Abstract
Background
Exercise stress testing for cardiovascular assessment in kidney transplant candidates has been shown to be a feasible alternative to pharmacologic methods. Exercise stress testing allows the additional assessment of exercise capacity, which may have prognostic value for long‐term cardiovascular outcomes in pre‐transplant recipients. This study aimed to evaluate the prognostic value of exercise capacity on long‐term cardiovascular outcomes in kidney transplant candidates.
Methods and Results
We retrospectively evaluated exercise capacity in 898 consecutive kidney transplant candidates between 2013 and 2020 who underwent symptom‐limited exercise stress echocardiography for pre‐transplant cardiovascular assessment. Exercise capacity was measured by age‐ and sex‐predicted metabolic equivalents (METs). The primary outcome was incident major adverse cardiovascular events, defined as cardiac death, non‐fatal myocardial infarction, and stroke. Cox proportional hazard multivariable modeling was performed to define major adverse cardiovascular events predictors with transplantation treated as a time‐varying covariate. A total of 429 patients (48%) achieved predicted METs. During follow‐up, 93 (10%) developed major adverse cardiovascular events and 525 (58%) underwent transplantation. Achievement of predicted METs was independently associated with reduced major adverse cardiovascular events (hazard ratio [HR] 0.49; [95% CI 0.29–0.82], P=0.007), as was transplantation (HR, 0.52; [95% CI 0.30–0.91], P=0.02). Patients achieving predicted METs on pre‐transplant exercise stress echocardiography had favorable outcomes that were independent (HR, 0.78; [95% CI 0.32–1.92], P=0.59) and of similar magnitude to subsequent transplantation (HR, 0.97; [95% CI 0.42–2.25], P=0.95).
Conclusions
Achievement of predicted METs on pre‐transplant exercise stress echocardiography confers excellent prognosis independent of and of similar magnitude to subsequent kidney transplantation. Future studies should assess the benefit on exercise training in this population.
Keywords: exercise testing, kidney transplantation, major adverse cardiovascular events, stress echocardiography
Subject Categories: Nephrology and Kidney, Cardiovascular Disease, Exercise Testing
Nonstandard Abbreviations and Acronyms
- ESE
exercise stress echocardiography
- LVEF
left ventricular ejection fraction
- MACE
major adverse cardiovascular events
- METs
metabolic equivalent
Clinical Perspective.
What Is New?
Better exercise capacity on pre‐transplant exercise stress echocardiography is associated with reduced major adverse cardiovascular events.
Patients who achieve predicted metabolic equivalents for age and sex had excellent long‐term prognosis of similar magnitude to and irrespective of future kidney transplantation.
Ability to achieve predicted metabolic equivalents for age and sex could be a better discriminator than an unadjusted threshold of 7 metabolic equivalents or achievement of target heart rate.
What Are the Clinical Implications?
Ability to achieve age and sex predicted metabolic equivalents could be used as a new metric to predict cardiovascular outcomes in kidney transplant candidates.
Future studies could evaluate exercise training to improve long‐term cardiovascular outcomes in patients with chronic kidney disease.
Cardiovascular disease (CVD) is the leading cause of mortality in patients with chronic kidney disease (CKD) regardless of transplantation status. 1 , 2 Therefore, risk stratification by cardiac stress testing is often performed in the work‐up for kidney transplantation with consideration of revascularization to reduce this risk. 3 , 4 However, the ISCHEMIA‐CKD study demonstrated that revascularization does not reduce death or non‐fatal myocardial infarction (MI) in patients with CKD, questioning the role of routine cardiac stress testing in this vulnerable population. 5 Furthermore, the majority of CKD patients referred for cardiac stress testing prior to transplantation are asymptomatic, and may not have improved angina‐related health status if they undergo coronary revascularization following abnormal cardiac stress testing results. 6 Although current literature suggests less need for cardiac stress testing to guide revascularization in patients with CKD, there may still be utility for cardiac stress testing to identify at risk patients who may benefit from lifestyle and medical preventative measures to improve long‐term cardiovascular outcomes.
Cardiac stress testing in the CKD population is routinely performed by pharmacological methods due to a perceived inability of this population to exercise adequately. 7 Despite this, exercise stress echocardiography (ESE) has been shown to be feasible, safe and well tolerated in patients with CKD 8 and allows measurement of exercise capacity, a recognized marker of long‐term cardiovascular risk in the general population. 9 , 10 Studies have reported that patients with CKD have reduced exercise capacity 11 due to a combination of sedentary lifestyle, chronic inflammation and maladaptive left ventricular (LV) remodeling. 12 , 13 , 14 However, the prognostic value of exercise capacity on long‐term cardiovascular outcomes in the CKD population remains unclear.
We aimed to evaluate the prognostic utility of exercise capacity, quantified by metabolic equivalents (METs) on pre‐operative ESE, on long‐term cardiovascular outcomes among kidney transplant candidates. This is with view of identifying a potentially modifiable risk factor that could be targeted with lifestyle intervention. We hypothesized that ability to achieve age and sex predicted METs on pre‐operative ESE is associated with better long‐term cardiovascular outcomes independent of other known cardiovascular risk factors and subsequent kidney transplantation.
METHODS
Data Availability Statement
Data are available from the corresponding author upon reasonable request.
Study Population
This was a retrospective analysis of a prospectively curated registry of consecutive patients above 18 years old with stage 4 or 5 CKD 7 (including those on dialysis) who were referred for ESE for cardiovascular risk stratification as part of routine assessment for kidney transplantation suitability at Monash Health, Melbourne, Australia. As part of local guidelines, all kidney transplant candidates were required to undergo non‐invasive coronary artery disease (CAD) screening prior to eligibility for waitlisting. All patients referred for ESE attempted ESE unless there were significant contraindications to ESE such as musculoskeletal disease affecting mobility, use of a gait aid, and/or prior leg amputation. Those without contraindications underwent a 40‐m gait assessment immediately prior to testing with the final decision on exercise versus pharmacologic testing at the discretion of the supervising cardiologist. Patients who were unable to exercise underwent dobutamine stress echocardiography and were excluded from the study. Prospective registry data collection of eligible patients commenced on February 1, 2013 and retrospective analysis was performed in July 2020, with follow‐up from the date of ESE to July 31, 2020. Patient consent was not required for this study. Institutional ethics approval was obtained for the study.
Exercise Stress Echocardiography
All ESE were performed according to American Society of Echocardiography guidelines. 15 Patients were asked to withhold any beta‐blocker use for at least 48 hours prior to the test, but beta‐blocker usage did not preclude testing. ESE was performed using the standard Bruce protocol to assess exercise capacity in METs. Exercise was not ceased when target heart rate was attained but was performed as a symptom‐limited test. The test was prematurely aborted at supervising physician discretion if any of the following occurred: limiting symptoms (angina, dyspnea), ST depression ≥3 mm, ventricular tachycardia, decline in blood pressure by ≥30 mm Hg, rise in systolic blood pressure to ≥230 mm Hg.
Baseline and stress imaging were performed using views from the parasternal long and short axis; apical 4 chamber, 2 chamber, and long axis. All echocardiographic studies were performed, supervised and reported by a specialist non‐invasive imaging cardiologist. Baseline LV dysfunction was defined as left ventricular ejection fraction (LVEF) <50% by modified Simpson biplane method on resting images prior to exercise. Tests were considered abnormal if they were non‐diagnostic due to poor imaging, had global failure of LV augmentation or fall in LVEF at peak stress, or inducible regional wall motion abnormalities. A decision on the necessity for post‐test coronary angiography and revascularization was discussed at a multi‐disciplinary cardiology and cardiothoracic surgical conference with additional input from the renal transplant department. Non‐MI revascularization was defined as revascularization for stable angina or asymptomatic patients following abnormal ESE results. All subjects with normal ESE results were allowed to be wait‐listed for kidney transplantation from a cardiovascular perspective without further cardiac testing, whilst those with abnormal ESE results were only cleared after review by a specialist cardiologist in clinic either with or without post‐test revascularization. If a patient underwent multiple ESE for pre‐transplant cardiovascular assessment, the earliest ESE was recorded for study purposes. Inter‐reader and intra‐reader variability were assessed in a subset of 20 randomly selected cases.
Clinical Data
Clinical data were collated from the patients' medical records and prospective local and national dialysis and transplant registries (ANZDATA [Australian and New Zealand Dialysis and Transplant Registry]). Achieved METs were automatically calculated by a computer‐generated algorithm according to the Bruce protocol. Predicted METs for age and sex for each patient was calculated using previously published formulas: for women, predicted METs=14.7−(0.13×age); and for men, predicted METs=18−(0.15×age). 16 Secondary analyses were performed using METs as a continuous variable, a non‐adjusted cut‐off of 7 METs which has previously been defined as threshold for “good” exercise capacity, 17 and ability to achieve 85% of maximal predicted heart rate (MPHR) which is the standard target heart rate for ESE. 15
End Points
The primary outcome of the study was major adverse cardiovascular events (MACE), defined as a composite of cardiac death, non‐fatal MI, and stroke. 18 Each individual endpoint was analyzed as a secondary outcome. Outcomes were obtained from ANZDATA and reported at the pre‐specified 7‐year follow‐up from start of enrolment, which represents the end of the study period from 2013 to 2020.
Statistical Analysis
Categorical data are presented as absolute numbers and percentages and compared with chi‐square test or Fishers exact test as appropriate. Continuous data are displayed as mean±SD if data were normally distributed, or medians (interquartile range [IQR]) for non‐Gaussian data and compared with t tests or Mann–Whitney tests as appropriate. Inter‐reader and intra‐reader variability were assessed by Kappa statistic.
Cox proportional hazards models were used to assess achievement of age and sex predicted METs, achieved METs, and achievement of 7 METs separately on time to first MACE. The date of ESE was used as time of study entry. If a patient experienced more than one MACE during follow‐up (eg, non‐fatal MI followed by death, the first event defined the MACE recorded, and the time of study exit). In patients without MACE, the date of last follow‐up or July 31, 2020, whichever came last, was considered to be the censoring date. Achievement of age and sex predicted METs and achievement of 7 METs were modeled as dichotomous variables, whilst achieved METs was modeled continuously. Kidney transplantation was treated as a time‐dependent covariate in order to account for the wait time between ESE and transplantation. Graphical time‐to‐event plots were constructed and the Mantel‐Byar test used to assess the differences in equality of curves due to the use of time‐varying data as previously recommended for transplant data. 19 Model covariates included those with P<0.20 on univariable assessment and variables of clinical relevance such as age. The final included covariates were: age, sex, diabetes, hypertension, hyperlipidemia, history of smoking, history of ischemic heart disease (IHD), previous kidney transplantation, body mass index (BMI), baseline LV dysfunction, abnormal ESE result, non‐MI revascularization prior to transplantation, achievement predicted METs, and transplantation. Multicollinearity between covariates was excluded by assessing variance inflation factors. Conditional proportional hazards assumptions were visually inspected by plotting Schoenfield residuals. Results were reported as hazard ratio (HR) with 95% CI. A two‐sided P‐value of <0.05 was considered statistically significant. Statistical analysis was performed using Stata MP/14 (StataCorp, College Station, TX).
RESULTS
Demographics
There were 974 patients with CKD referred for stress echocardiography for cardiovascular risk evaluation testing during the study period. A total of 76 patients were excluded due to an inability to exercise (musculoskeletal disease affecting mobility [n=49], use of a gait aid [n=14], leg amputation [n=13]), accordingly 898 patients were included in the study cohort. Patient characteristics are shown in Table 1. The mean age of the cohort was 51.8±11.3 years and 69% had renal replacement therapy at baseline.
Table 1.
Population Characteristics
| Demographics | Total (n=898) | Did not achieve predicted METs (n=469) | Achieved predicted METs (n=429) | P value |
|---|---|---|---|---|
| Age, y | 51.8±11.3 | 50.2±11.6 | 53.7±10.8 | <0.001 |
| Male sex | 586 (65%) | 331 (71%) | 255 (59%) | <0.001 |
| BMI, kg/m2 | 27.1±5.3 | 28.5±5.5 | 25.6±4.6 | <0.001 |
| Cardiovascular risk factors | ||||
| Diabetes | 351 (39%) | 221 (47%) | 130 (30%) | <0.001 |
| Hypertension | 791 (88%) | 414 (88%) | 377 (88%) | 0.856 |
| Hyperlipidemia | 421 (47%) | 222 (47%) | 199 (46%) | 0.776 |
| History of smoking | 303 (34%) | 180 (38%) | 123 (29%) | 0.002 |
| History of IHD | 198 (22%) | 109 (23%) | 89 (21%) | 0.368 |
| Previous kidney transplantation | 110 (12%) | 55 (12%) | 55 (13%) | 0.618 |
| On renal replacement therapy | 622 (69%) | 331 (71%) | 291 (68%) | 0.373 |
| Peritoneal dialysis | 199 (22%) | 100 (21%) | 99 (23%) | 0.527 |
| Hemodialysis | 423 (47%) | 231 (49%) | 192 (45%) | 0.177 |
| Cause of kidney disease | ||||
| Diabetes | 255 (28%) | 174 (37%) | 81 (19%) | <0.001 |
| IgA nephropathy | 150 (17%) | 69 (15%) | 81 (19%) | 0.094 |
| Reflux nephropathy | 71 (8%) | 30 (6%) | 41 (10%) | 0.080 |
| Polycystic kidney disease | 103 (11%) | 46 (10%) | 57 (13%) | 0.102 |
| Glomerulonephritis | 185 (20%) | 72 (15%) | 113 (26%) | <0.001 |
| Renovascular | 49 (5%) | 25 (5%) | 24 (6%) | 0.862 |
| Miscellaneous | 85 (9%) | 53 (11%) | 32 (7%) | 0.036 |
| Exercise stress echocardiography results | ||||
| Test during long interdialytic interval | 139 (15%) | 76 (36%) | 63 (39%) | 0.554 |
| Test performed on beta‐blockers | 379 (42%) | 223 (48%) | 156 (36%) | 0.001 |
| Exercise duration, min | 7.6±2.7 | 5.9±2.2 | 9.4±1.9 | <0.001 |
| Reached ≥85% MPHR | 535 (60%) | 221 (47%) | 314 (73%) | <0.001 |
| METs | 9.2±2.8 | 7.4±2.1 | 11.2±2.0 | <0.001 |
| Baseline LVEF <50% | 141 (16%) | 97 (21%) | 44 (10%) | <0.001 |
| Abnormal stress echocardiogram | 143 (16%) | 101 (22%) | 42 (10%) | <0.001 |
| Non‐diagnostic | 32 (4%) | 31 (7%) | 3 (1%) | 0.003 |
| Global failure in LV contractile reserve | 53 (6%) | 32 (7%) | 19 (4%) | 0.123 |
| Inducible regional wall motion abnormalities | 58 (6%) | 38 (8%) | 20 (5%) | 0.268 |
| Underwent coronary angiography | 56 (6%) | 40 (9%) | 16 (4%) | 0.866 |
| Non‐MI revascularization | 28 (3%) | 22 (5%) | 6 (1%) | 0.005 |
| Transplanted | 525 (58%) | 246 (52%) | 279 (65%) | <0.001 |
| Median time to transplantation | 1.5 [0.8–2.8] | 1.6 [0.7–2.7] | 1.5 [0.8–2.9] | 0.752 |
Values are mean±SD, median (Q1–Q3) or n (%). BMI indicates body mass index; IHD, ischemic heart disease; LV, left ventricular; LVEF, left ventricular ejection fraction; METs, metabolic equivalents; MI, myocardial infarction; and MPHR, maximum predicted heart rate.
There were 525 (58%) patients who received kidney transplantation during the study. Baseline characteristics stratified by transplantation status are shown in Table S1. Median time to transplantation was 1.5 years (IQR 0.8–2.8 years). Follow‐up duration was mean 5.0±1.9 years after ESE.
Exercise Capacity
The mean achieved exercise capacity was 9.2±2.8 METs (Table 1). At time of ESE, 139 (15%) patients were in their long interdialytic period (2‐day hemodialysis‐free interval due to weekend gap on a thrice weekly schedule), whilst 379 (42%) performed the test on beta‐blockers. A total of 429 (48%) patients achieved age and sex predicted METs, whilst 734 (82%) patients achieved ≥7 METs. Patients who achieved predicted METs were older (53.7±10.8 versus 50.2±11.6 years, P<0.001), more likely to be female (41% versus 29%, P<0.001), had lower BMI (25.6±4.6 versus 28.5±5.5 kg/m2, P<0.001), less beta‐blocker use (36% versus 48%, P=0.001), less diabetes (30% versus 47%, P<0.001), lower prevalence of smoking (29% versus 38%, P=0.002), and less baseline LV dysfunction on ESE (10% versus 21%, P<0.001). Patients who achieved predicted METs were less likely to have non‐MI revascularization (1% versus 5%, P=0.005) and were more likely to receive subsequent kidney transplantation (65% versus 52%, P<0.001). Population characteristics stratified by ability to achieve ≥7 METs are shown in Table S2.
The majority of ESE results were normal (755 [84%]). There was excellent inter‐reader (ĸ=0.93) and intra‐reader agreement (ĸ=0.95). Of the 143 abnormal ESE results, 32 (22%) were non‐diagnostic, 53 (37%) had a fall in post‐stress LVEF or a failure of LV contractile reserve, and 58 (41%) had inducible regional hypokinesis in a single coronary territory. A total of 56 patients with abnormal ESE results (39%) underwent coronary angiography after abnormal ESE results and 28 (50%) were subsequently revascularized. All remaining patients were treated with guideline directed medical therapy. Patients who achieved predicted METs had fewer abnormal ESE results (10% versus 22%, P<0.001) due to reduced non‐diagnostic studies (1% versus 7%, P=0.003), but similar incidences of global failure of LV contractile reserve and inducible regional wall motion abnormalities. Similar rates of coronary angiography were performed in both groups (4% versus 9%, P=0.87), but there was more non‐MI revascularization in the group that failed to achieve predicted METs (1% versus 5%, P=0.005).
Major Adverse Cardiovascular Events
A total of 106 MACE were recorded in 93 patients (21 cardiac deaths, 53 non‐fatal MI, and 32 strokes) over the follow‐up period (cumulative event rate of 2.4% per year). In the 525 patients who received a kidney transplant during the follow‐up period, there were 50 MACE (10%): 13 events (26%) prior to the transplant and 37 events (74%) post‐transplantation. Of the 37 post‐transplant events, 5 events occurred within 30 days of surgery (peri‐operative MACE incidence 1%) (Figure 1). In the 373 patients who did not receive a kidney transplant, there were 43 MACE (12%).
Figure 1. Major adverse cardiovascular events in patients stratified by transplantation status.
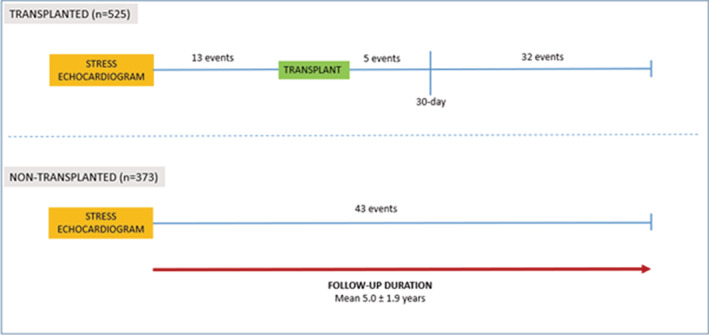
Results shown are for follow‐up duration from time of stress echocardiogram until July 2020.
Univariable and Multivariable Analysis
Several parameters were associated with future MACE at a univariable level (Table S3). Those with a crude increased risk of MACE included diabetes, hyperlipidemia, history of smoking, history of IHD, baseline LV dysfunction, and non‐MI revascularization. Variables associated with a reduction in MACE included female sex, ability to achieve predicted METs, and subsequent transplantation. Multivariable analysis demonstrated significant associations between MACE and diabetes, hyperlipidemia, ability to achieve predicted METs, and transplantation (Table 2). Both diabetes (HR, 1.78; [95% CI 1.11–2.87], P=0.02) and hyperlipidemia (HR, 1.70; [95% CI 1.03–2.82], P=0.04) were associated with an increased risk of MACE, while achievement of predicted METs (HR, 0.49; [95% CI 0.29–0.82], P=0.007) and subsequent kidney transplantation (HR, 0.52; [95% CI 0.30–0.91], P=0.02) conferred lower risk.
Table 2.
Multivariable Analysis for Major Adverse Cardiovascular Events
| Variable | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age | 1.00 | 0.98–1.03 | 0.890 |
| Sex (female referent) | 0.79 | 0.45–1.36 | 0.403 |
| Diabetes | 1.78 | 1.11–2.87 | 0.017 |
| Hypertension | 1.54 | 0.55–4.34 | 0.414 |
| Hyperlipidemia | 1.70 | 1.03–2.82 | 0.038 |
| History of smoking | 1.39 | 0.88–2.20 | 0.161 |
| History of ischemic heart disease | 1.14 | 0.69–1.87 | 0.616 |
| Previous kidney transplantation | 0.56 | 0.22–1.40 | 0.215 |
| Body mass index | 1.00 | 0.96–1.04 | 0.889 |
| LV ejection fraction<50% | 1.47 | 0.85–2.53 | 0.164 |
| Abnormal stress echocardiogram | 0.98 | 0.56–1.72 | 0.954 |
| Non‐MI revascularization | 2.07 | 0.97–4.43 | 0.061 |
| Achieved predicted METs | 0.49 | 0.29–0.82 | 0.007 |
| Kidney transplant* | 0.52 | 0.30–0.91 | 0.021 |
Hazard ratio for age was calculated per 1 year. Hazard ratio for body mass index was calculated per 1 kg/m2 increase. LV indicates left ventricle; METs, metabolic equivalents; and MI, myocardial infarction.
Transplantation was treated as a time‐dependent covariate.
Similar results were found on secondary analysis when achieved METs was analyzed as a continuous variable, with 12% reduction in MACE for each unit increment in achieved METs (HR, 0.88; [95% CI 0.80–0.96], P=0.007) (Table S4, Figure S1). Results were also unchanged when a cut‐off of ≥7 METs was analyzed, with the ability to achieve ≥7 METs associated with a significant reduction in MACE (HR, 0.55; [95% CI 0.32–0.95], P=0.03) (Table S5). Sensitivity analysis was performed with categorization of METs in groups <4 METs (very poor capacity), 4 to 7 METs (intermediate capacity), 7 to 10 METs (good capacity), and >10 METs (excellent capacity). This demonstrated a reduction in MACE with each increasing exercise capacity group (P<0.001) (Figure S2).
The Combined Impact of Exercise Capacity and Transplantation
When stratified according to achievement of predicted METs and subsequent kidney transplantation, the primary outcome occurred in 42 patients who did not achieve predicted METs and did not receive transplantation, 13 patients who did not achieve predicted METs but received transplantation, 14 patients who achieved predicted METs but did not receive transplantation, and 24 patients who achieved predicted METs and received transplantation (Figure 2). Patients who did not achieve predicted METs and did not receive a kidney transplant had the worst outcomes (Figure 2). In contrast, patients who did not receive transplantation but achieved predicted METs had better outcomes (HR, 0.33; [95% CI 0.18–0.61], P<0.001), which were similar to both other groups of patients who received transplantation (Figure 2). Differences in baseline demographics among the 4 patient groups are reported in Tables S6 and S7.
Figure 2. Cumulative major adverse cardiovascular event free proportion stratified by achievement of predicted METs and transplantation status.
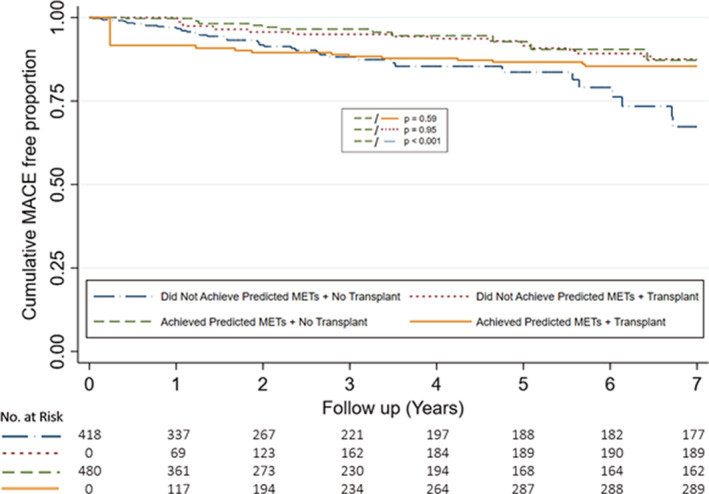
Graph demonstrates cumulative MACE free proportion stratified by achievement of predicted METs and transplantation status at 7 years. Transplantation was treated as a time‐dependent variable and curves reflect univariable modeling. MACE indicates major adverse cardiovascular event; and METs, metabolic equivalents.
When secondary analysis at ≥7 METs was performed, subsequent kidney transplantation conferred better outcomes irrespective of achievement of 7 METs on pre‐operative ESE (Figure 3). In patients who did not receive transplantation, patients who achieved ≥7 METs on pre‐transplant ESE had better outcomes than those who did not (HR, 0.41; [95% CI 0.24–0.71], P=0.001) (Figure 3, Table S8). Further secondary analysis using ability to achieve a target heart rate of 85% MPHR on pre‐operative ESE demonstrated that patients who received subsequent kidney transplantation had similar outcomes irrespective of achievement of 85% MPHR on pre‐operative ESE (HR, 0.74; [95% CI 0.34–1.60], P=0.44) (Figure 4). In patients who did not receive subsequent transplantation, ability to achieve 85% MPHR conferred better outcomes (HR, 0.49; [95% CI 0.29–0.83], P=0.01), although this benefit was declined after 5 years of follow‐up (Figure 4).
Figure 3. Cumulative major adverse cardiovascular event free proportion stratified by ≥7 MET threshold and transplantation status.
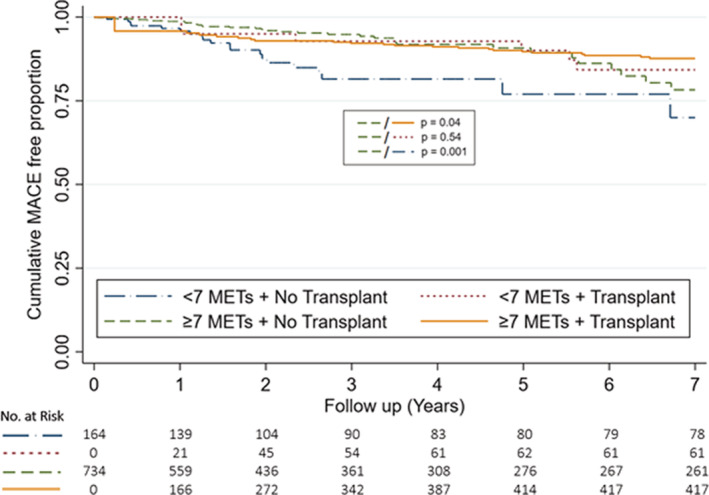
Graph demonstrates cumulative MACE free proportion stratified by achievement of 7 METs and transplantation status at 7 years. Transplantation was treated as a time‐dependent variable and curves reflect univariable modeling. MACE indicates major adverse cardiovascular event; and METs, metabolic equivalents.
Figure 4. Cumulative major adverse cardiovascular event free proportion stratified by ability to achieve 85% maximal predicted heart rate and transplantation status.
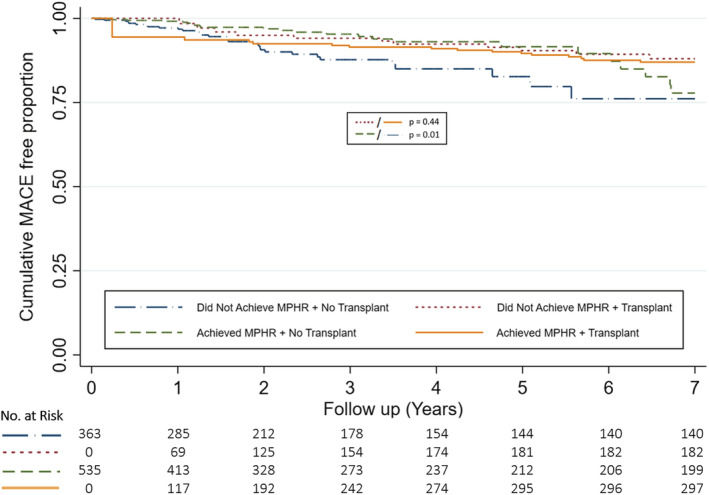
Graph demonstrates cumulative MACE free proportion stratified by achievement of 85% maximal predicted heart rate and transplantation status at 7 years. Transplantation was treated as a time‐dependent variable and curves reflect univariable modeling. MACE indicates major adverse cardiovascular event; and MPHR, maximal predicted heart rate.
Individual secondary outcomes stratified by ability to achieve predicted METs and transplantation status are shown in Table 3.
Table 3.
Primary and Secondary Outcomes
| Outcome |
Did not achieve predicted METs (n=469) |
Achieved predicted METs (n=429) |
||
|---|---|---|---|---|
|
Not transplanted (n=223) |
Transplanted* (n=246) |
Not transplanted (n=150) |
Transplanted* (n=279) |
|
| MACE | 42 (18%) | 13 (5%) | 14 (9%) | 24 (9%) |
| Cardiac death | 9 (4%) | 3 (1%) | 2 (1%) | 7 (3%) |
| Non‐fatal MI | 18 (8%) | 8 (3%) | 11 (7%) | 12 (4%) |
| Stroke | 15 (7%) | 2 (1%) | 1 (1%) | 5 (2%) |
MACE indicates major adverse cardiovascular event; METs, metabolic equivalents; and MI, myocardial infarction.
Transplantation was treated as a time‐dependent covariate.
DISCUSSION
In this analysis of 898 ambulatory patients with stage 4 or 5 CKD who underwent cardiovascular risk stratification for potential kidney transplantation with ESE, we have demonstrated the prognostic benefit of good exercise capacity on long‐term cardiovascular outcomes. The major findings of this study are: (1) ability to achieve predicted METs for age and sex on pre‐operative ESE confers excellent long‐term cardiovascular prognosis, (2) exercise capacity is an independent predictor of MACE with a 12% relative reduction for each 1‐unit increment in METs, and (3) ability to achieve predicted METs for age and sex may be a better discriminator than a threshold of 7 METs or ability to achieve 85% MPHR for long‐term cardiovascular outcomes in kidney transplant candidates. This is the largest contemporary study evaluating exercise stress testing in a CKD population.
Conventionally, the goals of pre‐operative kidney transplant cardiovascular risk assessment are to assess for the presence of significant CAD, to predict peri‐operative cardiovascular risk at transplantation, and to predict long‐term cardiovascular outcomes. 3 The role of pre‐operative cardiac stress testing with view of revascularization for stable or asymptomatic CAD has been recently challenged by the ISCHEMIA CKD trial, which demonstrated that revascularization for stable CAD in CKD patients did not reduce mortality and non‐fatal MI regardless of kidney transplant waitlist status. 5 , 20 Furthermore, revascularization following abnormal cardiac stress testing may not improve angina‐related health status in this patient population as kidney transplant candidates are often asymptomatic at the time of pre‐operative testing. 6 The ongoing CARSK trial will address this issue by evaluating the utility of screening for asymptomatic CAD after kidney transplant wait‐list entry. 21 Although the role of cardiac stress testing with goal of revascularization remains in contention, there may be a role for cardiac stress testing to identify patients with CKD at risk of CVD who may benefit from lifestyle intervention and risk factor modification, as well as patients of very high cardiovascular risk who may not prognostically benefit from transplantation.
The primary goal of this study was to assess the utility of pre‐operative exercise capacity assessment using ESE in predicting long‐term cardiovascular outcomes in kidney transplant candidates, which is a metric that is not assessed on pharmacological stress testing. Exercise capacity represents an integrated measure of multiple prognostic variables and has been suggested as a useful modality to assess long‐term cardiovascular risk in the general population. 22 Similarly, exercise capacity may be a more reliable metric in predicting long‐term cardiovascular outcomes in kidney transplant candidates. Poor exercise capacity is also a potential modifiable risk factor that could be improved with lifestyle measures and exercise training, an intervention which has previously been shown to be safe and effective in improving exercise capacity in patients with CKD without any adverse outcomes. 23
Although cardiac stress testing conventionally utilizes a target of 85% MPHR to improve detection of coronary ischemia, target heart rate may not be an adequate indicator of exercise capacity, which is a marker of functional status and better quantified with achievement of METs. Traditionally, a threshold of 7 METs has been described as “good” exercise capacity in pre‐operative assessment, however this is unadjusted for age and sex. 17 The findings of this study propose that the ability to achieve age and sex predicted METs may be a more practical discriminator for exercise capacity in predicting long‐term cardiovascular outcomes. In the study population, only 48% of patients achieved predicted METs, compared with 82% of patients achieving ≥7 METs and 60% of patients achieving 85% MPHR on pre‐transplant ESE.
The importance of exercise in potential kidney transplant candidates for long‐term cardiovascular prognosis has been investigated in previous studies. Patel et al performed exercise treadmill testing in 268 candidates as part of a cardiovascular screening program and reported a poorer survival in patients exercising <6 minutes. 24 Ting et al performed cardiopulmonary exercise testing (CPET) in 240 patients and demonstrated that reduced anerobic threshold <40% of alveolar oxygen uptake (VO2) conferred a significantly worse prognosis. 25 Other observational studies have also demonstrated the association of peak VO2 on CPET with future cardiac events and all‐cause mortality in kidney and/or pancreas transplant candidates 26 and patients receiving hemodialysis. 27 Our study supports and mirrors these findings and is further enhanced by a much larger sample size and consequently more events. However, the patients in this study achieved above expected exercise capacity when compared with a conventional CKD population, reflective of a fitter study cohort. This needs to be considered when interpreting this study's results.
The finding of better long‐term cardiovascular prognosis with achievement of age and sex predicted METs may not appear novel, but it is remarkable that patients who achieved predicted METs on pre‐operative ESE or received subsequent transplantation during follow‐up had similar favorable outcomes. These findings suggest that the prognostic benefit seen with achievement of predicted METs is independent of and has similar magnitude to receiving a kidney transplant in patients with advanced CKD. This result may provide clinicians with reassurance if there is delay to transplantation in patients who are able to achieve predicted METs whilst they remain on the wait‐list. Conversely, those with a poorer exercise capacity may warrant more expedited assessment for transplantation. Finally, this raises the possibility of using predicted METs for age and sex as a target for future studies exploring exercise training as a treatment modality to improve long‐term cardiovascular outcomes in CKD patients awaiting transplantation.
Study Limitations
Our results represent one of the largest kidney transplant centers in Australia, but are limited by the single‐center setting and observational design. Additionally, there could have been selection bias in the study cohort as we only included patients referred for stress echocardiography for pre‐transplant cardiovascular assessment but cannot account for patients who were referred solely for nuclear myocardial perfusion imaging. The cohort would also have excluded patients deemed unsuitable for transplantation on other clinical grounds and hence not referred for pre‐transplant cardiac stress testing. This selection bias may explain the younger patient population (52±11 years) with better exercise capacity (82% achieving 7 METs) in this study, leading to lower than expected MACE incidence which could affect the generalizability of these findings to an unselected CKD cohort. Hence, the results reported in this single‐center study may be indiscriminate and may not represent standard practice in other centers where standardized cardiovascular screening is performed for all‐comer CKD patients. Furthermore, some patients may not have been waitlisted for transplantation following ESE and cardiovascular clearance due to non‐cardiac reasons, which could introduce further selection bias into the transplanted cohort.
CONCLUSIONS
In patients with CKD undergoing cardiovascular assessment for kidney transplantation, exercise capacity as assessed on pre‐operative ESE is associated with reduced likelihood of long‐term MACE. Patients who are able to achieve predicted METs for age and sex have good long‐term cardiovascular prognosis that is independent of and of similar magnitude to receiving a kidney transplant. Further studies are required to prospectively assess exercise training as a treatment modality to improve long‐term cardiovascular outcomes in CKD patients awaiting transplantation.
Sources of Funding
This work was not supported by any funding. Dr Nerlekar is supported by an Emerging Leader Fellowship from the National Health and Medical Research Council.
Disclosures
The authors have no disclosures, conflicts of interest, or relationships to industry to report.
Supporting information
Tables S1–S8
Figures S1–S2
Acknowledgments
The authors wish to thank Professor Thomas Marwick (Baker Heart and Diabetes Institute, Melbourne, Australia) for his guidance, and Dr Caitlin Cheshire (Monash Heart, Melbourne, Australia), Dr Hashrul Rashid (Monash Heart, Melbourne, Australia), and Ms Orla Maney (Department of Nephrology, Monash Health, Melbourne, Australia) for their assistance in data collection.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.025862
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Johansen KL, Chertow GM, Foley RN, Gilbertson DT, Herzog CA, Ishani A, Israni AK, Ku E, Kurella Tamura M, Li S, et al. US renal data system 2020 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2021;77:A7–A8. doi: 10.1053/j.ajkd.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Briggs JD. Causes of death after renal transplantation. Nephrol Dial Transplant. 2001;16:1545–1549. doi: 10.1093/ndt/16.8.1545 [DOI] [PubMed] [Google Scholar]
- 3. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . Clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation. 2020;104:S11–S103. doi: 10.1097/TP.0000000000003136 [DOI] [PubMed] [Google Scholar]
- 4. Lentine KL, Costa SP, Weir MR, Robb JF, Fleisher LA, Kasiske BL, Carithers RL, Ragosta M, Bolton K, Auerbach AD, et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation: endorsed by the American Society of Transplant Surgeons, American Society of Transplantation, and National Kidney Foundation. Circulation. 2012;126:617–663. doi: 10.1161/CIR.0b013e31823eb07a [DOI] [PubMed] [Google Scholar]
- 5. Bangalore S, Maron DJ, O'Brien SM, Fleg JL, Kretov EI, Briguori C, Kaul U, Reynolds HR, Mazurek T, Sidhu MS, et al. Management of coronary disease in patients with advanced kidney disease. N Engl J Med. 2020;382:1608–1618. doi: 10.1056/NEJMoa1915925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spertus JA, Jones PG, Maron DJ, O'Brien SM, Reynolds HR, Rosenberg Y, Stone GW, Harrell FE Jr, Boden WE, Weintraub WS, et al. Health‐status outcomes with invasive or conservative care in coronary disease. N Engl J Med. 2020;382:1408–1419. doi: 10.1056/NEJMoa1916370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 8. Nerlekar N, Mulley W, Rehmani H, Ramkumar S, Cheng K, Vasanthakumar SA, Rashid H, Barton T, Nasis A, Meredith IT, et al. Feasibility of exercise stress echocardiography for cardiac risk assessment in chronic kidney disease patients prior to renal transplantation. Clin Transpl. 2016;30:1209–1215. doi: 10.1111/ctr.12796 [DOI] [PubMed] [Google Scholar]
- 9. Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila‐Roman VG, Gerhard‐Herman MD, Holly TA, Kane GC. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;130:2215–2245. doi: 10.1161/CIR.0000000000000105 [DOI] [PubMed] [Google Scholar]
- 10. Poldermans D, Bax JJ, Boersma E, De Hert S, Eeckhout E, Fowkes G, Gorenek B, Hennerici MG, Iung B, Kelm M. Guidelines for pre‐operative cardiac risk assessment and perioperative cardiac management in non‐cardiac surgery. Eur Heart J. 2009;30:2769–2812. doi: 10.1093/eurheartj/ehp337 [DOI] [PubMed] [Google Scholar]
- 11. Reese PP, Cappola AR, Shults J, Townsend RR, Gadegbeku CA, Anderson C, Baker JF, Carlow D, Sulik MJ, Lo JC, et al. Physical performance and frailty in chronic kidney disease. Am J Nephrol. 2013;38:307–315. doi: 10.1159/000355568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. doi: 10.1161/01.cir.0000042700.48769.59 [DOI] [PubMed] [Google Scholar]
- 13. Ting SM, Hamborg T, McGregor G, Oxborough D, Lim K, Koganti S, Aldridge N, Imray C, Bland R, Fletcher S, et al. Reduced cardiovascular reserve in chronic kidney failure: a matched cohort study. Am J Kidney Dis. 2015;66:274–284. doi: 10.1053/j.ajkd.2015.02.335 [DOI] [PubMed] [Google Scholar]
- 14. Gan GCH, Kadappu KK, Bhat A, Fernandez F, Eshoo S, Thomas L. Exercise E/e' is a determinant of exercise capacity and adverse cardiovascular outcomes in chronic kidney disease. JACC Cardiovasc Imaging. 2020;13:2485–2494. doi: 10.1016/j.jcmg.2020.05.044 [DOI] [PubMed] [Google Scholar]
- 15. Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–1041. doi: 10.1016/j.echo.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 16. Kim ES, Ishwaran H, Blackstone E, Lauer MS. External prognostic validations and comparisons of age‐ and gender‐adjusted exercise capacity predictions. J Am Coll Cardiol. 2007;50:1867–1875. doi: 10.1016/j.jacc.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 17. Patel AY, Eagle KA, Vaishnava P. Cardiac risk of noncardiac surgery. J Am Coll Cardiol. 2015;66:2140–2148. doi: 10.1016/j.jacc.2015.09.026 [DOI] [PubMed] [Google Scholar]
- 18. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, Solomon SD, Marler JR, Teerlink JR, Farb A, et al. 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018;137:961–972. doi: 10.1161/CIRCULATIONAHA.117.033502 [DOI] [PubMed] [Google Scholar]
- 19. Mantel N, Byar DP. Evaluation of response‐time data involving transient states: an illustration using heart‐transplant data. J Am Stat Assoc. 1974;69:81–86. doi: 10.2307/2285503 [DOI] [Google Scholar]
- 20. Herzog CA, Simegn MA, Xu Y, Costa SR, Mathew RO, El‐Hajjar MC, Gulati S, Maldonado RA, Daugas E, Madero M, et al. Kidney transplant list status and outcomes in the ISCHEMIA‐CKD trial. J Am Coll Cardiol. 2021;78:348–361. doi: 10.1016/j.jacc.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ying T, Gill J, Webster A, Kim SJ, Morton R, Klarenbach SW, Kelly P, Ramsay T, Knoll GA, Pilmore H, et al. Canadian‐Australasian randomised trial of screening kidney transplant candidates for coronary artery disease‐a trial protocol for the CARSK study. Am Heart J. 2019;214:175–183. doi: 10.1016/j.ahj.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 22. Dagianti A, Penco M, Agati L, Sciomer S, Dagianti A, Rosanio S, Fedele F. Stress echocardiography: comparison of exercise, dipyridamole and dobutamine in detecting and predicting the extent of coronary artery disease. J Am Coll Cardiol. 1995;26:18–25. doi: 10.1016/0735-1097(95)00121-F [DOI] [PubMed] [Google Scholar]
- 23. Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta‐analysis. Am J Kidney Dis. 2014;64:383–393. doi: 10.1053/j.ajkd.2014.03.020 [DOI] [PubMed] [Google Scholar]
- 24. Patel R, Mark P, Johnston N, McGeoch R, Lindsay M, Kingsmore D, Dargie H, Jardine A. Prognostic value of cardiovascular screening in potential renal transplant recipients: a single‐center prospective observational study. Am J Transplant. 2008;8:1673–1683. doi: 10.1111/j.1600-6143.2008.02281.x [DOI] [PubMed] [Google Scholar]
- 25. Ting SM, Iqbal H, Kanji H, Hamborg T, Aldridge N, Krishnan N, Imray CH, Banerjee P, Bland R, Higgins R, et al. Functional cardiovascular reserve predicts survival pre‐kidney and post‐kidney transplantation. J Am Soc Nephrol. 2014;25:187–195. doi: 10.1681/ASN.2013040348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chakkera HA, Angadi SS, Heilman RL, Kaplan B, Scott RL, Bollempalli H, Cha SS, Khamash HA, Huskey JL, Mour GK, et al. Cardiorespiratory fitness (peak oxygen uptake): safe and effective measure for cardiovascular screening before kidney transplant. J Am Heart Assoc. 2018;7:e008662. doi: 10.1161/JAHA.118.008662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sietsema KE, Amato A, Adler SG, Brass EP. Exercise capacity as a predictor of survival among ambulatory patients with end‐stage renal disease. Kidney Int. 2004;65:719–724. doi: 10.1111/j.1523-1755.2004.00411.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S8
Figures S1–S2
Data Availability Statement
Data are available from the corresponding author upon reasonable request.


