Abstract
Background
There is limited information regarding the clinical use and effectiveness of IV sotalol in pediatric patients and patients with congenital heart disease, including those with severe myocardial dysfunction. A multicenter registry study was designed to evaluate the safety, efficacy, and dosing of IV sotalol.
Methods and Results
A total of 85 patients (age 1 day–36 years) received IV sotalol, of whom 45 (53%) had additional congenital cardiac diagnoses and 4 (5%) were greater than 18 years of age. In 79 patients (93%), IV sotalol was used to treat supraventricular tachycardia and 4 (5%) received it to treat ventricular arrhythmias. Severely decreased cardiac function by echocardiography was seen before IV sotalol in 7 (9%). The average dose was 1 mg/kg (range 0.5–1.8 mg/kg/dose) over a median of 60 minutes (range 30–300 minutes). Successful arrhythmia termination occurred in 31 patients (49%, 95% CI [37%–62%]) with improvement in rhythm control defined as rate reduction permitting overdrive pacing in an additional 18 patients (30%, 95% CI [19%–41%]). Eleven patients (16%) had significant QTc prolongation to >465 milliseconds after the infusion, with 3 (4%) to >500 milliseconds. There were 2 patients (2%) for whom the infusion was terminated early.
Conclusions
IV sotalol was safe and effective for termination or improvement of tachyarrhythmias in 79% of pediatric patients and patients with congenital heart disease, including those with severely depressed cardiac function. The most common dose, for both acute and maintenance dosing, was 1 mg/kg over ~60 minutes with rare serious complications.
Keywords: IV sotalol, pediatrics, supraventricular tachycardia
Subject Categories: Electrophysiology
Clinical Perspective
What Is New?
IV sotalol is safe and effective for termination or improvement of tachyarrhythmias in pediatric patients and patients with congenital heart disease, including those with severely depressed cardiac function.
What Are the Clinical Implications?
The typical dose used, for both acute and maintenance dosing, across multiple centers was 1 mg/kg over ~60 minute with rare acute sotalol‐related complications.
IV sotalol, a beta blocker with Class III antiarrhythmic properties, was approved by the US Food and Drug Administration in 2015. Literature suggests that IV sotalol can be safely administered in adult patients achieving rapid, reliable therapeutic results with similar safety and efficacy to oral sotalol. 1 , 2 , 3 , 4 Before the availability of IV sotalol, amiodarone was the only IV Class III antiarrhythmic option for pediatric patients, which requires ongoing continuous infusion to achieve therapeutic levels as well as close monitoring for cytochrome P‐450 drug‐drug interactions. Sotalol given as an intermittent infusion has a more desirable administration and pharmacokinetic profile in critically ill patients who may require multiple medications. Additionally, unlike amiodarone, sotalol is not associated with serious noncardiac adverse effects such as thyroid dysfunction, blood dyscrasias, neuropathy, or pulmonary fibrosis, which may be of particular importance in pediatric patients and young adults with congenital heart disease (CHD) who may remain on antiarrhythmic therapy for many years.
Limited pediatric data support the safety and efficacy of IV sotalol in patients with both supraventricular and ventricular arrhythmias. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 The first reports included patients with structurally normal cardiac anatomy and normal to mildly depressed cardiac function, 5 , 6 , 7 with a few case reports or series of IV sotalol use in pediatric patients and adults with CHD. 8 , 9 , 12 , 13 The most recent studies have concluded that IV sotalol can be used safely and effectively for both acute and maintenance therapy in young patients with and without CHD including those with mildly to moderately depressed cardiac function. 10 , 11 However, there has not been a large multicenter study looking at IV sotalol use in a diverse population of pediatric patients and patients with CHD, and there are no studies to date that have addressed administering IV sotalol to patients with severe myocardial dysfunction. Thus, the purpose of this large multicenter registry study was to evaluate the safety, efficacy, and dosing of racemic IV sotalol (the Food and Drug Administration approved formulation) in pediatric patients and patients with CHD with tachyarrhythmias, including those with severely depressed ventricular function.
Methods
After completing individual site institutional review board approval and waiver of assent/consent because of the retrospective nature of the study and standard of care, 14 centers within the United States completed online de‐identified data collection forms using a REDcap database for pediatric patients and patients with CHD who received IV sotalol from August 2017 to May 2020. The data that support the findings of this study are available from the corresponding author upon reasonable request. The only inclusion criterion was pediatric patients or patients with CHD presenting with a tachyarrhythmia who had a clinical indication for the use of IV sotalol. Pediatric patients were defined as newborn to age 18 years. Tachyarrhythmias included reentrant supraventricular tachycardia (SVT) including atrioventricular reciprocating and atrioventricular nodal reentrant tachycardias, atrial tachycardia, atrial flutter/fibrillation, junctional ectopic tachycardia, hemodynamically significant frequent atrial or ventricular ectopy, and ventricular tachycardia. As this was a registry study on clinical use, there were no specific exclusion criteria. IV sotalol was given either in a stepdown unit (higher level monitoring than the general floor) or in the critical care setting with continuous telemetry and frequent vital sign monitoring at all institutions.
Data collected included patient demographics and cardiac history. Clinical parameters documented were vital signs (heart rate and blood pressure) before and after administration of IV sotalol, qualitative echocardiographic function assessment (defined as mild, moderate, or severe dysfunction determined by the reader based on standard quantitative shortening and ejection fraction measurement criteria), and pre‐ and postadministration ECG findings (including QTc changes and rate/rhythm changes). Providers at individual centers were responsible for echocardiographic and ECG assessment. Outcome measurements of efficacy included successful termination (during or after infusion), time to termination (if specified), and recurrence based on the first dose of IV sotalol given. The side effect profile, including acute hemodynamic changes, bradycardia, sinus arrest, proarrhythmic effects, and QTc prolongation with treatment was evaluated. Drug administration data were collected including the amount of drug administered (calculated in mg/kg or mg/m2) and the duration of administration (minutes). For the purposes of analysis, duration of administration was stratified as very short (≤30 minutes), short (>30 to ≤60 minutes), intermediate (>60–299 minutes), and long (≥300 minutes).
Statistical Analysis
SAS version 9.4 (SAS Institute Inc., Cary, NC) and R (R Core Team (2021)) were used for statistical analysis. Descriptive statistics are expressed as mean, SD, median, and interquartile range depending on the normality of the data. Outlier detection was performed, defined as values more than 3 or more normalized absolute deviations from the median as previously described. 14 Paired comparison was performed using estimation statistics and effect size using the package dabestr in R. The mean difference and the 95% CI of the mean difference were calculated by performing 5000 resamples with replacement (bootstrap) from a single set of paired measurements for each patient. The effect size was also computed on each of these resamples. 15 Paired comparisons were further stratified by IV sotalol dose.
Results
Demographics
There were 85 patients who received IV sotalol (age range 1 day–36 years), with 53 men (62%). Four patients (5%) were adults with CHD. The median age was 3 months (interquartile range 0.04–3.2 years), median weight 4.9 kg (interquartile range 3.2–20.1 kg), and the median height 56 cm (interquartile range 49–95 cm). Forty‐five patients (53%) had congenital cardiac diagnoses. Normal to mildly decreased cardiac function by qualitative echocardiographic assessment was seen before IV sotalol in 56 patients (72%), with moderate dysfunction in 15 (19%) and severely depressed function in 7 (9%) (Table 1).
Table 1.
Patient Demographic Data
| Characteristic | No. (%) or median (interquartile range) |
|---|---|
| Male sex | 53 (62) |
| Age, m | 0.3 [0.04–3.20] |
| Weight, kg | 4.9 [3.2–20.10] |
| Height, cm | 56 [49–95] |
| Arrythmia diagnosis | |
| Supraventricular tachycardia | 79 (93) |
| Hemodynamically significant premature atrial contractions | 2 (2) |
| Ventricular tachycardia/fibrillation | 4 (5) |
| Cardiac function (qualitative) | |
| Normal‐mildly decreased | 56 (72) |
| Moderately decreased | 15 (19) |
| Severely decreased | 7 (9) |
| Congenital heart disease | 45 (53) |
| Ebstein's anomaly | 3 |
| Atrioventricular canal defect | 4 |
| Atrial septal defect | 2 |
| VSD | 3 |
| Coarctation of the aorta | 1 |
| Cardiomyopathy ( including dilated, restrictive, and hypertrophic) | 8 |
| Single ventricle anatomy (double inlet left ventricle, double outlet right ventricle, hypoplastic left heart syndrome, heterotaxia syndrome) | 11 |
| D‐ Transposition of the great arteries | 2 |
| L‐ Transposition of the great arteries | 1 |
| Aortic stenosis | 3 |
| Tetralogy of Fallot, pulmonary stenosis | 2 |
| Pulmonary atresia, VSD‐MAPCA | 2 |
| Mitral stenosis, congenital | 2 |
| Cardiac, other | 1 |
| Adults with congenital heart disease | 4 (5) |
| Congenital mitral valve stenosis | 1 |
| Ebstein’s anomaly | 1 |
| Congenital aortic valve stenosis | 1 |
| Pulmonary atresia, VSD‐MAPCA | 1 |
MAPCA indicates major aortopulmonary collateral arteries; and VSD, ventricular septal defect.
Indications
In 79 patients (93%) IV sotalol was used to treat SVT, 2 (2%) for hemodynamically significant premature atrial contractions, and 4 (5%) received it to treat ventricular arrhythmias. The SVT group had several subtypes with the most common being ectopic atrial tachycardia (n=25, 31%), reentrant SVT (n=24, 30%), and atrial flutter/fibrillation (n=14, 17%) (see Table 2 for all subtypes). In 49 patients (58%) IV sotalol was used for acute treatment of a sustained tachyarrhythmia. The remaining patients (n=36, 42%) received IV sotalol for the following reasons: the need for IV medication/maintenance dosing (n=12, 14%), systemic organ dysfunction including liver and thyroid toxicity (n=3, 3.5%), achievement of therapeutic drug level more efficiently (n=3, 3.5%), risk of drug‐drug interactions (n=1, 1%), and other/not specified (n=17, 20%) (Figure 1).
Table 2.
Arrythmia Type and Conversion Rate
| Type of SVT | No. (%) | Sotalol conversion (%) |
|---|---|---|
| Ectopic atrial tachycardia | 25 (31) | 17/22 (77) |
| Reentrant SVT (atrioventricular reentry tachycardia/atrioventricular nodal reentry tachycardia) | 24 (30) | 14/15 (93) |
| Atrial flutter/fibrillation | 14 (17) | 6/12 (50) |
| Permanent junctional reciprocating tachycardia | 3 (4) | 1/2 (50) |
| Junctional ectopic tachycardia | 2 (2) | 2/2 (100) |
| Multifocal atrial tachycardia | 2 (2) | 2/2 (100) |
| Premature atrial contractions (hemodynamically significant)/atrial ectopy | 2 (2) | 1/2 (50) |
| Iintra‐atrial reentrant tachycardia | 1 (2) | N/A |
| Not specified | 8 (10) | 5/6 (83) |
SVT indicates supraventricular tachycardia.
Figure 1. Indications for use of IV sotalol.
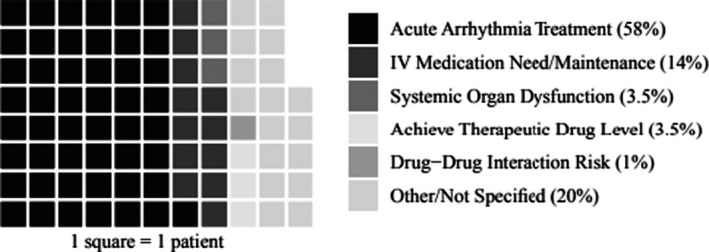
This graph depicts the primary indications for use of IV sotalol, including acute arrhythmia treatment (58%), need for IV antiarrhythmic/maintenance dosing (14%), systemic organ dysfunction (3.5%), to achieve therapeutic drug levels (3.5%), owing to drug‐drug‐interaction risk (1%) and other (20%).
Administration
The dosing and duration of infusion varied among centers. The average dose administered was 1 mg/kg (range 0.5–1.8 mg/kg/dose) over a median of 60 minutes (range 30–300 minutes) (Figure 2A). Sixteen patients (21%) had a very short infusion duration of ≤30 minutes, 25 (34%) had a short infusion duration of >30 to ≤60 minutes, 22 (29%) had an intermediate infusion duration of >60 to 299 minutes, and 12 (16%) had a long infusion duration of 300 minutes (Figure 2B). Thirty‐one patients (37%) received IV sotalol as first‐line therapy and 54 (63%) because they failed other antiarrhythmic medications orally and/or IV after a washout period at the discretion of the center (Table 3). Twenty‐six patients (31%) received a single dose of IV sotalol with 16 of these (62%) transitioned to oral sotalol, and 59 (69%) received more than 1 dose of IV sotalol with 37 of these patients (63%) transitioned to oral sotalol.
Figure 2. IV sotalol infusion duration.
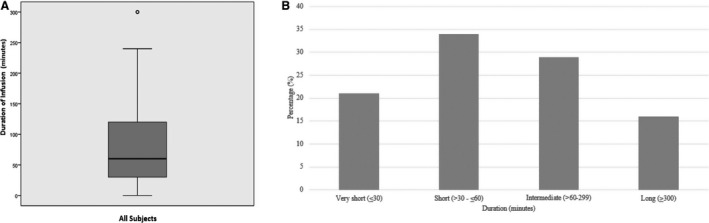
A, Time duration for IV sotalol drug infusion. This box‐whisker plot shows the median drug infusion duration of 60 minutes (range 30–300 minutes) for all‐comers. B, Percentage of patients who received IV sotalol infusions by duration. Drug duration infusions were categorized as very short (≤30 minutes), short (>30 to ≤60 minutes), intermediate (>60 to 299 minutes), and long (≥300 minutes). A total of 16 patients (21%) had a very short infusion duration, 25 (34%) had short infusion, 22 (29%) had an intermediate infusion duration, and 12 (16%) had a long infusion duration.
Table 3.
First‐Line Antiarrhythmic Medication Used
| Antiarrhythmic medication | No. of patients (%) |
|---|---|
| IV sotalol | 31 (37%) |
| Oral beta blockers | 16 (19%) |
| IV beta blockers | 18 (21%) |
| Flecainide | 1 (1%) |
| Digoxin oral/IV | 7 (8%) |
| Verapamil IV | 1 (1%) |
| Procainamide IV | 3 (4%) |
| Amiodarone oral/IV | 6 (7%) |
| Lidocaine IV | 2 (2%) |
Postinfusion Outcomes
After a single dose of IV sotalol, successful arrhythmia termination occurred in 31 patients (49%, 95% CI [37%–62%]), and an improvement in rhythm control occurred, defined as rate reduction permitting overdrive pacing based on patient age and hemodynamic tolerance in an additional 18 patients (30%, 95% CI [19%–41%]). Patients with reentrant SVT and ectopic atrial tachycardia had high conversion rates (Table 2). Patients who were not in an active arrythmia at the time of administration were not included. The median time to arrhythmia termination was 44 minutes after IV sotalol initiation when specified (29 patients). Recurrences occurred in 25 patients (35%, 95% CI [24%–46%]) with time to recurrence ranging from 30 minutes up to 2 weeks, with a median of 10 hours.
Adverse Events
Although many of the vital signs remained stable after IV sotalol infusion, there was, as expected, a significant decrease in mean heart rate of 29 beats per minute (−29 bpm) (95% CI [−39 to −19.5]) from baseline to end of treatment without severe hemodynamic compromising bradycardia reported. The mean initial heart rate before infusion was 151 bpm (N=77), with a mean final heart rate after infusion of 122 bpm. The mean difference in systolic blood pressure was minimal at −1 mm Hg (95% CI [−4.09 to 2.15]). The mean systolic blood pressure at baseline was 82 mm Hg (N=67) and the mean systolic blood pressure post infusion was 81 mm Hg. Finally, the mean difference in post‐infusion QTc (452 milliseconds, N=65) and baseline QTc (439) was 13 milliseconds (95% CI [0.85–23.2]). When including only patients with recorded IV sotalol dose, there was a significant decrease in heart rate post infusion (N=74; mean difference −30.5 bpm; 95% CI [−43.9 to −17.3]), no significant decrease in systolic blood pressure post infusion (N=64; mean difference was −0.516 mm Hg; 95% CI [−6.3 to 5.81]), and an increase in mean QTc post‐infusion (N=63; mean difference of 12.7 milliseconds; 95% CI [0.397–24]) (Figure 3). Eleven patients (16%) had significant QTc prolongation to >465 milliseconds after initial infusion. To account for baseline interventricular conduction delay/bundle‐branch block there had to be a >10 millisecond increase from the baseline QTC measurement. There was a marked QTc increase in 3 patients (4%) to >500 milliseconds with a dosing range of 30 to 300 minutes (Figure 4). Some patients experienced a substantial decrease in QTc after sotalol infusion likely confounded by the accuracy of measurement during tachycardia. Rare acute severe sotalol‐related complications were seen in 2 patients requiring early infusion termination: second‐degree heart block in a 1‐month‐old with single ventricle anatomy, common atrium, double outlet right ventricle, and torsades de pointes in a 19‐year‐old with ventricular tachycardia in the setting of congenital mitral valve stenosis status post valve replacement with severe mitral regurgitation.
Figure 3. Baseline and postinfusion measurements (heart rate, systolic blood pressure, corrected QTc).
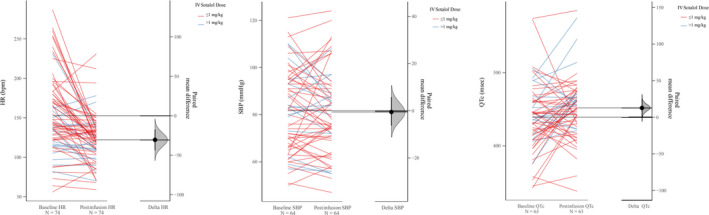
The slope graphs show the baseline and postinfusion values categorized by the IV sotalol dose received. The panel on the left contains paired measurements connected by lines (slopes) showing the direction of change between baseline and post infusion. The panel on the right shows the mean difference (black dot, delta) along with the bootstrap sampling distribution (shaded bell curve), and the 95% CI (black bar extending on both sides of the dot). There was a significant decrease in HR post infusion. There was an insignificant decrease in SBP. There was a slightly significant increase in QTc interval post infusion. HR indicates heart rate; and SBP, systolic blood pressure.
Figure 4. Impact of IV sotalol on QTc measurements.
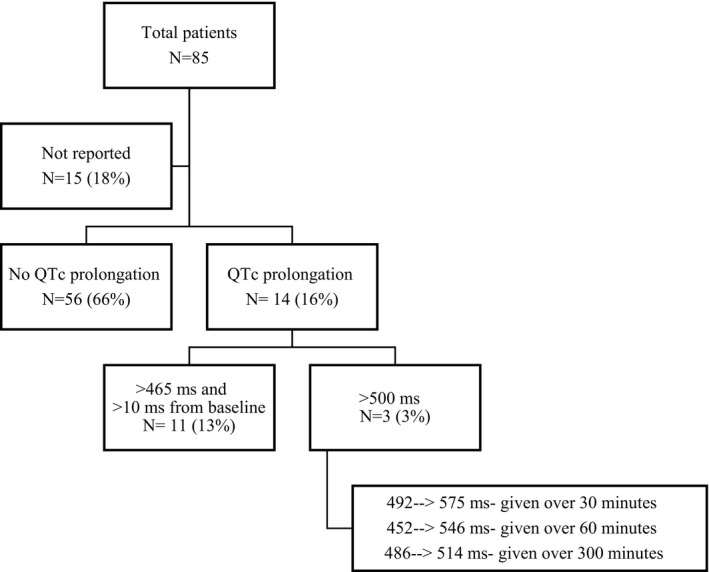
QTc measurements were assessed post IV sotalol infusion. There was no QTc prolongation noted in 56 (66%) patients. QTc prolongation was noted in 14 (16%) patients, with extreme prolongation of >500 milliseconds noted in 3 (3%) patients.
Discussion
This is the first large multicenter study to evaluate the efficacy, dosing, and safety of IV sotalol in pediatric patients and patients with CHD with tachyarrhythmias, including those with severely depressed ventricular function. Although most patients received IV sotalol for acute treatment of tachyarrhythmias, clinicians also chose IV sotalol because of clinical factors such as multiorgan system dysfunction as well as drug‐specific preferences including need for intermittent IV medication, avoidance of drug‐drug interactions, and desire to achieve therapeutic effect more quickly.
Novel to this study is the use of IV sotalol in 7 patients with severely depressed cardiac function. This was a small subgroup of the overall cohort; however, no significant adverse events were recorded in these patients. Similar to previous hemodynamic clinical studies of IV and oral sotalol 16 our study demonstrates that there were no deleterious hemodynamic sequelae attributable to negative inotropic effects, even in those with severely depressed ventricular function. There was not a statistically significant change in blood pressure before and after infusion. This is unlike the data recently reported with IV amiodarone administration and the risk of developing cardiovascular collapse in a significant subset of children, especially in young infants, patients with hemodynamic compromise, and patients receiving rapid amiodarone bolus administration. 17 Although this study did not specifically look at IV amiodarone versus IV sotalol, it suggests a need to reconsider the perceived advantage and safety of amiodarone and consider an expanded role of IV sotalol in pediatric arrhythmia management.
The preferred method of initiating IV sotalol is unclear at present. Valdes et al. used 1 mg/kg over an hour for their acute arrhythmia termination group and 80% to 90% of the patient’s prior daily oral maintenance dosage of sotalol divided every 8 to 12 hours over 5 hours in those receiving IV maintenance therapy. 10 Perry et al. reported a standardized target dose of 30 to 40 mg/m2 over 15 minutes for the acute arrythmia termination group and maintenance dosing based on a 1:1 ratio of the recommended oral dose with intermittent doses every 8 to 12 hours to reach a daily dose of 50 mg/m2 per day or 3 mg/kg per day infused over 2 hours. 11 Our data show, for both acute and maintenance dosing, that the mean administration of IV sotalol across multiple centers was 1 mg/kg over a median of 60 minutes. Some centers in this study used an IV sotalol infusion duration ≤30 minutes without complications consistent with published experience from Perry et al. 11 The median time to arrhythmia termination was longer (44 minutes) when compared with previous studies (time to termination 33 minutes, 14 minutes), 10 , 12 likely reflecting variable dosing regimens among participating centers. The most common arrythmia substrates were ectopic atrial tachycardia (31%), reentrant SVT (30%), and atrial flutter/fibrillation (17%). Patients with ectopic atrial tachycardia comprised a larger portion of recipients in our study compared with those of Valdes et al. (17%) and a smaller portion compared with Li et al. (43%). Of note, since the start of this study (March 2020), the Food and Drug Administration indications have expanded to include a 1‐day outpatient infusion approach for atrial fibrillation management that replaces the standard multiday oral load in adults. These new data could potentially lead to the increased use of IV sotalol, especially in patients with CHD who are at increased risk for atrial arrhythmias.
As with previous studies, our multicenter experience with IV sotalol demonstrates it to be efficacious in the termination, reduction, and/or rate control across a variety of tachyarrhythmias. 10 , 11 In some cases, IV sotalol was given as first‐line therapy; however, many patients were given IV sotalol following failure with other antiarrhythmic medications. Giving sotalol after other agents could have positive or negative effects on rhythm management. There are known limitations and adverse events specifically with pediatric IV amiodarone administration. Chang et al. noted if partial and full success were combined, procainamide was successful in 71% of cases compared with 34% for amiodarone (P=0.046). If partial success is considered a treatment failure, procainamide was successful in 50% compared with 15% for amiodarone (P=0.029). 18 Other IV options such as procainamide, digoxin, esmolol, and lidocaine have analogous limitations to amiodarone and to each other based on dosing, administration times, or efficacy for conversion of acute arrhythmias. 11 Given the variety of arrhythmias and the lack of comparison to alternative therapy, additional studies are needed to compare IV sotalol with other antiarrhythmic medications in specific arrhythmia substrates.
With the known risk of QTc prolongation and potential for proarrhythmic effects, an understanding of the safety of IV sotalol is vital. In our experience, IV sotalol appeared relatively safe for the treatment of atrial, ventricular, and junctional tachyarrhythmias in a heterogeneous population spanning a broad age range. This included patients that had a baseline QTc >460 milliseconds given the nature of this patient population (CHD with intraventricular conduction delay/bundle‐branch block). The 2% rate of serious adverse events in this patient cohort was lower than previously described by Valdes et al. (4%) but higher than reported by Perry et al. (0%). 10 , 12 The 2 patients who required early termination had significant issues including bradycardia with second‐degree heart block in the setting of other concomitant antiarrhythmic medications (amiodarone, procainamide, and esmolol), and torsades de pointes in the setting of QTc prolongation (up to 514 milliseconds) after 2 infusions (12 hours apart) of 1.4 mg/kg per dose each given over 300 minutes. Our study supports the prior literature that although rare, because of hemodynamic and proarrhythmic risks, IV sotalol should always be administered with close monitoring including heart rate, blood pressure, rhythm evaluation, and ECG assessment.
Limitations
Study limitations are related primarily to the challenges associated with multicenter data collection and interpretation. Data entry was rarely incomplete from participating centers, which may limit the results and conclusions. The maximum percentage of missing data in any single data field was 12% (noted for baseline systolic and diastolic BP with 2 reported as means and not used in calculations). All efforts were made to obtain missing data in the data collection set. Echocardiographic data were based on qualitative reader assessment, which has an element of subjectivity. Data review and validation were limited to the registry database because there was no access to individual centers’ medical records. Postinfusion outcomes were based on a single IV sotalol dose. More studies are needed to look at outcomes after several IV sotalol doses. In addition, the adult CHD data are limited in both number and type of underlying heart disease. The number of patients with severe ventricular dysfunction is also low and this means no major conclusions can be drawn about safety in this population of patients. Further multicenter studies could be useful to look specifically at these patient populations.
Conclusions
Based on the findings of this large multicenter registry, IV sotalol can be used safely to treat tachyarrhythmias in pediatric patients and patients with CHD. There were no significant adverse events noted in those with severely depressed ventricular function. The typical dose used, for both acute and maintenance dosing, across multiple centers was 1 mg/kg over ~60 minute with rare acute sotalol‐related complications. Further studies are needed in pediatric patients, as well as adults with CHD, to compare IV sotalol with other antiarrhythmic medications for specific arrhythmia substrates.
Sources of Funding
An unrestricted grant from Alta Thera was given to participating sites. The authors acknowledge the support and approval of the Pediatric and Congenital Electrophysiology Society (PACES).
Disclosures
Dr. Balaji has a research grant from Medtronic and is a consultant at yoR laboratories and Milestone Pharmaceuticals. Dr. Saul is a consultant for Pfizer–data and safety monitoring board, Bristol Myers Squibb–data and safety monitoring board, Supernus Pharm–cardiac issues with attention‐deficit/hyperactivity disorder drug, and ACI Clinical–ECG interpretation for drug study. The remaining authors have no disclosures to report.
Acknowledgments
This is a Pediatric and Congenital Electrophysiology Society (PACES) approved study.
This article was sent to Kerry‐Anne Rye, PhD, Senior Guest Editor, for review by expert referees, editorial decision, and final disposition.
These data have been presented at the Heart Rhythm Scientific Sessions, May 6–9, 2020.
For Sources of Funding and Disclosures, see page 8.
References
- 1. Ho DS, Zecchin RP, Cooper DA, Richards AB, Uther JB, Ross L. Rapid intravenous infusion of d‐1 sotalol: time to onset of effect on ventricular refractoriness, and safety. Eur Heart J. 1995;16:81–86. [DOI] [PubMed] [Google Scholar]
- 2. Ho DS, Zecchin RP, Richards DA, Uther JB, Ross DL. Double‐blind trial of lignocaine versus sotalol for acute termination of spontaneous sustained ventricular tachycardia. Lancet. 1994;344:18–23. doi: 10.1016/S0140-6736(94)91048-0 [DOI] [PubMed] [Google Scholar]
- 3. Somberg JC, Preston RA, Ranade V, Molnar J. Developing a safe intravenous sotalol dosing regimen. Am J Ther. 2010;17:365–372. doi: 10.1097/MJT.0b013e3181ea3184 [DOI] [PubMed] [Google Scholar]
- 4. Somberg JC, Preston RA, Ranade V, Molnar J. QT prolongation and serum sotalol concentration are highly correlated following intravenous and oral sotalol. Cardiology. 2010;116. doi: 10.1159/000316050 [DOI] [PubMed] [Google Scholar]
- 5. Zhang Y, Li X, Xu Z, Lui H. Efficacy of intravenous sotalol for treatment of incessant tachyarrhythmias in children. Chin J Pract Pediatr. 2013;28:4. doi: 10.1016/j.amjcard.2017.01.034 [DOI] [PubMed] [Google Scholar]
- 6. Li X, Zhang Y, Liu H, Jiang H, Ge H, Zhang Y. Efficacy of intravenous sotalol for treatment of incessant tachyarrhythmias in children. Am J Cardiol. 2017;119:1366–1370. doi: 10.1016/j.amjcard.2017.01.034 [DOI] [PubMed] [Google Scholar]
- 7. Kim H, Wolff J, Dalal A, Van Hare G, Avari SJ. Use of IV sotalol in newborns with SVT. Heart Rhythm Case Rep. 2017;10:332–335. doi: 10.1016/j.hrcr.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aljohani OA, Perry JC, Williams MR. Intravenous sotalol for conversion of atrial flutter in infants. Hear Case Rep. 2018;4:117–120. doi: 10.1016/j.hrcr.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pasierb MM, Seslar SP. Intravenous sotalol use in a complex critically ill child: balancing the systems in choosing antiarrhythmic medication. Cardiol Young. 2017;27:1857–1860. doi: 10.1017/S1047951117001408 [DOI] [PubMed] [Google Scholar]
- 10. Valdés SO, Miyake CY, Niu MC, de la Uz CM, Asaki SY, Landstrom AP, Schneider AE, Rusin CG, Patel R, Lam WW, et al. Early experience with intravenous sotalol in children with and without congenital heart disease. Heart Rhythm. 2018;15:1862–1869. doi: 10.1016/j.hrthm.2018.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borquez A, Aljohani O, Williams M; Perry J. IV Sotalol in the Young . Safe and effective treatment with standardized protocols. JACC Clin Electrophysiol. 2020;6:425–432. doi: 10.1016/j.jacep.2019.11.019 [DOI] [PubMed] [Google Scholar]
- 12. Jacobson JL, Somberg JC, Nguyen HH. Intravenous sotalol for the treatment of ventricular dysrhythmias in an infant on extracorporeal membrane oxygenation. Pediatr Cardiol. 2020;41:418–422. doi: 10.1007/s00246-019-02225-w [DOI] [PubMed] [Google Scholar]
- 13. Von Bergen N, Beshish A, Maginto K. Outpatient IV sotalol load to replace 3‐day admission oral sotalol load. Heart Rhythm Case Rep. 2019;5:382–383. doi: 10.1016/j.hrcr.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leys C, Ley C, Klein O, Bernard P, Licata L. Detecting outliers: do not use standard deviation around the mean, use absolute deviation around the median. J Exp Soc Psychol. 2013;49:764–766. doi: 10.1016/j.jesp.2013.03.013 [DOI] [Google Scholar]
- 15. Ho J, Tumkaya T, Aryal S, Choi H, Claridge‐Chang A. Moving beyond P values: data analysis with estimation graphics. Nat Methods. 2019;16:565–566. doi: 10.1038/s41592-019-0470-3 [DOI] [PubMed] [Google Scholar]
- 16. Anderson JL, Sotalol PEN. An important new antiarrhythmic. Am Heart J. 1999;137:388–409. doi: 10.1016/S0002-8703(99)70484-9 [DOI] [PubMed] [Google Scholar]
- 17. Maghrabi K, Uzun O, Kirsh JA, Balaji S, Von Bergen NH, Sanatani S. Cardiovascular collapse with intravenous amiodarone in children: a multi‐center retrospective cohort study. Pediatr Cardiol. 2019;40:925–933. doi: 10.1007/s00246-019-02090-7 [DOI] [PubMed] [Google Scholar]
- 18. Chang PM, Silka MJ, Moromisato DY, Bar‐Cohen Y. Amiodarone versus procainamide for the acute treatment of recurrent supraventricular tachycardia in pediatric patients. Circ Arrhythmia Electrophysiol. 2010;3:134–140. doi: 10.1161/CIRCEP.109.901629 [DOI] [PubMed] [Google Scholar]


