Abstract
Background
In the absence of obstructive coronary stenoses, abnormality of noninvasive stress tests (NIT) in patients with chronic coronary syndromes may indicate myocardial ischemia of nonobstructive coronary arteries (INOCA). The differential prognosis of INOCA according to the presence of coronary microvascular dysfunction (CMD) and incremental prognostic value of CMD with intracoronary physiologic assessment on top of NIT information remains unknown.
Methods and Results
From the international multicenter registry of intracoronary physiologic assessment (ILIAS [Inclusive Invasive Physiological Assessment in Angina Syndromes] registry, N=2322), stable patients with NIT and nonobstructive coronary stenoses with fractional flow reserve >0.80 were selected. INOCA was diagnosed when patients showed positive NIT results. CMD was defined as coronary flow reserve ≤2.5. According to the presence of INOCA and CMD, patients were classified into 4 groups: group 1 (no INOCA nor CMD, n=116); group 2 (only CMD, n=90); group 3 (only INOCA, n=41); and group 4 (both INOCA and CMD, n=40). The primary outcome was major adverse cardiovascular events, a composite of all‐cause death, target vessel myocardial infarction, or clinically driven target vessel revascularization at 5 years. Among 287 patients with nonobstructive coronary stenoses (fractional flow reserve=0.91±0.06), 81 patients (38.2%) were diagnosed with INOCA based on positive NIT. By intracoronary physiologic assessment, 130 patients (45.3%) had CMD. Regardless of the presence of INOCA, patients with CMD showed a significantly lower coronary flow reserve and higher hyperemic microvascular resistance compared with patients without CMD (P<0.001 for all). The cumulative incidence of major adverse cardiovascular events at 5 years were 7.4%, 21.3%, 7.7%, and 34.4% in groups 1 to 4. By documenting CMD (groups 2 and 4), intracoronary physiologic assessment identified patients at a significantly higher risk of major adverse cardiovascular events at 5 years compared with group 1 (group 2: adjusted hazard ratio [HRadjusted], 2.88; 95% CI, 1.52–7.19; P=0.024; group 4: HRadjusted, 4.00; 95% CI, 1.41–11.35; P=0.009).
Conclusions
In stable patients with nonobstructive coronary stenoses, a diagnosis of INOCA based only on abnormal NIT did not identify patients with higher risk of long‐term cardiovascular events. Incorporating intracoronary physiologic assessment to NIT information in patients with nonobstructive disease allowed identification of patient subgroups with up to 4‐fold difference in long‐term cardiovascular events.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04485234.
Keywords: coronary flow reserve, coronary microvascular disease, ischemia with nonobstructive coronary arteries, myocardial ischemia, prognosis
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Prognosis
Nonstandard Abbreviations and Acronyms
- CFR
coronary flow reserve
- CMD
coronary microvascular disease
- FFR
fractional flow reserve
- INOCA
ischemia with nonobstructive coronary arteries
- MACE
major adverse cardiovascular event
Clinical Perspective
What Is New?
In the absence of obstructive coronary stenoses, abnormality of noninvasive stress tests in patients with chronic coronary syndromes may indicate myocardial ischemia of nonobstructive coronary arteries (INOCA).
Coronary microvascular disease (CMD) with or without vasospastic angina is one of the major endotypes of INOCA.
Only limited data have been available on the prognostic implications of CMD defined by the universal definition among patients with INOCA.
What Are the Clinical Implications?
Among the patients with anginal symptoms and functionally nonobstructive coronary artery disease, 28.2% showed positive noninvasive stress test results and 45.3% had CMD; the presence of CMD was significantly associated with an increased risk of major adverse cardiovascular events, and the prognostic impact of CMD was higher than that of the presence of INOCA.
Among the 4 groups classified by INOCA and CMD, only patients with CMD showed a significantly increased risk of major adverse cardiovascular events at 5 years, regardless of the presence of INOCA.
Intracoronary physiologic assessment is necessary and incorporating it into noninvasive stress test information enables the identification of high‐risk subgroups with CMD for cardiovascular events.
Patients with symptoms and signs of ischemic heart disease (IHD) but found to have nonobstructive coronary arteries (INOCA) are increasingly recognized. Previous studies indicated that the prevalence of INOCA among patients referred to invasive coronary angiography was 20% to 65%. 1 , 2 Even among patients with positive noninvasive stress test (NIT) results, only 41.0% had obstructive coronary artery disease (CAD) defined by coronary stenoses ≥50%. 3 These findings indicate that a substantial proportion of stable IHD cases can be diagnosed as INOCA which is caused by functional abnormalities such as vasospastic angina or coronary microvascular disease (CMD) rather than obstructive CAD. Although previous studies have shown that INOCA is associated with a higher risk of adverse clinical outcome than the general population, 1 , 4 , 5 it has been under‐recognized because of limited understanding of disease entity and diagnostic challenges with heterogeneous criteria.
CMD is a consequence of reduced blood flow through the coronary microcirculation, 5 , 6 , 7 and CMD with or without vasospastic angina is one of the major endotypes of INOCA. 8 Recent Expert Consensus Documents on INOCA and the European Society of Cardiology guideline of Chronic Coronary Syndrome underlined an importance of evaluating CMD in patients with suspected INOCA and proposed a universal definition of CMD based on (1) functionally nonobstructive CAD defined by a fractional flow reserve (FFR)>0.80 and (2) impaired coronary microvascular function determined by abnormal coronary flow reserve (CFR) and/or microvascular resistance. 9 , 10
Nevertheless, only limited data have been available on the prognostic implications of CMD defined by the universal definition among patients with INOCA. Therefore, we sought to evaluate the long‐term prognostic impact of CMD and INOCA among the patients with typical angina but no obstructive coronary stenosis, using the international multicenter vessel‐level pooled registry of intracoronary pressure and flow assessment.
Methods
Study Design of ILIAS Registry
The ILIAS (Inclusive Invasive Physiological Assessment in Angina Syndromes) registry is an international multicenter vessel‐level pooled registry of intracoronary pressure and flow assessment. The registry is composed of 20 institutes from Korea, The Netherlands, Japan, Spain, Denmark, Italy, and the United States. All data were prospectively recorded according to each center’s protocols. Patients who underwent clinically indicated coronary angiography and comprehensive intracoronary physiologic assessment of at least 1 native coronary artery were enrolled. Patients’ symptoms and signs suggesting angina were collected by attending physicians based on the patients’ description. Typical angina was defined as constricting discomfort in the front of the chest or in the neck, jaw, shoulder, or arm, which was precipitated by physical exertion and relieved by rest or nitrates. 10 Patients with hemodynamic instability, significant valvular heart disease, prior coronary artery bypass graft surgery, or culprit vessels of acute coronary syndromes were excluded. Individual patient data were collected using standardized and anonymized spreadsheets by a fully compliant cloud‐based clinical data platform (Castor EDC, Amsterdam, The Netherlands). Standardized definitions were used for all variables including patient‐ and vessel‐level clinical outcomes. The study protocol was approved by the Institutional Review Board or Ethics Committee at each participating center and written informed consent was obtained from all participants. The study protocol was in accordance with the Declaration of Helsinki. The ILIAS Registry is registered at Clinicaltrials.gov (NCT04485234).
Study Population
A total of 2322 patients (3046 vessels) were enrolled in the ILIAS registry. Among them, 570 patients with anginal symptoms who were evaluated by NITs were selected in the current analysis (Figure 1). We excluded patients who underwent revascularization (n=207) or had functionally obstructive CAD with FFR≤0.80 (n=76). Finally, the current study included a total of 287 symptomatic patients with available NIT results in whom revascularization was deferred for functionally nonobstructive CAD (FFR>0.80).
Figure 1. Study flow.
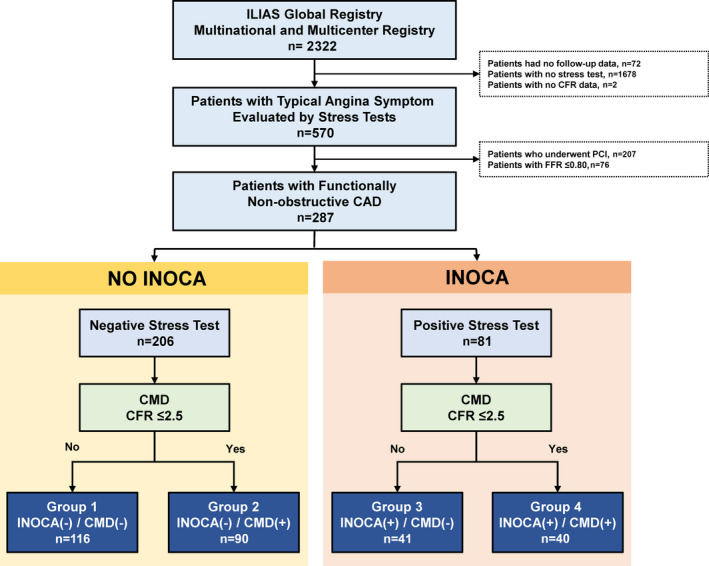
CAD indicates coronary artery disease; CFR, coronary flow reserve; CMD, coronary microvascular disease; FFR, fractional flow reserve; ILIAS, Inclusive Invasive Physiological Assessment in Angina Syndromes; INOCA, ischemia with nonobstructive coronary arteries; and PCI, percutaneous coronary intervention.
Noninvasive Stress Tests
All NITs were performed according to each participating center’s protocol and included exercise treadmill test, exercise or dobutamine stress echocardiography, single‐photon emission computed tomography, positron emission tomography, or cardiac magnetic resonance imaging. The selection of NITs was left to the discretion of the attending physicians based on patient characteristics, local expertise, and availability. The NITs were interpreted according to multicenter study protocols and in line with current guidelines. 10 , 11 The final results of NITs were interpreted locally and reported as a binary variable (positive or negative). The positive result was defined as moderate to severe reversible defect on nuclear perfusion imaging (≥10% ischemic myocardium) or high‐risk findings on exercise treadmill test without imaging (≤−11 Duke Treadmill Score). 10 , 11 , 12 Patients with anginal symptoms who had positive NITs but functionally nonobstructive CAD were diagnosed with INOCA. 9 Patients with anginal symptoms who had negative NITs and functionally nonobstructive CAD were classified into ‘no INOCA’ group (Figure 1). 9
Coronary Angiography and Intracoronary Physiologic Assessment
Coronary angiography was performed using standard techniques. Angiographic views were obtained following the administration of intracoronary nitrates (100 or 200 µg). After diagnostic coronary angiography, intracoronary physiologic assessment was performed by standard techniques using Doppler velocity‐equipped coronary guidewires (FloWire, Philips‐Volcano, San Diego, CA, USA) or dual pressure and Doppler velocity equipped guidewire (ComboWire, Philips‐Volcano, San Diego, CA, USA). For the patient using FloWire, another pressure wire (PressureWire, AbbottVascular, St. Paul, MN, USA) was used to measure FFR. Intracoronary nitrates (100 or 200 µg) was administered before physiologic measurements. Hyperemia was induced by intravenous infusion of adenosine (140 µg/kg per min) or adenosine triphosphate (150 µg/kg per min) through a peripheral or central vein, intracoronary bolus injection of adenosine (40–200 mcg), or intracoronary bolus injection of nicorandil (2 mg), according to local standards. 13 Doppler or pressure sensor was recommended to be positioned at the very distal part of the coronary artery. 13 Interrogated vessels were primarily the vessels with “nonsignificant” stenotic lesions defined by FFR>0.80. However, when there were no stenotic lesions (near‐normal), the left anterior descending artery was recommended to be used for FFR and CFR measurement. FFR was calculated as the ratio between the mean proximal aortic and mean distal coronary pressures during maximal hyperemia. After measurements were completed, the guidewire was pulled back to the guiding catheter, and the pressure drift was checked. In cases with a drift larger than >0.03 FFR unit, re‐equalizations and repeated measurements were recommended. Using the Doppler velocity technique, resting and hyperemic average peak flow velocities were measured, and CFR was calculated as the ratio of hyperemic to resting average peak flow velocities. Baseline microvascular resistance (BMR) was calculated by dividing the mean distal coronary pressure by average peak flow velocities during resting condition. Hyperemic microvascular resistance was calculated by dividing the mean distal coronary pressure by average peak flow velocities during hyperemia. In the current study, CMD was defined as CFR≤2.5 based on prior studies. 5 , 14 , 15 , 16
Treatment, Patient Follow‐up, and Clinical Outcomes
For vessels with functionally obstructive CAD with FFR≤0.80, percutaneous coronary intervention was recommended according to clinical practice guidelines at the time of the procedure. However, final decisions about revascularization were left at the discretion of the operator. Optimal medical treatments, including antiplatelet agents, statins, and antianginal medications, were provided based on guidelines.
Follow‐up was performed by outpatient visits or telephone contacts. The median follow‐up duration of the study population was 1194.0 days (interquartile range, 730.0–1826.0 days). Major adverse cardiac event (MACE) was defined as a composite of all‐cause death, target vessel‐related myocardial infarction, and clinically driven revascularization by means of coronary artery bypass graft surgery or percutaneous coronary intervention. Cardiac death was defined as death from any cardiac cause including sudden cardiac death, acute myocardial infarction, heart failure, stroke, arrhythmias, or other cardiovascular cause. 17 Revascularization events were separately assessed as target vessel revascularization (TVR) and non‐TVR. All adverse clinical events were verified by evaluating hospital records or contacting the treating cardiologist or general practitioner.
Classification of Patients According to Presence of INOCA and CMD
Based on the presence of INOCA and CMD, patients were classified into 4 groups: group 1 (no INOCA without CMD, n=116); group 2 (no INOCA with CMD, n=90); group 3 (INOCA without CMD, n=41); and group 4 (INOCA with CMD, n=40) (Figure 1).
Statistical Analysis
Data including clinical outcomes were analyzed on a per‐patient basis. Continuous variables were presented as means and standard deviations according to their distributions, which were checked by the Kolmogorov‐Smirnov test and visual inspection of Q‐Q plots. All categorical variables were presented as numbers and relative frequencies (percentages). Continuous variables were compared based on a one‐way analysis of variance, and dichotomous variables were compared using Chi‐square tests or Fisher exact tests. No post‐hoc adjustments were performed. Correlation coefficients between anatomical and physiologic indexes were analyzed by Pearson or Spearman methods according to the normality.
Restricted cubic spline curves with 3 knots were used to evaluate the continuous effects of CFR on the outcomes at 5 years. Event rates were calculated based on Kaplan–Meier censoring estimates and presented with cumulative incidences at the 5‐year follow‐up; the log‐rank test was used to compare survival curves between the groups. A Cox proportional hazard regression was used to calculate hazard ratio (HR) and 95% CIs. The assumption of proportionality was assessed graphically by the log‐minus‐log plot, and the Cox proportional hazard models for all clinical outcomes satisfied the proportional hazards assumption. Multivariable Cox proportional hazard models were constructed using all variables with a P value <0.1 from the univariable analyses and variables considered clinically relevant. The final model included age, sex, diabetes, hyperlipidemia, and previous percutaneous coronary intervention. All analyses were 2‐tailed, and clinical significance was defined as P<0.05. Statistical analyses were performed using SPSS 25.0 for Windows (SPSS‐PC, Chicago, IL, USA) and R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Clinical Characteristics
Among the patients who underwent NIT for typical anginal symptoms (n=570), 50.4% (n=287) did not have functionally significant coronary artery disease in invasive coronary angiogram (Figure 1). Table 1 summarizes baseline clinical characteristics of the study population. Among a total of 287 patients with anginal symptoms and functionally nonobstructive CAD, 81 patients (28.2%) were diagnosed with INOCA based on positive NIT and 130 patients (45.3%) had CMD based on CFR ≤2.5. All patients presented with stable IHD. There were no significant differences in the baseline clinical profiles among the 4 groups classified by the presence of INOCA and CMD, except for sex and hyperlipidemia. Angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers were more frequently used among patients with INOCA (groups 3 and 4) at baseline status, but there was no statistically significant difference in discharge medications among the groups.
Table 1.
Baseline Clinical Characteristics of the Study Population
| Variables |
Group 1 INOCA(−)/CMD(−) |
Group 2 INOCA(−)/CMD(+) |
Group 3 INOCA(+)/CMD(−) |
Group 4 INOCA(+)/CMD(+) |
P value |
|---|---|---|---|---|---|
| 116 (40.4%) | 90 (31.4%) | 41 (14.3%) | 40 (13.9%) | ||
| Demographics | |||||
| Age, y | 59.7±9.4 | 63.0±10.6 | 63.0±10.5 | 62.7±9.5 | 0.059 |
| Men | 74 (63.8) | 48 (53.3) | 30 (73.2) | 32 (80.0) | 0.015 |
| Body mass index, kg/m2 | 26.6±3.8 | 25.9±4.9 | 27.2±5.2 | 26.6±4.2 | 0.588 |
| Ejection fraction, % | 61.9±11.7 | 52.2±19.5 | 59.8±8.1 | 61.9±8.7 | 0.210 |
| Cardiovascular risk factors | |||||
| Hypertension | 56 (48.3) | 38 (42.2) | 25 (61.0) | 21 (53.8) | 0.222 |
| Diabetes | 21 (18.1) | 10 (11.1) | 9 (22.0) | 8 (20.0) | 0.347 |
| Hyperlipidemia | 59 (50.9) | 44 (48.9) | 29 (70.7) | 29 (72.5) | 0.011 |
| Family history of cardiovascular disease | 53 (45.7) | 50 (56.8) | 16 (40.0) | 17 (43.6) | 0.229 |
| Current smoking | 29 (25.9) | 23 (25.8) | 14 (34.1) | 8 (21.1) | 0.603 |
| Previous PCI | 14 (12.2%) | 11 (12.2%) | 11 (26.8%) | 10 (25.0%) | 0.055 |
| Baseline medications | |||||
| Antiplatelet agents | 99 (85.3) | 82 (92.1) | 36 (87.8) | 34 (85.0) | 0.447 |
| ACEI or ARBs | 29 (25.0) | 24 (27.0) | 20 (48.8) | 22 (55.0) | <0.001 |
| Beta blocker | 78 (67.2) | 59 (66.3) | 25 (61.0) | 20 (50.0) | 0.234 |
| Calcium channel blocker | 53 (45.7) | 41 (46.1) | 15 (36.6) | 17 (42.5) | 0.744 |
| Nitrates | 50 (43.1) | 36 (40.4) | 19 (46.3) | 13 (32.5) | 0.596 |
| Discharge medications | |||||
| Aspirin | 51 (79.7) | 41 (87.2) | 15 (83.3) | 16 (100.0) | 0.219 |
| P2Y12 inhibitor | 10 (22.2) | 4 (10.8) | 2 (18.2) | 6 (40.0) | 0.126 |
| ACEI or ARBs | 19 (29.7) | 13 (27.7) | 11 (61.1) | 6 (37.5) | 0.061 |
| Beta blocker | 41 (64.1) | 31 (66.0) | 12 (66.7) | 11 (68.8) | 0.985 |
| Calcium channel blocker | 21 (32.8) | 15 (31.9) | 4 (22.2) | 6 (37.5) | 0.793 |
| Nitrates | 15 (23.4) | 11 (23.4) | 5 (27.8) | 3 (18.8) | 0.959 |
| Statin | 38 (59.4) | 30 (63.8) | 12 (66.7) | 11 (68.8) | 0.873 |
Data are expressed as number (%) or mean±SD. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CMD, coronary microvascular disease; INOCA, ischemia with nonobstructive coronary arteries; and PCI, percutaneous coronary intervention.
Angiographic and Physiologic Parameters According to INOCA and CMD
Table 2 shows angiographic characteristics and intracoronary physiologic indexes. The overall study population showed functionally nonobstructive epicardial coronary disease with mean diameter stenosis of 48.1%±13.8% and mean FFR of 0.91±0.06. More patients in INOCA groups (groups 3 and 4) had no significant angiographic disease than did patients in no INOCA groups (groups 1 and 2). The distribution of diameter stenosis, FFR, CFR, and hyperemic microvascular resistance are shown in Figure S1. Angiographic stenosis severity (diameter stenosis) was significantly associated with FFR (R=−0.263, P<0.001), but not with CFR (R=−0.061, P=0.385). CFR was significantly associated with hyperemic microvascular resistance (R=−0.317, P<0.001), whereas no correlation was found between CFR and FFR (Figure S2). There was no significant difference in angiographic stenosis severity and functional significance of epicardial CAD (resting distal coronary pressure/aortic pressure and FFR) across the 4 groups. Regardless of the presence of INOCA, patients with CMD showed significantly lower CFR and higher hyperemic microvascular resistance than those without CMD (P<0.001 for all) (Table 2 and Figure S3).
Table 2.
Characteristics of Target Vessels According to Classification by INOCA and CMD
| Variables |
Group 1 INOCA(−)/CMD(−) |
Group 2 INOCA(−)/CMD(+) |
Group 3 INOCA(+)/CMD(−) |
Group 4 INOCA(+)/CMD(+) |
P value |
|---|---|---|---|---|---|
| 116 (40.4%) | 90 (31.4%) | 41 (14.3%) | 40 (13.9%) | ||
| Angiographic characteristics | |||||
| Angiographic disease extent | <0.001 | ||||
| No angiographic disease | 48 (41.4) | 41 (45.6) | 29 (70.7) | 26 (65.0) | |
| 1‐vessel disease | 30 (25.9) | 14 (15.6) | 10 (24.4) | 7 (17.5) | |
| 2‐vessel disease | 36 (31.0) | 29 (32.2) | 1 (2.4) | 5 (12.5) | |
| 3‐vessel disease | 2 (1.7) | 6 (6.7) | 1 (2.4) | 2 (5.0) | |
| Interrogated target vessel | 0.069 | ||||
| LAD | 57 (49.1) | 38 (42.2) | 24 (58.5) | 22 (55.0) | |
| LCX | 43 (37.1) | 41 (45.6) | 7 (17.1) | 12 (30.0) | |
| RCA | 16 (13.8) | 11 (12.2) | 10 (24.4) | 6 (15.0) | |
| Quantitative coronary angiography | |||||
| Reference vessel size, mm | 2.9±0.6 | 2.8±0.7 | 3.5±0.8 | 2.7±0.7 | 0.052 |
| Diameter stenosis, % | 46.8±12.8 | 45.6±12.8 | 47.2±15.9 | 51.2±9.1 | 0.360 |
| Lesion length, mm | 8.7±4.1 | 11.6±6.1 | 4.9±5.8 | 6.3±2.9 | 0.195 |
| Invasive hemodynamics | |||||
| Resting Pa, mm Hg | 96.9±13.3 | 98.8±15.1 | 100.5±15.1 | 96.5±15.4 | 0.503 |
| Hyperemic Pa, mm Hg | 91.6±13.2 | 93.2±15.5 | 91.4±20.4 | 87.5±16.3 | 0.302 |
| Resting Pd, mm Hg | 94.4±13.3 | 95.5±15.3 | 96.9±14.9 | 93.4±15.8 | 0.710 |
| Hyperemic Pd, mm Hg | 83.7±12.8 | 84.8±14.4 | 83.8±13.3 | 79.4±15.6 | 0.240 |
| Resting APV, cm/sec | 14.5±4.3 | 19.5±6.8 | 13.7±3.4 | 17.1±5.7 | <0.001 |
| Hyperemic APV, cm/sec | 42.9±11.9 | 38.3±11.7 | 41.9±11.0 | 32.6±9.9 | <0.001 |
| Invasive physiologic indexes | |||||
| Resting Pd/Pa | 0.97±0.03 | 0.97±0.03 | 0.96±0.03 | 0.97±0.03 | 0.292 |
| Fractional flow reserve | 0.91±0.07 | 0.91±0.06 | 0.90±0.05 | 0.91±0.06 | 0.509 |
| Coronary flow reserve | 3.1±0.6 | 2.0±0.3 | 3.3±0.8 | 2.0±0.4 | <0.001 |
| BMR, mm Hg/cm per sec | 7.1±2.1 | 5.4±1.7 | 7.5±2.1 | 6.0±2.3 | <0.001 |
| HMR, mmHg/cm per sec | 2.1±0.6 | 2.4±0.8 | 2.1±0.6 | 2.6±0.8 | <0.001 |
Data are expressed as number (%) or mean±SD. APV indicates averaged peak velocity; BMR, baseline microvascular resistance; CMD, coronary microvascular disease; HMR, hyperemic microvascular resistance; INOCA, ischemia with nonobstructive coronary arteries; LAD, left anterior descending artery; LCX, left circumflex artery; Pa, aortic pressure; Pd, distal coronary pressure; and RCA, right coronary artery.
Clinical Outcomes of Patients According to INOCA and CMD
There was an inverse association between CFR values and the risk of MACE (per 1 increase; adjusted HR, 0.40; 95% CI, 0.22‒0.72; P=0.002) (Figure S4). Although the cumulative incidence of MACE at 5 years was numerically higher in INOCA groups than in no INOCA groups, it was not statistically significant (20.1% versus 13.2%; adjusted HR, 1.45; 95% CI, 0.68–3.09; P=0.340). In contrast, the presence of CMD was significantly associated with a higher risk of MACE at 5 years than those without CMD (24.0% versus 7.8%; adjusted HR, 2.97; 95% CI, 1.39‒6.34; P=0.005), which was mainly driven by a higher rate of any revascularization (Table 3 and Figure 2).
Table 3.
Clinical Outcomes According to Presence of INOCA or CMD
| No INOCA | INOCA | Multivariable* HR (95% CI) | P value | No CMD | CMD | Multivariable* HR (95% CI) | P value | |
|---|---|---|---|---|---|---|---|---|
| Total (N=287) | n=206 | n=81 | n=157 | n=130 | ||||
| All cause death | 11 (7.6%) | 4 (10.3%) | 1.30 (0.39–4.33) | 0.666 | 5 (4.3%) | 10 (14.4%) | 2.21 (0.72–6.80) | 0.165 |
| Cardiac death | 8 (5.4%) | 3 (7.2%) | 1.39 (0.35–5.55) | 0.640 | 3 (2.4%) | 8 (11.1%) | 3.05 (0.77–12.13) | 0.114 |
| Target‐vessel MI | 1 (0.8%) | 2 (2.6%) | 0.87 (0.61–160.44) | 0.108 | 3 (2.5%) | 0 (0%) | NA | 0.998 |
| Any revascularization | 13 (7.0%) | 7 (10.9%) | 1.47 (0.56–3.84) | 0.434 | 5 (3.7%) | 15 (12.7%) | 3.70 (1.34–10.25) | 0.012 |
| TVR † | 8 (4.4%) | 6 (9.8%) | 1.85 (0.60–5.68) | 0.285 | 3 (2.5%) | 11 (9.1%) | 4.26 (1.18–15.39) | 0.027 |
| Non‐TVR | 5 (2.7%) | 1 (1.2%) | 0.67 (0.76–5.85) | 0.713 | 2 (1.3%) | 4 (3.9%) | 2.75 (0.50–15.21) | 0.246 |
| MACE ‡ | 22 (13.2%) | 11 (20.1%) | 1.45 (0.68–3.09) | 0.340 | 10 (7.8%) | 23 (24.0%) | 2.97 (1.39–6.34) | 0.005 |
The cumulative incidences of clinical outcomes were presented as Kaplan‒Meier estimates during the median follow‐up of 1194.0 days (Q1–Q3, 730.0–1826.0 days). CMD indicates coronary microvascular disease; HR, hazard ratio; INOCA, ischemia with no obstructive coronary artery disease; MACE, major adverse cardiovascular events; MI, myocardial infarction; NA, not applicable; PCI, percutaneous coronary intervention; and TVR, target vessel revascularization.
Adjusted for age, sex, diabetes, hyperlipidemia, and previous percutaneous coronary intervention.
Defined as clinically driven revascularization of the target vessel by means of coronary bypass grafting or percutaneous coronary intervention.
Defined as the composite of all‐cause death, acute myocardial infarction not clearly attributable to a nontarget vessel, and any revascularization by means of coronary bypass grafting or percutaneous coronary intervention.
Figure 2. Prognostic impact of ischemia with nonobstructive coronary arteries and coronary microvascular disease.
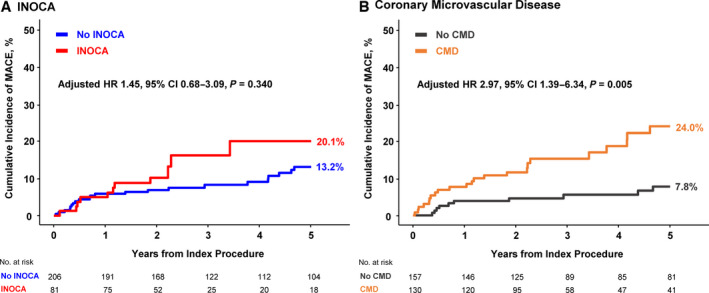
The cumulative incidence of major adverse cardiovascular event at 5 years were compared according to presence of (A) ischemia with nonobstructive coronary arteries and (B) coronary microvascular disease. Adjusted covariates in the multivariable model included age, sex, diabetes, hyperlipidemia, and previous percutaneous coronary intervention. CMD indicates coronary microvascular disease; HR, hazard ratio; INOCA, ischemia with nonobstructive coronary arteries; and MACE, major adverse cardiovascular event.
In the comparison of clinical outcomes across the 4 groups classified by INOCA and CMD, the cumulative incidences of MACE at 5 years were 7.4%, 21.3%, 7.7%, and 34.4% in groups 1 to 4, respectively (Table 4 and Figure 3). Compared with the reference group (group 1: no INOCA and no CMD), patients with CMD (groups 2 and 4) showed a significantly higher risk of MACE at 5 years regardless of the presence of INOCA (group 2: adjusted HR, 2.88; 95% CI, 1.51–7.19; P=0.024; group 4: adjusted HR, 4.00; 95% CI, 1.41–11.35; P=0.009) (Table 5 and Figure 3). This result remained consistent, when the patients were stratified using different cut‐off value of 2.0 for depressed CFR (Figure S5).
Table 4.
Clinical Outcomes According to Stress Tests Results and Presence of CMD
|
Group 1 INOCA(−)/CMD(−) |
Group 2 INOCA(−)/CMD(+) |
Group 3 INOCA(+)/CMD(−) |
Group 4 INOCA(+)/CMD(+) |
P value | |
|---|---|---|---|---|---|
| Total (N=287) | 116 (40.4%) | 90 (31.4%) | 41 (14.3%) | 40 (13.9%) | |
| All‐cause death | 3 (3.5%) | 8 (14.0%) | 2 (5.3%) | 2 (18.8%) | 0.151 |
| Cardiac death | 1 (1.3%) | 7 (11.6%) | 2 (5.3%) | 1 (12.5%) | 0.056 |
| Target‐vessel MI | 1 (1.4%) | 0 (0%) | 2 (5.3%) | 0 (0%) | 0.037 |
| Any revascularization | 4 (4.0%) | 9 (10.7%) | 1 (2.4%) | 6 (19.2%) | 0.025 |
| TVR* | 2 (2.3%) | 6 (6.8%) | 1 (2.4%) | 5 (17.1%) | 0.024 |
| Non‐TVR | 2 (1.8%) | 3 (4.1%) | 0 (0%) | 1 (2.6%) | 0.636 |
| MACE † | 7 (7.4%) | 15 (21.3%) | 3 (7.7%) | 8 (34.4%) | 0.007 |
Data expressed as number of events (%). The cumulative incidence of clinical outcomes is presented as Kaplan–Meier estimates during the median follow‐up of 1194.0 days (Q1–Q3, 730.0–1826.0 days). The P values were log‐rank P value in survival analysis. CMD indicates coronary microvascular disease; INOCA, ischemia with nonobstructive coronary arteries; MACE, major adverse cardiac event; MI, myocardial infarction; and TVR, target vessel revascularization.
Defined as clinically driven revascularization of the target vessel by means of coronary bypass grafting or percutaneous coronary intervention.
Defined as cardiac death, acute myocardial infarction not clearly attributable to a nontarget vessel, and clinically driven revascularization of the target vessel by means of coronary bypass grafting or percutaneous coronary intervention.
Figure 3. Comparison of major adverse cardiovascular event at 5 years according to stress tests results and presence of coronary microvascular disease.
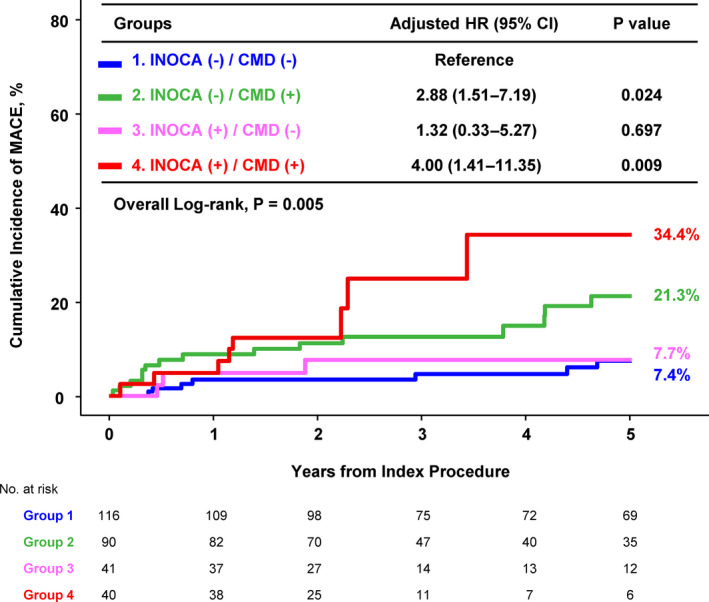
Kaplan–Meier curve are shown for the 4 groups of patients according to ischemia with nonobstructive coronary arteries and coronary microvascular disease. Adjusted covariates in multivariable model included age, sex, diabetes, hyperlipidemia, and previous percutaneous coronary intervention. CMD indicates coronary microvascular disease; HR, hazard ratio; INOCA, ischemia with nonobstructive coronary arteries; and MACE, major adverse cardiovascular event.
Table 5.
Comparison of MACE* at 5 Years According to Stress Tests Results and Presence of CMD
| Patient number | Cumulative incidence | Univariable | Multivariable † | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |||
| Group 1: INOCA(−)/CMD(−) | 116 (40.4%) | 7 (7.4%) | Reference | Reference | ||
| Group 2: INOCA(−)/CMD(+) | 90 (31.4%) | 15 (21.3%) | 3.18 (1.30–7.82) | 0.012 | 2.88 (1.51–7.19) | 0.024 |
| Group 3: INOCA(+)/CMD(−) | 41 (14.3%) | 3 (7.7%) | 1.56 (0.40–6.04) | 0.523 | 1.32 (0.33–5.27) | 0.697 |
| Group 4: INOCA(+)/CMD(+) | 40 (13.9%) | 8 (34.4%) | 4.78 (1.71–13.40) | 0.003 | 4.00 (1.41–11.35) | 0.009 |
Data expressed as number of events (%). The cumulative incidence of clinical outcomes is presented as Kaplan–Meier estimates during median follow‐up of 1194.0 days (Q1–Q3, 730.0–1826.0 days). CMD indicates coronary microvascular disease; HR, hazard ratio; INOCA, ischemia with no obstructive coronary arteries; and MACE, major adverse cardiac event.
Defined as the composite of all‐cause death, acute myocardial infarction not clearly attributable to a nontarget vessel, and any revascularization by means of coronary bypass grafting or percutaneous coronary intervention.
Adjusted for age, sex, diabetes, hyperlipidemia, and previous percutaneous coronary intervention.
Discussion
The present study evaluated the long‐term prognosis of INOCA and CMD defined by NITs and intracoronary physiologic assessment according to the current expert consensus. 9 The major findings were as follows: First, among patients with anginal symptoms and functionally nonobstructive CAD, 28.2% showed positive NIT results and 45.3% had CMD. Second, the presence of CMD was significantly associated with an increased risk of MACE, and the prognostic impact of CMD was higher than that of the presence of INOCA. Third, among the 4 groups classified by INOCA and CMD, only patients with CMD showed a significantly increased risk of MACE at 5 years, regardless of the presence of INOCA. Patients with INOCA but without CMD did not show a significantly higher risk of MACE compared with those without both INOCA and CMD (Figure 4).
Figure 4. Long‐term prognostic implication of ischemia with nonobstructive coronary arteries and coronary microvascular disease.
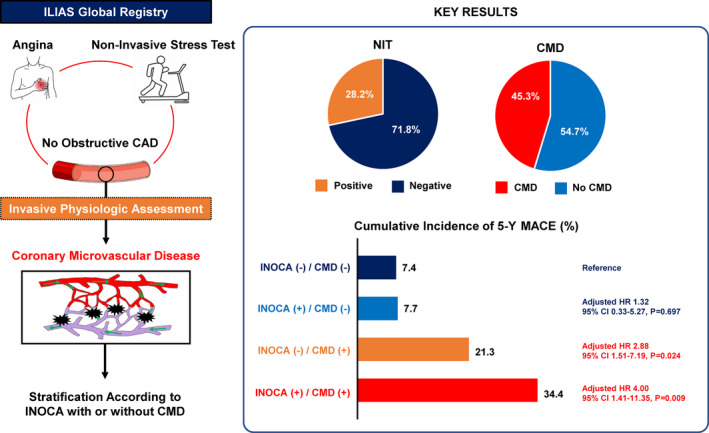
Symptomatic patients with stable ischemic heart disease who underwent of noninvasive stress tests and intracoronary coronary physiologic assessment were evaluated. The patients were stratified according to the presence of ischemia with nonobstructive coronary arteries and coronary microvascular disease. In the overall population, 28.2% showed positive of noninvasive stress tests results and 45.3% had coronary microvascular disease. Patients with coronary microvascular disease showed a significantly increased risk of major adverse cardiovascular event at 5 years, regardless of noninvasive stress tests results. These findings indicate that the differential prognostic impact of endotype of ischemia with nonobstructive coronary arteries which support the necessity of intracoronary physiologic assessment. CAD indicates coronary artery disease; CMD, coronary microvascular disease; HR, hazard ratio; ILIAS, Inclusive Invasive Physiological Assessment in Angina Syndromes; INOCA, ischemia with nonobstructive coronary arteries; MACE, major adverse cardiovascular event; and NIT, noninvasive stress test.
Diagnostic Challenge of INOCA and Its Endotypes
NITs have been recommended as the first line diagnostic test for the patients with suspected IHD and intermediate pretest probability of CAD. 18 , 19 However, in the ILIAS registry which included patients who underwent coronary angiogram and physiologic assessment, NIT was performed before the invasive tests only in about a quarter of patients, which could suggest underutilization of upstream NIT as described in the prior study. 20 Moreover, a substantial portion of patients with anginal symptoms and abnormal NIT results do not have obstructive CAD on coronary angiography. 3 Despite the absence of obstructive epicardial lesions requiring revascularization, however, multiple reports showed that these patients continued to experience recurrent angina leading to repeat hospitalization and unnecessary coronary angiography, impaired quality of life, and adverse cardiovascular outcomes. 1 , 21 , 22 In this regard, patients with anginal symptoms and abnormal NIT results but without obstructive CAD are increasingly recognized to have important disease entity called INOCA. 9 In INOCA, an abnormal NIT result may suggest the mismatch between blood supply and myocardial oxygen demand, and functional disturbance of coronary blood flow attributable to vasospasm or CMD has been suspected as the major underlying mechanism. 4 Nevertheless, heterogeneous diagnostic criteria and limited evidence‐based treatment options have been challenging issues in daily practice.
Recently, the Expert Consensus Document for INOCA suggested a systematic approach for diagnosing INOCA based on the evolution of invasive techniques for assessment of CMD. 9 The universal definition of CMD included symptoms of myocardial ischemia, objective abnormality of NIT, absence of functionally obstructive CAD (FFR>0.80), and evidence of impaired coronary microvascular function such as impaired CFR, elevated microvascular resistance, or coronary microvascular spasm. 9 In the current study, there was a substantial discrepancy among patients’ symptoms, NIT results, and coronary microvascular function. For example, among patients with negative NIT results, 43.7% of patients showed depressed CFR, while 50.6% of patients with positive NIT results had preserved CFR. These results indicate that NIT and intracoronary physiologic assessment are not exclusive diagnostic modalities, but complementary tools in evaluating patients with symptoms of myocardial ischemia. In addition, intracoronary physiologic assessment can be useful in patients with negative NITs, if clinical suspicion for IHD remains high.
CMD as a Major Endotype of INOCA
CMD is one of the endotypes of INOCA, and a previous meta‐analysis reported that patients with CMD diagnosed by positron emission tomography or trans‐thoracic Doppler echocardiography‐derived depressed CFR showed a 2‐ to 4‐fold higher risk of adverse cardiovascular outcome. 23 Similarly, previous studies that used intracoronary physiologic assessment to define CMD also revealed that patients with functionally nonobstructive CAD (FFR>0.80) and CMD defined by depressed CFR had a significantly higher risk of MACE. 24 , 25 , 26 , 27 CMD is related to traditional cardiovascular risk factors including age, hypertension, hyperlipidemia, diabetes, or smoking. 5 , 6 , 7 , 8 , 27 , 28 The COVADIS (Coronary Vasomotor Disorders International Study) group reported that more than half of patients with microvascular angina had hypertension and dyslipidemia, whereas the prevalence of diabetes and current smoking were relatively low. 5 The current study population with CMD also showed a similar profile of cardiovascular comorbidities.
However, it should be noted that patients with CMD could not be differentiated based on clinical characteristics and demographics. Furthermore, the current study showed that anatomical severity (diameter stenosis) or functional significance of epicardial CAD (FFR) were not significantly associated with the presence of CMD. The recent CorMicA (Coronary Microvascular Angina) trial presented that stratified medical treatment based on a systematic interventional diagnostic procedure to define INOCA endotypes (CMD and vasospastic angina) significantly improved anginal symptoms and quality of life. 8 Considering the limited diagnostic predictability of clinical risk factors and angiographic findings for CMD, these results support the need of intracoronary coronary physiologic assessment to evaluate CMD in patients with anginal symptoms despite the lack of obstructive CAD on angiogram or positive NIT results.
Prognostic Implications of INOCA and CMD
Although previous studies reported a higher risk of adverse cardiovascular events in patients with INOCA, it should be noted that the definitions of INOCA were heterogeneous across these studies and the endotypes of INOCA were not specified. 1 , 21 , 22 Recently, the COVADIS group 5 reported the clinical outcomes of 686 patients with microvascular angina, a major endotype of INOCA, using the standardized diagnostic criteria recommended by the Expert Consensus Document for INOCA. 9 In this report, the 1‐year MACE rate, including cardiovascular death, myocardial infarction, stroke, and hospitalization because of heart failure or unstable angina, was 7.7% per patient‐year and there was no sex or ethnic difference in prognosis. 5 However, the differential prognosis according to endotypes of INOCA has not been studied.
In the current study patients with INOCA showed numerically higher MACE rate than those without INOCA, but the difference was not statistically significant. Conversely, patients with CMD showed a 3‐fold higher risk of MACE compared with those without CMD. It has been well known that CMD, defined by depressed CFR, is associated with an increased risk of adverse cardiovascular events, regardless of measurement methods. 24 , 25 , 26 , 27 , 29 Furthermore, previous studies presented pathophysiologic mechanisms between CMD and adverse clinical outcome. 7 , 30 , 31 CMD is associated with the structural remodeling of the coronary microvasculature and/or functional dysregulation of arterioles, which ultimately cause reduced vasodilatory capacity and limited oxygen supply to myocardium. The mismatch between oxygen supply and demand causes angina and is also associated with endothelial dysfunction, smooth muscle cell dysfunction, and vascular remodeling. 7 Depressed CFR and/or elevated microvascular resistance is a phenotypic manifestation of the structural remodeling and/or functional dysregulation. These are key components related to atherosclerosis and plaque formation in both micro‐ and macrovascular systems. 32 , 33 , 34 , 35 Indeed, previous studies presented that CMD was associated with endothelial dysfunction and inflammatory activity that precede intimal thickening, lipid deposition in the macrovascular system, coronary vasomotor dysfunction, and thin‐cap fibroatheroma. 7 , 36 , 37 , 38
When the study population was stratified into 4 groups by the presence of INOCA and CMD, only patients with CMD showed significantly increased risk of MACE at 5 years, regardless of the presence of INOCA. Patients with INOCA but without CMD, who might have different endotypes of INOCA such as vasospastic angina, did not show a significantly higher risk of MACE compared with the reference group. Our findings indicate that the long‐term clinical outcomes of patients with INOCA depend on the endotypes. Indeed, previous studies showed that patients with vasospastic angina had relatively good long‐term prognosis with appropriate medical treatment, 39 , 40 unless the initial presentation was acute coronary syndrome and/or aborted cardiac arrest 41 or patients had concomitant obstructive CAD. 42 Yasue et al. evaluated 245 patients with vasospastic angina with 10 years of follow‐up. The survival rate at 10 years was 93%, and patients with appropriate medical treatment showed significantly better survival rates than those without. 39 Hung et al. reported that patients with vasospastic angina without obstructive CAD (n=284) did not show a significantly increased risk of cardiac death or myocardial infarction compared with the control group (n=239) during a median follow‐up of 49 months. 42
Another important message from the current study is that a diagnosis of INOCA based only on abnormal NIT results would not effectively identify patients with higher risk of long‐term cardiovascular events. This suggests the importance of intracoronary physiologic assessment to identify CMD in patients with INOCA, which supports the current Expert Consensus. Incorporating intracoronary physiologic assessment to the NIT results would allow better risk stratification and tailored management of patients with INOCA depending on its endotypes. Therefore, as the guidelines recommend, physiologic evaluation with CFR and/or microcirculatory resistance measurements needs to be considered in patients with persistent symptoms or evidence of ischemia but no obstructive disease on coronary angiogram. 10
Study Limitations
Several limitations of the study should be acknowledged. First, although the current study used the latest universal definitions of INOCA and CMD using intracoronary physiologic assessments, provocation test for vasospasm was not systematically performed. Thus, possible etiology of ischemia in patients with INOCA but no CMD could not be further evaluated. Second, given the limited diagnostic accuracy of NIT, false positive results of NITs (because of either attenuation artifacts or misinterpretation of the stress tests) might have been translated into false positive INOCA. Furthermore, there could be an interobserver variability, since NITs were interpreted locally rather than centrally in the core laboratory. This might be another reason for the limited prognostic implication of INOCA without information on CMD, which, in turn, further supports the incremental value of intracoronary physiologic assessment for CMD. Conversely, the etiology of symptoms in patients without INOCA and CMD was not further evaluated in the current registry. Furthermore, we could not evaluate the degree of NIT abnormality. Third, since the ILIAS registry excluded culprit vessel of acute coronary syndrome and all patients in the current analysis presented with stable IHD, the current results cannot be extended to patients with acute coronary syndrome. In addition, ILIAS registry included selected patients who underwent invasive coronary angiography with physiologic study, which could have caused selection bias and in part explain the low proportion of patients who had upstream NIT in the current registry (24.5%). Fourth, since intravascular imaging was not systematically performed, the possibility of diffuse atherosclerotic narrowing as a cause of depressed CFR cannot be excluded. However, more than half of the study population showed no evidence of angiographic disease and the mean FFR was 0.91±0.06. Fifth, differential prognosis following specific medical treatment for INOCA endotypes could not be evaluated. Sixth, there were numerical differences in the interrogated target vessels among 4 groups classified by INOCA and CMD. Seventh, although multiple centers participated in this study, the final sample size and clinical events were relatively small. Eighth, subjectivity of anginal symptoms could have caused some degree of heterogeneity among the study population. However, typicality of anginal symptoms was assessed according to the study protocol at each participating site with a widely used definition from the guidelines, reflecting a routine approach to patients with chest pain in a real‐world practice. Ninth, there was a lack of information about renal function including creatinine level. Lastly, since this was a registry‐based observational study, patient’s or physician’s blinding to the results of physiologic evaluation was not possible. In addition, persistent symptoms in patients with CMD might be possible because of increased risk of repeat revascularization.
Conclusions
Among patients with anginal symptoms and functionally nonobstructive CAD, incorporating intracoronary physiologic assessment to the NIT results allowed identification of high‐risk patients, since long‐term prognosis was mainly determined by the presence of CMD, regardless of NIT results. Patients with INOCA without CMD showed a similar long‐term prognosis with patients without INOCA. Differential prognosis by endotypes of INOCA warrants intracoronary physiologic assessment in patients with INOCA and supports the current guidelines. Further studies are needed to clarify prognostic impact of specific treatments based on endotypes of INOCA.
Appendix
ILIAS Registry Investigators
Tim P. van de Hoef (Department of Cardiology, Amsterdam UMC – location AMC, Amsterdam; Department of Cardiology, Amsterdam UMC – location VUmc, Amsterdam; Department of Cardiology, NoordWest Ziekenhuisgroep, The Netherlands); Joo Myung Lee, Ki Hong Choi, David Hong (Division of Cardiology, Department of Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea); Seung Hun Lee (Division of Cardiology, Department of Internal Medicine, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea); Doosup Shin (Division of Cardiovascular Medicine, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA); Masahiro Hoshino, Tadashi Murai, Tsunekazu Kakuta (Tsuchiura Kyodo General Hospital, Department of Cardiology, Tsuchiura City, Japan); Bon Kwon Koo, Doyeon Hwang (Seoul National University Hospital, Department of Internal Medicine, Cardiovascular Center, Seoul, Korea); Coen K. M. Boerhout, Jan JPiek(Department of Cardiology, Amsterdam UMC – location AMC, Amsterdam, The Netherlands); Guus A. de Waard, Steven A. J. Chamuleau, Koen Marques, Paul Knaapen (Department of Cardiology, Amsterdam UMC – location VUmc, Amsterdam, The Netherlands); Ji‐Hyun Jung (Sejong General Hospital, Sejong Heart Institute, Bucheon, Korea); Hernan Mejia‐Renteria, Javier Escaned (Hospital Clínico San Carlos, IDISSC, and Universidad Complutense de Madrid, Madrid, Spain); Mauro Echavarria‐Pinto (Hospital General ISSSTE Querétaro ‐ Facultad de Medicina, Universidad Autónoma de Querétaro, Querétaro, México); Martijn Meuwissen (Department of Cardiology, Amphia Hospital, Breda, The Netherlands); Hitoshi Matsuo (Gifu Heart Center, Department of Cardiovascular Medicine, Gifu, Japan); Maribel Madera‐Cambero (Tergooi Hospital, Department of Cardiology, Blaricum, The Netherlands); Ashkan Eftekhari, Evald HChristiansen (Aarhus University Hospital, Department of Cardiology, Aarhus, Denmark); Mohamed AEffat (Division of Cardiovascular Health and Disease, University of Cincinnati, Cincinnati, Ohio, USA); Joon‐Hyung Doh (Department of Medicine, Inje University Ilsan Paik Hospital, Goyang, Korea); Rupak Banerjee (Department of Mechanical and Materials Engineering, University of Cincinnati, Veterans Affairs Medical Center, Cincinnati, Ohio, USA); Hyun Kuk Kim (Department of Internal Medicine and Cardiovascular Center, Chosun University Hospital, University of Chosun College of Medicine, Gwangju, Korea); Chang‐Wook Nam (Department of Medicine, Keimyung University Dongsan Medical Center, Daegu, Korea); Giampaolo Niccoli (University of Parma, Parma, Italy); Masafumi Nakayama (Toda Central General Hospital, Cardiovascular Center, Toda, Japan); Nobuhiro Tanaka (Tokyo Medical University Hachioji Medical Center, Department of Cardiology, Tokyo, Japan); Eun‐Seok Shin (Department of Cardiology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea); NielsRoyen (Department of Cardiology, Radboud University Medical Center, Nijmegen, The Netherlands).
Sources of Funding
None.
Disclosures
T.v.d.H. has received speaker fees and institutional research grants from Abbott and Philips. J.M.L received research grants from Abbott and Philips. M.E.P. has received speaker fees from Abbott and Philips. N.v.R. has received speaker fees and institutional research grants from Abbott and Philips. B.K.K. has received institutional research grants from Abbott Vascular and Philips Volcano. J.J.P. has received support as consultant for Philips/Volcano and has received institutional research grants from Philips. The remaining authors have no disclosures to report.
Supporting information
Figures S1–S5
This manuscript was sent to N.A. Mark Estes III, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 13.
Contributor Information
Joo Myung Lee, Email: joomyung.lee@samsung.com.
The ILIAS Registry Investigators:
Tim P. van de Hoef, Joo Myung Lee, Ki Hong Choi, David Hong, Seung Hun Lee, Doosup Shin, Masahiro Hoshino, Tadashi Murai, Tsunekazu Kakuta, Bon Kwon Koo, Doyeon Hwang, Coen K. M. Boerhout, Jan J Piek, Guus A. de Waard, Steven A. J. Chamuleau, Koen Marques, Paul Knaapen, Ji‐Hyun Jung, Hernan Mejia‐Renteria, Javier Escaned, Mauro Echavarria‐Pinto, Martijn Meuwissen, Hitoshi Matsuo, Maribel Madera‐Cambero, Ashkan Eftekhari, Evald H Christiansen, Mohamed A Effat, Joon‐Hyung Doh, Rupak Banerjee, Hyun Kuk Kim, Chang‐Wook Nam, Giampaolo Niccoli, Masafumi Nakayama, Nobuhiro Tanaka, Eun‐Seok Shin, and Niels Royen
References
- 1. Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–744. doi: 10.1093/eurheartj/ehr331 [DOI] [PubMed] [Google Scholar]
- 2. Reynolds HR, Picard MH, Spertus JA, Peteiro J, Lopez Sendon JL, Senior R, El‐Hajjar MC, Celutkiene J, Shapiro MD, Pellikka PA, et al. Natural history of patients with ischemia and no obstructive coronary artery disease: the CIAO‐ISCHEMIA study. Circulation. 2021. doi: 10.1161/CIRCULATIONAHA.120.046791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. doi: 10.1056/NEJMoa0907272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL, Camici PG, Chilian WM, Clayton JA, Cooper LS, Crea F, Di Carli M, et al. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence‐based therapies and research agenda for the next decade. Circulation. 2017;135:1075–1092. doi: 10.1161/circulationaha.116.024534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shimokawa H, Suda A, Takahashi J, Berry C, Camici PG, Crea F, Escaned J, Ford T, Yii E, Kaski JC, et al. Clinical characteristics and prognosis of patients with microvascular angina: an international and prospective cohort study by the Coronary Vasomotor Disorders International Study (COVADIS) Group. Eur Heart J. 2021. doi: 10.1093/eurheartj/ehab282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889 [DOI] [PubMed] [Google Scholar]
- 7. Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–1111. doi: 10.1093/eurheartj/eht513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ford TJ, Stanley B, Sidik N, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, et al. 1‐Year outcomes of angina management guided by invasive coronary function testing (CorMicA). JACC Cardiovasc Interv. 2020;13:33–45. doi: 10.1016/j.jcin.2019.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas A, Prescott E, Karam N, Appelman Y, Fraccaro C, et al. An EAPCI expert consensus document on ischaemia with non‐obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504–3520. doi: 10.1093/eurheartj/ehaa503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 11. Authors/Task Force m , Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, et al. ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;2014(35):2541–2619. doi: 10.1093/eurheartj/ehu278 [DOI] [PubMed] [Google Scholar]
- 12. Shaw LJ, Berman DS, Picard MH, Friedrich MG, Kwong RY, Stone GW, Senior R, Min JK, Hachamovitch R, Scherrer‐Crosbie M, et al. Comparative definitions for moderate‐severe ischemia in stress nuclear, echocardiography, and magnetic resonance imaging. JACC Cardiovasc Imaging. 2014;7:593–604. doi: 10.1016/j.jcmg.2013.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toth GG, Johnson NP, Jeremias A, Pellicano M, Vranckx P, Fearon WF, Barbato E, Kern MJ, Pijls NH, De Bruyne B. Standardization of fractional flow reserve measurements. J Am Coll Cardiol. 2016;68:742–753. doi: 10.1016/j.jacc.2016.05.067 [DOI] [PubMed] [Google Scholar]
- 14. Serruys PW, di Mario C, Piek J, Schroeder E, Vrints C, Probst P, de Bruyne B, Hanet C, Fleck E, Haude M, et al. Prognostic value of intracoronary flow velocity and diameter stenosis in assessing the short‐ and long‐term outcomes of coronary balloon angioplasty: the DEBATE Study (Doppler Endpoints Balloon Angioplasty Trial Europe). Circulation. 1997;96:3369–3377. doi: 10.1161/01.cir.96.10.3369 [DOI] [PubMed] [Google Scholar]
- 15. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1445–1453. doi: 10.1016/j.jcin.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 16. AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook‐Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, et al. Impact of abnormal coronary reactivity on long‐term clinical outcomes in women. J Am Coll Cardiol. 2019;73:684–693. doi: 10.1016/j.jacc.2018.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia‐Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel M‐A, van Es G‐A, Zuckerman B, et al. Standardized end point definitions for coronary intervention trials: the academic research consortium‐2 consensus document. Circulation. 2018;137:2635–2650. doi: 10.1161/CIRCULATIONAHA.117.029289 [DOI] [PubMed] [Google Scholar]
- 18. Neumann F‐J, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J‐P, Falk V, Head SJ, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 19. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477: doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 20. Lin GA, Dudley RA, Lucas FL, Malenka DJ, Vittinghoff E, Redberg RF. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. JAMA. 2008;300:1765–1773. doi: 10.1001/jama.300.15.1765 [DOI] [PubMed] [Google Scholar]
- 21. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jespersen L, Abildstrom SZ, Hvelplund A, Prescott E. Persistent angina: highly prevalent and associated with long‐term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol. 2013;102:571–581. doi: 10.1007/s00392-013-0568-z [DOI] [PubMed] [Google Scholar]
- 23. Brainin P, Frestad D, Prescott E. The prognostic value of coronary endothelial and microvascular dysfunction in subjects with normal or non‐obstructive coronary artery disease: a systematic review and meta‐analysis. Int J Cardiol. 2018;254:1–9. doi: 10.1016/j.ijcard.2017.10.052 [DOI] [PubMed] [Google Scholar]
- 24. Echavarria‐Pinto M, Escaned J, Macías E, Medina M, Gonzalo N, Petraco R, Sen S, Jimenez‐Quevedo P, Hernandez R, Mila R, et al. Disturbed coronary hemodynamics in vessels with intermediate stenoses evaluated with fractional flow reserve: a combined analysis of epicardial and microcirculatory involvement in ischemic heart disease. Circulation. 2013;128:2557–2566. doi: 10.1161/CIRCULATIONAHA.112.001345 [DOI] [PubMed] [Google Scholar]
- 25. van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SAJ, Voskuil M, Henriques JPS, Koch KT, de Winter RJ, et al. Physiological basis and long‐term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014;7:301–311. doi: 10.1161/CIRCINTERVENTIONS.113.001049 [DOI] [PubMed] [Google Scholar]
- 26. Lee JM, Choi KH, Hwang D, Park J, Jung JH, Kim HY, Jung HW, Cho YK, Yoon HJ, Song YB, et al. Prognostic implication of thermodilution coronary flow reserve in patients undergoing fractional flow reserve measurement. JACC Cardiovasc Interv. 2018;11:1423–1433. doi: 10.1016/j.jcin.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 27. Lee JM, Jung JH, Hwang D, Park J, Fan Y, Na SH, Doh JH, Nam CW, Shin ES, Koo BK. Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol. 2016;67:1158–1169. doi: 10.1016/j.jacc.2015.12.053 [DOI] [PubMed] [Google Scholar]
- 28. Camici PG, Tschöpe C, Di Carli MF, Rimoldi O, Van Linthout S. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc Res. 2020;116:806–816. doi: 10.1093/cvr/cvaa023 [DOI] [PubMed] [Google Scholar]
- 29. Lee SH, Lee JM, Park J, Choi KH, Hwang D, Doh J‐H, Nam C‐W, Shin E‐S, Hoshino M, Murai T, et al. Prognostic implications of resistive reserve ratio in patients with coronary artery disease. J Am Heart Assoc. 2020;9:e015846. doi: 10.1161/jaha.119.015846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Escaned J, Flores A, García‐Pavía P, Segovia J, Jimenez Jesús, Aragoncillo P, Salas C, Alfonso F, Hernández R, Angiolillo DJ, et al. Assessment of microcirculatory remodeling with intracoronary flow velocity and pressure measurements: validation with endomyocardial sampling in cardiac allografts. Circulation. 2009;120:1561–1568. doi: 10.1161/CIRCULATIONAHA.108.834739 [DOI] [PubMed] [Google Scholar]
- 31. Pries AR, Badimon L, Bugiardini R, Camici PG, Dorobantu M, Duncker DJ, Escaned J, Koller A, Piek JJ, de Wit C. Coronary vascular regulation, remodelling, and collateralization: mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur Heart J. 2015;36:3134–3146. doi: 10.1093/eurheartj/ehv100 [DOI] [PubMed] [Google Scholar]
- 32. Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/atvbaha.107.159327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gimbrone MA Jr, García‐Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. doi: 10.1161/circresaha.115.306301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dhawan SS, Corban MT, Nanjundappa RA, Eshtehardi P, McDaniel MC, Kwarteng CA, Samady H. Coronary microvascular dysfunction is associated with higher frequency of thin‐cap fibroatheroma. Atherosclerosis. 2012;223:384–388. doi: 10.1016/j.atherosclerosis.2012.05.034 [DOI] [PubMed] [Google Scholar]
- 37. Taqueti VR, Ridker PM. Inflammation, coronary flow reserve, and microvascular dysfunction: moving beyond cardiac syndrome X. JACC Cardiovasc Imaging. 2013;6:668–671. doi: 10.1016/j.jcmg.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Camici PG, Olivotto I, Rimoldi OE. The coronary circulation and blood flow in left ventricular hypertrophy. J Mol Cell Cardiol. 2012;52:857–864. doi: 10.1016/j.yjmcc.2011.08.028 [DOI] [PubMed] [Google Scholar]
- 39. Yasue H, Takizawa A, Nagao M, Nishida S, Horie M, Kubota J, Omote S, Takaoka K, Okumura K. Long‐term prognosis for patients with variant angina and influential factors. Circulation. 1988;78:1–9. doi: 10.1161/01.cir.78.1.1 [DOI] [PubMed] [Google Scholar]
- 40. Japanese Circulation Society . Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779–2801. doi: 10.1253/circj.cj-66-0098 [DOI] [PubMed] [Google Scholar]
- 41. Cho SW, Park TK, Gwag HB, Lim AY, Oh MS, Lee DH, Seong CS, Yang JH, Song YB, Hahn J‐Y, et al. Clinical outcomes of vasospastic angina patients presenting with acute coronary syndrome. J Am Heart Assoc. 2016;5. doi: 10.1161/jaha.116.004336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hung MJ, Hung MY, Cheng CW, Yang NI, Cherng WJ. Comparison of clinical characteristics and prognosis in Taiwanese patients with coronary vasospastic angina pectoris without significant fixed coronary artery disease versus patients with significant fixed coronary artery disease and either stable angina pectoris or acute coronary syndromes. Am J Med Sci. 2007;334:160–167. doi: 10.1097/MAJ.0b013e3181405b30 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S5


