2016 to 2019 to those seen among all live births in the main analysis (Table S2). The average annual percent change for hypertensive disorders of pregnancy among nulliparous individuals was 7.3% per year (95% CI, 5.9–8.7) in rural areas and 8.6% per year (95% CI, 6.1–11.2) in urban areas.
Age‐Specific Trends in Hypertensive Disorders of Pregnancy
The incidence of hypertensive disorders of pregnancy per 1000 live births approximately doubled in each age group from 2007 to 2019 in both rural and urban areas (Figure 2, Table S3). Within each age group, the average annual percent change was higher in urban compared with rural areas, and was greater in 2014 to 2019 versus 2007 to 2014 (Table S4). The highest incidence of hypertensive disorders of pregnancy was in the older age group (aged 40–44 years) in both rural and urban areas, but the greatest increases over the study period were observed among 20‐ to 24‐year‐old individuals in urban areas from 2014 to 2019 at 10.5% per year (95% CI, 9.5–11.5). Point values for rate ratios comparing the incidence of hypertensive disorders of pregnancy in rural versus urban areas were greatest among individuals aged 30 to 34 and 35 to 39 years. As in the overall population, urban‐to‐rural rate ratios decreased from 2007 to 2019 in all age groups (Table 3).
Figure 2. Trends in the incidence of new‐onset hypertensive disorders of pregnancy per 1000 live births in rural and urban areas by age group in the United States, 2007 to 2019.
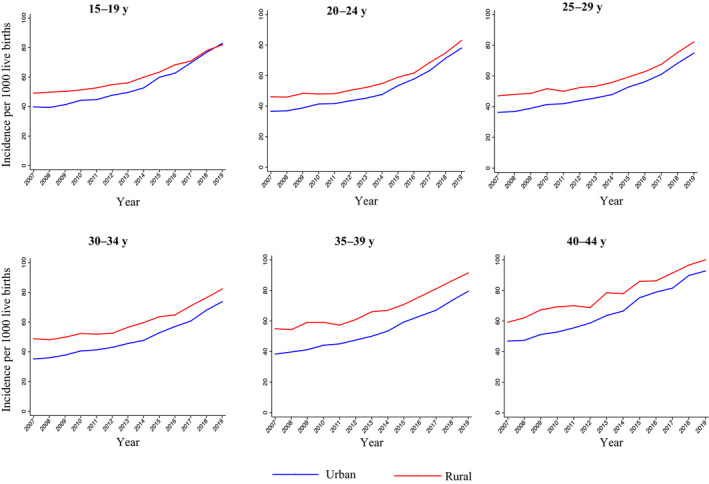
The incidence of hypertensive disorders of pregnancy increased from 2007 to 2019 in each age group.
Race‐ and Ethnicity‐Specific Trends in Hypertensive Disorders of Pregnancy
The incidence of hypertensive disorders of pregnancy differed by racial and ethnic identity within both rural and urban areas. The highest age‐adjusted incidence of hypertensive disorders of pregnancy was observed among individuals who identify as American Indian/Alaskan Native followed by those who identify as non‐Hispanic Black, non‐Hispanic White, Hispanic/Latina, and Asian American/Pacific Islander in both rural and urban areas over the study period (Figure 3, Table S5). In 2019, the incidence ranged from 45.7 (95% CI, 44.3–47.1 ) per 1000 live births among Asian American/Pacific Islander individuals in urban areas to 102.0 (95% CI, 96.9–107.1) per 1000 live births among American Indian/Alaskan Native individuals in rural areas. In each racial and ethnic group, the average annual percent change was higher in urban compared with rural areas (Table S5). The greatest overall average annual percent change from 2007 to 2019 was observed among Hispanic/Latina individuals (7.7% per year [95% CI, 7.0–8.4]) in urban areas (Table S6). Rate ratios comparing the incidence of hypertensive disorders of pregnancy in rural and urban areas declined from 2007 to 2019 in all race and ethnicity groups (Table 3).
Figure 3. Trends in the incidence of new‐onset hypertensive disorders of pregnancy per 1000 live births in rural and urban areas by race and ethnicity in the United States, 2007 to 2019.
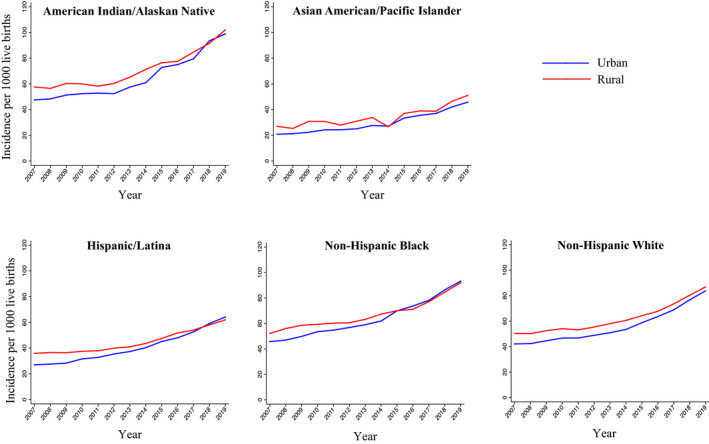
The incidence of hypertensive disorders of pregnancy increased from 2007 to 2019 in each racial and ethnic group. The incidence was highest among individuals with live births who identified as American Indian/Alaskan Native or Non‐Hispanic Black.
Census Region‐Specific Trends in Hypertensive Disorders of Pregnancy
The incidence of hypertensive disorders of pregnancy differed by US census region ranging from 67.7 (95% CI, 67.0–68.3) in the urban West to 93.4 (95% CI, 92.6–94.2) in the urban Midwest in 2019 (Table S7). Within each region, incidence increased significantly over the time period in both rural and urban areas (Figure 4). Point values for average annual percent change were higher in urban areas compared with rural areas, contributing to decreasing rate ratios comparing the incidence of hypertensive disorders of pregnancy in rural versus urban areas from 2007 to 2019 in each US region (Table S8 ). Incidence of hypertensive disorders of pregnancy in the urban Midwest surpassed rates in the rural Midwest starting in 2016.
Figure 4. Trends in the incidence of new‐onset hypertensive disorders of pregnancy per 1000 live births in rural and urban areas by geographic region in the United States, 2007 to 2019.
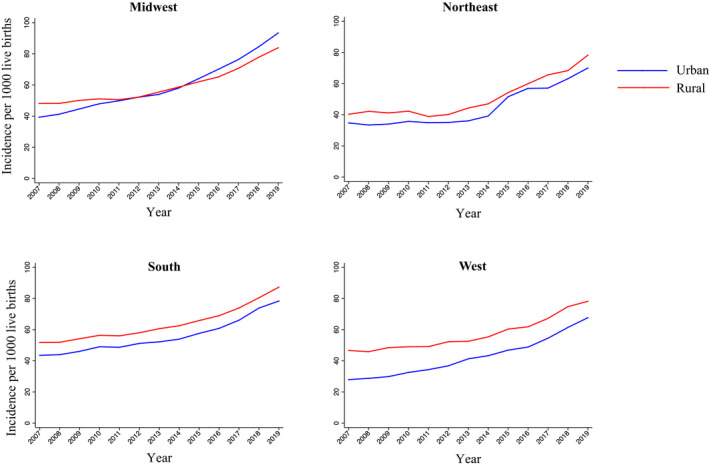
The incidence of hypertensive disorders of pregnancy increased from 2007 to 2019 in rural and urban areas in each United States census region.
Discussion
The age‐standardized incidence of hypertensive disorders of pregnancy (gestational hypertension and preeclampsia/eclampsia) increased significantly during the study period in both rural and urban areas in each self‐identified racial and ethnic group and US region. Nevertheless, from 2007 to 2019, rural‐urban differences persisted such that individuals with live births in rural areas were 9% more likely to have hypertensive disorders of pregnancy than those in urban areas in 2019. Although the rural‐urban gap in hypertensive disorders of pregnancy decreased over the study period, this was attributable to more rapid increases in the rates of hypertensive disorders of pregnancy among individuals in urban compared with rural areas. Racial and ethnic differences in the incidence of hypertensive disorders of pregnancy were observed throughout the study period as well.
Our work expands upon prior studies by describing contemporary estimates and trends in the incidence of hypertensive disorders of pregnancy in rural compared with urban areas. We identified rural‐urban differences in hypertensive disorders of pregnancy, demonstrating that individuals in rural areas were between 9% and 30% more likely to have hypertensive disorders of pregnancy than those in urban areas. Our results complement previous work demonstrating a higher prevalence of chronic hypertension and severe maternal morbidity and mortality in rural compared with urban areas. 12 , 13 Possible explanations for these rural‐urban gaps in maternal health include place‐based differences in obesity, physical activity, access to gyms and recreational areas, dietary patterns, poverty, and access to care. 19 , 20 , 21 Between 2004 and 2014, ≈9% of rural counties in the United States lost access to hospital obstetrics services with >50% lacking these services in 2014. 20 Given these gaps in rural healthcare, the American Heart Association and the American College of Obstetricians and Gynecologists issued calls to action to eliminate health differences in rural counties. 10 , 11 Strategies proposed by the Centers for Medicare and Medicaid Services to improve maternal health among rural individuals include expanding the obstetrics workforce in rural areas, extending Medicaid beyond the 60‐day postpartum period, and partnering with community services to coordinate medical, behavioral, and social support for pregnant individuals. 22
Although the rural‐urban gap in hypertensive disorders of pregnancy declined from 2007 to 2019, rates of hypertensive disorders of pregnancy increased in both rural and urban areas. Therefore, the narrowing gap does not represent improvements in rural maternal health, but rather points to more rapid acceleration of the incidence of hypertensive disorders of pregnancy in urban areas. This pattern is similar to trends in the prevalence of chronic hypertension, which has accelerated in recent years in both rural and urban areas. Additionally, the prevalence of chronic hypertension 13 and hypertensive disorders of pregnancy have increased among individuals in all age groups in both rural and urban areas, suggesting that increases in the incidence of hypertensive disorders of pregnancy are not simply attributable to increases in maternal age. Rather, unfavorable patterns in maternal risk factors in both rural and urban areas such as obesity, physical activity, and diet are likely contributing to accelerating rates of hypertensive disorders of pregnancy in recent years. 23 , 24 , 25 Obesity, physical activity, and nutrition represent modifiable risk factors that can be targeted to improve maternal health regardless of location. Furthermore, given rapidly accelerating rates of hypertensive disorders of pregnancy in urban areas compared with rural areas, it is essential to identify and target factors unique to individuals living in urban areas that may influence rates of hypertensive disorders of pregnancy such as air pollution, environmental noise, crowding, urban planning, and availability of green spaces. 26 , 27 , 28 Even within a single urban county, there is significant heterogeneity at the neighborhood level (ie, racial segregation, physical disorder) that are associated with hypertensive disorders of pregnancy. 29 , 30 Further research is needed to understand these neighborhood level factors within urban areas to help develop targeted programs to slow the acceleration of rates in hypertensive disorders of pregnancy.
Racial and ethnic differences in the incidence of hypertensive disorders of pregnancy persisted over the study period within both rural and urban areas. The incidence was greatest among self‐identified non‐Hispanic Black and American Indian/Alaskan Native individuals, with up to 1 out of 10 individuals from these groups diagnosed with hypertensive disorders of pregnancy in 2019. Our results support prior work demonstrating higher rates of hypertensive disorders of pregnancy among non‐Hispanic Black and American Indian/Alaskan Native individuals 31 , 32 and a larger body of work reporting racial and ethnic differences in maternal health. 13 , 33 , 34 Our results further highlight the complex interaction between urbanization, structural racism, and other social determinants of health such as poverty and education in shaping cardiometabolic health. 35 , 36 Future work is needed to investigate the specific risk factors at all ecologic levels, including rurality, that contribute to hypertensive disorders of pregnancy in racial and ethnic groups.
Strengths of this study include use of data from the CDC WONDER and Natality Database, the largest repository of data describing individuals with live births in the United States. Given the large sample size, we were able to describe age, race‐ and ethnicity‐specific trends in hypertensive disorders of pregnancy, including among Asian American/Pacific Islander and American Indian/Alaskan Native individuals, groups that have been previously underrepresented in the literature. We also uniquely identified inflection points using Joinpoint software to precisely describe changes in trends over time.
Limitations of the CDC WONDER and Natality Database include the possibility of misclassification bias. Although the sensitivity of hypertensive disorders of pregnancy recorded on birth certificates ranges from ≈30% to 60%, specificity is high (99%). 37 Therefore, we may have underestimated the incidence of hypertensive disorders of pregnancy in rural and urban areas in the United States. In addition, because this was a serial cross‐sectional analysis of individuals with live births, our data included some individuals with >1 birth during the study period and did not include individuals with pregnancy loss. However, in a sensitivity analysis that analyzed only nulliparous individuals, the incidence of hypertensive disorders of pregnancy was higher and temporal trends were similar compared with all individuals in our primary analysis. This suggests that the overall trends in our analysis are not attributable to individuals prone to hypertensive disorders of pregnancy having >1 birth over time. The Natality Database also does not include information on fetal deaths and only includes individuals with live births. However since perinatal mortality rates are ≈0.6%, this should not have significantly affected our results. 38
Finally, available data used the extant definitions of hypertensive disorders of pregnancy at the time of birth, and definitions have changed over time. In 2013, the American College of Obstetricians and Gynecologists Task Force changed the definition of preeclampsia to include hypertension during pregnancy in association with organ dysfunction, even if proteinuria was not present. 1 By changing diagnostic criteria and increasing awareness of hypertensive disorders of pregnancy among practitioners, the Task Force may have contributed to accelerating rates of hypertensive disorders of pregnancy and the observed inflection point in 2014. 39 , 40 However, incidence of hypertensive disorders of pregnancy was increasing before the Task Force recommendations, suggesting that unfavorable patterns in other maternal risk factors are also likely contributing to accelerating rates in recent years. We were unable to quantify the contribution of these maternal risk factors (ie, obesity, physical inactivity, poor nutrition) to trends in hypertensive disorders of pregnancy because data were not available in the Natality Database. In addition, because our study included both individuals with gestational hypertension and preeclampsia, and did not distinguish between these 2 conditions, the changing definition of preeclampsia should not have affected our trends substantially.
Conclusions
We report a doubling in the incidence of new‐onset hypertensive disorders of pregnancy that arise during pregnancy from 2007 to 2019 in both rural and urban areas with accelerating rates since 2014. Although the rural‐urban gap decreased across the study period, this reflected greater increases in rates of hypertensive disorders of pregnancy among individuals in urban areas, rather than improvements in rural outcomes. Differences in the incidence and rural‐urban gap among racial and ethnic groups highlight the need to identify and target the individual and environmental factors that uniquely shape maternal and neonatal health in both rural and urban areas.
Sources of Funding
This work was supported by grants from the National Institutes of Health (P30AG059988; P30DK092939) and the American Heart Association (#19TPA34890060) to SSK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
None.
Supporting information
Tables S1–S8
Acknowledgments
The funding sponsor did not contribute to design and conduct of the study, collection, management, analysis, or interpretation of the data or preparation, review, or approval of the manuscript. The authors take responsibility for the decision to submit the manuscript for publication. Dr Khan had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023791
For Sources of Funding and Disclosures, see page 9.
References
- 1. American College of Obstetricians, Task Force on Hypertension in Pregnancy . Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. In: Obstetrics and Gynecology. 2013:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88 [DOI] [PubMed] [Google Scholar]
- 2. Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, Smith GN, Gore GC, Ray JG, Nerenberg K, et al. Cardiovascular disease‐related morbidity and mortality in women with a history of pregnancy complications: systematic review and meta‐analysis. Circulation. 2019;139:1069–1079. doi: 10.1161/CIRCULATIONAHA.118.036748 [DOI] [PubMed] [Google Scholar]
- 3. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew‐Graham CA, et al. Preeclampsia and future cardiovascular health. Circ Cardiovasc Qual Outcomes. 2017;10:1–9. doi: 10.1161/CIRCOUTCOMES.116.003497 [DOI] [PubMed] [Google Scholar]
- 4. Petersen EE, Davis NL, Goodman D, Cox S, Mayes N, Johnston E, Syverson C, Seed K, Shapiro‐Mendoza CK, et al. Vital signs: pregnancy‐related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68:2013–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis EF, Lewandowski AJ, Aye C, Williamson W, Boardman H, Huang RC, Mori TA, Newnham J, Beilin LJ, Leeson P. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: insights from a 20‐year prospective follow‐up birth cohort. BMJ Open. 2015;5:1–8. doi: 10.1136/bmjopen-2015-008136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21:521–526. doi: 10.1038/ajh.2008.20 [DOI] [PubMed] [Google Scholar]
- 7. Hirshberg A, Srinivas SK. Epidemiology of maternal morbidity and mortality. Semin Perinatol. 2017;41:332–337. doi: 10.1053/j.semperi.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 8. Hoyert DL. Maternal mortality rates in the United States, 2019. NCHS Health E‐Stats. 2021. doi: 10.15620/cdc:103855 [DOI] [Google Scholar]
- 9. Brown HL, Warner JJ, Gianos E, Gulati M, Hill AJ, Hollier LM, Rosen SE, Rosser ML, Wenger NK; American Heart Association and the American College of Obstetricians and Gynecologists . Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American Heart Association and the American college of obstetricians and gynecologists. Circulation. 2018;137:e843–e852. doi: 10.1161/CIR.0000000000000582 [DOI] [PubMed] [Google Scholar]
- 10. Harrington RA, Califf RM, Balamurugan A, Brown N, Benjamin RM, Braund WE, Hipp J, Konig M, Sanchez E, Joynt Maddox KE. Call to action: rural health: a presidential advisory from the American Heart Association and American Stroke Association. Circulation. 2020;141:E615–E644. doi: 10.1161/CIR.0000000000000753 [DOI] [PubMed] [Google Scholar]
- 11. The American College of Obstetricians and Gynecologists . Committee Opinion: health disparities in rural women. Obstet Gynecol. 2014;123:384–388. [DOI] [PubMed] [Google Scholar]
- 12. Kozhimannil KB, Interrante JD, Henning‐Smith C, Admon LK. Rural‐urban differences in severe maternal morbidity and mortality in the us, 2007–15. Health Aff. 2019;38:2077–2085. doi: 10.1377/hlthaff.2019.00805 [DOI] [PubMed] [Google Scholar]
- 13. Cameron NA, Molsberry R, Pierce JB, Perak AM, Grobman WA, Allen NB, Greenland P, Lloyd‐Jones DM, Khan SS. Pre‐pregnancy hypertension among women in rural and urban areas of the United States. J Am Coll Cardiol. 2020;76:2611–2619. doi: 10.1016/j.jacc.2020.09.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United Stated Department of Health and Human Services (US DHSS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), Division of Vitality Statistics. Natality public‐use data on CDC WONDER Online Database. Available at https://wonder.cdc.gov/natality.html. Accessed December 20, 2020.
- 15. Ingram D, Franco S. NCHS urban – rural classification scheme for counties. National center for health statistics. Vital Health Stat. 2014;2:1–7 [PubMed] [Google Scholar]
- 16. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2001;20:655. doi: 10.1002/sim.811 [DOI] [PubMed] [Google Scholar]
- 17.Joinpoint Regression Program, Version 4.8.0.1 ‐ April 2020; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute.
- 18. StataCorp . Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 19. Cohen SA, Greaney ML, Sabik NJ. Assessment of dietary patterns, physical activity and obesity from a national survey: rural‐urban health disparities in older adults. PLoS One. 2018;13:1–15. doi: 10.1371/journal.pone.0208268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hung P, Henning‐Smith CE, Casey MM, Kozhimannil KB. Access to obstetric services in rural counties still declining, with 9 percent losing services, 2004–14. Health Aff. 2017;36:1663–1671. [DOI] [PubMed] [Google Scholar]
- 21. Kershaw KN, Marsh DJ, Crenshaw EG, McNeil RB, Pemberton VL, Cordon SA, Haas DM, Debbink MP, Mercer BM, Parry S, et al. Associations of the neighborhood built environment with physical activity across pregnancy. J Phys Act Health. 2021;18:541–547. doi: 10.1123/jpah.2020-0510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Medicare and Medicaid Services. Improving access to maternal health care in rural communities: an issue brief. 2020.
- 23. Bicocca MJ, Mendez‐Figueroa H, Chauhan SP, Sibai BM. Maternal obesity and the risk of early‐onset and late‐onset hypertensive disorders of pregnancy. Obstet Gynecol. 2020;136:118–127. doi: 10.1097/AOG.0000000000003901 [DOI] [PubMed] [Google Scholar]
- 24. Sorensen TK, Williams MA, Lee IM, Dashow EE, Lou TM, Luthy DA. Recreational physical activity during pregnancy and risk of preeclampsia. Hypertension. 2003;41:1273–1280. doi: 10.1161/01.HYP.0000072270.82815.91 [DOI] [PubMed] [Google Scholar]
- 25. Schoenaker DAJM, Soedamah‐Muthu SS, Callaway LK, Mishra GD. Prepregnancy dietary patterns and risk of developing hypertensive disorders of pregnancy: results from the Australian Longitudinal Study on Women’s Health. Am J Clin Nutr. 2015;102:94–101. doi: 10.3945/ajcn.114.102475 [DOI] [PubMed] [Google Scholar]
- 26. National Toxicology Program . NTP monograph on the systematic review of traffic‐related air pollution and hypertensive disorders of pregnancy. NTP Monogr. 2019;7:1–115. doi: 10.22427/NTP-MGRAPH-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weber KA, Lyons E, Yang W, Stevenson C, Stevenson DK, Shaw GM. Residential proximity to green space and preeclampsia in California. Environ Epidemiol. 2020;4:e120. doi: 10.1097/EE9.0000000000000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bhatnagar A. Environmental determinants of cardiovascular disease. Circ Res. 2017;121:162–180. doi: 10.1161/CIRCRESAHA.117.306458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salow AD, Pool LR, Grobman WA, Kershaw KN. Associations of neighborhood‐level racial residential segregation with adverse pregnancy outcomes. Am J Obstet Gynecol. 2018;218:351.e1–351.e7. doi: 10.1016/j.ajog.2018.01.022 [DOI] [PubMed] [Google Scholar]
- 30. Mayne SL, Pellissier BF, Kershaw KN. Neighborhood physical disorder and adverse pregnancy outcomes among women in Chicago: a cross‐sectional analysis of electronic health record data. J Urban Health. 2019;96:823–834. doi: 10.1007/s11524-019-00401-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ghosh G, Grewal J, Männistö T, Mendola P, Chen Z, Xie Y, Laughon SK. Racial/ethnic differences in pregnancy‐related hypertensive disease in nulliparous women. Ethn Dis. 2014;24:283–289. [PMC free article] [PubMed] [Google Scholar]
- 32. Zamora‐Kapoor A, Nelson LA, Buchwald DS, Walker LR, Mueller BA. Pre‐eclampsia in American Indians/Alaska Natives and Whites: the significance of body mass index. Matern Child Health J. 2016;20:2233–2238. doi: 10.1007/s10995-016-2126-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gad MM, Elgendy IY, Mahmoud AN, Saad AM, Isogai T, Mathias IS, Rameez RM, Chahine J, Jneid H, Kapadia SR. Disparities in cardiovascular disease outcomes among pregnant and post‐partum women. J Am Heart Assoc. 2021;10:1–15. doi: 10.1161/JAHA.120.017832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hauspurg A, Lemon L, Cabrera C, Javaid A, Binstock A, Quinn B, Larkin J, Watson AR, Beigi RH, Simhan H. Racial differences in postpartum blood pressure trajectories among women after a hypertensive disorder of pregnancy. JAMA Netw Open. 2020;3:e2030815. doi: 10.1001/jamanetworkopen.2020.30815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bell CN, Owens‐Young JL. Self‐rated health and structural racism indicated by county‐level racial inequalities in socioeconomic status: the role of urban‐rural classification. J Urban Health. 2020;97:52–61. doi: 10.1007/s11524-019-00389-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williams AD, Shenassa E, Slopen N, Rossen L. Cardiometabolic dysfunction among U.S. adolescents and area‐level poverty: race/ethnicity‐specific associations. J Adolesc Health. 2018;63:546–553. doi: 10.1016/j.jadohealth.2018.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dietz P, Bombard J, Mulready‐Ward C, Gauthier J, Sackoff J, Brozicevic P, Gambatese M, Funke M, England L, Harrison L, et al. Validation of selected items on the 2003 U.S. Standard certificate of live birth: New York city and Vermont. Public Health Rep. 2015;130:60–70. doi: 10.1177/003335491513000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gregory ECW, Drake P, Martin JA. Lack of change in perinatal mortality in the United States, 2014‐2016. NCHS Data Brief. 2018;316:1–8. [PubMed] [Google Scholar]
- 39. Kallela J, Jääskeläinen T, Kortelainen E, Heinonen S, Kajantie E, Kere J, Kivinen K, Pouta A, Laivuori H. The diagnosis of pre‐eclampsia using two revised classifications in the Finnish Pre‐eclampsia Consortium (FINNPEC) cohort. BMC Pregnancy Childbirth. 2016;16:1–7. doi: 10.1186/s12884-016-1010-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roberts JM, August PA, Bakris G, Barton JR, Bernstein IM, Druzin M, Gaiser RR. Hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–1131.24150027 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S8


