Abstract
Background
Early reports from the COVID‐19 pandemic identified coronary thrombosis leading to ST‐segment–elevation myocardial infarction (STEMI) as a complication of COVID‐19 infection. However, the epidemiology of STEMI in patients with COVID‐19 is not well characterized. We sought to determine the incidence, diagnostic and therapeutic approaches, and outcomes in STEMI patients hospitalized for COVID‐19.
Methods and Results
Patients with data on presentation ECG and in‐hospital myocardial infarction were identified from January 14, 2020 to November 30, 2020, from 105 sites participating in the American Heart Association COVID‐19 Cardiovascular Disease Registry. Patient characteristics, resource use, and clinical outcomes were summarized and compared based on the presence or absence of STEMI. Among 15 621 COVID‐19 hospitalizations, 54 (0.35%) patients experienced in‐hospital STEMI. Among patients with STEMI, the majority (n=40, 74%) underwent transthoracic echocardiography, but only half (n=27, 50%) underwent coronary angiography. Half of all patients with COVID‐19 and STEMI (n=27, 50%) did not undergo any form of primary reperfusion therapy. Rates of all‐cause shock (47% versus 14%), cardiac arrest (22% versus 4.8%), new heart failure (17% versus 1.4%), and need for new renal replacement therapy (11% versus 4.3%) were multifold higher in patients with STEMI compared with those without STEMI (P<0.050 for all). Rates of in‐hospital death were 41% in patients with STEMI, compared with 16% in those without STEMI (P<0.001).
Conclusions
STEMI in hospitalized patients with COVID‐19 is rare but associated with poor in‐hospital outcomes. Rates of coronary angiography and primary reperfusion were low in this population of patients with STEMI and COVID‐19. Adaptations of systems of care to ensure timely contemporary treatment for this population are needed.
Keywords: acute myocardial infarction, coronavirus disease 2019, ST‐segment–elevation myocardial infarction
Subject Categories: Cardiovascular Disease, Complications
Patients with established cardiovascular disease who develop COVID‐19 have high rates of morbidity and mortality. SARS‐CoV‐2 has been shown to promote inflammation and thrombosis. 1 Early single‐center series reported patients with COVID‐19 presenting with ST‐segment elevation on the ECG, and some of whom had obstructive coronary disease and thrombosis whereas others did not. 2 , 3 To date, the epidemiology, care patterns, treatment strategies, and outcomes in patients hospitalized for COVID‐19 who develop ST‐segment–elevation myocardial infarction (STEMI) have not been described in a large multicenter population. Therefore, we sought to examine these characteristics among hospitalized patients with COVID‐19 experiencing STEMI within the American Heart Association (AHA) COVID‐19 Cardiovascular Disease Registry.
Methods
Given the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the AHA. The design of the AHA COVID‐19 Cardiovascular Disease Registry has been reported. 4 In this voluntary registry of consecutive admissions for adults with active COVID‐19 in the United States from 105 participating health centers, clinical information, including demographics, medical history, presentation details, laboratory markers, and in‐hospital outcomes were captured using standardized definitions. Individual informed consent was not required. Personnel at participating hospitals are instructed to abstract data from all consecutive patients hospitalized with active COVID‐19 infection, regardless of cardiovascular disease status, and data are entered into an electronic case record form in the Patient Management Tool, powered by IQVIA (Parsippany, NJ). Patients with missing data on the presence of STEMI or the presenting ECG were not eligible for this analysis.
Statistical Analysis
The incidence of STEMI among patients hospitalized with COVID‐19 was assessed from January 14, 2020 to November 30, 2020. Characteristics of the presenting illness were summarized and compared between patients based on the presence or absence of STEMI. STEMI was indicated by trained personnel inputting data into electronic case report forms using standardized definitions, consistent with established diagnostic criteria for STEMI. STEMI was noted if it occurred anytime during the hospitalization, including at hospital admission, and was not required to be a principal reason for hospital admission. Diagnostic strategies, in‐hospital resource use, and clinical outcomes were described. Resource use included use of intensive care unit or mechanical ventilation. Clinical outcomes included the development of shock, cardiac arrest, sustained ventricular arrhythmias, de novo heart failure, and mortality, among others. Where available, angiographic findings and reperfusion strategies were reported. To assess for differences in early and later periods of the pandemic, we evaluated diagnostic and therapeutic evaluations for patients admitted on or after June 1, 2020, as compared with those admitted earlier in the pandemic. In‐hospital outcomes were compared in patients with and without STEMI. The association between STEMI and in‐hospital mortality was evaluated using multivariable logistic regression. Models were adjusted for age and sex (Model 1) and for age, sex, history of coronary artery disease, heart failure, chronic kidney disease, diabetes, pulmonary disease, immune disorder, pulmonary embolism/deep venous thrombosis, and obesity (Model 2). Continuous variables are presented as medians and interquartile ranges, and categorical variables are presented as counts and percentages. Differences in continuous variables were assessed across groups using the Mann‐Whitney U test. Differences in categorical variables were evaluated across groups using the χ2 test and Fisher exact test, where appropriate. Statistical significance was assessed at a nominal 2‐sided α level of 0.05. All reported P values were 2‐sided. The protocol was approved by the Mass General Brigham Institutional Review Board, and informed consent was deemed not to be required.
Results
Incidence and Clinical Characteristics
Among 15 621 admissions for COVID‐19 from 105 participating centers, site‐reported ST‐segment elevation on the admission ECG was present in 243 (1.6%) cases, of which 196 (80.7%) did not have a final site‐reported diagnosis of acute myocardial infarction during the hospitalization. Among included hospitalizations, 54 (0.35%) patients experienced STEMI during hospitalization, of whom 47 had STEMI presentation at the time of hospital admission. Data on presenting ECG features and on the presence or absence of myocardial infarction (with or without ST elevation) were missing in 95 patients in the overall registry, and therefore STEMI status was unable to be discerned in those patients.
Patients with STEMI had a median age of 70 years (25th–75th percentiles, 62–78 years) and were predominantly men (n=37, 68.5%) (Table 1). Compared with COVID‐19 patients without STEMI, patients with STEMI had similar rates of prior myocardial infarction and coronary risk factors, including diabetes, hypertension, and hyperlipidemia. Systemic COVID‐19 symptoms are described in Table 1. Patients with STEMI had higher white blood cell counts (10.3 K/µL [25th–75th percentiles, 7.6–13.7 K/µL] versus 6.9 K/µL [25th–75th percentiles, 5.0–9.6K/µL], P<0.001) and greater elevations in aspartate transaminase (51.5 µ/L [25th–75th percentiles, 30.5–164.0 µ/L] versus 40.0 µ/L [25th–75th percentiles, 27.0–61.0 µ/L], P=0.004) and alanine aminotransferase (34.0 µ/L [25th–75th percentiles, 26.0–57.0 µ/L] versus 30.0 µ/L [25th–75th percentiles, 19.0–49.0 µ/L], P=0.042), compared with those without STEMI. Available natriuretic peptide concentrations were numerically greater in those with STEMI compared with those without, though it did not reach statistical significance. Available high‐sensitivity troponin values at hospital admission were markedly higher in those with STEMI versus those without (74 ng/L [23–600 ng/L] versus 10 ng/L [0–42 ng/L], P<0.001). Among patients with available information surviving to hospital discharge, median length of stay was 6 days (25th‐75th percentiles, 3–12 days) for all patients and 4 days (25th–75th percentiles, 3–15 days) for those with in‐hospital STEMI.
Table 1.
Baseline Characteristics and Presenting Variables According to STEMI Status
| No STEMI, N=15 567 | STEMI, N=54 | P value | |
|---|---|---|---|
| Demographics | |||
| Age, y, median (25th–75th) | 63 (51–75) | 70 (62–78) | 0.017 |
| Female sex | 6935 (44.5) | 17 (31.5) | 0.054 |
| White race | 5919 (38.0) | 23 (42.6) | 0.490 |
| Comorbidities | |||
| Diabetes | 5821 (37.4) | 22 (40.7) | 0.612 |
| Dyslipidemia | 5825 (37.4) | 24 (44.4) | 0.287 |
| Hypertension | 9634 (61.9) | 38 (70.4) | 0.200 |
| Prior MI | 909 (5.8) | 6 (11.1) | 0.133 |
| Prior PCI | 778 (5.0) | 3 (5.6) | 0.751 |
| Prior heart failure | 1945 (12.5) | 0 (0.0) | 0.006 |
| Chronic kidney disease | 2197 (14.1) | 4 (7.4) | 0.157 |
| Before admit medication use | |||
| ACEI/ARB | 4579/15 208 (30.1) | 17/52 (32.7) | 0.685 |
| β‐Blocker | 4254/15 208 (28.0) | 10/52 (19.2) | 0.161 |
| Statin | 5906/15 208 (38.8) | 19/52 (36.5) | 0.734 |
| Aspirin | 3999/15 565 (25.7) | 12/54 (22.2) | 0.560 |
| Anticoagulant | 1847/15 208 (12.1) | 4/52 (7.7) | 0.326 |
| Admission presentation | |||
| Hemoglobin, g/dL | 13.0 (11.4–14.4) | 13.2 (12.0–14.7) | 0.308 |
| WBC, K/µL | 6.9 (5.0–9.6) | 10.3 (7.6–13.7) | <0.001 |
| Platelets, K/µL | 204.0 (156.0–266.0) | 214.9 (162.0–300.0) | 0.194 |
| Absolute lymphocyte count, ×109 | 1.0 (0.7–1.4) | 1.1 (0.7–1.8) | 0.347 |
| AST, µ/L | 40.0 (27.0–61.0) | 51.5 (30.5–164.0) | 0.004 |
| ALT, µ/L | 30.0 (19.0–49.0) | 34.0 (26.0–57.0) | 0.042 |
| Serum creatinine, mg/dL | 1.0 (0.8–1.5) | 1.2 (0.9–1.7) | 0.139 |
| CRP, mg/L | 60.8 (15.2–114.0) | 61.0 (13.7–147.7) | 0.429 |
| Interleukin‐6, pg/mL | 19.0 (5.0–61.6) | 8.1 (5.0–25.0) | 0.299 |
| D‐dimer, ng/mL | 770 (340–1530) | 1000 (584–3050) | 0.058 |
| Ferritin, ng/mL | 576 (259–1174) | 784.9 (315–1483) | 0.318 |
| BNP, pg/mL | 53.0 (17.0–190.0) | 173.0 (34.0–687.0) | 0.050 |
| NT‐proBNP, pg/mL | 291.0 (67.1–1535.0) | 826.0 (259.0–2397.0) | 0.099 |
| Troponin, ng/L | 10.0 (0.0–42.0) | 74.0 (22.9–600.0) | <0.001 |
| Time from COVID‐19 symptom onset to admission, d, median (25th–75th) | 6.0 (2.0–9.0) | 7.0 (1.0–12.0) | 0.533 |
| Resource use | |||
| Intensive care unit use | 5152 (33.1) | 44 (81.5) | <0.001 |
| Mechanical ventilation use | 3237 (20.8) | 26 (48.1) | <0.001 |
| New renal replacement therapy | 676 (4.3) | 6 (11.1) | 0.030 |
| In‐hospital events | |||
| All‐cause mortality | 2449 (15.7) | 22 (40.7) | <0.001 |
| Cause of death | <0.001 | ||
| Respiratory | 1815 (75.4) | 13 (59.1) | |
| Cardiovascular* | 193 (8.0) | 7 (31.8) | |
| Other | 398 (16.5) | 2 (9.1) | |
| Shock | 2056 (13.5) | 25 (47.2) | <0.001 |
| Cardiogenic | 110 (5.4) | 6 (24.0) | |
| Distributive | 1544 (75.3) | 9 (36.0) | |
| Mixed | 173 (8.4) | 9 (36.0) | |
| Other | 224 (10.9) | 1 (4.0) | |
| Cardiac arrest | 752 (4.8) | 12 (22.2) | <0.001 |
| Sustained ventricular arrhythmias | 191 (1.2) | 7 (13.0) | <0.001 |
| De novo heart failure | 217 (1.4) | 9 (16.7) | <0.001 |
| Intracranial hemorrhage/stroke | 239 (1.5) | 3 (5.6) | 0.051 |
| Deep vein thrombosis | 417 (2.7) | 2 (3.7) | 0.657 |
| Pulmonary embolism | 300 (1.9) | 4 (7.4) | 0.021 |
Values reflect count (number) and proportion (percent) unless otherwise specified. All ranges represent the 25th–75th quartiles. Differences in categorical variables were assessed across study groups using the χ2 test or Fisher exact test as appropriate. Differences in continuous variables were assessed using the Mann‐Whitney U test. ACEI indicates angiotensin‐converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin receptor blockers; AST, aspartate transaminase; BNP, brain natriuretic peptide; CRP, C‐reactive protein; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; STEMI, ST‐segment–elevation myocardial infarction; and WBC, white blood cell count.
Includes death attributable to acute MI, arrhythmia, heart failure, or stroke.
Diagnostic Evaluation and Reperfusion
Among patients with STEMI (n=54), the majority (n=40, 74%) underwent transthoracic echocardiogram during hospitalization, where the median left ventricular ejection fraction was 45% (25th–75th percentiles, 35%–57%; Table 2). Among STEMI patients, coronary angiography was pursued in half (n=27) of patients. In all cases, angiographic assessment was invasive rather than via coronary computed tomography angiography. Angiographic findings included no areas of obstructive coronary disease (defined as >50% stenosis) in 3 (11%) patients. Primary percutaneous coronary intervention (PCI) was performed in 21 (38.9%) patients, whereas fibrinolytic therapy was used in 6 (11.1%) patients. Of patients who received fibrinolytic therapy, 2 (33.3%) underwent coronary angiography, and 1 (16.7%) underwent PCI during hospitalization. Among these patients (n=6), 3 (50.0%) developed shock, 1 (16.7%) had cardiac arrest, and 2 (33.3%) died during hospitalization. Half (n=27) of all patients with COVID‐19 and STEMI did not undergo any reperfusion therapy. Rates of diagnostic evaluation with transthoracic echocardiogram and coronary angiography and therapeutic intervention with primary PCI were higher in patients admitted on or after June 1, 2020, as compared with those admitted earlier in the pandemic (Table S1).
Table 2.
Diagnostic and Therapeutic Evaluations Among Patients With STEMI
| Diagnostic and therapeutic evaluations | STEMI, N=54 |
|---|---|
| Diagnostic evaluations | |
| Transthoracic echocardiography | 40 (74.1) |
| LVEF, %, median (25th–75th quartiles), n=39 | 45.0 (35.0–57.0) |
| Coronary angiography | 27 (50.0) |
| CCTA | 0 (0.0) |
| Invasive | 27 (100.0) |
| Angiographic findings, n=27 | |
| No. of vessels with >50% stenosis | |
| 0 | 3 (11.1) |
| 1 | 8 (29.6) |
| 2 | 7 (25.9) |
| ≥3 | 8 (29.6) |
| Left main coronary disease | 1 (3.7) |
| STEMI reperfusion | |
| Primary PCI | 21 (38.9) |
| Fibrinolytic therapy | 6 (11.1) |
| No reperfusion | 27 (50.0) |
CCTA indicates coronary computed tomography angiography; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; and STEMI, ST‐segment–elevation myocardial infarction.
Resource Use and In‐Hospital Events
Patients hospitalized for COVID‐19 with STEMI had significantly higher rates of intensive care unit admission (81% versus 33%) and mechanical ventilation (48% versus 21%) compared with those without STEMI (P<0.001 for both). Rates of all‐cause shock (47% versus 14%), cardiac arrest (22% versus 4.8%), new heart failure (17% versus 1.4%), sustained ventricular arrhythmias (13% versus 1.2%), and new initiation of renal replacement therapy (11% versus 4.3%) were multifold higher in patients with STEMI compared with those without STEMI (P<0.050 for all) (Table 1, Figure). Among STEMI patients, all‐cause in‐hospital mortality was 41%, and cardiovascular mortality was 32%, compared with 16% and 8%, respectively, for those without STEMI (P<0.01 for both comparisons). In multivariate regression adjusted for age and sex (Model 1), STEMI was associated with greater odds of in‐hospital mortality (adjusted odds ratio [aOR], 3.14 [95% CI, 1.80–5.63]; P<0.001). After further adjustment for clinical characteristics (Model 2), STEMI remained associated with greater odds of in‐hospital mortality (aOR, 2.95 [95% CI, 1.59–5.49]; P<0.001) (Table S2).
Figure 1. In‐hospital events among COVID‐19 patients with and without STEMI.
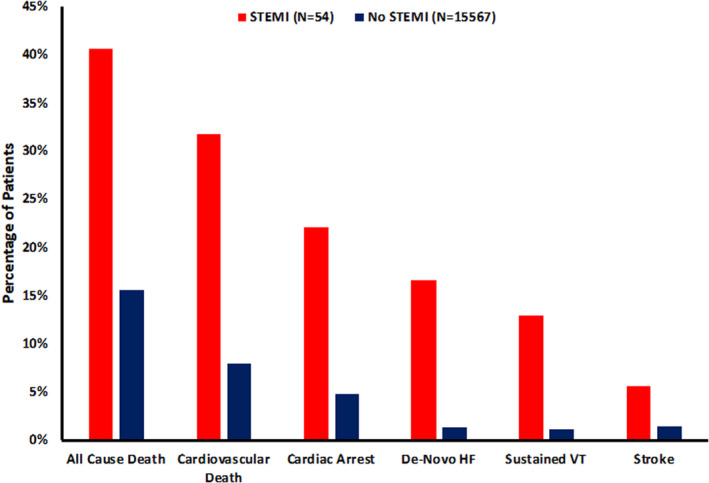
Stroke includes ischemic stroke and intracranial hemorrhage. HF indicates heart failure; STEMI, ST‐segment–elevation myocardial infarction; and VT, ventricular tachycardia.
Discussion
In this large national cohort of patients hospitalized for COVID‐19 in the United States, STEMI was rare, occurring in <0.5% of patients, but was associated with poor in‐hospital outcomes and high mortality. Diagnostic evaluations, including coronary angiography, were pursued in only half of patients with STEMI, and primary PCI was performed in a minority of patients.
We assessed the incidence of STEMI in patients hospitalized with COVID‐19 across a large, multisite US national registry. Early case reports and case series raised concerns that COVID‐19 might be associated with high rates of coronary thrombosis and STEMI. 3 Publication bias and concerns over other thrombotic events seen during COVID‐19 illness may have inflated concerns about the incidence of STEMI in this population. When we assessed the incidence of STEMI in consecutive hospitalizations for COVID‐19 in a large multicenter US registry, we found it to be a relatively infrequent event occurring in <0.5% of patients. Our findings are consistent with the limited data available from randomized controlled trials, which have to date demonstrated low rates of type 1 myocardial infarction events. 5 , 6 , 7
We also found that coronary angiography was pursued at rates far lower than in contemporary practice for patients without COVID‐19, but higher than reported in international case series among COVID‐19 patients earlier in the pandemic, in which 24% of patients were treated with primary PCI and 76% with fibrinolysis. 8 Lower rates of coronary angiography could be related to infection control concerns or concerns about diminished overall clinical benefit in the setting of severe COVID‐19. 9 Finally, diagnostic uncertainty may have played a role in low rates of reperfusion therapy. In addition, our findings suggest important differences in the diagnostic use of coronary angiography and primary PCI for STEMI during early versus subsequent waves of the pandemic. Particularly, rates of transthoracic echocardiography and coronary angiography were higher in those admitted during later stages of the pandemic, whereas fibrinolytic therapy was used less frequently. This finding may represent increasing familiarity with the disease state, established center‐specific or global protocols in management of STEMI in patients with COVID‐19, 10 and greater availability of personal protective equipment for the catherization laboratory and echocardiography staff later in the pandemic, 11 among other reasons. The majority of patients in this cohort identified as having ST‐elevations on presentation ECG were ultimately not diagnosed with STEMI. Prior case reports have described STEMI mimics in the absence of epicardial coronary disease in patients with COVID‐19, the pathophysiology of which may include stress cardiomyopathy, pericarditis, myocarditis, and other entities. 2 , 3 Importantly, however, of those diagnosed with STEMI and undergoing coronary angiography in our study, ≈90% underwent primary PCI, suggesting similar pathophysiology as in non–COVID‐19 STEMI. Professional societies, including the AHA and others, have offered guidance encouraging effective and timely STEMI systems of care during the pandemic. 10 These findings may have pertinence in future waves of the current pandemic or in other instances where resource use needs shift drastically.
Despite the low overall incidence of STEMI, patients hospitalized with COVID‐19 and STEMI had markedly increased ventilatory, intensive care, and renal replacement needs and multifold increases in in‐hospital adverse clinical events compared with patients with COVID‐19 without STEMI. Myocardial injury and resultant heart failure and arrhythmia may have contributed to the worse outcomes, exaggerated by a predilection for more severe COVID‐19 and high overall thrombotic burden in those presenting with STEMI. 12 , 13 Prior reports have also demonstrated delays in door‐to‐device times in STEMI during the COVID‐19 pandemic, possibly furthering STEMI‐related mortality among patients with COVID‐19. 14 , 15
This analysis has several limitations that should be acknowledged. First, this study was observational, and therefore associations should be interpreted as hypothesis generating. Analysis relied on site‐level characterization and reporting of events and lacked centralized adjudication. Second, a detailed characterization of the severity of illness at the time of STEMI diagnosis and reliable information linking the timing of symptom onset to STEMI diagnosis or reperfusion times are not available, thereby limiting the ability to assess the timeliness of reperfusion in patients presenting with COVID‐19 and STEMI. Third, the AHA COVID‐19 Registry is a voluntary program among hospitals interested in a quality improvement program in the United States, affecting the generalizability of these findings to other areas similarly affected by the COVID‐19 pandemic.
Conclusions
In a large US registry of COVID‐19 hospitalizations, the incidence of STEMI was <0.5%. Diagnostic evaluation and primary PCI were pursued in a relative minority of patients. Although rare, STEMI was associated with poor in‐hospital outcomes, including multifold higher shock rates, cardiac arrest, intensive care unit admission, and all‐cause mortality.
Sources of Funding
AHA’s suite of registries is funded by multiple industry sponsors. AHA’s COVID‐19 Cardiovascular Disease Registry is partially supported by the Gordon and Betty Moore Foundation. The Get With The Guidelines programs are provided by the AHA.
Disclosures
Dr Bhatt reports consulting fees from Sanofi Pasteur, Verve Therapeutics, and Clarivate and is supported by the National Heart, Lung, and Blood Institute T32 postdoctoral training grant T32HL007604. Dr Varshney is on the Advisory Board for Broadview Ventures and is supported by the National Heart, Lung, and Blood Institute T32 postdoctoral training grant T32HL007604. E.L. Goodrich is a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from Abbott Laboratories, Amgen, Anthos Therapeutics, Arca Biopharma, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi‐Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Regeneron, Roche, Siemens, Takeda, The Medicines Company, and Zora Biosciences. Dr de Lemos reports grant support from Roche Diagnostics and Abbott Diagnostics and consulting income from Amgen, Regneneron, Siemen’s Health Care Diagnostics, Ortho Clinical Diagnostics, and Quidel. Dr Morrow is a member of the TIMI (Thrombolysis In Myocardial Infarction) Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from Abbott Laboratories, Amgen, Anthos Therapeutics, Arca Biopharma, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi‐Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Regeneron, Roche, Siemens, Takeda, The Medicines Company, and Zora Biosciences. Dr Morrow has received consulting fees from Bayer Pharma, InCarda, Merck, Novartis, and Roche Diagnostics. Dr Bohula is a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from Abbott Laboratories, Amgen, Anthos Therapeutics, Arca Biopharma, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi‐Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Regeneron, Roche, Siemens, Takeda, The Medicines Company, and Zora Biosciences. Dr Bohula has received consulting fees from Novo Nordisk, Amgen, Medscape, Servier, and Kowa. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Acknowledgments
The Get With The Guidelines programs are provided by the AHA. The AHA Precision Medicine Platform (https://precision.heart.org/) was used for data analysis. IQVIA (Parsippany, NJ) serves as the data collection and coordination center.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024451
For Sources of Funding and Disclosures, see page 6.
References
- 1. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stefanini GG, Montorfano M, Trabattoni D, Andreini D, Ferrante G, Ancona M, Metra M, Curello S, Maffeo D, Pero G, et al. ST‐elevation myocardial infarction in patients with COVID‐19: clinical and angiographic outcomes. Circulation. 2020;141:2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, Ibrahim H, Friedman GH, Thompson C, Alviar CL, et al. ST‐segment elevation in patients with Covid‐19—a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alger HM, Rutan C, Williams JH, Walchok JG, Bolles M, Hall JL, Bradley SM, Elkind MSV, Rodriguez F, Wang TY, et al. American Heart Association COVID‐19 CVD registry powered by get with the guidelines. Circ Cardiovasc Qual Outcomes. 2020;13:e006967. doi: 10.1161/CIRCOUTCOMES.120.006967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mazloomzadeh S, Khaleghparast S, Ghadrdoost B, Mousavizadeh M, Baay MR, Noohi F, Sharifnia H, Ahmadi A, Tavan S, Malekpour Alamdari N, et al. Effect of intermediate‐dose vs standard‐dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID‐19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopes RD, de Barros e Silva PGM, Furtado RHM, Macedo AVS, Bronhara B, Damiani LP, Barbosa LM, de Aveiro Morata J, Ramacciotti E, de Aquino Martins P, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID‐19 and elevated D‐dimer concentration (ACTION): an open‐label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. REMAP‐CAP Investigators, ACTIV‐4a Investigators, ATTACC Investigators , Goligher EC, Bradbury CA, McVerry BJ, Lawler P, Berger J, Gong M, Carrier M, Reynolds HR, Kumar A, Turgeon AF, et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid‐19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamadeh A, Aldujeli A, Briedis K, Tecson KM, Sanz‐Sánchez J, Al dujeili M, Al‐Obeidi A, Diez JL, Žaliūnas R, Stoler RC, et al. Characteristics and outcomes in patients presenting with COVID‐19 and ST‐segment elevation myocardial infarction. Am J Cardiol. 2020;131:1–6. doi: 10.1016/j.amjcard.2020.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campo G, Rapezzi C, Tavazzi L, Ferrari R. Priorities for Cath labs in the COVID‐19 tsunami. Eur Heart J. 2020;41:1784–1785. doi: 10.1093/eurheartj/ehaa308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The American Heart Association’s Get With The Guidelines‐Coronary Artery Disease Advisory Work Group And Mission Lifeline Program, The American Heart Association’s Council On Clinical Cardiology, The American Heart Association’s Council On Clinical Cardiology’s Committee On Acute Cardiac Care And General Cardiology Committee, The American Heart Association’s Council On Clinical Cardiology’s Committee Interventional Cardiovascular Care Committee . Temporary emergency guidance to STEMI systems of care during the COVID‐19 pandemic: AHA’s mission: lifeline. Circulation. 2020;142:199–202. doi: 10.1161/CIRCULATIONAHA.120.048180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Welt FGP, Shah PB, Aronow HD, Bortnick AE, Henry TD, Sherwood MW, Young MN, Davidson LJ, Kadavath S, Mahmud E, et al. Catheterization laboratory considerations during the coronavirus (COVID‐19) pandemic: from the ACC’s Interventional Council and SCAI. J Am Coll Cardiol. 2020;75:2372–2375. doi: 10.1016/j.jacc.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choudry FA, Hamshere SM, Rathod KS, Akhtar MM, Archbold RA, Guttmann OP, Woldman S, Jain AK, Knight CJ, Baumbach A, et al. High thrombus burden in patients with COVID‐19 presenting with ST‐segment elevation myocardial infarction. J Am Coll Cardiol. 2020;76:1168–1176. doi: 10.1016/j.jacc.2020.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lowenstein CJ, Solomon SD. Severe COVID‐19 is a microvascular disease. Circulation. 2020;142:1609–1611. doi: 10.1161/CIRCULATIONAHA.120.050354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tam C‐C, Cheung K‐S, Lam S, Wong A, Yung A, Sze M, Lam Y‐M, Chan C, Tsang T‐C, Tsui M, et al. Impact of coronavirus disease 2019 (COVID‐19) outbreak on ST‐segment‐elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13:e006631. doi: 10.1161/CIRCOUTCOMES.120.006631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiang D, Xiang X, Zhang W, Yi S, Zhang J, Gu X, Xu Y, Huang K, Su XI, Yu BO, et al. Management and outcomes of patients with STEMI during the COVID‐19 pandemic in China. J Am Coll Cardiol. 2020;76:1318–1324. doi: 10.1016/j.jacc.2020.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


