Abstract
Background
Ongoing exercise intolerance of unclear cause following COVID‐19 infection is well recognized but poorly understood. We investigated exercise capacity in patients previously hospitalized with COVID‐19 with and without self‐reported exercise intolerance using magnetic resonance–augmented cardiopulmonary exercise testing.
Methods and Results
Sixty subjects were enrolled in this single‐center prospective observational case‐control study, split into 3 equally sized groups: 2 groups of age‐, sex‐, and comorbidity‐matched previously hospitalized patients following COVID‐19 without clearly identifiable postviral complications and with either self‐reported reduced (COVIDreduced) or fully recovered (COVIDnormal) exercise capacity; a group of age‐ and sex‐matched healthy controls. The COVIDreducedgroup had the lowest peak workload (79W [Interquartile range (IQR), 65–100] versus controls 104W [IQR, 86–148]; P=0.01) and shortest exercise duration (13.3±2.8 minutes versus controls 16.6±3.5 minutes; P=0.008), with no differences in these parameters between COVIDnormal patients and controls. The COVIDreduced group had: (1) the lowest peak indexed oxygen uptake (14.9 mL/minper kg [IQR, 13.1–16.2]) versus controls (22.3 mL/min per kg [IQR, 16.9–27.6]; P=0.003) and COVIDnormal patients (19.1 mL/min per kg [IQR, 15.4–23.7]; P=0.04); (2) the lowest peak indexed cardiac output (4.7±1.2 L/min per m2) versus controls (6.0±1.2 L/min per m2; P=0.004) and COVIDnormal patients (5.7±1.5 L/min per m2; P=0.02), associated with lower indexed stroke volume (SVi:COVIDreduced 39±10 mL/min per m2 versus COVIDnormal 43±7 mL/min per m2 versus controls 48±10 mL/min per m2; P=0.02). There were no differences in peak tissue oxygen extraction or biventricular ejection fractions between groups. There were no associations between COVID‐19 illness severity and peak magnetic resonance–augmented cardiopulmonary exercise testing metrics. Peak indexed oxygen uptake, indexed cardiac output, and indexed stroke volume all correlated with duration from discharge to magnetic resonance–augmented cardiopulmonary exercise testing (P<0.05).
Conclusions
Magnetic resonance–augmented cardiopulmonary exercise testing suggests failure to augment stroke volume as a potential mechanism of exercise intolerance in previously hospitalized patients with COVID‐19. This is unrelated to disease severity and, reassuringly, improves with time from acute illness.
Keywords: cardiopulmonary exercise testing, cardiovascular magnetic resonance imaging, COVID‐19, exercise, stroke volume
Subject Categories: Exercise Testing, Magnetic Resonance Imaging (MRI), Physiology
Nonstandard Abbreviations and Acronyms
- ∆avO2
arteriovenous oxygen content gradient
- CO
cardiac output
- COi
indexed cardiac output
- COVIDnormal
normal exercise capacity following COVID‐19
- COVIDreduced
reduced exercise capacity following COVID‐19
- CPET
cardiopulmonary exercise testing
- iVO2
indexed oxygen uptake
- MR
magnetic resonance
- MR‐CPET
magnetic resonance‐cardiopulmonary exercise testing
- SV
stroke volume
- VO2
oxygen consumption
Clinical Perspective
What Is New?
The importance of stroke volume augmentation in the normal response to exercise is emphasized by this magnetic resonance–augmented cardiopulmonary exercise test study.
The magnetic resonance–augmented cardiopulmonary exercise test demonstrated reduced systolic blood pressure augmentation in patients who had previously been hospitalized with COVID‐19 infection and with self‐reported ongoing reduced exercise capacity.
There were no magnetic resonance–augmented cardiopulmonary exercise test findings to suggest impaired contractile reserve, exercise diastolic dysfunction, or abnormalities of peak afterload in these patients, suggesting that inadequate preload could be a possible mechanism underlying reduced systolic blood pressure augmentation and, consequently, exercise intolerance.
What Are the Clinical Implications?
The comprehensive evaluation of unexplained dyspnea and exercise intolerance is pivotal to help correctly identify potential therapeutic targets in these patients.
Although the causes of failure to augment systolic blood pressure on exercise require further investigation in these patients, the findings do raise the possibility of interventions tailored to volume expansion being explored in future studies.
Reassuringly, abnormalities in peak magnetic resonance–augmented cardiopulmonary exercise test metrics may improve with time from the acute illness and are unrelated to markers of disease severity.
Ongoing exercise intolerance is recognized following COVID‐19, with a significant proportion of hospitalized patients reporting reduced exercise capacity after discharge. 1 In addition, reduced peak oxygen consumption (VO2) has been demonstrated after resolution of acute COVID‐19 infection. 2 This can partly be explained by residual lung parenchymal damage, myocardial injury, or sequelae from pulmonary thromboembolic disease. 3 , 4 , 5 , 6 However, exercise intolerance is also described in patients without clearly identifiable postviral complications. 7 In this group, possible causes include: (1) failure to augment cardiac output (CO) attributable to reduced contractile or preload reserve and; (2) impaired skeletal muscle oxygen extraction caused by skeletal muscle dysfunction. Unfortunately, identifying and understanding the relative importance of these potential mechanisms is difficult with conventional cardiopulmonary exercise testing (CPET) as only VO2 is directly measured.
We have developed a novel technique that combines exercise cardiovascular magnetic resonance (MR) with CPET (MR‐CPET), allowing the simultaneous measurement of VO2 and CO during exercise. 8 , 9 These data can then be used to calculate arteriovenous oxygen content gradient (∆avO2), a recognized marker of tissue oxygen extraction. 10 MR‐CPET provides a comprehensive evaluation of the cardiopulmonary exercise response, affording a better understanding of exercise intolerance. In addition, MR‐CPET also allows the accurate evaluation of biventricular volumes and function during exercise. These data can be useful in understanding CO augmentation in terms of both contractile and preload reserve.
The aim of this study was to use MR‐CPET to investigate exercise capacity in patients hospitalized due to COVID‐19 infection, with and without self‐reported exercise intolerance, through comparison with a healthy control group.
Methods
Study Population
Sixty subjects were prospectively recruited into this single‐center prospective observational case‐control study and split into 3 equally sized groups: (1) self‐reported reduced exercise capacity following COVID‐19 infection (COVIDreduced); (2) normal exercise capacity following COVID‐19 infection (COVIDnormal); and (3) age‐ and sex‐matched normal controls. Patients who had recovered from COVID‐19 were recruited from the Royal Free London NHS Foundation Trust COVID‐19 follow‐up clinic between January and May 2021. Patients in the post–COVID‐19 groups were matched for age, sex, and the presence of diabetes and hypertension. Inclusion criteria for patients following COVID‐19 were: (1) previous hospitalization with COVID‐19 diagnosed by positive combined oro/nasopharyngeal swab for severe acute respiratory syndrome coronavirus 2 by reverse‐transcriptase‐polymerase chain reaction; (2) age 18 to 80 years. Exclusion criteria were: (1) radiological evidence of residual lung parenchymal disease secondary to COVID‐19; (2) troponin positivity during hospital admission; (3) admission to an intensive care unit for invasive ventilation; (4) diagnosis of pulmonary embolism during acute COVID‐19 illness; (5) impaired left ventricular (LV) or right ventricular (RV) systolic function on resting cardiovascular magnetic resonance (CMR); (6) general contraindications to MR scanning; (7) pre‐existing contraindications to performing exercise (eg, unstable symptoms including angina, exertional syncope); (8) previous known cardiovascular disease (including ischemic, nonischemic, and moderate to severe valvular disease); and (9) significant lung parenchymal disease that may confound CPET results, such as emphysema or interstitial lung disease (defined as >20% lung volume on computed tomography imaging).
We also recruited 20 uninfected age‐ and sex‐matched healthy control subjects who had no history of COVID‐19 and no history of cardiovascular disease, hypertension, or diabetes through advertisement by our institution. Fourteen (70%) control subjects underwent MR‐CPET before the pandemic. The 6 postpandemic controls had not experienced any prior symptoms or previously been diagnosed with COVID‐19.
The study was approved by national ethics committee (IRAS project ID 226101; REC reference 17/LO/1499, National Health Service Health Research Authority UK CRN 058274). All subjects provided written informed consent. The study data set, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure upon reasonable request to the corresponding author, subject to institutional and ethical committee approvals.
Clinical Assessment
All patients underwent a patient symptom perception questionnaire, 6‐minute walk test, blood tests, and spirometry. Patients gave an overall percentage score of how they felt at the time of MR‐CPET study compared with premorbid baseline. Patient clinical histories, comorbidities, and in‐patient blood test results were obtained from patient electronic records systems. Physician‐determined severity of acute COVID‐19 disease was defined by World Health Organization guidelines. 11
MR‐Augmented Cardiopulmonary Exercise Testing
Imaging was performed on a 1.5 Tesla MR scanner (Magnetom Aera, Siemens Healthcare, Erlangen, Germany) in a temperature‐controlled suite. Full resuscitation facilities were available with peripheral venous access sited for emergency and ECG continuously monitored on the MR scanner. Systolic blood pressure was recorded at rest and peak using a MR‐compatible blood pressure monitor (Expression MR400, Philips Healthcare, Best, The Netherlands).
MR Imaging Techniques (Real‐Time Flow and Volume Imaging)
Before exercise, subjects underwent routine CMR with long‐ and short‐axis cine imaging and myocardial native T1 and T2 mapping. 12 , 13 T1 mapping used the modified Look‐Locker inversion recovery sequence after regional shimming with 5s(3s)3s sampling. 14 T2 mapping used single‐shot T2‐prepared images with varying T2 preparation. 15
All exercise CMR was performed with real‐time sequences and was acquired during free breathing. Aortic flow was measured using real‐time phase‐contrast MR at baseline and at peak exercise using a uniform‐density golden‐angle spiral sequence, with a compressive sensing reconstruction. 9 , 16 The following parameters were used: matrix=192×192, slice thickness=7 mm, repetition time/echo time=9.8/1.6 ms, flip angle=25°, velocity encoding=300 cm/s, temporal resolution=~41 ms, spatial resolution=2.3×2.3 mm, and acceleration factor=6. Real‐time assessment of biventricular volumes was performed immediately after real‐time flow acquisition at rest and at peak exercise using a 2‐dimensional multislice, real‐time, tiny golden‐angle spiral compressive sensing balanced steady‐state free precession sequence. 9 , 17 The following parameters were used: matrix=208×208, slice thickness=8 mm, repetition time/echo time=3.4/0.7 ms, flip angle=67°, temporal resolution=~31 ms, spatial resolution=1.7×1.7 mm, and acceleration factor=8. Up to 14 slices were used in each acquisition, with each slice being acquired over 2 R‐R intervals.
Aortic flow and short‐axis image data were reconstructed offline (MATLAB R2018a, MathWorks Inc, Natick, MA), using the Berkeley Advanced Reconstruction Toolbox. 18 Reconstructed images were exported as Digital Imaging and Communications in Medicine files and analyzed on reporting workstations.
Respiratory Gas Analysis
Breath‐by‐breath gas exchange analysis was performed using a commercial CPET system (Ultima, MedGraphics, St Paul, MN). The analyzer was placed in the MR control room and attached to the facemask (Hans Rudolph, Kansas City, KS) via bespoke MR‐compatible sampling tubes passed through the waveguide. The sampling tubes were modified as previously described 8 , 9 and manufacturer‐tested to meet all quality control standards. Gas and flow calibrations were performed before each test and at least 30 minutes after system initiation. All measurements were taken at body temperature and ambient pressure.
Exercise Protocol
Subjects exercised on a supine MR‐compatible cycle ergometer (MR Cardiac Ergometer Pedal, Lode, Groningen, the Netherlands). Exercise workload (power measured in watts) was controlled by altering resistance depending on cadence. The exercise protocol (Figure 1) was followed until exhaustion (peak exercise) with MR measurements performed at rest and peak and VO2 measured continuously.
Figure 1. Magnetic resonance‐cardiopulmonary exercise testing (MR‐CPET) exercise protocol.

A, The protocol was split in 2‐minute stages. During each stage, workload was increased at 0, 30, and 60 seconds. B, Workload increments by stage. Smaller increments at the start of the protocol were designed to ensure that subjects with significant exercise intolerance were able to complete at least 2 exercise stages. C, Cumulative workload as a function of time.
Data Processing
All postprocessing of reconstructed images was performed using “in‐house” plug‐ins for OsiriX DICOM software version 9.0.1 (OsiriX Foundation, Geneva, Switzerland). 19 , 20 , 21 Aortic phase‐contrast MR flow data was segmented using a semiautomatic method with manual operator correction. Stroke volume (SV) was calculated by integrating the flow curve across a single R‐R interval; cardiac output was given by stroke volume×heart rate (CO=SV×HR). Biventricular endocardial borders were traced manually on short‐axis images at end‐diastole and end‐systole, identified by visual assessment of the largest and smallest cavity areas, respectively. Biventricular SV, ejection fraction, and mass were calculated as previously described, with papillary muscles and trabeculae excluded from the blood pool. 9 Postprocessing of SV data obtained by aortic phase‐contrast MR flow imaging and by volumetry of cine images was performed in tandem to maintain internal consistency as suggested by Society for Cardiovascular Magnetic Resonance Task Force recommendations. 22 Biatrial areas were traced on a 4‐chamber cine image at end‐systole. Native myocardial T1 and T2 relaxation times were measured by drawing a single region of interest within the midportion of the interventricular septum on the midcavity short‐axis maps. 13 All measurements were reported by an experienced clinical CMR specialist (D.K.) blinded to clinical information. All volumetric data and CO were indexed to body surface area and denoted by the suffix ‐i.
VO2 and respiratory exchange ratio measurements were time registered to CMR data. The VO2 was indexed to body weight and denoted by the prefix ‐i. Arteriovenous oxygen content gradient was calculated as ∆avO2=VO2/CO (using nonindexed data) at rest and peak exercise for all subjects.
Statistical Analysis
All statistical analysis was performed using R version 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria). Data were examined for normality using the Shapiro‐Wilk normality test. Descriptive statistics were expressed as mean (±SD) for normally distributed data and median (interquartile range) for non‐normally distributed data. Between‐group differences in demographic data, clinical metrics, and MR‐CPET metrics (at rest and exercise) were assessed using either 1‐way ANOVA for normal data or the Kruskal‐Wallis test for non‐normal data. Post hoc comparisons were performed using pairwise t tests (normal data) and Mann‐Whitney tests (non‐normal data) with Benjamini Hochberg correction for multiple comparisons. When only comparing post–COVID‐19 groups, t tests (normal data) and Mann‐Whitney tests (non‐normal data) were used. Sex distribution between the groups was assessed using the chi‐squared test. Correlation between metrics corrected for diagnosis was computed using multilevel Spearman’s rank partial correlation coefficient. A P value <0.05 was considered statistically significant.
Results
Demographics and Clinical Data
There were no differences in age, sex, height, weight, or body surface area among the 3 groups and no differences in comorbidities between post–COVID‐19 patient groups (Table 1). Clinical data for the post–COVID‐19 groups are shown in Table 2. The only differences were significantly lower perceived functional recovery (70% of baseline [Interquartile range (IQR), 60–80] versus 98% [IQR, 90–100]; P<0.001) and 6‐minute walk test (470±87meters versus 560±117 meters; P=0.009) in the COVIDreduced group. There were no differences in any markers of disease severity between post–COVID‐19 patient groups. There were also no significant differences in blood biomarkers at the time of MR‐CPET between patient groups. However, there was a trend toward a greater length of time from discharge to MR‐CPET in the COVIDnormal group (115 days [IQR, 96–151] versus 97 days [IQR, 80–116]; P=0.07).
Table 1.
Patient Characteristics
| Control | COVIDreduced | COVIDnormal | P value | |
|---|---|---|---|---|
| Age, y | 51±4 | 52±14 | 52±8 | 0.98 |
| Weight, kg | 73±13 | 79±13 | 80±20 | 0.23 |
| Height, m | 1.7±0.1 | 1.7±0.1 | 1.7±0.1 | 0.87 |
| BSA, m2 | 1.8±0.2 | 1.9±0.2 | 1.9±0.3 | 0.19 |
| Female, n (%) | 12 (60) | 11 (55) | 11 (55) | >0.99 |
| Diabetes, n (%) | … | 1 (5) | 1 (5) | >0.99 |
| Hypertension, n (%) | … | 6 (30) | 3 (15) | 0.45 |
Normally distributed data displayed as mean±SD. BSA indicates body surface area; COVIDnormal, normal exercise capacity following COVID‐19; and COVIDreduced, reduced exercise capacity following COVID‐19.
Table 2.
Clinical Data for Patient Groups
| COVIDreduced | COVIDnormal | P value | |
|---|---|---|---|
| Perception of recovery (% of normal) | 70 (60–80) | 98 (90–100) | <0.001* |
| 6‐min walk distance (m) | 470±87 | 560±117 | 0.0089* |
| Duration from discharge to MR‐CPET (d) | 97 (80–116) | 115 (96–151) | 0.068 |
| Severity of acute COVID‐19 illness | |||
| Length of admission (d) | 3.5 (1.0–5.2) | 4.0 (0.8–5.0) | 0.87 |
| Severity score | 4.0 (2.0–4.0) | 4.0 (2.0–4.0) | 0.69 |
| Peak d‐dimer, ng/mL | 576 (373–1160) | 496 (463–1020) | 0.91 |
| Peak CRP, mg/L | 64 (21–116) | 45 (23–133) | 0.95 |
| Current investigations | |||
| FEV1 (% predicted) | 97 (91–102) | 93 (88–100) | 0.49 |
| FVC (% predicted) | 88 (79–93) | 80 (76–93) | 0.39 |
| Hemoglobin, g/L | 141±13 | 137±16 | 0.42 |
| Creatinine, μmol/L | 73±13 | 67±15 | 0.16 |
| CK, units/L | 93 (71–110) | 107 (73–157) | 0.29 |
| NT‐proBNP, ng/L | 49 (49–122) | 49 (49–64) | 0.38 |
Normally distributed data displayed as mean±SD and non‐normally distributed data shown as median (interquartile range). CK indicates creatine kinase; COVIDnormal, normal exercise capacity following COVID‐19; COVIDreduced, reduced exercise capacity following COVID‐19; CRP, C‐reactive protein; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; MR‐CPET, magnetic resonance–augmented cardiopulmonary exercise test; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Significant difference between post‐COVID‐19 patient groups.
There were no significant differences in spirometry metrics at the time of MR‐CPET between the 2 post–COVID‐19 patient groups. Two patients in the COVIDreduced group and 4 patients in the COVIDnormal group had normal index chest radiographs. As per clinical patient follow‐up protocol, they did not have follow‐up chest imaging. The remaining 34(85%) patients had outpatient follow‐up chest radiographs, all of which were normal.
Resting MR‐CPET
Resting CMR metrics are shown in Table 3. The main differences in functional metrics between the 2 post‐COVID‐19 patient groups and controls were higher LV ejection fraction (COVIDreduced 68%±7% versus COVIDnormal 69%±6% versus controls 61%±7%; P≤0.004) and RV mass (COVIDreduced 25±5 g versus COVIDnormal 27±5 g versus controls 21±6 g; P≤0.02). There was also higher native myocardial T1 in the COVIDnormal group versus controls (COVIDnormal 1016±25 ms versus controls 994±22ms; P=0.04). There were no significant group differences in systolic blood pressure , biventricular size, biatrial size, indexed SV, heart rate, indexed oxygen uptake (iVO2), or ∆avO2 at rest.
Table 3.
Resting MR‐CPET Data
| Variable | Control | COVIDreduced | COVIDnormal | P value |
|---|---|---|---|---|
| iVO2, mL/min per kg | 3.2±0.5 | 3.1±0.8 | 3.6±0.7 | 0.14 |
| COi, L/min per m2 | 2.4 (2.2–2.8) | 2.5 (2.3–3.0) | 2.9 (2.4–3.2) | 0.14 |
| avO2, mlO2/100 mL | 5.2±1.0 | 4.8±1.2 | 5.1±0.9 | 0.44 |
| SVi, mL/min per m2 | 39±9 | 36±7 | 40±7 | 0.35 |
| Heart rate, bpm | 67±12 | 73±12 | 73±13 | 0.21 |
| Systolic BP, mm Hg | 124 (117–128) | 128 (121–138) | 120 (116–126) | 0.24 |
| RVEDVi, mL/m2 | 65 (54–75) | 59 (52–69) | 64 (56–69) | 0.52 |
| RVESVi, mL/m2 | 24 (21–30) | 20 (18–30) | 22 (20–28) | 0.29 |
| RVSVi, mL/m2 | 38±9 | 37±7 | 40±8 | 0.48 |
| LVEDVi, mL/m2 | 64±15 | 56±11 | 59±10 | 0.13 |
| LVESVi, mL/m2 | 25±7 | 18±6† | 19±4.6† | 0.0026 * |
| LVSVi, mL/m2 | 39±9 | 37±7 | 40±7 | 0.55 |
| LVEF (%) | 61±7 | 68±7† | 69±6† | <0.001 * |
| RVEF (%) | 58±7 | 62±8 | 63±6 | 0.087 |
| Septal T2, ms | 46 (44–46) | 46 (44–47) | 46 (45–48) | 0.58 |
| Septal T1, ms | 994±22 | 1007±29 | 1016±25† | 0.045 * |
| LV mass, g/m2 | 56±15 | 54±9 | 58±10 | 0.61 |
| RV mass, g/m2 | 21±6 | 25±5 † | 27±5† | 0.003 * |
| LA area, cm/m2 | 11±2 | 10±2 | 11±2 | 0.14 |
| RA area, cm/m2 | 11 (9–12) | 9 (8–11) | 10 (8–11) | 0.17 |
The P value is for the appropriate omnibus test. Normally distributed data displayed as mean±SD and non‐normally distributed data shown as median (interquartile range). avO2 indicates tissue oxygen extraction; BP, blood pressure; COi, cardiac output indexed to body surface area (BSA); COVIDnormal, normal exercise capacity following COVID‐19; COVIDreduced, reduced exercise capacity following COVID‐19; iVO2, oxygen consumption indexed to weight; LA, left atrial; LVEDVi, BSA indexed left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESVi, BSA indexed left ventricular end‐systolic volume; LVSVi, BSA indexed left ventricular stroke volume; MR‐CPET, magnetic resonance‐cardiopulmonary exercise testing; RA, right atrial; RVEDVi, BSA indexed right ventricular end‐diastolic volume; RVEF, right ventricular ejection fraction; RVESVi, BSA indexed right ventricular end‐systolic volume; RVSVi, BSA indexed right ventricular stroke volume; SVi, BSA indexed stoke volume.
Significant P value for the omnibus test.
Significant difference between controls and indicated patient groups.
Exercise Feasibility
All subjects successfully completed the exercise protocol. No subjects required medical intervention. All subjects achieved maximal respiratory exchange ratio ≥1.0. Comparisons of exercise duration and peak workload are shown in Table 4. The COVIDreduced group had the lowest peak workload, which was significantly different from controls (COVIDreduced 79W [IQR, 65–100] versus controls 104W [IQR, 86–148]; P=0.01) and shortest exercise duration (COVIDreduced 13.3±2.8 minutes versus controls 16.6±3.5 minutes; P=0.008). There were no differences in peak workload or exercise duration patients between patients with COVIDnormal and controls.
Table 4.
Exercise MR‐CPET Data
| Variable | Control | COVIDreduced | COVIDnormal | P value |
|---|---|---|---|---|
| Metrics of exercise performance | ||||
| Exercise duration (min) | 16.6±3.5 | 13.3±2.8† | 15.1±3.8 | 0.01 * |
| Peak workload (W) | 104 (86–148) | 79 (65–100)† | 104 (71–134) | 0.017 * |
| Maximum RER | 1.6±0.2 | 1.4±0.2† | 1.5±0.2 | 0.043 * |
| MR‐CPET metrics | ||||
| iVO2, mL/min per kg | 22.3 (16.9–27.6) | 14.9 (13.1–16.2)† , ‡ | 19.1 (15.4–23.7) | 0.0037 * |
| COi, L/min per m2 | 6.0±1.2 | 4.7±1.2† , ‡ | 5.7±1.5 | 0.0041 * |
| avO2, mlO2/100 mL | 14.8±3.9 | 13.5±3.4 | 13.8±2.8 | 0.46 |
| SVi, mL/min per m2 | 47.7±10.4 | 39.1±10.0† | 43.2±7.4 | 0.02 * |
| Heart rate, bpm | 130±22 | 122±22 | 132±24 | 0.35 |
| Systolic BP, mm Hg | 145±22 | 150±26 | 154±16 | 0.37 |
| RVEDVi, mL/m2 | 59 (53–66) | 52 (50–56) | 59 (48–63) | 0.098 |
| LVEDVi, mL/m2 | 62±13 | 52±11† | 56±11 | 0.023 * |
| LVEF (%) | 76±8 | 74±10 | 78±8 | 0.31 |
| RVEF (%) | 76 (69–84) | 75 (70–79) | 76 (72–81) | 0.55 |
The P value is for the appropriate omnibus test. Normally distributed data displayed as mean±SD and non‐normally distributed data shown as median (interquartile range). avO2 indicates tissue oxygen extraction; BP, blood pressure; COi, cardiac output indexed to body surface area (BSA); COVIDnormal, normal exercise capacity following COVID‐19; COVIDreduced, reduced exercise capacity following COVID‐19; iVO2, oxygen consumption indexed to weight; LVEDVi, BSA indexed left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; MR‐CPET, magnetic resonance‐cardiopulmonary exercise testing; RER, Respiratory Exchange Ratio; RVEDVi, BSA indexed right ventricular end‐diastolic volume; RVEF, right ventricular ejection fraction; and SVi, BSA indexed stoke volume.
Significant P value for the omnibus test.
Significant difference compared with controls.
Significant difference between COVID patient groups.
Exercise MR‐CPET Metrics
MR‐CPET metrics at peak exercise are shown in Table 4 and Figure 2. The COVIDreduced group had the lowest peak iVO2 (14.9 mL/min per kg [IQR, 13.1–16.2]), significantly different from healthy controls (22.3 mL/min/kg [16.9–27.6]; P=0.003) and patients with COVIDnormal (19.1 mL/min per kg [IQR, 15.4–23.7]; P=0.04). The COVIDreduced group also had lower peak indexed cardiac output (COi) (4.7±1.2 L/min per m2) compared with controls (6.0±1.2 L/min per m2; P=0.004) and patients with COVIDnormal (5.7±1.5 L/min per m2; P=0.02). Lower peak COi during exercise was associated with lower indexed SV in patients with COVIDreduced (39±10 mL/min per m2) compared with controls (48±10 mL/min per m2; P=0.02). Patients with COVIDreduced also had lower body surface area indexed LV end‐diastolic volume than controls (52±11 mL/min per m2 versus 62±13 mL/min per m2; P=0.02) and a trend toward lower indexed RV end diastolic volume (52 mL/min per m2 [IQR, 50–56] versus 59 mL/min per m2 [53–66]; P=0.1). There were no differences in peak ∆avO2, systolic blood pressure, heart rate, LV ejection fraction, or RV ejection fraction between groups.
Figure 2. MR‐CPET metrics at rest and peak exercise for each subject group.
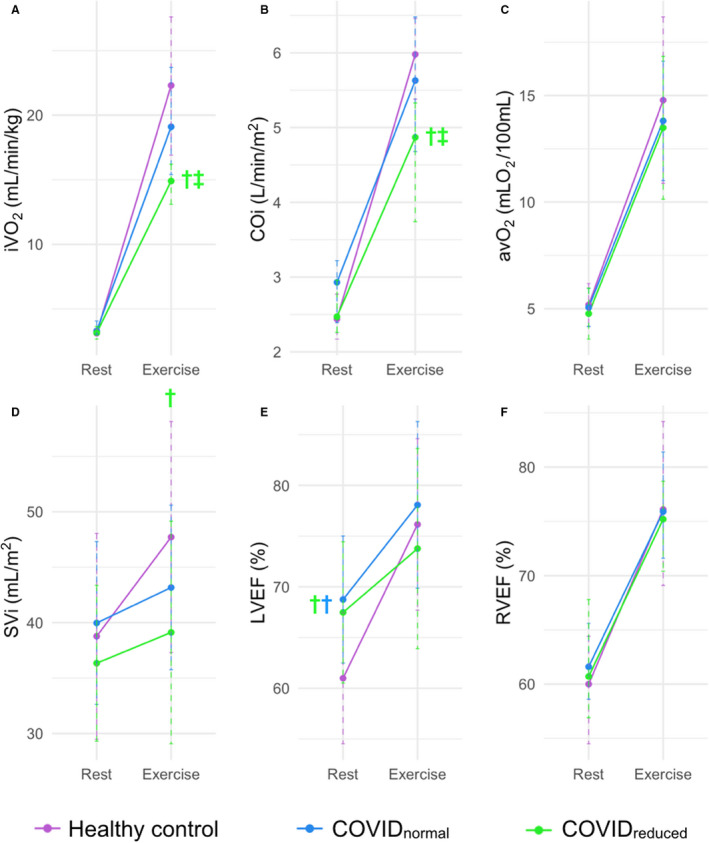
A, Oxygen consumption indexed to weight (iVO2). B, Cardiac output indexed to body surface area (COi). C, Arteriovenous oxygen gradient (∆avO2). D, Stroke volume indexed to body surface area (SVi). E, Left ventricular ejection fraction (LVEF). F, Right ventricular ejection fraction (RVEF). †Significant difference between the color‐coded group and controls. ‡Significant difference between COVID patient groups. COVIDnormal indicates normal exercise capacity following COVID‐19; and COVIDreduced, reduced exercise capacity following COVID‐19.
Current Clinical Status and MR‐CPET Metrics
Peak iVO2 (r=0.73, P<0.001), COi (r=0.41, P=0.008), ∆avO2 (r=0.46, P=0.003), and heart rate (r=0.49, P=0.001) all correlated with the 6‐minute walk test after adjusting for diagnosis. There were no significant correlations between the 6‐minute walk test and peak RV ejection fraction, LV ejection fraction, or indexed SV. There were also no significant correlations between predicted forced vital capacity or forced expiratory volume during the first second and any peak‐exercise MR‐CPET metrics.
Disease Severity and MR‐CPET Metrics
There was no association between measures of acute COVID‐19 illness severity (length of admission and disease severity score) and any peak exercise MR‐CPET metrics. However, peak iVO2 (r=0.37, P=0.02), COi (r=0.37, P=0.02) and indexed SV (r=0.32, P<0.05) all correlated with length of time from discharge to MR‐CPET.
Discussion
The COVID‐19 pandemic has caused significant mortality and morbidity worldwide. 23 Following resolution of acute infection, ongoing exercise intolerance and failure to return to functional baseline are commonly described. 2 , 3 However, many patients have no obvious causes to account for these symptoms. 7 We used MR‐CPET to evaluate potential mechanisms of exercise limitation in patients previously hospitalized with COVID‐19 and without obvious resting cardiopulmonary pathological sequelae. The main findings of this study were: (1) the COVIDreduced patient group had significantly lower peak iVO2 than the COVIDnormal group and healthy controls; (2) this was attributable to lower peak COi during exercise, resulting from a failure to augment SV; (3) there were no abnormalities in cardiac contractile reserve, lung function, or ∆avO2 to account for lower peak iVO2 in COVIDreduced patients; and (4) the deleterious effect on exercise capacity was unrelated to acute COVID‐19 illness severity and lessened over time.
Peak VO2 is an objective marker of exercise capacity that is determined by lung capacity, cardiac function, and peripheral tissue oxygen extraction. Studies have demonstrated reduced peak VO2 in patients who have recovered from acute COVID‐19 infection. 2 , 24 Although residual cardiovascular and pulmonary damage could explain these findings, 3 , 4 our study was designed to investigate exercise intolerance in patients without immediately attributable causes. Thus, we hoped to identify “subclinical” pathophysiology that might explain decreased exercise capacity in COVIDreduced patients.
We demonstrated no differences in spirometry metrics between the 2 post–COVID‐19 patient groups, implying that persistent lung damage was not the cause of exercise limitation. We also found no difference in peak ∆avO2 between any of the groups, suggesting that skeletal muscle function was normal in our patients following COVID‐19. This contrasts with a recent invasive CPET study that demonstrated impaired ∆avO2 in a group of patients who were exercise intolerant following COVID‐19. 24 However, there were some notable differences between the studied cohorts. First, most patients in the invasive CPET study had not been hospitalized during their acute COVID‐19 illness. Second, the time from illness to examination was markedly longer in the invasive CPET study (≈11 months) compared with our medium‐term follow‐up cohort (≈3 months). Nevertheless, the differences suggest heterogeneity in the pathophysiology of persistent exercise intolerance following COVID‐19. This is in keeping with the finding of different exercise phenotypes in patients with postviral chronic fatigue syndrome, characterized as either low flow (with underlying low biventricular filling) or high flow (associated with impaired systemic oxygen extraction). 25 Finally, while both studies use a cycle ergometer, MR‐CPET is performed supine rather than conventional upright cycling. This affects exercise physiology, such as aerobic work efficiency and muscle deoxygenation response, 26 , 27 and may account for some differences between study findings.
In our study, reduced iVO2 in COVIDreduced patients appeared to be attributable to a failure to augment CO. This was caused by an inability to increase SV during exercise, with no evidence of chronotropic incompetence. The most obvious reason for reduced SV augmentation is impaired contractile reserve. This is particularly pertinent considering the high prevalence of post–COVID‐19 CMR abnormalities, including myocarditis. 28 However, we excluded patients with evidence of troponin positivity during their hospital admission. It is worth noting that both patient groups had the higher resting LV ejection fraction, RV mass, and native myocardial T1. While these parameters were in keeping with reference ranges in the general population, 4 , 29 the differences versus the controls indicate that the patient groups did not have completely “normal” hearts. This might represent the background health status of these previously hospitalized patients and could possibly be suggestive of subclinical cardiovascular disease. Nevertheless, peak exercise biventricular ejection fractions were the same in all groups, and there were no differences in RV mass and T1 between the patients with and without exercise intolerance. These findings go against the idea of contractile dysfunction being the cause of reduced CO augmentation in COVIDreduced patients. Another reason for failure to augment SV on exertion could be diastolic dysfunction, but the similar left atrial sizes and LV masses among all the groups make this less likely. In the absence of abnormalities of systolic or diastolic function, we propose that inadequate preload could be a remaining cause of reduced SV augmentation to consider in the COVIDreduced patient group.
Factors that control ventricular filling (not directly assessed in this study) include blood volume and venous compliance. Indeed, blood volume is one of the most important factors in maintaining ventricular filling. Blood volume depletion is known to reduce exercise capacity, 30 is depleted in postviral chronic fatigue syndrome, and correlates with exercise symptoms. 31 , 32 Furthermore, volume expansion in these patients results in improved exercise tolerance. 30 It is possible that similar mechanisms could occur in some patients following COVID‐19, reducing ventricular filling during exercise and contributing to exercise intolerance. Similar mechanistic origins of disease would perhaps be unsurprising given the symptom overlap between postviral chronic fatigue syndrome and patients following COVID‐19. Possible causes of reduced blood volume following COVID‐19 that could be evaluated in future studies include detraining after severe illness, abnormal renal regulation of fluid balance, and reduced fluid or salt intake. 33 , 34 Although these causes require further investigation, they do raise the possibility of interventions tailored to volume expansion, such as increased hydration and salt intake. The other important contributor to preload is venous compliance, which controls the proportion of the total blood volume that is “stressed.” It is stressed that blood volume that directly contributes to preload and dynamic reduction of venous compliance is required to increase preload during exercise. Venous compliance is controlled by the autonomic system, and there is some evidence of dysautonomia in patients with postviral chronic fatigue syndrome 34 along with small studies demonstrating dysautonomia in patients following COVID‐19. 35 , 36 Thus, an inability to dynamically reduce compliance may contribute to reduced exercise capacity in the COVIDreduced group and also requires further investigation.
Irrespective of the cause of reduced SV and CO at peak exercise, we did show that exercise capacity appears to improve with time since discharge. This suggests that reduced exercise capacity following COVID‐19 is not permanent and that a return to baseline is possible. Of course, this requires long‐term surveillance, as well as identification of interventions that may hasten recovery. In this regard, future work should include the longitudinal serial assessment of exercise capacity over a sufficient time period to ensure adequate time for recovery (eg, at 1 year). Interestingly, we also showed no association between exercise capacity and disease severity, which is consistent with other studies of patients with previous COVID‐19 infection. 37 This strengthens the idea that exercise intolerance is not simply the result of residual organ damage but a specific response to COVID‐19 infection.
One of the unique aspects of this study is the use of MR‐CPET. This technique enables simultaneous quantification of peak VO2 and CO, with subsequent calculation of ∆avO2, but is dependent on real‐time MR sequences. These can be challenging to use and require optimization to ensure robustness, particularly during exercise. We have previously shown that one of the most important factors in determining the reproducibility of real‐time MR is spatial and temporal resolution. 38 Thus, we used highly accelerated spiral acquisitions, reconstructed using compressed sensing. These techniques provide spatial and temporal resolution only slightly lower than clinical sequences. We have previously validated accelerated real‐time spiral sequences with compressed sensing reconstruction for assessment of aortic flow and ventricular volumes. 16 , 17 Unfortunately, validation at peak exercise with conventional gated breath‐hold or respiratory averaged MR is not possible. It should also be noted that these techniques are not widely available and can be challenging to use, meaning that MR‐CPET cannot be considered a clinical tool at the moment. Nevertheless, MR‐CPET does allow more sophisticated investigation of pathophysiology enabling generation of important new hypotheses. Furthermore, high‐resolution real‐time sequences are starting to become more clinically available, 39 opening the possibility of MR‐CPET being used as a clinical test. Of course, this will require further validation both of real‐time sequences (at peak exercise) and MR‐CPET in general. Simultaneous right heart catheterization and CPET (invasive CPET) is the reference standard direct method of measuring of ∆avO2. 24 , 30 Furthermore, invasive CPET can be performed using conventional metabolic carts and upright exercise ergometers, and allows traditional catheter‐based estimation of CO. We have previously shown that VO2 measured during MR‐CPET correlates well with VO2 measured during conventional CPET. 8 In addition, resting CO by phase‐contrast MR correlates well with CO measured invasively. 40 However, MR‐CPET measurements of exercise CO and ∆avO2 have not been directly compared with invasive CPET. This is mainly attributable to the significant difficulties in performing simultaneous MR and invasive CPET. Nevertheless, validating our novel technique is desirable, and future work should investigate a way of overcoming the logistical barriers to a direct comparison.
There are limitations of the study to consider. The COVIDreduced and COVIDnormal groups, but not the control group, were matched for hypertension and diabetes. While this is an important limitation, it does not account for the differences in MR‐CPET metrics between the 2 post–COVID‐19 patient groups given their matching for these comorbidities. Furthermore, there were no differences in peak iVO2 and peak COi between the COVIDnormal group and healthy controls. These results suggest that the absence of comorbidity matching in the control group did not significantly affect the results. As patients with significant cardiac and respiratory diseases were excluded, we only matched post–COVID‐19 patient groups by age, sex, hypertension, and diabetes. We believe that this covers most confounders of exercise capacity, but in future studies with larger recruitment, it may be beneficial to more extensively match groups. Neither of the patient groups were matched for pre–COVID‐19 exercise history because of the difficulty in ascertaining accurate exercise histories. Future studies may be able to overcome this problem by excluding subjects who are unable to provide an accurate exercise history, although this does risk introducing a significant selection bias. We did not have spirometry data for our healthy control group to compare against patient groups. However, this cohort was particularly advantageous as most data were acquired before the pandemic and, therefore, would not have been confounded by prior COVID‐19 infection. We have presented CMR‐derived data of left atrial size and LV mass but no specific measures of diastolic function, which should also be evaluated in future work. Further studies should also include the assessment of exercise capacity in response to intravenous fluid challenge, as we propose blood volume depletion as a potential cause of impaired SV augmentation.
Our MR‐CPET study emphasizes the importance of SV augmentation in the normal response to exercise. There were no differences in contractile reserve, left atrial size or LV mass (suggestive of diastolic impairment), or peak afterload that could explain reduced SV augmentation in COVIDreduced patients. Therefore, we propose that inadequate preload could be a possible mechanism of reduced SV augmentation and, consequently, of exercise intolerance in these patients. However, this is a hypothesis of exclusion, and larger, more statistically powered studies are required to fully understand the origin of exercise intolerance in these patients. Our data show no relationship between disease severity and peak MR‐CPET metrics and, reassuringly, that abnormalities in peak MR‐CPET metrics may improve with time from the acute illness. We believe that the comprehensive evaluation of unexplained dyspnea and exercise intolerance is pivotal to help correctly identify potential therapeutic targets in these patients.
Sources of Funding
Drs Muthurangu and Knight are supported by a British Heart Foundation Project Grant (grant number PG/17/47/32963). Dr Knight is supported by a British Heart Foundation Clinical Research Leave Fellowship (FS/CRLF/20/23004), by the National Institute for Health Research University College London Hospitals Biomedical Research Centre, and by research funding from Johnson & Johnson. Dr Fontana is supported by a British Heart Foundation Intermediate Fellowship (FS/18/21/33447).
Disclosures
Dr Knight reports consulting fees, speaker bureau fees, and research funding from Janssen. The remaining authors have no disclosures to report.
For Sources of Funding and Disclosures, see page 11.
References
- 1. Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty‐day outcomes among patients hospitalized with COVID‐19. Ann Intern Med. 2021;174:576–578. doi: 10.7326/m20-5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raman B, Cassar MP, Tunnicliffe EM, Filippini N, Griffanti L, Alfaro‐Almagro F, Okell T, Sheerin F, Xie C, Mahmod M, et al. Medium‐term effects of SARS‐CoV‐2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post‐hospital discharge. EClinicalMedicine. 2021;31. doi: 10.1016/j.eclinm.2020.100683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo LI, Liu M, Zhou X, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/s0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, Patel R, Chacko L, Brown JT, Coyle C, et al. Patterns of myocardial injury in recovered troponin‐positive COVID‐19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42:1866–1878. doi: 10.1093/eurheartj/ehab075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S. Pulmonary embolism in COVID‐19 patients: awareness of an increased prevalence. Circulation. 2020. doi: 10.1161/circulationaha.120.047430 [DOI] [PubMed] [Google Scholar]
- 6. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, et al. Post‐acute COVID‐19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lam GY, Befus AD, Damant RW, Ferrara G, Fuhr DP, Stickland MK, Varughese RA, Wong EY, Smith MP. Exertional intolerance and dyspnea with preserved lung function: an emerging long COVID phenotype? Respir Res. 2021;22:222. doi: 10.1186/s12931-021-01814-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barber NJ, Ako EO, Kowalik GT, Steeden JA, Pandya B, Muthurangu V. MR augmented cardiopulmonary exercise testing‐a novel approach to assessing cardiovascular function. Physiol Meas. 2015;36:N85–N94. doi: 10.1088/0967-3334/36/5/n85 [DOI] [PubMed] [Google Scholar]
- 9. Brown JT, Kotecha T, Steeden JA, Fontana M, Denton CP, Coghlan JG, Knight DS, Muthurangu V. Reduced exercise capacity in patients with systemic sclerosis is associated with lower peak tissue oxygen extraction: a cardiovascular magnetic resonance‐augmented cardiopulmonary exercise study. J Cardiovasc Magn Reson. 2021;23:118. doi: 10.1186/s12968-021-00817-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mottram RF. The interrelationship of blood flow, oxygen utilization of blood and oxygen consumption of human skeletal muscle. J Physiol. 1958;142:314–322. doi: 10.1113/jphysiol.1958.sp006018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization . WHO R&D Blueprint: COVID‐19 Therapeutic Trial Synopsis. Accessed January 28, 2021. https://www.who.int/blueprint/priority‐diseases/key‐action/COVID‐19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf
- 12. Kramer CM, Barkhausen J, Bucciarelli‐Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson. 2020;22:17. doi: 10.1186/s12968-020-00607-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Messroghli DR, Moon JC, Ferreira VM, Grosse‐Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson. 2017;19:75. doi: 10.1186/s12968-017-0389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kellman P, Hansen MS. T1‐mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16:2. doi: 10.1186/1532-429x-16-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. doi: 10.1186/1532-429x-11-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kowalik GT, Knight D, Steeden JA, Muthurangu V. Perturbed spiral real‐time phase‐contrast MR with compressive sensing reconstruction for assessment of flow in children. Magn Reson Med. 2020;83:2077–2091. doi: 10.1002/mrm.28065 [DOI] [PubMed] [Google Scholar]
- 17. Steeden JA, Kowalik GT, Tann O, Hughes M, Mortensen KH, Muthurangu V. Real‐time assessment of right and left ventricular volumes and function in children using high spatiotemporal resolution spiral bSSFP with compressed sensing. J Cardiovasc Magn Reson. 2018;20:79. doi: 10.1186/s12968-018-0500-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tamir JI, Ong F, Cheng JY, Uecker M, Lustig M. Generalized Magnetic Resonance Image Reconstruction Using the Berkeley Advanced Reconstruction Toolbox. Sedona, AZ: ISMRM Workshop on Data Sampling & Image Reconstruction; 2016. [Google Scholar]
- 19. Muthurangu V, Lurz P, Critchely JD, Deanfield JE, Taylor AM, Hansen MS. Real‐time assessment of right and left ventricular volumes and function in patients with congenital heart disease by using high spatiotemporal resolution radial k‐t SENSE. Radiology. 2008;248:782–791. doi: 10.1148/radiol.2482071717 [DOI] [PubMed] [Google Scholar]
- 20. Odille F, Steeden JA, Muthurangu V, Atkinson D. Automatic segmentation propagation of the aorta in real‐time phase contrast MRI using nonrigid registration. J Magn Reson Imaging. 2011;33:232–238. doi: 10.1002/jmri.22402 [DOI] [PubMed] [Google Scholar]
- 21. Rosset A, Spadola L, Ratib O. OsiriX: an open‐source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–216. doi: 10.1007/s10278-004-1014-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schulz‐Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff‐Brenkenhoff F, Kramer CM, Pennell DJ, et al. Standardized image interpretation and post‐processing in cardiovascular magnetic resonance ‐ 2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post‐Processing. J Cardiovasc Magn Reson. 2020;22:19. doi: 10.1186/s12968-020-00610-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/s1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh I, Joseph P, Heerdt PM, Cullinan M, Lutchmansingh DD, Gulati M, Possick JD, Systrom DM, Waxman AB. Persistent exertional intolerance after COVID‐19: insights from invasive cardiopulmonary exercise testing. Chest. 2021. doi: 10.1016/j.chest.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Joseph P, Arevalo C, Oliveira RKF, Faria‐Urbina M, Felsenstein D, Oaklander AL, Systrom DM. Insights from invasive cardiopulmonary exercise testing of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Chest. 2021;160:642–651. doi: 10.1016/j.chest.2021.01.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goulding RP, Okushima D, Fukuoka Y, Marwood S, Kondo N, Poole DC, Barstow TJ, Koga S. Impact of supine versus upright exercise on muscle deoxygenation heterogeneity during ramp incremental cycling is site specific. Eur J Appl Physiol. 2021;121:1283–1296. doi: 10.1007/s00421-021-04607-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wehrle A, Waibel S, Gollhofer A, Roecker K. Power output and efficiency during supine, recumbent, and upright cycle ergometry. Front Sports Act Living. 2021;3. doi: 10.3389/fspor.2021.667564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim JY, Han K, Suh YJ. Prevalence of abnormal cardiovascular magnetic resonance findings in recovered patients from COVID‐19: a systematic review and meta‐analysis. J Cardiovasc Magn Reson. 2021;23:100. doi: 10.1186/s12968-021-00792-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kawel‐Boehm N, Maceira A, Valsangiacomo‐Buechel ER, Vogel‐Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluemke DA. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. doi: 10.1186/s12968-015-0111-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oldham WM, Lewis GD, Opotowsky AR, Waxman AB, Systrom DM. Unexplained exertional dyspnea caused by low ventricular filling pressures: results from clinical invasive cardiopulmonary exercise testing. Pulm Circ. 2016;6:55–62. doi: 10.1086/685054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hurwitz BE, Coryell VT, Parker M, Martin P, Laperriere A, Klimas NG, Sfakianakis GN, Bilsker MS. Chronic fatigue syndrome: illness severity, sedentary lifestyle, blood volume and evidence of diminished cardiac function. Clin Sci (Lond). 2009;118:125–135. doi: 10.1042/cs20090055 [DOI] [PubMed] [Google Scholar]
- 32. Newton JL, Finkelmeyer A, Petrides G, Frith J, Hodgson T, Maclachlan L, MacGowan G, Blamire AM. Reduced cardiac volumes in chronic fatigue syndrome associate with plasma volume but not length of disease: a cohort study. Open Heart. 2016;3:e000381. doi: 10.1136/openhrt-2015-000381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miwa K. Down‐regulation of renin‐aldosterone and antidiuretic hormone systems in patients with myalgic encephalomyelitis/chronic fatigue syndrome. J Cardiol. 2017;69:684–688. doi: 10.1016/j.jjcc.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 34. Natelson BH, Brunjes DL, Mancini D. Chronic fatigue syndrome and cardiovascular disease: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2021;78:1056–1067. doi: 10.1016/j.jacc.2021.06.045 [DOI] [PubMed] [Google Scholar]
- 35. Barizien N, Le Guen M, Russel S, Touche P, Huang F, Vallée A. Clinical characterization of dysautonomia in long COVID‐19 patients. Sci Rep. 2021;11:14042. doi: 10.1038/s41598-021-93546-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Becker RC. Autonomic dysfunction in SARS‐COV‐2 infection acute and long‐term implications COVID‐19 editor's page series. J Thromb Thrombolysis. 2021;52:692–707. doi: 10.1007/s11239-021-02549-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rinaldo RF, Mondoni M, Parazzini EM, Baccelli A, Pitari F, Brambilla E, Luraschi S, Balbi M, Guazzi M, Di Marco F, et al. Severity does not impact on exercise capacity in COVID‐19 survivors. Respir Med. 2021;187:106577. doi: 10.1016/j.rmed.2021.106577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lurz P, Muthurangu V, Schievano S, Nordmeyer J, Bonhoeffer P, Taylor AM, Hansen MS. Feasibility and reproducibility of biventricular volumetric assessment of cardiac function during exercise using real‐time radial k‐t SENSE magnetic resonance imaging. J Magn Reson Imaging. 2009;29:1062–1070. doi: 10.1002/jmri.21762 [DOI] [PubMed] [Google Scholar]
- 39. Vermersch M, Longère B, Coisne A, Schmidt M, Forman C, Monnet A, Pagniez J, Silvestri V, Simeone A, Cheasty E, et al. Compressed sensing real‐time cine imaging for assessment of ventricular function, volumes and mass in clinical practice. Eur Radiol. 2020;30:609–619. doi: 10.1007/s00330-019-06341-2 [DOI] [PubMed] [Google Scholar]
- 40. Razavi R, Hill DLG, Keevil SF, Miquel ME, Muthurangu V, Hegde S, Rhode K, Barnett M, van Vaals J, Hawkes DJ, et al. Cardiac catheterisation guided by MRI in children and adults with congenital heart disease. Lancet. 2003;362:1877–1882. doi: 10.1016/s0140-6736(03)14956-2 [DOI] [PubMed] [Google Scholar]


