Abstract
Background
Previous literature about the effect of heart rate on poststroke outcomes is limited. We attempted to elucidate (1) whether heart rate during the acute period of ischemic stroke predicts subsequent major clinical events, (2) which heart rate parameter is best for prediction, and (3) what is the estimated heart rate cutoff point for the primary outcome.
Methods and Results
Eight thousand thirty‐one patients with acute ischemic stroke who were hospitalized within 48 hours of onset were analyzed retrospectively. Heart rates between the 4th and 7th day after onset were collected and heart rate parameters including mean, time‐weighted average, maximum, and minimum heart rate were evaluated. The primary outcome was the composite of recurrent stroke, myocardial infarction, and mortality up to 1 year after stroke onset. All heart rate parameters were associated with the primary outcome (P’s<0.001). Maximum heart rate had the highest predictive power. The estimated cutoff point for the primary outcome was 81 beats per minute for mean heart rate and 100 beats per minute for maximum heart rate. Patients with heart rates above these cutoff points had a higher risk of the primary outcome (adjusted hazard ratio, 1.80 [95% CI, 1.57–2.06] for maximum heart rate and 1.65 [95% CI, 1.45–1.89] for mean heart rate). The associations were replicated in a separate validation dataset (N=10 000).
Conclusions
These findings suggest that heart rate during the acute period of ischemic stroke is a predictor of major clinical events, and optimal heart rate control might be a target for preventing subsequent cardiovascular events.
Keywords: acute ischemic stroke, cohort study, heart rate, prognosis
Subject Categories: Ischemic Stroke, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- AIS
acute ischemic stroke
- bpm
beats per minute
- CRCS‐K
Clinical Research Collaboration for Stroke in Korea registry
- PROFESS
Prevention Regimen for Effectively Avoiding Second Strokes trial
- SBP
systolic blood pressure
Clinical Perspective
What Is New?
In this retrospective analysis of 8031 acute ischemic stroke cases, heart rate between the fourth and seventh day after stroke onset was nonlinearly associated with the composite of recurrent stroke, myocardial infarction, and death.
Maximum heart rate had the best predictive power for adverse events.
Maximum heart rate of 100 beats per minute and mean rate of 81 beats per minute were determined as cutoffs.
What Are the Clinical Implications?
Heart rate during the acute period of ischemic stroke was associated with future adverse cardiovascular events.
A specific cutoff could be implemented in clinical practice or clinical trials.
In contradistinction to blood pressure, where there are specific guidelines for management of this metric after acute ischemic stroke (AIS), 1 guidance for the management of heart rate after AIS is not well established. The largest study on this topic was a post‐hoc analysis of the PROFESS (Prevention Regimen for Effectively Avoiding Second Strokes) trial, which included >20 000 ischemic stroke cases and reported that a higher heart rate was associated with increased mortality. 2 However, this post‐hoc analysis had limitations. First, the PROFESS trial enrolled patients who had experienced stroke within 90 days of randomization; therefore, this study could not analyze the effect of heart rate obtained during the acute period of stroke. Second, because the PROFESS trial excluded patients with indications for anticoagulants with intention to compare aspirin and dipyridamole versus clopidogrel, the study results might not be applicable to patients with cardioembolic stroke. 3
In a recent analysis that we carried out for patients with AIS and atrial fibrillation (AF), heart rate during the acute period was associated with poststroke mortality. 4 Considering this result together with the post‐hoc analysis of the PROFESS trial, it may be reasonable to generalize the effect of heart rate on poststroke outcomes to all AIS populations, although there is no direct evidence.
Our study aimed to elucidate whether heart rate obtained during the acute period of stroke is associated with subsequent major clinical events up to 1‐year poststroke and the nature of the association. Furthermore, we also aimed to determine which heart rate parameter best predicts outcomes and its optimal target for clinical practice and future trials.
Methods
Data Availability
The anonymized data from this study may be shared after approval from the local institutional review board with qualified researchers carrying out legitimate research by contacting the lead investigator (H‐JB at braindoc@snu.ac.kr).
Study Population
Patients with AIS admitted to 14 hospitals participating in the CRCS‐K (Clinical Research Collaboration for Stroke in Korea) registry between January 2011 and November 2014 were eligible for the study. Among them, the following participants were included for the study. Those who: (1) were admitted within 48 hours of symptom onset, (2) had a relevant ischemic brain lesion on magnetic resonance imaging or computed tomography of the head, and (3) had heart rate data between the 4th and 7th day from onset of stroke. Those whose heart rate was collected <5 times and had no outcome data were excluded. Baseline characteristics, including demographics, risk factors, and acute treatment including reperfusion therapy and discharge medication, were collected during hospitalization. Stroke severity at admission was measured using the National Institutes of Health Stroke Scale, and stroke subtypes were classified according to the Trial of Org 10172 in Acute Stroke Treatment classification with some modification. 5 A separate dataset consisting of 10 000 patients with AIS who were hospitalized and registered in the CRCS‐K registry between September 2015 and October 2018 and met the identical eligibility criteria was established for validation.
Collection of Heart Rate Data
Heart rate data collected through routine clinical practice during hospitalization because of index stroke were extracted retrospectively from the electronic medical record systems of the participating centers. Heart rate data between the 4th and 7th day after stroke onset were analyzed. Heart rate during the first 3 days after stroke was not analyzed because it might reflect the effect of a stress reaction to stroke. 4 For those who were discharged within 7 days of stroke onset, heart rate data until discharge were used. Heart rate data were summarized into 4 parameters: arithmetic mean, time‐weighted average, and minimum and maximum heart rate. The time‐weighted average was calculated by summing the average heart rate between 2 time points by the time interval between 2 time points, which is the area under the curve in the time–heart rate graph, and dividing it by total elapsed time. 6 Minimum and maximum heart rates were the highest and lowest heart rates among the heart rates that were captured between the 4th and 7th day after stroke onset.
Outcomes and Ascertainment of Outcomes
The primary outcome of the study was the composite of recurrent stroke, myocardial infarction, and all‐cause mortality. Secondary outcomes were all‐cause mortality, recurrent stroke, and the composite of recurrent stroke, myocardial infarction, and vascular death. Vascular death was defined as any death during the index stroke admission, death caused by recurrent stroke, myocardial infarction or congestive heart failure, or sudden death without an identifiable nonvascular cause. 7 Outcome events were ascertained prospectively for 1 year after the index stroke by surveillance of medical records or structured telephone interviews conducted by trained stroke coordinators at each participating center. Because heart rate data were collected during the first 7 days after stroke, outcome ascertainment was started at the 8th day of stroke. The detailed protocols were described elsewhere. 8 , 9 The collection of clinical information for the CRCS‐K registry was approved by the local institutional review boards in all participating centers with a waiver for patient consent because of the use of anonymized data and minimal risk to participants. We obtained additional approval for this study. This study was reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline. 10
Statistical Analysis
Missing values were treated with multiple imputations by creating 10 multiple imputed datasets, and the pooled estimate was presented as a result. Each heart rate parameter was categorized as deciles and the cumulative incidence of outcomes was estimated using the Kaplan–Meier product‐limit method and compared among the deciles using the log‐rank test. Since a nonlinear association was previously suggested between heart rate and outcomes, 2 , 4 a multivariable Cox proportional hazards model with restricted cubic spines of 4 knots was constructed with each heart rate parameter and predetermined covariates. Predetermined covariates included age, sex, time from symptom onset to hospital arrival, initial stroke severity measured as National Institutes of Health Stroke Scale score, history of hypertension, diabetes, dyslipidemia, coronary artery disease, heart failure, previous history of stroke or transient ischemic attack, premorbid modified Rankin scale, current smoking status, whether the patient received recanalization therapy including intravenous thrombolysis or endovascular treatment, antithrombotic administration (antiplatelet agents and/or anticoagulants), statin administration at discharge, symptomatic steno‐occlusion of the intra‐ or extracranial major cerebral arteries, initial systolic blood pressure (SBP), mean value of SBP between the 4th and 7th days after stroke onset, initial glucose level, and ischemic stroke subtype.
Nonlinearity of the associations between the heart rate parameters and outcomes was assessed using the cubic term of the heart rate parameters, while the overall association was assessed using both the linear and cubic terms. The predictive power of each heart rate parameter for the primary outcome and all‐cause mortality was assessed using Akaike information criterion, Bayes Information Criterion, and Harrell’s C‐statistics. An optimal target (ie, cutoff point) for each heart rate parameter was determined using the Contal and O’Quigley method, which was developed to determine cutoff points in survival data by choosing the point that maximizes the log‐rank statistics. 11 Hazards below and above the cutoff were derived from a multivariable Cox‐frailty model with adjustment for clustering within hospitals. The robustness of the study results was tested by subgroup analysis according to SBP tertiles collected between the 4th and 7th day after stroke onset, age (>70 and ≤70 years), and presence and absence of AF or hypertension. Furthermore, a landmark analysis within or >30 days after starting outcome ascertainment using the time‐dependent Cox model was performed. The validation analysis using a separate dataset (2015–2018) was performed.
All statistical analyses were performed using SAS software (version 9.4, SAS Institute Inc, Cary, NC, USA) and R software version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria). A 2‐sided P value <0.05 was considered statistically significant.
Results
Among 18 093 patients with AIS registered between 2011 and 2014, 8031 met the eligibility criteria (Figure S1). The mean age was 68 years, ≈60% of the patients were men, 1 fourth had AF, half had steno‐occlusion of the major cerebral artery corresponding to the acute ischemic lesions, and the median National Institutes of Health Stroke Scale score at admission was 4 (Table 1). The median (interquartile range) number of heart rate measurements was 18 (12–35), and the mean and SD of the 4 heart rate parameters were 74.0±13.01 beats per minute (bpm) for the arithmetic mean, 73.9±13.01 for the time‐weighted average, 91.9±20.9 for maximum heart rate, and 60.3±10.5, for minimum heart rate (Figure S2).
Table 1.
Patients’ Baseline Characteristics: Comparison Between Main Dataset and Validation Dataset
| Variables | Main dataset (N=8031) | Validation dataset (N=10 000) |
|---|---|---|
| Age, y | 68.1±13.0 | 68.4±12.9 |
| Male sex | 4703 (58.6) | 5946 (59.5) |
| Hypertension | 5631 (70.1) | 6310 (63.1) |
| Diabetes | 2603 (32.4) | 3043 (30.4) |
| Dyslipidemia | 2482 (30.9) | 2544 (25.4) |
| Atrial fibrillation | 1966 (24.5) | 2250 (22.5) |
| Previous stroke or TIA | 1791 (22.3) | 2077 (20.8) |
| Current smoking | 2050 (25.5) | 2291 (22.9) |
| Coronary heart disease | 720 (9.0) | 922 (9.2) |
| Heart failure | 171 (2.1) | 178 (1.8) |
| Premorbid mRS | ||
| 0 | 6006 (74.8) | 8242 (82.4) |
| 1 | 589 (7.3) | 616 (6.2) |
| 2 | 463 (5.8) | 464 (4.6) |
| 3 | 491 (6.1) | 395 (3.9) |
| 4 | 264 (3.3) | 211 (2.1) |
| 5 | 218 (2.7) | 73 (0.7) |
| Onset to arrival time | 5 (2–17) | 5 (2–17) |
| Initial NIHSS score | 4 (2–11) | 4 (2–9) |
| Hyperacute treatment | ||
| Intravenous thrombolysis | 1378 (17.2) | 1642 (16.4) |
| Endovascular thrombectomy | 788 (9.8) | 1101 (11.0) |
| Stroke subtype | ||
| Large artery atherosclerosis | 2765 (34.4) | 3369 (33.7) |
| Small vessel occlusion | 1367 (17.0) | 1724 (17.2) |
| Cardioembolism | 2101 (26.2) | 2364 (23.6) |
| Other‐determined | 218 (2.7) | 368 (3.7) |
| Undetermined | 1580 (19.7) | 2175 (21.8) |
| Symptomatic steno‐occlusion of relevant artery | 4094 (51.0) | 4701 (47.0) |
| Discharge antiplatelet | 6111 (76.1) | 7435 (74.4) |
| Discharge anticoagulant | 1798 (22.4) | 1974 (19.7) |
| Discharge statin | 6711 (83.6) | 8708 (87.1) |
| Initial SBP, mm Hg | 149.4±27.8 | 148.4±27.7 |
| Mean SBP during 3rd to 7th day after onset, mm Hg | 134.0±14.7 | 135.9±15.6 |
| Initial glucose, mg/dL | 142.7±61.2 | 145.8±60.6 |
Values are number of patients (%), mean±SD, or median (interquartile range) unless otherwise indicated. mRS indicates modified Rankin’s scale; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood pressure; and TIA, transient ischemic attack.
The median follow‐up duration was 370 days (interquartile range, 356–392). The 1‐year cumulative incidence of outcome events was 13.8% for the primary outcome, 10.9% for all‐cause mortality, 3.7% for recurrent stroke, and 5.3% for the vascular composite, the composite of recurrent stroke, myocardial infarction, and vascular death. When the arithmetic mean of heart rate was divided into deciles, the cumulative incidence of the primary outcome, all‐cause mortality, and the vascular composite tended to increase with increasing deciles, respectively, and the results were most distinct in the highest 2 deciles. There were no significant differences, however, among deciles for recurrent stroke (Figure 1 and Table S1).
Figure 1. Survival curve of primary and secondary outcomes by mean heart rate deciles.
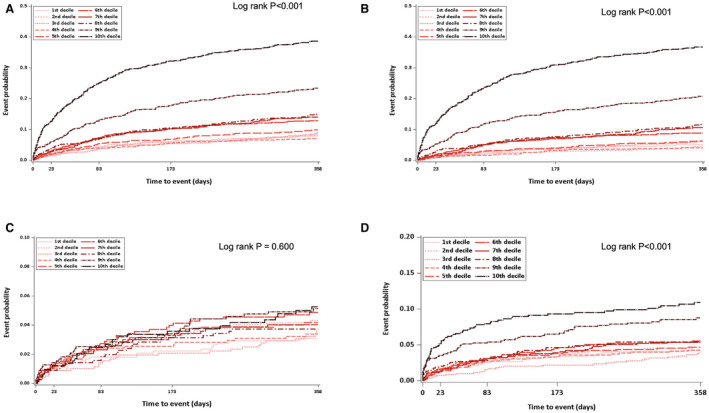
A, Composite of stroke recurrence, myocardial infarction, and all‐cause mortality. B, All‐cause mortality. C, Stroke recurrence. D, Composite of stroke recurrence, myocardial infarction, and vascular death.
The overall associations between all 4 heart rate parameters and the primary outcome were statistically significant (Figure 2A). Regarding nonlinearity, the relationships between maximum and minimum heart rate and the primary outcome were significant (P’s for nonlinear terms=0.008 for maximum heart rate and <0.001 for minimum heart rate), and the relationship appeared more “J‐shaped” for minimum heart rate than for maximum heart rate. The nadir was ≈55 bpm for minimum heart rate. The nonlinear relationship was more evident for all‐cause mortality in that all 4 heart rate parameters had a significant nonlinear effect on all‐cause mortality, and the relationships appeared more “J‐shaped” (Figure 2B). However, none of the 4 parameters were associated with recurrent stroke (Figure 2C), and the relationships with the vascular composite were similar to those with the primary outcome (Figure 2D).
Figure 2. Association between heart rate parameters and outcomes.
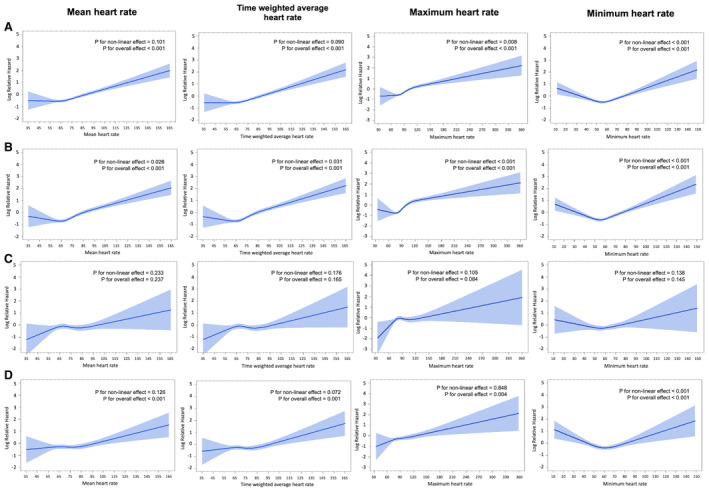
A, Composite of stroke recurrence, myocardial infarction, and all‐cause mortality. B, All‐cause mortality. C, Stroke recurrence. D, Composite of stroke recurrence, myocardial infarction, and vascular death.
We compared heart rate parameters regarding the predictive power of the constructed models. The primary outcome and all‐cause mortality were selected as outcomes for prediction based on the analysis results above showing the prominent relationships between heart rate parameters and these 2 outcomes. The model including maximum heart rate had the highest Harrell’s C‐index for the primary outcome and all‐cause mortality, and compared with this model, the models with no heart rate parameters or minimum heart rate were significantly inferior, although the models with arithmetic mean and time‐weighted average were comparable (Table 2). The model including time‐weighted average heart rate had the lowest Akaike information criterion and Bayes Information Criterion for the primary outcome and the model including maximum heart rate had the lowest Akaike information criterion and Bayes Information Criterion for all‐cause mortality.
Table 2.
Comparison of the Predictive Power for Primary Outcome and All‐Cause Mortality Between Heart Rate Parameters
| Composite of stroke recurrence, myocardial infarction, and all‐cause mortality | All‐cause mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| AIC | BIC | Harrell’s C index | P value for compared with Model 3 (paired differences in the Harrell’s C index) | AIC | BIC | Harrell’s C index | P value for compared with Model 3 (paired differences in the Harrell’s C index) | |
| Model 0 | 18 571.48 | 18 706.83 | 0.7639 | <0.001 | 14 089.51 | 14 218.35 | 0.8302 | <0.001 |
| Model 1 | 18 468.39 | 18 618.78 | 0.7740 | 0.213 | 13 958.29 | 14 101.45 | 0.8433 | 0.062 |
| Model 2 | 18 460.64 | 18 611.03 | 0.7748 | 0.504 | 13 950.16 | 14 093.32 | 0.8441 | 0.164 |
| Model 3 | 18 469.49 | 18 619.88 | 0.7758 | Ref | 13 946.48 | 14 089.64 | 0.8464 | Ref |
| Model 4 | 18 506.46 | 18 656.86 | 0.7693 | 0.002 | 14 013.48 | 14 156.64 | 0.8364 | <.001 |
AIC indicates Akaike information criterion; BIC, Bayes Information Criterion; and SBP, systolic blood pressure.
Model 0: predetermined covariates only; Model 1: Model 0+mean heart rate; Model 2: Model 0+time‐weighted average heart rate; Model 3: Model 0+maximum heart rate; Model 4: Model 0+minimum heart rate.
Predetermined covariates included admitted hospital, age, sex, time from symptom onset to hospital arrival, initial stroke severity, history of hypertension, diabetes, dyslipidemia, coronary artery disease, heart failure, previous history of stroke or transient ischemic attack, premorbid modified Rankin’s scale, current smoking, whether the patient received recanalization therapy (intravenous thrombolysis or endovascular treatment), antithrombotic (antiplatelet agents and/or anticoagulants) and statin administration at discharge, symptomatic steno‐occlusion of the intra‐ or extracranial major cerebral arteries, initial SBP, mean value of SBP between the 4th and 7th days after symptom onset, initial glucose level, and ischemic stroke subtype.
Regarding the primary outcome, the Contal and O’Quigley method yielded a cutoff point of 81 bpm for mean heart rate and time‐weighted average, 100 bpm for maximum heart rate, and 65 bpm for minimum heart rate (Table 3). The adjusted hazard ratio (HR) of each parameter above the obtained cutoff point was highest for maximum heart rate followed by time‐weighted average, mean, and minimum heart rate. Similar patterns were observed for all‐cause mortality.
Table 3.
Heart Rate Cutoff and HR of Primary Outcome and All‐Cause Mortality
| Heart rate cutoff by Contal and O’Quigley Method (beats per minute) | Main dataset | Validation dataset | |||
|---|---|---|---|---|---|
| Adjusted HR* (95% CI) | P value |
Adjusted HR* (95% CI) |
P value | ||
| Composite of stroke recurrence, myocardial infarction, and all‐cause mortality | |||||
| Mean heart rate | >81 | 1.65 (1.45–1.89) | <0.0001 | 1.50 (1.31–1.73) | <0.0001 |
| Time‐weighted average heart rate | >81 | 1.67 (1.46–1.91) | <0.0001 | 1.54 (1.34–1.77) | <0.0001 |
| Maximum heart rate | >100 | 1.80 (1.57–2.06) | <0.0001 | 1.69 (1.46–1.95) | <0.0001 |
| Minimum heart rate | >65 | 1.36 (1.20–1.54) | <0.0001 | 1.23 (1.08–1.40) | 0.0020 |
| All‐cause mortality | |||||
| Mean heart rate | >81 | 1.88 (1.62–2.17) | <0.0001 | 1.70 (1.45–1.98) | <0.0001 |
| Time‐weighted average heart rate | >80 | 1.89 (1.63–2.19) | <0.0001 | 1.65 (1.41–1.93) | <0.0001 |
| Maximum heart rate | >100 | 2.15 (1.84–2.51) | <0.0001 | 1.84 (1.57–2.17) | <0.0001 |
| Minimum heart rate | >69 | 1.68 (1.45–1.95) | <0.0001 | 1.22 (1.04–1.44) | 0.0156 |
Predetermined covariates included age, sex, time from symptom onset to hospital arrival, initial stroke severity, history of hypertension, diabetes, dyslipidemia, coronary artery disease, heart failure, previous history of stroke or transient ischemia attack, premorbid modified Rankin’s scale, current smoking, whether the patient received recanalization therapy (intravenous thrombolysis or endovascular treatment), antithrombotic (antiplatelet agents and/or anticoagulants) and statin administration at discharge, symptomatic steno‐occlusion of the intra‐ or extracranial major cerebral arteries, initial SBP, mean value of SBP between the 4th and 7th days after symptom onset, initial glucose level, and ischemic stroke subtype. HR indicates hazard ratio; and SBP, systolic blood pressure.
Derived from multivariable Cox‐frailty model.
The subgroup analysis according to mean SBP tertiles and history of hypertension showed that both SBP and history of hypertension did not affect the associations between heart rate parameters and outcomes (Figure S3 through S4). However, patients with AF had a higher risk of the primary outcome at the lower end of each heart rate parameter and a lower risk at the higher end (Figure S5). A similar pattern of effect modification was observed in the subgroup analysis by age for all‐cause mortality (Figure S6). In the landmark analysis, risk of the primary outcome consistently increased regardless of heart rate parameters before and after the landmark (Table S2 and Figure S7).
In the validation dataset, the frequency of most vascular risk factors was numerically lower than in the main dataset (Table 1). The 1‐year cumulative incidence of the primary outcome or all‐cause mortality in the validation dataset was lower than that in the main dataset (10.1% versus 13.8% for the primary outcome and 7.6% versus 10.9% for all‐cause mortality). Most results were reproduced with the validation dataset (Table 3 and Figure S8), but the overall strength of the associations between heart rate parameters and outcomes seemed to be attenuated.
Discussion
Our findings indicate that the heart rate parameters obtained between the 4th and 7th day after stroke onset were associated with major clinical events during the first year after AIS. The association was most evident for all‐cause mortality, which appears to be the main contributor to the association between heart rate parameters and the primary outcome. Among the heart rate parameters, maximum heart rate was the strongest predictor, while the mean and time‐weighted average heart rates were comparable. The cutoff value of 100 bpm for maximum heart rate and 81 bpm for mean and time‐weighted average heart rate best predicted outcomes with a >1.5‐fold increase in risk, which was reproduced in the validation dataset.
Contrary to plentiful evidence for the effect of heart rate on cardiovascular outcomes in the general population and patients with coronary artery disease, 12 , 13 , 14 , 15 only a few studies have evaluated the effect of heart rate on poststroke outcomes. The post‐hoc analysis of the PROFESS trial might be the largest study on this topic to date. 2 In this study, higher baseline heart rate was associated with higher risk of mortality but was not associated with stroke recurrence as reproduced here. The adjusted HR of the highest quintile, >82 bpm, was 1.74, which was also similar to the adjusted HR of the mean heart rate >81 bpm in our study (1.88 in the main dataset and 1.70 in the validation dataset). However, the generalizability of the PROFESS post‐hoc analysis is limited; it included only patients who had an indication for antiplatelet therapy; therefore, only 3% had AF. Our recent study reported that the mean heart rate had a nonlinear, “J‐shaped” relationship with 1‐year mortality in patients with AF‐related AIS. 4 Furthermore, the patients in the PROFESS trial were enrolled after the acute period of stroke, within 90 days of stroke onset. Our study collected heart rates during days 4 to 7 after stroke onset and tracked major clinical events for up to 1‐year poststroke.
Although we demonstrated an association between heart rate and major clinical events following AIS, a claim of causality cannot be concluded based solely on this association. For example, patients with elevated heart rates may have comorbid conditions, such as infection, dehydration, hyperthyroidism, or arrhythmia that underlie the elevation of the heart rate. 16 Furthermore, higher heart rate may be a marker of elevated sympathetic activity (eg, a stress response to stroke), which leads to pathophysiologic consequences such as endothelial dysfunction, cardiac remodeling, and renin‐angiotensin‐aldosterone system activation, which may be associated with poor outcomes. 17 , 18 , 19 A high heart rate itself can cause either hypoperfusion or hyperperfusion to ischemic brain regions where cerebral autoregulation is diminished or absent and result in further brain damage and adverse outcomes. 20 , 21 Otherwise, elevated heart rate was reported to be associated with poststroke disability and cognitive decline. 2 , 22 Elevated heart rate could induce oxidative stress and endothelial dysfunction, leading to atherosclerosis. 23 Ventricular dysfunction caused by prolonged tachycardia, decreased coronary perfusion, and renal dysfunction also may be a possible explanation for the association between elevated heart rate and adverse outcomes. 23 , 24 , 25 Whether lowering heart rate to a specific target range would be beneficial cannot be answered by our study and requires additional proof from a high‐level epidemiological study such as a randomized clinical trial.
The efficacy of β‐blockers (antihypertensive agents that lower heart rate) was proven by clinical trials in patients with acute myocardial infarction for prevention of re‐infarction and mortality, 26 , 27 , 28 and their use is currently recommended. 14 , 15 However, for patients with AIS, the efficacy of β‐blockers has been deemed inconclusive. 29 , 30 , 31 , 32 Our results suggest that it is time to re‐open the study of β‐blocker administration in patients with AIS with heart rates above our specified cutoff points. Maximum heart rate had the best predictive power among the 4 heart rate parameters. Patients who had ever experienced an episode of heart rate exceeding 100 bpm were at >200% risk of mortality during the first year after stroke.
Interestingly, our findings suggest that even transient tachycardia may be a predictor of future adverse events, a phenomenon that was reported in a study published in 1945. 33 A recent study based on a Chinese cohort also showed that even 1 measurement of high resting heart rate might be associated with higher risk of future mortality. 34 Maximum heart rate of >100 bpm can easily be applied to routine clinical practice and clinical trials.
Nonlinear “J‐shaped” relationships between heart rate parameters and the primary outcome or all‐cause mortality suggest an increased risk of major clinical events for patients with relatively lower heart rates. Sensitivity analysis according to AF status suggests that the increased risk observed at the lower end of the heart rate continuum may be driven by patients with AF (Figure S5). This phenomenon is consistent with our previous study of patients with AF‐related stroke. 4 Decreased cardiac contractility in patients with AF and a low heart rate may be an underlying explanatory mechanism. Moreover, low heart rate itself is known to be a predictor of incident AF. 35
The attenuated strength of the associations between heart rate and outcomes in the validation dataset could be explained by improvement in poststroke outcomes over time (2011–2014 versus 2015–2018). Also, this improvement might be attributed to lower prevalence of vascular risk factors, better premorbid functional status (Table 1), and improvement in quality of stroke care in South Korea over time. 36 , 37
Our study has several limitations. First, this was an observational study and the causal relationship between heart rate and outcomes could not be determined. Second, the participating centers of the CRCS‐K registry were mostly tertiary hospitals, limiting the generalizability of the study results. However, the age and sex distribution of the CRCS‐K registry was comparable to that of nationwide data of South Korea. 38 Third, heart rate was measured during routine practice, and therefore not by a standardized protocol. Fourth, heart rates and medications after discharge were not considered in our study.
Sources of Funding
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (HI10C2020), by a fund (code 2017ER620100) from the Research of Korea Centers for Disease Control and Prevention, and by a grant from Jeil Pharmaceutical Co., Ltd. However, the sponsors do not have any role in the analysis, interpretation, or publication of the study results.
Disclosures
Philip B. Gorelick reports participation in data safety monitoring boards, study steering committees, and MACE adjudication panels for industry. Hee‐Joon Bae reports grants from Astrazeneca, AstraZeneca Korea, Ltd., Bayer Korea, Boehringer Ingelheim Korea, Boryung Pharmaceutical, Bristol Myers Squibb, Bristol Myers Squibb Korea, Chong Gun Dang Pharmaceutical Corp., Daiichi Sankyo, Daiichi Sankyo Korea, Dong‐A ST, Esai, Jeil Pharmaceutical Co., Ltd., JLK, Korean Drug Co., Ltd., SAMJIN Pharm., Servier Korea, Shinpoong Pharm. Co. Ltd, Shire International GmbH, and Yuhan Corporation, and personal fees from Amgen Korea, Esai Korea, Otsuka Korea, Takeda Korea, and Viatris Korea outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Appendix S1
Tables S1–S2
Figures S1–S8
For Sources of Funding and Disclosures, see page 8.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.025861
References
- 1. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 2. Böhm M, Cotton D, Foster L, Custodis F, Laufs U, Sacco R, Bath PMW, Yusuf S, Diener HC. Impact of resting heart rate on mortality, disability and cognitive decline in patients after ischaemic stroke. Eur Heart J. 2012;33:2804–2812. doi: 10.1093/eurheartj/ehs250 [DOI] [PubMed] [Google Scholar]
- 3. Sacco RL, Diener H‐C, Yusuf S, Cotton D, Ôunpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, et al. Aspirin and extended‐release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238–1251. doi: 10.1056/NEJMoa0805002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee K‐J, Kim BJ, Han M‐K, Kim J‐T, Choi K‐H, Shin D‐I, Yeo M‐J, Cha J‐K, Kim D‐H, Nah H‐W, et al. Effect of heart rate on stroke recurrence and mortality in acute ischemic stroke with atrial fibrillation. Stroke. 2020;51:162–169. doi: 10.1161/STROKEAHA.119.026847 [DOI] [PubMed] [Google Scholar]
- 5. Ko Y, Lee SJ, Chung J‐W, Han M‐K, Park J‐M, Kang K, Park TH, Park S‐S, Cho Y‐J, Hong K‐S, et al. MRI‐based algorithm for acute ischemic stroke subtype classification. J Stroke. 2014;16:161. doi: 10.5853/jos.2014.16.3.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aslanyan S, Fazekas F, Weir CJ, Horner S, Lees KR. Effect of blood pressure during the acute period of ischemic stroke on stroke outcome: a tertiary analysis of the GAIN International Trial. Stroke. 2003;34:2420–2425. doi: 10.1161/01.STR.0000091233.04524.0C [DOI] [PubMed] [Google Scholar]
- 7. Kang K, Park TH, Kim N, Jang MU, Park S‐S, Park J‐M, Ko Y, Lee SJ, Lee KB, Lee J, et al. Recurrent stroke, myocardial infarction, and major vascular events during the first year after acute ischemic stroke: the multicenter prospective observational study about recurrence and its determinants after acute ischemic stroke I. J Stroke Cerebrovasc Dis. 2016;25:656–664. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.036 [DOI] [PubMed] [Google Scholar]
- 8. Kim BJ, Park J‐M, Kang K, Lee SJ, Ko Y, Kim JG, Cha J‐K, Kim D‐H, Nah H‐W, Han M‐K, et al. Case characteristics, hyperacute treatment, and outcome information from the clinical research center for stroke‐fifth division registry in South Korea. J Stroke. 2015;17:38–53. doi: 10.5853/jos.2015.17.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim BJ, Han M‐K, Park TH, Park S‐S, Lee KB, Lee B‐C, Yu K‐H, Cha JK, Kim D‐H, Lee J, et al. Current status of acute stroke management in Korea: a report on a multicenter, comprehensive acute stroke registry. Int J Stroke. 2014;9:514–518. doi: 10.1111/ijs.12199 [DOI] [PubMed] [Google Scholar]
- 10. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 11. Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30:253–270. doi: 10.1016/S0167-9473(98)00096-6 [DOI] [Google Scholar]
- 12. Cook S, Togni M, Schaub MC, Wenaweser P, Hess OM. High heart rate: a cardiovascular risk factor? Eur Heart J. 2006;27:2387–2393. doi: 10.1093/eurheartj/ehl259 [DOI] [PubMed] [Google Scholar]
- 13. Kolloch R, Legler UF, Champion A, Cooper‐DeHoff RM, Handberg E, Zhou Q, Pepine CJ. Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: findings from the INternational VErapamil‐SR/ trandolapril STudy (INVEST). Eur Heart J. 2008;29:1327–1334. doi: 10.1093/eurheartj/ehn123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e344–e426. doi: 10.1161/CIR.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 15. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al., American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6 [DOI] [PubMed] [Google Scholar]
- 16. Olshansky B, Sullivan RM. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013;61:793–801. doi: 10.1016/j.jacc.2012.07.074 [DOI] [PubMed] [Google Scholar]
- 17. Jia G, Aroor AR, Hill MA, Sowers JR. Role of renin‐angiotensin‐aldosterone system activation in promoting cardiovascular fibrosis and stiffness. Hypertension. 2018;72:537–548. doi: 10.1161/HYPERTENSIONAHA.118.11065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res. 2014;114:1804–1814. doi: 10.1161/CIRCRESAHA.114.302524 [DOI] [PubMed] [Google Scholar]
- 19. Bruno RM, Ghiadoni L, Seravalle G, Dell’Oro R, Taddei S, Grassi G. Sympathetic regulation of vascular function in health and disease. Front Physiol. 2012;3:1–15. doi: 10.3389/fphys.2012.00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castillo J, Leira R, García MM, Serena J, Blanco M, Dávalos A. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35:520–527. doi: 10.1161/01.STR.0000109769.22917.B0 [DOI] [PubMed] [Google Scholar]
- 21. Saglietto A, Scarsoglio S, Ridolfi L, Gaita F, Anselmino M. Higher ventricular rate during atrial fibrillation relates to increased cerebral hypoperfusions and hypertensive events. Sci Rep. 2019;9:1–9. doi: 10.1038/s41598-019-40445-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Böhm M, Schumacher H, Leong D, Mancia G, Unger T, Schmieder R, Custodis F, Diener H‐C, Laufs U, Lonn E, et al. Systolic blood pressure variation and mean heart rate is associated with cognitive dysfunction in patients with high cardiovascular risk. Hypertension. 2015;65:651–661. doi: 10.1161/HYPERTENSIONAHA.114.04568 [DOI] [PubMed] [Google Scholar]
- 23. Custodis F, Schirmer SH, Baumhkel M, Heusch G, Bhm M, Laufs U. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol. 2010;56:1973–1983. doi: 10.1016/j.jacc.2010.09.014 [DOI] [PubMed] [Google Scholar]
- 24. Huizar JF, Ellenbogen KA, Tan AY, Kaszala K. Arrhythmia‐induced cardiomyopathy: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019;73:2328–2344. doi: 10.1016/j.jacc.2019.02.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Böhm M, Schumacher H, Schmieder RE, Mann JFE, Teo K, Lonn E, Sleight P, Mancia G, Linz D, Mahfoud F, et al. Resting heart rate is associated with renal disease outcomes in patients with vascular disease: results of the ONTARGET and TRANSCEND studies. J Intern Med. 2015;278:38–49. doi: 10.1111/joim.12333 [DOI] [PubMed] [Google Scholar]
- 26. Freemantle N, Cleland J, Young P, Mason J, Harrison J. β blockade after myocardial infarction: systematic review and meta regression analysis. Br Med J. 1999;318:1730–1737. doi: 10.1136/bmj.318.7200.1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Z, Xie J, COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group . Early intravenous then oral metoprolol in 45 852 patients with acute myocardial infarction: randomised placebo‐controlled trial. Lancet. 2005;366:1622–1632. doi: 10.1016/S0140-6736(05)67661-1 [DOI] [PubMed] [Google Scholar]
- 28. Puymirat E, Riant E, Aissoui N, Soria A, Ducrocq G, Coste P, Cottin Y, Aupetit JF, Bonnefoy E, Blanchard D, et al. β blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ. 2016;354. doi: 10.1136/bmj.i4801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Lima LG, Saconato H, Atallah ÁN, da Silva EMK. Beta‐blockers for preventing stroke recurrence. Cochrane Database Syst Rev. 2014;2014: doi: 10.1002/14651858.CD007890.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sykora M, Siarnik P, Diedler J, Lees KR, Alexandrov A, Bath PM, Bluhmki E, Bornstein N, Claesson L, Davis SM, et al. β‐blockers, pneumonia, and outcome after ischemic stroke. Stroke. 2015;46:1269–1274. doi: 10.1161/STROKEAHA.114.008260 [DOI] [PubMed] [Google Scholar]
- 31. Dziedzic T, Slowik A, Pera J, Szczudlik A. Beta‐blockers reduce the risk of early death in ischemic stroke. J Neurol Sci. 2007;252:53–56. doi: 10.1016/j.jns.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 32. Starr JB, Tirschwell DL, Becker KJ. Labetalol use is associated with increased in‐hospital infection compared with nicardipine use in intracerebral hemorrhage. Stroke. 2017;48:2693–2698. doi: 10.1161/STROKEAHA.117.017230 [DOI] [PubMed] [Google Scholar]
- 33. Levy RL. Transient tachycardia. J Am Med Assoc. 1945;129(9):585. doi: 10.1001/jama.1945.02860430001001 [DOI] [Google Scholar]
- 34. Zhao Q, Li H, Wang A, Guo J, Yu J, Luo Y, Chen S, Tao L, Li Y, Li A, et al. Cumulative resting heart rate exposure and risk of all‐cause mortality: results from the Kailuan Cohort Study. Sci Rep. 2017;7:1–9. doi: 10.1038/srep40212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grundvold I, Skretteberg PT, Liestøl K, Erikssen G, Engeseth K, Gjesdal K, Kjeldsen SE, Arnesen H, Erikssen J, Bodegard J. Low heart rates predict incident atrial fibrillation in healthy middle‐aged men. Circ Arrhythmia Electrophysiol. 2013;6:726–731. doi: 10.1161/CIRCEP.113.000267 [DOI] [PubMed] [Google Scholar]
- 36. Kim JY, Kang K, Kang J, Koo J, Kim D‐H, Kim BJ, Kim W‐J, Kim E‐G, Kim JG, Kim J‐M, et al. Executive summary of stroke statistics in Korea 2018: a report from the epidemiology research council of the Korean Stroke Society. J Stroke. 2019;21:42–59. doi: 10.5853/jos.2018.03125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park H‐K, Kim S‐E, Cho Y‐J, Kim JY, Oh H, Kim BJ, Kang J, Lee K‐J, Jang MU, Park J‐M, et al. Quality of acute stroke care in Korea (2008–2014): retrospective analysis of the nationwide and nonselective data for quality of acute stroke care. Eur Stroke J. 2019;4:337–346. doi: 10.1177/2396987319849983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park TH, Ko Y, Lee SJ, Lee KB, Lee J, Han M‐K, Park J‐M, Kim D‐E, Cho Y‐J, Hong K‐S, et al. Gender differences in the age‐stratified prevalence of risk factors in Korean ischemic stroke patients: a nationwide stroke registry‐based cross‐sectional study. Int J Stroke. 2014;9:759–765. doi: 10.1111/ijs.12146 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Tables S1–S2
Figures S1–S8
Data Availability Statement
The anonymized data from this study may be shared after approval from the local institutional review board with qualified researchers carrying out legitimate research by contacting the lead investigator (H‐JB at braindoc@snu.ac.kr).


