Abstract
Background and Objectives:
Although polychlorinated biphenyls (PCBs) were banned decades ago, populations are continuously exposed to PCBs due to their persistence and bioaccumulation/biomagnification in the environment. Results from limited epidemiologic studies linking PCBs to thyroid cancer have been inconclusive. This study aimed to investigate the association between individual PCBs and PCB mixture and papillary thyroid cancer (PTC), the most common thyroid cancer histologic subtype.
Methods:
We carried out a nested case-control study including 742 histologically confirmed PTC cases diagnosed in 2000–2013 and 742 individually matched controls from among U.S. military service members. Pre-diagnostic serum samples that were collected on average nine years before PTC diagnosis were used to measure PCB congeners by gas chromatography isotope dilution high resolution mass spectrometry (GC/ID-HRMS). Conditional logistic regression, Bayesian kernel machine regression (BKMR), and weighted quantile sum (WQS) regression were employed to estimate the association between single PCB congeners as well as their mixture and PTC.
Results:
Four PCB congeners (PCB-74, PCB-99, PCB-105, PCB-118) had significant associations and dose-response relationships with increased risk of PTC in single congener models. When considering PCB congeners as a mixture in the BKMR model, PCB-118 showed positive trends of association with PTC. Increased exposure to the PCB mixture identified by WQS was also associated with an increased risk of PTC, with the mixture dominated by PCB-118, followed by PCB-74 and PCB-99.
Discussion:
This study suggests that exposure to certain PCBs as well as a mixture of PCBs were associated with an increased risk of PTC. The observed association was mainly driven by PCB-118, and to a lesser extent by PCB-74 and PCB-99. The findings warrant further investigation.
Keywords: Papillary thyroid cancer, Polychlorinated biphenyls, PCBs, Persistent organic pollutants, Endocrine disrupting chemicals, Mixtures
1. Introduction
Polychlorinated biphenyls (PCBs) were manufactured and heavily used in electrical equipment such as capacitors and transformers, industrial fluids like lubricants and heat transfer fluids, as well as tiles and sealants in both comercial and residential settings in the U.S. since 1929; their production was banned in the U.S. in 1979 and in most other countries by 1980s (IARC, 2016). However, environmental persistence of PCBs has hindered their complete degradation in the ecosystem for decades, resulting in continuous human exposure to PCBs mainly through diet and, to a lesser extent, through air and skin contact (ATSDR, 2000; Holford et al., 2000; IARC, 2016). Dioxin-like PCBs were classified as Group 1 Human Carcinogens by the International Agency for Research on Cancer (IARC) in 2013 (IARC, 2016), based on strong evidence from animal and epidemiologic studies (ATSDR, 2000; Lauby-Secretan et al., 2013; Van den Berg et al., 2006).
The incidence of thyroid cancer has risen significantly since the early 1980s in many countries, largely driven by the papillary subtype of thyroid cancer (PTC) (Miranda-Filho et al., 2021). In the U.S., the incidence rate more than tripled in both overall thyroid cancer (6.5 to 21.8 per 100,000 person years) and in PTC subtype (4.6 to 18.0 per 100,000 person years) from 1975 to 2018 in the general population (SEER, 2021). A similar increasing trend of thyroid cancer incidence was also observed in the military personnel, with a higher incidence among military women compared to the general population (Enewold et al., 2011). Heightened medical surveillance in the military may only partially explain the higher incidence (Enewold et al., 2011), indicating a potentially important role from other risk factors such as exposure to endocrine disrupting chemicals, including PCBs (Alsen et al., 2021).
The association between PCBs and thyroid cancer risk has been widely hypothesized but not well investigated to date. In animal studies, PCBs have disrupted thyroid hormones, altered thyroid functions, and increased thyroid tumors (Akoso et al., 1992; Benson et al., 2018; Capen and Martin, 1989; Collins et al., 1977; National Toxicology Program, 2006; Ness et al., 1993; Saeed and Hansen, 1997; Zoeller, 2001). In humans, few epidemiologic studies have been conducted and the association between PCB exposures and thyroid cancer remains inconclusive. Most studies were conducted in small populations and/or utilized either proxies or post-diagnosis serum concentration for PCB exposure assessment (Deziel et al., 2021; Han et al., 2019; Haslam et al., 2016; Lerro et al., 2018; Mallin et al., 2004; Pavuk et al., 2004). Three epidemiologic studies have investigated non-occupational PCB exposures and observed mixed effects on thyroid cancer. One study observed both positive and negative associations with post-diagnosis serum PCB concentrations with a non-monotonic trend (Han et al., 2019), while two other studies reported positive associations with a few PCB congeners among partcipants whose early-life period coincided with PCB peak production (Deziel et al., 2021; Lerro et al., 2018). One study investigated the multi-pollutant effect of PCB mixtures on PTC subtype and found a null association (Deziel et al., 2021).
Although interest in identifying the impact of environmental mixtures on adverse health outcomes is emerging (Carlin et al., 2013; NIEHS, 2015), the single-exposure analysis approach is still most commonly applied, and the health impact of chemical mixtures is relatively unknown (Taylor Kyla et al., 2016). Studying individual exposures in isolation may lead to the underestimation of the true health impact of chemical mixtures, as molecular toxicology studies have found individual chemical which is considered harmless in isolation could be harmful in aggregate chemical mixtures (Monosson, 2005). Therefore, studying health impacts of exposure mixtures is essential for public health intervention, and also vital for enlightening exposome studies where the totality of exposures from conception to demise is of great interest.
In light of the ubiquitous human exposures to PCBs and inconclusive evidence from previous epidemiologic studies of their association with thyroid cancer, as well as the insufficient health impact of PCB mixtures, we conducted a large nested case-control study of PTC to investigate risk associated with pre-diagnostic serum concentrations of individual PCBs and a PCB mixture among United States (U.S.) military personnel. We utilized serum samples from the Department of Defense Serum Repository (DoDSR). While there may be specific point sources of PCB exposures in the U.S. military (e.g, old commissioned Navy vessels, electrical transformers and capacitors on military installations), the main exposure route for military service members is likely to be ingestion of contaminated food, similar to the general population (Still et al., 2003; U.S. Department of Veterans Affairs; US Army Corps of Engineers, 2012).
2. Materials and Methods
2.1. Study population
Our study population was nested within a U.S. military cohort of service members with serum samples stored in the DoDSR, established in 1989 as a repository for storing serum that remained following mandatory HIV testing within the active and reserve components of the US military (Perdue et al., 2015). PTC cases were identified via the DoD Automated Central Tumor Registry (ACTUR) using International Classification of Diseases for Oncology (ICD-O) [3rd edition] codes: 8050, 8260, 8340–8343. A total of 742 PTC cases who had stored serum samples in the DoDSR, were aged 21 years or older at the time of diagnosis (1994–2009), and who had no recorded prior cancers (except for non-melanoma skin cancer), were included in this study. Individually matched controls were randomly selected from the DoDSR cohort based on date of birth (DOB) (±1 year), sex, race/ethnicity, and average collection date of a case’s serum sample (±1 year), and component at diagnosis/matching. Demographics and other characteristics for all cases and controls were abstracted from the Defense Medical Surveillance System (DMSS), including matching variables (birth year (±1 year), sex, race/ethnicity, dates of serum collection), height and weight at the time of joining the military, and military branch.
All study procedures were approved and were given the determination of Exempt Human Subjects Research Protocol by the Uniformed Services University Institutional Review Board (IRB), the Walter Reed National Military Medical Center, the DoD Joint Pathology Center, the Human Investigation Committee of Yale University, and the Centers for Disease Control and Prevention (CDC).
2.2. Measurement of Pre-diagnostic PCBs exposure
Serum concentrations of 24 PCB congeners (28, 66, 74, 99, 105, 114, 118, 138/158, 146, 153, 156, 157, 167, 170, 178, 180, 183, 187, 189, 194, 196/203, 199, 206, 209) were measured in samples collected on average nine years before the diagnosis of PTC cases, and measured at the Persistent Organic Pollutants Laboratory of the CDC, using methods that have been described previously (Jones et al., 2012; Sjödin et al., 2004; Zhuo, 2018). Briefly, serum samples of 0.5 mL were first fortified with internal standards and extracted by automated liquid-liquid extraction (LLE) using Gilson 215 liquid handler (Gilson, Inc.; Middleton, WI). Then the co-extracted lipids were removed on a ‘silica: silica/sulfuric’ acid column using the automated Rapid Trace equipment (Biotage; Uppsala, Sweden). Finally, the analytical determinations of PCB congeners were performed with gas chromatography isotope dilution high resolution mass spectrometry (GC-ID/HRMS) employing a DFS instrument (Thermo DFS, Bremen, Germany) (Huang et al., 2017). Measurement of serum lipid concentrations (total triglycerides and total cholesterol) were determined by commercially available test kits (Roche Diagnostics Corp.; Indianapolis, IN) and final determinations were made on a Hitachi 912 Chemistry Analyzer (Tokyo, Japan). Laboratory personnel were blinded to case and control status of PTC. Method blanks (n=3) and quality controls (n=3) were used as internal laboratory quality control samples in every set of 24 study samples. Serum concentrations of PCB congeners were reported as lipid-corrected serum concentration in nanograms per gram (ng/g) lipid after subtracting background concentrations (i.e., the median concentration of contaminants measured in blank samples). The range of coefficient of variation (CV) for PCB congeners measured in quality control samples were 3.2% (PCB180) to 11.6% (PCB74). Due to the similar retention time on the gas chromatograph (Robertson and Hansen, 2015), four PCBs congeners were measured as congener-pairs. Specifically, PCB-138/158 pair refers to the mixture of PCB-138 and PCB-158; PCB-196/203 pair refers to the mixture of PCB-196 and PCB-203. The other PCBs were measured as separate congeners. All values under the limit of detection (LOD) were considered as undetectable except that imputation was implemented in PCB mixtures analysis, and PCB analytes (PCB-66, PCB-114, PCB-189) with >80% of values under the LOD were excluded from all statistical analyses.
2.3. Statistical analysis
Demographic and military characteristics were compared between PTC cases and controls by chi-squared tests and one-way analysis of variance (ANOVA). Body mass index (BMI) was calculated by dividing weight (kg) by the square of height (m2); approximately 43% of both cases and controls had missing BMI data. A multiple imputation approach with a regression on covariates (age, sex, race/ethnicity, and military branch), PCB congeners, and the PTC outcome (cases vs. controls) for the entire population was applied to estimate BMI for those missing observations (Allison, 2012). Lipid-corrected serum concentrations of PCB congeners were log-transformed to adjust for right skewness, and Pearson correlation coefficients were calculated among PCB congeners to examine correlations.
2.3.1. Single PCB congener analysis
In single-chemical analyses, 21 PCB congeners with at least 20% of detectable values were included; categorized concentrations with four strata were created based on the quartile or tertile of the distribution of detectable values in the control group. Specifically, PCB congeners with ≥75% of detectable values were categorized into quartiles with the first quartile serving as the reference group. The rest, with 20–75% of detectable values, were categorized into tertiles based on detectable values, and a category of undetectable values served as the reference group. A conditional logistic regression model was developed for each congener to estimate odds ratios (OR) and 95% confidence intervals (CI), with adjustment for potential confounders of BMI and military branch.
A sensitivity analysis was conducted, with adjustment for military branch only in models to account for the possibility of BMI serving as a mediator, as opposed to a confounder. A test for linear trend was implemented to investigate dose-response relationships by modeling the median value of each exposure category as a continuous variable. Additionally, we conducted stratified analyses by tumor size (microcarcinoma: ≤10 mm; macrocarcinoma: >10 mm), sex (female; male) and histological subtype (classical PTC; follicular variant PTC); p-values for interaction were calculated to evaluate heterogeneity. Another sensitivity analysis was conducted by excluding diagnosed PTC cases within five years since serum sample collection and their matched controls, to account for the potential latent time period of PTC development.
2.3.2. PCB mixtures analysis
Various statistical approaches available to evaluate the health effects of chemical mixtures were compared in a 2015 NIEHS workshop, but no standard method has been identified with the best performance to comprehensively study environmental mixtures (Taylor Kyla et al., 2016). However, two important perspectives of mixtures analyses were proposed since the NIEHS workshop: the health effect of individual chemical components within a mixture and the joint effect of the overall mixtures (Braun et al., 2016). In this study, Bayesian kernel machine regression (BKMR) was used to investigate the individual chemical component effects within a non-linear and/or non-additive PCB mixture while considering other congeners simultaneously. Weighted quantile sum (WQS) regression was used to estimate the joint effect of a PCB mixture and identify the most important chemical components within the mixture. Although both BKRM and WQS can be employed to estimate the joint effects, WQS was chosen because its strength in easier interpretations using OR and useful output of contribution of each PCB component within the mixture.
For the mixtures analyses, we excluded PCBs with <50% detectable values (PCB-28, 105, 157, 167, 178, 206, 209). This left 14 congeners in the mixtures analyses (PCB-74, 99, 118, 138/158, 146, 153, 156, 170, 180, 183, 187, 194, 196/203, 199) and their values below the LOD for these PCBs were imputed by using a maximum likelihood procedure with the assumption of a lognormal distribution defined by the detected measurements of PCBs above the LOD (Lubin et al., 2004).
2.3.2. PCB mixtures analysis: Bayesian kernel machine regression (BKMR)
BKMR is a relatively new approach for studying mixtures in which the health outcome is regressed on a flexible function of the exposures (Bobb et al., 2015). It is particularly useful in highly correlated chemicals (PCB congeners in our case) and their interactions in the mixture, which otherwise could potentially bias the estimate and mask non-linear or non-additive relationship using traditional logistic regression with co-adjustment for all congeners. We implemented a probit link function-based BKMR model to consider the binary outcome, using the R package “bkmr” (Bobb et al., 2018; Bobb et al., 2015). A Gaussian kernel function was used to flexibly model the exposure-outcome relationship, allowing for potential non-linear and/or non-additive effects. To account for the high correlation (>0.5) among PCBs, we incorporated a hierarchical variable selection by classifying PCB congeners into five groups (group 1- group 5) based on either their hormonal activities and biological effects using the Wolff criteria (Wolff et al., 1997) or on their high correlation. Group 1 included one congener PCB-187 in Wolff’s group 1 (potential estrogenic and weak phenobarbital inducers); Group 2 included PCB-74, 118, and 156 in Wolff’s group 2A (non-ortho and mono-ortho substituted, potentially antiestrogenic, immunotoxic or dioxin-like); Group 3 included PCB-138/158 and 170 in Wolff’s group 2B (di-ortho substituted, with more limited toxicity than group 2A); Group 4 included PCB-99, 153, 180, 183, and 196/203 in Wolff’s group 3 (phenobarbital and CYP1A/CYP2B inducers); Group 5 included PCB-146, PCB-194, and PCB-199 (which did not consider Wolff’s groupings but were highly correlated with each other).
In this context, variable selection was simultaneously conducted across groups and among individual PCBs within each group. Using a Markov chain Monte Carlo (MCMC) sampling algorithm with default prior settings in the “bkmr” package, we collected 10,000 posterior samples after discarding the first 10,000 iterations during a burn-in period, and estimated: 1) the PTC risk difference (denoted by posterior mean estimate) and 95% credible intervals (CrI) between the 75th and 25th percentiles of a target PCB, while fixing all other congeners and BMI at their median level, and military branch at “Air Force” (arbitrary selection); 2) the exposure-outcome relationship curve across the entire range of the target PCB’s concentrations, conditional on the median level of all the other PCBs, median BMI, and the “Air Force” branch. A match-level random effect was included to account for our individually-matched case-control design (Bobb et al., 2018; Bobb et al., 2015).
Sensitivity analyses were conducted by changing the fixed level of other PCBs to their 25th percentiles and 75th percentiles, while keeping the fixed level of covariates unchanged. We further investigated the individual PCB component effects within the mixture among subgroups stratified by tumor size (macrocarcinoma; microcarcinoma) and sex (males; females). BKMR was not applied to subgroups on histological type of PTC because of the small sample size of follicular variant thyroid cancer.
2.3.3. PCB mixtures analysis: Weighted quantile sum (WQS) regression
We evaluated the joint effect of the PCB mixture on PTC risk via a generalized WQS approach in conjunction with logistic regression for a binary outcome using the R package “gWQS” (Renzetti et al., 2019). This approach took all PCB congeners into consideration with constraints to have the same effect direction for the PTC association. The WQS regression constructed a weighted index (the WQS Index) of quartile-based categorical individual components to estimate the joint/cumulative effect of the PCB mixture. The estimated weights of the individual components in the WQS Index indicated the contribution of each PCB congener in the PCB mixture. Simultaneous estimation of the coefficient of the WQS Index and unknown weights was achieved through: 1) 1,000 times bootstrap sampling on the full data with constraints of positive coefficient; 2) averaging significant samples from bootstrap sets to get empirical weights and re-applied to the full data to get the estimated coefficient of the WQS Index and its statistical significance (p-value) with adjustment for BMI and military branch. The ORs and 95% CIs of the joint effect on PTC risk were derived by exponentiating the estimated coefficient of the WQS Index. To account for the individual matched design in our study, we first used the R package “gWQS” to obtain the WQS Index, and then a conditional logistic regression was implemented to analyze the effect of the identified mixture on PTC risk.
We first ran a model including all 14 PCBs with restricted positive effects on PTC to investigate a harmful joint effect. As we did not suspect protective effects of PCB congeners on PTC, a model with negative effect constraints on all congeners was not implemented. However, in order to consider the potential negative association between one PCB congener (PCB-187) and PTC from the results of single-congener analyses and the BKMR mixture analyses, we then ran another model of 13 PCBs with restricted positive effects while adjusting for PCB187 as an additional covariate other than BMI and military branch.
We also implemented the WQS models in subgroups by tumor size (macrocarcinoma; microcarcinoma) and sex (males; females), but not in subgroups of PTC histological type because of the small sample size of follicular variant thyroid cancer.
All tests related to conditional logistic regression were carried out using a 2-sided significance level of 0.05 and conducted using SAS software, version 9.4 (SAS Institute, Inc.; Cary, NC). The mixtures analyses were conducted using R, version 3.6.0.
3. Results
3.1. Population characteristics
Among the matched 742 case-control pairs (Table 1), cases were more likely to have higher BMI, although statistical significance was only observed for BMI with imputation. Cases were also more likely to serve in the Army and Air Force, and less likely to serve in the Marines, Coast Guard, and Navy. The distributions of age (at both serum collection and diagnosis), sex and race/ethnicity were similar between cases and controls because of matching. Serum samples were collected on average 8.89 years prior to the diagnosis of PTC. Nearly half of the cases (48.52%) were diagnosed with PTC 10–13 years after serum collection. A majority of the diagnosed cases had microcarcinoma with tumor size >10 mm (62.40%) and were classical PTC subtypes (80.86%).
Table 1.
Selected characteristic of the study population
| Characteristics | Cases | Controls | P-value† | ||
|---|---|---|---|---|---|
|
| |||||
| N=742 | % | N=742 | % | ||
|
| |||||
| Age at serum sample collection (yr) | 0.99 | ||||
| 17–19 | 124 | 16.71 | 121 | 16.31 | |
| 20–29 | 348 | 46.90 | 352 | 47.44 | |
| 30–39 | 220 | 29.65 | 221 | 29.78 | |
| 40–51 | 50 | 6.74 | 48 | 6.47 | |
| Mean ± SD | 26.25 ± 7.39 | 26.25 ± 7.34 | |||
| Age at PTC diagnosis (yr) ¶ | 0.92 | ||||
| 20–29 | 210 | 28.30 | 203 | 27.36 | |
| 30–39 | 310 | 41.78 | 323 | 43.53 | |
| 40–49 | 185 | 24.93 | 179 | 24.12 | |
| 50–62 | 37 | 4.99 | 37 | 4.99 | |
| Mean ± SD | 35.14 ± 8.11 | 35.14 ± 8.05 | |||
| Sex | >0.99 | ||||
| Female | 341 | 45.96 | 341 | 45.96 | |
| Male | 401 | 54.04 | 401 | 54.04 | |
| Race/ethnicity | >0.99 | ||||
| White | 468 | 63.07 | 467 | 62.94 | |
| Black | 131 | 17.65 | 132 | 17.79 | |
| Hispanic | 68 | 9.16 | 68 | 9.16 | |
| Other | 55 | 7.41 | 55 | 7.41 | |
| Unknown | 20 | 2.70 | 20 | 2.70 | |
| BMI (kg/m2) | 0.19 | ||||
| 16–25 | 261 | 35.18 | 286 | 38.54 | |
| 25–29.9 | 143 | 19.27 | 128 | 17.25 | |
| 30–34.8 | 16 | 2.16 | 10 | 1.35 | |
| missing | 322 | 43.40 | 318 | 42.86 | |
| Mean ± SD | 24.02 ± 3.17 | 23.77 ± 3.06 | 0.24 | ||
| BMI with imputation (kg/m2) ☨ | 0.07 | ||||
| 14–25 | 452 | 61.00 | 494 | 66.58 | |
| 25–29.9 | 260 | 35.09 | 227 | 30.59 | |
| 30–34.8 | 29 | 3.91 | 21 | 2.83 | |
| Mean ± SD | 24.14 ± 3.17 | 23.79 ± 3.16 | 0.03 | ||
| Service | <0.0001 | ||||
| Army | 299 | 40.30 | 253 | 34.10 | |
| Air Force | 193 | 26.10 | 150 | 20.20 | |
| Navy | 186 | 25.00 | 248 | 33.30 | |
| Marines & Coast Guard, combined | 64 | 8.60 | 91 | 12.30 | |
| Years from serum sample collection to diagnosis (yr) | |||||
| 3–5 | 115 | 15.50 | |||
| 6–9 | 267 | 35.98 | |||
| 10–13 | 360 | 48.52 | |||
| Mean ± SD | 8.89 ± 2.67 | ||||
| Tumor size (mm) | |||||
| ≤10 | 235 | 31.67 | |||
| >10 | 463 | 62.40 | |||
| Missing | 44 | 5.93 | |||
| Histological type | |||||
| Classical PTC | 600 | 80.86 | |||
| Follicular variant of PTC | 142 | 19.14 | |||
Abbreviation: BMI, body mass index; PTC, papillary thyroid cancer.
For controls, the age was at the diagnosed year of matched cases
Estimated by Chi-squared test for categorical variable and one-way ANOVA for continuous variables
Missing values calculated by multiple imputation
3.2. PCBs serum concentrations and correlations
The lipid-corrected serum concentrations of the 24 PCB congeners measured in our study are shown in Table 2. The median level of LOD ranged from 1.00–2.30 ng/g lipid. Three of the measured congeners (PCB-66, PCB-114, PCB-189) had less than 20% of detection. Among the remaining 21 congeners, the proportion of detection ranged from 20.55% (PCB-157) to 97.57% (PCB-180) and 14 congeners had at least 50% of detection. The detection rate and median levels of PCBs were similar between cases and controls (data not shown). Except for the weak to moderate correlation for PCB-28, PCB-157, PCB-167 and PCB-209 with all other congeners (for which all were ≤0.5), Pearson correlation coefficients among other PCBs varied widely, ranging from 0.3 to 0.9 (Supplementary Figure 1).
Table 2.
Lipid-corrected serum concentrations of PCBs (ng/g lipid) among cases and controls.
| congener | Detected (%) | Median LOD | Cases | Controls | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Median | IQR | Max | Median | IQR | Max | |||
|
| ||||||||
| PCB28** | 26.15 | 1.50 | <LOD | <LOD - 1.4 | 61.5 | <LOD | <LOD - 0.38 | 66.7 |
| PCB66* | 7.95 | 1.60 | <LOD | <LOD - <LOD | 11.1 | <LOD | <LOD - <LOD | 36.79 |
| PCB74 | 62.53 | 1.60 | 2.4 | <LOD - 4.3 | 32.2 | 2.1 | <LOD - 3.7 | 24.0 |
| PCB99 | 82.01 | 1.00 | 2.7 | 1.5 – 4.7 | 26.8 | 2.5 | 1.3– 4.3 | 40.8 |
| PCB105** | 38.34 | 1.00 | <LOD | <LOD - 1.4 | 10.2 | <LOD | <LOD - 1.1 | 9.7 |
| PCB114* | 7.08 | 1.00 | <LOD | <LOD - <LOD | 5.3 | <LOD | <LOD - <LOD | 8.1 |
| PCB118 | 92.65 | 1.00 | 4.6 | 2.8 – 7.5 | 46.2 | 4.2 | 2.4 – 6.7 | 53.6 |
| PCB138~158 | 97.16 | 1.00 | 9.8 | 5.7 – 17.4 | 95.4 | 9.6 | 5.4 – 16.2 | 176.3 |
| PCB146 | 60.38 | 1.00 | 1.4 | <LOD - 2.8 | 19.0 | 1.3 | <LOD - 2.6 | 29 |
| PCB153 | 97.44 | 1.00 | 16.8 | 9.8 – 28.2 | 141.1 | 16 | 9.2 – 27.2 | 234.4 |
| PCB156 | 67.92 | 1.00 | 1.9 | <LOD - 3.7 | 27.3 | 1.8 | <LOD - 3.4 | 50.3 |
| PCB157** | 20.55 | 1.00 | <LOD | <LOD - <LOD | 7.5 | <LOD | <LOD - <LOD | 10.3 |
| PCB167** | 22.37 | 1.00 | <LOD | <LOD - <LOD | 6.7 | <LOD | <LOD - <LOD | 8.7 |
| PCB170 | 88.88 | 1.00 | 4.5 | 2.3 – 7.8 | 40.8 | 4.3 | 2.3 – 7.6 | 55.2 |
| PCB178** | 32.35 | 1.00 | <LOD | <LOD - 1.3 | 8.6 | <LOD | <LOD - 1.1 | 13.2 |
| PCB180 | 97.57 | 1.00 | 11.8 | 6.3 – 21.2 | 89.0 | 11.4 | 6.4 – 20.3 | 153.9 |
| PCB183 | 54.11 | 1.00 | 1.1 | <LOD - 2.4 | 13.0 | 1.1 | <LOD - 2.3 | 19.4 |
| PCB187 | 78.77 | 1.00 | 3.4 | 1.6 – 6.2 | 35.3 | 3.4 | 1.6 – 6.1 | 77.6 |
| PCB189* | 5.46 | 1.00 | <LOD | <LOD - <LOD | 2.9 | <LOD | <LOD - <LOD | 6.9 |
| PCB194 | 71.70 | 1.00 | 2.3 | <LOD - 4.6 | 24.7 | 2.3 | <LOD - 4.5 | 35.6 |
| PCB196~203 | 83.61 | 1.00 | 2.6 | 1.3 – 4.8 | 27.4 | 2.7 | <LOD - 4.7 | 45.0 |
| PCB199 | 73.45 | 1.00 | 2.4 | <LOD - 4.8 | 32.4 | 2.4 | 1.1 – 4.6 | 60.9 |
| PCB206** | 38.95 | 2.30 | <LOD | <LOD - 3.1 | 21.1 | <LOD | <LOD - 3.0 | 30.9 |
| PCB209** | 29.78 | 1.00 | <LOD | <LOD - 1.0 | 6.8 | <LOD | <LOD - 1.0 | 15.6 |
Abbreviation: LOD, limit of detection concentration.
PCBs have <20% detected values, excluded from any regression analyses
PCBs have <50% detected values, excluded from mixture analyses
3.3. Single PCB congener analyses: Conditional logistic regressions
Twenty-one PCB congeners with at least 20% of detection were included in the single-congener analyses. After adjusting for BMI and military branch (Table 3), significant positive associations with PTC were observed in quartile 4 (Q4) of PCB-74 (OR=1.48, 95% CI: 1.12, 1.96) and PCB-99 (OR=1.37, 95% CI: 1.02, 1.84), quartile 3 (Q3) of PCB-105 (OR=1.42, 95% CI: 1.05, 1.92), and quartile 2 (Q2) as well as Q4 of PCB-118 (OR=1.54, 95% CI: 1.14, 2.09; OR=1.71, 95% CI: 1.27, 2.31, respectively). Significant exposure-response relationships were observed for PCB-74, PCB-99, PCB-105, and PCB-118, with all test for trend P-values < 0.05. No significant associations were observed between other PCB congeners and PTC.
Table 3.
Associations between lipid-corrected serum concentrations of PCBs and papillary thyroid cancer overall and by tumor size
| Overall | Stratified by Tumor size | P for interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Cases/Controls=742 pairs | ≤10 mm † Cases/Controls=235 pairs | >10 mm † Cases/Controls=462 | ||||||||
|
| ||||||||||
| Crude OR | ORf | 95% CI | Crude OR | OR | 95% CI | Crude OR | OR | 95% CI | ||
|
| ||||||||||
| PCB28** | ||||||||||
| <LOD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.993 | |||
| >LOD - 1.90 | 0.89 | 0.80 | (0.54, 1.19) | 0.94 | 0.80 | (0.40, 1.58) | 0.93 | 0.81 | (0.49, 1.33) | |
| 1.91 – 3.89 | 1.32 | 1.26 | (0.89, 1.80) | 1.47 | 1.31 | (0.70, 2.44) | 1.26 | 1.15 | (0.74, 1.79) | |
| 3.90 – 66.74 | 1.15 | 1.10 | (0.76, 1.59) | 1.26 | 1.09 | (0.57, 2.07) | 1.20 | 1.12 | (0.70, 1.79) | |
| P for trend☨ | 0.636 | 0.634 | 0.625 | |||||||
| PCB74** | ||||||||||
| <LOD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.992 | |||
| >LOD - 2.47 | 1.07 | 1.03 | (0.77, 1.39) | 1.12 | 1.11 | (0.64, 1.94) | 1.10 | 1.02 | (0.71, 1.49) | |
| 2.48 – 4.20 | 1.20 | 1.16 | (0.87, 1.54) | 1.20 | 1.11 | (0.66, 1.86) | 1.24 | 1.16 | (0.81, 1.66) | |
| 4.21 – 24.00 | 1.50 | 1.48 | (1.12, 1.96) | 1.34 | 1.27 | (0.78, 2.06) | 1.72 | 1.63 | (1.13, 2.35) | |
| P for trend☨ | 0.020 | 0.348 | 0.020 | |||||||
| PCB99 | ||||||||||
| <LOD - 1.48 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.992 | |||
| 1.49 – 2.46 | 1.19 | 1.17 | (0.87, 1.58) | 1.13 | 1.03 | (0.58, 1.83) | 1.28 | 1.24 | (0.85, 1.81) | |
| 2.47 – 4.34 | 1.25 | 1.22 | (0.91, 1.64) | 1.04 | 0.97 | (0.56, 1.68) | 1.34 | 1.25 | (0.86, 1.82) | |
| 4.35 – 40.79 | 1.37 | 1.37 | (1.02, 1.84) | 1.25 | 1.15 | (0.68, 1.95) | 1.49 | 1.49 | (1.02, 2.17) | |
| P for trend☨ | 0.049 | 0.522 | 0.043 | |||||||
| PCB105** | ||||||||||
| <LOD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.981 | |||
| >LOD - 1.16 | 0.88 | 0.85 | (0.60, 1.21) | 0.93 | 0.92 | (0.50, 1.69) | 1.87 | 0.80 | (0.52, 1.23) | |
| 1.17 – 1.89 | 1.47 | 1.42 | (1.05, 1.92) | 0.97 | 0.86 | (0.51, 1.44) | 1.89 | 1.79 | (1.21, 2.63) | |
| 1.90 – 9.68 | 1.32 | 1.36 | (0.99, 1.86) | 1.46 | 1.43 | (0.84, 2.46) | 1.34 | 1.34 | (0.89, 2.02) | |
| P for trend☨ | 0.036 | 0.391 | 0.034 | |||||||
| PCB118 | ||||||||||
| <LOD - 2.42 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.989 | |||
| 2.43 – 4.10 | 1.56 | 1.54 | (1.14, 2.09) | 1.65 | 1.56 | (0.87, 2.80) | 1.51 | 1.47 | (1.00, 2.17) | |
| 4.11 – 6.60 | 1.32 | 1.28 | (0.94, 1.75) | 1.34 | 1.28 | (0.73, 2.27) | 1.25 | 1.17 | (0.78, 1.73) | |
| 6.61 – 43.68 | 1.70 | 1.71 | (1.27, 2.31) | 1.74 | 1.64 | (0.93, 2.86) | 1.72 | 1.70 | (1.15, 2.50) | |
| P for trend☨ | 0.010 | 0.176 | 0.034 | |||||||
| PCB138/158 | ||||||||||
| <LOD - 5.45 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.991 | |||
| 5.46 – 9.64 | 1.16 | 1.15 | (0.85, 1.55) | 1.34 | 1.19 | (0.67, 2.13) | 1.11 | 1.11 | (0.76, 1.61) | |
| 9.65 – 16.22 | 1.13 | 1.12 | (0.83, 1.51) | 1.19 | 1.10 | (0.61, 1.97) | 1.08 | 1.04 | (0.71, 1.51) | |
| 16.23 – 176.30 | 1.23 | 1.22 | (0.91, 1.64) | 1.24 | 1.12 | (0.65, 1.94) | 1.26 | 1.26 | (0.86, 1.83) | |
| P for trend☨ | 0.313 | 0.877 | 0.251 | |||||||
| PCB146** | ||||||||||
| <LOD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.993 | |||
| >LOD - 1.71 | 1.05 | 1.01 | (0.76, 1.35) | 1.17 | 1.20 | (0.70, 2.06) | 1.05 | 1.00 | (0.70, 1.43) | |
| 1.72 – 3.05 | 0.97 | 0.92 | (0.69, 1.23) | 1.04 | 0.95 | (0.55, 1.63) | 0.91 | 0.86 | (0.60, 1.23) | |
| 3.06 – 29.0 | 1.16 | 1.18 | (0.89, 1.56) | 1.14 | 1.20 | (0.74, 1.94) | 1.21 | 1.22 | (0.84, 1.78) | |
| P for trend☨ | 0.418 | 0.660 | 0.490 | |||||||
| PCB153 | ||||||||||
| <LOD - 9.09 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | >0.99 | |||
| 9.10 – 15.78 | 0.98 | 0.97 | (0.72, 1.30) | 1.09 | 0.98 | (0.55, 1.73) | 0.96 | 0.95 | (0.66, 1.39) | |
| 15.79 – 26.93 | 1.15 | 1.12 | (0.84, 1.50) | 1.17 | 1.13 | (0.65, 1.97) | 1.07 | 1.01 | (0.70, 1.40) | |
| 26.94 – 234.40 | 1.12 | 1.13 | (0.85, 1.52) | 1.00 | 0.98 | (0.57, 1.69) | 1.19 | 1.19 | (0.82, 1.74) | |
| P for trend☨ | 0.337 | 0.949 | 0.302 | |||||||
| PCB156** | ||||||||||
| <LOD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.990 | |||
| >LOD - 1.98 | 1.10 | 1.02 | (0.76, 1.36) | 1.06 | 0.98 | > (0.57, 1.70) | 1.27 | 1.14 | (0.79, 1.64) | |
| 1.99 – 3.67 | 1.01 | 0.99 | (0.74, 1.31) | 1.08 | 1.01 | (0.60, 1.72) | 1.01 | 0.97 | (0.68, 1.39) | |
| 3.68 – 50.3 | 1.25 | 1.21 | (0.92, 1.61) | 1.17 | 1.16 | (0.71, 1.92) | 1.36 | 1.29 | (0.90, 1.85) | |
| P for trend☨ | 0.206 | 0.595 | 0.232 | |||||||
| PCB157** | ||||||||||
| <LOD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | >0.99 | |||
| >LOD - 1.12 | 1.05 | 1.00 | (0.65, 1.53) | 0.79 | 0.76 | (0.38, 1.54) | 1.21 | 1.09 | (0.61, 1.92) | |
| 1.13 – 1.69 | 1.33 | 1.29 | (0.87, 1.91) | 1.13 | 1.09 | (0.58, 2.05) | 1.48 | 1.46 | (0.86, 2.48) | |
| 1.70 – 10.31 | 1.09 | 1.07 | (0.70, 1.64) | 0.89 | 0.92 | (0.46, 1.85) | 1.20 | 1.14 | (0.65, 2.00) | |
| P for trend☨ | 0.347 | 0.798 | 0.274 | |||||||
| PCB167** | ||||||||||
| <LOD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | >0.99 | |||
| >LOD - 1.12 | 0.91 | 0.89 | (0.59, 1.36) | 0.67 | 0.65 | (0.32, 1.32) | 1.07 | 1.12 | (0.64, 1.96) | |
| 1.12 – 1.63 | 1.17 | 1.19 | (0.80, 1.77) | 0.85 | 0.86 | (0.44, 1.67) | 1.44 | 1.52 | (0.91, 2.54) | |
| 1.64 – 7.43 | 1.33 | 1.32 | (0.90, 1.93) | 1.02 | 1.01 | (0.54, 1.87) | 1.59 | 1.57 | (0.94, 2.64) | |
| P for trend☨ | 0.150 | 0.783 | 0.032 | |||||||
| PCB170 | ||||||||||
| <LOD - 2.28 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.99 | |||
| 2.29 – 4.20 | 0.96 | 0.96 | (0.71, 1.28) | 1.04 | 0.99 | (0.56, 1.74) | 0.96 | 0.98 | (0.68, 1.43) | |
| 4.21 – 7.53 | 1.02 | 1.02 | (0.76, 1.36) | 1.31 | 1.29 | (0.75, 2.22) | 0.90 | 0.92 | (0.63, 1.33) | |
| 7.54 – 55.16 | 1.07 | 1.07 | (0.80, 1.43) | 0.93 | 0.94 | (0.55, 1.59) | 1.16 | 1.17 | (0.80, 1.71) | |
| P for trend☨ | 0.808 | 0.183 | 0.329 | |||||||
| PCB178** | ||||||||||
| <LOD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | >0.99 | |||
| >LOD - 1.25 | 0.73 | 0.72 | (0.50, 1.04) | 0.66 | 0.71 | (0.36, 1.41) | 0.77 | 0.74 | (0.47, 1.17) | |
| 1.26 – 2.18 | 1.35 | 1.29 | (0.94, 1.77) | 1.23 | 1.15 | (0.65, 2.01) | 1.41 | 1.35 | (0.91, 2.02) | |
| 2.19 – 13.23 | 0.93 | 0.91 | (0.65, 1.29) | 0.82 | 0.85 | (0.48, 1.53) | 0.98 | 0.93 | (0.59, 1.47) | |
| P for trend☨ | 0.576 | >0.99 | 0.599 | |||||||
| PCB180 | ||||||||||
| <LOD - 6.08 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.988 | |||
| 6.09 – 11.10 | 0.91 | 0.91 | (0.68, 1.23) | 1.04 | 1.04 | (0.59, 1.84) | 0.85 | 0.88 | (0.61, 1.27) | |
| 11.11 – 20.20 | 0.99 | 0.98 | (0.74, 1.32) | 1.41 | 1.36 | (0.79, 2.35) | 0.85 | 0.86 | (0.59, 1.25) | |
| 20.21 – 153.90 | 1.02 | 1.02 | (0.76, 1.37) | 0.88 | 0.90 | (0.53, 1.53) | 1.08 | 1.09 | (0.75, 1.59) | |
| P for trend☨ | 0.656 | 0.702 | 0.519 | |||||||
| PCB183** | ||||||||||
| <LOD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.989 | |||
| >LOD - 1.56 | 0.97 | 0.92 | (0.68, 1.24) | 1.11 | 1.07 | (0.62, 1.86) | 0.88 | 0.83 | (0.57, 1.21) | |
| 1.57 – 2.86 | 1.12 | 1.09 | (0.83, 1.44) | 1.37 | 1.33 | (0.80, 2.22) | 1.04 | 1.01 | (0.72, 1.43) | |
| 2.87 – 19.4 | 1.02 | 0.99 | (0.74, 1.33) | 0.82 | 0.83 | > (0.50, 1.36) | 1.17 | 1.12 | (0.76, 1.60) | |
| P for trend☨ | 0.804 | 0.772 | 0.540 | |||||||
| PCB187 | ||||||||||
| <LOD - 1.57 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.983 | |||
| 1.58– 2.46 | 1.00 | 0.99 | (0.74, 1.33) | 0.74 | 0.71 | (0.40, 1.27) | 1.15 | 1.13 | (0.78, 1.64) | |
| 3.47 – 6.11 | 0.97 | 0.95 | (0.71, 1.28) ^ | 1.13 | 1.08 | (0.62, 1.89) | 0.88 | 0.87 | (0.60, 1.26) | |
| 6.12 – 77.57 | 1.02 | 1.01 | (0.75, 1.36) | 1.02 | 1.02 | (0.59, 1.76) | 1.01 | 0.98 | (0.67, 1.43) | |
| P for trend☨ | 0.952 | 0.655 | 0.624 | |||||||
| PCB194** | ||||||||||
| <LOD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.987 | |||
| >LOD - 2.27 | 1.02 | 1.00 | (0.74, 1.34) | 1.50 | 1.41 | (0.79, 2.50) | 0.98 | 0.96 | (0.66, 1.38) | |
| 2.28 – 4.69 | 1.04 | 1.03 | (0.77, 1.37) | 1.44 | 1.36 | (0.80, 2.32) | 0.98 | 0.99 | (0.68, 1.43) | |
| 4.70 – 35.55 | 0.93 | 0.92 | (0.69, 1.24) | 0.79 | 0.79 | (0.46, 1.34) | 1.05 | 1.03 | (0.70, 1.51) | |
| P for trend☨ | 0.768 | 0.344 | 0.847 | |||||||
| PCB196/203 | ||||||||||
| <LOD - 1.30 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.987 | |||
| 1.30 – 2.78 | 1.06 | 1.01 | (0.76, 1.35) | 1.11 | 1.09 | (0.59, 2.03) | 1.19 | 1.17 | (0.79, 1.76) | |
| 2.78 – 4.98 | 0.98 | 0.90 | (0.67, 1.20) | 1.35 | 1.36 | (0.77, 2.42) | 0.89 | 0.87 | (0.59, 1.28) | |
| 4.98 – 45.0 | 1.06 | 0.95 | (0.71, 1.27) | 0.95 | 0.97 | (0.56, 1.70) | 1.21 | 1.19 | (0.80, 1.76) | |
| P for trend☨ | 0.604 | 0.593 | 0.821 | |||||||
| PCB199** | ||||||||||
| <LOD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.988 | |||
| >LOD - 2.39 | 1.08 | 1.03 | (0.71, 1.37) | 1.06 | 0.99 | (0.56, 1.74) | 1.29 | 1.19 | (0.83, 1.72) | |
| 2.40 – 4.74 | 1.04 | 1.03 | (0.72, 1.52) | 1.16 | 1.07 | (0.63, 1.84) | 1.06 | 1.06 | (0.73, 1.52) | |
| 4.75 – 60.87 | 1.11 | 1.09 | (0.68, 1.95) | 0.91 | 0.89 | (0.53, 1.52) | 1.31 | 1.25 | (0.86, 1.81) | |
| P for trend☨ | 0.605 | 0.671 | 0.414 | |||||||
| PCB206** | ||||||||||
| <LOD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.993 | |||
| >LOD - 3.00 | 1.19 | 1.23 | (0.90, 1.68) | 1.16 | 1.09 | (0.63, 1.86) | 1.23 | 1.28 | (0.85, 1.91) | |
| 3.01 – 4.17 | 0.94 | 0.93 | (0.67, 1.28) | 0.71 | 0.74 | (0.42, 1.31) | 1.00 | 0.99 | (0.66, 1.50) | |
| 4.18 – 30.89 | 1.26 | 1.26 | (0.92, 1.72) | 0.95 | 0.98 | (0.58, 1.65) | 1.53 | 1.55 | (1.03, 2.34) | |
| P for trend☨ | 0.195 | 0.658 | 0.064 | |||||||
| PCB209** | ||||||||||
| <LOD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | >0.99 | |||
| >LOD - 1.27 | 0.85 | 0.82 | (0.57, 1.17) | 1.27 | 1.23 | (0.65, 2.33) | 0.70 | 0.70 | (0.44, 1.10) | |
| 1.28 – 1.98 | 0.84 | 0.81 | (0.57, 1.16) | 0.68 | 0.65 | (0.36, 1.19) | 0.91 | 0.86 | (0.54, 1.37) | |
| 1.99 – 15.58 | 1.13 | 1.08 | (0.77, 1.52) | 1.02 | 1.09 | (0.61, 1.96) | 1.17 | 1.09 | (0.71, 1.69) |
|
| P for trend☨ | 0.936 | 0.840 | 0.861 | |||||||
PCBs have >25% undetectable values, using <LOD as reference group
Conditional logistic regression, adjusted for BMI and military branch
Trend test: estimated by treating median values of PCBs categories as continuous variables
After stratifying by tumor size, significant associations were only observed for PTC macrocarcinoma but not microcarcinoma (Table 3). Specifically, an increased risk of PTC macrocarcinoma was associated with Q4 of PCB-74 (OR=1.63, 95% CI: 1.13, 2.35), PCB-99 (OR=1.49, 95% CI: 1.02, 2.17) and PCB-206 (OR=1.55, 95% CI: 1.03, 2.34), Q3 of PCB105 (OR=1.79, 95% CI: 1.21, 2.63), Q2 and Q4 of PCB-118 (OR=1.47, 95% CI: 1.01, 2.17; OR=1.70, 95% CI: 1.15, 2.50, respectively). Additionally, significant exposure-response relationships were observed for PCB-74, PCB-99, PCB-105, and PCB-118, with all test for trend P-values < 0.05. However, none of the p-values for interaction between microcarcinoma and macrocarcinoma was statistically significant, indicating no effect modification by tumor size.
We also stratified the single-congener analyses by sex (men; women) and histological subtype (classical PTC; follicular variant PTC), and no statistically significant differences were observed among subgroups with tests for interaction having P-values > 0.05. Significant associations were found for PCB-105 and PCB-118 with PTC among females as well as for the classical PTC subtype (Supplementary Table 1). After restricting the study population to cases diagnosed more than five years after serum sample collection and the matched controls (N=696 case/control pairs) and further stratifying this sample by tumor size, the results were similar to the overall population (Supplementary Table 2). Removing BMI from the adjusted covariates in the conditional logistic regression models showed similar results (data not shown).
3.4. Individual effects within the PCB mixture: BKMR
The relative importance of the mixture components, denoted by the posterior inclusion probability (PIPs) in the model across the five groups and across individual PCB congeners within each group (Supplementary Figure 2). In the analyzed PCB mixture, Group 2 which consists of PCB-74, PCB-118 and PCB-156 played the most important group role while PCB-118 played the most important individual role within Group 2, indicated by the highest PIP values among groups and within groups respectively.
The PTC risk differences between the 75th and 25th percentiles of the targeted PCBs, while holding all other PCBs at their median levels, from the BKMR model are shown in Figure 1. We found a significant positive PTC risk difference for PCB-118 (posterior mean estimate=0.091, 95% CrI: 0.002, 0.180) and a suggestive negative PTC risk difference for PCB-187 (posterior mean estimate=−0.054, 95% CrI: −0.135, 0.026). The estimated risk differences for other PCBs were close to null. Similar results were observed in sensitivity analyses where we fixed the levels of other PCBs at their 25th and 75th percentiles (Supplementary Figure 3).
Figure 1.
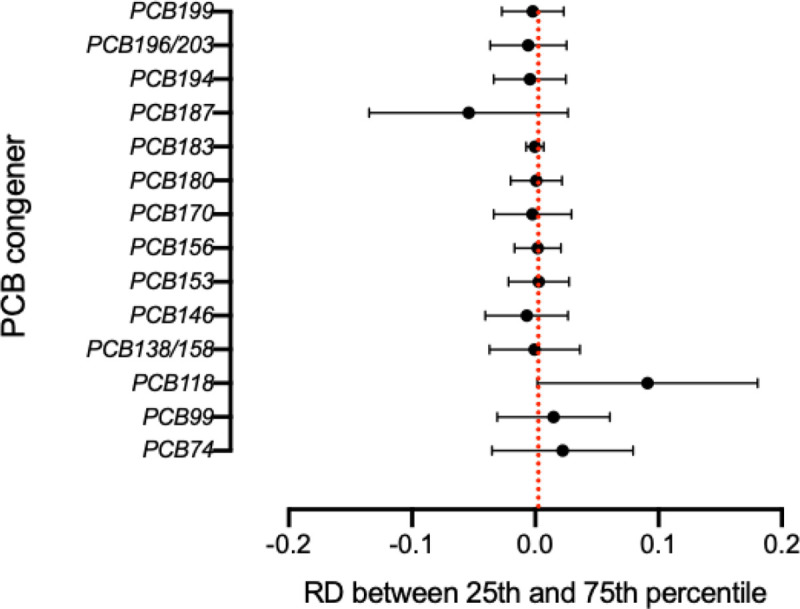
PTC Risk differences (RD) and 95% credible intervals between serum concentrations of PCB congeners at the 75th and 25th percentiles when other PCBs and BMI were fixed at median level, military branch at Air Force service, adjusted for BMI and military branch, using BKMR
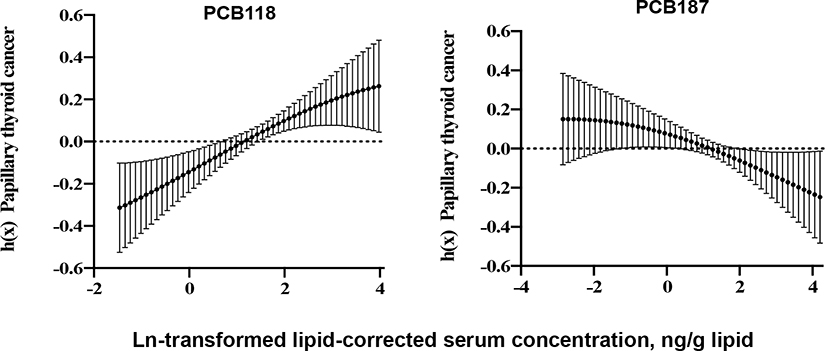
The exposure-outcome relationship curves across the whole concentration range of the target PCB and risk of PTC are presented for PCB-118 and PCB-187 in Figure 2. When fixing other congeners at their median levels, the exposure-outcome curve of PCB-118 showed an increasing trend with PTC, whereas PCB-187 showed a decreasing trend with PTC. Other PCBs had relatively flatter trends with PTC across different congener concentrations (Supplementary Figure 4).
Figure 2.
Exposure-outcome function h and 95% credible intervals between selected PCB congeners and PTC while fixing other PCBs at median level, adjusted for BMI and military branch using BKMR.
We also implemented BKMR models on stratified subgroups by tumor size and sex. PTC microcarcinomas had a similar PCB mixture structure (dominant Group 2 and dominant congener PCB-118 within Group 2) as the overall population (Supplementary Figure 5(a)), while PTC macrocarcinomas had more comparable weights across groups and within groups (Supplementary Figure 5(b)). A suggestive positive PTC risk difference between the 75th and 25th percentiles for PCB-118 was observed among PTC microcarcinomas, and a suggestive negative risk difference for PCB-187 among PTC macrocarcinomas (Supplementary Figures 6(a) and 6(b)). When stratified by sex, males had a similar dominant Group 2 within the mixture and PCB-118 within Group 2, while females had comparable weights across groups and within groups (Supplementary Figures 7(a) and 7(b)). For risk difference estimates, a suggestive positive PTC risk difference for PCB-118 was observed among males, while null effects with wider CrIs were observed among females (Supplementary Figures 8(a) and 8(b)).
3.5. Joint effect of the PCB mixture and relative chemical importance: WQS
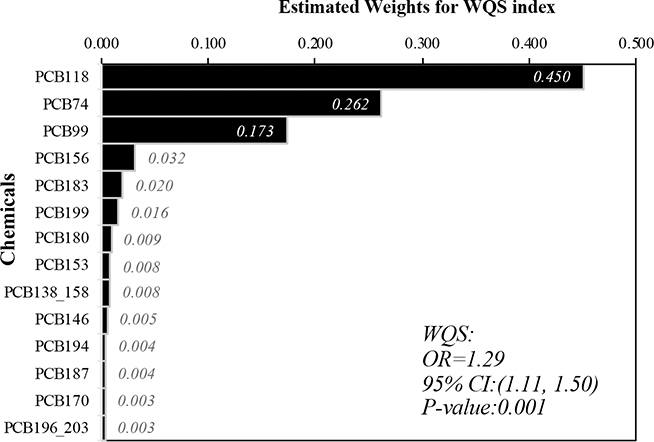
The joint effect of the 14 PCBs with a forced positive association with PTC (i.e., the joint harmful effect) and their relative importance in the mixture are presented in Figure 3. A significant overall joint effect of the PCB mixture was identified between the WQS index and PTC (OR=1.29, 95% CI: 1.11, 1.50). This finding indicates that a one quartile increase in the overall PCBs mixture exposure was associated with a 29% increase in PTC risk, conditional on BMI and military branch. Among the 14 included congeners, PCB-118 was the component with the highest weight for this positive association with PTC (weight=0.450), followed by PCB-74 (weight = 0.262) and PCB-99 (weight = 0.173). In the model with the WQS Index consisting of 13 PCBs while adjusting for PCB-187 as a covariate in addition to BMI and military branch, the joint effect of the remaining 13 PCBs was significantly associated with PTC and had a higher magnitude (OR=1.41, 95% CI: 1.18, 1.67); the adjusted PCB-187 was significantly negatively associated with PTC (OR=0.83, 95%CI: 0.71, 0.98). In this analysis, PCB-118, PCB-99, and PCB-74 were the “most important” components, with the highest weights in the mixture (weights = 0.312, 0.203, and 0.177, respectively) (Supplementary Figure 9).
Figure 3.
Estimated weights for individual PCBs in the WQS index, adjusted for BMI and military branch; Association between weighted quantile sum (WQS) regression index and PTC while forcing all effect direction of PCBs congeners to be positive.
When we stratified by tumor size and by sex, the positive WQS index of the 14 PCBs was significantly associated with PTC macrocarcinomas (OR=1.31, 95% CI=1.08–1.58), PTC among males (OR=1.25, 95% CI=1.03–1.53) and PTC among females (OR=1.40, 95% CI= 1.09, 1.80) (Supplementary Table 4). A similar association was also observed for PTC microcarcinomas though it was not significant (OR=1.30, 95% CI =0.98, 1.71). Similar to the overall population results, PCB-74, PCB-99, PCB-118 had the highest weights in the mixture for PTC macrocarcinomas and PTC among males and females. PCB-118 had the highest weights in the mixture with PTC microcarcinomas (data not shown).
4. DISCUSSION
Our study found that exposure to higher levels of PCB congeners 118, 74, 99, and 105 was associated with an increased risk of PTC in single congener models. After considering the effects from other PCBs and their potential interactions in mixtures analyses (BKMR), only PCB-118 showed a significant association with PTC. In joint mixtures analyses (WQS regression), PTC was associated with the overall PCB mixture that was mainly driven by PCB-118 and to a lesser extent by PCB-74 and PCB-99.
To our knowledge, this is the first epidemiologic study investigating the individual effect of PCB congeners within a PCB mixture on PTC risk via BKMR, a model that considers high correlations among mixture components and potential nonlinear exposure-outcome relationships. We also applied WQS to investigate the joint effect of the PCB mixture and to identify important components within the mixture in relation to PTC risk. Our findings for PCB-118 were consistent among the single congener model, BKMR and WQS regression, indicating that the association with PTC might act either through an independent exposure or as a component within a PCB mixture, or both. Significant associations were also observed in single congener models with PCB-74 and PCB-99 as independent exposures, but not supported by the mixtures analyses in our findings. Currently, only three other epidemiologic studies have investigated PCBs measured in serum samples and the risk of thyroid cancer (Deziel et al., 2021; Han et al., 2019; Lerro et al., 2018). Of these, one was conducted in a cohort in Norway (108 thyroid cancer cases and 316 controls) (Lerro et al., 2018), one was conducted in the U.S. (250 PTC cases and 250 controls, Caucasian women in Connecticut) (Deziel et al., 2021), and one was conducted in China (137 thyroid cancer cases and 186 controls) (Han et al., 2019). Null association for PCB-118 was found in the Norway study (Lerro et al., 2018) as an individual congener and in the U.S. study of Caucasian women (Deziel et al., 2021) as both an individual congener and within a chemical mixture; inverse association was reported in the China study (Han et al., 2019). The U.S. study also found a similar positive association with PCB-74 among younger women (Deziel et al., 2021) as our study, while a null association was reported for both PCB-74 and PCB-99 in the Norway study (Lerro et al., 2018). The observed inconsistency might reflect the differences across studies in the study population, timing of exposure assessment, exposure concentration, and the sample size. Specifically, the U.S. and China studies used post-diagnostic serum samples and a traditional case-control study design, while the Norway study used pre-diagnostic samples and a nested case-control study design. The exposure concentration of was higher in the U.S. and Norway studies while lower in China study. The Norwegian cohort included 324 participants who were born in 1923–1957 with a mean age of 41 at exposure measurement, while our study cohort included 1,484 military personnel who were born in 1946–1991 with a mean age of 26. The sample size of U.S. study and China study were also smaller than ours as stated previously.
An animal study from the National Toxicology Program found that PCB-118 caused a dose-dependent decrease in serum T4 levels and induced an increased incidence of thyroid gland lesions in female rats (National Toxicology Program, 2010). Epidemiologic studies also found that PCB-118 was inversely associated with free T4 levels (Alvarez-Pedrerol et al., 2008) and positively associated with TSH (Osius et al., 1999). In addition, PCB-118 has been identified as one of the nine congeners most strongly associated with thyroid disruptive effects (Patrick, 2009) as well as a Group 1 Human Carcinogen by the IARC, given its dioxin-like properties (IARC, 2016). Taken together, it is biologically plausible that PCB-118 may increase the risk of PTC. PCB-74 is in the same Wolff group (Group 2A) and shares some similar toxicologic characteristics with PCB-118. PCB-99 and PCB-118 also have shown similar thyroid disruptive effects in causing reductions of T4 levels in animal studies (Heiger-Bernays et al., 2020). A study of mothers in California found that PCB-99 was positively associated with increasing neonatal TSH level (Patrick, 2009), supporting a possible association between PCB-99 and PTC in our study.
A suggestive inverse association between PCB-187 and PTC risk was observed in mixtures analyses but not in single congener models. The mixtures analysis including PCB-187 was only performed in the U.S. study (Deziel et al., 2021), which did not observe a significant association. Compared with our study, which used BMKR and WQS regression for evaluation of 14 PCBs, the U.S. study applied hierarchical Bayesian logistics regression and principal components analysis and evaluated 31 PCBs + OCPs. Additionally, PCB-187 concentrations were lower in our study (median: 3.4 ng/g lipid) compared to the U.S. study (median: 6.1 ng/g lipid). The Norway and the U.S. study populations found a consistent null association between PCB-187 and PTC in single congener models. As the carcinogenecity of PCB-187 in epidemiologic studies is still unclear and the impact within mixtures is yet to be identified, it is possible that the suggestive inverse association in mixture analyses is due to chance.
The possibility that the observed associations were due to other unmeasured PCB congeners is unlikely. Based on the CDC’s Fourth National Report on Human Exposures to Environmental Chemicals, 38 out of all 209 PCB congeners are commonly found in humans (full list in Supplementary Table 5) (Crinnion, 2010). Among these 38 congeners, 15 were not measured in our study (PCB-44, 49, 52, 81, 87, 101, 110, 126, 128, 149, 151, 169, 172, 177, 195). However, these 15 have not been measured in recent National Health and Nutrition Examination Survey (NHANES) biomarker panels, as they are not detected in high enough frequency. However, with the exception of PCB-126, the other PCBs that were not measured in our study do not have documented animal and/or human health effects (Faroon and Ruiz, 2016).
While we cannot rule out potential confounding from other chemicals that may be correlated with PCBs, such as organochlorine pesticids (OCP), polychlorinated dibenzo-p-dioxins (dioxins), and polychlorinated dibenzofurans (furans) (Control and Prevention, 2009; EPA, 2017), we consider this potential to be limited. Using data from our study, we investigated this potential. A pairwise Pearson correlation test yielded only weak to moderate correlations (correlation coefficients < 0.53) between three OCPs that were found associated with thyroid cancer in a previous study (DDE, DDT, and trans-nonachlor) (Lerro et al., 2018) and PCB congeners. Although further adjustment by each of these OCP exposures in our mixtures analyses somewhat attenuated our results, they did not appreciably change the patterns of association which we report here (data not shown). We did not measure dioxins/furans in our study, however, the serum concentrations of dioxins/furans in the general US population from NHANES during the similar period of exposure were under the limit of detection, and the correlation between PCBs and detected dioxins/furans in serum samples have been reported to be moderate to weak (<0.5) (Control and Prevention, 2009; Gladen et al., 1999). Therefore, we consider the possibility of the associations we found between PCBs and PTC being explained by confounding by other chemicals to be limited.
A major strength of our study was the use of pre-diagnostic serum samples for measurements of PCB exposures, which minimized potential reverse causality. In particular, the average 9 years between serum samples of PCBs measurement and the diagnosis of PTC cases was appropriate, given that the latency period of thyroid cancer has been observed to be approximately 5–10 years (albeit, post-radiation exposure) (Iglesias et al., 2017). Our study population of US active-duty military personnel also minimized the potential for selection bias related to differential access to medical care or health insurance, since the Military Health System is a healthcare system designed for equal access. A large sample size allowed us to perform stratified analyses by important subgroups: sex, tumor size, and histological subtypes. Because the incidence rate in females is about three times higher than in males, previous studies have had limited statistical power to investigate risk factors in males separately (Rahbari et al., 2010). In addition, more advanced-stage cancers tend to present in males, and the recurrence rate of thyroid cancer among males is higher than that among females (Zahedi et al., 2019). It is therefore important to study sex-specific risk factors for PTC, and the sample size of our study enabled that. The investigation of PCB mixtures is another strength of our study, as our multiple pollutant models reflect more realistic exposure scenarios than single congener models. The implemented BKMR and WQS analyses provided new insights into the health impact of PCB congeners on PTC risk. Further studies of mixtures of PCBs and other chemical exposures are needed to reveal the complex and realistic exposure scenarios that occur.
Although exposure to PCBs was measured at one time point prior to the disease diagnosis, the average half-life of PCBs within the human body is 10–15 years (Ritter et al., 2011). Additionally, Meeker and colleagues (2009) examined temporal reliability of organochlorine compounds in serum samples over 2–24 months and found strong intraclass correlations (0.86 to 0.98) of serum PCBs during the follow-up time. This suggests that using a single serum sample to represent PCB exposures over time is highly reliable in epidemiologic studies (Meeker et al., 2009). However, potential measurement error from a single sample might still exist, which is likely to be non-differential and result in an underestimation of observed associations. Another limitation was the lack of information on other potential confounders such as ionizing radiation and iodine consumption, though their potential to introduce confounding is debatable, since there is not strong evidence that these exposures are associated with PCBs. Some medical equipment for radiation treatment might have old transformers and capacitors that contain PCBs, however, this is not considered a major source of PCB exposure (Institute of Medicine Committee on Curriculum Development in Environmental, 1995). Although medical ionizing radiation exposure is an important risk factor for PTC, there is a lack of evidence that ionizing radiation from medical treatment is associated with PCB exposures in this population. Compared to the general population, military personnel might have higher radiation exposure from nuclear testing or depleted uranium. However, military personnel who have been reported to be exposed to nuclear testing radiation largely served in 1940s-1960s (U.S. Department of Veterans Affairs, 2016), which was generally earlier than the service time period in our study population. Although our study population might be exposed to depleted uranium which has been frequently reported in the US since the 1990s (U.S. Department of Veterans Affairs, 2016), an expert working group from the UK indicated that the possibility of excess cancer risk from this exposure is minimal (The Royal Society, 2001). Iodine consumption is largely from seafood, and studies have suggested a protective effect of seafood on thyroid cancer (Dal Maso et al., 2009). However, it is likely that not controlling for the potentially confounding effect of iodine consumption would result in a more conservative estimation of PCBs and PTC association.
Sources and pathways of exposure to PCBs among military service members were likely to have been generally similar to the general population, with ingestion of contaminated food as the main exposure route (U.S. Department of Veterans Affairs; US Army Corps of Engineers, 2012). Although PCBs have been found in old Navy vessels (Still et al., 2003), this additional exposure source might be less likely associated with PTC than other sources since the incidence rate of PTC among those in the Navy was lower than in those from other military branches in our study (Enewold et al., 2011). Our study of US military personnel may be generalizable to the general population with respect to exposure sources, exposure concentration, and PTC incidence rates. Besides the similar exposure sources and routes stated previously, PCB exposure concentrations among the general population from the NHANES III (exposure in 1994–1999) and annual NHANES panels (exposures in 1999–2009) are comparable to those from our study population in the same time period of 1994–2009 (National Center for Health Statistics). Although the incidence rates of PTC among military service women were higher than those in the U.S. general population between 1990 and 2004, an increasing trend of PTC incidence was observed in both the military and the general population (Enewold et al., 2011).
5. Conclusion
Our large nested case-control study among U.S. military service members supports that exposure to certain PCBs congeners might be associated with an increased risk of PTC. Four PCB congeners (PCB-118, PCB-74, PCB-99, PCB-105) had significant associations and dose-response relationships with PTC risk in single congener models. In a PCB mixtures analysis, PTC was predominately associated with PCB-118, followed by PCB-74 and PCB-99. One PCB congener, PCB-187, showed an inverse trend in the mixture analyses. Future studies are needed to confirm our study findings and to investigate the health impacts of real-life complex exposure scenarios.
Supplementary Material
Highlights.
Humans are continously exposed to polychlorinated biphenyls (PCBs)
Pre-diagnosis PCBs were measured in serum samples of U.S. military personnel
Exposure to certain PCBs or mixtures were associated with PTC risk
Acknowledgement
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Uniformed Services University of the Health Sciences, the Department of Defense, or the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the Department of Defense, the Centers for Disease Control and Prevention, the Public Health Service, or the US Department of Health and Human Services.
Source of funding
This research was supported by the National Institutes of Health (NIH) grant R01ES020361 (Y. Zhang and J. Rusiecki) and National Cancer Institute (NCI) grant P30CA016359 (J. Warren and S. Ma).
ABBREVIATION
- BKMR
Bayesian kernel machine regression
- BMI
Body mass index
- CI
Confidence interval
- CrI
Credible interval
- DMSS
Defense Medical Surveillance System
- DoDSR
Department of Defense Serum Repository
- DOB
Date of birth
- ICD-O
International Classification of Diseases for Oncology code
- LOD
Limit of detection
- OR
Odds ratio
- PCB
Polychlorinated biphenyls
- PTC
Papillary thyroid cancer
- WQS
Weighted quantile sum
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akoso BT, et al. , 1992. Effects of Purified Polybrominated Biphenyl Congeners on the Thyroid and Pituitary Glands in Rats. Journal of the American College of Toxicology. 1, 23–36. [Google Scholar]
- Allison PD, Handling missing data by maximum likelihood. SAS global forum, 2012, pp. 1038–21. [Google Scholar]
- Alsen M, et al. , 2021. Endocrine Disrupting Chemicals and Thyroid Cancer: An Overview. Toxics. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Pedrerol M, et al. , 2008. Effects of PCBs, p,p’-DDT, p,p’-DDE, HCB and beta-HCH on thyroid function in preschool children. Occup Environ Med. 65, 452–7. [DOI] [PubMed] [Google Scholar]
- ATSDR, Toxicology profile for polychlorinated biphenyls (PCBs). Atlanta, GA: Agency for Toxic Substances and Disease Registry (ATSDR), U.S. Department of Health and Human Services, Public Health Service, 2000. [Google Scholar]
- Benson K, et al. , 2018. Polychlorinated biphenyls, indicators of thyroid function and thyroid autoantibodies in the Anniston Community Health Survey I (ACHS-I). Chemosphere. 195, 156–165. [DOI] [PubMed] [Google Scholar]
- Bobb JF, et al. , 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environmental Health. 17, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, et al. , 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 16, 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, et al. , 2016. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environmental health perspectives. 124, A6–A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capen CC, Martin SL, 1989. The effects of xenobiotics on the structure and function of thyroid follicular and C-cells. Toxicol Pathol. 17, 266–93. [DOI] [PubMed] [Google Scholar]
- Carlin DJ, et al. , 2013. Unraveling the health effects of environmental mixtures: an NIEHS priority. Environmental health perspectives. 121, A6–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WT, et al. , 1977. Effect of Polychlorinated Biphenyl (PCB) on the Thyroid Gland of Rats: Ultrastructural and Biochemical Investigations. The American Journal of Pathology. 89, 119–136. [PMC free article] [PubMed] [Google Scholar]
- Control, C. f. D., Prevention, Fourth national report on human exposure to environmental chemicals. Atlanta, GA, 2009.
- Crinnion WJ, 2010. The CDC Fourth National Report on Human Exposure to Environmental Chemicals: What it Tells Us About our Toxic Burden and How it Assists Environmental Medicine Physicians. Alternative medicine review. 15. [PubMed] [Google Scholar]
- Dal Maso L, et al. , 2009. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control. 20, 75–86. [DOI] [PubMed] [Google Scholar]
- Deziel NC, et al. , 2021. Exposure to polychlorinated biphenyls and organochlorine pesticides and thyroid cancer in connecticut women. Environmental Research. 192, 110333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enewold LR, et al. , 2011. Thyroid Cancer Incidence among Active Duty U.S. Military Personnel, 1990–2004. Cancer Epidemiology Biomarkers & Prevention. 20, 2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA, Studies of Fish Tiuss Contamination. Vol. 2020. Environmental Protection Agency, 2017. [Google Scholar]
- Faroon O, Ruiz P, 2016. Polychlorinated biphenyls: New evidence from the last decade. Toxicology and industrial health. 32, 1825–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladen BC, et al. , 1999. Correlations among polychlorinated biphenyls, dioxins, and furans in humans. Am J Ind Med. 35, 15–20. [DOI] [PubMed] [Google Scholar]
- Han X, et al. , 2019. Associations between Exposure to Persistent Organic Pollutants and Thyroid Function in a Case-Control Study of East China. Environmental Science & Technology. 53, 9866–9875. [DOI] [PubMed] [Google Scholar]
- Haslam A, et al. , 2016. Polychlorinated biphenyls and omega-3 fatty acid exposure from fish consumption, and thyroid cancer among New York anglers. J Environ Sci (China). 41, 270–277. [DOI] [PubMed] [Google Scholar]
- Heiger-Bernays WJ, et al. , 2020. Human health risks due to airborne polychlorinated biphenyls are highest in New Bedford Harbor communities living closest to the harbor. Science of The Total Environment. 710, 135576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holford TR, et al. , 2000. Joint effects of nine polychlorinated biphenyl (PCB) congeners on breast cancer risk. International Journal of Epidemiology. 29, 975–982. [DOI] [PubMed] [Google Scholar]
- Huang H, et al. , 2017. Thyroid-Stimulating Hormone, Thyroid Hormones, and Risk of Papillary Thyroid Cancer: A Nested Case-Control Study. Cancer Epidemiol Biomarkers Prev. 26, 1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC, IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. Polychlorinated Biphenyls and Polybrominated Biphenyls. Lyon (FR): International Agency for Research on Cancer; 2016. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 107.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK361696/. 2016. [PMC free article] [PubMed] [Google Scholar]
- Iglesias ML, et al. , 2017. Radiation exposure and thyroid cancer: a review. Arch Endocrinol Metab. 61, 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine Committee on Curriculum Development in Environmental, M. In: Pope AM, Rall DP, Eds.), Environmental Medicine: Integrating a Missing Element into Medical Education. National Academies Press (US). Washington (DC), 1995. [PubMed] [Google Scholar]
- Jones R, et al. , 2012. Semi-automated extraction and cleanup method for measuring persistent organic pollutants in human serum. [Google Scholar]
- Lauby-Secretan B, et al. , 2013. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 14, 287–8. [DOI] [PubMed] [Google Scholar]
- Lerro CC, et al. , 2018. A nested case-control study of polychlorinated biphenyls, organochlorine pesticides, and thyroid cancer in the Janus Serum Bank cohort. Environ Res. 165, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin JH, et al. , 2004. Epidemiologic evaluation of measurement data in the presence of detection limits. Environmental health perspectives. 112, 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallin K, et al. , 2004. Cohort mortality study of capacitor manufacturing workers, 1944–2000. J Occup Environ Med. 46, 565–76. [DOI] [PubMed] [Google Scholar]
- Meeker JD, et al. , Serum and follicular fluid organochlorine concentrations among women undergoing assisted reproduction technologies. Environmental health : a global access science source, Vol. 8, 2009, pp. 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Filho A, et al. , 2021. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol. 9, 225–234. [DOI] [PubMed] [Google Scholar]
- Monosson E, 2005. Chemical mixtures: considering the evolution of toxicology and chemical assessment. Environmental health perspectives. 113, 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics, National Health and Nutrition Examination Survey. Center for Disease Control and Prevention. [Google Scholar]
- National Toxicology Program, 2006. Toxicology and carcinogenesis studies of a binary mixture of 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) (Cas No. 57465–28-8) and 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB 153) (CAS No. 35065–27-1) in female Harlan Sprague-Dawley rats (gavage studies). National Toxicology Program technical report series. 1–258. [PubMed] [Google Scholar]
- National Toxicology Program, 2010. Toxicology and carcinogenesis studies of 2,3’,4,4’,5-pentachlorobiphenyl (PCB 118) (CAS No. 31508–00-6) in female harlan Sprague-Dawley rats (gavage studies). Natl Toxicol Program Tech Rep Ser. 1–174. [PubMed] [Google Scholar]
- Ness DK, et al. , 1993. Effects of perinatal exposure to specific PCB congeners on thyroid hormone concentrations and thyroid histology in the rat. Toxicol Lett. 68, 311–23. [DOI] [PubMed] [Google Scholar]
- NIEHS, Statistical Approaches for Assessing Health Effects of Environmental Chemical Mixtures in Epidemiology Studies [Workshop], 13–14 July 2015, National Research of Environmental Health Sciences, Research Triangle Park, North Carolina., Vol. 2020, 2015. [Google Scholar]
- Osius N, et al. , 1999. Exposure to polychlorinated biphenyls and levels of thyroid hormones in children. Environmental Health Perspectives. 107, 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick L, 2009. Thyroid disruption: mechanism and clinical implications in human health. Altern Med Rev. 14, 326–46. [PubMed] [Google Scholar]
- Pavuk M, et al. , 2004. Environmental exposure to PCBs and cancer incidence in eastern Slovakia. Chemosphere. 54, 1509–20. [DOI] [PubMed] [Google Scholar]
- Perdue CL, et al. , 2015. A Brief Description of the Operation of the DoD Serum Repository. Mil Med. 180, 10–2. [DOI] [PubMed] [Google Scholar]
- Rahbari R, et al. , 2010. Thyroid cancer gender disparity. Future oncology (London, England). 6, 1771–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzetti S, et al. , 2019. gWQS: An R Package for Linear and Generalized Weighted Quantile Sum (WQS) Regression. Journal of Statistical Software. [Google Scholar]
- Ritter R, et al. , 2011. Intrinsic human elimination half-lives of polychlorinated biphenyls derived from the temporal evolution of cross-sectional biomonitoring data from the United Kingdom. Environmental health perspectives. 119, 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LW, Hansen LG, 2015. PCBs: recent advances in environmental toxicology and health effects. University Press of Kentucky. [Google Scholar]
- Saeed A, Hansen LG, 1997. Morphometric changes in the prepubertal female rat thyroid gland following acute exposure to 2,2’,4,4’-tetrachlorobiphenyl and Aroclor 1242. J Toxicol Environ Health. 51, 503–13. [DOI] [PubMed] [Google Scholar]
- SEER, SEER Cancer Statistics Review, 1975–2018, National Cancer Institute. Bethesda, MD. 2021. [Google Scholar]
- Sjödin A, et al. , 2004. Semiautomated High-Throughput Extraction and Cleanup Method for the Measurement of Polybrominated Diphenyl Ethers, Polybrominated Biphenyls, and Polychlorinated Biphenyls in Human Serum. Analytical Chemistry. 76, 1921–1927. [DOI] [PubMed] [Google Scholar]
- Still KR, et al. , 2003. Estimation of the Health Risks Associated with Polychlorinated Biphenyl (PCB) Concentrations Found Onboard Older U.S. Navy Vessels. Applied Occupational and Environmental Hygiene. 18, 737–758. [DOI] [PubMed] [Google Scholar]
- Taylor Kyla W, et al. , 2016. Statistical Approaches for Assessing Health Effects of Environmental Chemical Mixtures in Epidemiology: Lessons from an Innovative Workshop. Environmental Health Perspectives. 124, A227–A229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Royal Society, The health hazards of depleted uranium munitions. Part I. London, UK: The Royal Society, 2001. [Google Scholar]
- U.S. Department of Veterans Affairs, Polychloriniated Biphenyls (PCBs).
- U.S. Department of Veterans Affairs, Environmental exposures programs and services for veterans. Washington D.C, US; March, 2006., 2016. [Google Scholar]
- US Army Corps of Engineers, PCBs in caulk and paint. Washington, DC. 2012. [Google Scholar]
- Van den Berg M, et al. , 2006. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicological sciences : an official journal of the Society of Toxicology. 93, 223–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, et al. , 1997. Proposed PCB congener groupings for epidemiological studies. Environ Health Perspect. 105, 13–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi A, et al. , 2019. Risk for Thyroid Cancer Recurrence Is Higher in Men Than in Women Independent of Disease Stage at Presentation. Thyroid. 30, 871–877. [DOI] [PubMed] [Google Scholar]
- Zhuo H, Exposure to Polychlorinated Biphenyl (PCBs) and Risk of Papillary Thyroid Cancer (PTC): A Nested Case-Control Study. Vol. Master Thesis. Master Thesis. Yale University, 2018. [Google Scholar]
- Zoeller RT, 2001. Polychlorinated biphenyls as disruptors of thyroid hormone action. PCBs: Recent Advances in Environmental Toxicology and Health Effects. Lexington: University Press of Kentucky. 265–271. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.