Abstract
Background
The CHA2DS2‐VASc (congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke or TIA [transient ischemic attack], vascular disease, age 65 to 74 years, sex category female; 2 indicates 2 points, otherwise 1 point) scoring system is recommended to guide decisions on oral anticoagulation therapy for stroke prevention in patients with nonsurgery atrial fibrillation. A score ≥1 in men and ≥2 in women, corresponding to an annual stroke risk exceeding 1%, warrants long‐term oral anticoagulation provided the bleeding risk is acceptable. However, in patients with new‐onset postoperative atrial fibrillation, the optimal risk stratification method is unknown. The aim of this study was therefore to evaluate the CHA2DS2‐VASc scoring system for estimating the 1‐year ischemic stroke risk in patients with new‐onset postoperative atrial fibrillation after coronary artery bypass grafting.
Methods and Results
All patients with new‐onset postoperative atrial fibrillation and without oral anticoagulation after first‐time isolated coronary artery bypass grafting performed in Sweden during 2007 to 2017 were eligible for this registry‐based observational cohort study. The 1‐year ischemic stroke rate at each step of the CHA2DS2‐VASc score was estimated using a Kaplan‐Meier estimator. Of the 6368 patients included (mean age, 69.9 years; 81% men), >97% were treated with antiplatelet drugs. There were 147 ischemic strokes during the first year of follow‐up. The ischemic stroke rate at 1 year was 0.3%, 0.7%, and 1.5% in patients with CHA2DS2‐VASc scores of 1, 2, and 3, respectively, and ≥2.3% in patients with a score ≥4. A sensitivity analysis, with the inclusion of patients on anticoagulants, was performed and supported the primary results.
Conclusions
Patients with new‐onset atrial fibrillation after coronary artery bypass grafting and a CHA2DS2‐VASc score <3 have such a low 1‐year risk for ischemic stroke that oral anticoagulation therapy should probably be avoided.
Keywords: CHA2DS2‐VASc, coronary artery bypass grafting, new‐onset postoperative atrial fibrillation
Subject Categories: Cardiovascular Surgery, Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- CHA2DS2‐VASc
congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke or TIA [transient ischemic attack], vascular disease, age 65 to 74 years, sex category female; 2 indicates 2 points, otherwise 1 point
- OAC
oral anticoagulation
- POAF
postoperative atrial fibrillation
Clinical Perspective
What Is New?
In a cohort exceeding 6000 patients with new‐onset postoperative atrial fibrillation after coronary artery surgery, the CHA2DS2‐VASc (congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke or TIA [transient ischemic attack], vascular disease, age 65 to 74 years, sex category female) score was applicable for identifying those with a low risk for ischemic stroke during 1 year following hospital discharge.
In patients with new‐onset postoperative atrial fibrillation after coronary artery surgery, a CHA2DS2‐VASc score of <3 was associated with a low 1‐year stroke risk (ie, <1% risk).
What Are the Clinical Implications?
The CHA2DS2‐VASc scoring system is suitable in patients with new‐onset postoperative atrial fibrillation after coronary artery bypass for identification of those with a low stroke risk during the first year following hospital discharge.
Oral anticoagulation therapy should probably be avoided in patients with new‐onset atrial fibrillation after coronary bypass surgery and a CHA2DS2‐VASc score of <3, at least in the 1‐year perspective.
Atrial fibrillation (AF) is the most common clinically significant arrhythmia in the general population. 1 , 2 It also occurs in 20% to 40% of cardiac surgery patients without history of AF. 3 Prevention of ischemic stroke is a primary objective in the management of patients with AF. 4 , 5 , 6 International guidelines provide explicit recommendations on how to estimate and manage the risk of ischemic stroke and other thromboembolic events in patients with nonsurgery AF. However, the optimum management of new‐onset postoperative AF (POAF) is not clear. 4 , 5
The CHA2DS2‐VASc (congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke or TIA [transient ischemic attack], vascular disease, age 65 to 74 years, sex category female; 2 indicates 2 points, otherwise 1 point) scoring system was introduced in 2010 to refine risk stratification in patients with low thromboembolic risk. 7 It has since gained wide acceptance and become the most commonly used tool for stroke risk stratification and treatment recommendations in patients with nonsurgery AF. 4 , 5 , 6 The system classifies patients with nonsurgery AF into those at low risk (<1%), intermediate risk (1–2%), and high risk (>2%) for ischemic stroke in a 1‐year perspective. 5 , 7 , 8 , 9 Low risk corresponds to 0 points in men and 1 point in women, intermediate risk to 1 point in men and 2 points in women, and high risk to ≥2 points in men and ≥3 points in women. Current guidelines recommend oral anticoagulation (OAC) for patients at intermediate and high risk for ischemic stroke, provided the bleeding risk is acceptable, but not for those at low risk. 4 , 5 , 6
Several studies have shown that POAF is associated with an increased long‐term risk of stroke, 10 , 11 but current guidelines only offer weak recommendations for OAC in patients with POAF. 1 , 2 There is therefore an ongoing debate both about how best to estimate the stroke risk and the risk/benefit ratio of OAC therapy in patients with POAF. 4 , 5 Whether the CHA2DS2‐VASc scoring system is useful for stroke risk assessment in patients with POAF after cardiac surgery thus remains unclear. The main purpose of this study was therefore to explore whether the CHA2DS2‐VASc score could be used to estimate the 1‐year risk for ischemic stroke in patients with POAF after coronary artery bypass grafting (CABG).
METHODS
Study Design and Patient Cohort
This was an observational, nationwide, registry‐based cohort study. Patients with a first‐time isolated CABG (without any concomitant procedure) between January 1, 2007, and December 31, 2017, were eligible for inclusion. The patients were identified in the Swedish Cardiac Surgery Registry, 12 which is a part of the SWEDEHEART (Swedish Web System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies) registry. 13 POAF was defined as any new‐onset AF during the index hospitalization for CABG using data obtained from the Swedish Cardiac Surgery Registry and the National Patient Registry, as previously described. 10 Patients who died during the index hospitalization and those who had a history of AF recorded in the National Patient Registry from 1997 to the admission date for the index CABG were excluded.
We focused on the question of whether CHA2DS2‐VASc could be used to differentiate low‐risk from intermediate‐ and high‐risk patients with POAF at hospital discharge, and this date was the baseline for follow‐up. Patients who had a filled prescription for OAC within 6 months before and up to 7 days after baseline were excluded from the main analysis. However, patients with POAF with a filled prescription for OAC beyond day 7 but within the first year after hospital discharge were included but censored when OAC was dispensed (n=653). In addition, one sensitivity analysis comprising patients with at least 1 year’s follow‐up and another including patients with oral anticoagulation at discharge were performed.
Data Sources
Preoperative AF status, baseline characteristics, and data on POAF were obtained from the Swedish Cardiac Surgery Registry and the National Patient Registry. Data on postoperative morbidity, including end points, were taken from the National Patient Registry; data on medications dispensed preoperatively and postoperatively were taken from the Swedish Prescribed Drug Registry; and mortality data were taken from the National Cause of Death Registry. Data linkage was performed using the unique personal identification number given to all Swedish citizens at birth or shortly after immigration. The Swedish Cardiac Surgery Registry contains detailed information on all cardiac surgical procedures since 1992. 12 , 13 The National Patient Registry records all diagnoses in conjunction with hospital admissions and hospital outpatient visits since 1987. This registry has full coverage and a validity of 85% to 95%, depending on diagnosis. 14 Preoperative data, including AF status and CHA2DS2‐VASc score as well as end points, were identified using the International Classification of Diseases, Tenth Revision (ICD‐10). A list of ICD‐10 codes used to identify diagnoses is provided in Table S1.
All information on medication both preoperatively and following discharge was obtained from the Swedish Prescribed Drug Registry, which holds detailed information about all dispensed prescription drugs since July 2005. 15 Anatomical Therapeutic Chemical Classification codes were used to identify medications, as listed in Table S2. Finally, all deaths in Sweden are reported to the National Cause of Death Registry, from which information on deaths was obtained. The authors declare that all supporting data are available within the article and the supplementary file.
Study End Points
The primary end point was the ischemic stroke rate during a 1‐year follow‐up, in accordance with previous studies on the CHA2DS2‐VASc scoring system. 7 , 8 , 9 Secondary end points were the rates of any thromboembolic event (ischemic stroke, TIA, and/or systemic embolism) and major bleeding during the 1‐year follow‐up. Finally, the event rates for ischemic stroke and any thromboembolic event during the entire follow‐up period were calculated. End points were only counted if they occurred after discharge and were associated with hospital admission or death. All patients were followed up until the occurrence of end points, initiation of OAC, death, emigration, or the end of the study period (December 31, 2017).
Statistical Analysis
Descriptive statistics are presented as means and SDs for continuous variables and as counts and percentages for categorical variables. Associations between CHA2DS2‐VASc score and the risk of stroke within 1 year of follow‐up in patients with POAF without oral anticoagulation (the primary end point) are presented as percentages of patients with events (with 95% exact Poisson CIs) estimated using a Kaplan‐Meier estimator. 16 Associations between CHA2DS2‐VASc score and risks during the complete follow‐up are presented as events per 100 patient‐years with 95% exact Poisson CIs, and cumulative incidence curves are estimated as one minus the Kaplan‐Meier curve using the complete follow‐up time.
The sensitivity and specificity of the CHA2DS2‐VASc score in estimating the risk of events during the 1‐year follow‐up was assessed using a logistic regression model with CHA2DS2‐VASc score as an independent categorical variable and presented as a receiver operating characteristic curve. The receiver operating characteristic curve was summarized using the area under the curve with a 95% CI 17 using the algorithm developed by Sun and Xu. 18
All statistical analyses were performed in version 4.0.2 of R (R Foundation for Statistical Computing, Vienna, Austria; https://www.R‐project.org/), using the pROC package for the calculations of area under the receiver operating characteristic curves.
Ethical Approval
The study complies with the Declaration of Helsinki and was approved by the regional Human Research Ethics Committee in Gothenburg, Sweden (approval number 139‐16). The need for individual consent from the patients was waived by the Ethics Committee.
RESULTS
Study Cohort
During the study period, 32 109 patients underwent first‐time isolated CABG in Sweden. After excluding patients who died before discharge (n=546; 1.7%) and those with a history of AF (n=2854; 8.9%), a total of 28 709 patients remained. POAF was diagnosed in 8348 of them (29.1%). Patients with POAF and preoperative use of OAC (n=116; 1.4%) and those prescribed OAC at discharge (n=1864; 22.6%) were excluded as per protocol (Figure 1). The final primary study population comprised 6368 patients with POAF and without OAC.
Figure 1. Selection of study cohort.
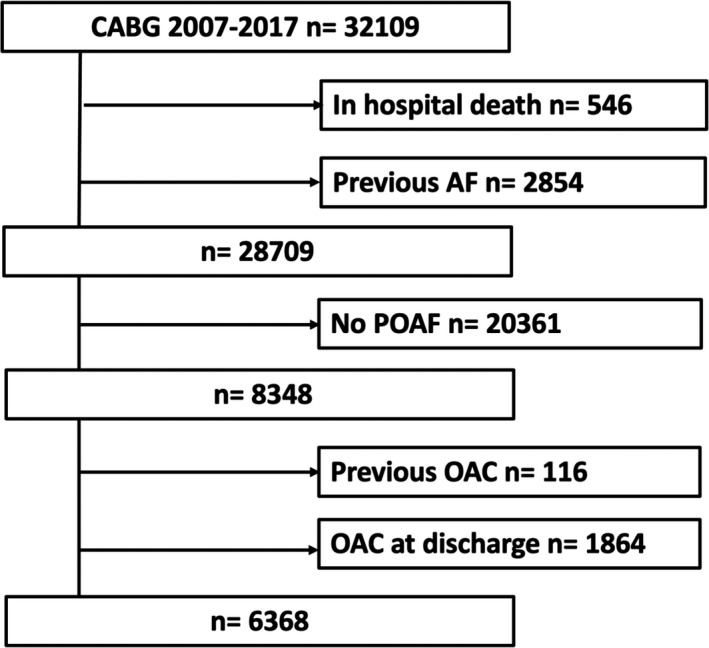
Selection process for the study cohort of 6368 patients with new‐onset postoperative atrial fibrillation (POAF) after first‐time isolated coronary artery bypass grafting (CABG) not treated with oral anticoagulation (OAC) at hospital discharge. AF indicates atrial fibrillation.
Baseline characteristics and medications at discharge for all included patients are presented in Table 1 for the total group and in Tables S3 and S4 divided per step in CHA2DS2‐VASc score. The mean (SD) age was 69.9 (7.9) years, and 81.2% were men. At discharge, 97.1% were prescribed at least one antiplatelet drug, and 20.5% had dual antiplatelet therapy. Because all were patients undergoing CABG, the lowest possible CHA2DS2‐VASc score was 1 (for vascular disease). Furthermore, a stroke before or during the index hospitalization would add 2 more points and put the patient in the categories of ≥3 points for men and ≥4 points for women. Mean (SD) CHA2DS2‐VASc score was 3.7 (1.5). All patients with CHA2DS2‐VASc score of 1 were men, and only 2.3% of patients with CHA2DS2‐VASc score of 2 were women.
Table 1.
Baseline Characteristics and Medications at Discharge for 6368 Patients With New‐Onset POAF After Isolated First‐Time Coronary Bypass Surgery and Without OAC
| Variable | Value |
|---|---|
| Male sex | 5170 (81.2) |
| Age, y | 69.9 (7.9) |
| Body mass index, kg/m2 | 27.6 (5.0) |
| Acute coronary syndrome | 3196 (50.2) |
| Heart failure | 825 (13.0) |
| Hypertension | 4727 (74.2) |
| Diabetes | 1915 (30.1) |
| Peripheral vascular disease | 658 (10.3) |
| Previous stroke | 438 (6.9) |
| Previous transitory ischemic attack | 388 (6.1) |
| Arterial embolism | 28 (0.4) |
| Pulmonary embolism | 39 (0.6) |
| Deep vein thrombosis | 129 (2.0) |
| Respiratory disease | 678 (10.6) |
| Renal failure | 233 (3.7) |
| Renal replacement therapy | 26 (0.4) |
| Liver disease | 56 (0.9) |
| History of cancer | 969 (15.2) |
| Antiplatelet therapy | 6181 (97.1) |
| DAPT | 1304 (20.5) |
| Lipid‐lowering agents | 6107 (95.9) |
| β blockers | 5909 (92.8) |
| ACE‐i/ARB | 4888 (76.8) |
| MRA | 750 (11.8) |
| CCB | 1774 (27.9) |
| Diuretics | 3091 (48.5) |
| Digoxin | 135 (2.1) |
| Sotalol | 712 (11.2) |
| Amiodarone | 763 (12.0) |
| Antidiabetics | 1222 (19.2) |
Data are given as number (percentage) or mean (SD). ACE‐i indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; DAPT, dual‐antiplatelet therapy; MRA, mineralocorticoid receptor antagonist; OAC, oral anticoagulation; and POAF, postoperative atrial fibrillation.
CHA2DS2‐VASc and 1‐Year Rates for Ischemic Stroke and Any Thromboembolism
In the primary analysis, 5875 (92.3%) of 6368 included patients with POAF had a follow‐up of at least 1 year. A total of 147 ischemic strokes occurred within the first year following discharge, 42 (28.6%) of them within the first month. The ischemic stroke rates at 1 year were 0.3%, 0.7%, and 1.5% in patients with a CHA2DS2‐VASc score of 1, 2, and 3, respectively, and ≥2.3% for patients with a score ≥4 (Table 2 and Figure 2). No woman with a CHA2DS2‐VASc score <3 had a diagnosis of ischemic stroke at 1‐year follow‐up (Table S5). A sensitivity analysis restricted to patients with POAF and at least 1‐year follow‐up (n=5875) did not alter the results of the primary analysis (Table S6).
Table 2.
Number of Events and Event Rates Within 1 Year per 100 Patient‐Years With Exact 95% Poisson CIs by CHA2DS2‐VASc Score at Discharge Among 6368 Patients With New‐Onset POAF After Isolated First‐Time Coronary Bypass Surgery and Without OAC
| CHA2DS2‐VASc score | No. (%) of patients | Ischemic stroke | Any thromboembolism | Major bleeding | |||
|---|---|---|---|---|---|---|---|
| No. of events (n=147) | Event rate/100 patient‐years (95% CI) | No. of events (n=200) | Event rate/100 patient‐years (95% CI) | No. of events (n=174) | Event rate/100 patient‐years (95% CI) | ||
| 1 | 328 (5.2) | 1 | 0.3 (0.0–1.8) | 2 | 0.7 (0.8–2.4) | 5 | 1.7 (0.5–3.9) |
| 0 | … | 1 | … | 3 | … | ||
| 2 | 1066 (16.7) | 7 | 0.7 (0.3–1.5) | 13 | 1.4 (0.7–2.3) | 16 | 1.7 (1.0–2.7) |
| 2 | … | 4 | … | 9 | … | ||
| 3 | 1662 (26.1) | 22 | 1.5 (0.9–2.3) | 33 | 2.2 (1.5–3.1) | 39 | 2.7 (1.9–3.6) |
| 5 | … | 7 | … | 14 | … | ||
| 4 | 1621 (25.5) | 33 | 2.3 (1.6–3.3) | 43 | 3.0 (2.2–4.1) | 57 | 4.1 (3.1–5.3) |
| 12 | … | 6 | … | 28 | … | ||
| 5 | 1038 (16.3) | 39 | 4.4 (3.1–6.0) | 50 | 5.6 (4.2–7.4) | 31 | 3.5 (2.4–4.9) |
| 10 | … | 6 | … | 16 | … | ||
| 6 | 423 (6.6) | 24 | 6.7 (4.3–9.9) | 31 | 8.7 (5.9–12.4) | 12 | 3.3 (1.7–5.8) |
| 5 | … | 2 | … | 7 | |||
| 7 | 179 (2.8) | 16 | 11.4 (6.5–18.5) | 21 | 15.2 (9.4–23.3) | 9 | 6.1 (2.8–11.6) |
| 7 | … | 3 | … | 2 | … | ||
| 8–9 | 51 (0.8) | 5 | 11.3 (3.7–26.3) | 7 | 15.8 (6.4–32.6) | 5 | 11.6 (3.7–27.0) |
| 1 | … | 1 | … | 1 | … | ||
Gray cells indicate the number of events within the first 30 days after discharge. CHA2DS2‐VASc indicates congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke or TIA [transient ischemic attack], vascular disease, age 65 to 74 years, sex category female; OAC, oral anticoagulation; and POAF, postoperative atrial fibrillation.
Figure 2. Ischemic stroke rates within 1 year, divided by CHA2DS2‐VASc score.
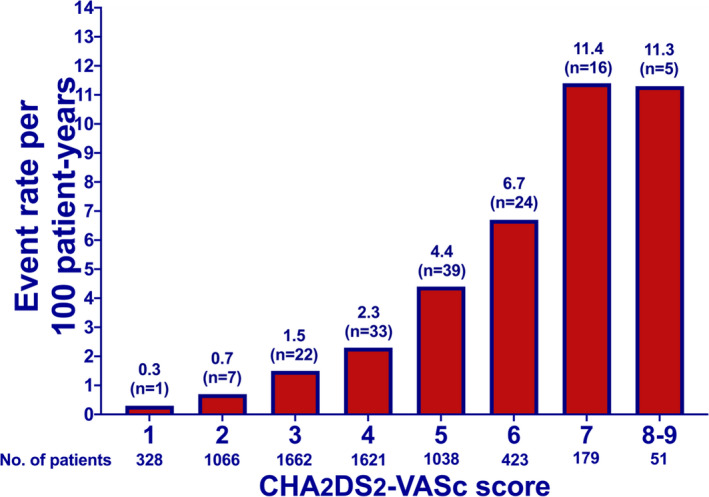
Ischemic stroke rates per 100 patient‐years within 1 year, divided by CHA2DS2‐VASc score, among 6368 patients with new‐onset atrial fibrillation following coronary artery bypass grafting. Numbers above the bars denote ischemic strokes. CHA2DS2‐VASc indicates congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke or TIA [transient ischemic attack], vascular disease, age 65 to 74 years, sex category female.
Overall, 200 thromboembolic events were diagnosed within 1‐year follow‐up (147 ischemic strokes, 47 TIAs, and 6 systemic embolisms). The rates of any thromboembolic event at 1 year are presented in Table 2.
The area under the receiver operating characteristic curve was 0.67 (95% CI, 0.64–0.69) for ischemic stroke and 0.64 (95% CI, 0.61–0.66) for any thromboembolic event (Figure S1). The sensitivity and specificity of the CHA2DS2‐VASc score are given in Table S7. The specificity at 1 year was ≥98% at scores of ≤2.
Another sensitivity analysis included 1864 patients who had OAC at hospital discharge (total n=8232) and were consequently not among those censored at the initiation of OAC therapy during follow‐up. This analysis showed similar results as the main analysis (Table S8). The proportions of patients who received OAC at discharge for each step in the score are presented in Figure S2. An additional analysis focusing only on the 1864 patients with POAF who were discharged on OAC therapy yielded fairly similar results (Table S9). 19
Major Bleedings Within 1 Year
During the first year following hospital discharge, 174 major bleeding events were diagnosed: 81 (46.6%) of them during the first month. The 1‐year rate was 1.7% in patients with POAF with CHA2DS2‐VASc scores 1 and 2 and increased with higher scores (Table 2).
CHA2DS2‐VASc and Ischemic Stroke and Thromboembolism Rates During the Entire Follow‐Up Period
During the entire follow‐up period (median, 5.2 years; range, 0–10 years), 1044 patients died (16.4%), 473 were diagnosed with ischemic stroke (7.4%), and 639 were diagnosed with any thromboembolic event (10.0%). The event rate per 100 patient‐years for ischemic stroke was 0.2, 0.7, 1.2, and 1.7 in patients with POAF with a CHA2DS2‐VASc score of 1, 2, 3, and 4, respectively, and ≥2.9 in patients with a score of ≥5 (Table 3). The rates of ischemic stroke in each step of the score were largely linear during follow‐up for CHA2DS2‐VASc scores <7 (Figure 3). The rates of any thromboembolism during the entire follow‐up period are presented in Table 3.
Table 3.
Number of Events, Patient‐Years, and Event Rate per 100 Patient‐Years With Exact 95% Poisson CIs by CHA2DS2‐VASc Score at Discharge Among 6368 Patients With New‐Onset POAF Followed Up on Average 5 Years
| CHA2DS2‐VASc score | Ischemic stroke | Any thromboembolism | ||||
|---|---|---|---|---|---|---|
| No. of events (n=473) | Patient‐years (n=27 472) | Event rate/100 patient‐years (95% CI) | No. of events (n=639) | Patient‐years (n=26 990) | Event rate/100 patient‐years (95% CI) | |
| 1 | 3 | 1823 | 0.2 (0.0–0.5) | 13 | 1806 | 0.7 (0.4–1.2) |
| 2 | 36 | 5325 | 0.7 (0.5–0.9) | 60 | 5241 | 1.1 (0.9–1.5) |
| 3 | 87 | 7422 | 1.2 (0.9–1.5) | 128 | 7314 | 1.8 (1.5–2.0) |
| 4 | 116 | 6792 | 1.7 (1.4–2.0) | 148 | 6700 | 2.2 (1.9–2.6) |
| 5 | 114 | 3968 | 2.9 (2.4–3.5) | 143 | 3874 | 3.7 (3.1–4.4) |
| 6 | 70 | 1448 | 4.8 (3.8–6.1) | 87 | 1402 | 6.2 (5.0–7.7) |
| 7 | 34 | 550 | 6.2 (4.3–8.6) | 41 | 515 | 8.0 (5.7–10.8) |
| 8–9 | 13 | 144 | 9.0 (4.8–15.4) | 19 | 138 | 13.7 (8.3–21.5) |
CHA2DS2‐VASc indicates congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke or TIA [transient ischemic attack], vascular disease, age 65 to 74 years, sex category female; and POAF, postoperative atrial fibrillation.
Figure 3. Cumulative incidence of ischemic stroke, divided by CHA2DS2‐VASc score.
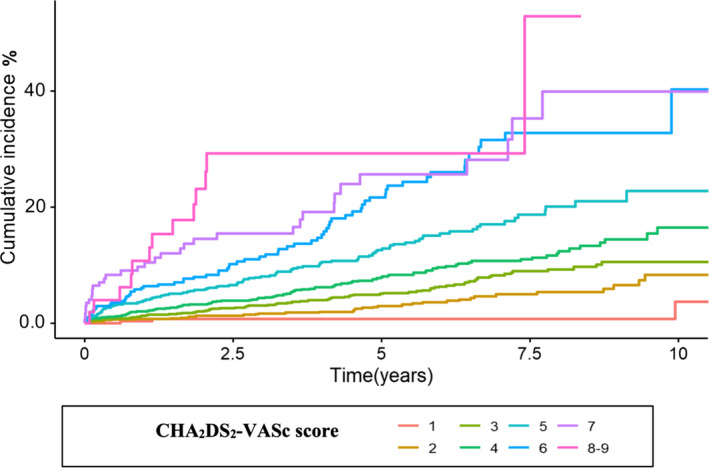
Cumulative incidence of ischemic stroke, divided by CHA2DS2‐VASc score, in 6368 patients with new‐onset postoperative atrial fibrillation after first‐time isolated coronary artery bypass grafting during the entire follow‐up period (median, 5.2 years; range, 0–10 years). CHA2DS2‐VASc indicates congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke or TIA [transient ischemic attack], vascular disease, age 65 to 74 years, sex category female.
DISCUSSION
The ischemic stroke rate was ≤0.7% for patients with CHA2DS2‐VASc scores <3 in a cohort of 6358 patients with POAF after first‐time isolated CABG who were not receiving OAC. The CHA2DS2‐VASc scores thus identified patients with POAF and low risk for ischemic stroke during follow‐up, in whom OAC probably should be avoided, at least in the 1‐year perspective.
International AF guidelines state that patients with nonsurgery AF and low risk for stroke, defined as a 1‐year risk of <1% (corresponding to a CHA2DS2‐VASc score of 0 in men and 1 in women), should not be recommended OAC for stroke prevention. 4 , 5 , 6 In our study, the 1‐year rate for ischemic stroke in patients with POAF was >1% for CHA2DS2‐VASc score ≥3. These results suggest that if the CHA2DS2‐VASc score is used in patients with POAF to identify those with <1% 1‐year risk for ischemic stroke, a higher cutoff level should be applied than in patients with nonsurgery AF. The rates of ischemic stroke and any thromboembolism in this cohort with POAF were apparently lower for any given CHA2DS2‐VASc score than the rates observed in studies conducted on patients with nonsurgery AF. 7 , 8 , 20 , 21 Our results therefore partly corroborate those of a recently published study where the risk for a stroke in patients with POAF was comparable to that in patients with sinus rhythm for CHA2DS2‐VASc score <4, and significantly higher in patients with POAF and CHA2DS2‐VASc score ≥4. 22 Likewise, our study is in line with the findings of Gialdini et al, who reported low stroke risk in relation to CHA2DS2‐VASc score in a mixed population of patients with POAF. 23 However, the latter study excluded patients with POAF and previous stroke, which might explain their low stroke rates. Our findings are also in accordance with the observations of a recent study in patients undergoing CABG, showing that the risk for thromboembolism is lower in patients with POAF than in matched patients with nonsurgery AF. 24
One possible explanation for the lower event rates for ischemic stroke and any thromboembolism observed in our study, compared with studies on patients with nonsurgery AF, might be a beneficial effect of antiplatelet therapy, which in a previous study was associated with a 22% reduction in stroke risk compared with no antithrombotic treatment. 19 Antiplatelet therapy was present in 97.1% of the patients in our cohort versus 31% to 74% in previous studies evaluating the CHA2DS2‐VASc score in nonsurgery AF. 7 , 8 , 21 In addition, patients undergoing CABG are most likely subjected to more rigorous medical examinations and closer follow‐up, which might result in improved cardiovascular risk factor modification also reducing the ischemic stroke risk. Although the exact mechanism cannot be decided in individual cases, POAF presumably occurs because of various perioperative and postoperative triggers and might have a different pathophysiology to that in nonsurgery AF. The thromboembolic risk might therefore be different in patients with POAF despite the fact that patients undergoing CABG share many risk factors with patients with nonsurgery AF.
Nonsurgical AF is generally associated with a 5‐fold increase in the risk for ischemic stroke, 25 which is reduced by ≈65% with OAC. 19 The decision to recommend OAC is, however, based not solely on the thromboembolic risk, but also on balancing this risk reduction with the risk for bleeding on OAC therapy. Patients with POAF following CABG are at increased risk for bleeding complications, because at the time of the POAF diagnosis they have recently undergone major cardiac surgery and are being treated with antiplatelet drugs. 26 Studies conducted on patients with AF who have undergone percutaneous coronary intervention showed that the combination of OAC and antiplatelet therapy is associated with a higher risk for major bleeding (2.9%) compared with antiplatelet therapy alone (0.8%). 27 Retrospective studies investigating the consequences of early‐initiated OAC therapy in patients with POAF have shown conflicting results. Two studies showed an association between OAC and reduced stroke risk, 24 , 28 whereas another 2 showed no reduction in the stroke risk but reported an increased risk of bleeding both within 30 days of hospital discharge and during long‐term follow‐up. 10 , 29 We acknowledge that an increased bleeding risk for any particular reason should not automatically lead to withholding OAC in patients with intermediate or high stroke risk. 5 , 9 However, initiation of OAC therapy in patients with POAF after CABG would presumably increase the bleeding risk further in patients with low stroke risk. This elevated risk for bleeding might not be balanced by the potential benefit, and therefore OAC should probably be avoided in patients with low stroke risk, at least in the 1‐year perspective.
In patients with nonsurgery AF, there is a consensus that OAC should be recommended when the CHA2DS2‐VASc score is ≥2 in men and ≥3 in women because of a proven benefit of OAC therapy with risk reduction for stroke exceeding the risk for severe bleedings with such therapy. 4 , 5 Unfortunately, data are not yet available at which risk level defined by CHA2DS2‐VASc benefit of OAC therapy exceeds risk in patients with POAF. Consequently, our results only allow us to express an opinion on at which score the risk for stroke and any thromboembolism is so low that OAC therapy is unlikely to offer a benefit. We cannot state that OAC therapy should be recommended in all patients with POAF with a score exceeding that level.
The CHA2DS2‐VASc score in this cohort showed a limited overall ability to estimate the risk of ischemic stroke and thromboembolism, with an area under the curve of 0.67. 30 This result is similar to that for the CHA2DS2‐VASc score in patients with nonsurgery AF. 31 On the other hand, the specificity in patients with a CHA2DS2‐VASc score of <3 was high (≥98%), emphasizing the ability of this scoring system to identify patients with low stroke and thromboembolic risk.
Our study suggests that if CHA2DS2‐VASc is used to identify patients with low ischemic stroke risk, a higher cutoff score for identification of low‐risk patients should be used for patients with POAF after CABG than for patients with nonsurgical AF. However, only randomized controlled studies can provide information about the net clinical benefit of OAC therapy in patients with POAF after CABG.
Methodological Aspects and Limitations
This study carries the inherent weaknesses of any observational study, including selection bias and the potential effect of unaccounted confounding. However, previous stroke risk stratification schemes, including the CHA2DS2‐VASc score, were also developed and validated using retrospective studies. 1 , 7 , 8 , 32
Ischemic stroke, rather than any thromboembolism, was chosen as the primary end point. The reason was that ischemic stroke is a specific outcome measure with consequences most likely leading to hospitalization and therefore registered in available databases. Furthermore, of 200 thromboembolic events that were diagnosed within 1‐year follow‐up, 47 (23.5%) were attributable to TIA. The accuracy in diagnosing TIA is often questioned because of the short symptom duration, retrospective evaluation, and interobserver variability, 33 , 34 making TIA a less reliable end point to study. Because our focus was on the association between the CHA2DS2‐VASc score and the risk of stroke within a 1‐year time period, we calculated the cause‐specific survival function estimated by a Kaplan‐Meier estimator, rather than estimating the cumulative incidence function (adjusted for competing risks).
Our study population was composed of patients with POAF and without OAC, which constitutes a potential selection bias. Censoring of patients who received OAC during follow‐up was performed to obtain the true ischemic stroke rates in patients with POAF after CABG without such therapy. However, inclusion of patients with OAC therapy at discharge in a sensitivity analysis yielded similar results.
The rates of major bleedings were explored in the different levels of the CHA2DS2‐VASc scoring system merely to illustrate the true bleeding rates in patients with POAF after CABG and without OAC.
Because of the retrospective nature of this study, an underestimation of the presence of risk factors composing the CHA2DS2‐VASc score, such as hypertension, is possible. However, this would have driven our results toward an even lower risk at a higher score, rather than the opposite. We cannot exclude the possibility that some patients had undiagnosed AF before surgery or that an AF episode went unrecognized during the index hospitalization after the initial period of continuous electrocardiography telemetry. In addition, we were not able to evaluate the clinical course of AF during the hospitalization period and the heart rhythm at discharge, information that potentially influenced the decision to prescribe OAC or not. Finally, we focused on events occurring during the first postoperative year, similar to the original CHA2DS2‐VASc study. 7 Longer follow‐up periods would presumably lead to changes in patient characteristics and risk profiles, altering the CHA2DS2‐VASc score and hence attenuating the relative risk estimates. However, the 5‐year results emphasize the relatively lower risks in patients with POAF compared with observations in patients with nonsurgery AF.
CONCLUSIONS
Among patients with new‐onset atrial fibrillation after coronary bypass surgery, the CHA2DS2‐VASc score was suitable for identifying those with a 1‐year risk for ischemic stroke so low that oral anticoagulation therapy should probably be avoided.
Sources of Funding
The study was supported by the Swedish Heart‐Lung Foundation (grants 20150587 and 20180560 to Dr Jeppsson) and the Swedish State (grant ALFGBG‐725131 to Dr Jeppsson) under the agreement between the Swedish government and the county councils concerning economic support of research and education of doctors (ALF agreement), Region Västra Götaland (grant VGFOUREG‐847811 to Dr Jeppsson and grant VGFOUREG‐648981 to Dr Taha), and Wilhelm and Martina Lundgrens Foundation (grant 2019‐3110 to Dr Taha). The supporting bodies had no influence on the analysis and interpretation of data, on the writing of the report, or on the decision to submit the article for publication.
Disclosures
Dr Jeppsson reports personal fees from Boehringer‐Ingelheim, XVIVO, and LFB outside the submitted work. Dr Taha reports personal fees from Bayer outside the submitted work. Drs Friberg and Bergfeldt report personal fees from Bayer, Boehringer Ingelheim, and Sanofi outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S9
Figures S1–S2
For Sources of Funding and Disclosures, see page 8.
REFERENCES
- 1. Björck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population‐based study. Stroke. 2013;44:3103–3108. doi: 10.1161/STROKEAHA.113.002329 [DOI] [PubMed] [Google Scholar]
- 2. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 3. Filardo G, Damiano RJ Jr, Ailawadi G, Thourani VH, Pollock BD, Sass DM, Phan TK, Nguyen H, da Graca B. Epidemiology of new‐onset atrial fibrillation following coronary artery bypass graft surgery. Heart. 2018;104:985–992. doi: 10.1136/heartjnl-2017-312150 [DOI] [PubMed] [Google Scholar]
- 4. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2020:42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 5. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. doi: 10.1161/CIR.0000000000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 7. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 8. Olesen JB, Lip GYH, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen AM, Gislason GH, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379:648–661. doi: 10.1016/S0140-6736(11)61514-6 [DOI] [PubMed] [Google Scholar]
- 10. Taha A, Nielsen SJ, Bergfeldt L, Ahlsson A, Friberg L, Björck S, Franzén S, Jeppsson A. New‐onset atrial fibrillation after coronary artery bypass grafting and long‐term outcome: a population‐based nationwide study from the SWEDEHEART registry. J Am Heart Assoc. 2021;10:e017966. doi: 10.1161/JAHA.120.017966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin MH, Kamel H, Singer DE, Wu YL, Lee M, Ovbiagele B. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality. Stroke. 2019;50:1364–1371. doi: 10.1161/STROKEAHA.118.023921 [DOI] [PubMed] [Google Scholar]
- 12. Vikholm P, Ivert T, Nilsson J, Holmgren A, Freter W, Ternström L, Ghaidan H, Sartipy U, Olsson C, Granfeldt H, et al. Validity of the Swedish Cardiac Surgery Registry. Interact Cardiovasc Thorac Surg. 2018;27:67–74. doi: 10.1093/icvts/ivy030 [DOI] [PubMed] [Google Scholar]
- 13. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish web‐system for enhancement and development of evidence‐based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart. 2010;96:1617–1621. doi: 10.1136/hrt.2010.198804 [DOI] [PubMed] [Google Scholar]
- 14. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish National Inpatient Register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Swedish National Board of Health and Welfare . The Swedish Prescribed Drug Registry: reporting procedures and quality of data. Available at: https://www.socialstyrelsen.se/en/statistics‐and‐data/registers/register‐information/the‐swedish‐prescribed‐drug‐register/. Accessed February 14, 2021.
- 16. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 17. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 18. Sun X, Xu W. Fast implementation of Delong’s algorithm for comparing the areas under correlated receiver operating characteristic curves. IEEE Signal Process Lett. 2014;21:1389–1393. doi: 10.1109/LSP.2014.2337313 [DOI] [Google Scholar]
- 19. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007 [DOI] [PubMed] [Google Scholar]
- 20. Friberg L, Skeppholm M, Terént A. Benefit of anticoagulation unlikely in patients with atrial fibrillation and a CHA2DS2‐VASc score of 1. J Am Coll Cardiol. 2015;65:225–232. doi: 10.1016/j.jacc.2014.10.052 [DOI] [PubMed] [Google Scholar]
- 21. Fauchier L, Clementy N, Bisson A, Ivanes F, Angoulvant D, Babuty D, Lip GY. Should atrial fibrillation patients with only 1 nongender‐related CHA2DS2‐VASc risk factor be anticoagulated? Stroke. 2016;47:1831–1836. doi: 10.1161/STROKEAHA.116.013253 [DOI] [PubMed] [Google Scholar]
- 22. Benedetto U, Gaudino MF, Dimagli A, Gerry S, Gray A, Lees B, Flather M, Taggart DP, Westaby S, Cook J, et al. Postoperative atrial fibrillation and long‐term risk of stroke after isolated coronary artery bypass graft surgery. Circulation. 2020;142:1320–1329. doi: 10.1161/CIRCULATIONAHA.120.046940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gialdini G, Nearing K, Bhave PD, Bonuccelli U, Iadecola C, Healey JS, Kamel H. Perioperative atrial fibrillation and the long‐term risk of ischemic stroke. JAMA. 2014;312:616–622. doi: 10.1001/jama.2014.9143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Butt JH, Xian Y, Peterson ED, Olsen PS, Rørth R, Gundlund A, Olesen JB, Gislason GH, Torp‐Pedersen C, Køber L, et al. Long‐term thromboembolic risk in patients with postoperative atrial fibrillation after coronary artery bypass graft surgery and patients with nonvalvular atrial fibrillation. JAMA Cardiol. 2018;3:417–424. doi: 10.1001/jamacardio.2018.0405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.STR.22.8.983 [DOI] [PubMed] [Google Scholar]
- 26. Sato H, Ishikawa K, Kitabatake A, Ogawa S, Maruyama Y, Yokota Y, Fukuyama T, Doi Y, Mochizuki S, Izumi T, et al. Low‐dose aspirin for prevention of stroke in low‐risk patients with atrial fibrillation: Japan Atrial Fibrillation Stroke Trial. Stroke. 2006;37:447–451. doi: 10.1161/01.STR.0000198839.61112.ee [DOI] [PubMed] [Google Scholar]
- 27. Sra S, Tan MK, Mehta SR, Fisher HN, Déry J‐P, Welsh RC, Eisenberg MJ, Overgaard CB, Rose BF, Siega AJD, et al. Ischemic and bleeding events in patients with myocardial infarction undergoing percutaneous coronary intervention who require oral anticoagulation: insights from the Canadian Observational Antiplatelet Study. Am Heart J. 2016;180:82–89. doi: 10.1016/j.ahj.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 28. El‐Chami MF, Kilgo P, Thourani V, Lattouf OM, Delurgio DB, Guyton RA, Leon AR, Puskas JD. New‐onset atrial fibrillation predicts long‐term mortality after coronary artery bypass graft. J Am Coll Cardiol. 2010;55:1370–1376. doi: 10.1016/j.jacc.2009.10.058 [DOI] [PubMed] [Google Scholar]
- 29. Matos JD, McIlvaine S, Grau‐Sepulveda M, Jawitz OK, Brennan JM, Khabbaz KR, Sellke FW, Yeh R, Zimetbaum P. Anticoagulation and amiodarone for new atrial fibrillation after coronary artery bypass grafting: prescription patterns and 30‐day outcomes in the United States and Canada. J Thorac Cardiovasc Surg. 2021;162:616–624.e3. doi: 10.1016/j.jtcvs.2020.01.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315–1316. doi: 10.1097/JTO.0b013e3181ec173d [DOI] [PubMed] [Google Scholar]
- 31. Borre E, Goode A, Raitz G, Shah B, Lowenstern A, Chatterjee R, Sharan L, Allen LaPointe N, Yapa R, Davis J, et al. Predicting thromboembolic and bleeding event risk in patients with non‐valvular atrial fibrillation: a systematic review. Thromb Haemost. 2018;118:2171–2187. doi: 10.1055/s-0038-1675400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao‐Melacini P, Zhang X, Pais P, Agapay S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case‐control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2 [DOI] [PubMed] [Google Scholar]
- 33. Castle J, Mlynash M, Lee K, Caulfield AF, Wolford C, Kemp S, Hamilton S, Albers GW, Olivot JM. Agreement regarding diagnosis of transient ischemic attack fairly low among stroke‐trained neurologists. Stroke. 2010;41:1367–1370. doi: 10.1161/STROKEAHA.109.577650 [DOI] [PubMed] [Google Scholar]
- 34. Lee W, Frayne J. Transient ischaemic attack clinic: an evaluation of diagnoses and clinical decision making. J Clin Neurosci. 2015;22:645–648. doi: 10.1016/j.jocn.2014.09.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S9
Figures S1–S2


