A patient is referred for chronic total occlusion (CTO) percutaneous coronary intervention (PCI). Should CTO PCI be offered? It should, if we knew that the patient would derive benefit and would not be harmed (Figure). 1 , 2 Unfortunately, we do not always have a window to the future, as the assessment of risks and benefits can be challenging and subjective.
Figure . Overview of the potential risks and benefits of chronic total occlusion (CTO) percutaneous coronary intervention (PCI).
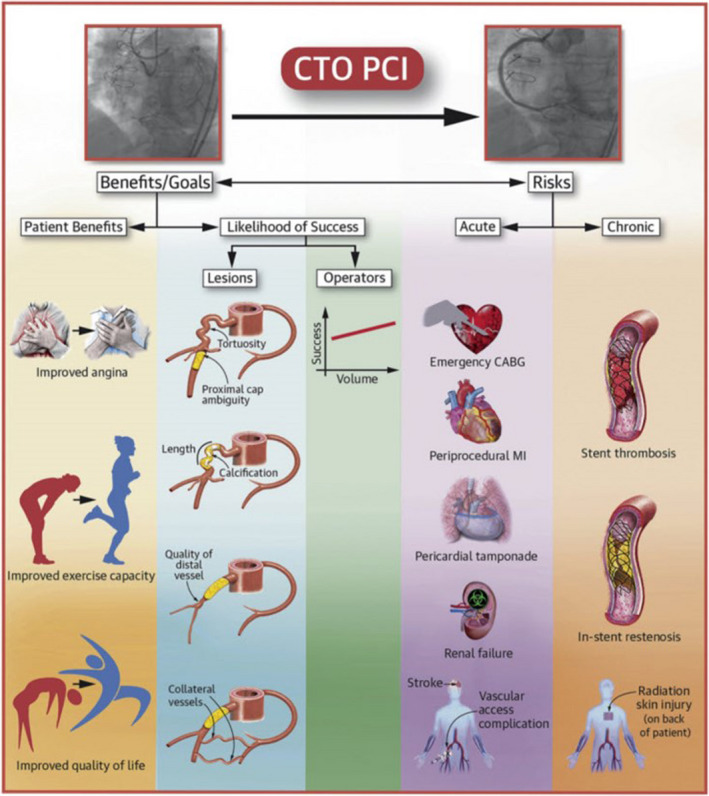
Parameters that can help determine the risks and benefits of chronic total occlusion percutaneous coronary intervention. Reprinted from Tajti et al 1 with permission. Copyright ©2018, Elsevier. CABG indicates coronary artery bypass grafting; and MI, myocardial infarction.
RISKS
CTO PCI carries increased risk of complications compared with non‐CTO PCI, including perforation, periprocedural myocardial infarction, and radiation skin injury. 3 The average periprocedural complication risk is ≈3%. 4 The risk increases with increasing angiographic complexity, use of advanced CTO techniques, such as the retrograde approach, older patient age, and comorbidities.
BENEFITS
Symptom relief is currently the main indication for CTO PCI. 2 , 5 Several observational studies and 3 6 , 7 , 8 of 4 9 randomized‐controlled trials showed symptom improvement with CTO PCI compared with optimal medical therapy (OMT) alone. In the EuroCTO (randomized multicenter trial to compare revascularization with OMT for the treatment of chronic total coronary occlusions) trial, 396 patients were randomized to OMT versus OMT+PCI. At 12 months, patients who underwent CTO PCI had greater improvements in angina frequency, quality of life, and physical limitation, as assessed by Seattle Angina Questionnaire. 3 In the Impactor‐CTO (Impact on Inducible Myocardial Ischemia of Percutaneous Coronary Intervention Versus Optimal Medical Therapy in Patients With Right Coronary Artery Chronic Total Occlusion) trial, 94 patients with angina and isolated dominant right coronary artery CTOs were randomized to OMT versus OMT+PCI. At 12 months, the CTO PCI group had significantly lower myocardial ischemia burden, improved 6‐minute walk distance, and improved health, as assessed by the 36‐Item Short Form Survey. 4 In the COMET‐CTO (Randomized Controlled Comparison of Optimal Medical Therapy With Percutaneous Recanalization of Chronic Total Occlusion) trial, 100 patients were randomized to OMT versus OMT+PCI; at 9‐month follow‐up, patients who underwent CTO PCI had significantly improved physical limitation, angina, treatment satisfaction, and quality of life, whereas the OMT only group had no change in symptoms. 5 The DECISION‐CTO (Drug‐Eluting Stent Implantation Versus Optimal Medical Treatment in Patients With Chronic Total Occlusion) trial randomized 834 patients to CTO PCI versus no CTO PCI and found no difference in quality of life or in the incidence of major adverse cardiac events during a median follow‐up of 4 years. 9 However, the DECISION‐CTO trial had several limitations, such as mild baseline symptoms, concomitant PCI of non‐CTO lesions, and 20% crossover from no CTO PCI to CTO PCI within 3 days of randomization, that hinder interpretation of the study findings.
Because of the conflicting results of the aforementioned trials, the 2021 American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions guidelines for coronary artery revascularization downgraded the recommendation for CTO PCI to class IIb (level of evidence B): “In patients with suitable anatomy who have refractory angina on medical therapy, after treatment of non‐CTO lesions, the benefit of PCI of a CTO to improve symptoms is uncertain.” 10 In contrast, CTO PCI is given a class IIa (level of evidence B) recommendation for CTO PCI in the 2018 European Society of Cardiology/European Association for Cardiothoracic Surgery coronary revascularization guidelines: “Percutaneous revascularization of CTOs should be considered in patients with angina resistant to medical therapy or with a large area of documented ischemia in the territory of the occluded vessel.” 11
CTO SCORES
Several prediction models have been developed to predict the time required for CTO crossing (Japan‐CTO: J‐CTO) (Table), 12 the likelihood of technical success (PROGRESS‐CTO: Prospective Global Registry for the Study of CTO, 13 RECHARGE: Registry of CrossBoss and Hybrid Procedures in France, the Netherlands, Belgium, and United Kingdom, 14 CASTLE‐CTO: CABG, Age, Stump Anatomy, Tortuosity Degree, Length of Occlusion, Extent of Calcification‐CTO), 15 and the likelihood of complications (PROGRESS‐CTO Complications score 16 and OPEN‐CLEAN: CABG, CTO Length, Ejection Fraction <50%, Age, Calcification Score for Perforations). 17 Calculating ≥1 of those scores (https://www.ctomanual.org/cto‐scores) helps the operator focus on important aspects of angiography and plan CTO PCI, but their predictive capacity has been limited. 18
Table .
Comparison of Various Scores for Estimating the Success and Complication Rates of CTO PCI
| Variables/scores | J‐CTO 9 | PROGRESS‐CTO 10 | RECHARGE 12 | CASTLE 11 | PROGRESS‐CTO complications 13 | OPEN‐CLEAN 14 |
|---|---|---|---|---|---|---|
| Year of publication | 2011 | 2016 | 2018 | 2019 | 2016 | 2017 |
| No. of variables | 5 | 4 | 6 | 6 | 3 | 5 |
| No. of cases | 494 | 781 | 880 | >20 000 | 1569 (44 Events) | 1000 (89 Perforations) |
| Setup | 12 Japanese centers | 7 US centers | European centers | Expert European operators | 12 US centers | 12 US centers |
| Dates | 2006–2007 | 2012–2015 | 2014–2015 | 2008–2016 | 2012–2016 | 2014–2015 |
| Technical success, % | 88.6 | 92.9 | 84 | 87.8 | 90 | 86 |
| Clinical | ||||||
| Age, y | ≥70 (+1) | >65 (+3) |
50–<70 (+1) ≥70 (+2) |
|||
| Prior CABG | +1 | +1 | +1 | |||
| Prior CTO PCI failure | +1 | |||||
| Left ventricular ejection fraction, % | <50 (+1) | |||||
| Angiographic | ||||||
| Proximal cap ambiguity | +1 | |||||
| Blunt stump | +1 | +1 | +1 | |||
| Calcification | +1 | +1 | +1 | +1 | ||
| Proximal tortuosity | +1 | +1 | ||||
| Within occlusion tortuosity | +1 | +1 | ||||
| CTO length, mm | ≥20 (+1) | ≥20 (+1) | ≥20 (+1) | ≥23 (+2) |
≥20 (+1) ≥20 (+2) |
|
| Retrograde approach | +1 | |||||
| Diseased distal landing zone | +1 | |||||
| CTO target vessel | Circumflex (+1) | |||||
| Collaterals | Absent interventional (+1) | |||||
CABG indicates coronary artery bypass graft surgery; CASTLE, Coronary Artery Bypass Graft History, Age (≥70 Years), Stump Anatomy [Blunt or Invisible], Tortuosity Degree [Severe or Unseen], Length of Occlusion [≥20 mm], and Extent of Calcification [Severe]; CTO, chronic total occlusion; J‐CTO, Multicenter CTO Registry in Japan Score; OPEN‐CLEAN, CABG, CTO Length, EF [Ejection Fraction] <50%, Age, Calcification; PCI, percutaneous coronary intervention; PROGRESS‐CTO, Prospective Global Registry for the Study of Chronic Total Occlusion Intervention Score; and RECHARGE, Registry of CrossBoss and Hybrid Procedures in France, the Netherlands, Belgium, and United Kingdom.
NOVEL ANGINA SCORE
Can symptom relief be predicted? Symptom relief depends on the presence and severity of symptoms at baseline, coronary anatomy, comorbidities, and the outcome of CTO PCI. In this issue of the Journal of the American Heart Association (JAHA), Butala et al used data from 901 patients participating in the OPEN‐CTO (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures) registry and used elegant statistical techniques to create a model for predicting angina frequency at 6 months using the Seattle Angina Questionnaire. 19 Most (81%) patients were men with multiple comorbidities (40% diabetes and 38% prior coronary artery bypass graft surgery). Many patients had symptoms: 54% were taking at least 2 antianginal medications, and 41% of patients reported weekly or daily angina within the past month. Six months after CTO PCI, 78% of the patients had no angina, indicating considerable improvement; the remaining 22% continued to have angina: daily (3%), weekly (8%), or monthly (12%).
The angina prediction model included 7 variables (baseline angina frequency, baseline nitroglycerin use, Rose Dyspnea Score ≥2, Patient Health Questionnaire 8 ≥10, number of antianginal medications, PCI indication, and the presence of multiple CTOs) and had a c‐statistic of 0.78, indicating good discrimination to predict 6‐month postprocedural angina frequency score. The model’s c‐statistic for detecting ≥20‐point improvement in Seattle Angina Questionnaire angina frequency score at 6 months was also good (0.81). Patients who were more symptomatic at baseline (depression, more frequent angina, dyspnea, or on more antianginals/more frequent nitroglycerin preprocedure) were more likely to improve after CTO PCI.
Butala et al should be congratulated for improving our understanding on symptom improvement after CTO PCI, but should the novel angina frequency model be used in everyday clinical practice? Should it be routinely calculated and discussed with each patient? Probably not, at least for now, for several reasons. First, the findings of the study are pretty clear: the more symptomatic the patient, the higher the potential benefit of CTO PCI. Perhaps the patients do not need a numeric estimate of the likelihood of symptom improvement; if they have severe and frequent angina and require multiple sublingual tablets, they are likely to experience significant amelioration with CTO PCI. Second, the Seattle Angina Questionnaire is proprietary, takes time to complete, and may be difficult to understand (by both physicians and patients), limiting its adoption. Third, the angina frequency score needs to be validated in independent populations. Fourth, the model applies to experienced operators and centers that can achieve high success rates (85%–90%) and may not be applicable to less experienced centers that often have much lower success rates (50%–60%). 4
CONCLUSIONS
“Looking into the future” is key for deciding whether a patient should undergo CTO PCI or not. The novel angina score provides a window to the future by quantifying the likelihood of symptomatic improvement and reaffirms that the worse the baseline symptom severity, the higher the likelihood of improvement. Simplifying and validating the model in various patient populations will be key for its future adoption. After all, a window is only useful if one can see clearly through it.
DISCLOSURES
Dr. Brilakis reports consulting/speaker honoraria from Abbott Vascular, American Heart Association (associate editor Circulation), Amgen, Asahi Intecc, Biotronik, Boston Scientific, Cardiovascular Innovations Foundation (Board of Directors), ControlRad, CSI, Elsevier, GE Healthcare, IMDS, InfraRedx, Medicure, Medtronic, Opsens, Siemens, and Teleflex; research support from Boston Scientific, GE Healthcare; owner, Hippocrates LLC; shareholder: MHI Ventures, Cleerly Health, Stallion Medical. The remaining authors have no disclosures to report.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
See Article by Butala et al.
For Disclosures, see page 4.
REFERENCES
- 1. Tajti P, Burke MN, Karmpaliotis D, Alaswad K, Werner GS, Azzalini L, Carlino M, Patel M, Mashayekhi K, Egred M, et al. Update in the percutaneous management of coronary chronic total occlusions. JACC Cardiovasc Interv. 2018;11:615–625. doi: 10.1016/j.jcin.2017.10.052 [DOI] [PubMed] [Google Scholar]
- 2. Azzalini L, Karmpaliotis D, Santiago R, Mashayekhi K, Di Mario C, Rinfret S, Nicholson WJ, Carlino M, Yamane M, Tsuchikane E, et al. Contemporary issues in chronic total occlusion percutaneous coronary intervention. JACC Cardiovasc Interv. 2022;15:1–21. doi: 10.1016/j.jcin.2021.09.027 [DOI] [PubMed] [Google Scholar]
- 3. Brilakis ES, Banerjee S, Karmpaliotis D, Lombardi WL, Tsai TT, Shunk KA, Kennedy KF, Spertus JA, Holmes DR Jr, Grantham JA. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2015;8:245–253. doi: 10.1016/j.jcin.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 4. Assali M, Buda KG, Megaly M, Hall AB, Burke MN, Brilakis ES. Update on chronic total occlusion percutaneous coronary intervention. Prog Cardiovasc Dis. 2021;69:27–34. doi: 10.1016/j.pcad.2021.11.004 [DOI] [PubMed] [Google Scholar]
- 5. Brilakis ES, Mashayekhi K, Tsuchikane E, Abi Rafeh N, Alaswad K, Araya M, Avran A, Azzalini L, Babunashvili AM, Bayani B, et al. Guiding principles for chronic total occlusion percutaneous coronary intervention. Circulation. 2019;140:420–433. doi: 10.1161/CIRCULATIONAHA.119.039797 [DOI] [PubMed] [Google Scholar]
- 6. Werner GS, Martin‐Yuste V, Hildick‐Smith D, Boudou N, Sianos G, Gelev V, Rumoroso JR, Erglis A, Christiansen EH, Escaned J, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39:2484–2493. doi: 10.1093/eurheartj/ehy220 [DOI] [PubMed] [Google Scholar]
- 7. Obedinskiy AA, Kretov EI, Boukhris M, Kurbatov VP, Osiev AG, Ibn Elhadj Z, Obedinskaya NR, Kasbaoui S, Grazhdankin IO, Prokhorikhin AA, et al. The IMPACTOR‐CTO Trial. JACC Cardiovasc Interv. 2018;11:1309–1311. doi: 10.1016/j.jcin.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 8. Juricic SA, Tesic MB, Galassi AR, Petrovic ON, Dobric MR, Orlic DN, Vukcevic VD, Stankovic GR, Aleksandric SB, Tomasevic MV, et al. Randomized controlled comparison of optimal medical therapy with percutaneous recanalization of chronic total occlusion (COMET‐CTO). Int Heart J. 2021;62:16–22. doi: 10.1536/ihj.20-427 [DOI] [PubMed] [Google Scholar]
- 9. Lee S‐W, Lee PH, Ahn J‐M, Park D‐W, Yun S‐C, Han S, Kang H, Kang S‐J, Kim Y‐H, Lee CW, et al. Randomized trial evaluating percutaneous coronary intervention for the treatment of chronic total occlusion. Circulation. 2019;139:1674–1683. doi: 10.1161/circulationaha.118.031313 [DOI] [PubMed] [Google Scholar]
- 10. Lawton JS, Tamis‐Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e18–e114. doi: 10.1161/CIR.0000000000001038 [DOI] [PubMed] [Google Scholar]
- 11. Neumann F‐J, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J‐P, Falk V, Head SJ, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 12. Morino Y, Abe M, Morimoto T, Kimura T, Hayashi Y, Muramatsu T, Ochiai M, Noguchi Y, Kato K, Shibata Y, et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J‐CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4:213–221. doi: 10.1016/j.jcin.2010.09.024 [DOI] [PubMed] [Google Scholar]
- 13. Christopoulos G, Kandzari DE, Yeh RW, Jaffer FA, Karmpaliotis D, Wyman MR, Alaswad K, Lombardi W, Grantham JA, Moses J, et al. Development and validation of a novel scoring system for predicting technical success of chronic total occlusion percutaneous coronary interventions: the PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) Score. JACC Cardiovasc Interv. 2016;9:1–9. doi: 10.1016/j.jcin.2015.09.022 [DOI] [PubMed] [Google Scholar]
- 14. Maeremans J, Spratt JC, Knaapen P, Walsh S, Agostoni P, Wilson W, Avran A, Faurie B, Bressollette E, Kayaert P, et al. Towards a contemporary, comprehensive scoring system for determining technical outcomes of hybrid percutaneous chronic total occlusion treatment: the RECHARGE score. Catheter Cardiovasc Interv. 2018;91:192–202. doi: 10.1002/ccd.27092 [DOI] [PubMed] [Google Scholar]
- 15. Szijgyarto Z, Rampat R, Werner GS, Ho C, Reifart N, Lefevre T, Louvard Y, Avran A, Kambis M, Buettner H‐J, et al. Derivation and validation of a chronic total coronary occlusion intervention procedural success score from the 20,000‐Patient EuroCTO Registry: the EuroCTO (CASTLE) Score. JACC Cardiovasc Interv. 2019;12:335–342. doi: 10.1016/j.jcin.2018.11.020 [DOI] [PubMed] [Google Scholar]
- 16. Danek BA, Karatasakis A, Karmpaliotis D, Alaswad K, Yeh RW, Jaffer FA, Patel MP, Mahmud E, Lombardi WL, Wyman MR, et al. Development and validation of a scoring system for predicting periprocedural complications during percutaneous coronary interventions of chronic total occlusions: the Prospective Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS CTO) Complications Score. J Am Heart Assoc. 2016;5:e004272. doi: 10.1161/jaha.116.004272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hirai T, Grantham JA, Sapontis J, Nicholson WJ, Lombardi W, Karmpaliotis D, Moses J, Nugent K, Gosch KL, Salisbury AC, et al. Development and validation of a prediction model for angiographic perforation during chronic total occlusion percutaneous coronary intervention: OPEN‐CLEAN perforation score. Catheter Cardiovasc Interv. 2022;99:280–285. doi: 10.1002/ccd.29466 [DOI] [PubMed] [Google Scholar]
- 18. Karacsonyi J, Stanberry L, Alaswad K, Krestyaninov O, Choi JW, Rangan BV, Nikolakopoulos I, Vemmou E, Ungi I, Brilakis ES. Predicting technical success of chronic total occlusion percutaneous coronary intervention: comparison of 3 scores. Circ Cardiovasc Interv. 2021;14:e009860. doi: 10.1161/circinterventions.120.009860 [DOI] [PubMed] [Google Scholar]
- 19. Butala NM, Tamez H, Secemsky EA, Grantham JA, Spertus JA, Cohen DJ, Jones P, Salisbury AC, Arnold SV, Harrell F, et al. Predicting residual angina after chronic total occlusion percutaneous coronary intervention: insights from the OPEN‐CTO Registry. J Am Heart Assoc. 2022;11:e024056. doi: 10.1161/JAHA.121.024056 [DOI] [PMC free article] [PubMed] [Google Scholar]


