Abstract
The lactose operon from Lactobacillus casei is regulated by very tight glucose repression and substrate induction mechanisms, which made it a tempting candidate system for the expression of foreign genes or metabolic engineering. An integrative vector was constructed, allowing stable gene insertion in the chromosomal lactose operon of L. casei. This vector was based on the nonreplicative plasmid pRV300 and contained two DNA fragments corresponding to the 3′ end of lacG and the complete lacF gene. Four unique restriction sites were created, as well as a ribosome binding site that would allow the cloning and expression of new genes between these two fragments. Then, integration of the cloned genes into the lactose operon of L. casei could be achieved via homologous recombination in a process that involved two selection steps, which yielded highly stable food-grade mutants. This procedure has been successfully used for the expression of the E. coli gusA gene and the L. lactis ilvBN genes in L. casei. Following the same expression pattern as that for the lactose genes, β-glucuronidase activity and diacetyl production were repressed by glucose and induced by lactose. This integrative vector represents a useful tool for strain improvement in L. casei that could be applied to engineering fermentation processes or used for expression of genes for clinical and veterinary uses.
Lactic acid bacteria (LAB) have been used for centuries in the preparation and processing of foods and beverages. Due to its great economic importance for the agrofeed sector and its alleged importance for human and animal health, research on the characterization, metabolism, and genetics of the genus Lactobacillus has increased over the last decade. Several vectors have been developed to express genes and to secrete proteins in Lactobacillus (12, 26, 36, 37, 47, 48). However, if these vectors are to be considered safe for humans, animals, or the environment, only DNA from organisms generally regarded as safe should be used, and no antibiotic resistance markers should remain after genetic manipulation. The integration of foreign genes into the genome constitutes an interesting option for stably maintaining cloned genes without the need for selective markers. Technically, foreign gene integration could be achieved by homologous recombination through cloned DNA fragments (randomly cloned fragments or target genes) and by self-integrative elements (insertion sequences or an attachment site and integrase gene). In the genus Lactobacillus, stabilization of cloned genes is normally achieved by chromosomal integration, based on the use of cloned DNA fragments in nonreplicating plasmids. Stable chromosomal integration of the genes encoding the α-amylase from Bacillus stearothermophilus and a cellulase from Clostridium thermocellum was obtained in Lactobacillus plantarum using a randomly cloned chromosomal fragment as the integration target (42). A similar strategy was used to construct an integrative vector for Lactobacillus acidophilus (25). Other ingenious systems have been developed using a phage integrase-mediated site-specific insertion in the host chromosome (4, 6, 28). There are also examples of stable integration in target genes, such as cbh, which encodes a bile salt hydrolase, and pepXP, which encodes an X-prolyl-dipeptidyl aminopeptidase, from L. plantarum and L. helveticus, respectively (9, 22). All foreign genes integrated by these procedures are normally expressed from their own promoters, which makes more difficult the control of their regulation. The α-amylase gene from Bacillus licheniformis (amyL) was satisfactorily expressed in L. plantarum only when the amyL promoter was replaced by an L. plantarum promoter (42).
Very efficient expression systems based on antimicrobial peptide (nisin), sugar utilization, or nonsense suppressors have been developed for Lactococcus lactis (12, 14, 23, 40). However, besides the nisin system, these approaches could not be transferred to species of Lactobacillus. In lactobacilli, the regulation of gene expression has been studied mainly for carbon catabolism pathways, such as those of lactose, xylose, ribose, sorbose, and arginine deiminase (1, 2, 3, 10, 18, 19, 30, 34, 35, 43, 49, 50). In Lactobacillus casei, the best-characterized sugar transport is the lactose-specific phosphoenolpyruvate-dependent phosphotransferase system (PTS). The lac operon, lacTEGF, encodes an antiterminator protein (LacT), lactose-specific PTS proteins (LacE and LacF), and a phospho-β-galactosidase (P-β-Gal) (LacG) (1, 2, 3, 18, 34). It has been previously reported (3, 19, 30) that the expression of the lac operon in L. casei ATCC393 (pLZ15−) is subject to dual regulation: carbon catabolite repression (CCR) mediated by the general regulator CcpA and induction by lactose through transcriptional antitermination. LacT, whose activity is modulated by the EII elements of the lactose PTS (LacE and/or LacF), mediates the latter mechanism. Furthermore, HPr, a general component of PTS, and LacT are involved in an additional CcpA-independent CCR effect (19, 39, 44).
In this report, an integrative expression vector that allowed the selection of stable mutants that express Escherichia coli gusA and L. lactis ilvBN genes through the lactose regulon is described. This integrative vector represents the first specific expression system developed for L. casei that has great potential for food industry and health applications.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used in this work are listed in Table 1. L. casei cells were grown in MRS medium (Oxoid) and MRS fermentation broth (Adsa-Micro; Scharlau S.A., Barcelona, Spain) plus 0.5% of the different carbohydrates at 37°C under static conditions. E. coli DH5α was grown with shaking at 37°C in Luria-Bertani medium. Plating of bacteria was performed on the respective media solidified with 1.5% agar. When required, the concentrations of antibiotics used were 100 μg of ampicillin per ml to select E. coli transformants and 5 μg of erythromycin per ml for L. casei.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| L. casei CECTa 5275 | Wild type | B. Chassy (University of Illinois, Urbana) |
| L. casei CECT 5276 | L. casei CECT 5275 with a frameshift in lacF | 19 |
| L. casei CECT 5290 | L. casei CECT 5275 with gusA gene integrated | This work |
| L. casei CECT 5291 | L. casei CECT 5275 with ilvBN genes integrated | This work |
| L. lactis MG1363 | Source of ilvBN genes | 16 |
| Plasmids | ||
| pRV300 | Err from pAMβ1 | 24 |
| pNZ272 | gusA Cmr | 33 |
| pIlacF | lacF gene cloned as EcoRV/KpnI fragment in pRV300 | This work |
| pIlac | lacG 3′ end and lacF gene in pRV300 | This work |
| pIlacgus | pIlac containing gusA gene | This work |
| pIlacilv | pIlac containing ilvBN genes | This work |
CETC, Colección Española de Cultivos Tipo.
Recombinant DNA procedures.
Genomic DNA from L. casei and L. lactis strains was purified using a Puregene DNA isolation kit (Gentra Systems, Inc., Minneapolis, Minn.), following the procedure described by the manufacturer. Restriction and modifying enzymes were used according to the recommendations of the manufacturers. General cloning procedures were performed as described by Sambrook et al. (41). L. casei was transformed by electroporation with a gene-pulser apparatus (Bio-Rad Laboratories, Richmond, Calif.). For Southern blot hybridization, L. casei DNA was digested with BglII and PstI endonucleases, separated on agarose gel, and blotted to a Hybond nylon membrane (Amersham). The probes used in the three hybridization experiments were Perm, Pilv, and Plac. The Perm probe consists of an erm gene from the pRV300 plasmid digested with BamHI endonuclease. Pilv corresponds to ilvBN genes from L. lactis that were obtained by PCR using ilv1 and ilv2 oligonucleotides as primers (for sequence, see below), with the genomic DNA of a Lactococcus strain as a template. The Plac probe comprised a fragment of 537 bp that corresponds to the 3′ end of the lacG gene. This DNA fragment was obtained by PCR using genomic DNA from L. casei as a template and the oligonucleotides lac46 (5′TGCGTGCCTATCATGGC) and lac6 (5′CTTGCTGTCTAAATAGCC) as primers. The DNA probes were prepared using the reagents from the Boehringer digoxigenin-DNA labeling kit as recommended by the manufacturer. Hybridization, washing, and staining were done as described by the supplier. PCR was performed using the Expand High fidelity PCR system (Roche Molecular Biochemicals), containing 200 μM concentrations of each deoxynucleoside triphosphate and 10 pmol of each primer. Upon agarose gel electrophoresis, the amplified DNA was recovered with the GFX PCR kit (Amersham Pharmacia Biotech).
Construction of integration plasmids.
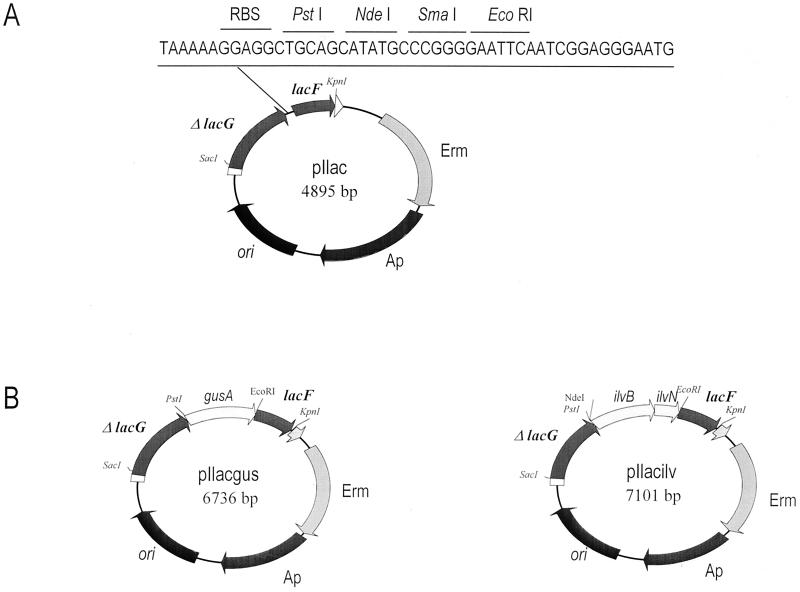
The integrative vector, pIlac, is based on the vector pRV300 (24), which does not replicate in Lactobacillus, and it carries the erm gene from pAMβ1. This vector was constructed in a two-step cloning experiment. A 461-bp DNA fragment containing the lacF gene was amplified by PCR from an L. casei chromosome with the primers lac43 (5′TACATATGCCCGGGGAATTCAATCGGAGGGAAAATG) and lac45 (5′TTGAGGTACCGCTAACAGC). The Lac43 primer showed several substitutions (boldface) to introduce the new NdeI, SmaI, and EcoRI sites (underlined) in the 45-bp region between lacG and lacF. Lac45 contained three substitutions (boldface), generating a new KpnI site (underlined). The blunt-ended fragment amplified was digested with KpnI and cloned into pRV300 that had been previously digested with EcoRV and KpnI. This construction, pIlacF, was used to clone an 878-bp DNA fragment, containing the 3′ end of the lacG gene and 23 bp of the 45-bp intergenic region between the lacG and lacF genes. This fragment was amplified by PCR from an L. casei chromosome using the primers lac49 (5′ATAAGAGCTCCCAAGCTGA) and lac42 (5′TGCATATGCTGCAGCCTCCTTTTTAATCCGGAATG). Lac49 had three substitutions (boldface), generating a SacI site (underlined). The Lac42 primer contained several substitutions (boldface), creating new NdeI and PstI sites and a ribosome-binding site (RBS) (underlined). Then this fragment was cloned into the NdeI/SacI sites of pIlacF. Fig. 1A shows the physical map of the resulting integrative vector, pIlac.
FIG. 1.
Restriction maps of integrative vectors. (A) Integrative vector pIlac. Erm and Ap are erythromycin and ampicillin resistance genes: ori represents the E. coli replicon. lacG and lacF genes encode P-β-Gal and EIIAlac of the lac operon. The 45 bp of intergenic region are shown with the newly created restriction sites and an RBS. (B) Vector pIlacgus. The gusA gene was cloned into PstI/EcoRI pIlac. Vector pIlacilv was constructed by cloning ilvBN genes into NdeI/EcoRI pIlac.
A DNA fragment containing the E. coli gusA gene was amplified by PCR using the plasmid pNZ272 (33) as a template and the primers gus1 (5′AAAACTGCAGTATTATTATCTTAATGAGG) (a newly created PstI site is underlined) and gus2 (5′CGGAATTCTCATTGTTTGCCTCCC) (a newly created EcoRI site is underlined). The amplified DNA fragment was purified, digested with the endonucleases EcoRI and PstI, and ligated into EcoRI/PstI-digested pIlac. This construction was named pIlacgus (Fig. 1B).
The ilvBN genes were amplified from the genomic DNA of L. lactis using the oligonucleotides ilv1 (5′CGATCATATGAAAAAAATAAAGTTAGAAAAACCTACTTCC) and ilv2 (5′CCGAATTCTTAGCCACGCTCAAAACCTGC) as primers, containing NdeI and EcoRI sites (underlined), respectively. The amplified fragment was cloned into NdeI/EcoRI-digested pIlac to give pIlacilv (Fig. 1B).
Enzymatic assays.
P-β-Gal and β-glucuronidase activities were assayed as previously described (33, 46) in permeabilized L. casei cells.
Total nitrogen determination.
The procedure used was based on the method described by Doi et al. (15).
Determination of end metabolites.
The metabolites released by wild-type and mutant strains grown on glucose plus lactose, lactose, or ribose have been analyzed in a resting cell system (11). The analysis of volatile compounds such as ethanol, acetaldehyde, acetone, 1-butanol, acetoin, and diacetyl was performed using a purge-and-trap apparatus equipped with a Vocarb 3000 trap (Supelco) to concentrate the analytes and coupled to a gas chromatographer equipped with a mass spectrometer (Hewlett-Packard 7695) (Barcelona, Spain) as described by Dauneau et al. (11). The α-acetolactate (ALA) determination is based on its oxidative decarboxylation to diacetyl as previously reported, with some modification (38). The samples (2 ml each) were pretreated in a 4-ml vial by addition of 150 μl of 1.85 M FeCl3 and 1 ml of 80% lactate buffer (pH 2.8) and vigorously stirred for 5 s. The vials were hermetically sealed with teflon-lined rubber seals and heated at 75°C for 30 min. The concentration of ALA was calculated by subtraction of the diacetyl concentrations found before and after decarboxylation of the samples.
Lactic acid produced in the resting cell system was measured with a d-lactic acid/l-lactic acid enzymatic bioanalysis kit (Boehringer-Mannheim) as described by the supplier. The total amount of lactic acid produced corresponds to the addition of the concentrations of both isomers determined.
RESULTS
Integration strategy with vector pIlac.
This vector (Fig. 1A) contains two regions of homology that are physically close in the L. casei chromosome, a 3′ fragment of lacG (ΔlacG) and lacF. The sequence of the intergenic region has been modified, keeping intact the spacing between both genes, to introduce a typical RBS for Lactobacillus and a multiple cloning site (PtsI, NdeI, SmaI, and EcoRI), allowing the cloning of new genes, so that after integration, the transcription of these genes would take place from the lac promoter and the newly created RBS would facilitate their translation initiation.
For the integration of the cloned genes in the chromosome, two recombination events should take place, one in each of the homologous regions (ΔlacG and lacF). The use of a lacF frameshift mutant (19), L. casei CECT 5276, as the host strain (Lac−) facilitated the selection of the clones that had undertaken the second recombination as Lac+ clones among the Lac− background, with the following procedure. After electroporation of L. casei CECT 5276 with pIlac derivatives, Lac+ Er+ and Lac− Er+ transformants were recovered, depending on the region where the Campbell-like recombination had occurred. A transformant with the Lac− Err phenotype was grown for 200 generations in MRS broth without antibiotic in order to allow the second recombination event, which would excise the plasmid rendering Lac+ Ers colonies (one out of 20 viables). This strategy can be applied for the insertion of any gene of interest in the lac operon of L. casei and rendered mutants totally deprived of any DNA sequence from the plasmid and erm gene.
Chromosomal integration of E. coli gusA into L. casei.
In order to evaluate the potential of the integrative vector pIlac as a vehicle for chromosomal gene insertion, the β-glucuronidase-encoding gene of E. coli, gusA, was cloned into it. The plasmid obtained, pIlacgus (Fig. 1B), was used to transform L. casei CECT 5276. Following the procedure described above, colonies that had undergone a second recombination event suffered the excision of the vector, giving rise to Erms Lac+ colonies which had the gusA gene integrated into the lac operon. The first and second recombination events were confirmed by Southern blot hybridization of the integrants' chromosomal DNA (data not shown). The resulting new structure of the lac operon contained gusA between lacG and lacF, and as a consequence, the expression of gusA was subject to the same regulation as the lac genes. This was confirmed by measuring β-glucuronidase activity in one of the colonies selected, L. casei CECT 5290, when it was grown on ribose, lactose, and glucose plus lactose (Table 2). Greater P-β-Gal activity was detected for the gusA integrant on lactose than for the wild type and ilvBN integrant (described below), possibly due to the partial cleavage of ONPG-6-P (the P-β-Gal substrate) by β-glucuronidase. It could also be noticed that the growth rate of both integrants on glucose was identical to that of the wild type (data not shown). On lactose, duplication times were not substantially different during early growth stages (95.5 ± 2.6 min, 91.8 ± 3.4 min, and 90.3 ± 5.4 min for wild-type, CECT 5290, and CECT5291 strains, respectively); however, it was observed that both mutants (gus and ilvBN) would reach only an optical density at 550 nm of 0.8.
TABLE 2.
Enzymatic activities in different strains of L. caseia
| Strain | Relevant genotype | Sugar | Activity (nmol min−1 mg−1 dry wt)
|
|
|---|---|---|---|---|
| P-β-Gal | β-glucuronidase | |||
| CECT 5275 | Wild type | Ribose | 2.03 ± 0.86 | 0.98 ± 0.15 |
| Lactose | 23.91 ± 2.93 | 1.89 ± 0.19 | ||
| Glucose + lactose | 0.78 ± 0.24 | 1.16 ± 0.23 | ||
| CECT 5290 | gusA | Ribose | 10.040 ± 1.69 | 43.13 ± 11.64 |
| Lactose | 57.84 ± 8.51 | 119.05 ± 8.91 | ||
| Glucose + lactose | 1.26 ± 0.12 | 1.23 ± 0.2 | ||
| CECT 5291 | ilvBN | Ribose | 6.43 ± 0.63 | |
| Lactose | 27.11 ± 3.56 | |||
| Glucose + lactose | 1.56 ± 0.26 | |||
The values and standard deviations are from at least three independent experiments.
Construction of a food-grade ilvBN integrant of L. casei.
Diacetyl is an important compound related to the characteristic flavor of many fermented milk products. Only a few LAB could produce this metabolite from the citrate of milk. During citrate fermentation, ALA synthase converts pyruvate to ALA, which could be converted spontaneously to diacetyl in the presence of oxygen. The L. lactis ilvBN genes encode the catalytic and regulatory subunits of acetohydroxy acid synthase (17). This enzyme is involved in biosynthesis of branched-chain amino acids, isoleucine and valine, converting pyruvate to ALA with higher affinity for pyruvate than ALA synthase. In this work, in order to increase the cellular pool of ALA that could be turned into diacetyl by oxidative decarboxylation, ilvBN genes of L. lactis were integrated into the chromosome of L. casei. Both genes were cloned into pIlac to give pIlacilv (Fig. 1B), which was used to transform L. casei CECT 5276. The selection strategy for recombinant colonies was identical to that described above. A double recombinant (Ers and Lac+) mutant of L. casei (ivlBN integrant) was selected for further analysis and designated L. casei CECT 5291. The pattern of P-β-Gal activity in this strain was similar to that in the wild-type strain, since it also is induced by lactose and repressed by glucose (Table 2).
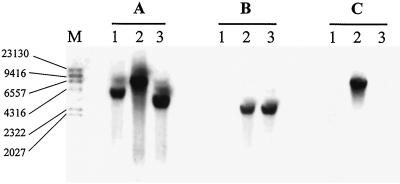
Integration of the ilvBN genes into the chromosomal lac operon of L. casei was confirmed by Southern hybridization using the probe Plac, corresponding to the 3′ end of lacG (Fig. 2A). A hybridization band was detected on the genomic DNA of the L. casei CECT 5276 (host strain), the Err Lac− integrant of pIlacilv (first recombination event), and L. casei CECT 5291 (double recombinant) (Fig. 2A, lanes 1, 2, and 3, respectively) when digested with BglII/PstI. The fragment detected in L. casei CECT 5276 was larger than in the double recombinant because the fragment integrated containing ilvBN carries an additional PstI site. The chromosomal integration of ilvBN was confirmed using a Pilv probe (Fig. 2B). Evidence that the antibiotic resistance gene (erm) had been excised from the L. casei genome was demonstrated using a Perm probe. In this Southern blot, the hybridization signal was detected only with the genomic DNA from the first integrant (Fig. 2C). Moreover, the ilvBN genes remained stably integrated on the genome after 50 overnight transfers in MRS medium without selective pressure (data not shown).
FIG. 2.
Southern blot of total DNA digested with BglII/PstI from L. casei CECT 5276 (lane 1), an integrant of pIlacilv (lane 2), and L. casei CECT 5291 (food-grade integrant) (lane 3). The probes used were Plac (A), Pilv (B), and Perm (C). M represents digoxigenin-labeled λ phage DNA digested with HindIII as a molecular size marker.
Determination of metabolic products in the ilvBN mutant.
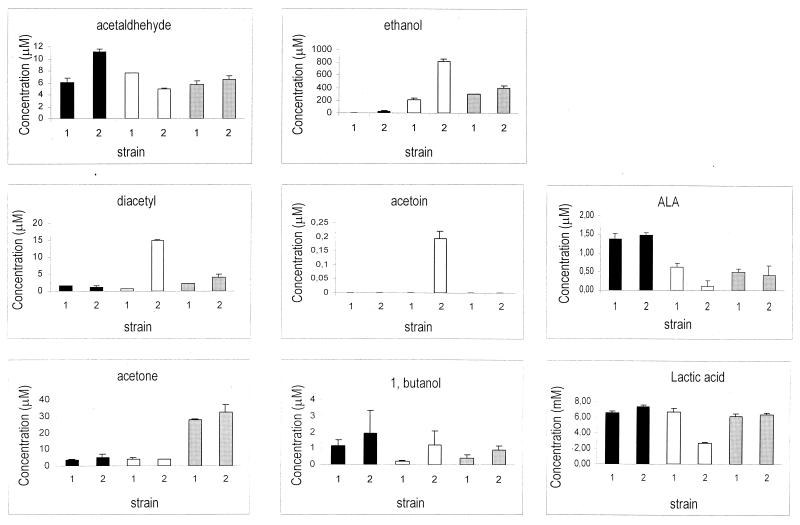
Metabolites released by the L. casei wild type and L. casei CETC 5291, carrying the integrated ilvBN genes in the lactose operon (ilvBN integrant), have been analyzed in a resting cell system when cells were grown on glucose plus lactose, lactose, and ribose (Fig. 3). Besides lactic acid, which is by far the most abundant compound, ethanol and acetone were the predominant metabolites accumulated by cells grown on ribose, on which L. casei becomes heterofermentative. Remarkable differences could be noticed in the ilvBN integrant on lactose, regarding the production of ethanol, 1-butanol, acetoin, and diacetyl. In particular, the amount of diacetyl accumulated by the lactose-induced ilvBN integrant was 23-fold greater than that for the wild type. Other significant differences found in the ilvBN integrant are related to the lower level of accumulation of ALA and lactate on lactose, possibly due to the diversion of pyruvate and ALA towards the synthesis of branched-chain amino acids (isoleucine, leucine, and valine). In order to test this hypothesis, total soluble nitrogen (amino acids) was determined in the supernatant of the resting cell systems on lactose, obtaining 1.25 ± 0.07 mM concentrations for the wild type, and 2.01 ± 0.26 mM concentrations for the ilvBN integrant. This difference (0.76 mM) could partially be explained by the secretion of a proportion of the excess amino acids synthesized by the integrant. Regarding acetaldehyde production, only small differences were observed between the two strains with different carbon sources. Unexpectedly, slightly higher concentrations of acetaldehyde and 1-butanol could be observed when the ilvBN integrant was grown on glucose plus lactose. This different behavior was clearly related to the presence of the ilvBN genes, indicating that some degree of expression of the acetohydroxy acid synthase was taking place on glucose plus lactose, altering the proportions in metabolites derived from acetyl coenzyme A.
FIG. 3.
End metabolite production (acetaldehyde, ethanol, diacetyl, acetoin, ALA, acetone, 1, butanol, and lactic acid) by the wild type (1) and ilvBN integrant (2) from cells grown on glucose plus lactose (black), lactose (white), and ribose (grey). The values are from at least three independent experiments, and the coefficient of variation for each mean was less than 10%.
DISCUSSION
The present study describes the construction of an integrative expression vector for L. casei that allowed the obtainment of stable food-grade integrants capable of expressing foreign genes under the tight control of the well-characterized lac operon promoter (Fig. 1A). Lactose genes have been used in other LAB for different biotechnological purposes, such as the construction of food-grade vectors in L. lactis, addressing integration in Lactobacillus helveticus, and gene expression in Streptococcus thermophilus (9, 27, 29, 31). However, both structural organization and regulation of the lac operon in L. casei are very different from those described for the other LAB (1, 2, 3, 13, 18, 19, 30, 34); it displays very tight glucose repression and lactose induction mechanisms, which were very promising for the expression of foreign genes. The integrative vector designed in this work, pIlac, allowed cloning of DNA fragments between the two target genes (ΔlacG and lacF). Then, through Campbell-like recombination, the genes of interest could be inserted in the lac operon, obtaining a food-grade construct in which the foreign genes became a functional part of the operon and were subject to the same regulation (Table 2). However, it could be observed that the insertion of foreign genes, gusA and ilvBN, led to some induction of the operon on ribose, as was shown by P-β-Gal and β-glucuronidase activities (Table 2). In a previous work it was shown that mutants in the genes encoding either of the lactose-specific PTS elements (lacE and lacF) showed constitutive transcription of the lac operon (19). It could be tentatively proposed that transcription of the new constructions gave a longer, more unstable mRNA, where perhaps lacF—placed at the end of it—could be less efficiently translated. This could be confirmed by the fact that neither of the integrants could grow on lactose for more than a few generations, possibly because of an inefficient lactose-PTS transport that allowed growth only at high lactose concentrations.
L. casei is frequently used as the starter culture in many fermentation processes, especially in cheese making or, recently, as a probiotic in fermented milk products. However, L. casei is not a good producer of diacetyl, and this is a very desirable compound in dairy fermentations. This product is normally synthesized in LAB from the glycolytic intermediate pyruvate, which is converted to ALA by the ALA synthase. Then, ALA is transformed to acetoin through ALA decarboxylase activity or to diacetyl in the presence of oxygen. Also, acetoin yields diacetyl by the action of acetoin reductase. Different approaches have been used with L. lactis to improve diacetyl production, such as deletion of acetolactate decarboxylase, mutation of lactate dehydrogenase, or overexpression of acetohydroxy acid synthase (8, 20, 21, 32, 45). This biotechnological approach was also considered in the present work. Induction of ilvBN genes by lactose in the food-grade system developed in this work yielded, in 3 h, a total amount of diacetyl comparable to that in overnight cultures of L. lactis overexpressing ilvBN (7, 8, 20, 45). However, further optimization of the diacetyl production through detailed fermentation studies could be achieved because, in our resting cell system, the high cell density and static incubation conditions in a sealed tube were possibly generating an adverse environment—poor in oxygen—where a lower diacetyl reductase activity favored a certain amount of accumulation of acetoin.
Another objective of this work was the evaluation of the balance of metabolites during the overexpression of “cross-road” enzymes, such as acetohydroxy acid synthase (encoded by ilvBN). The accumulation of ALA on glucose-grown cells suggests that the biosynthesis of diacetyl from this ketoacid could be subject to CCR. However, a major interference with an even greater overproduction of diacetyl in L. casei CETC 5291 can be attributed to the fact that pyruvate is also a substrate of acetohydroxy acid synthase in the anabolic pathway of branched-chain amino acids. Furthermore, both compounds, pyruvate and ALA, could be inducers of subsequent steps in these pathways (5), for which the overexpression of ilvBN was probably leading to a drainage of pyruvate for the synthesis of leucine, isoleucine, and valine. This was demonstrated by the smaller amount of lactate and higher concentration of amino acids detected in the supernatant of the lactose-induced mutant during the resting cell assay. However, a metabolite balance could not be calculated. This is an intricate part of the metabolic map, and many more compounds should be analyzed to get a clearer picture of the carbon fluxes at this metabolic level.
The kind of experiment described in this work has never before been performed with lactobacilli. Due to its food-grade nature, the system developed here has a great potential for the metabolic engineering of intracellular metabolites and the production of different enzymes during dairy fermentation. However, due to the regular presence of lactobacilli in higher vertebrate mucosae, other applications could be envisaged, such as the delivery of antigens in the gut, mouth, or vagina as well as a variety of clinical and veterinary products.
ACKNOWLEDGMENTS
We thank M. C. Miralles for her skillful technical assistance.
This work was financed by the EU project BIO4-CT96-0380 and by funds of the Spanish CICyT (Interministerial Commission for Science and Technology) (Ref. ALI 98-0714). C.D.E. was the recipient of a fellowship from the Spanish government.
REFERENCES
- 1.Alpert C-A, Chassy B M. Molecular cloning and nucleotide sequence of the factor IIIlac gene of Lactobacillus casei. Gene. 1988;62:277–288. doi: 10.1016/0378-1119(88)90565-3. [DOI] [PubMed] [Google Scholar]
- 2.Alpert C-A, Chassy B M. Molecular cloning and DNA sequence of lacE, the gene encoding the lactose-specific enzyme II of the phosphotransferase system of Lactobacillus casei. J Biol Chem. 1990;265:22561–22568. [PubMed] [Google Scholar]
- 3.Alpert C-A, Siebers U. The lac operon of Lactobacillus casei contains lacT, a gene coding for a protein of the BglG family of transcriptional antiterminators. J Bacteriol. 1997;179:1555–1562. doi: 10.1128/jb.179.5.1555-1562.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez M A, Herrero M, Suárez J E. The site-specific recombination system of Lactobacillus species bacteriophage A2 integrates in Gram-positive and Gram-negative bacteria. Virology. 1998;250:185–193. doi: 10.1006/viro.1998.9353. [DOI] [PubMed] [Google Scholar]
- 5.Arfin S M, Umbarger H E. The metabolism of valine and isoleucine in Escherichia coli. XVII. The role of induction in the derepression of acetohydroxy acid isomeroreductase. Biochem Biophys Res Commun. 1969;37:902–908. doi: 10.1016/0006-291x(69)90216-2. [DOI] [PubMed] [Google Scholar]
- 6.Auvray F, Coddeville M, Ritzenthaler P, Dupont L. Plasmid integration in a wide range of bacteria mediated by integrase of Lactobacillus delbrueckii bacteriophage mv4. J Bacteriol. 1997;179:1837–1845. doi: 10.1128/jb.179.6.1837-1845.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassit N, Boquien C-Y, Picque D, Corrieu G. Effect of initial oxygen concentration on diacetyl and acetoin production by Lactobacillus lactis subsp. lactis biovar diacetylactis. Appl Environ Microbiol. 1993;59:1893–1897. doi: 10.1128/aem.59.6.1893-1897.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benson K H, Godon J-J, Renault P, Griffin H G, Gasson M J. Effect of ilvBN-encoded α-acetolactate synthase expression on diacetyl production in Lactobacillus lactis. Appl Microbiol Biotechnol. 1996;45:107–111. [Google Scholar]
- 9.Bhowmik T, Fernandez L, Steele J L. Gene replacement in Lactobacillus helveticus. J Bacteriol. 1993;175:6341–6344. doi: 10.1128/jb.175.19.6341-6344.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Champomier Vergès M C, Zuñiga M, Morel-Deville F, Pérez-Martínez G, Zagorec M, Ehrlich S D. Relationships between arginine degradation, pH and survival in Lactobacillus sakei. FEMS Microbiol Lett. 1999;180:297–304. doi: 10.1111/j.1574-6968.1999.tb08809.x. [DOI] [PubMed] [Google Scholar]
- 11.Dauneau P, Pérez-Martínez G. Fractional factorial and multiple linear regression to optimise extraction of volatiles from a Lactobacillus plantarum bacterial suspension using purge and trap. J Chromatogr A. 1997;775:225–230. [Google Scholar]
- 12.de Vos W M. Safe and suitable system for food-grade fermentations by genetically modified lactic acid bacteria. Int Dairy J. 1999;9:3–10. [Google Scholar]
- 13.de Vos W M, Vaughan E E. Genetics of lactose utilization in lactic acid bacteria. FEMS Microbiol Rev. 1994;15:217–237. doi: 10.1111/j.1574-6976.1994.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 14.Dickely F, Nilsson D, Hansen E B, Johansen E. Isolation of Lactococcus lactis nonsuppressors and construction of food-grade cloning vector. Mol Microbiol. 1995;15:839–847. doi: 10.1111/j.1365-2958.1995.tb02354.x. [DOI] [PubMed] [Google Scholar]
- 15.Doi E, Shibata D, Matoba T. Modified colorimetric ninhydrin methods for peptidase assay. Anal Biochem. 1981;118:173–184. doi: 10.1016/0003-2697(81)90175-5. [DOI] [PubMed] [Google Scholar]
- 16.Gasson M J. Plasmid complements of Streptococcus lactis NCDO712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godon J-J, Chopin M-C, Ehrlich S D. Branched-chain amino acid biosynthesis genes in Lactococcus lactis subsp. lactis. J Bacteriol. 1992;174:6580–6589. doi: 10.1128/jb.174.20.6580-6589.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gosalbes M J, Monedero V, Alpert C-A, Pérez-Martínez G. Establishing a model to study the regulation of the lactose operon in Lactobacillus casei. FEMS Microbiol Lett. 1997;148:83–89. doi: 10.1111/j.1574-6968.1997.tb10271.x. [DOI] [PubMed] [Google Scholar]
- 19.Gosalbes M J, Monedero V, Pérez-Martínez G. Elements involved in catabolite repression and substrate induction of lactose operon in Lactobacillus casei. J Bacteriol. 1999;181:3928–3934. doi: 10.1128/jb.181.13.3928-3934.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goupil N, Corthier G, Ehrlich S D, Renault P. Imbalance of leucine flux in Lactococcus lactis and its use for the isolation of diacetyl-overproducing strains. Appl Environ Microbiol. 1996;62:2636–2640. doi: 10.1128/aem.62.7.2636-2640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goupil-Feuillerat N, Cocaign-Bousquet M, Godon J-J, Ehrlich S D, Renault P. Dual role of α-acetolactate decarboxylase in Lactococcus lactis subsp. lactis. J Bacteriol. 1997;179:6285–6293. doi: 10.1128/jb.179.20.6285-6293.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hols P, Ferain T, Garmyn D, Bernard N, Delcour J. Use of homologous expression-secretion signals and vector-free stable chromosomal integration in engineering of Lactobacillus plantarum for α-amylase and levanase expression. Appl Environ Microbiol. 1994;60:1401–1413. doi: 10.1128/aem.60.5.1401-1413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leenhouts K, Bolhuis A, Venema G, Kok J. Construction of a food-grade multicopy integration system in Lactococcus lactis. Appl Microbiol Biotechnol. 1998;49:417–423. doi: 10.1007/s002530051192. [DOI] [PubMed] [Google Scholar]
- 24.Leloup L, Ehrlich S D, Zagorec M, Morel-Deville F. Single crossing-over integration in the Lactobacillus sake chromosome and insertional inactivation of the pts and the lacI genes. Appl Environ Microbiol. 1997;63:2117–2123. doi: 10.1128/aem.63.6.2117-2123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin M-Y, Harlander S, Savaiano D. Construction of an integrative food-grade cloning vector for Lactobacillus acidophilus. Appl Microbiol Biotechnol. 1999;45:484–489. doi: 10.1007/s002530050717. [DOI] [PubMed] [Google Scholar]
- 26.Maassen C B M, Laman J D, den Bak-Glashouwer M J H, Tielen F J, van Holten-Neelen J C P A, Hoogteijling L, Antonissen C, Leer R J, Pouwels P H, Boersma W J A, Shaw D M. Instruments for oral disease-intervention strategies: recombinant Lactobacillus casei expressing tetanus toxin fragment C for vaccination or myelin proteins for oral tolerance induction in multiple sclerosis. Vaccine. 1999;17:2117–2128. doi: 10.1016/s0264-410x(99)00010-9. [DOI] [PubMed] [Google Scholar]
- 27.MacCormick C A, Griffin H G, Gasson M J. Construction of a food-grade host/vector system for Lactococcus lactis based on lactose operon. FEMS Microbiol Lett. 1995;127:105–109. doi: 10.1111/j.1574-6968.1995.tb07457.x. [DOI] [PubMed] [Google Scholar]
- 28.Martin M C, Alonso J C, Suárez J E, Alvarez M A. Generation of food-grade recombinant lactic acid bacterium strains by site-specific recombination. Appl Environ Microbiol. 2000;66:2599–2604. doi: 10.1128/aem.66.6.2599-2604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mollet B, Knol J, Poolman B, Marciset O, Delley M. Directed genomic integration, gene replacement, and integrative gene expression in Streptococcus thermophilus. J Bacteriol. 1993;175:4315–4324. doi: 10.1128/jb.175.14.4315-4324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monedero V, Gosalbes M J, Pérez-Martínez G. Catabolite repression in Lactobacillus casei ATCC 393 is mediated by CcpA. J Bacteriol. 1997;179:6657–6664. doi: 10.1128/jb.179.21.6657-6664.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platteeuw C, van Alen-Boerrigter I, van Schalkwijk S, de Vos W M. Food-grade cloning and expression systems for Lactococcus lactis. Appl Environ Microbiol. 1996;62:1008–1013. doi: 10.1128/aem.62.3.1008-1013.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Platteeuw C, Hugenholtz J, Starrenburg M, van Alen-Boerrigter I, de Vos W M. Metabolic engineering of Lactococcus lactis: influence of the overproduction of α-acetolactate synthase in strains deficient in lactate dehydrogenase as a function of culture conditions. Appl Environ Microbiol. 1995;61:3967–3971. doi: 10.1128/aem.61.11.3967-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Platteeuw C, Simons G, de Vos W M. Use of the Escherichia coli β-glucuronidase (gusA) gene as a reporter gene for analyzing promoters in lactic acid bacteria. Appl Environ Microbiol. 1994;60:587–593. doi: 10.1128/aem.60.2.587-593.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porter E V, Chassy B M. Nucleotide sequence of the β-D-phospho-galactosidase gene of Lactobacillus casei: comparison to analogous pbg genes of other Gram-positive organisms. Gene. 1988;62:263–276. doi: 10.1016/0378-1119(88)90564-1. [DOI] [PubMed] [Google Scholar]
- 35.Posno M, Heuvelmans P T, van Giezen M J, Lokman B C, Leer R J, Pouwels P H. Complementation of the inability of Lactobacillus strains to utilize d-xylose with d-xylose catabolism-encoding genes of Lactobacillus pentosus. Appl Environ Microbiol. 1991;57:2764–2766. doi: 10.1128/aem.57.9.2764-2766.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pouwels P H, Leer R J. Genetics of lactobacilli: plasmids and gene expression. Antonie Leeuwenhoek. 1993;64:85–107. doi: 10.1007/BF00873020. [DOI] [PubMed] [Google Scholar]
- 37.Pouwels P H, Leer R J, Boersma W J A. The potential of Lactobacillus as a carrier for oral immunization: development and preliminary characterization of vector systems for targeted delivery of antigens. J Biotechnol. 1996;44:183–192. doi: 10.1016/0168-1656(95)00140-9. [DOI] [PubMed] [Google Scholar]
- 38.Richelieu M, Houlberg U, Nielsen J C. Determination of α-acetolactic acid and volatile compounds by headspace gas chromatography. J Dairy Sci. 1997;80:1918–1925. [Google Scholar]
- 39.Rutberg B. Antitermination of transcription of catabolic operons. Mol Microbiol. 1997;23:413–421. doi: 10.1046/j.1365-2958.1997.d01-1867.x. [DOI] [PubMed] [Google Scholar]
- 40.Ruyter P G G A, Kuipers O, de Vos W M. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol. 1996;62:3662–3667. doi: 10.1128/aem.62.10.3662-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Scheirlinck T, Mahillon J, Joos H, Dahese P, Michiels F. Integration and expression of α-amylase and endoglucanase genes in the Lactobacillus plantarum chromosome. Appl Environ Microbiol. 1989;55:2130–2137. doi: 10.1128/aem.55.9.2130-2137.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stentz R, Zagorec M. Ribose utilization in Lactobacillus sakei: analysis of the regulation of rbs operon and putative involvement of a new transporter. J Mol Microbiol Biotechnol. 1999;1:165–173. [PubMed] [Google Scholar]
- 44.Stülke J, Arnaud M, Rapoport G, Martin-Verstraete I. PRD-α protein domain involved in PTS-dependent induction and carbon catabolite repression of catabolic operons in bacteria. Mol Microbiol. 1998;28:865–874. doi: 10.1046/j.1365-2958.1998.00839.x. [DOI] [PubMed] [Google Scholar]
- 45.Swindell S R, Benson K H, Griffin H G, Renault P, Ehrlich S D, Gasson M J. Genetic manipulation of the pathway for diacetyl metabolism in Lactococcus lactis. Appl Environ Microbiol. 1996;62:2641–2643. doi: 10.1128/aem.62.7.2641-2643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veyrat A, Monedero V, Pérez-Martínez G. Glucose transport by the phosphoenolpyruvate: mannose phosphotransferase system in Lactobacillus casei ATCC 393 and its role in carbon catabolite repression. Microbiology. 1994;140:1141–1149. doi: 10.1099/13500872-140-5-1141. [DOI] [PubMed] [Google Scholar]
- 47.Vogel R F, Ehrmann M. Genetics of lactobacilli in foods fermentations. Biotechnol Annu Rev. 1996;2:123–150. doi: 10.1016/s1387-2656(08)70008-5. [DOI] [PubMed] [Google Scholar]
- 48.Wang T-T, Lee B H. Plasmids in Lactobacillus. Crit Rev Biotechnol. 1997;17:227–272. doi: 10.3109/07388559709146615. [DOI] [PubMed] [Google Scholar]
- 49.Yebra M J, Veyrat A, Santos M A, Pérez-Martínez G. Genetics of l-sorbose transport and metabolism in Lactobacillus casei. J Bacteriol. 2000;182:155–163. doi: 10.1128/jb.182.1.155-163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuñiga M, Champomier-Vergès M, Zagorec M, Pérez-Martínez G. Structural and functional analysis of the gene cluster encoding the enzymes of the arginine deiminase pathway of Lactobacillus sake. J Bacteriol. 1998;180:4154–4159. doi: 10.1128/jb.180.16.4154-4159.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]