Abstract
Background
Estimated pulse wave velocity (ePWV) calculated by equations using age and blood pressure has been suggested as a new marker of mortality and cardiovascular risk. However, the prognostic potential of ePWV during long‐term follow‐up in patients with symptoms of stable angina remains unknown.
Methods and Results
In this study, ePWV was calculated in 25 066 patients without diabetes, previous myocardial infarction (MI), stroke, heart failure, or valvular disease (mean age 63.7±10.5 years, 58% male) with stable angina pectoris undergoing elective coronary angiography during 2003 to 2016. Multivariable Cox models were used to assess the association with incident all‐cause mortality, MI, and stroke. Discrimination was assessed using Harrell´s C‐index. During a median follow‐up period of 8.5 years (interquartile range 5.5–11.3 years), 779 strokes, 1233 MIs, and 4112 deaths were recorded. ePWV was associated with all‐cause mortality (hazard ratio [HR] per 1 m/s, 1.13; 95% CI, 1.05–1.21) and MI (HR per 1 m/s 1.23, 95% CI, 1.09–1.39) after adjusting for age, systolic blood pressure, body mass index, smoking, estimated glomerular filtration rate, Charlson Comorbidity Index score, antihypertensive treatment, statins, aspirin, and number of diseased coronary arteries. Compared with traditional risk factors, the adjusted model with ePWV was associated with a minor but likely not clinically relevant increase in discrimination for mortality, 76.63% with ePWV versus 76.56% without ePWV, P<0.05.
Conclusions
In patients with stable angina pectoris, ePWV was associated with all‐cause mortality and MI beyond traditional risk factors. However, the added prediction of mortality was not improved to a clinically relevant extent.
Keywords: all‐cause mortality, blood pressure measurement, estimated pulse wave velocity, myocardial infarction, stroke
Subject Categories: Cardiovascular Disease, High Blood Pressure, Hypertension
Nonstandard Abbreviations and Acronyms
- CAG
coronary angiography
- Cf‐PWV
carotid‐femoral pulse wave velocity
- ePWV
estimated pulse wave velocity
- FRS
Framingham Risk Score
- PP
pulse pressure
- SCORE
Systematic Coronary Risk Evaluation
- SPRINT
Systolic Blood Pressure Intervention Trial
Clinical Perspective
What Is New?
Estimated pulse wave velocity is associated with all‐cause mortality and myocardial infarction in high‐risk patients during long‐term follow‐up even with extensive adjustment for potential confounders including invasively measured blood pressure.
What Are the Clinical Implications?
The study shows that nonlinear estimated pulse wave velocity seems to incorporate prognostic information from quadratic terms and interactions between age and blood pressure, which are not accounted for in traditional risk modeling based on linear associations between age, blood pressure, and outcomes.
In the present study, even though the association was robust for extensive adjustment for confounders, discrimination was not improved to a clinically relevant extent.
Arterial stiffness assessed by carotid‐femoral pulse wave velocity (cf‐PWV) is a strong marker of cardiovascular disease and mortality beyond traditional risk factors. 1 , 2 , 3 , 4 , 5 , 6 Current hypertension guidelines list cf‐PWV as a marker for detection of hypertension‐mediated organ damage. 7 However, cf‐PWV measurement requires specialized equipment and trained staff. Hence, the modality has not been widely implemented in clinical practice. In healthy individuals, it was recently shown that an estimated pulse wave velocity (ePWV) calculated from age and mean blood pressure (BP) using an equation generated from the Reference Values for Arterial Stiffness Collaboration 8 had similar predictive value as cf‐PWV for a combined cardiovascular end point. 9 Even though ePWV and cf‐PWV were correlated, it was also evident that ePWV cannot substitute for cf‐PWV. ePWV seems to incorporate other aspects of risk information than cf‐PWV as both parameters remained significant when included in the same prediction models. 9 Rather, ePWV seems to incorporate prognostic information from quadratic terms and interactions between age and BP, which are not accounted for in traditional risk modeling based on linear associations between age, BP, and outcomes. 10 In a subsequent study, ePWV was associated with all‐cause mortality, cardiovascular mortality, and a composite cardiovascular end point beyond Systematic Coronary Risk Evaluation (SCORE) and Framingham Risk Score (FRS) in a large European cohort. 11 Similarly, ePWV was associated with cardiovascular mortality and all‐cause mortality in adults from the general US population in the NHANES (National Health and Nutrition Examination Survey) cohort beyond traditional risk factors. 12 A post hoc analysis of the high‐risk patients from SPRINT (Systolic Blood Pressure Intervention Trial) showed that ePWV was associated with a composite cardiovascular end point and all‐cause mortality during 3.26 years follow‐up beyond the FRS. 13 The analysis also showed that intensive BP‐lowering treatment was superior to standard treatment only in responders to ePWV (patients with an increase in ePWV at 12 months of 0.15 m/s or less). The long‐term prognostic potential of ePWV in high‐risk patients remains to be elucidated.
In this study we assessed the long‐term association between ePWV and all‐cause mortality, myocardial infarction (MI), and stroke in patients with stable angina undergoing elective coronary angiography.
Methods
Setting
This study was conducted using Danish medical databases. In Denmark, linkage of all medical registries is possible through the unique central personal registry number. 14 In this study, we included patients without diabetes, previous MI, stroke, heart failure, or valvular disease with stable angina pectoris undergoing elective coronary angiography (CAG) in Western Denmark from January 1, 2003 to December 31, 2016, who were registered in the Western Denmark Heart Registry. 15 The registry collects patient and procedure data from all cardiac intervention centers in Western Denmark covering more than 3 million inhabitants. In case of multiple examination of the same patient during the inclusion period, the first CAG was used as the index CAG.
Owing to Danish data protection regulations, the data cannot be made publicly available.
Data were linked to outcome variables in the Danish National Patient Registry 16 and the Danish Civil Registration System. 14
Patient Inclusion
As the ePWV equation from the Reference Values for Arterial Stiffness Collaboration (discussed later) was derived in subjects without prevalent cardiovascular disease or diabetes, patients with previous diagnoses of diabetes, MI, stroke, heart failure, or valvular disease were excluded. (Figure S1).
Data Characterizations
Cuff BP data from the Western Denmark Heart Registry were used in the analysis. The cuff BP registered in the Western Denmark Heart Registry was measured by the referring general practitioner or during the CAG admission.
Hypertension was defined as receipt of treatment for hypertension at the time of CAG as recorded in the Western Denmark Heart Registry or a diagnosis of hypertension registered in the Danish National Patient Registry.
Prescription records for statins, aspirin, and antihypertensive drugs were obtained from the Danish National Prescription Registry.
Extent of coronary artery disease (CAD), that is, the number of coronary arteries with obstructive CAD (defined as ≥50% angiographic stenosis), was recorded as 0‐, 1‐, 2‐, or 3‐vessel disease, or diffuse nonobstructive vessel disease.
Comorbidities were evaluated using the Charlson Comorbidity Index score based on discharge diagnoses registered in the Danish National Patient Registry. The original Charlson Comorbidity Index is a weighted index including 19 conditions categorizing comorbidities of patients based on the International Classification of Diseases (ICD) diagnosis to predict risk of death within 1 year of hospitalization for patients with comorbid conditions. Each comorbidity category has an associated weight (from 1 to 6), based on the adjusted risk of mortality or resource use. The sum of all the weights results in a single comorbidity score for the patient. A score of zero indicates that no comorbidities were found. The higher the score, the more likely the predicted outcome will result in mortality or higher resource use. 17 , 18 We used a full look‐back period of patient history before the study inclusion date.
Diabetes was defined as either (1) treatment with insulin±oral glucose‐lowering drugs, oral glucose‐lowering drugs alone, or nonpharmacological dietary treatment for diabetes, as recorded in the Western Denmark Heart Registry; (2) a diabetes diagnosis recorded in the Danish National Patient Registry before or 1 month after CAG; or (3) redemption of ≥1 prescription(s) for diabetes medication within 6 months before or 1 month after CAG, as recorded in the Danish National Prescription Registry. 19
Outcome Definition
The Danish National Patient Registry was used to identify admissions for MI (ICD, Tenth Revision [ICD‐10] codes DI21‐21.9) and stroke (ICD‐10 DI60‐61, DI629, DI63‐64). Analyses were made separately for the first stroke and the first MI event occurring after the index CAG date. Information on all‐cause death was obtained from the Civil Registration System. 14
The study was approved by the Danish Data Protection Agency (record no. 1‐16‐02‐193‐18). According to Danish law, approval from an ethics committee and informed consent from the patients are not required for this registry study.
Statistical Analysis
The ePWV was calculated by the formula as described in the study by Greve et al 9 that was derived by the Reference Values for Arterial Stiffness Collaboration. 8 The ePWV was calculated from age and mean arterial BP:
Mean arterial BP was calculated as diastolic BP+0.4*(systolic BP–diastolic BP). 20
Continuous variables are reported as mean with SD for normally distributed data and as median (range) for skewed data. Normality was assessed by histograms and QQ‐plots. The associations between ePWV with stroke, MI, and death were assessed in Cox regression models and are reported as hazard ratio (HR) per m/s. The proportional hazards assumption was assessed by log‐log plots and found to be satisfied. Two multivariable models were tested. Model 1: Adjusted for age and systolic BP. Model 2: model 1 plus the following predefined variables: sex, smoking (never/former/active), body mass index, estimated glomerular filtration rate, a categorized Charlson Comorbidity index 17 (0 points, 1 point, 2 points, or >2 points), antihypertensive treatment (yes/no), lipid‐lowering treatment (yes/no), aspirin treatment, and extent of vessel disease (none, diffuse nonobstructive, 1‐, 2‐, or 3‐vessel disease). The ePWV was also evaluated as a dichotomized parameter as above versus below or equal to the median value (10.93 m/s). We tested for interaction between ePWV and sex and ePWV and antihypertensive treatment. We conducted supplementary exploratory analyses evaluating the effect of replacing systolic BP in the multivariable analysis with cuff pulse pressure (PP), invasively measured systolic BP and invasively measured PP.
Missing data regarding estimated glomerular filtration rate (n=2326), smoking (n=1022), antihypertensive treatment (n=274), and body mass index (n=658) were imputed by 20 imputations using chained equations as recommended for Cox models by White et al. 21 To avoid double registration of procedure‐related events, follow‐up and event registration was initiated 30 days after CAG.
The added prediction and discrimination by ePWV beyond traditional risk factors, comorbidity, and extent of CAD was assessed with Harrell´s C‐index on complete cases (n=21 724) 22 using bootstrapping with 50 replications. A 2‐tailed P value <0.05 was considered to indicate statistical significance. All data were analyzed using Stata version 16.1 (StataCorp LP, TX, USA).
Results
Baseline patient characteristics are shown in Table 1. There was a majority of slightly overweight male patients, and the majority did not have any comorbidities. Approximately half the patients were diagnosed with 1–, 2–, or 3– vessel disease CAD at the CAG. The majority of patients received statin and aspirin treatment and more than half received antihypertensive treatment.
Table 1.
Baseline Characteristics
| (n=25 066) | |
|---|---|
| Age, y | 63.7±10.5 |
| Male sex, n (%) | 14556 (58) |
| Weight, kg | 80.3±15.8 |
| Body mass index, kg/m2 | 27.2±4.5 |
| Estimated glomerular filtration rate, m:/min per 1.73 m2 | 87±23 |
| Smoking, n (%) | |
| Never | 8.235 (33) |
| Previous | 10.199 (41) |
| Current | 5610 (22) |
| Missing | 1022 (4) |
| Medical history, no. (%) | |
| Antihypertensive treatment | 13859 (55) |
| Missing | 273 (1) |
| Lipid lowering treatment | 18.927 (76) |
| Aspirin treatment | 19435 (78) |
| Atrial fibrillation | 1902 (8) |
| Modified Charlson Comorbidity Index, n (%) | |
| 0 | 19923 (79) |
| 1 | 3005 (12) |
| 2 | 1545 (6) |
| ≥2 | 593 (2) |
| Coronary angiography findings ‐ n (%) | |
| Coronary vessels with >50% stenosis | |
| 0 | 10351 (41) |
| 1 | 5546 (22) |
| 2 | 3249 (13) |
| 3 | 3075 (12) |
| Diffuse coronary vessel disease without significant stenoses | 2845 (11) |
| Cuff systolic blood pressure, mm Hg | 143±20 |
| Cuff diastolic blood pressure, mm Hg | 82±11 |
| Cuff pulse pressure, mm Hg | 62±17 |
| Invasive systolic blood pressure, mm Hg | 145±23 |
| Invasive diastolic blood pressure, mm Hg | 73±12 |
| Invasive pulse pressure, mm Hg | 73±21 |
| Estimated pulse wave velocity, m/s | 10.0±1.9 |
During a median follow‐up of 8.5 years (interquartile range 5.5–11.3 years), 779 strokes, 1233 Mis, and 4112 deaths were recorded.
Associations Between ePWV and All‐Cause Mortality
The ePWV was associated with death in crude analyses (Table 2 and Figure–Panel A; figure: ePWV below versus above the median value 10.93 m/s). The association remained significant in the adjusted models for continuous ePWV. No interaction between ePWV and sex and between ePWV and antihypertensive treatment was observed.
Table 2.
Association Between Estimated Pulse Wave Velocity and the Risk of Death, Stroke, and Acute Myocardial Infarction
| n=25 066 |
Continuous ePWV Hazard ratio per 1 m/s (95% CI) |
Dichotomized ePWV Hazard ratio above vs below or equal to the median value 10.93 m/s (95% CI) |
|---|---|---|
| All‐cause mortality | ||
| Crude | 1.46 (1.44–1.49) | 3.37 (3.15–3.61) |
| Model 1 | 1.13 (1.05–1.21) | 1.02 (0.92–1.13) |
| Model 2 | 1.13 (1.05–1.21) | 1.00 (0.90–1.11) |
| Myocardial infarction | ||
| Crude | 1.17 (1.14–1.21) | 1.54 (1.38–1.73) |
| Model 1 | 1.24 (1.09–1.40) | 0.93 (0.77–1.11) |
| Model 2 | 1.23 (1.09–1.39) | 0.92 (0.77–1.11) |
| Stroke | ||
| Crude | 1.33 (1.29–1.39) | 2.63 (2.26–3.06) |
| Model 1 | 1.02 (0.88–1.20) | 1.13 (0.89–1.43) |
| Model 2 | 0.99 (0.85–1.16) | 1.11 (0.87–1.41) |
Hazard ratios (HR) per 1 m/s increase in ePWV with 95% CIs (first column) and above vs below or equal to the median value 10.93 m/s (second column). Model 1: Adjusted for age and systolic blood pressure. Model 2: Adjusted for age, sex, systolic blood pressure, body mass index, smoking (never, previous vs current), a categorized Charlson Comorbidity Index (0, 1, 2, or above 2), estimated glomerular filtration ratio, antihypertensive treatment (yes/no), statin treatment (yes/no), aspirin treatment (yes/no) and number of diseased vessels. (0, diffuse, 1, 2, or 3). ePWV indicates estimated pulse wave velocity.
Figure 1. Kaplan‐Meier plots.
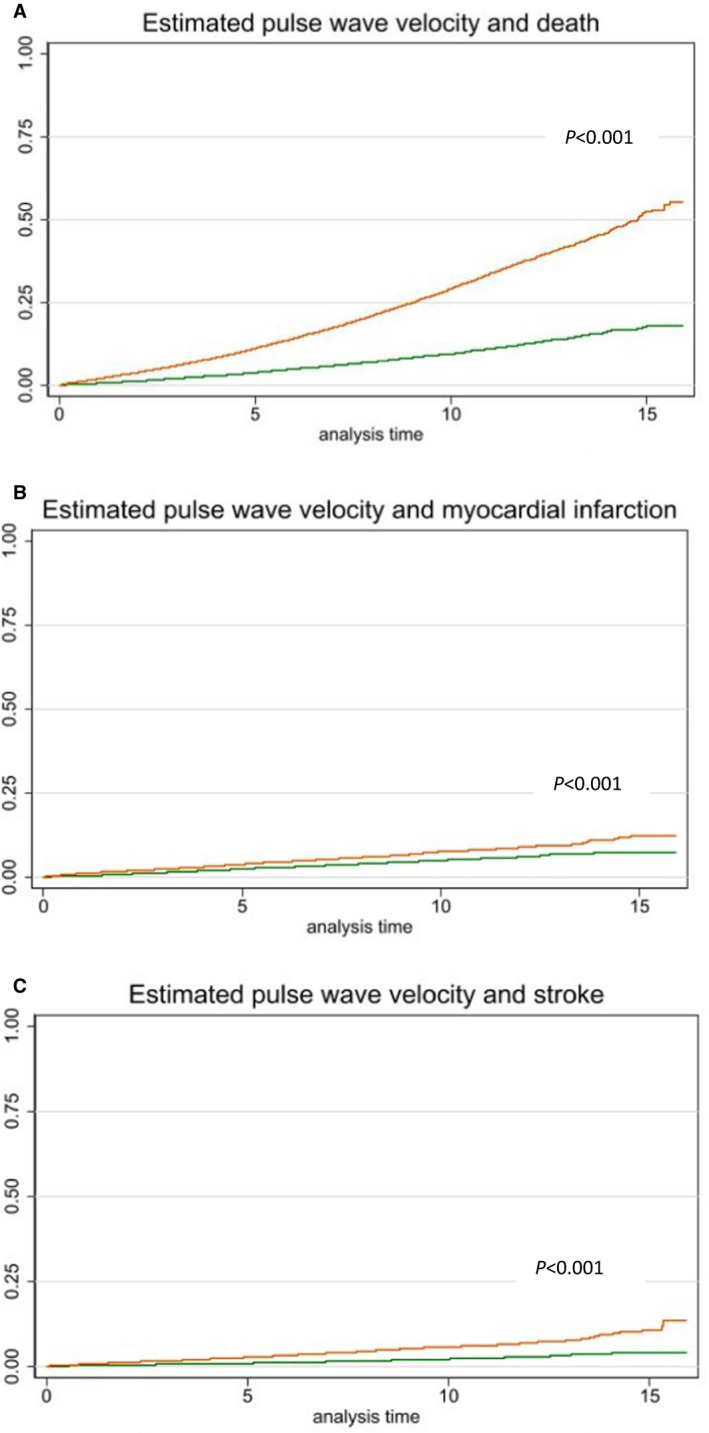
Association between estimated pulse wave velocity above (orange) or below or equal to (green) the median value (10.93 m/s) and risk of death, stroke, and myocardial infarction. Analysis time in years. A, Estimated pulse wave velocity and death. B, Estimated pulse wave velocity and myocardial infarction. C, Estimated pulse wave velocity and stroke.
Associations Between ePWV and MI
The ePWV was associated with MI in crude analyses (Table 2 and Figure–Panel B). Continuous ePWV remained associated with MI in the adjusted models. We observed interaction between ePWV and sex, P for interaction <0.001. In stratified crude analyses, the HR was 1.30 (95% CI, 1.23–1.37) per m/s increase in ePWV for women, and 1.13 (95% CI, 1.08–1.17) per m/s increase in ePWV for men. However, in multivariable analyses, the association between ePWV and MI became insignificant for women (HR, 0.94; 95% CI, 0.75–1.17) and remained significant for men (HR, 1.35; 95% CI, 1.16–1.57) per m/s increase in ePWV. No interaction between ePWV and antihypertensive treatment was observed.
Associations Between ePWV and Stroke
The ePWV was associated with stroke in crude analyses (Table 2 and Figure–Panel C) but became insignificant in the adjusted models. We observed interaction between ePWV and antihypertensive treatment. Hence, in crude analyses HR was 1.41 (95% CI, 1.32–1.50) per m/s increase in ePWV for patients not in antihypertensive treatment versus HR 1.26 (95% CI, 1.20–1.32) per m/s increase in ePWV for patients in antihypertensive treatment. In adjusted analysis ePWV became insignificant in both groups, data not shown. No interaction between ePWV and sex was observed.
Supplementary Analyses
The association between ePWV and mortality, MI, and stroke was also evaluated on complete cases (n=21 724) with similar results, data not shown. When replacing systolic BP in the multivariate analyses with cuff PP, continuous ePWV remained associated with mortality and MI (Table S1). With adjustment for invasively measured systolic BP or PP, the association with mortality became nonsignificant and the association with MI remained significant for continuous ePWV. The association with stroke became significant for continuous ePWV with adjustment for invasively measured systolic BP and for both continuous and dichotomized ePWV when adjusted for invasively measured PP (Table S1).
Discriminative Properties of ePWV for All‐Cause Mortality and MI
The ePWV improved C‐statistics statistically significantly for mortality; however, the improvement was minor (Table 3).
Table 3.
C‐Statistics for Models With and Without ePWV
| N=21 724 | ePWV | |||
|---|---|---|---|---|
| Outcome | Model with ePWV | Model without ePWV | Difference (95% CI) | P value |
| All‐cause mortality | 76.63% | 76.56% | 0.07 (0.0008 to 0.13)% | <0.05 |
| Myocardial infarction | 71.18% | 70.90% | 0.27 (−0.02 to 0.56)% | 0.07 |
ePWV indicates estimated pulse wave velocity.
Discussion
We assessed the association between ePWV and stroke, MI, and all‐cause mortality in patients with symptoms of stable angina without diabetes, previous MI, stroke, heart failure, or valvular disease undergoing elective CAG. We found that ePWV was associated with all‐cause mortality and MI beyond traditional risk factors in patients with and without CAD and across levels of comorbidity. This indicates that cardiovascular risk and mortality risk may be better estimated if quadratic term and interaction terms between major risk factors are allowed than with linear terms only. However, discrimination was not improved to any clinically meaningful extent beyond traditional risk factors including the linear effects of age and BP. Hence, in this high‐risk cohort, the linear effects of age or BP seemed to capture the majority of the prognostic information carried by these parameters and our data do not indicate that ePWV would improve risk classification during long term follow‐up.
Our results in this high‐risk population adds to the findings from previous studies in low‐ and high‐risk populations.
In a large low‐risk European cohort ePWV was associated with all‐cause mortality, cardiovascular mortality, and a composite cardiovascular end point beyond SCORE and FRS and ePWV increased C‐statistics. 11 However, adjusted for traditional cardiovascular risk factors, ePWV remained associated only with all‐cause mortality and c‐statistics were not improved. The authors concluded that their data did not support changing the SCORE or FRS by including ePWV in the risk equation. 11 The ePWV was associated with cardiovascular mortality, all‐cause mortality, and residual‐specific mortality in the NHANES cohort, 12 , 23 with all‐cause mortality, cardiovascular mortality and stroke in the Kuopio Ischemic Heart Disease Study cohort, 24 , 25 and with cardiovascular events and all‐cause mortality in the Chinese Kailuan Study cohort. 26 The ePWV improved the C‐statistics for the primary outcome in the Kailuan study slightly (from 0.676; 95% CI, 0.65–0.70; to 0.683; 95% CI, 0.66–0.71) 26 but not in the Kuopio study. 24 , 25 In high‐risk patients from the SPRINT trial, ePWV was associated with a composite cardiovascular end point and all‐cause mortality during 3.26 years follow‐up and improved C‐statistics beyond the Framingham Risk Score (from 0.67; 95% CI, 0.64–0.69; to 0.69; 95% CI, 0.66–0.72). 13 Similarly, ePWV was associated with cardiovascular and all‐cause mortality in 2 smaller high‐risk Asian cohorts 27 , 28 and in a high‐risk European cohort by Hametner et al. ePWV was associated with a combined end point (mortality, stroke, MI, and unplanned revascularization). 29
In comparison with the SPRINT cohort 13 and the study by Hametner et al, 29 our analysis (1) includes substantially more participants (25 066 versus 9361 in the SPRINT study versus 1040 in the Hametner study), (2) has markedly longer follow‐up time (median 8.5 years versus median 3.26 years in the SPRINT study versus median 4.3 years in the Hametner study), (3) has extensive adjustment for potential confounders, including Charlson Comorbidity Index and invasive BP indices.
The HRs for mortality and stroke were reduced in the multivariable analyses compared with the crude model, whereas the HR for MI increased slightly. The multivariable analyses showed that the declines in hazard ratios were mainly driven by the adjustment of age and systolic BP. The HRs were markedly reduced in model 1, whereas further adjustment for other covariates did not change HRs further in model 2. The reduction in HRs in model 1 may not be surprising as both age and mean BP are used for the calculation of ePWV. The association with stroke became statistically insignificant in the adjusted model. Our finding may relate to the fewer stroke events compared with deaths and MI or that the linear effects of age and BP capture the majority of the stroke risk prediction. We observed an interaction effect between ePWV and sex for the association with MI. The association between ePWV and MI was attenuated in the multivariable analysis in women and increased in men. The lack of attenuation in the HR for MI observed in men in the multivariable regression model could indicate a sex‐specific synergistic effect between the linear and nonlinear effects of age, mean BP, and systolic BP. However, C‐statistics did not improve for MI by inclusion of ePWV. The previous studies have not reported data specifically on the association between ePWV and MI. Hence, it remains to be clarified whether this finding is reproducible in other populations.
In contrast to continuous ePWV, the dichotomized ePWV became nonsignificant with adjustment for age and systolic BP. This indicate that increases in ePWV are associated with increased risk across both low and high levels of ePWV, and that dichotomizing the nonlinear age and BP effects captured by the ePWV is too crude a measure to capture the added prognostic information beyond the linear effects of age and systolic BP.
When replacing systolic BP with cuff PP, ePWV remained associated with mortality and MI. When replacing cuff systolic BP with invasive systolic BP or invasive PP, the association between ePWV and mortality was weakened, whereas the association with stroke became significant. This could indicate that ePWV may mediate predictive information from the effects of invasive BP on mortality. We have previously demonstrated that invasively measured systolic BP and PP did not add predictive information beyond cuff systolic BP and cuff PP in mortality prediction. 30 , 31 However, the current data suggest that the interaction terms and quadratic terms in the ePWV may reflect information from the invasive BP not captured by cuff BP. These findings warrant further investigations in future studies. The association between ePWV and stroke became significant with adjustment for invasive systolic BP or PP instead of cuff BP, which is in line with our previous studies where no added prediction from invasive systolic BP was observed, and cuff PP, but not invasively measured PP, remained significantly associated with stroke in multivariate analyses. 30 , 31
Strengths and Limitations
The study was conducted in a country with a tax‐based health care system, reducing selection bias caused by selective inclusion of specific hospitals, health insurance systems, or age groups. High‐quality baseline data were available from the Western Denmark Heart Registry. 15 , 32 Follow‐up and outcome ascertainment were standardized through high‐quality nationwide registers, and patients were followed until death, emigration, or end of follow‐up. Information bias or differential outcome misclassification are therefore unlikely to have affected our results. The study population consisted of patients referred for elective CAG on suspicion of obstructive CAD. Thus, our results may not be directly applicable to patients in the general population. Information regarding the BP equipment used for BP measurement (oscillometric, manometry, auscultation) was not available. Race and ethnicity data were not available. However, the majority of the Danish population is of White origin with <10% being immigrants and descendants from nonwestern countries. 33
The cf‐PWV data were not available, and hence the prognostic potential of ePWV versus cf‐PWV could not be assessed. As stated previously, ePWV cannot substitute for cf‐PWV. 9 Hence, the 2 indices cannot be used interchangeably and one may argue that another term than “estimated PWV” would more clearly indicate that this concept captures different risk information than cf‐PWV.
Conclusions
In conclusion, ePWV was associated with all‐cause mortality and MI beyond traditional risk factors in patients undergoing CAG for suspected CAD during long‐term follow‐up. However, discrimination was not improved to a pivotal clinical influence beyond traditional risk factors including the linear effects of age and BP.
Sources of Funding
This work was supported by a research grant from the Central Region Midt, Denmark, The Novo Nordisk Foundation, and the A.P. Møller Foundation for the Advancement of Medical Science. The funding entities were not involved in any aspects of the design or conduct of the study or collection, analysis or interpretation of the data nor in the review or approval of the article.
Disclosures
None.
Supporting information
Table S1
Figure S1
For Sources of Funding and Disclosures, see page 8.
See Editorial for Boutouyrie et al.
References
- 1. Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse‐wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.CIR.0000033824.02722.F7 [DOI] [PubMed] [Google Scholar]
- 2. Willum‐Hansen T, Staessen JA, Torp‐Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342 [DOI] [PubMed] [Google Scholar]
- 3. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.HYP.37.5.1236 [DOI] [PubMed] [Google Scholar]
- 4. Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031 [DOI] [PubMed] [Google Scholar]
- 5. Greve SV, Blicher MK, Blyme A, Sehestedt T, Hansen TW, Rassmusen S, Vishram JK, Ibsen H, Torp‐Pedersen C, Olsen MH. Association between albuminuria, atherosclerotic plaques, elevated pulse wave velocity, age, risk category and prognosis in apparently healthy individuals. J Hypertens. 2014;32:1034–1041. discussion 1041. doi: 10.1097/HJH.0000000000000147 [DOI] [PubMed] [Google Scholar]
- 6. Ben‐Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al, ESC Scientific Document Group . 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 8. Reference Values for Arterial Stiffness' Collaboration Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'Establishing normal and reference values'. Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greve SV, Blicher MK, Kruger R, Sehestedt T, Gram‐Kampmann E, Rasmussen S, Vishram JK, Boutouyrie P, Laurent S, Olsen MH. Estimated carotid‐femoral pulse wave velocity has similar predictive value as measured carotid‐femoral pulse wave velocity. J Hypertens. 2016;34:1279–1289. doi: 10.1097/HJH.0000000000000935 [DOI] [PubMed] [Google Scholar]
- 10. Greve SV, Laurent S, Olsen MH. Estimated pulse wave velocity calculated from age and mean arterial blood pressure. Pulse (Basel). 2017;4:175–179. doi: 10.1159/000453073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vishram‐Nielsen JKK, Laurent S, Nilsson PM, Linneberg A, Sehested TSG, Greve SV, Pareek M, Palmieri L, Giampaoli S, Donfrancesco C, et al. Does estimated pulse wave velocity add prognostic information?: Morgam prospective cohort project. Hypertension. 2020;75:1420–1428. doi: 10.1161/HYPERTENSIONAHA.119.14088 [DOI] [PubMed] [Google Scholar]
- 12. Heffernan KS, Jae SY, Loprinzi PD. Association between estimated pulse wave velocity and mortality in U.S. Adults. J Am Coll Cardiol. 2020;75:1862–1864. doi: 10.1016/j.jacc.2020.02.035 [DOI] [PubMed] [Google Scholar]
- 13. Vlachopoulos C, Terentes‐Printzios D, Laurent S, Nilsson PM, Protogerou AD, Aznaouridis K, Xaplanteris P, Koutagiar I, Tomiyama H, Yamashina A, et al. Association of estimated pulse wave velocity with survival: a secondary analysis of sprint. JAMA Netw Open. 2019;2:e1912831. doi: 10.1001/jamanetworkopen.2019.12831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish civil registration system. A cohort of eight million persons. Dan Med Bull. 2006;53:441–449. [PubMed] [Google Scholar]
- 15. Schmidt M, Maeng M, Jakobsen CJ, Madsen M, Thuesen L, Nielsen PH, Botker HE, Sorensen HT. Existing data sources for clinical epidemiology: the western Denmark heart registry. Clin Epidemiol. 2010;2:137–144. doi: 10.2147/clep.s10190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish national hospital register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46:263–268. [PubMed] [Google Scholar]
- 17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 18. Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sorensen HT. The predictive value of icd‐10 diagnostic coding used to assess Charlson comorbidity index conditions in the population‐based Danish national registry of patients. BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pottegard A, Schmidt SAJ, Wallach‐Kildemoes H, Sorensen HT, Hallas J, Schmidt M. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2017;46:798–798f. doi: 10.1093/ije/dyw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bos WJ, Verrij E, Vincent HH, Westerhof BE, Parati G, van Montfrans GA. How to assess mean blood pressure properly at the brachial artery level. J Hypertens. 2007;25:751–755. doi: 10.1097/HJH.0b013e32803fb621 [DOI] [PubMed] [Google Scholar]
- 21. White IR, Royston P. Imputing missing covariate values for the cox model. Stat Med. 2009;28:1982–1998. doi: 10.1002/sim.3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Newson RB. Comparing the predictive powers of survival models using Harrell's C or Somers' D. Stata Journal. 2010;10:339–358. doi: 10.1177/1536867X1001000303 [DOI] [Google Scholar]
- 23. Heffernan KS, Jae SY, Loprinzi PD. Estimated pulse wave velocity is associated with residual‐specific mortality: findings from the national health and nutrition examination survey. J Hypertens. 2021;39:698–702. doi: 10.1097/HJH.0000000000002691 [DOI] [PubMed] [Google Scholar]
- 24. Jae SY, Heffernan KS, Kurl S, Kunutsor SK, Laukkanen JA. Association between estimated pulse wave velocity and the risk of stroke in middle‐aged men. Int J Stroke. 2020;1747493020963762. doi: 10.1177/1747493020963762 [DOI] [PubMed] [Google Scholar]
- 25. Jae SY, Heffernan KS, Park JB, Kurl S, Kunutsor SK, Kim JY, Laukkanen JA. Association between estimated pulse wave velocity and the risk of cardiovascular outcomes in men. Eur J Prev Cardiol. 2021;28:e25–e27. doi: 10.1177/2047487320920767 [DOI] [PubMed] [Google Scholar]
- 26. Ji C, Gao J, Huang Z, Chen S, Wang G, Wu S, Jonas JB. Estimated pulse wave velocity and cardiovascular events in Chinese. Int J Cardiol Hypertens. 2020;7:100063. doi: 10.1016/j.ijchy.2020.100063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hsu P‐C, Lee W‐H, Tsai W‐C, Chen Y‐C, Chu C‐Y, Yen H‐W, Lin T‐H, Voon W‐C, Lai W‐T, Sheu S‐H, et al. Comparison between estimated and brachial‐ankle pulse wave velocity for cardiovascular and overall mortality prediction. J Clin Hypertens (Greenwich). 2021;23:106–113. doi: 10.1111/jch.14124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hsu PC, Lee WH, Tsai WC, Chi NY, Chang CT, Chiu CA, Chu CY, Lin TH, Lai WT, Sheu SH., et al. Usefulness of estimated pulse wave velocity in prediction of cardiovascular mortality in patients with acute myocardial infarction. Am J Med Sci. 2021;361:479‐484. doi: 10.1016/j.amjms.2020.10.023 [DOI] [PubMed] [Google Scholar]
- 29. Hametner B, Wassertheurer S, Mayer CC, Danninger K, Binder RK, Weber T. Aortic pulse wave velocity predicts cardiovascular events and mortality in patients undergoing coronary angiography: a comparison of invasive measurements and noninvasive estimates. Hypertension. 2021;77:571–581. doi: 10.1161/HYPERTENSIONAHA.120.15336 [DOI] [PubMed] [Google Scholar]
- 30. Laugesen E, Knudsen ST, Hansen KW, Rossen NB, Jensen LO, Hansen MG, Munkholm H, Thomsen KK, Søndergaard H, Bøttcher M, et al. Invasively measured aortic systolic blood pressure and office systolic blood pressure in cardiovascular risk assessment: a prospective cohort study. Hypertension. 2016;68:768–774. doi: 10.1161/HYPERTENSIONAHA.116.07495 [DOI] [PubMed] [Google Scholar]
- 31. Laugesen E, Knudsen ST, Hansen KW, Rossen NB, Jensen LO, Hansen MS, Andersen LK, Thomsen KK, Søndergaard H, Böttcher M, et al. Invasive aortic pulse pressure is not superior to cuff pulse pressure in cardiovascular risk prediction. J Hypertens. 2021;39:607–613. doi: 10.1097/HJH.0000000000002694 [DOI] [PubMed] [Google Scholar]
- 32. Rasmussen LA, Botker HE, Jensen LO, Ravkilde J, Riber L, Nielsen PH, Andreasen JJ, Jakobsen CJ. Quality assurance of the western Denmark heart registry, a population‐based healthcare register. Dan Med J. 2017;64. [PubMed] [Google Scholar]
- 33. Statistics Denmark. Immigrants and their descendants . 2021. Accessed 09 February 2022. https://www.dst.dk/en/Statistik/emner/borgere/befolkning/indvandrere‐og‐efterkommere
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1


