Abstract
Background
Although rare, classic viral myocarditis in the pediatric population is a disease that carries significant morbidity and mortality. Since 2020, myocarditis has been a common component of multisystem inflammatory syndrome in children (MIS‐C) following SARS‐CoV‐2 infection. In 2021, myocarditis related to mRNA COVID‐19 vaccines was recognized as a rare adverse event. This study aims to compare classic, MIS‐C, and COVID‐19 vaccine‐related myocarditis with regard to clinical presentation, course, and outcomes.
Methods and Results
In this retrospective cohort study, we compared patients aged <21 years hospitalized at our institution with classic viral myocarditis from 2015 to 2019, MIS‐C myocarditis from March 2020 to February 2021, and vaccine‐related myocarditis from May 2021 to June 2021. Of 201 total participants, 43 patients had classic myocarditis, 149 had MIS‐C myocarditis, and 9 had vaccine‐related myocarditis. At presentation, ejection fraction was lowest for those with classic myocarditis, with ejection fraction <55% present in 58% of patients. Nearly all patients with MIS‐C myocarditis (n=139, 93%) and all patients with vaccine‐related myocarditis (n=9, 100%) had normal left ventricular ejection fraction at the time of discharge compared with 70% (n=30) of the classic myocarditis group (P<0.001). At 3 months after discharge, of the 21 children discharged with depressed ejection fraction, none of the 10 children with MIS‐C myocarditis had residual dysfunction compared with 3 of the 11 (27%) patients in the classic myocarditis group.
Conclusions
Compared with classic myocarditis, those with MIS‐C myocarditis had better clinical outcomes, including rapid recovery of cardiac function. Patients with vaccine‐related myocarditis had prompt resolution of symptoms and improvement of cardiac function.
Keywords: COVID‐19, MIS‐C, mRNA vaccine, myocarditis
Subject Categories: Pediatrics
Nonstandard Abbreviations and Acronyms
- cMRI
cardiac magnetic resonance imaging
- MIS‐C
multisystem inflammatory syndrome in children
Clinical Perspective
What Is New?
Patients with classic myocarditis had higher rates of persistent ventricular dysfunction at discharge and at their 3‐month follow‐up visits when compared with those with multisystem inflammatory syndrome in children myocarditis and vaccine‐related myocarditis.
Those with COVID‐19 vaccine‐related myocarditis generally had a milder clinical course with a lower likelihood of cardiac dysfunction at presentation and more rapid recovery when present.
What Are the Clinical Implications?
Compared with classic myocarditis, patients with multisystem inflammatory syndrome in children myocarditis have a greater likelihood of full recovery of cardiac function with a faster time to recovery, even when patients present as critically ill, requiring extracorporeal membrane oxygenation or mechanical ventilatory support.
COVID‐19 vaccine‐related myocarditis is mild, and patients typically have evidence of normal ventricular function recovery at discharge from the hospital.
Myocarditis is an inflammatory disease of the myocardium with a variety of causes and a spectrum of presentations, ranging from subclinical disease to acute fulminant myocarditis that leads to cardiogenic shock. 1 In the pediatric population, myocarditis is rare with an approximate annual incidence from 2007 to 2016 of 0.8 per 100 000 among the US pediatric population and is most commonly virally mediated, with high prevalence of detection of enterovirus, adenovirus, parvovirus B19, and Epstein‐Barr virus. 1 , 2 , 3 Regardless of initial presentation, some patients with so‐called “classic” myocarditis have full recovery of cardiac function, whereas others may develop residual cardiomyopathy and chronic congestive heart failure. A recent single‐center study demonstrated that within a population of 41 pediatric patients with myocarditis, 66% made a full recovery, 10% had an incomplete recovery of function, and 24% either died or underwent transplantation. 4 Long‐term follow‐up in the pediatric population of classic myocarditis is limited, but myocarditis has been shown to be the cause of dilated cardiomyopathy in 27% to 46% of newly diagnosed cases. 5 , 6
Although classic viral myocarditis was the most common cause of myocarditis in children before 2020, since the onset of the COVID‐19 pandemic, multisystem inflammatory syndrome in children (MIS‐C) linked to SARS‐CoV‐2 has proven to be another important cause of myocarditis. In children with MIS‐C, myocarditis has been found to be a common complication, with an estimated incidence of 17% to 75% in affected children. 7 , 8 , 9 Little is currently known about the long‐term cardiac sequelae of MIS‐C myocarditis, specifically with regard to improvement in cardiac function, given the novelty of the disease. Existing studies evaluating changes to cardiac function in this patient population over time are limited but have shown near‐complete recovery of left ventricular ejection fraction (LVEF) in most children with MIS‐C myocarditis. 10 , 11 , 12
In recent months, a new etiology of myocarditis related to the mRNA‐based COVID‐19 vaccines has been proposed in the pediatric population. This type of myocardial injury has typically presented in adolescent and young adult male patients, most commonly after a second dose of the vaccine. 13 , 14 , 15 Information about this new entity is rapidly evolving, and little is known about how this vaccine‐mediated myocarditis differs from classic myocarditis.
These 3 types of myocarditis appear to be distinct, and little is currently known about the differences between these etiologies. We aimed to compare findings and outcomes among classic myocarditis, MIS‐C myocarditis, and COVID‐19 vaccine‐related myocarditis.
Methods
In this retrospective cohort study, we evaluated patients aged <21 years with classic myocarditis, MIS‐C myocarditis, and COVID‐19 vaccine‐related myocarditis at our institution. In our study, patients were considered to have myocarditis if they presented with elevated troponin without any evidence of another cause for the troponin elevation. The classic myocarditis group consisted of patients who were diagnosed with viral or idiopathic myocarditis from 2015 to 2019. The MIS‐C myocarditis cohort included patients from March 2020 to February 2021 who met the Centers for Disease Control and Prevention case definition of MIS‐C and had evidence of myocardial involvement with troponin elevation. 16 The COVID‐19 vaccine‐related group consisted of patients from May 2021 to June 2021 with myocarditis after the vaccine, with no other etiology of myocarditis identified. The study was approved by the Institutional Review Board of Children’s Healthcare of Atlanta. The data that support the findings of this study are available from the corresponding author upon reasonable request. Informed consent was waived in the setting of a retrospective chart‐based study.
For eligible patients, key outcomes of interest included LVEF at the time of presentation, discharge, and at clinic follow‐up; peak troponin I, BNP (B‐type natriuretic peptide) levels, and CRP (C‐reactive protein); and medical treatment for myocarditis. Other variables of interest were symptomatology at presentation, ECG findings, and laboratory evidence of anemia, thrombocytopenia, and lymphopenia. In addition, clinical status at 2 time points, time of hospital discharge and 3‐month follow‐up after initial presentation, were included when available. Clinical status outcomes included follow‐up without oral heart failure medications, follow‐up with oral heart failure medications, left ventricular assist device placement, and transplant or death. Oral heart failure medications included diuretics, angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, aldosterone antagonists, and β‐blockers.
Statistical Analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), and statistical significance was assessed at the 0.05 level. Normality of continuous variables was assessed using histogram, normal probability plots, and the Anderson–Darling test for normality. Descriptive statistics are presented as counts and percentages for categorical variables and median (25th percentile–75th percentile) for continuous data with skewed distributions. Continuous data were compared between myocarditis groups using Kruskal–Wallis tests, and comparisons between categorical variables were performed using χ2 tests or the Fisher exact test when expected cell counts were <5. The time‐dependent outcome of normal LVEF was analyzed with survival analysis. Because in‐hospital mortality was considered as a competing event for normal LVEF, a competing‐risk analysis was performed.
After performing the overall analyses, similar measures were undertaken to do subanalyses comparing patients aged ≥12 years in all 3 groups. These comparisons were completed given that vaccines were not authorized during the study period in those aged <12 years.
Results
Demographics and Presenting Symptoms
A total of 201 patients with myocarditis were included in this study, 43 of whom were in the classic myocarditis arm, 149 in the MIS‐C myocarditis arm, and 9 in the vaccine‐related arm (Table 1). In the vaccine‐related group, 8 of the 9 patients presented with myocarditis after the second vaccine dose; among those patients, there was a mean of 5.4 days and median of 3 days between vaccination dose number 2 and hospital admission.
Table 1.
Demographics and Cardiac Symptomology and Signs Among Patients With Classic Myocarditis, MIS‐C Myocarditis, and Vaccine‐Related Myocarditis
| Variable | Level | No. | Overall (N=201) | Classic myocarditis (n=43) | MIS‐C (n=149) | Vaccine‐related myocarditis (n=9) | P value |
|---|---|---|---|---|---|---|---|
| Age, y | 201 | 10.9 (7.1, 15.1) | 14.7 (9.0, 16.6) | 9.5 (6.9, 13.2) | 15.7 (14.5, 16.6) | <0.001 * | |
| Age group | 1: <1 y | 201 | 4 (2.0) | 1 (2.3) | 3 (2.0) | 0 (0) | <0.001 * |
| 2: 1–5 y | 36 (17.9) | 8 (18.6) | 28 (18.8) | 0 (0) | |||
| 3: 6–11 y | 73 (36.3) | 2 (4.7) | 71 (47.7) | 0 (0) | |||
| 4: ≥12 y | 88 (43.8) | 32 (74.4) | 47 (31.5) | 9 (100.0) | |||
| Sex | Female sex | 201 | 67 (33.3) | 12 (27.9) | 55 (36.9) | 0 (0) | 0.052 |
| Male sex | 134 (66.7) | 31 (72.1) | 94 (63.1) | 9 (100.0) | |||
| Race or ethnicity | Black race | 200 | 110 (55.0) | 23 (53.5) | 86 (58.1) | 1 (11.1) | 0.008 * |
| Asian race | 4 (2.0) | 1 (2.3) | 3 (2.0) | 0 (0) | |||
| Hispanic or Latino ethnicity | 36 (18.0) | 7 (16.3) | 29 (19.6) | 0 (0) | |||
| Other race or ethnicity | 11 (5.5) | 4 (9.3) | 5 (3.4) | 2 (22.2) | |||
| White race | 39 (19.5) | 8 (18.6) | 25 (16.9) | 6 (66.7) | |||
| Comorbidities | Asthma | 201 | 24 (11.9) | 6 (14.0) | 16 (10.7) | 2 (22.2) | 0.187 |
| Obesity | 25 (12.4) | 1 (2.3) | 23 (15.4) | 1 (11.1) | |||
| Both | 7 (3.5) | 0 (0) | 7 (4.7) | 0 (0) | |||
| None | 138 (68.7) | 35 (81.4) | 97 (65.1) | 6 (66.7) | |||
| Other | 7 (3.5) | 1 (2.3) | 6 (4.0) | 0 (0) | |||
| Chest pain | Yes | 200 | 57 (28.5) | 32 (76.2) | 16 (10.7) | 9 (100.0) | <0.001 * |
| Dyspnea | Yes | 201 | 49 (24.4) | 15 (34.9) | 30 (20.1) | 4 (44.4) | 0.050 * |
Data are provided as number, median (quartile 1, quartile 3), or number (percentage). MIS‐C indicates multisystem inflammatory syndrome in children.
Indicates significance at P < 0.05 level.
Those with MIS‐C were younger than those with classic myocarditis (median 9.5 years versus 14.7 years) and vaccine‐related myocarditis (median 15.7 years). Patients in all 3 groups were predominantly male patients. More than half of the children with classic myocarditis or MIS‐C were Black non‐Hispanic race or ethnicity, whereas two‐thirds of the vaccine‐related myocarditis group were White non‐Hispanic race or ethnicity. Among presenting symptoms, more patients with classic myocarditis and vaccine‐related myocarditis presented with chest pain (76.2% and 100%, respectively) compared with patients with MIS‐C myocarditis (10.7%; P<0.001).
Laboratory Findings
There were significant differences between the 3 groups when comparing cardiac biomarkers and inflammatory markers (Figure 1). Peak troponin was highest in the classic myocarditis group (median, 16 ng/mL; interquartile range [IQR], 8.1–30.6; normal value, <0.045 ng/mL), whereas the MIS‐C myocarditis group had the highest recorded BNP (median, 932 pg/mL; IQR, 459–1672; normal value, <100.0 pg/mL). The MIS‐C group also had the most prominent hematologic derangements, including lymphopenia (median, 0.6×103/µL; IQR, 0.4–0.99) with leukocytosis (median, 15.1×103/µL; IQR, 11–20), thrombocytopenia (median, 145×103/µL; IQR, 110–189), and anemia (median, 9.4 g/dL; IQR, 8.6–10.4).
Figure 1. Comparison of laboratory testing among those with classic myocarditis, multisystem inflammatory syndrome in children (MIS‐C), or COVID‐19 vaccine‐related myocarditis.
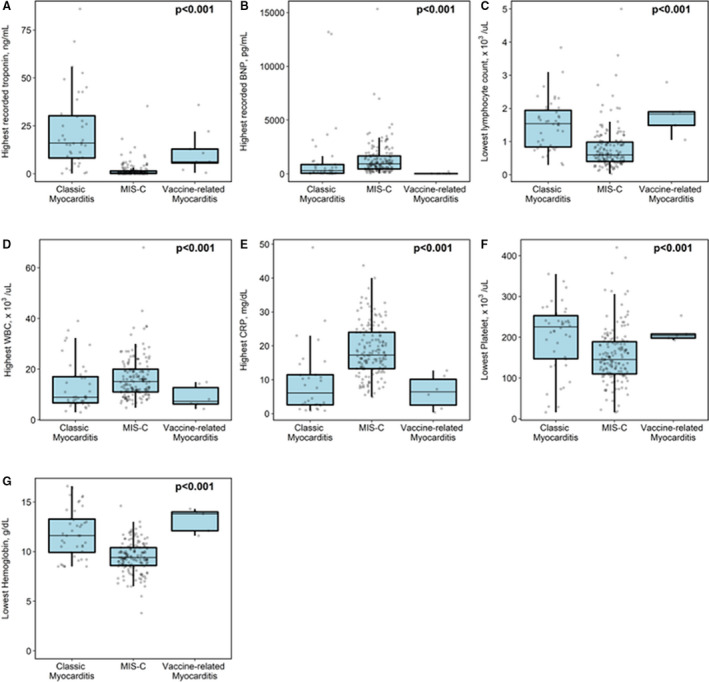
Peak troponin was highest in the classic myocarditis group (A), whereas the MIS‐C myocarditis group had highest recorded BNP (B‐type natriuretic peptide) (B), most profound lymphopenia (C), highest white blood cell count (WBC) (D), highest CRP (C‐reactive protein) (E), lowest platelet level (F), and lowest hemoglobin level (G).
ECG and Echocardiogram Findings
ECG abnormalities were common in those with classic myocarditis (74%) and vaccine‐related myocarditis (67%), but fewer than half of the children with MIS‐C myocarditis had ECG abnormalities (Table 2). The most common ECG abnormality was a repolarization abnormality, present in 58% of those with classic myocarditis, 18.5% of those with MIS‐C, and 67% of those with vaccine‐related myocarditis.
Table 2.
Cardiac Testing Among Patients With Classic Myocarditis, MIS‐C Myocarditis, and Vaccine‐Related Myocarditis
| Variable | Level | No. | Overall (N=201) | Classic myocarditis (n=43) | MIS‐C (n=149) | Vaccine‐related myocarditis (n=9) | P value |
|---|---|---|---|---|---|---|---|
| Left ventricular ejection fraction at presentation, categorized | 1: <30% | 201 | 7 (3.5) | 4 (9.3) | 3 (2.0) | 0 (0) | 0.066 |
| 2: 30%–55% | 82 (40.8) | 21 (48.8) | 59 (39.6) | 2 (22.2) | |||
| 3: >55% | 112 (55.7) | 18 (41.9) | 87 (58.4) | 7 (77.8) | |||
| Left ventricular ejection fraction at presentation | 201 | 56 (46, 64) | 51.0 (38.0, 61.0) | 56.2 (48, 64.8) | 59.7 (57.8, 67.2) | 0.019 * | |
| Lowest recorded EF | 201 | 50.2 (39, 58.6) | 45.3 (29.4, 60) | 50.2 (40.0, 57.6) | 59.7 (57.8, 67.2) | 0.013 * | |
| Normal EF at the time of discharge | Yes * | 201 | 178 (88.6) | 30 (73.2) | 139 (93.3) | 9 (100.0) | <0.001 * |
| Coronary artery dilation at time of presentation | No | 199 | 186 (93.5) | 42 (97.7) | 135 (91.8) | 9 (100.0) | 0.440 |
| Coronary artery dilation at discharge | No | 199 | 191 (96.0) | 41 (97.6) | 141 (95.3) | 9 (100.0) | 0.786 |
| Presence of pericardial effusion | Absent | 201 | 102 (50.7) | 22 (51.2) | 72 (48.3) | 8 (88.9) | 0.005 * |
| Present | 99 (49.3) | 21 (48.8) | 77 (51.7) | 1 (11) | |||
| ECG findings at admission | Normal | 198 | 125 (63.1) | 12 (27.9) | 110 (73.8) | 3 (33.3) | <0.001 * |
| Complete AV block | 3 (1.5) | 3 (7.0) | 0 (0) | 0 (0) | |||
| Repolarization abnormalities | 58 (29.3) | 25 (58.1) | 27 (18.5) | 6 (66.7) | |||
| Other † | 12 (6.1) | 3 (7.0) | 9 (6.2) | 0 (0) | |||
| ECG findings at clinic follow‐up, 1–2 wk after discharge | Normal | 183 | 168 (91.8) | 29 (74.4) | 134 (97.1) | 5 (83.3) | <0.001 * |
| Repolarization abnormalities | 5 (2.7) | 3 (7.7) | 1 (0.7) | 1 (16.7) | |||
| Other † | 10 (5.5) | 7 (17.9) | 3 (2.2) | 0 (0) |
Data are provided as number, median (quartile 1, quartile 3), or number (percentage). AV indicates atrioventricular; EF, ejection fraction; and MIS‐C, multisystem inflammatory syndrome in children.
Indicates significance at P < 0.05 level.
The EF at the time of discharge for 2 additional patients with classic myocarditis, 1 who died and 1 who progressed to transplant, are not included in this row of the table.
Other ECG findings included Brugada pattern, atrial tachyarrhythmia, markedly abnormal ventricular axis, junctional tachycardia, and abnormal intervals (ie, prolonged QTc and PR).
On echocardiogram, there were notable differences in LVEF and in the presence of pericardial effusion between groups, but there was no significant difference in the incidence of coronary artery dilation (Table 2). At presentation, LVEF was lowest for those with classic myocarditis, with ejection fraction (EF) <55% present in 58% of patients compared with 42% of MIS‐C myocarditis and 22% of vaccine‐related myocarditis. Patients with classic myocarditis also had the lowest recorded EF (median, 45%; IQR, 29%–60%). Among those with transplant‐free survival at the time of discharge, 26% of patients with classic myocarditis and 7% of patients with MIS‐C had EF <55%; all patients with vaccine‐related myocarditis had EF >55% at discharge. Pericardial effusion was present in roughly half of the patients with classic and MIS‐C myocarditis (49% and 52%, respectively), but was rare in the patients with vaccine‐related myocarditis (1 patient with trivial pericardial effusion).
Treatment
Treatment of myocarditis varied depending on the type (Table 3). In patients with MIS‐C myocarditis, intravenous immunoglobulin (IVIG) and steroids were frequently used in accordance with our hospital protocol for MIS‐C management. 17 IVIG was commonly used in classic myocarditis (65%), but in only 1 patient with vaccine‐related myocarditis. Steroids were occasionally used in classic myocarditis (33%) and were not used at all in vaccine‐related myocarditis. Vasopressors were most often used in those with classic myocarditis (42%) or MIS‐C (52%) and rarely used in vaccine‐related myocarditis (22%), although this difference was not statistically significant. Patients with classic myocarditis were more likely to require mechanical ventilation (28%) compared with only 4% of patients with MIS‐C. Extracorporeal membrane oxygenation (ECMO) was used in 21% of patients with classic myocarditis and 2% (n=3) of the patients with MIS‐C. Only 2 children with vaccine‐related myocarditis had intensive care unit admission; neither received mechanical ventilation or ECMO.
Table 3.
Treatment Variation Among Patients With Classic Myocarditis, MIS‐C Myocarditis, and Vaccine‐Related Myocarditis
| Variable | Level | No. | Overall (N=201) | Classic myocarditis (n=43) | MIS‐C (n=149) | Vaccine‐related myocarditis (n=9) | P value |
|---|---|---|---|---|---|---|---|
| IVIG while admitted | Yes | 201 | 151 (75.1) | 28 (65.1) | 122 (81.9) | 1 (11.1) | <0.001 * |
| Steroids while admitted | Yes | 201 | 152 (75.6) | 14 (32.6) | 138 (92.6) | 0 (0) | <0.001 * |
| Vasopressors | Yes | 201 | 97 (48.3) | 18 (41.9) | 77 (51.7) | 2 (22.2) | 0.155 |
| Aspirin while admitted | Yes | 201 | 164 (81.6) | 24 (55.8) | 139 (93.3) | 1 (11.1) | <0.001 * |
| Other NSAID if no aspirin | N/A | 200 | 166 (83.0) | 23 (54.8) | 142 (95.3) | 1 (11.1) | <0.001 * |
| No | 8 (4.0) | 1 (2.4) | 7 (4.7) | 0 (0) | |||
| Yes | 26 (13.0) | 18 (42.9) | 0 (0) | 8 (88.9) | |||
| ECMO | Yes | 201 | 12 (6.0) | 9 (20.9) | 3 (2.0) | 0 (0) | <0.001 * |
| Mechanical ventilation | Yes | 201 | 18 (9.0) | 12 (27.9) | 6 (4.0) | 0 (0) | <0.001 * |
| ICU stay during admission | Yes | 201 | 121 (60.2) | 18 (41.9) | 101 (67.8) | 2 (22.2) | <0.001 * |
Data are provided as number or number (percentage). ECMO indicates extracorporeal membrane oxygenation; ICU, intensive care unit; IVIG, intravenous immunoglobulin; MIS‐C, multisystem inflammatory syndrome in children; and N/A, not applicable.
Indicates significance at P < 0.05 level.
Outcomes
There was 1 death and 1 heart transplant among the classic myocarditis group, with all other patients in this study discharged to home (Figure 2). Approximately 44% of the patients with classic myocarditis were discharged on oral heart failure medications, whereas only 3% of the patients with MIS‐C myocarditis were discharged on oral heart failure therapy; no patients with vaccine‐related myocarditis required oral heart failure therapy at discharge. These proportions were similar at the 3‐month follow‐up (Figure 2). Of note, among the 21 patients with classic myocarditis and MIS‐C myocarditis who were discharged from the hospital with depressed function, all 10 of the patients with MIS‐C myocarditis had ventricular recovery 3 months after discharge, whereas 3 of the 11 (27%) patients with classic myocarditis had persistent ventricular dysfunction. Length of stay was significantly shorter for the vaccine‐related myocarditis group (median, 2 days; IQR, 1–3 days) than for the classic myocarditis group (median, 5 days; IQR, 2–10 days) and the MIS‐C myocarditis group (median, 6 days; IQR, 4–8 days; P=0.0002).
Figure 2. Comparison of discharge outcomes and 3‐month outcomes among those with classic myocarditis, multisystem inflammatory syndrome in children (MIS‐C), or COVID‐19 vaccine‐related myocarditis.
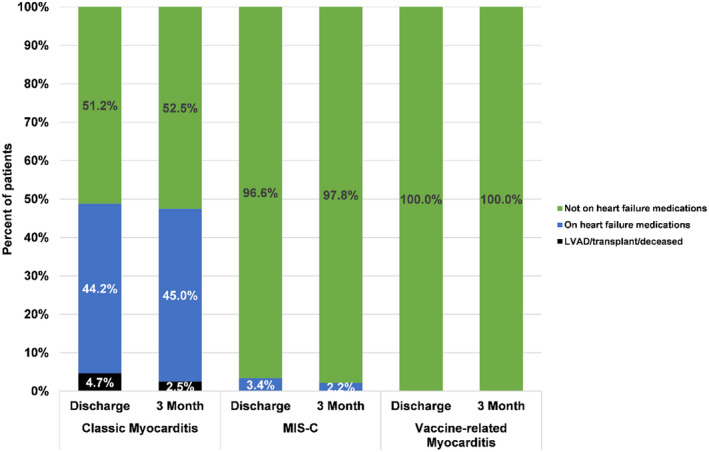
LVAD indicates left ventricular assist device.
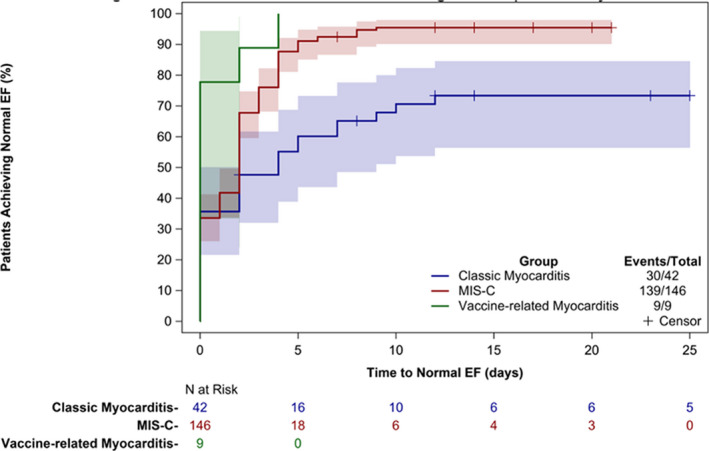
Timing of ventricular recovery varied among the 3 groups (Figure 3). Within 3 days of hospitalization, only 47% of those with classic myocarditis had regained normal function (EF >55%), whereas 76% of those with MIS‐C myocarditis and all of those with vaccine‐related myocarditis had achieved that target. Within 10 days of presentation, 70% of those with classic myocarditis and 94% of those with MIS‐C had regained normal cardiac function.
Figure 3. Comparison of time to return to normal cardiac ejection fraction (EF) by echocardiogram among those with classic myocarditis, multisystem inflammatory syndrome in children (MIS‐C), or COVID‐19 vaccine‐related myocarditis.
Subanalyses
When restricting the analysis to children who were aged ≥12 years, we found similar findings to the total cohort. Notable differences included higher rates of comorbidities and greater need for vasoactive support in the older children with MIS‐C when compared with the 2 other groups (Tables S1 through S3, Figure S1). Differently than the full cohort, in patients aged ≥12 years, the patients with MIS‐C tended to have the lowest recorded median EF. The majority of affected patients with MIS‐C had full recovery of cardiac function at the time of discharge, similar to the analysis of children of all ages (Table S2).
Discussion
To our knowledge, this is the first analysis comparing findings in patients with classic myocarditis, MIS‐C myocarditis, and COVID‐19 vaccine‐related myocarditis. Compared with classic myocarditis, patients with MIS‐C had a greater likelihood of full recovery of cardiac function with a faster time to recovery, even when they presented with fulminant myocarditis (sudden and severe diffuse cardiac inflammation that leads to cardiogenic shock, ventricular arrhythmias, or multiorgan system failure). 18 Those with COVID‐19 vaccine‐related myocarditis generally had a milder clinical course, with a lower likelihood of cardiac dysfunction at presentation and more rapid recovery when present.
Similar to our study, other analyses have also highlighted the return of normal systolic cardiac function in the follow‐up of patients with MIS‐C myocarditis. 12 , 19 This ventricular recovery is a key finding and is distinct from the pattern of recovery seen in patients with classic myocarditis in whom ventricular recovery tends to be slow. Other children in the classic myocarditis population never recovered function, progressing to heart transplantation or death. Thus, although patients with MIS‐C myocarditis can present with severely depressed cardiac function requiring intensive care, mechanical ventilation, and even ECMO, reassuringly patients in the short term regain normal cardiac function and continue to have evidence of full cardiac recovery at the time of their 3‐month follow‐up visits.
One explanation for this rapid recovery for children with MIS‐C myocarditis compared with classic myocarditis is that cardiac dysfunction is a result of the systemic inflammatory state associated with MIS‐C; once that has been addressed, cardiac function recovers. Recovery may be slower in classic myocarditis because of direct myocyte injury as well as immune mediation attributed to a viral trigger in the case of viral myocarditis. 20 Another factor to consider in the MIS‐C population is the impact that treatment with IVIG and steroids may have had in the return to full recovery of cardiac function. There was a statistically significant difference between the MIS‐C myocarditis and classic myocarditis group in that the MIS‐C group received treatment more readily when compared with the classic myocarditis group, and thus this could play a role in the difference in return to recovery. However, in the treatment of classic myocarditis, IVIG is not routinely recommended given its variable efficacy in studies, so its role in the improvement of EF in the setting of MIS‐C myocarditis is also unclear. 6
In our study, MIS‐C myocarditis tended to affect a younger population, similar to findings in a large‐scale analysis of patients with MIS‐C with a median age of 8 years. 21 Interestingly, patients with classic myocarditis in our study tended to be older and to present with chest pain when compared with the MIS‐C myocarditis group. This difference could be explained by the fact that the older patient population in the classic myocarditis group was more readily able to express chest discomfort compared with the younger population in the MIS‐C myocarditis group. The COVID‐19 vaccine‐related myocarditis group was exclusively male patients, similar to the other reports of this disease in which there is a male predominance. 13 , 14 , 22 With regard to the race and ethnicity of our cohort, the classic and MIS‐C myocarditis groups had a Black majority, whereas the majority of the vaccine‐related group was White race. Adjusted analyses for race and sex were considered, but the limited observations in the vaccine‐related myocarditis group precluded this. These differences are important areas to be examined in a future larger series.
There are significant laboratory differences between classic myocarditis and MIS‐C myocarditis, with the MIS‐C myocarditis group having higher CRP and higher BNP with more prominent anemia, thrombocytopenia, and lymphopenia despite a prominent leukocytosis. These findings are congruent with the level of systemic inflammation associated with MIS‐C. The etiology of the elevated BNP is unclear, but this finding may be related to the consistent administration of IVIG in the MIS‐C cohort compared with the classic myocarditis group in our study; this volume load may have caused an increase in myocardial stress, reflected by the elevated BNP. With regard to the more significant troponin elevation in the classic myocarditis group, abnormal troponin levels within the first 72 hours have been associated with increased ECMO use. 23 This is similar to what we found in our study in the classic myocarditis group with higher rates of ECMO usage and higher troponin values for this subset, although we used peak troponin values rather than baseline within the first 72 hours. 24 The patients with vaccine‐related myocarditis had similar laboratory studies to the classic myocarditis group, mainly with troponin elevation, without significant laboratory evidence of systemic inflammation.
Notably, all individuals in the COVID‐19 vaccine‐related myocarditis group who presented with depressed ventricular function regained normal cardiac function at the time of discharge. This finding provides further reassurance that although myocarditis is a possible, albeit rare, sequela of the mRNA COVID‐19 vaccine, affected patients have recovery of cardiac function in the short term.
Limitations
There are important limitations in our study. Our data reflect patients hospitalized at a single tertiary pediatric institution. Thus, it represents our center’s approach to classic myocarditis, MIS‐C myocarditis, and vaccine‐related myocarditis and may not be generalizable to all children with myocarditis. By including only hospitalized patients, we also may have missed patients with subclinical findings in whom the clinical course may have been different. In addition, we did not have cardiac magnetic resonance imaging (cMRI) data for comparisons between the groups. Given the concerns for active infection and potential hemodynamic instability, patients with MIS‐C myocarditis did not undergo cMRI at the time of presentation. cMRI is the gold standard for noninvasive diagnosis of myocarditis and thus, even in the setting of elevated troponin and symptoms, some patients with MIS‐C myocarditis included in this study may not have met cMRI criteria for the diagnosis of myocarditis. 6 The inclusion of patients with signs of mild myocardial inflammation but not true myocarditis based on cMRI may therefore have overrepresented the number of patients who had full cardiac recovery. The patients with vaccine‐related myocarditis did undergo cMRI during their initial hospitalization, but most have not yet undergone follow‐up cMRI testing. This testing will be important to assess for evidence of ongoing myocardial inflammation and risk for sequelae other than decreased function. Finally, 3‐month outcome data are limited as some of the patients were lost to follow‐up.
Conclusions
This study highlights the important differences among classic myocarditis, MIS‐C myocarditis, and COVID‐19 vaccine‐related myocarditis. Compared with those with classic myocarditis, those with MIS‐C myocarditis had more significant hematologic derangements and worse inflammation at presentation, but had better clinical outcomes, including rapid recovery of cardiac function. Although patients with COVID‐19 vaccine‐related myocarditis had similar clinical presentation to patients with classic myocarditis, their pattern of recovery was similar to those with MIS‐C, with prompt resolution of symptoms and improvement of cardiac function. These findings are a crucial foundation for health care providers, public health officials, and affected patients and families as knowledge of the cardiac impact of MIS‐C and COVID‐19 vaccines continues to grow.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S3
Figure S1
Acknowledgments
We thank Deborah Whitaker (Sibley Heart Center) for her help in extracting the data for this study along with the report writers and analysts on the Children’s Healthcare of Atlanta Business Intelligence Team.
Preprint posted on MedRxiv October 7, 2021. doi: https://doi.org/10.1101/2021.10.05.21264581.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Klugman D, Berger JT, Sable CA, He J, Khandelwal SG, Slonim AD. Pediatric patients hospitalized with myocarditis: a multi‐institutional analysis. Pediatr Cardiol. 2010;31:222–228. doi: 10.1007/s00246-009-9589-9 [DOI] [PubMed] [Google Scholar]
- 2. Canter CE, Simpson KE. Diagnosis and treatment of myocarditis in children in the current era. Circulation. 2014;129:115–128. doi: 10.1161/CIRCULATIONAHA.113.001372 [DOI] [PubMed] [Google Scholar]
- 3. Vasudeva R, Bhatt P, Lilje C, Desai P, Amponsah J, Umscheid J, Parmar N, Bhatt N, Adupa R, Pagad S, et al. Trends in acute myocarditis related pediatric hospitalizations in the United States, 2007–2016. Am J Cardiol. 2021;149:95–102. doi: 10.1016/j.amjcard.2021.03.019 [DOI] [PubMed] [Google Scholar]
- 4. English RF, Janosky JE, Ettedgui JA, Webber SA. Outcomes for children with acute myocarditis. Cardiol Young. 2004;14:488–493. doi: 10.1017/S1047951104005049 [DOI] [PubMed] [Google Scholar]
- 5. Gagliardi MG, Bevilacqua M, Bassano C, Leonardi B, Boldrini R, Camassei FD, Fierabracci A, Ugazio AG, Bottazzo GF. Long term follow up of children with myocarditis treated by immunosuppression and of children with dilated cardiomyopathy. Heart. 2004;90:1167–1171. doi: 10.1136/hrt.2003.026641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dasgupta S, Iannucci G, Mao C, Clabby M, Oster ME. Myocarditis in the pediatric population: a review. Congenit Heart Dis. 2019;14:868–877. doi: 10.1111/chd.12835 [DOI] [PubMed] [Google Scholar]
- 7. Aronoff SC, Hall A, Del Vecchio MT. The natural history of SARS‐Cov‐2 related multisystem inflammatory syndrome in children (MIS‐C): a systematic review. J Pediatric Infect Dis Soc. 2020;9:746–751. doi: 10.1093/jpids/piaa112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, Barranco MA, Maxted AM, Rosenberg ES, Easton D, et al.; Centers for Disease C and Prevention Multisystem Inflammatory Syndrome in Children Investigation T . Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McMurray JC, May JW, Cunningham MW, Jones OY. Multisystem inflammatory syndrome in children (MIS‐C), a post‐viral myocarditis and systemic vasculitis‐a critical review of its pathogenesis and treatment. Front Pediatr. 2020;8:626182. doi: 10.3389/fped.2020.626182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Belhadjer Z, Meot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, Grimaud M, Oualha M, Beghetti M, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS‐C) in the context of global SARS‐CoV‐2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360 [DOI] [PubMed] [Google Scholar]
- 11. Blondiaux E, Parisot P, Redheuil A, Tzaroukian L, Levy Y, Sileo C, Schnuriger A, Lorrot M, Guedj R, Ducou le Pointe H. Cardiac MRI of children with multisystem inflammatory syndrome (MIS‐C) associated with COVID‐19: case series. Radiology. 2020;297:E283–E288. doi: 10.1148/radiol.2020202288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vukomanovic VA, Krasic S, Prijic S, Ninic S, Minic P, Petrovic G, Nesic D. Differences between pediatric acute myocarditis related and unrelated to SARS‐CoV‐2. Pediatr Infect Dis J. 2021;40:e173–e178. doi: 10.1097/INF.0000000000003094 [DOI] [PubMed] [Google Scholar]
- 13. Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS, Shaughnessya R, Carona R, Fuss C, Corbin KJE, et al. Symptomatic acute myocarditis in seven adolescents following Pfizer‐BioNTech COVID‐19 vaccination. Pediatrics. 2021;148:e2021052478. doi: 10.1542/peds.2021-052478 [DOI] [PubMed] [Google Scholar]
- 14. Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, et al. Myocarditis following immunization with mRNA COVID‐19 vaccines in members of the US military. JAMA Cardiol. 2021;6:1202–1206. doi: 10.1001/jamacardio.2021.2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, Broder KR, Gee J, Weintraub E, Shimabukuro T, et al. Use of mRNA COVID‐19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–982. doi: 10.15585/mmwr.mm7027e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Godfred‐Cato S, Bryant B, Leung J, Oster ME, Conklin L, Abrams J, Roguski K, Wallace B, Prezzato E, Koumans EH, et al. COVID‐19‐associated multisystem inflammatory syndrome in children—United States, March–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dove ML, Jaggi P, Kelleman M, Abuali M, Ang JY, Ballan W, Basu SK, Campbell MJ, Chikkabyrappa SM, Choueiter NF, et al. Multisystem inflammatory syndrome in children: survey of protocols for early hospital evaluation and management. J Pediatr. 2021;229:33–40. doi: 10.1016/j.jpeds.2020.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, Shah RV, Sims DB, Thiene G, Vardeny O; American Heart Association Heart F and Transplantation Committee of the Council on Clinical C . Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141:e69–e92. doi: 10.1161/CIR.0000000000000745 [DOI] [PubMed] [Google Scholar]
- 19. Matsubara D, Kauffman HL, Wang Y, Calderon‐Anyosa R, Nadaraj S, Elias MD, White TJ, Torowicz DL, Yubbu P, Giglia TM, et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID‐19 in the United States. J Am Coll Cardiol. 2020;76:1947–1961. doi: 10.1016/j.jacc.2020.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738–747. doi: 10.1016/S0140-6736(11)60648-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abrams JY, Oster ME, Godfred‐Cato SE, Bryant B, Datta SD, Campbell AP, Leung JW, Tsang CA, Pierce TJ, Kennedy JL, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS‐C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5:323–331. doi: 10.1016/S2352-4642(21)00050-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, Darty SN, Parker MA, Kim RJ. Patients with acute myocarditis following mRNA COVID‐19 vaccination. JAMA Cardiol. 2021;6:1196–1201. doi: 10.1001/jamacardio.2021.2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kobayashi D, Aggarwal S, Kheiwa A, Shah N. Myopericarditis in children: elevated troponin I level does not predict outcome. Pediatr Cardiol. 2012;33:1040–1045. doi: 10.1007/s00246-012-0222-y [DOI] [PubMed] [Google Scholar]
- 24. Butto A, Rossano JW, Nandi D, Ravishankar C, Lin KY, O'Connor MJ, Shaddy RE, Shamszad P. Elevated troponin in the first 72 h of hospitalization for pediatric viral myocarditis is associated with ECMO: an analysis of the PHIS+ database. Pediatr Cardiol. 2018;39:1139–1143. doi: 10.1007/s00246-018-1871-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figure S1


