Abstract
Background
This study aimed to evaluate the cardiovascular risk and outcomes after lipid reduction in patients with severe hypercholesterolemia using a nationwide cohort.
Methods and Results
This study used the database from the National Health Insurance Service of Korea. Among individuals who underwent regular health examination and follow‐up, 2 377 918 were enrolled and categorized into 3 groups with severe hypercholesterolemia according to low‐density lipoprotein cholesterol (LDL‐C) levels, namely, ≥260, 225 to 259, and 190 to 224 mg/dL groups, and a control group (<160 mg/dL). Risks of composite cardiovascular events (myocardial infarction, coronary revascularization, and ischemic stroke) and total mortality were compared. In statin new users, the outcomes after statin use were further analyzed according to posttreatment LDL‐C levels. The prevalence of individuals with LDL‐C≥190 mg/dL was 1 of 106. Adjusted hazard ratios of composite events and total mortality (median follow‐up, 6.1 years) in the groups ranged up to 2.4 (log‐rank P<0.0001) and 2.3 (log‐rank P=0.0002), respectively, and were dependent on LDL‐C levels. The risks of each event were up to 4.1‐, 3.8‐, and 1.9‐fold higher, respectively, in these groups. The risk of composite events (median follow‐up, 6.2 years) was lower after lipid lowering; particularly, the risk was lowest in the group showing LDL‐C<100 mg/dL after treatment (hazard ratio, 0.56, log‐rank P=0.043).
Conclusions
Using large Korean cohort data, our study proved incrementally elevated cardiovascular risk and clinical benefit associated with LDL‐C<100 mg/dL in individuals with severe hypercholesterolemia. These results support aggressive lipid lowering and provide evidence for the LDL‐C target in this population.
Keywords: Asian continental ancestry group, atherosclerosis, health care, lipoproteins, LDL, outcome assessment
Subject Categories: Primary Prevention, Coronary Artery Disease, Risk Factors
Nonstandard Abbreviations and Acronyms
- FH
familial hypercholesterolemia
- NHIS
National Health Insurance Service
Clinical Perspective
What Is New?
Incremental risk elevation and its dependency on low‐density lipoprotein cholesterol levels were demonstrated in severe hypercholesterolemia by large‐scale analysis.
A significant association between ischemic stroke and severe hypercholesterolemia was observed.
Lower risk was observed more clearly when low‐density lipoprotein cholesterol <100 mg/dL was achieved after statin therapy in these patients.
What Are the Clinical Implications?
By providing evidence on vascular risk and target low‐density lipoprotein cholesterol levels from large cohort data, this study strongly helps clinicians to choose more aggressive lipid‐lowering therapy in this population.
Severe hypercholesterolemia, frequently accompanying familial hypercholesterolemia (FH), has been spotlighted for its global clinical burden. 1 Many academic societies recently concentrate on it to promote its awareness and improve timely diagnosis and treatment; however, the results remain insufficient. 2 The risk of atherosclerotic cardiovascular disease (ASCVD) is ≈3 times higher in these individuals than in the general population, 3 although the data were inconsistent in different studies. Because elevated low‐density lipoprotein cholesterol (LDL‐C) is the major contributor to ASCVD development 2 in this population, special attention to its severe form is suggested. 4
Since the early 2010s, guidelines on lipid‐lowering therapy have been revised in many areas worldwide. 5 , 6 , 7 Along with the introduction of new lipid‐modifying agents, such as proprotein convertase subtilisin/kexin type 9 inhibitors, 8 some guidelines have lowered target LDL‐C levels, 5 , 7 European and American guidelines recommended LDL‐C <70 mg/dL for high‐risk groups and the former advised LDL‐C <55 mg/dL for very‐high‐risk individuals. Furthermore, European guidelines classified patients with FH into specific risk groups according to the presence of other cardiovascular conditions. 7 This called attention to the clinical importance of patients with FH and appropriate lipid management for them.
To date, few studies have evaluated the effect of lipid‐lowering therapy, mostly by statins, on cardiovascular risk or mortality in patients with severe hypercholesterolemia. 9 , 10 Although they provided evidence to support lipid management in this population, it is insufficient to suggest specific target LDL‐C levels in these individuals. This study aimed to evaluate the prevalence, cardiovascular risk, and total mortality of individuals with severe hypercholesterolemia. In addition, the effect associated with lipid‐lowering therapy on the risks was analyzed in a subgroup receiving statins. A large nationwide cohort of >2 million individuals, >20 000 with LDL‐C≥190 mg/dL, including >10 000 statin users, was enrolled and followed up.
Methods
The present study was performed according to the Declaration of Helsinki and approved by the Institutional Review Board of Severance Hospital (4‐2019‐0516) and National Health Insurance Service (NHIS) of Korea (NHIS‐2020‐4‐160). The NHIS database has been made for wider use of the health screening data with anonymous and deidentified information. Therefore, the requirement for informed consent was waived. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data Source and Study Population
This nationwide population‐based cohort study used the database from the NHIS of Korea, 11 , 12 containing demographic data, diagnoses encoded by the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10), use of in‐ and outpatient services, pharmacy claims, and mortality. The NHIS of Korea provides a biennial health examination for all Korean adults aged ≥20 years. This includes self‐questionnaires on medical history, physical examinations, and blood tests, such as complete blood counts and cholesterol profile. Health examination results are included in the NHIS database anonymously.
The study enrollment flow is presented in Figure 1. Subjects who underwent the first NHIS health examination between January and December 2009 were screened. Among them, those who underwent >1 follow‐up health examination were identified. The last follow‐up occurred in December 2018. The exclusion criteria were prior cardio‐ or cerebrovascular disease or coronary revascularization, prior statin use, no timely follow‐up health examination, missing laboratory values, suspicious errors in cholesterol levels, starting statins at 0‐ to 1‐year follow‐up, or death or cardio‐/cerebrovascular events in <1‐year follow‐up. Finally, 2 377 918 individuals were enrolled: 2 261 332 statin nonusers and 116 586 statin new users.
Figure 1. Flow of patient enrollment.
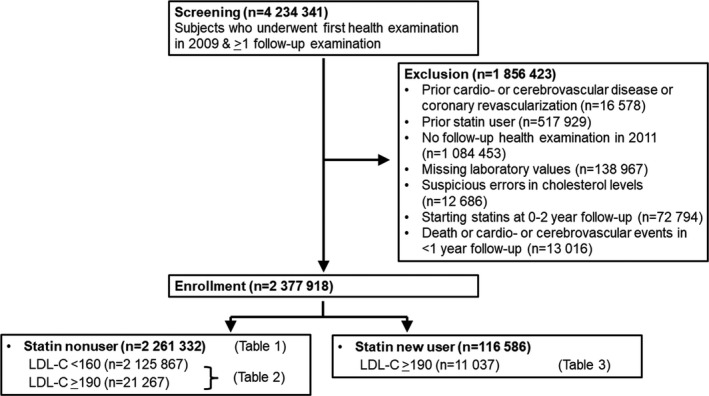
LDL‐C indicates low‐density lipoprotein cholesterol.
The study subjects were categorized by LDL‐C levels, which are as follows: ≥260, 225 to 259, 190 to 224, 160 to 189, and <160 mg/dL. The 260 mg/dL level is from the American Make Early Diagnosis to Prevent Early Death criteria for people with FH aged ≥40 years, 13 which is the age range of most individuals in our cohort. The 225‐mg/dL level is from the LDL‐C threshold for carriers of putative FH‐associated mutations from our previous analysis. 14 The 190‐mg/dL level is from the cutoff value of severe hypercholesterolemia in the latest American guidelines for lipid‐lowering therapy. 5 Because LDL‐C ≥160 mg/dL is considered “high,” 15 we used individuals with LDL‐C <160 mg/dL as a reference group.
Variables and Definitions
Subjects’ demographic information, including age and sex, and cardiovascular risk factors are summarized in Table 1. Diabetes, hypertension, and body mass index were assessed as baseline characteristics. Subjects’ smoking status was assessed on the basis of self‐questionnaires. Blood samples for the lipid profiles were obtained after overnight fasting, and the levels were measured using an enzymatic method. Diabetes and hypertension were defined as previous diagnoses (ICD‐10 codes) and use of ≥1 antidiabetic or antihypertensive agent, respectively.
Table 1.
Clinical Characteristics of the Patient Groups Classified by LDL‐C Levels Without Statin Therapy
| Variables | Patient group by LDL‐C levels, mg/dL (N=2 261 332) | P value | ||||
|---|---|---|---|---|---|---|
|
≥260 (N=487; 0.02%) |
225–259 (N=1936; 0.09%) |
190–224 (N=18 844; 0.83%) |
160–189 (N=114 198; 5.1%) |
<160 (N=2 125 867; 94%) |
||
| Age, y | ||||||
| 20–39 | 172 (33.3) | 561 (29.0) | 5044 (26.8) | 29 359 (25.7) | 761 720 (35.8) | <0.0001 |
| 40–49 | 128 (26.8) | 499 (25.8) | 5073 (26.9) | 32 539 (28.5) | 592 368 (27.9) | |
| 50–59 | 111 (22.8) | 517 (26.7) | 5264 (27.9) | 31 940 (28.0) | 441 218 (20.8) | |
| >60 | 76 (15.6) | 359 (18.5) | 3463 (18.4) | 20 360 (17.8) | 330 561 (15.6) | |
| Male sex | 283 (58.1) | 1067 (55.1) | 11 303 (60.0) | 70 057 (61.4) | 1 278 196 (60.1) | <0.0001 |
| Medical history | ||||||
| Diabetes | 61 (12.5) | 174 (9.0) | 1287 (6.8) | 5971 (5.2) | 109 350 (5.1) | <0.0001 |
| Hypertension | 117 (24.0) | 406 (21.0) | 4107 (21.8) | 23 074 (20.2) | 394 907 (18.6) | <0.0001 |
| Current smoker | 158 (32.4) | 346 (28.2) | 5597 (29.7) | 31 702 (27.8) | 581 068 (27.3) | <0.0001 |
| Body mass index, kg/m2 | 24.8±3.5 | 25.0±3.3 | 24.9±3.1 | 24.6±3.1 | 23.5±3.2 | <0.0001 |
| Lipid profile, mg/dL | ||||||
| Total cholesterol | 398±106 | 320±22 | 282±19 | 250±17 | 188±29 | <0.0001 |
| Triglyceride | 138 (95–204) | 135 (99–184) | 128 (94–174) | 119 (87–162) | 103 (71–154) | <0.0001 |
| HDL‐C | 57.6±44.9 | 55.1±14.2 | 54.0±17.0 | 53.8±17.6 | 55.6±21.1 | <0.0001 |
| LDL‐C | 308±82 | 236±9 | 201±9 | 170±8 | 107±26 | <0.0001 |
| Antiplatelet agent | 17 (3.5) | 61 (3.2) | 684 (3.6) | 4430 (3.9) | 102 690 (4.8) | <0.0001 |
Data are presented as number (%), mean±SD, or median (interquartile range) unless defined otherwise. HDL‐C indicates high‐density lipoprotein‐cholesterol; and LDL‐C, low‐density lipoprotein‐cholesterol.
The primary end point was a composite of incident myocardial infarction (MI), coronary revascularization, and ischemic stroke. The secondary end points were each component of the primary end point and total mortality. MI was defined by ICD‐10 codes (121–122) during hospitalization or these diagnostic codes documented at least twice in the outpatient records. Coronary revascularization included percutaneous coronary intervention and coronary artery bypass graft. Percutaneous coronary intervention was defined as procedure codes M655*‐M657* in NHIS, and coronary artery bypass graft was defined as the codes OA631*‐OA639*, OB631*‐OB639*, OA641*, OA642*, O0161*‐O0171*, and O1641*‐O1647*. Ischemic stroke was defined by ICD‐10 codes during hospitalization plus claims for brain imaging studies, including magnetic resonance imaging and computed tomography. Total mortality was defined as those included in the NHIS linked to mortality data provided by Statistics Korea. 16 The study population was followed up until the date of vascular events or death.
Statistical Analysis
Continuous variables were tested for normality using the Shapiro‐Wilk normality test. Variables with normal distribution are presented as mean±SD, those with skewed distribution as median (interquartile ranges), and categorical variables as numbers (percentages). For between‐group comparisons, Student’s t test and the chi‐square test were used for continuous and categorical variables, respectively.
Cox proportional hazards models were used to evaluate the association between categorized baseline LDL‐C levels and risk of composite events and total mortality. Hazard ratios (HRs) and 95% CIs were calculated in an unadjusted model 1 and adjusted for covariates. Eight prespecified potential confounders were adjusted in model 2, which are as follows: age, sex, body mass index, diabetes, hypertension, smoking, triglycerides, and antiplatelet agents. The trend of risk was presented as Kaplan‐Meier curves and compared using the log‐rank test. Risk of events and mortality was compared between the population with baseline LDL‐C <160 mg/dL and those with baseline LDL‐C >190 mg/dL. Individuals in the latter group who started statin therapy were further analyzed. Finally, the risk of events and mortality was analyzed according to posttreatment LDL‐C levels as <100, 100 to 129, 130 to 159, and ≥160 mg/dL.
Results
Baseline Characteristics
Of all individuals included, 2 261 332 did not undergo statin therapy. Among them, the percentages of individuals with LDL‐C ≥260, ≥225, and ≥190 mg/d were 0.02%, 0.11%, and 0.94%, respectively (Table 1), which translates to 1 of 5000, 909, and 106 individuals, respectively. Individuals aged 20 to 39 years were more common in the LDL‐C ≥260 mg/dL group than in the LDL‐C 190 to 259 mg/dL group, whereas those aged >60 years were less common in the former group (Table 1). Frequencies of diabetes, hypertension, and current smoker were higher in groups with higher LDL‐C levels.
Cardiovascular Risk and Total Mortality in Individuals With Different LDL‐C Levels
During follow‐up (median, 6.1 years), the rates of composite events/1000 person‐years were 5.91, 4.68, 4.08, and 2.06 in the LDL‐C >260, 225 to 259, 190 to 224, and <160 mg/dL (reference) groups, respectively. Unadjusted HRs of composite events in the first 3 groups were 2.90, 2.30, and 2.01, respectively. When adjusted by prespecified confounding factors, HRs were 2.40, 2.04, and 1.79, respectively, which were significantly higher than that of the reference group (P<0.0001) (Table 2).
Table 2.
Risk of Composite Cardiovascular Events and Total Mortality in the Patient Groups Classified by LDL‐C Levels Without Statin Therapy
| Variables | LDL‐C, mg/dL | Number of patients | Events | Duration, person‐year | Rate, /1000 person‐year |
HR (95% CI) (Model 1) |
P value |
HR (95% CI) (Model 2) |
P value |
|---|---|---|---|---|---|---|---|---|---|
| Composite events | ≥260 | 487 | 13 | 2198 | 5.91 | 2.90 (1.69–5.00) | <0.0001 | 2.40 (1.39–4.13) | <0.0001 |
| 225–259 | 1936 | 40 | 8551 | 4.68 | 2.30 (1.69–5.14) | 2.04 (1.50–2.78) | |||
| 190–224 | 18 844 | 354 | 86 679 | 4.08 | 2.01 (1.81–2.23) | 1.79 (1.61–1.99) | |||
| <160 | 2 125 867 | 26 118 | 12 672 647 | 2.06 | 1 | 1 | |||
| MI | ≥260 | 487 | 6 | 2201 | 2.73 | 4.93 (2.22–10.95) | <0.0001 | 4.06 (1.83–9.00) | <0.0001 |
| 225–259 | 1936 | 16 | 8563 | 2.22 | 4.01 (2.56–6.29) | 3.41 (2.17–5.35) | |||
| 190–224 | 18 844 | 135 | 86 761 | 1.56 | 2.81 (2.37–3.33) | 2.42 (2.04–2.88) | |||
| <160 | 2 125 867 | 7203 | 12 688 468 | 0.57 | 1 | 1 | |||
| Coronary revascularization | ≥260 | 487 | 6 | 2202 | 2.73 | 4.09 (1.84–9.11) | <0.0001 | 3.43 (1.54–7.64) | <0.0001 |
| 225–259 | 1936 | 24 | 8564 | 2.80 | 4.22 (2.82–6.29) | 3.76 (2.52–5.62) | |||
| 190–224 | 18 844 | 171 | 86 775 | 1.97 | 2.96 (2.55–3.45) | 2.67 (2.29–3.11) | |||
| <160 | 2 125 867 | 8529 | 12 692 356 | 0.67 | 1 | 1 | |||
| Ischemic stroke | ≥260 | 487 | 6 | 2199 | 2.73 | 2.35 (1.06–5.23) | <0.0001 | 1.89 (0.85–4.20) | <0.0001 |
| 225–259 | 1936 | 16 | 8554 | 1.87 | 1.61 (0.99–2.63) | 1.44 (0.88–2.35) | |||
| 190–224 | 18 844 | 163 | 96 714 | 1.88 | 1.62 (1.38–1.89) | 1.44 (1.23–1.68) | |||
| <160 | 2 125 867 | 14 918 | 12 679 185 | 1.18 | 1 | 1 | |||
| Total mortality | ≥260 | 487 | 15 | 2201 | 6.81 | 2.36 (1.42–3.91) | <0.0001 | 2.25 (1.35–3.73) | 0.0002 |
| 225–259 | 1936 | 30 | 8565 | 3.50 | 1.21 (0.85–1.73) | 1.25 (0.88–1.79) | |||
| 190–224 | 18 844 | 279 | 86 788 | 3.21 | 1.11 (0.99–1.25) | 1.11 (0.99–1.25) | |||
| <160 | 2 125 867 | 37 663 | 12 693 956 | 2.97 | 1 | 1 |
Composite events: MI, coronary revascularization, or ischemic stroke. Model 1: unadjusted. Model 2: adjusted for age, sex, body mass index, diabetes, hypertension, smoking, triglycerides, antiplatelet agents.
P values are from the Wald test for HRs of patient groups. HR indicates hazard ratio; LDL‐C, low‐density lipoprotein‐cholesterol; and MI, myocardial infarction.
Adjusted HRs of each event component in the first 3 groups were 2.42 to 4.06 for MI (P<0.0001), 2.67 to 3.76 for coronary revascularization (P<0.0001), and 1.44 to 1.89 for ischemic stroke, respectively (P<0.0001). Adjusted HR of total mortality in the first 3 groups was 1.11 to 2.25 (P=0.0002) (Table 2). The Kaplan‐Meier curves for the composite events, each event component, and total mortality are presented in Figure 2.
Figure 2. Kaplan‐Meier curves for clinical outcomes according to hyper–LDL‐cholesterolemia severity in individuals with LDL‐C≥190 mg/dL.
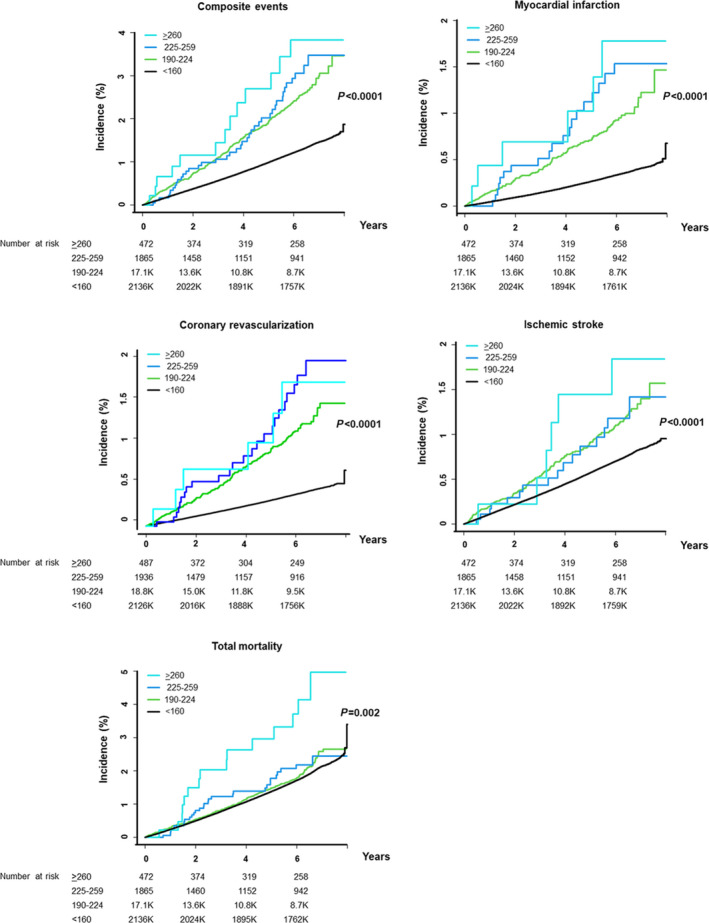
Clinical outcomes include composite events, each component of the events, and total mortality. LDL‐C indicates low‐density lipoprotein cholesterol.
Benefit Associated With Statin Therapy According to Post‐Treatment LDL‐C Levels
Among the study population receiving statin therapy, those with LDL‐C≥190 mg/dL and followed up ≥1 year with laboratory work‐up (n=11 037) were analyzed for composite events and total mortality. During follow‐up (median 6.2 years), the rates of composite events/1000 person‐years in individuals with posttreatment LDL‐C <100, 100 to 129, 130 to 159, and ≥160 mg/dL (the reference group) were 4.88, 6.81, 4.90, and 6.40, respectively. The risk in individuals achieving <70 mg/dL was not separately analyzed as their proportion was only 2.9%. Unadjusted HRs of composite events in the first 3 groups were 0.75, 1.05, and 0.76, respectively, whereas when adjusted for confounding factors, the HRs were 0.56, 0.84, and 0.69, respectively (P=0.0007) (Table 3). Adjusted HRs of each event component in these groups were 0.71 to 0.97 for MI (P=0.69), 0.64 to 0.74 for coronary revascularization (P=0.058), and 0.51 to 1.00 for ischemic stroke (P=0.0056). Adjusted HRs of total mortality in these groups were 0.81 to 0.96 (P=0.53) (Table 3). In log‐rank tests, the risks of composite events (log‐rank P=0.043) and ischemic stroke (log‐rank P=0.014) were significantly lower in the group achieving <100 mg/dL than in the reference group.
Table 3.
Effect of Lipid‐Lowering Therapy Intensity and Posttreatment LDL‐C on Composite Cardiovascular Events in the Population With LDL‐C ≥190 mg/dL but Without ASCVD
| Variables | Posttreatment LDL‐C, mg/dL | Number of patients | Events | Duration, person‐years | Rate per 1000 person‐years | HR (95% CI) (Model 1) | P value | HR (95% CI) (Model 2) | P value |
|---|---|---|---|---|---|---|---|---|---|
| Composite events | <100 | 1975 | 56 | 11 485 | 4.88 | 0.75 (0.56, 1.02) | 0.044 | 0.56 (0.41–0.76) | 0.0007 |
| 100–129 | 2111 | 92 | 13 515 | 6.81 | 1.05 (0.82–1.35) | 0.85 (0.66–1.09) | |||
| 130–159 | 2448 | 77 | 15 724 | 4.90 | 0.76 (0.58–0.99) | 0.69 (0.53–0.91) | |||
| >160 | 4685 | 192 | 29 720 | 6.46 | 1 | 1 | |||
| MI | <100 | 1975 | 16 | 11 603 | 1.38 | 0.83 (0.47–1.46) | 0.86 | 0.71 (0.40–1.26) | 0.69 |
| 100–129 | 2111 | 25 | 13 707 | 1.82 | 1.09 (0.68–1.77) | 0.97 (0.59–1.58) | |||
| 130–159 | 2448 | 26 | 15 851 | 1.64 | 0.99 (0.62–1.59) | 0.93 (0.58–1.49) | |||
| >160 | 4685 | 50 | 30 188 | 1.66 | 1 | 1 | |||
| Coronary revascularization | <100 | 1793 | 35 | 11 546 | 3.03 | 0.87 (0.59–1.28) | 0.58 | 0.64 (0.43–0.95) | 0.058 |
| 100–129 | 2111 | 40 | 13 660 | 2.93 | 0.84 (0.58–1.21) | 0.68 (0.47–0.99) | |||
| 130–159 | 2448 | 44 | 15 792 | 2.79 | 0.80 (0.56–1.14) | 0.74 (0.52–1.06) | |||
| >160 | 4685 | 104 | 29 979 | 3.47 | 1 | 1 | |||
| Ischemic stroke | <100 | 1975 | 21 | 11 575 | 1.81 | 0.70 (0.43–1.14) | 0.016 | 0.51 (0.31–0.83) | 0.0056 |
| 100–129 | 2111 | 45 | 13 642 | 3.30 | 1.27 (0.88–1.84) | 1.00 (0.68–1.45) | |||
| 130–159 | 2448 | 26 | 15 858 | 1.64 | 0.63 (0.41–0.99) | 0.57 (0.37–0.90) | |||
| >160 | 4685 | 78 | 30 134 | 2.59 | 1 | 1 | |||
| Total mortality | <100 | 1975 | 59 | 11 636 | 5.07 | 1.30 (0.95–1.78) | 0.35 | 0.81 (0.58–1.11) | 0.53 |
| 100–129 | 2111 | 65 | 13 764 | 4.72 | 1.21 (0.89–1.63) | 0.85 (0.62–1.15) | |||
| 130–159 | 2448 | 67 | 15 914 | 4.21 | 1.08 (0.80–1.46) | 0.96 (0.71–1.29) | |||
| >160 | 4685 | 118 | 30 356 | 3.89 | 1 | 1 |
Composite events: MI, coronary revascularization, or ischemic stroke. ASCVD indicates atherosclerotic cardiovascular disease; Model 1: unadjusted. Model 2: adjusted for age, sex, body mass index, diabetes, hypertension, smoking, triglycerides, LDL‐C, and antiplatelet agents.
P values are from the Wald test for HRs of patient groups. R, hazard ratio; LDL‐C, low‐density lipoprotein‐cholesterol; and MI, myocardial infarction.
Discussion
The major findings of this study were (1) the prevalence of individuals with severe LDL‐C elevation was estimated up to 1 of 106 but varied according to LDL‐C cutoffs; (2) adjusted HRs of composite cardiovascular events and total mortality reached up to 2.40 and 2.25, respectively, which were dependent on cutoff LDL‐C levels. The risks of coronary and cerebrovascular events were also higher in individuals with this phenotype; and (3) in the group with statin therapy, HR of composite events was as low as 0.56, and the risk was lower in the group achieving LDL‐C <100 mg/dL. HR of total mortality was as low as 0.80. Although general association between high LDL‐C and cardiovascular risk has been well reported, the current study is remarkable to analyze the risk in the groups with LDL‐C ≥225 mg/dL and ≥260 mg/dL using a large‐scale analysis. Furthermore, this study suggested the clinical benefits associated with an LDL‐C level <100 mg/dL after statin use in this population.
We estimated the prevalence of severe hypercholesterolemia only from individuals without statin use. Because we excluded patients who received statins before or after enrollment, a substantial portion of them are likely to have elevated cholesterol levels. Therefore, it is possible that our calculation in the study population could have underestimated the real prevalence of this phenotype.
In this study, HRs of composite events in patients with severe hypercholesterolemia not receiving statin therapy were 1.79 to 2.40 compared with the general population. The risks of composite events and total mortality were incrementally higher according to LDL‐C levels, even in individuals with LDL‐C ≥190 mg/dL. This finding confirms the concept of greater cardiovascular risk by exposure of the arteries to higher cholesterol loading. 17 Adjusted risks of MI and coronary revascularization in our study were 2.4 to 4.1 and 2.7 to 4.5, respectively, which are in accordance with prior studies that analyzed coronary heart disease–associated events: coronary heart disease mortality of patients with FH enrolled in the Simon Broome register was 3.2‐fold higher than that of the general population in the prestatin era. 3 A Norwegian study showed that the risks of events related to acute MI or coronary heart disease were 4.2‐ and 4.7‐fold higher in men and women with FH. 18 An American study reported 6‐ to 22‐fold higher risk of coronary artery disease in individuals with LDL‐C >190 mg/dL. 19 However, that analysis used population with LDL‐C <130 mg/dL, whereas ours used those with LDL‐C <160 mg/dL as controls. In that study, the coronary risk was ≈42% lower in individuals with LDL‐C <130 mg/dL than in those with LDL‐C 130 to 159 mg/L. Accordingly, different control groups might have over‐ or underestimated the risk of those with LDL‐C ≥190 mg/dL.
To our best knowledge, this is the first study to identify the higher risk of future ischemic stroke in a population with FH‐compatible cholesterol levels without underlying ASCVD using large‐scale analysis. To date, the results on the relationship between these LDL‐C levels and ischemic stroke have been inconsistent. In the Copenhagen General Population Study, no difference was noted in the risk of ischemic stroke between participants with FH mutations and mutation‐negative individuals. 20 However, that study had a limitation of including a small number of participants with mutations. In addition, the Spanish Familial Hypercholesterolemia Cohort Study from Spain that analyzed 2752 patients with FH mutations reported no difference in cerebrovascular event risk compared with controls 21 ; the cross‐sectional design of the study might have been a limitation. However, our study showed elevated ischemic stroke risk in a much larger number of individuals with severe hypercholesterolemia using longitudinal analysis.
The HR of total mortality in our study population with LDL‐C ≥190 mg/dL was 1.15–2.25, which was slightly lower than that reported in a previous Korean study that included participants as per the modified Make Early Diagnosis to Prevent Early Death criteria. 22 Although the reason for the difference is uncertain, it may partly depend on different inclusion criteria or the control group. In our study, the inclusion of individuals without prior ASCVD might have lowered the death rate and a slightly higher LDL‐C cut‐off level for the control group might have affected the relative risk.
Achievement rates of LDL‐C target levels, such as <70 or <100 mg/dL, were very low in our data: 2.9% and 16.2%, respectively. A previous Korean study enrolling patients with FH revealed the rate of 28% for achieving <100 mg/dL, and their treatment protocol might have led to better results 23 than the real‐world data in the present study. A study from the Netherlands showed a rate of 21% in achieving LDL‐C <100 mg/dL, similar to ours, 24 while another from Spain reported the rate of 11%. 21 Achievement rates may be improved when proprotein convertase subtilisin/kexin type 9 inhibitors are more widely used in the near future.
Our study has potential limitations. The primary outcome variable was composite events, including MI, coronary revascularization, and ischemic stroke. However, data on cardiovascular death were not available. Although composite events similar to those used in this study have been commonly used previously, data on cardiovascular death might have strengthened our study’s clinical power. Cardiovascular risk elevation was less obvious in our population compared with some of previous studies. As mentioned earlier, this might have been influenced by the reference group in our study with LDL‐C <160 mg/dL; this was higher than the level in the other groups and could have attenuated the risk of individuals with severe hypercholesterolemia. However, our reference group could better represent the general population and contribute to more appropriate risk estimation for the patient group. In addition, individuals with posttreatment LDL‐C <70 mg/dL were not analyzed because of the small sample size. In this regard, it is difficult to evaluate the risk of individuals showing LDL‐C <70 mg/dL or <55 mg/dL after statin use in the present study.
In conclusion, the estimated prevalence of severe hypercholesterolemia was 1 of 106 in this cohort including only statin‐naïve individuals. The cardiovascular risk was higher in individuals with severe hypercholesterolemia, incrementally in those with higher LDL‐C levels within the population. Lower risk in individuals receiving statin therapy, particularly those showing LDL‐C <100 mg/dL, in this large‐scale analysis supports aggressive lipid lowering and provides evidence for LDL‐C target in this population.
Sources of Funding
This research was financially supported by the Korean Society of Lipid and Atherosclerosis and the National Research Foundation grant funded by the Korean government (2019R1F1A1057952). The sponsors had no involvement in study design, collection, analysis and interpretation of data, writing of the report, and the decision to submit the article for publication.
Disclosures
None.
Acknowledgments
Author contributions: Dr C.J. Lee contributed to the investigation, methodology, and writing of the original manuscript. S. Park contributed to the data curation, formal analysis, investigation, visualization, and writing of the original manuscript. Dr Han contributed to the conceptualization, formal analysis, investigation, methodology, supervision, and writing—review and editing. Dr S.H. Lee contributed to the conceptualization, funding acquisition, methodology, project administration, supervision, and writing of the original manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Kyungdo Han, Email: hkd0917@naver.com.
Sang‐Hak Lee, Email: shl1106@yuhs.ac.
References
- 1. Representatives of the Global Familial Hypercholesterolemia Community ; Wilemon KA, Patel J, Aguilar‐Salinas C, Ahmed CD, Almahmeed MAW, Alonso R, Al‐Rasadi K, Badimon L, Bernal LM, Bogsrud MP Reducing the clinical and public health burden of familial hypercholesterolemia: a global call to action. JAMA Cardiol. 2020;5:217–229. doi: 10.1001/jamacardio.2019.5173 [DOI] [PubMed] [Google Scholar]
- 2. Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Humphries SE, Cooper JA, Seed M, Capps N, Durrington PN, Jones B, McDowell IFW, Soran H, Neil HAW; Simon Broome Familial Hyperlipidaemia Register Group . Coronary heart disease mortality in treated familial hypercholesterolaemia: update of the UK Simon Broome FH register. Atherosclerosis. 2018;274:41–46. doi: 10.1016/j.atherosclerosis.2018.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Santos RD, Gidding SS, Hegele RA, Cuchel MA, Barter PJ, Watts GF, Baum SJ, Catapano AL, Chapman MJ, Defesche JC, et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 2016;4:850–861. doi: 10.1016/S2213-8587(16)30041-9 [DOI] [PubMed] [Google Scholar]
- 5. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139:E1082–E1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhee E‐J, Kim HC, Kim JH, Lee EY, Kim BJ, Kim EM, Song Y, Lim JH, Kim HJ, Choi S, et al. 2018 guidelines for the management of dyslipidemia in Korea. J Lipid Atheroscler. 2019;8:78–131. doi: 10.12997/jla.2019.8.2.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi: 10.1016/j.atherosclerosis.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 8. Choi JY, Na JO. Pharmacological strategies beyond statins: ezetimibe and PCSK9 inhibitors. J Lipid Atheroscler. 2019;8:183–191. doi: 10.12997/jla.2019.8.2.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benn M, Watts GF, Tybjaerg‐Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol‐lowering medication. J Clin Endocrinol Metab. 2012;97:3956–3964. doi: 10.1210/jc.2012-1563 [DOI] [PubMed] [Google Scholar]
- 10. Besseling J, Hovingh GK, Huijgen R, Kastelein JJP, Hutten BA. Statins in familial hypercholesterolemia: consequences for coronary artery disease and all‐cause mortality. J Am Coll Cardiol. 2016;68:252–260. doi: 10.1016/j.jacc.2016.04.054 [DOI] [PubMed] [Google Scholar]
- 11. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, Park JY, Lee KU, Ko KS, Lee BW. Background and data configuration process of a nationwide population‐based study using the Korean national health insurance system. Diabetes Metab J. 2014;38:395–403. doi: 10.4093/dmj.2014.38.5.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang Y, Han K, Park SH, Kim MK, Yoon KH, Lee SH. High‐density lipoprotein cholesterol and the risk of myocardial infarction, stroke, and cause‐specific mortality: a nationwide cohort study in Korea. J Lipid Atheroscler. 2021;10:74–87. doi: 10.12997/jla.2021.10.1.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hovingh GK, Davidson MH, Kastelein JJ, O’Connor AM. Diagnosis and treatment of familial hypercholesterolaemia. Eur Heart J. 2013;34:962–971. doi: 10.1093/eurheartj/eht015 [DOI] [PubMed] [Google Scholar]
- 14. Shin DG, Han SM, Kim DI, Rhee M‐Y, Lee B‐K, Ahn YK, Cho BR, Woo J‐T, Hur S‐H, Jeong J‐O, et al. Clinical features of familial hypercholesterolemia in Korea: predictors of pathogenic mutations and coronary artery disease ‐ a study supported by the Korean Society of Lipidology and Atherosclerosis. Atherosclerosis. 2015;243:53–58. doi: 10.1016/j.atherosclerosis.2015.08.033 [DOI] [PubMed] [Google Scholar]
- 15. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 16. Sung J, Hong KP. Descriptive study on the Korean status of percutaneous coronary intervention using National Health Insurance Service‐National Sample Cohort (NHIS‐NSC) database: focused on temporal trend. Korean Circ J. 2019;49:1155–1163. doi: 10.4070/kcj.2019.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmidt EB, Hedegaard BS, Retterstøl K. Familial hypercholesterolaemia: history, diagnosis, screening, management and challenges. Heart. 2020;106:1940–1946. doi: 10.1136/heartjnl-2019-316276 [DOI] [PubMed] [Google Scholar]
- 18. Mundal LJ, Igland J, Veierød MB, Holven KB, Ose L, Selmer RM, Wisloff T, Kristiansen IS, Tell GS, Leren TP, et al. Impact of age on excess risk of coronary heart disease in patients with familial hypercholesterolaemia. Heart. 2018;104:1600–1607. doi: 10.1136/heartjnl-2017-312706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khera AV, Won H‐H, Peloso GM, Lawson KS, Bartz TM, Deng X, van Leeuwen EM, Natarajan P, Emdin CA, Bick AG, et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. 2016;67:2578–2589. doi: 10.1016/j.jacc.2016.03.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beheshti S, Madsen CM, Varbo A, Benn M, Nordestgaard BG. Relationship of familial hypercholesterolemia and high low‐density lipoprotein cholesterol to ischemic stroke: Copenhagen general population study. Circulation. 2018;138:578–589. doi: 10.1161/CIRCULATIONAHA.118.033470 [DOI] [PubMed] [Google Scholar]
- 21. Perez de Isla L, Alonso R, Watts GF, Mata N, Saltijeral Cerezo A, Muñiz O, Fuentes F, Diaz‐Diaz JL, de Andrés R, Zambón D, et al. Attainment of LDL‐cholesterol treatment goals in patients with familial hypercholesterolemia: 5‐year SAFEHEART registry follow‐up. J Am Coll Cardiol. 2016;67:1278–1285. doi: 10.1016/j.jacc.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 22. Jung KJ, Koh H, Choi Y, Lee SJ, Ji E, Jee SH. Familial hypercholesterolemia and atherosclerotic cardiovascular mortality among Korean adults with low levels of serum cholesterol. Atherosclerosis. 2018;278:103–109. doi: 10.1016/j.atherosclerosis.2018.09.012 [DOI] [PubMed] [Google Scholar]
- 23. Oh J, Lee CJ, Kim DI, Rhee M‐Y, Lee B‐K, Ahn Y, Cho BR, Woo J‐T, Hur S‐H, Jeong J‐O, et al. Target achievement with maximal statin‐based lipid‐lowering therapy in Korean patients with familial hypercholesterolemia: a study supported by the Korean Society of Lipid and Atherosclerosis. Clin Cardiol. 2017;40:1291–1296. doi: 10.1002/clc.22826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pijlman AH, Huijgen R, Verhagen SN, Imholz BP, Liem AH, Kastelein JJ, Abbink EJ, Stalenhoef AF, Visseren FL. Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: a large cross‐sectional study in the Netherlands. Atherosclerosis. 2010;209:189–194. doi: 10.1016/j.atherosclerosis.2009.09.014 [DOI] [PubMed] [Google Scholar]


