Abstract
Background
There is a paucity of evidence regarding the association between visit‐to‐visit blood pressure variability and residual cardiovascular risk. We aimed to provide relevant evidence by determining whether high systolic blood pressure (SBP) variability in the optimal SBP levels still influences the risk of cardiovascular disease.
Methods and Results
We studied 7065 participants (aged 59.3±5.6 years; 44.3% men; and 82.9% White) in the ARIC (Atherosclerosis Risk in Communities) study with optimal SBP levels from visit 1 to visit 3. Visit‐to‐visit SBP variability was measured by variability independent of the mean in the primary analysis. The primary outcome was the major adverse cardiovascular event (MACE), defined as the first occurrence of all‐cause mortality, coronary heart disease, stroke, and heart failure. During a median follow‐up of 19.6 years, 2691 participants developed MACEs. After multivariable adjustment, the MACE risk was higher by 21% in participants with the highest SBP variability (variability independent of the mean quartile 4) compared with the lowest SBP variability participants (variability independent of the mean quartile 1) (hazard ratio, 1.21; 95% CI, 1.09–1.35). The restricted cubic spline showed that the hazard ratio for MACE was relatively linear, with a higher variability independent of the mean being associated with higher risk. These association were also found in the stratified analyses of participants with or without hypertension.
Conclusions
In adults with optimal SBP levels, higher visit‐to‐visit SBP variability was significantly associated with a higher risk of MACE regardless of whether they had hypertension. Therefore, it may be necessary to further focus on the visit‐to‐visit SBP variability even at the guideline‐recommended optimal blood pressure levels.
Keywords: major adverse cardiovascular event, residual cardiovascular risk, risk factor, systolic blood pressure variability
Subject Categories: Hypertension, High Blood Pressure, Cardiovascular Disease, Risk Factors
Nonstandard Abbreviations and Acronyms
- ALLHAT
Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial
- ARIC
Atherosclerosis Risk in Communities
- ARV
average real variability
- ASCOT‐BPLA
Anglo‐Scandinavian Cardiac Outcomes Trial Blood Pressure Lowering Arm
- CARDIA
Coronary Artery Risk Development in Young Adults
- CV
coefficient of variation
- MACE
major adverse cardiovascular event
- SBP
systolic blood pressure
- VIM
variability independent of the mean
Clinical Perspective
What Is New?
The present study is the first to report the association of visit‐to‐visit systolic blood pressure (SBP) variability and residual cardiovascular risk and further emphasize the significance of SBP variability.
In adults with optimal SBP levels, greater visit‐to‐visit SBP variability was significantly associated with a higher risk of a major adverse cardiovascular event. This association remained significant for the participants with or without hypertension.
What Are the Clinical Implications?
To optimize clinical and public health strategies toward minimizing the cardiovascular burden, it may be necessary to further focus on the visit‐to‐visit SBP variability even at the guideline‐recommended optimal blood pressure levels.
Elevated blood pressure (BP) is a well‐recognized leading risk factor for cardiovascular events. 1 , 2 BP‐lowering treatment was considered best practice to reduce the risks of cardiovascular events and death, irrespective of previous diagnoses of cardiovascular disease (CVD), and even at normal or high‐normal BP values. 3 , 4 Current guidelines recommend treating high BP to a systolic BP (SBP) goal <140 mm Hg to achieve optimal BP levels. 5 , 6 However, large cohort studies have shown persistent residual cardiovascular risk despite BP lowering to the optimal levels. 7 , 8 Of note, BP does not remain steady, but instead fluctuates continually over the short and long term. 9 Thus, a single measurement or the average of BP might fail to represent the full spectrum of BP‐related cardiovascular risk. Historically, BP fluctuations have been perceived as inhibiting accurate BP measurement and as a phenomenon to be overcome by improved monitoring. 10 Recently, ample evidence 11 , 12 , 13 and our previous work 14 both indicated that elevated visit‐to‐visit BP variability could be a new risk factor for CVD. Unfortunately, the contribution of visit‐to‐visit BP variability to the residual cardiovascular risk remains unknown, which impedes the development of an optimum approach to the long‐term reduction of CVD events. Therefore, to provide evidence regarding the association between BP variability and residual cardiovascular risk, we conducted a secondary analysis of data from the ARIC (Atherosclerosis Risk in Communities) study 15 to determine whether high SBP variability in the optimal SBP levels still influences CVD risk.
Methods
The data, analytic methods, or study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The ARIC study’s data and materials are publicly available to qualified investigators.
Study Populations
The ARIC study is an epidemiological, prospective cohort study designed to assess the risk factors for atherosclerosis and CVD in the general population. Between 1987 and 1989 (visit 1), 15 792 middle‐aged subjects were recruited in 4 communities in the United States: Jackson, Mississippi; Forsyth County, North Carolina; northwestern suburbs of Minneapolis, Minnesota; and Washington County, Maryland. After the baseline examination, subsequent visits were conducted in visit 2 (1990–1992), visit 3 (1993–1995), visit 4 (1996–1998), and visit 5 (2011–2013). The study continuously followed the participants for cardiovascular events through examinations of death certificates and hospitalization records and telephone interviews, and collected information about demographic and medical variables through examinations and questionnaires. Details of the study design have been published elsewhere. 15
This analysis included 7065 participants with optimal SBP levels from visit 1 to visit 3 and without coronary heart disease (CHD), stroke, and heart failure (HF) before or at visit 3, excluding those missing data in the public‐access data sets (n=809); those missing outcome data (n=675) or information on covariates in the regression model (n=323); those who were deemed to have CHD, stroke, and HF before or at visit 3 (n=1126); and those without optimal SBP levels at visit 1, 2, or 3 (n=2981) (Figure S1). The institutional review boards at all participating institutions approved the ARIC study protocol, and all participants provided informed consent.
Definition of Optimal SBP Level and Hypertension
Technicians obtained 3 seated BP readings of participants after a 5‐minute rest in a quiet room. The average of the last 2 measures was used for analysis. Based on the BP management from European Society of Cardiology/European Society of Hypertension guideline, 5 optimal SBP level was defined as 90 mm Hg≤SBP<140 mm Hg. Hypertension was defined as SBP≥140 mm Hg, diastolic BP ≥90 mm Hg, or use of antihypertensive medication.
Assessment of Visit‐to‐Visit SBP Variability
Visit‐to‐visit SBP variability was calculated using the measured SBP across the first 3 visits and assessed using 4 indices: (1) SD, (2) coefficient of variation (CV), (3) average real variability (ARV), and (4) variability independent of the mean (VIM). The formulas of each SBP variability are shown in Figure S2. The mean SBP level was calculated across the first 3 visits for each participant. In the Pearson correlation, VIM had a strong correlation with SD, CV, and ARV (Pearson r=0.878–0.999) but was poorly correlated with mean SBP (Pearson r=0.021) (Table S1). Therefore, VIM was used to measure visit‐to‐visit SBP variability in the primary analysis aimed to distinguish the impact of SBP variability from that of mean SBP on outcomes. The SD, CV, and ARV were used only in the sensitivity analyses.
Outcome Ascertainment
The primary outcome was the incidence of major adverse cardiovascular event (MACE, composite of 4 outcomes), defined as the first occurrence of all‐cause mortality, CHD, stroke, and HF. Secondary outcomes were the 4 individual components of the primary outcome.
In the ARIC study, the ascertainment of deaths and methods of assessing incident CHD, stroke, and HF have been published elsewhere. 16 , 17 , 18 , 19 All‐cause mortality was ascertained by reviewing death certificates and hospital discharge records, supplemented by informant interviews or physician questionnaires for out‐of‐hospital deaths. 16 Incident CHD events included fatal CHD, definite or probable myocardial infarction, and silent myocardial infarction (as determined by ECG). 17 The physician reviewers obtained hospital records for possible stroke‐related hospitalizations and collected information on fatal stroke through linkage with the National Death Index. Definite or probable stroke events were identified by a computer algorithm and adjudicated by physician reviewers. 19 The ARIC study defined incident hospitalized HF by diagnostic code from hospital discharges until 2005 18 and by additional adjudication by an expert panel after 2005. 16
Statistical Analysis
All statistical analyses were performed using Stata version 14 (StataCorp, College Station, TX). After calculating SBP variability for each participant, we categorized SBP variability into quartiles on the basis of the sample distribution. Clinical characteristics at visit 3 are presented as the mean (SD) for continuous variables and as number (%) for categorical variables. To determine the differences among the 4 groups, we used the 1‐way ANOVA test or the Kruskal‐Wallis test for continuous values and the chi‐square test for categorical variables.
Cumulative incidences were estimated for MACEs and 4 secondary outcomes in 4 groups categorized by the quartiles of SBP variability using the Kaplan‐Meier method. The multivariable‐adjusted Cox proportional hazards models with robust standard errors were used to estimate hazard ratios (HRs) and 95% CIs for the associations of SBP variability (assessed by VIM) on MACE and 4 secondary outcomes, adjusted for the following covariates: Model 1 included age, sex, and race at visit 3; model 2 included the variables in model 1 plus education level, body mass index, smoking status, drinking status, total cholesterol, high‐density lipoprotein cholesterol, prevalent diabetes, and use of aspirin and statins at visit 3; model 3 included the variables in model 2 plus prevalent hypertension, use of antihypertensive drugs, diastolic BP at visit 3, and trend and mean of SBP from visit 1 to visit 3. Each participant’s trend was calculated as the slope of the linear regression of SBP measures by the first 3 visits, 20 and each participant’s mean SBP level was calculated across the first 3 visits. To assess the continuous association between SBP variability (assessed by VIM) and MACEs, we conducted a restricted cubic spline with 3 knots and presented it graphically along with the best‐fitted straight line. The associations between SBP variability (assessed by SD, CV, and ARV) and outcomes were also estimated by multivariable‐adjusted Cox models and restricted cubic spline in the sensitivity analyses. To determine whether the association between SBP variability and MACEs varies for the participants with or without hypertension in optimal SBP levels, we performed a stratified analysis of hypertension and assessed the interaction between hypertension and SBP variability. An interaction term between hypertension and SBP variability was individually added to the adjusted Cox model 3, and the P values for these associations were calculated. All the tests were 2‐sided, with P<0.05 considered significant.
Results
Clinical Characteristics of Study Population
Among the 7065 participants included in the present study, the mean age (SD) was 59.3 (5.6) years at visit 3, 44.3% were men, 82.9% were White, and 24.3% had hypertension, as seen in Table 1. Compared with the lower VIM of SBP, the participants with the highest quartile of VIM (quartile 4) were more likely to be female, Black, current smokers, and never drinkers and to have hypertension and a high SBP level.
Table 1.
Clinical Characteristics at Visit 3 of Each Group Categorized by the VIM of SBP
| Clinical characteristics |
Total (n=7065) |
VIM quartile 1 (n=1766) |
VIM quartile 2 (n=1767) |
VIM quartile 3 (n=1766) |
VIM quartile 4 (n=1766) |
P value |
|---|---|---|---|---|---|---|
| Age, y | 59.3 (5.6) | 59.1 (5.6) | 59.3 (5.4) | 59.2 (5.7) | 59.5 (5.5) | 0.137 |
| Sex | <0.001 | |||||
| Men | 3129 (44.3) | 873 (49.4) | 816 (46.2) | 755 (42.8) | 685 (38.8) | |
| Women | 3936 (55.7) | 893 (50.6) | 951 (53.8) | 1011 (57.2) | 1081 (61.2) | |
| Race | 0.001 | |||||
| Black | 1207 (17.1) | 272 (15.4) | 276 (15.6) | 307 (17.4) | 352 (19.9) | |
| White | 5858 (82.9) | 1494 (84.6) | 1491 (84.4) | 1459 (82.6) | 1414 (80.1) | |
| Body mass index, kg/m2 | 27.9 (5.1) | 28.0 (5.1) | 27.7 (4.9) | 27.9 (5.1) | 28.1 (5.4) | 0.181 |
| SBP, mm Hg | 117.2 (11.4) | 115.3 (11.0) | 115.9 (10.9) | 117.6 (11.1) | 120.2 (11.4) | <0.001 |
| Diastolic BP, mm Hg | 69.9 (8.7) | 69.6 (8.5) | 69.3 (8.5) | 70.0 (8.9) | 70.5 (8.9) | <0.001 |
| Total cholesterol, mmol/L | 5.4 (1.0) | 5.4 (0.9) | 5.3 (1.0) | 5.4 (1.0) | 5.4 (1.0) | 0.893 |
| HDL‐C, mmol/L | 1.4 (0.5) | 1.3 (0.4) | 1.4 (0.5) | 1.4 (0.5) | 1.4 (0.5) | <0.001 |
| LDL‐C, mmol/L | 3.3 (0.9) | 3.3 (0.9) | 3.3 (0.9) | 3.3 (0.9) | 3.2 (0.9) | 0.158 |
| Triglyceride, mmol/L | 1.6 (0.9) | 1.6 (0.9) | 1.6 (0.9) | 1.6 (1.0) | 1.6 (0.9) | 0.969 |
| Diabetes | 794 (11.2) | 207 (11.7) | 196 (11.1) | 189 (10.7) | 202 (11.4) | 0.794 |
| Hypertension, n (%) | 1718 (24.3) | 391 (22.1) | 376 (21.3) | 418 (23.7) | 533 (30.2) | <0.001 |
| Atrial fibrillation | 17 (0.2) | 3 (0.2) | 3 (0.2) | 5 (0.3) | 6 (0.3) | 0.659 |
| Education level | 0.019 | |||||
| Basic or 0 y | 1138 (16.1) | 263 (14.9) | 262 (17.3) | 306 (17.3) | 307 (17.4) | |
| Intermediate | 3005 (42.5) | 774 (43.8) | 733 (41.5) | 723 (40.9) | 775 (43.9) | |
| Advanced | 2922 (41.4) | 729 (41.3) | 772 (43.7) | 737 (41.7) | 684 (38.7) | |
| Smoking | 0.034 | |||||
| Current smoker | 1237 (17.5) | 279 (15.8) | 290 (16.4) | 318 (18.0) | 350 (19.8) | |
| Former smoker | 2878 (40.7) | 738 (41.8) | 742 (42.0) | 694 (39.3) | 704 (39.9) | |
| Never smoker | 2950 (41.8) | 749 (42.4) | 735 (41.6) | 754 (42.7) | 712 (40.3) | |
| Drinking | 0.006 | |||||
| Current drinker | 3985 (56.4) | 1065 (60.3) | 1001 (56.6) | 957 (54.2) | 962 (54.5) | |
| Former drinker | 1441 (20.4) | 316 (17.9) | 362 (20.5) | 382 (21.6) | 381 (21.6) | |
| Never drinker | 1639 (23.3) | 385 (21.8) | 404 (22.9) | 427 (24.2) | 423 (24.0) | |
| Antihypertensive | 1664 (23.6) | 376 (21.3) | 367 (20.8) | 403 (22.8) | 518 (29.3) | <0.001 |
| Aspirin | 3566 (50.5) | 854 (48.4) | 889 (50.3) | 895 (50.7) | 928 (52.5) | 0.100 |
| Statin | 301 (4.3) | 70 (4.0) | 76 (4.3) | 72 (4.1) | 83 (4.7) | 0.713 |
Continuous variables are presented as mean (SD), and categorical variables are presented as percentage. HDL‐C indicates high density lipoprotein cholesterol; LDL‐C, low density lipoprotein cholesterol; SBP, systolic blood pressure; and VIM, variability independent of the mean.
Association Between SBP Variability Measured by VIM and Cardiovascular Outcomes in Optimal SBP Levels
During a median follow‐up of 19.6 years, 2691 participants developed MACEs: 1973 deaths, 888 HF, 670 CHD, and 388 stroke events. From the first to fourth quartiles of VIM, the cumulative incidences (95% CIs) for MACE increased progressively, from 38.8% (35.9%–41.8%) to 41.9% (37.6%–46.5%) to 41.3% (38.8%–43.9%) and to 45.6% (42.7%–48.7%) (Table 2). In the multivariable‐adjusted model with robust standard errors, the HRs (95% CIs) for MACEs comparing the second through fourth quartiles (VIM quartile 2–4) to the first quartile (VIM quartile 1) were 1.05 (0.94–1.17), 1.12 (1.00–1.24), and 1.21 (1.08–1.35), respectively (P for trend<0.001) (Table 2). In the analyses for secondary outcomes, participants with the highest SBP variability (VIM quartile 4) were associated with a 26% higher risk of all‐cause mortality (HR, 1.26; 95% CI, 1.11–1.44) and a 28% higher risk of HF (HR, 1.28; 95% CI, 1.06–1.55) but not with the risk of CHD (HR, 1.11; 95% CI, 0.90–1.37) and stroke (HR, 1.00; 95% CI, 0.75–1.34), compared with the lowest SBP variability participants (VIM quartile 1) (Table S2).
Table 2.
Risk of MACEs Associated With SBP Variability Measured by VIM in Participants With Optimal SBP Levels
| SBP variability | No. of events/Total No. |
Cumulative incidence % (95% CI) |
Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
Model 3 HR (95% CI) |
|---|---|---|---|---|---|
| VIM quartile 1 | 627/1766 | 38.8 (35.9–41.8) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| VIM quartile 2 | 646/1767 | 41.9 (37.6–46.5) | 1.05 (0.94–1.17) | 1.04 (0.93–1.16) | 1.05 (0.94–1.17) |
| VIM quartile 3 | 683/1766 | 41.3 (38.8–43.9) | 1.11 (1.00–1.24) | 1.10 (0.99–1.22) | 1.11 (1.00–1.24) |
| VIM quartile 4 | 735/1766 | 45.6 (42.7–48.7) | 1.23 (1.10–1.37) | 1.19 (1.07–1.32) | 1.21 (1.08–1.35) |
| P for trend | … | <0.001 | <0.001 | 0.001 | <0.001 |
MACE was defined as the first occurrence of all‐cause mortality, coronary heart disease, stroke, and heart failure. HR indicates hazard ratio; MACE, major adverse cardiovascular event; SBP, systolic blood pressure; and VIM, variability independent of the mean.
Model 1: adjusted for age, sex, and race at visit 3; model 2: adjusted for model 1 + education level, body mass index, smoking status, drinking status, total cholesterol, high‐density lipoprotein cholesterol, prevalent diabetes, and use of aspirin and statins at visit 3; model 3: adjusted for model 2 + prevalent hypertension, use of antihypertensive drugs, diastolic blood pressure at visit 3, and trend and mean of SBP from visit 1 to visit 3.
In the multivariable‐adjusted model that measured SBP variability (VIM) as a continuous rather than a categorical variable, an increase of 3.73 in VIM (corresponding to 1 SD) was also associated with a higher risk of a MACE (HR, 1.06; 95% CI, 1.02–1.10) (Table S3). The restricted cubic spline was conducted to assess the fully adjusted continuous association between SBP variability (VIM) and the risk of a MACE, with the 25th percentile of sample set as the reference point. As expected, the HR for MACEs was relatively linear, with a higher VIM being associated with higher risk (Figure 1).
Figure 1. Multivariable‐adjusted HRs of MACE according to visit‐to‐visit SBP variability measured by VIM in adults with optimal SBP levels.
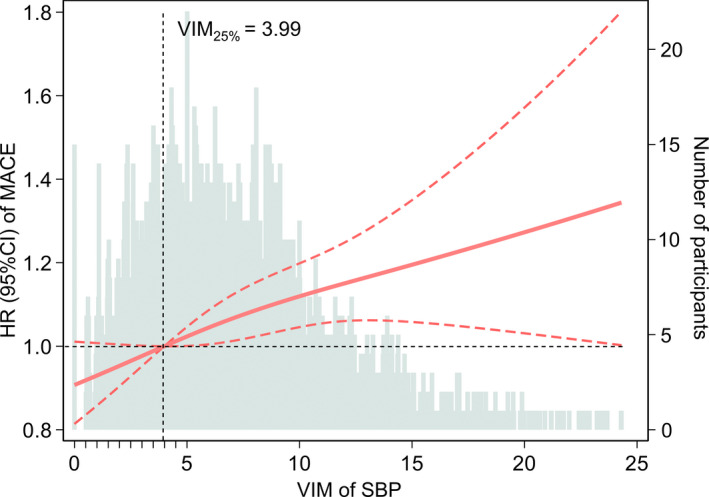
The HRs (orange‐red solid line) and 95% CIs (orange‐red dotted lines) are derived from the Cox model 3 that adjusted for age, sex, race, education level, body mass index, smoking status, alcohol use status, total cholesterol, high‐density lipoprotein cholesterol, prevalent diabetes, use of aspirin and statins, prevalent hypertension, use of antihypertensive drugs, diastolic blood pressure at visit 3, and trend and mean of SBP from visit 1 to visit 3. SBP variability was centered at the 25th percentile of the sample (VIM=3.99) and modeled using a restricted cubic spline with knots at the 5th, 50th, and 95th percentiles. Histograms represent the frequency distribution of SBP variability (VIM). HR indicates hazard ratio; MACE, major adverse cardiovascular event; SBP, systolic blood pressure; and VIM, variability independent of the mean.
Sensitivity Analyses for SBP Variability Measured by SD, CV, or ARV
We performed the sensitivity analyses to determine the association between SBP variability assessed by SD, CV, or ARV and the risk of MACEs. In the 4 groups categorized by the quartiles of SD, CV, or ARV, the cumulative incidences for MACEs also increased progressively from the first to fourth quartiles (Table S4). Consistent with the results of the primary analysis, both the multivariable‐adjusted analyses (Table S4) and restricted cubic splines (Figure S3) revealed the robust association between SBP variability and the risk of MACEs in optimal SBP levels.
Stratified Analyses of Participants With or Without Hypertension
Stratified analyses were conducted to evaluate the effects of hypertension on the association between SBP variability and incident MACE in participants with optimal SBP levels. Compared with the lowest SBP variability participants (VIM quartile 1), participants with the highest SBP variability (VIM quartile 4) were still associated with the higher risk of MACEs in those with hypertension (HR, 1.24; 95% CI, 1.02–1.50) or normotension (HR, 1.17; 95% CI, 1.02–1.34), with a negative interaction (P for interaction=0.558) (Figure 2). Therefore, the association between SBP variability and incident MACEs was similar in the participants with and without hypertension.
Figure 2. Association between visit‐to‐visit SBP variability measured by VIM and MACE in adults with optimal SBP levels and with or without hypertension.
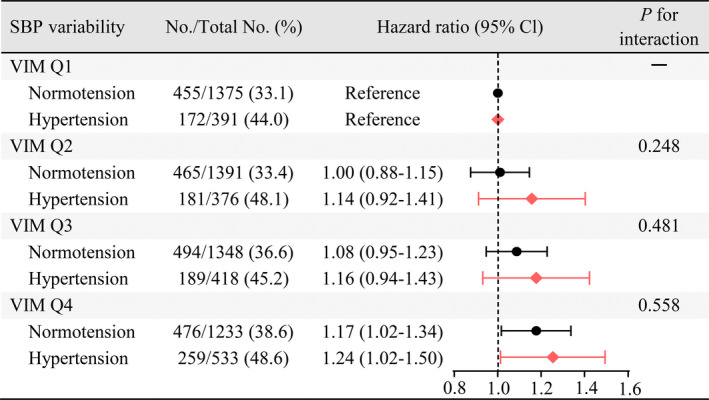
The HRs and 95% CIs were obtained from Cox proportional hazard regression models adjusted for age, sex, race, education level, body mass index, smoking status, alcohol use status, total cholesterol, high‐density lipoprotein cholesterol, prevalent diabetes, use of aspirin and statins, use of antihypertensive drugs, diastolic blood pressure at visit 3, and trend and mean of SBP from visit 1 to visit 3. P for interaction between hypertension and SBP variability (VIM) from the fully adjusted model is also displayed. HR indicates hazard ratio; MACE, major adverse cardiovascular event; Q, quartile; SBP, systolic blood pressure; and VIM, variability independent of the mean.
Discussion
In this prospective community‐based cohort study, including 7065 participants with optimal SBP levels, we found that greater visit‐to‐visit SBP variability was significantly associated with a higher risk of MACEs independent of mean SBP and traditional cardiovascular risk factors after a median follow‐up of almost 20 years. Furthermore, this association remained significant for the participants with or without hypertension. These findings suggest that, for the adults with optimal SBP levels, higher visit‐to‐visit SBP variability over a period of several years is potentially a novel risk factor of residual cardiovascular events. Therefore, lowering visit‐to‐visit SBP variability might still provide additional benefit in terms of reducing residual cardiovascular risk even at the guideline‐recommended optimal SBP levels.
As evidence is firm on the benefits of BP management, current guidelines suggest clear‐cut target BP levels helping clinicians easily manage hypertension in clinical practice. 5 However, the residual cardiovascular risk for the patients with effective BP management is still worthy of note. Several prior cohort studies, including the Framingham Offspring Cohort, UK Biobank cohort, and Korean National Health Insurance Service cohort, have demonstrated that individuals with controlled hypertension showed a markedly higher risk of adverse outcomes than people with normotension. 7 , 8 These findings have shown persistent residual cardiovascular risk despite aggressive BP lowering, leading to efforts to identify determinants of residual cardiovascular risk. To the best of our knowledge, this is the first study to evaluate the association between SBP variability and residual cardiovascular risk. Our results suggest that visit‐to‐visit SBP variability may provide incremental value to evaluate BP‐related residual cardiovascular risk. Therefore, long‐term BP monitoring and attention to the cumulative effect of BP fluctuations may be necessary to robustly assess certain patients with optimal BP levels for their risk of adverse cardiovascular outcomes.
In recent years, BP variability has been recognized as a powerful risk factor in its own right. 21 Two secondary analyses of the CARDIA (Coronary Artery Risk Development in Young Adults) study indicated that greater visit‐to‐visit BP variability was associated with adverse alterations in cardiac structure 22 and higher CVD risk 23 in young adults aged 18 to 30 years. For middle‐aged people, the association between BP variability and CVD risk was still observed in a large cohort of 3 285 684 US veterans 12 and a high‐quality meta‐analysis including 19 observational cohort studies and 17 clinical trial cohorts. 11 According to these findings, higher BP variability is undoubtedly a novel risk factor for cardiovascular events. However, what is confusing is whether BP variability still matters for patients with effective BP management. Our results confirm and strengthen the importance of long‐term SBP variability for health‐related outcomes from the perspective of residual cardiovascular risk. What the present study emphasizes is that it is still necessary to focus on visit‐to‐visit BP variability for patients with effective BP management, although they may be considered to have optimal BP levels. Lowering BP variability might contribute to further improving cardiovascular health for adults with optimal BP levels. Such information is potentially crucial for optimizing clinical and public health strategies toward minimizing the burden of CVD.
A large meta‐analysis including 389 studies has suggested that drug‐class effects on visit‐to‐visit BP variability can account for differences in effects of antihypertensive drugs on the risk of stroke, independently of changes in mean BP level. 24 Therefore, developing clinical strategies for simultaneously lowering BP and BP variability might be essential for long‐term BP management. In the ASCOT‐BPLA (Anglo‐Scandinavian Cardiac Outcomes Trial Blood Pressure Lowering Arm), including 19 257 patients with hypertension and other vascular risk factors, the visit‐to‐visit SBP variability was lower in the amlodipine group than in the atenolol group. 25 The lower SBP variability was also found in participants who received amlodipine compared with those who received chlorthalidone or lisinopril among 24 004 participants from the ALLHAT (Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial). 26 Some systematic reviews also suggested that compared with other classes of antihypertensive medication (eg, angiotensin receptor blockers, angiotensin‐converting enzyme inhibitors, non–loop diuretic drugs and β blockers), calcium channel blockers have a stronger effect in reducing visit‐to‐visit SBP variability. 24 , 27 Our study emphasized residual cardiovascular risk associated with high SBP variability. Therefore, future research is needed to determine whether the calcium channel blockers can reduce residual cardiovascular risk by controlling BP variability. The potential for lowering SBP variability with existing and emerging therapeutic agents holds promise for reducing residual cardiovascular risk in adults with optimal BP levels.
Strengths of this study include the large community‐based cohort with a long follow‐up of almost 20 years and the assessment of visit‐to‐visit SBP variability based on 3 equally spaced medical measurements. Furthermore, our study included the participants who had optimal BP levels from visit 1 to visit 3, not just one BP value.
The current study has several limitations. First, the use, classification, and drug dose of antihypertensive medication and the adherence to antihypertensive therapy might be associated with SBP variability. In this study, it is difficult to evaluate the effects of these associations on results because of a lack of relevant data. However, our results remained consistent even among a subset of participants with normotension who never used antihypertensive medications. Second, there is no consensus on a standard approach to assessing visit‐to‐visit BP variability. 28 In the current study, we used 4 indices to assess SBP variability and found consistent results. Third, although various common confounding factors had been adjusted in Cox proportional hazards models, the effects of residual measured or unmeasured confounders on our results might not be ruled out. Thus, the interpretation of the findings should be cautious. Fourth, although this study included a biracial cohort, it is unclear if the associations between SBP variability and BP‐related residual cardiovascular risk can be extrapolated to Asian, Hispanic, or other racial and ethnic groups. Fifth, because of the analysis of observational data, the findings are unable to establish a causality but are merely hypotheses generating. Therefore, further research is warranted to determine if lowering visit‐to‐visit BP variability would ensure greater benefits in adults with optimal BP levels.
Conclusions
In adults with optimal SBP levels, higher visit‐to‐visit SBP variability was significantly associated with a higher risk of MACEs independent of mean SBP and traditional cardiovascular risk factors regardless of whether they had hypertension. Therefore, to optimize clinical and public health strategies toward minimizing the burden of CVD, it may be necessary to further focus on the visit‐to‐visit SBP variability even at the guideline‐recommended optimal BP levels.
Sources of Funding
The ARIC study is performed as a collaborative trial supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This study is supported by the National Natural Science Foundation of China (81870195 to Dr Liao), and Natural Science Foundation of Guangdong Province (2016A020220007 and 2019A1515011582 to Dr Liao). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures
None.
Supporting information
Tables S1–S4
Figures S1–S3
Acknowledgments
The authors thank the staff and participants of the ARIC study for their significant contributions.
For Sources of Funding and Disclosures, see page 7.
References
- 1. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair‐Rohani H, AlMazroa MA, Amann M, Anderson HR, Andrews KG, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285–292. doi: 10.1161/HYPERTENSIONAHA.119.14240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 4. Blood Pressure Lowering Treatment Trialists’ Collaboration . Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant‐level data meta‐analysis. Lancet. 2021;397:1625–1636. doi: 10.1016/S0140-6736(21)00590-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 6. Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, Flack JM, Carter BL, Materson BJ, Ram CVS, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32:3–15. doi: 10.1097/HJH.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 7. Lieb W, Enserro DM, Sullivan LM, Vasan RS. Residual cardiovascular risk in individuals on blood pressure–lowering treatment. J Am Heart Assoc. 2015;4:e002155. doi: 10.1161/JAHA.115.002155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park S, Han K, Lee S, Kim Y, Lee Y, Kang MW, Park S, Kim YC, Han SS, Lee H, et al. Cardiovascular or mortality risk of controlled hypertension and importance of physical activity. Heart. 2021;107:1472–1479. doi: 10.1136/heartjnl-2020-318193 [DOI] [PubMed] [Google Scholar]
- 9. Grassi G, Bombelli M, Brambilla G, Trevano FQ, Dell'Oro R, Mancia G. Total cardiovascular risk, blood pressure variability and adrenergic overdrive in hypertension: evidence, mechanisms and clinical implications. Curr Hypertens Rep. 2012;14:333–338. doi: 10.1007/s11906-012-0273-8 [DOI] [PubMed] [Google Scholar]
- 10. Takahashi O, Glasziou PP, Perera R, Shimbo T, Fukui T. Blood pressure re‐screening for healthy adults: what is the best measure and interval? J Hum Hypertens. 2012;26:540–546. doi: 10.1038/jhh.2011.72 [DOI] [PubMed] [Google Scholar]
- 11. Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: systematic review and meta‐analysis. BMJ. 2016;354:i4098. doi: 10.1136/bmj.i4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar‐Zadeh K, Kovesdy CP. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol. 2016;68:1375–1386. doi: 10.1016/j.jacc.2016.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diaz KM, Tanner RM, Falzon L, Levitan EB, Reynolds K, Shimbo D, Muntner P. Visit‐to‐visit variability of blood pressure and cardiovascular disease and all‐cause mortality: a systematic review and meta‐analysis. Hypertension. 2014;64:965–982. doi: 10.1161/HYPERTENSIONAHA.114.03903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu M, Chen X, Zhang S, Lin Y, Xiong Z, Zhong X, Guo Y, Sun X, Zhou H, Xu X, et al. Long‐term visit‐to‐visit mean arterial pressure variability and the risk of heart failure and all‐cause mortality. Front Cardiovasc Med. 2021;8:665117. doi: 10.3389/fcvm.2021.665117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 16. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA, The ARIC Investigators . Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0 [DOI] [PubMed] [Google Scholar]
- 18. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061 [DOI] [PubMed] [Google Scholar]
- 19. Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle‐aged adults: 9‐year follow‐up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.STR.30.4.736 [DOI] [PubMed] [Google Scholar]
- 20. Kalani R, Bartz TM, Suchy‐Dicey A, Elkind M, Psaty BM, Leung LY, Rice K, Tirschwell D, Longstreth WJ. Cholesterol variability and cranial magnetic resonance imaging findings in older adults: the cardiovascular health study. Stroke. 2020;51:69–74. doi: 10.1161/STROKEAHA.119.026698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Messerli FH, Hofstetter L, Rimoldi SF, Rexhaj E, Bangalore S. Risk factor variability and cardiovascular outcome: JACC review topic of the week. J Am Coll Cardiol. 2019;73:2596–2603. doi: 10.1016/j.jacc.2019.02.063 [DOI] [PubMed] [Google Scholar]
- 22. Nwabuo CC, Yano Y, Moreira HT, Appiah D, Vasconcellos HD, Aghaji QN, Viera A, Rana JS, Shah RV, Murthy VL, et al. Association between visit‐to‐visit blood pressure variability in early adulthood and myocardial structure and function in later life. Jama Cardiol. 2020;5:795–801. doi: 10.1001/jamacardio.2020.0799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yano Y, Reis JP, Lewis CE, Sidney S, Pletcher MJ, Bibbins‐Domingo K, Navar AM, Peterson ED, Bancks MP, Kanegae H, et al. Association of blood pressure patterns in young adulthood with cardiovascular disease and mortality in middle age. JAMA Cardiol. 2020;5:382–389. doi: 10.1001/jamacardio.2019.5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive‐drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta‐analysis. Lancet. 2010;375:906–915. doi: 10.1016/S0140-6736(10)60235-8 [DOI] [PubMed] [Google Scholar]
- 25. Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlöf B, Poulter NR, Sever PS. Effects of beta blockers and calcium‐channel blockers on within‐individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–480. doi: 10.1016/S1474-4422(10)70066-1 [DOI] [PubMed] [Google Scholar]
- 26. Muntner P, Levitan EB, Lynch AI, Simpson LM, Whittle J, Davis BR, Kostis JB, Whelton PK, Oparil S. Effect of chlorthalidone, amlodipine, and lisinopril on visit‐to‐visit variability of blood pressure: results from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. J Clin Hypertens (Greenwich). 2014;16:323–330. doi: 10.1111/jch.12290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kollias A, Stergiou GS, Kyriakoulis KG, Bilo G, Parati G. Treating visit‐to‐visit blood pressure variability to improve prognosis: is amlodipine the drug of choice? Hypertension. 2017;70:862–866. doi: 10.1161/HYPERTENSIONAHA.117.10087 [DOI] [PubMed] [Google Scholar]
- 28. Yano Y. Visit‐to‐visit blood pressure variability‐what is the current challenge? Am J Hypertens. 2017;30:112–114. doi: 10.1093/ajh/hpw124 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S3


