Abstract
Background
Given that percutaneous coronary intervention (PCI) of a chronic total occlusion (CTO) is indicated primarily for symptom relief, identifying patients most likely to benefit is critically important for patient selection and shared decision‐making. Therefore, we identified factors associated with residual angina frequency after CTO PCI and developed a model to predict postprocedure anginal burden.
Methods and Results
Among patients in the OPEN‐CTO (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures) registry, we evaluated the association between patient characteristics and residual angina frequency at 6 months, as assessed by the Seattle Angina Questionnaire Angina Frequency Scale. We then constructed a prediction model for angina status after CTO PCI using ordinal regression. Among 901 patients undergoing CTO PCI, 28% had no angina, 31% had monthly angina, 30% had weekly angina, and 12% had daily angina at baseline. Six months later, 53% of patients had a ≥20‐point increase in Seattle Angina Questionnaire Angina Frequency Scale score. The final model to predict residual angina after CTO PCI included baseline angina frequency, baseline nitroglycerin use frequency, dyspnea symptoms, depressive symptoms, number of antianginal medications, PCI indication, and presence of multiple CTO lesions and had a C index of 0.78. Baseline angina frequency and nitroglycerin use frequency explained 71% of the predictive power of the model, and the relationship between model components and angina improvement at 6 months varied by baseline angina status.
Conclusions
A 7‐component OPEN‐AP (OPEN‐CTO Angina Prediction) score can predict angina improvement and residual angina after CTO PCI using variables commonly available before intervention. These findings have implications for appropriate patient selection and counseling for CTO PCI.
Keywords: angina, angina frequency, chronic total occlusion, patient selection, percutaneous coronary intervention, prediction, score
Subject Categories: Percutaneous Coronary Intervention, Catheter-Based Coronary and Valvular Interventions, Revascularization, Stent, Quality and Outcomes, Chronic Ischemic Heart Disease
Nonstandard Abbreviations and Acronyms
- CTO
chronic total occlusion
- OPEN‐AP
OPEN‐CTO Angina Prediction
- OPEN‐CTO
Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures
- PHQ‐8
Patient Health Questionnaire‐8
- SAQ
Seattle Angina Questionnaire
Clinical Perspective
What Is New?
The OPEN‐AP score, a 7‐component score including baseline angina frequency, nitroglycerin use frequency, dyspnea symptoms, depressive symptoms, number of antianginal medications, percutaneous coronary intervention (PCI) indication, and presence of multiple chronic total occlusion (CTO) lesions, can predict angina improvement and residual angina after CTO PCI.
Baseline angina frequency and nitroglycerin use frequency explained 71% of the predictive power of the model, and the relationship between model components and angina improvement at 6 months varied by baseline angina status.
What Are the Clinical Implications?
The novel OPEN‐AP score presented in this study can enable clinicians to take patient heterogeneity of benefit into account in clinical decision‐making for CTO PCI.
Coupled with the knowledge of a patient’s baseline symptoms and risk tolerance, the OPEN‐AP tool can inform both the expected magnitude of angina reduction from attempted CTO PCI, as well as the amount of residual angina patients might expect at 6 months after the procedure.
These findings have important implications for appropriate patient selection and counseling for CTO PCI.
Percutaneous coronary intervention (PCI) is often performed for patients with chronic stable coronary artery disease, 1 and has been shown to improve angina symptoms in large randomized controlled trials. 2 Chronic total occlusion (CTO) of a coronary artery is often found on routine diagnostic angiography, but PCI of such lesions is not frequently attempted. 3 , 4 , 5 , 6 , 7 Newer approaches have made it possible to achieve technical success in crossing CTO lesions, 8 , 9 , 10 though complication rates are higher. 11
Understanding the expected angina relief from CTO PCI is essential to support shared decision‐making with patients, particularly given the increased procedural risks associated with CTO PCI. Many studies have shown an improvement in anginal symptoms after CTO PCI, 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 but the patient response is not uniform. 13 Although there are models to predict technical success in CTO PCI, 20 , 21 , 22 , 23 data on patient‐level predictors of angina improvement after CTO PCI are lacking. Identifying which patients may be the most likely to experience angina improvement, and which others may have residual angina, is critically important to inform appropriate patient selection for these higher risk procedures.
To address this gap in knowledge, we leveraged a large registry of consecutive CTO PCI procedures to describe factors associated with residual angina after CTO PCI and developed a prediction model for postprocedure angina. Such results can guide individualized clinical decision‐making, patient counseling, and patient selection.
Methods
Study Population
The OPEN‐CTO (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures) registry is an investigator‐initiated, observational cohort of 1000 consecutive patients undergoing CTO PCI at 12 high‐volume centers in the United States. As previously described, all adults scheduled for a CTO PCI by an experienced CTO operator proficient with the hybrid approach were included if they were able to comply with telephone follow‐up. 24 CTO was defined as a 100% occlusion of a coronary artery known to be occluded for at least 3 months with an antegrade intraluminal TIMI (Thrombolysis In Myocardial Infarction) flow grade of 0. Informed consent was obtained from every participant, and each institution’s own institutional review board approved the study. The analytic cohort for this study included all patients in the OPEN‐CTO registry with complete baseline and 6‐month Seattle Angina Questionnaire (SAQ) Angina Frequency Scale scores. Anonymized data that support the findings of this study can be made available from the corresponding author upon reasonable request.
Study Outcomes and Variables
Baseline data collection was performed by on‐site research nurses who were trained by the project coordinator. An experienced, centralized call center with staff trained in health‐status interviewing techniques performed all follow‐up assessments at 6 months. In addition to a detailed health‐status assessment, patients were queried about current medications, medication discontinuance and reason, and rehospitalization events and their causes. Patient‐reported health status was captured using the SAQ (4‐week recall, scale 0–100, with higher scores indicating less frequent angina, better function, and better quality of life), 25 the Rose Dyspnea Scale (scale 0 to 4, with higher scores indicating dyspnea with less strenuous activities), 26 and the Patient Health Questionnaire‐8 (scale 0–24, with higher scores indicating more depressive symptoms). 27
The primary outcome of this study was residual angina 6 months after CTO PCI, as assessed by the SAQ Angina Frequency Scale score (scale 0–100, with higher scores indicating less frequent angina). 28 The SAQ Angina Frequency Scale score has been used previously to assess symptoms in a CTO population. 29 , 30 For the purposes of prediction modeling, the SAQ Angina Frequency Scale score at 6 months was selected as the primary outcome in lieu of predicting change in angina scores to avoid the limitations imparted by floor and ceiling effects of the 0‐ to 100‐point scale of the SAQ. 31 , 32 Nevertheless, baseline angina frequency was included in the model so a clinically interpretable improvement in angina frequency could be calculated from the model’s output. As a secondary analysis, we examined a binary indicator for a ≥20‐point increase in the SAQ Angina Frequency Scale score, representing a clinically large improvement in angina, 28 6 months after CTO PCI.
Statistical Analysis
We evaluated the unadjusted associations between baseline characteristics and SAQ Angina Frequency Scale score at 6 months using the linear trend test for continuous variables and the Mantel‐Haenszel trend test for categorical variables.
We then used an ordinal regression model to predict 6‐month SAQ Angina Frequency Scale score while considering a range of candidate predictor variables. To mitigate the risk of overfitting, we divided these variables into those that were judged a priori to be likely associated with residual angina and those for which the association was less certain. The first group included the 2 SAQ Angina Frequency Scale questions (frequency of symptoms and frequency of nitroglycerin use), the individual items of the Rose Dyspnea Scale, presence of depressive symptoms (Patient Health Questionnaire‐8 score ≥10), number of antianginal medications on arrival, indication for CTO PCI (symptom relief/ischemia reduction versus other), presence of multiple CTO lesions, and presence of any non‐CTO lesions. These variables comprised the primary model. The remaining candidate predictors included age, sex, body mass index, current smoking status, diabetes, prior myocardial infarction, prior PCI, prior coronary artery bypass grafting, prior stroke/transient ischemic attack, chronic heart failure, chronic kidney disease, chronic lung disease, left ventricular ejection fraction, and SAQ Physical Limitation and Quality of Life scores. We assessed the combined contribution of these secondary predictors as a whole by a likelihood ratio test comparing a model with all primary and secondary candidate predictors to one with only the primary predictors. The P value for this test was 0.80, suggesting no evidence of additional explanatory power provided by the secondary predictors. Notably, we did not consider procedural success as a candidate variable for inclusion in our model, because we wanted all potential predictors to be available before the procedure to aid in clinical decision‐making about whether to pursue CTO PCI intervention.
Examination of observed versus predicted outcomes and model Akaike information criterion suggested that a complementary log‐log link provided reasonable fit to the data. Effects of numerical variables were fit using restricted cubic splines to allow for nonlinear relationships. Ordinal regression yielded predicted probabilities of each possible value of the outcome (SAQ Angina Frequency Scale score) for each patient. We converted these into an expected score for each patient by integrating the possible scores over their probabilities. We examined model calibration by smoothed plots of observed versus predicted values, and discrimination by the C index. We performed internal model validation using bootstrap methods and examined the bootstrap‐corrected C index and calibration slope to assess the degree of overfitting risk. Finally, we constructed a simplified prediction rule using backward selection on the linear predictor, dropping variables with the smallest contribution to the model, as measured by the F value, until the reduced model explained no <95% of the predictive capacity of the full model. 32 We took the β weights from the simplified model and converted them into integer points, and then mapped the total points to expected 6‐month Angina Frequency Scale scores.
We also assessed discrimination by the C statistic for observed and predicted values of our secondary outcome of a ≥20‐point increase in the SAQ Angina Frequency Scale score at 6 months.
In supplemental analysis, we assessed the possible contribution of the 5 J‐CTO (Multicenter CTO Registry of Japan) score components (blunt cap, occlusion length ≥20mm, bending >45°, calcification, retry of lesion) 21 as a whole by a likelihood ratio test comparing a model with all primary and J‐CTO candidate predictors to one with only the primary predictors. The P value for this test was 0.51, suggesting no evidence of additional explanatory power provided by the J‐CTO predictors. We additionally assessed performance of our model only on the subset of patients with procedural success to better understand the chance for angina improvement after a successful procedure. We performed multiple imputation using chained equations for missing values. Statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Patient Population
Among 1000 patients enrolled between January 21, 2014, and July 22, 2015, 901 patients (90%) had SAQ data available at baseline and 6 months and were included in this analysis. Patients included in the analyses were similar to those excluded with respect to baseline angina frequency, though excluded patients were more likely to have diabetes, prior MI, congestive heart failure, chronic kidney disease, and non‐CTO lesions, and were less likely to have a CTO indication for symptom relief (Table S1).
Mean age was 65.7±9.9 years, 81% were men, 40% had diabetes, and 38% had prior coronary artery bypass grafting (Table). Fifty‐four percent of patients were taking at least 2 antianginal medications. Mean baseline SAQ Angina Frequency Scale score was 70±27, and mean Rose Dyspnea Scale score was 2.2±1.5, with 41% of patients reporting weekly or daily angina within the prior 4 weeks.
Table 1.
Baseline Patient Characteristics by Angina Frequency at 6 Months
| Variable | Total | SAQ angina frequency, 6 mo | P value* | |||
|---|---|---|---|---|---|---|
| n=901 | Daily, 0–30, n=23 | Weekly, 40–60, n=70 | Monthly, 70–90, n=104 | None, 100, n=704 | ||
| Sociodemographics | ||||||
| Age, y | 65.7±9.9 | 63.1±13.0 | 63.9±10.4 | 66.2±10.3 | 65.8±9.7 | 0.111 |
| Sex | 0.303 | |||||
| Men | 728 (80.8%) | 20 (87.0%) | 51 (72.9%) | 82 (78.8%) | 575 (81.7%) | |
| Women | 173 (19.2%) | 3 (13.0%) | 19 (27.1%) | 22 (21.2%) | 129 (18.3%) | |
| Marital status | 0.121 | |||||
| Married | 630 (70.2%) | 15 (68.2%) | 46 (65.7%) | 62 (59.6%) | 507 (72.2%) | |
| Divorced/separated | 134 (14.9%) | 2 (9.1%) | 14 (20.0%) | 25 (24.0%) | 93 (13.2%) | |
| Widowed | 81 (9.0%) | 3 (13.6%) | 5 (7.1%) | 9 (8.7%) | 64 (9.1%) | |
| Single/other | 53 (5.9%) | 2 (9.1%) | 5 (7.1%) | 8 (7.7%) | 38 (5.4%) | |
| Missing | 3 | 1 | 2 | |||
| Living alone | 179 (20.1%) | 2 (9.1%) | 17 (24.6%) | 27 (26.2%) | 133 (19.1%) | 0.551 |
| Missing | 12 | 1 | 1 | 1 | 9 | |
| Completed high school | 807 (90.3%) | 19 (86.4%) | 62 (88.6%) | 88 (85.4%) | 638 (91.3%) | 0.124 |
| Missing | 7 | 1 | 1 | 5 | ||
| Finances at the end of the month | <0.001 | |||||
| Some money left over | 537 (60.3%) | 9 (40.9%) | 32 (47.1%) | 56 (53.8%) | 440 (63.2%) | |
| Just enough to make ends meet | 247 (27.8%) | 7 (31.8%) | 21 (30.9%) | 28 (26.9%) | 191 (27.4%) | |
| Not enough to make ends meet | 106 (11.9%) | 6 (27.3%) | 15 (22.1%) | 20 (19.2%) | 65 (9.3%) | |
| Missing | 11 | 1 | 2 | 8 | ||
| Working status | 0.002 | |||||
| No | 547 (61.0%) | 19 (86.4%) | 50 (72.5%) | 74 (71.2%) | 404 (57.5%) | |
| Yes, full time | 278 (31.0%) | 1 (4.5%) | 13 (18.8%) | 22 (21.2%) | 242 (34.5%) | |
| Yes, part time | 72 (8.0%) | 2 (9.1%) | 6 (8.7%) | 8 (7.7%) | 56 (8.0%) | |
| Missing | 4 | 1 | 1 | 2 | ||
| Insurance coverage for medications | 853 (95.0%) | 21 (95.5%) | 65 (92.9%) | 97 (93.3%) | 670 (95.4%) | 0.347 |
| Missing | 3 | 1 | 2 | |||
| Have avoided health care because of costs | <0.001 | |||||
| Always | 25 (2.8%) | 1 (4.5%) | 5 (7.1%) | 4 (3.8%) | 15 (2.2%) | |
| Frequently | 38 (4.3%) | 1 (4.5%) | 8 (11.4%) | 3 (2.9%) | 26 (3.7%) | |
| Occasionally | 56 (6.3%) | 0 (0.0%) | 3 (4.3%) | 7 (6.7%) | 46 (6.6%) | |
| Rarely | 80 (9.0%) | 5 (22.7%) | 15 (21.4%) | 9 (8.7%) | 51 (7.3%) | |
| Never | 694 (77.7%) | 15 (68.2%) | 39 (55.7%) | 81 (77.9%) | 559 (80.2%) | |
| Missing | 8 | 1 | 7 | |||
| Clinical characteristics | ||||||
| BMI | 30.4±5.9 | 29.9±5.3 | 32.1±6.8 | 30.7±6.8 | 30.2±5.7 | 0.899 |
| Smoking history | 0.027 | |||||
| Current smoker | 111 (12.5%) | 2 (8.7%) | 14 (20.3%) | 15 (14.7%) | 80 (11.5%) | |
| Former smoker | 459 (51.6%) | 12 (52.2%) | 35 (50.7%) | 64 (62.7%) | 348 (50.0%) | |
| Never smoked | 320 (36.0%) | 9 (39.1%) | 20 (29.0%) | 23 (22.5%) | 268 (38.5%) | |
| Missing | 11 | 1 | 2 | 8 | ||
| Diabetes | 362 (40.2%) | 15 (65.2%) | 32 (45.7%) | 41 (39.4%) | 274 (38.9%) | 0.024 |
| Hypertension | 771 (85.6%) | 23 (100.0%) | 63 (90.0%) | 88 (84.6%) | 597 (84.8%) | 0.049 |
| Prior MI | 423 (46.9%) | 14 (60.9%) | 35 (50.0%) | 57 (54.8%) | 317 (45.0%) | 0.044 |
| Prior PCI | 594 (65.9%) | 19 (82.6%) | 47 (67.1%) | 75 (72.1%) | 453 (64.3%) | 0.059 |
| Prior CABG | 338 (37.5%) | 19 (82.6%) | 39 (55.7%) | 44 (42.3%) | 236 (33.5%) | <0.001 |
| Prior stroke/TIA | 82 (9.1%) | 3 (13.0%) | 4 (5.7%) | 11 (10.6%) | 64 (9.1%) | 0.938 |
| Congestive heart failure | 194 (21.5%) | 3 (13.0%) | 17 (24.3%) | 20 (19.2%) | 154 (21.9%) | 0.626 |
| Chronic kidney disease | 112 (12.4%) | 5 (21.7%) | 6 (8.6%) | 20 (19.2%) | 81 (11.5%) | 0.264 |
| Chronic lung disease | 126 (14.0%) | 5 (21.7%) | 16 (22.9%) | 11 (10.6%) | 94 (13.4%) | 0.061 |
| No. of antianginal medications on arrival | <0.001 | |||||
| 0 | 72 (8.0%) | 0 (0.0%) | 1 (1.4%) | 8 (7.7%) | 63 (8.9%) | |
| 1 | 342 (38.0%) | 5 (21.7%) | 16 (22.9%) | 33 (31.7%) | 288 (40.9%) | |
| 2 | 342 (38.0%) | 4 (17.4%) | 29 (41.4%) | 43 (41.3%) | 266 (37.8%) | |
| 3 | 122 (13.5%) | 9 (39.1%) | 22 (31.4%) | 16 (15.4%) | 75 (10.7%) | |
| 4 | 23 (2.6%) | 5 (21.7%) | 2 (2.9%) | 4 (3.8%) | 12 (1.7%) | |
| Ischemia on stress test | 438 (89.2%) | 9 (100.0%) | 27 (90.0%) | 61 (92.4%) | 341 (88.3%) | 0.230 |
| Missing | 410 | 14 | 40 | 38 | 318 | |
| LV ejection fraction | 51.4±13.3 | 55.1±14.6 | 51.1±12.9 | 51.7±13.0 | 51.2±13.4 | 0.242 |
| Missing | 81 | 3 | 8 | 7 | 63 | |
| CTO characteristics | ||||||
| Indication for CTO intervention | <0.001 | |||||
| Symptom relief | 659 (73.1%) | 21 (91.3%) | 64 (91.4%) | 86 (82.7%) | 488 (69.3%) | |
| Ischemia reduction | 97 (10.8%) | 1 (4.3%) | 5 (7.1%) | 9 (8.7%) | 82 (11.6%) | |
| Staged procedure | 48 (5.3%) | 1 (4.3%) | 0 (0.0%) | 3 (2.9%) | 44 (6.3%) | |
| Reduced ejection fraction | 42 (4.7%) | 0 (0.0%) | 0 (0.0%) | 2 (1.9%) | 40 (5.7%) | |
| Other | 55 (6.1%) | 0 (0.0%) | 1 (1.4%) | 4 (3.8%) | 50 (7.1%) | |
| Primary CTO vessel | 0.210 | |||||
| LAD | 182 (20.2%) | 6 (26.1%) | 9 (12.9%) | 13 (12.5%) | 154 (21.9%) | |
| LCX | 153 (17.0%) | 4 (17.4%) | 11 (15.7%) | 23 (22.1%) | 115 (16.3%) | |
| LM | 8 (0.9%) | 0 (0.0%) | 1 (1.4%) | 1 (1.0%) | 6 (0.9%) | |
| RCA | 558 (61.9%) | 13 (56.5%) | 49 (70.0%) | 67 (64.4%) | 429 (60.9%) | |
| Multiple CTO lesions | 48 (5.3%) | 3 (13.0%) | 6 (8.6%) | 12 (11.5%) | 27 (3.8%) | <0.001 |
| Any non‐CTO lesions | 117 (13.0%) | 2 (8.7%) | 8 (11.4%) | 13 (12.5%) | 94 (13.4%) | 0.444 |
| J‐CTO score components | ||||||
| Blunt cap | 574 (63.7%) | 18 (78.3%) | 43 (61.4%) | 71 (68.3%) | 442 (62.8%) | 0.272 |
| Length >20 | 553 (61.4%) | 19 (82.6%) | 44 (62.9%) | 69 (66.3%) | 421 (59.8%) | 0.040 |
| Bending | 494 (54.8%) | 11 (47.8%) | 40 (57.1%) | 65 (62.5%) | 378 (53.7%) | 0.559 |
| Calcification | 297 (33.0%) | 11 (47.8%) | 23 (32.9%) | 35 (33.7%) | 228 (32.4%) | 0.294 |
| Retry | 182 (20.2%) | 6 (26.1%) | 12 (17.1%) | 22 (21.2%) | 142 (20.2%) | 0.930 |
| Procedural success | 745 (82.7%) | 12 (52.2%) | 53 (75.7%) | 83 (79.8%) | 597 (84.8%) | <0.001 |
| Baseline health status | ||||||
| SAQ Angina Frequency Scale score | 70.3±27.0 | 38.7±24.0 | 46.6±20.7 | 58.7±25.0 | 75.4±25.4 | <0.001 |
| SAQ Angina Frequency Scale score categories | <0.001 | |||||
| Daily, 0–30 | 104 (11.5%) | 9 (39.1%) | 20 (28.6%) | 19 (18.3%) | 56 (8.0%) | |
| Weekly, 40–60 | 266 (29.5%) | 11 (47.8%) | 37 (52.9%) | 45 (43.3%) | 173 (24.6%) | |
| Monthly, 70–90 | 275 (30.5%) | 3 (13.0%) | 12 (17.1%) | 29 (27.9%) | 231 (32.8%) | |
| None, 100 | 256 (28.4%) | 0 (0.0%) | 1 (1.4%) | 11 (10.6%) | 244 (34.7%) | |
| SAQ Question 3: Angina frequency | <0.001 | |||||
| 4 or more times per d | 93 (10.4%) | 5 (21.7%) | 15 (21.4%) | 16 (15.4%) | 57 (8.1%) | |
| 1–3 times per d | 174 (19.4%) | 9 (39.1%) | 25 (35.7%) | 25 (24.0%) | 115 (16.4%) | |
| 3 or more times per wk | 157 (17.5%) | 7 (30.4%) | 21 (30.0%) | 30 (28.8%) | 99 (14.1%) | |
| 1–2 times per wk | 109 (12.1%) | 0 (0.0%) | 8 (11.4%) | 12 (11.5%) | 89 (12.7%) | |
| Less than once a wk | 98 (10.9%) | 1 (4.3%) | 0 (0.0%) | 7 (6.7%) | 90 (12.8%) | |
| None over the past 4 wk | 267 (29.7%) | 1 (4.3%) | 1 (1.4%) | 14 (13.5%) | 251 (35.8%) | |
| Missing | 3 | 3 | ||||
| SAQ Question 4: Nitroglycerin frequency | <0.001 | |||||
| 4 or more times per d | 18 (2.0%) | 1 (4.5%) | 2 (2.9%) | 1 (1.0%) | 14 (2.0%) | |
| 1–3 times per d | 64 (7.1%) | 5 (22.7%) | 10 (14.5%) | 12 (11.7%) | 37 (5.3%) | |
| 3 or more times per wk | 48 (5.4%) | 5 (22.7%) | 12 (17.4%) | 11 (10.7%) | 20 (2.8%) | |
| 1–2 times per wk | 55 (6.1%) | 5 (22.7%) | 9 (13.0%) | 14 (13.6%) | 27 (3.8%) | |
| Less than once a wk | 111 (12.4%) | 3 (13.6%) | 12 (17.4%) | 11 (10.7%) | 85 (12.1%) | |
| None over the past 4 wk | 600 (67.0%) | 3 (13.6%) | 24 (34.8%) | 54 (52.4%) | 519 (73.9%) | |
| Missing | 5 | 1 | 1 | 1 | 2 | |
| SAQ quality‐of‐life score, baseline | 49.7±27.1 | 30.8±20.0 | 27.9±18.7 | 40.7±23.3 | 53.8±27.0 | <0.001 |
| Missing | 3 | 0 | 0 | 0 | 3 | |
| SAQ physical limitation score, baseline | 65.8±26.1 | 48.3±23.8 | 49.0±23.3 | 57.9±26.3 | 69.3±25.3 | <0.001 |
| Missing | 57 | 2 | 1 | 9 | 45 | |
| SAQ summary score, baseline | 61.9±22.5 | 38.7±18.1 | 41.2±15.9 | 52.0±19.3 | 66.2±21.5 | <0.001 |
| PHQ‐8 score, baseline | 6.2±5.5 | 10.8±6.7 | 8.9±6.2 | 7.8±5.9 | 5.6±5.1 | <0.001 |
| Missing | 21 | 2 | 2 | 0 | 17 | |
| Rose Dyspnea Scale, baseline | 2.2±1.5 | 2.7±1.5 | 3.0±1.2 | 2.8±1.4 | 2.0±1.5 | 0.023 |
| Missing | 12 | 1 | 3 | 1 | 7 | |
BMI indicates body mass index; CABG, coronary artery bypass graft; CTO, chronic total occlusion; J‐CTO, Multicenter CTO Registry of Japan; MI, myocardial infarction; PCI, percutaneous coronary intervention; PHQ‐8, Patient Health Questionnaire‐8; SAQ, Seattle Angina Questionnaire; TIA, transient ischemic attack; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; LM, left main coronary artery; and RCA, right coronary artery.
Continuous variables compared using linear trend test. Categorical variables compared using Mantel‐Haenszel trend test.
Angina Frequency After CTO PCI
At 6 months after CTO PCI, 78% of patients had no angina, whereas 3% had daily angina, 8% weekly angina, and 12% monthly angina (Table). Patients with more frequent angina at 6 months were more likely to have traditional risk factors including diabetes, hypertension, prior myocardial infarction, or prior coronary artery bypass grafting as well as social stressors (difficulty with finances at the end of the month, no employment). Additionally, patients with more angina at 6 months were more likely to have multiple CTO lesions, to have undergone CTO PCI for symptom relief, and to have been taking more antianginal medications before CTO PCI. Patients with greater residual angina were also much more likely to have had more angina at baseline (Figure 1). Specifically, 61% of those with daily angina after CTO had angina daily before CTO (with 22% of patients with 4 or more times per day), whereas only 25% of those with no angina after CTO PCI had daily angina at baseline (8% with angina 4 or more times/day). Similarly, 27% of patients with daily angina used nitroglycerin at least daily at baseline, whereas only 7% of patients with no angina after CTO PCI used nitroglycerin at least daily at baseline. Patients with higher 6‐month angina frequency also had higher baseline Patient Health Questionnaire‐8 scores and greater dyspnea as well as worse physical limitation and angina‐related quality of life as assessed by the SAQ.
Figure 1. Observed 6‐month angina frequency by baseline angina.
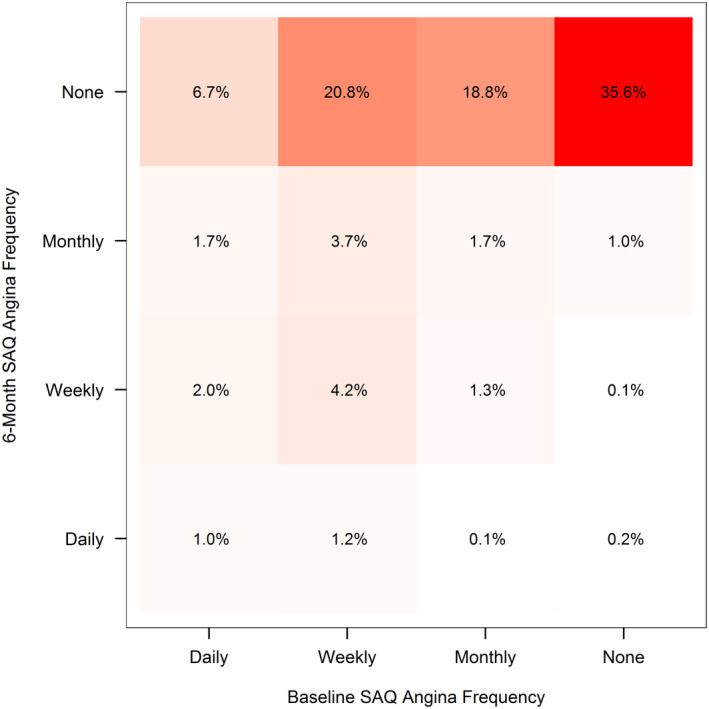
SAQ indicates Seattle Angina Questionnaire.
Angina Prediction Model
The full model to predict 6‐month Angina Frequency Scale score including all primary predictors had an apparent R 2 of 0.25 and a C index of 0.78, as well as a bootstrap validation‐adjusted R 2 of 0.22, C index of 0.77, and calibration slope of 0.92, suggesting good model fit and discrimination. The final simplified model included 7 variables: baseline angina frequency and baseline nitroglycerin use (ie, baseline SAQ Angina Frequency Scale score), Rose Dyspnea Scale score ≥ 2 (shortness of breath when walking with others), Patient Health Questionnaire‐8 score ≥10, number of antianginal medications, indication for PCI (symptom/ischemia reduction or not), and the presence of multiple CTO lesions (Figure 2; online calculator available at https://myhealthoutcomes.org). The reduced model had an R 2 of 0.24 and a C index of 0.78. Baseline SAQ Angina Frequency Scale score explained 71% of the predictive power of the model. The median predicted risk of having at least weekly angina symptoms in this cohort was 6%, and 7% of patients had at least a 33% chance of daily or weekly angina after CTO PCI.
Figure 2. Chronic total occlusion percutaneous coronary intervention (PCI) angina prediction model.
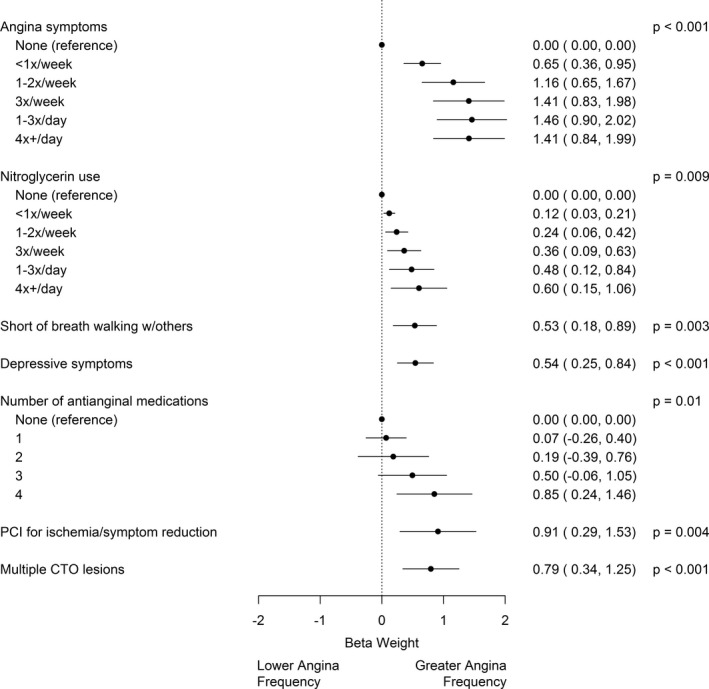
The β coefficients are displayed for each variable in the chronic total occlusion (CTO) angina prediction model and indicate the relative weights of different variables. Coefficients are included in an ordinal regression model to generate the OPEN‐CTO Angina Prediction (OPEN‐AP) score, which indicates the predicted amount of residual angina after CTO PCI.
The OPEN‐CTO Angina Prediction (OPEN‐AP) score was able to separate patients into groups with different levels of residual angina at 6 months angina (Figure 3). Among those with the lowest preprocedure score (0–9), 97% of patients had no angina at 6 months, 3% of patients had monthly angina, and 0.5% of patients had daily or weekly angina. In contrast, among those with the highest preprocedure score (30–40), 42% of patients had no angina at 6 months, 19% of patients had monthly angina, and 40% of patients had daily or weekly angina. Similar results were obtained when stratified by baseline angina frequency status (Figure S1) and when limited to patients only with procedural success (C index 0.76 for 6‐month Angina Frequency Scale score; Figure S2).
Figure 3. Observed 6‐month angina frequency by OPEN‐CTO Angina Prediction (OPEN‐AP) score.
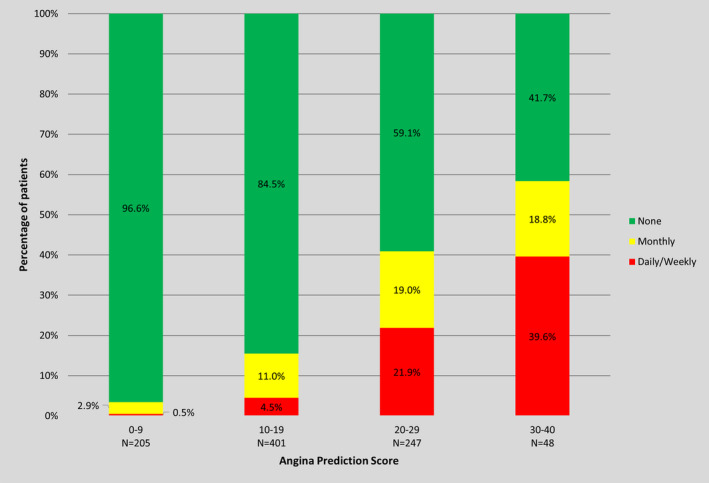
Higher values of the OPEN‐CTO Angina Prediction (OPEN‐AP) score are associated with more frequent angina in a graded manner. OPEN‐CTO indicates Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures.
The OPEN‐AP score was also able to predict whether patients would have a ≥20‐point improvement in SAQ Angina Frequency Scale score at 6 months based on baseline angina frequency status with a C statistic of 0.81 (Figure S3). Patients with more frequent angina at baseline, depressive symptoms, or dyspnea symptoms, as well as those taking greater numbers of antianginals or more frequent nitroglycerin preprocedure, were generally more likely to improve after CTO PCI, and those with multiple CTO lesions were less likely to improve after CTO PCI (Table S2). However, the relationship between several model components and angina improvement at 6 months varied by baseline angina status. Among those with daily or weekly baseline angina, those on a greater number of antianginal medications were less likely to improve, whereas, among those with monthly baseline angina, patients on a greater number of antianginal medications were more likely to improve. Among those with daily baseline angina, patients with depressive symptoms were more likely to improve, whereas depressed patients with less frequent baseline angina were less likely to improve. Conversely, among those with daily or weekly baseline angina, dyspneic patients were less likely to improve, whereas among those with monthly angina, dyspneic patients were more likely to improve.
Discussion
Given the higher risks of CTO PCI, engaging patients in shared decision‐making about whether or not to undergo the procedure mandates providing an estimated outcome from treatment. As the primary benefit of PCI in stable coronary artery disease, including CTO, is to alleviate patients’ angina, we leveraged a large prospective CTO PCI registry to identify specific patient characteristics, identifiable before the procedure, that are associated with residual angina 6 months after treatment. To facilitate the use of these insights in clinical care, we developed the OPEN‐AP score to predict angina frequency after CTO PCI based on information obtained from patients at the bedside. Coupled with knowledge of a patient’s baseline symptoms and risk tolerance, this tool could inform both the expected magnitude of angina reduction from attempted CTO PCI, as well as the amount of residual angina patients might expect at 6 months after the procedure. Such results have implications for appropriate patient selection and counseling for CTO PCI.
This study adds to our understanding of patient characteristics that are most likely to affect improvement in angina frequency after CTO PCI. Although there is significant variation in patient‐reported angina frequency after CTO PCI, 13 few studies have identified specific factors responsible for this variation. A subanalysis of the FlowCardia Approach to CTO Recanalization study found that improvements in patient symptoms, function, and quality of life differed based on whether patients had symptoms at baseline. 33 Prior studies from the OPEN‐CTO registry have found greater improvements in angina among patients with depression at baseline, but no clinically meaningful differences in improvements by sex or diabetes status. 34 , 35 , 36 We extend this work and demonstrate that the relationship between patient characteristics and anginal improvement depends on baseline angina status. Broadly, those patients with more frequent angina before PCI, those taking greater numbers of antianginals and more frequent nitroglycerin, and those with dyspnea were the most likely to have significant improvements in angina with CTO PCI. However, some subtleties in bivariate relationships also emerged after accounting for baseline angina. For instance, among patients with more frequent baseline angina, those on a greater number of antianginal medications were less likely to improve, perhaps suggesting a more refractory anginal syndrome among this patient subset. Additionally, depressed patients with more frequent baseline angina were more likely to improve, but depressed patients with less frequent baseline angina were less likely to improve, suggesting that depression can compound the effects and expected relief from angina.
The OPEN‐AP score can identify patients who might be the most likely to improve after CTO PCI using variables commonly available before intervention. The performance metrics of our model are generally similar in magnitude to other models using ordinal regression analysis in clinical medicine. 31 , 37 , 38 , 39 , 40 Our model is similar to a previously published prediction model for presence of residual angina after PCI of all types in that both models include baseline angina frequency, depressive symptoms, and number of anti‐anginal medications as key components. 41 However, the general post‐PCI residual angina prediction model includes additional variables such as age and avoidance of self‐reported care because of costs, whereas the OPEN‐AP score includes PCI indication and the presence of multiple CTO lesions as predictors. These differences make the OPEN‐AP score uniquely suited to the CTO PCI context.
The OPEN‐AP model predicts the specific frequency of angina for a particular patient at 6 months after CTO PCI, and, combined with knowledge about baseline angina, the predicted improvement in angina. This model provides additional detail beyond simply the presence or absence of angina predicted in the general post‐PCI residual angina prediction model. 41 This additional granularity is crucial for CTO PCI in particular, because many patients with CTO may still have angina after PCI but will experience a clinically relevant improvement compared with their baseline state, which is important to consider. Importantly, among patients starting with severe angina at baseline, patients with lower scores derived significantly greater improvement in symptoms compared with those with higher scores.
This novel score has implications for counseling patients with CTO PCI. In a potentially high‐risk procedure that is primarily indicated for symptom benefit, it is crucial to understand heterogeneity in patient symptoms to identify those patients who are most likely to benefit. Currently, patient conversations about the risks and benefits for CTO PCI are individualized to account for heterogeneity in procedural risk, because existing tools can identify likelihood of technical success for a particular patient based on their coronary anatomy. 21 , 22 However, clinicians previously did not have tools to account for heterogeneity in patient benefit. The OPEN‐AP score enables clinicians to identify the predicted frequency of angina at the end of the procedure for a given patient based on their characteristics and individualize treatment decisions and patient counseling. In our cohort, 7% of patients would have been counseled that there would remain at least a 33% likelihood that they would have significant anginal symptoms even after CTO PCI and may reconsider their options. Notably, the overall complication rate in this same cohort was 14.5%. 42 Interestingly, factors included in existing scores to predict technical success did not have additional predictive ability for predicting residual angina after CTO PCI, 21 suggesting that factors that predict technical success are distinct from factors that predict patient‐reported outcome. Thus, if both technical success and angina prediction tools are applied in concert, clinicians can identify patients for CTO PCI who are most likely to benefit with the highest likelihood of technical success.
This study must be interpreted in context of its limitations. First, there were a limited number of patients with weekly or daily angina after CTO PCI, largely as a result of presumed efficacy of CTO PCI in our sample. As a result, model predictions of patients with frequent angina after CTO PCI may be less reliable. Second, although our sample included real‐world data from 12 hospitals, all centers and operators performed a high‐volume of CTO PCIs, and thus our model needs to be externally validated. Furthermore, the high success rate of CTO PCI in OPEN‐CTO reflects both careful patient selection and the technical skill of the participating operators, both of which could impact the outcomes of patients who undergo CTO PCI outside of these centers. As such, practitioners will need to modify interpretation of angina improvement based on this model based on their own likelihood of technical success for a particular procedure. Importantly, the OPEN‐CTO registry mandated consecutive enrollment of patients, which reduces some selection bias and increases generalizability. Finally, given that this model was derived from a real‐world registry, it is possible we did not include certain potentially relevant variables, such as ischemic burden, because such diagnostic tests were not mandated for all patients for inclusion in the registry.
In conclusion, we used a large observational cohort to identify patient factors associated with residual angina after CTO PCI and developed an angina frequency prediction model. The novel OPEN‐AP score presented in this study can enable clinicians to take patient heterogeneity of benefit into account in clinical decision‐making and counseling on CTO PCI.
Sources of Funding
The OPEN‐CTO study was funded by an unrestricted grant from Boston Scientific.
Disclosures
Dr Secemsky receives grants from AstraZeneca, BD Bard, Boston Scientific, Cook Medical, CSI, Medtronic, and Philips. He receives consulting/speaking fees from Abbott, Bayer, Boston Scientific, Cook, CSI, Medtronic, Inari, Janssen, and Philips, outside the submitted work. Dr Grantham reports speaking fees and honoraria from Abbott Vascular, Asahi Intecc, and Boston Scientific; institutional research grants from Boston Scientific; part time employment with Corindus, a Siemens Healthineers Company; and on the advisory board for Boston Scientific, outside the submitted work. Dr Spertus has been a consultant for Novartis, Amgen, Bayer, Janssen, United Healthcare, and AstraZeneca; has served on the board of directors for Blue Cross Blue Shield of Kansas City; and has equity in Health Outcomes Sciences and copyright ownership of Kansas City Cardiomyopathy Questionnaire, SAQ, and Peripheral Artery Questionnaire. Dr Cohen receives research grant support and consulting income from Edwards Lifesciences, Medtronic, Boston Scientific, and Abbott, outside the submitted work. Dr Salisbury reports institutional research grant support from Boston Scientific and Gilead Sciences and consultancy/advisory board fees from Medtronic, outside the submitted work. Dr Lombardi reports speaking fees and honoraria from Boston Scientific, Abbott Vascular, and Abiomed; consultancy for Vascular Solutions, Abbott Vascular, Boston Scientific, Abiomed, and Roxwood Medical; equity in Roxwood Medical and Bridgepoint Medical; and his wife is an employee of Phillips. Dr Karmpaliotis reports honoraria from Abiomed, Boston Scientific, and Abbott Vascular; and equity in Saranas, Soundbite, and Traverse Vascular, outside the submitted work. Dr Sapontis reports speaking fees and honoraria from Boston Scientific, outside the submitted work. Dr Yeh reports grant/contracts support from Abiomed, Astra Zeneca, BD Bard, Boston Scientific, Cook Medical, Medtronic, and Philips; and consulting fees from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and Shockwave Medical outside the submitted work. The other authors report no conflicts.
Supporting information
Tables S1–S2
Figures S1–S3
For Sources of Funding and Disclosures, see pages 10 and 11.
See Editorial by Simsek et al.
References
- 1. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes: the task force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2019. doi: 10.15829/1560-4071-2020-2-3757 [DOI] [Google Scholar]
- 2. Spertus JA, Jones PG, Maron DJ, O’Brien SM, Reynolds HR, Rosenberg Y, Stone GW, Harrell FE, Boden WE, Weintraub WS, et al. Health‐status outcomes with invasive or conservative care in coronary disease. N Engl J Med. 2020;382:1408–1419. doi: 10.1056/NEJMoa1916370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fefer P, Knudtson ML, Cheema AN, Galbraith PD, Osherov AB, Yalonetsky S, Gannot S, Samuel M, Weisbrod M, Bierstone D, et al. Current perspectives on coronary chronic total occlusions: the Canadian multicenter chronic total occlusions registry. J Am Coll Cardiol. 2012;59:991–997. doi: 10.1016/j.jacc.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 4. Christofferson RD, Lehmann KG, Martin GV, Every N, Caldwell JH, Kapadia SR. Effect of chronic total coronary occlusion on treatment strategy. Am J Cardiol. 2005;95:1088–1091. doi: 10.1016/j.amjcard.2004.12.065 [DOI] [PubMed] [Google Scholar]
- 5. Jeroudi OM, Alomar ME, Michael TT, Sabbagh AE, Patel VG, Mogabgab O, Fuh E, Sherbet D, Lo N, Roesle M, et al. Prevalence and management of coronary chronic total occlusions in a tertiary veterans affairs hospital. Catheter Cardiovasc Interv. 2014;84:637–643. doi: 10.1002/ccd.25264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Werner GS, Gitt AK, Zeymer U, Juenger C, Towae F, Wienbergen H, Senges J. Chronic total coronary occlusions in patients with stable angina pectoris: impact on therapy and outcome in present day clinical practice. Clin Res Cardiol. 2009;98:435–441. doi: 10.1007/s00392-009-0013-5 [DOI] [PubMed] [Google Scholar]
- 7. Secemsky EA, Gallagher R, Harkness J, Pomerantsev E, Gewirtz H, Jaffer FA, Yeh RW. Target vessel revascularization and territory of myocardial ischemia in patients with chronic total occlusions. J Am Coll Cardiol. 2017;70:1196–1197. doi: 10.1016/j.jacc.2017.07.713 [DOI] [PubMed] [Google Scholar]
- 8. Christopoulos G, Karmpaliotis D, Alaswad K, Yeh RW, Jaffer FA, Wyman RM, Lombardi WL, Menon RV, Grantham JA, Kandzari DE, et al. Application and outcomes of a hybrid approach to chronic total occlusion percutaneous coronary intervention in a contemporary multicenter US registry. Int J Cardiol. 2015;198:222–228. doi: 10.1016/j.ijcard.2015.06.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maeremans J, Walsh S, Knaapen P, Spratt JC, Avran A, Hanratty CG, Faurie B, Agostoni P, Bressollette E, Kayaert P, et al. The hybrid algorithm for treating chronic total occlusions in Europe: the recharge registry. J Am Coll Cardiol. 2016;68:1958–1970. doi: 10.1016/j.jacc.2016.08.034 [DOI] [PubMed] [Google Scholar]
- 10. Habara M, Tsuchikane E, Muramatsu T, Kashima Y, Okamura A, Mutoh M, Yamane M, Oida A, Oikawa Y, Hasegawa K. Comparison of percutaneous coronary intervention for chronic total occlusion outcome according to operator experience from the Japanese retrograde summit registry. Catheter Cardiovasc Interv. 2016;87:1027–1035. doi: 10.1002/ccd.26354 [DOI] [PubMed] [Google Scholar]
- 11. Brilakis ES, Banerjee S, Karmpaliotis D, Lombardi WL, Tsai TT, Shunk KA, Kennedy KF, Spertus JA, Holmes DR Jr, Grantham JA. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2015;8:245–253. doi: 10.1016/j.jcin.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 12. Tomasello SD, Boukhris M, Giubilato S, Marzà F, Garbo R, Contegiacomo G, Marzocchi A, Niccoli G, Gagnor A, Varbella F, et al. Management strategies in patients affected by chronic total occlusions: results from the Italian registry of chronic total occlusions. Eur Heart J. 2015;36:3189–3198. doi: 10.1093/eurheartj/ehv450 [DOI] [PubMed] [Google Scholar]
- 13. Sapontis J, Salisbury AC, Yeh RW, Cohen DJ, Hirai T, Lombardi W, McCabe JM, Karmpaliotis D, Moses J, Nicholson WJ, et al. Early procedural and health status outcomes after chronic total occlusion angioplasty: a report from the open‐CTO registry (outcomes, patient health status, and efficiency in chronic total occlusion hybrid procedures). JACC Cardiovasc Interv. 2017;10:1523–1534. doi: 10.1016/j.jcin.2017.05.065 [DOI] [PubMed] [Google Scholar]
- 14. Obedinskiy AA, Kretov EI, Boukhris M, Kurbatov VP, Osiev AG, Ibn Elhadj Z, Obedinskaya NR, Kasbaoui S, Grazhdankin IO, Prokhorikhin AA, et al. The IMPACTOR‐CTO trial. JACC Cardiovasc Interv. 2018;11:1309–1311. doi: 10.1016/j.jcin.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 15. Werner GS, Martin‐Yuste V, Hildick‐Smith D, Boudou N, Sianos G, Gelev V, Rumoroso JR, Erglis A, Christiansen EH, Escaned J, et al. A randomized Multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39:2484–2493. doi: 10.1093/eurheartj/ehy220 [DOI] [PubMed] [Google Scholar]
- 16. Borgia F, Viceconte N, Ali O, Stuart‐Buttle C, Saraswathyamma A, Parisi R, Mirabella F, Dimopoulos K, Di Mario C. Improved cardiac survival, freedom from mace and angina‐related quality of life after successful percutaneous recanalization of coronary artery chronic total occlusions. Int J Cardiol. 2012;161:31–38. doi: 10.1016/j.ijcard.2011.04.023 [DOI] [PubMed] [Google Scholar]
- 17. Ybarra LF, Dautov R, Gibrat C, Dandona S, Rinfret S. Midterm angina‐related quality of life benefits after percutaneous coronary intervention of chronic total occlusions. Can J Cardiol. 2017;33:1668–1674. doi: 10.1016/j.cjca.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 18. Joyal D, Afilalo J, Rinfret S. Effectiveness of recanalization of chronic total occlusions: a systematic review and meta‐analysis. Am Heart J. 2010;160:179–187. doi: 10.1016/j.ahj.2010.04.015 [DOI] [PubMed] [Google Scholar]
- 19. Christakopoulos GE, Christopoulos G, Carlino M, Jeroudi OM, Roesle M, Rangan BV, Abdullah S, Grodin J, Kumbhani DJ, Vo M, et al. Meta‐analysis of clinical outcomes of patients who underwent percutaneous coronary interventions for chronic total occlusions. Am J Cardiol. 2015;115:1367–1375. doi: 10.1016/j.amjcard.2015.02.038 [DOI] [PubMed] [Google Scholar]
- 20. Galassi AR, Boukhris M, Azzarelli S, Castaing M, Marza F, Tomasello SD. Percutaneous coronary revascularization for chronic total occlusions: a novel predictive score of technical failure using advanced technologies. JACC Cardiovasc Interv. 2016;9:911–922. doi: 10.1016/j.jcin.2016.01.036 [DOI] [PubMed] [Google Scholar]
- 21. Morino Y, Abe M, Morimoto T, Kimura T, Hayashi Y, Muramatsu T, Ochiai M, Noguchi Y, Kato K, Shibata Y, et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J‐CTO (multicenter CTO registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4:213–221. doi: 10.1016/j.jcin.2010.09.024 [DOI] [PubMed] [Google Scholar]
- 22. Christopoulos G, Kandzari DE, Yeh RW, Jaffer FA, Karmpaliotis D, Wyman MR, Alaswad K, Lombardi W, Grantham JA, Moses J, et al. Development and validation of a novel scoring system for predicting technical success of chronic total occlusion percutaneous coronary interventions: the progress CTO (prospective global registry for the study of chronic total occlusion intervention) score. JACC Cardiovasc Interv. 2016;9:1–9. doi: 10.1016/j.jcin.2015.09.022 [DOI] [PubMed] [Google Scholar]
- 23. Alessandrino G, Chevalier B, Lefevre T, Sanguineti F, Garot P, Unterseeh T, Hovasse T, Morice MC, Louvard Y. A clinical and angiographic scoring system to predict the probability of successful first‐attempt percutaneous coronary intervention in patients with total chronic coronary occlusion. JACC Cardiovasc Interv. 2015;8:1540–1548. doi: 10.1016/j.jcin.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 24. Sapontis J, Marso SP, Cohen DJ, Lombardi W, Karmpaliotis D, Moses J, Nicholson WJ, Pershad A, Wyman RM, Spaedy A, et al. The outcomes, patient health status, and efficiency in chronic total occlusion hybrid procedures registry: rationale and design. Coron Artery Dis. 2017;28:110–119. doi: 10.1097/MCA.0000000000000439 [DOI] [PubMed] [Google Scholar]
- 25. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9 [DOI] [PubMed] [Google Scholar]
- 26. Rose GA, Blackburn H, Gillum R, Prineas R. Cardiovascular Survey Methods. Geneva, Switzerland: WHO; 1982. [Google Scholar]
- 27. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359:677–687. doi: 10.1056/NEJMoa072771 [DOI] [PubMed] [Google Scholar]
- 29. Safley DM, Grantham JA, Hatch J, Jones PG, Spertus JA. Quality of life benefits of percutaneous coronary intervention for chronic occlusions. Catheter Cardiovasc Interv. 2014;84:629–634. doi: 10.1002/ccd.25303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bruckel JT, Jaffer FA, O'Brien C, Stone L, Pomerantsev E, Yeh RW. Angina severity, depression, and response to percutaneous revascularization in patients with chronic total occlusion of coronary arteries. J Invasive Cardiol. 2016;28:44–51. [PubMed] [Google Scholar]
- 31. Harrell FE. Regression models for continuous y and case study in ordinal regression. In: Harrell FE, ed. Regression Modeling Strategies. New York: Springer; 2015:359–387. [Google Scholar]
- 32. Harrell FE. Regression modeling strategies, with applications to linear models, survival analysis and logistic regression. GET ADDRESS: New York: Springer; 2001. [Google Scholar]
- 33. Grantham JA, Jones PG, Cannon L, Spertus JA. Quantifying the early health status benefits of successful chronic total occlusion recanalization: results from the Flowcardia's Approach to Chronic Total Occlusion Recanalization (FACTOR) trial. Circ Cardiovasc Qual Outcomes. 2010;3:284–290. doi: 10.1161/CIRCOUTCOMES.108.825760 [DOI] [PubMed] [Google Scholar]
- 34. Yeh RW, Tamez H, Secemsky EA, Grantham JA, Sapontis J, Spertus JA, Cohen DJ, Nicholson WJ, Gosch K, Jones PG. Depression and angina among patients undergoing chronic total occlusion percutaneous coronary intervention: the open‐CTO registry. JACC Cardiovasc Interv. 2019;12:651–658. doi: 10.1016/j.jcin.2018.12.029 [DOI] [PubMed] [Google Scholar]
- 35. Salisbury AC, Sapontis J, Grantham JA, Qintar M, Gosch KL, Lombardi W, Karmpaliotis D, Moses J, Cohen DJ, Spertus JA, et al. Outcomes of chronic total occlusion percutaneous coronary intervention in patients with diabetes. Insights from the OPEN CTO Registry. 2017;10:2174–2181. doi: 10.1016/j.jcin.2017.08.043 [DOI] [PubMed] [Google Scholar]
- 36. Pershad A, Gulati M, Karmpaliotis D, Moses J, Nicholson WJ, Nugent K, Tang Y, Sapontis J, Lombardi W, Grantham JA, et al. A sex stratified outcome analysis from the open‐CTO registry. Catheter Cardiovasc Interv. 2019;93:1041–1047. doi: 10.1002/ccd.28023 [DOI] [PubMed] [Google Scholar]
- 37. Harrell FE Jr, Margolis PA, Gove S, Mason KE, Mulholland EK, Lehmann D, Muhe L, Gatchalian S, Eichenwald HF. Development of a clinical prediction model for an ordinal outcome: the World Health Organization Multicentre study of Clinical Signs and Etiological agents of pneumonia, sepsis and meningitis in young infants. Stat Med. 1998;17:909–944. doi: [DOI] [PubMed] [Google Scholar]
- 38. Rundell SD, Pennings JS, Nian H, Harrell FE Jr, Khan I, Bydon M, Asher AL, Devin CJ, Archer KR. Adding 3‐month patient data improves prognostic models of 12‐month disability, pain, and satisfaction after specific lumbar spine surgical procedures: development and validation of a prediction model. Spine J. 2020;20:600–613 doi: 10.1016/j.spinee.2019.12.010 [DOI] [PubMed] [Google Scholar]
- 39. Williams DJ, Zhu Y, Grijalva CG, Self WH, Harrell FE, Reed C, Stockmann C, Arnold SR, Ampofo KK, Anderson EJ, et al. Predicting severe pneumonia outcomes in children. Pediatrics. 2016;138. doi: 10.1542/peds.2016-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McGirt MJ, Bydon M, Archer KR, Devin CJ, Chotai S, Parker SL, Nian H, Harrell FE Jr, Speroff T, Dittus RS, et al. An analysis from the quality outcomes database, part 1. Disability, quality of life, and pain outcomes following lumbar spine surgery: predicting likely individual patient outcomes for shared decision‐making. J Neurosurg Spine. 2017;27:357–369. doi: 10.3171/2016.11.SPINE16526 [DOI] [PubMed] [Google Scholar]
- 41. Arnold SV, Jang JS, Tang F, Graham G, Cohen DJ, Spertus JA. Prediction of residual angina after percutaneous coronary intervention. Euro Heart J Qual Care Clin Outcomes. 2015;1:23–30. doi: 10.1093/ehjqcco/qcv010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salisbury AC, Karmpaliotis D, Grantham JA, Sapontis J, Meng Q, Magnuson EA, Gada H, Lombardi W, Moses J, Li H, et al. In‐hospital costs and costs of complications of chronic total occlusion angioplasty: insights from the open‐CTO registry. JACC Cardiovasc Interv. 2019;12:323–331. doi: 10.1016/j.jcin.2018.10.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S3


