Abstract
Background
There is little evidence on the relationship between statin use and the risk of hospitalization attributable to COVID‐19.
Methods and Results
The French National Healthcare Data System database was used to conduct a matched‐cohort study. For each adult aged ≥40 years receiving statins for the primary prevention of cardiovascular diseases, one nonuser was randomly selected and matched for year of birth, sex, residence area, and comorbidities. The association between statin use and hospitalization for COVID‐19 was examined using conditional Cox proportional hazards models, adjusted for baseline characteristics, comorbidities, and long‐term medications. Its association with in‐hospital death from COVID‐19 was also explored. All participants were followed up from February 15, 2020, to June 15, 2020. The matching procedure generated 2 058 249 adults in the statin group and 2 058 249 in the control group, composed of 46.6% of men with a mean age of 68.7 years. Statin users had a 16% lower risk of hospitalization for COVID‐19 than nonusers (adjusted hazard ratio [HR], 0.84; 95% CI, 0.81–0.88). All types of statins were significantly associated with a lower risk of hospitalization, with the adjusted HR ranging from 0.75 for fluvastatin to 0.89 for atorvastatin. Low‐ and moderate‐intensity statins also showed a lower risk compared with nonusers (HR, 0.78 [95% CI, 0.71–0.86] and HR, 0.84 [95% CI, 0.80–0.89], respectively), whereas high‐intensity statins did not (HR, 1.01; 95% CI, 0.86–1.18). We found similar results with in‐hospital death from COVID‐19.
Conclusions
Our findings support that the use of statins for primary prevention is associated with lower risks of hospitalization for COVID‐19 and of in‐hospital death from COVID‐19.
Keywords: COVID‐19, hospitalization, mortality, SARS‐CoV‐2, statins
Subject Categories: Epidemiology
Nonstandard Abbreviations and Acronyms
- ASD
absolute standardized difference
- CCAM
French medical classification for clinical procedures (Classification Commune des Actes Médicaux)
- CIP
French coding scheme for identifying a single drug package (Code Identifiant de Présentation)
- CNIL
French Data Protection Office (Commission Nationale de l'Informatique et des Libertés)
- IPTW
inverse probability of treatment weighting
- MERS‐CoV
Middle East respiratory syndrome coronavirus
- PMSI
National Hospital Discharge Database (Programme de Médicalisation des Systèmes d’Information)
- SNDS
French National Healthcare Data System (Système National des Données de Santé)
- UCD
French coding scheme used for identifying hospital drugs (Unité Commune de Dispensation)
Clinical Perspective
What Is New?
The evidence about statins and serious in‐hospital COVID‐19 outcomes is abundant but is scarce for initial outcomes in the disease course such as hospitalization for COVID‐19. We conducted a population‐based matched cohort study including 2 million adults aged ≥40 years who used statins for the primary prevention of cardiovascular diseases compared with 2 million nonusers.
Our finding supports that statin use was associated with a lower risk of hospitalization for COVID‐19, and we found similar results with all types of statins.
Low‐ and moderate‐intensity statins were also associated with a lower risk compared with nonuse, whereas high‐intensity statins were not.
What Are the Clinical Implications?
Statins are now known to be beneficial in primary prevention, decreasing all‐cause mortality, cardiovascular diseases, coronary heart disease, and stroke without any evidence of serious harm caused by their use.
Since the beginning of the COVID‐19 pandemic, many clinicians have suggested that statins could be used as an adjunctive treatment for SARS‐CoV infection.
Our finding supports the hypothesis that low‐ and moderate‐intensity statin use might contribute to a small risk reduction of hospitalization for COVID‐19.
A better understanding of the determinants associated with COVID‐19 helps to identify vulnerable individuals and to provide satisfactory health care management. Given the absence of a specific treatment for COVID‐19, several existing drugs were thought to be beneficial, in particular those with anti‐inflammatory or immunomodulatory activities such as statins. 1 Besides their well‐known lipid‐lowering effect, statins have been reported to have pleiotropic beneficial actions by regulating numerous biological pathways implicated in anti‐inflammatory, immune‐modulatory, or anticoagulant actions. These drugs were found to be effective in previous outbreaks, namely those of hemagglutinin type 1 and neuraminidase type 1 (H1N1) influenza 2 , 3 and the Ebola virus. 3 , 4 It has also been shown that they have been useful for survival in SARS‐CoV and Middle East respiratory syndrome coronavirus (MERS‐CoV) infections. 5 Statins exert an anti‐inflammatory effect by directly inhibiting the toll‐like receptor MYD88‐NF‐kB pathway and by upregulating angiotensin‐converting enzyme 2 (ACE‐2) expression. 6 , 7 , 8 , 9 , 10
Numerous epidemiological studies demonstrate that individuals who were previously treated with statins had a lower risk of experiencing severe COVID‐19 outcomes, including admission into an intensive care unit, invasive mechanical intubation, acute respiratory distress syndrome, and in‐hospital death, compared with nonexposed individuals. In total, 36 of 49 studies (73%) show a lower risk of severe COVID‐19 outcomes––in particular mortality––among statin users compared with nonusers. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 In 11 studies, ratio measures were close to 1. 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 In 2 studies, an increased risk was observed. 58 , 59 Most of these studies were conducted in hospitalized patients and/or patients tested for COVID‐19. This could have led to a collider bias––also known as admission bias––which could have distorted the association between statin exposure and severe COVID‐19 outcomes compared with that observed in the general population. That is, both the cause of using statins and the risk for COVID‐19–related hospitalization may influence the likelihood of being selected for the study. 60 Furthermore, in the literature, there is little evidence on statin use with hospitalization for COVID‐19.
In this context, we conducted a matched‐cohort study in a general population aimed at studying the relationship between statin use before the start of the COVID‐19 pandemic and symptomatic COVID‐19 leading to hospitalization, using a French nationwide database. In addition, we examined its association with in‐hospital death from COVID‐19, frequently investigated in published studies.
Methods
According to data protection and French regulation, the authors cannot publicly release data from the SNDS (French National Healthcare Data System [Système National des Données de Santé]. However, any person or structure, public or private, for‐profit or nonprofit, can access SNDS data on authorization from the CNIL (French Data Protection Office [Commission Nationale de l'Informatique et des Libertés]) to perform a study, research, or an evaluation of public interest (https://www.snds.gouv.fr/SNDS/Processus‐d‐acces‐aux‐donnees and https://www.indsante.fr/).
Data Source
This cohort study used data from the SNDS, formerly known as SNIIRAM, established in 2006. 61
SNDS covers the entire population of France (67 million residents). Each person is identified by a unique and anonymous number. Since 2006, SNDS has recorded all reimbursement data on: (1) outpatient care including drugs, imaging, and laboratory tests; (2) inpatient care (including diagnoses and procedures performed) from the national hospital discharge database (PMSI [Programme de Médicalisation des Systèmes d’Information]); and (3) health expenditure for patients with long‐term diseases, such as cancer and diabetes, which is fully reimbursed. SNDS has been extensively used in France to conduct real‐life pharmacoepidemiological studies including those on the COVID‐19 pandemic. 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 SNDS also contains sociodemographic data and, when applicable, the date of death.
As a routine, information on hospital stays is collected monthly in the PMSI and integrated annually into the SNDS the following year. In April 2020, the French government encouraged hospitals to report all hospital stays attributable to COVID‐19 once or twice a week through an exceptional fast‐tracking procedure (“fast‐track” PMSI). The present study was based on the fast‐track PMSI database available as of September 30, 2020. A cutoff discharge date of June 15, 2020, was chosen to ensure completeness of data over the study period, which covers the first epidemic wave in France. At this date, 87 809 participants were admitted with a principal diagnosis of COVID‐19, and 95% of them were linked to outpatient data using anonymized identifiers. Of these 87 809 participants, 15 661 died in hospital.
All variables used in this study were defined based on International Statistical Classification of Diseases, Tenth Revision (ICD‐10), codes for primary and secondary diagnosis; the French common classification of medical procedures Classification Commune des Actes Médicaux (CCAM) codes for procedures; and Anatomical Therapeutic Chemical, Code Identifiant de Présentation (CIP), or Unité Commune de Dispensation (UCD) codes for drugs. We used algorithms developed by the national health insurance in the Diseases and Health Expenditures Mapping, 68 , 74 which are detailed in Tables S1 through S4. For the ICD‐10 and CCAM codes, any occurrence in the 5 years preceding inclusion is used. For the anatomical therapeutic chemical and CIP codes, at least 3 drug dispensing (or 2 when at least one concerned the dispensing of large pack size) during 2019 are used. A small pack size usually contains a sufficient number of pills for a 1‐month treatment and a large one for 3 months. For the exposure variable, statins, we added another condition: at least one dispensing in the last month (if small pack size) or 3 months (if large pack size) preceding inclusion. The inclusion or index date was defined as February 15, 2020, considered the start date of the epidemic in France.
Study Population
Individuals receiving at least 1 health care reimbursement after February 15, 2019, and aged ≥40 years were included in this study. The exposed group was composed of those using statins in monotherapy for the primary prevention of cardiovascular diseases to avoid confounding biases related to these conditions.
The statin group was further studied according to statin type (atorvastatin, fluvastatin, pravastatin, rosuvastatin, simvastatin) and intensity (low, moderate, high), based on information (international nonproprietary name and dose) from the most recently dispensed statin between November 15, 2019, and February 15, 2020 (index date). Statin intensity on low‐density lipoprotein cholesterol reduction was defined by the American College of Cardiology/American Heart Association. 75
For each statin user, we randomly selected one nonuser (ratio 1:1) matched for year of birth, sex, residence area (101 French departments, administrative divisions), hypertension, diabetes, and chronic respiratory condition to further control for main confounding biases.
Noninclusion Criteria
The noninclusion criteria were all individuals: (1) aged <40 years, (2) using a statin combined with another statin or a lipid‐lowering drug other than a statin (eg, fibrates, ezetemib, and PCSK9 inhibitors), (3) with a history of cardiovascular diseases including coronary artery disease, heart failure, and stroke (statins used as secondary prevention), cancer, kidney condition (chronic transplant, or dialysis), and dementia.
Covariates
The following baseline characteristics were described according to statin use status: social deprivation index categorized into quintiles as a marker of socioeconomic status based on the residence area’s median household income; percentage of high school graduates in the population aged ≥15 years; percentage of manual workers in the labor force; and unemployment in the individual’s city of residence. Other variables included smoking‐, alcohol‐, and obesity‐related conditions; liver and pancreas disorder; and concomitant medications (eg, NSAID, low‐dose aspirin, antiplatelet agent, heparin, anticoagulant, oral corticosteroid, anxiolytic, hypnotic, antidepressant, and antipsychotic).
Outcomes Definition
The primary outcome was COVID‐19–related hospitalization defined based on 1 of the following principals or secondary diagnosis discharge codes derived from the ICD‐10 codes: U07.10 (COVID‐19, respiratory form, virus identified), U07.11 (COVID‐19, respiratory form, virus not identified), U07.14 (COVID‐19, other clinical forms, virus identified), U07.15 (COVID‐19, other clinical forms, virus not identified), and U04.9 (severe acute respiratory syndrome). The secondary outcome was in‐hospital mortality from COVID‐19. The latter allowed us to compare results from our study with those of other published studies. The individuals were followed up from the index date (February 15, 2020) until the occurrence of the outcome of interest or until the closure of the study on June 15, 2020.
Statistical Analysis
Categorical variables are reported as frequencies with percentages and continuous variables as means with SDs. To report the balance in each covariate between statin users and nonusers, the difference in proportions for categorical variables and means for continuous variables is standardized. 76 , 77 , 78 The imbalance between the groups is defined as an absolute value >0.10. 77
Conditional Cox proportional hazards models were used to take into account the matched design and to compare the incidence of events between the various groups: (1) statin and control groups (nonusers) for the main analysis; (2) atorvastatin, fluvastatin, pravastatin, rosuvastatin, simvastatin, and control groups; (3) low, moderate, high statin intensity, and control groups; and (4) statin intensity and type (low [fluvastatin 20/40, pravastatin 10/20, simvastatin 10], moderate [atorvastatin 10/20, fluvastatin 80, pravastatin 40, rosuvastatin 5/10, simvastatin 20/40], and high [atorvastatin 40/80, rosuvastatin 20]), and control groups.
We ran 4 types of conditional Cox proportional hazards models: (1) unadjusted (model 1); (2) adjusted for all baseline characteristics described in the Covariates section (model 2); (3) stabilized inverse probability of treatment weighting (IPTW) using the propensity score (model 3) 79 ; and (4) stabilized IPTW further adjusted with all covariates (model 4). Models 3 and 4 were run after trimming the IPTWs at the first and 99th percentiles, as extremely large weights may disproportionately influence results and yield estimates with high variance. 80
We performed a subanalysis of only patients hospitalized for COVID‐19 to evaluate the association between statin use and in‐hospital death using a conventional multivariable Cox model because of a small number of paired individuals in this subsample.
Two sensitivity analyses were also conducted to examine the effect of excluding participants with their matched pairs who had a highly imbalanced covariate (absolute standardized difference [ASD] >0.20) between statin users and their matched nonusers, and the robustness of the association between statins and COVID‐19–related hospitalization to unmeasured confounding using E‐value methodology developed by VanderWeele and Ding. 81
All analyses were performed with SAS Enterprise Guide version 4.3 software (SAS Institute Inc). A 2‐sided P value <0.05 indicated significance.
Regulatory and Ethical Considerations
SNDS is a strictly anonymous database, comprising all reimbursement data derived from mandatory health insurance. The authors had access to the SNDS database, in the application of the provisions of articles R. 1461‐12 et seq. of the French Public Health Code and the French data protection authority decision CNIL‐2016‐316, to process personal health data in retrospective cohort studies designed to describe possible statistical associations between the use of a drug product and the development of a health outcome. Therefore, informed consent from the study participants was not required. EPI‐PHARE staff, individually authorized to access SNDS, extracted and analyzed the data.
Results
Of the 27 250 310 eligible individuals, 2 071 465 were identified as statin users for the primary prevention of cardiovascular diseases. The 1:1 matching procedure generated 4 116 498 participants aged ≥40 years: 2 058 249 in the statin group and 2 058 249 in the control group (Figure).
Figure 1. Flowchart of participants’ inclusion.
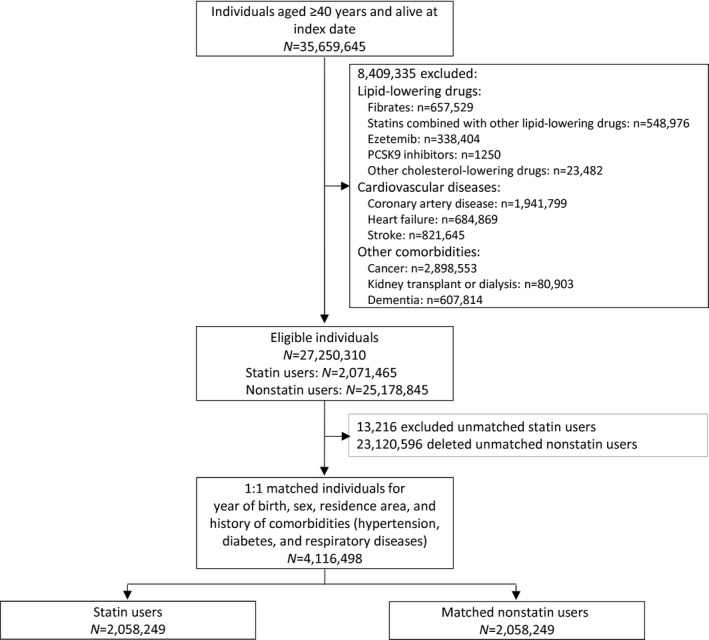
Table 1 shows that the comparison groups were well balanced according to matching variables: the participants were aged 68.7 years on average (SD, 10.4), and 46.6% were men. The participants’ distribution according to residence area was similar to that of the general population (Table S5). Hypertension was present in 42% of the population, diabetes in 34%, and a chronic respiratory condition in 9%.
Table 1.
Baseline Characteristics According to Statin Exposure
|
No exposure (n=2 058 249) |
Statin exposure (n=2 058 249) |
Standardized difference | |
|---|---|---|---|
| Matching variables | |||
| Age, y | |||
| Mean (SD) | 68.65 (10.36) | 68.65 (10.36) | 0.00000 |
| Age categories, y | |||
| 40–59 | 395 018 (19.2) | 395 018 (19.2) | |
| 60–69 | 683 378 (33.2) | 683 378 (33.2) | |
| 70–79 | 660 264 (32.1) | 660 264 (32.1) | |
| ≥80 | 319 589 (15.5) | 319 589 (15.5) | |
| Sex | |||
| Men | 958 989 (46.6) | 958 989 (46.6) | 0.00000 |
| Women | 1 099 260 (53.4) | 1 099 260 (53.4) | |
| Residence area* | |||
| Auvergne‐Rhône‐Alpes | 213 640 (10.4) | 213 640 (10.4) | 0.00000 |
| Bourgogne‐Franche‐Comté | 98 693 (4.8) | 98 693 (4.8) | |
| Bretagne | 104 714 (5.1) | 104 714 (5.1) | |
| Centre‐Val de Loire | 95 625 (4.6) | 95 625 (4.6) | |
| Corse | 8697 (0.4) | 8697 (0.4) | |
| Grand Est | 192 826 (9.4) | 192 826 (9.4) | |
| Hauts‐de‐France | 234 718 (11.4) | 234 718 (11.4) | |
| Ile‐de‐France | 317 010 (15.4) | 317 010 (15.4) | |
| Normandie | 121 260 (5.9) | 121 260 (5.9) | |
| Nouvelle‐Aquitaine | 199 285 (9.7) | 199 285 (9.7) | |
| Occitanie | 164 959 (8.0) | 164 959 (8.0) | |
| Pays de la Loire | 125 184 (6.1) | 125 184 (6.1) | |
| Provence‐Alpes‐Côte d’Azur | 133 389 (6.5) | 133 389 (6.5) | |
| Overseas departments | 47 939 (2.3) | 47 939 (2.3) | |
| Overseas territories | 310 (0.0) | 310 (0.0) | |
| Hypertension | |||
| No | 1 198 186 (58.2) | 1 198 186 (58.2) | 0.00000 |
| Yes | 860 063 (41.8) | 860 063 (41.8) | |
| Diabetes | |||
| No | 1 364 924 (66.3) | 1 364 924 (66.3) | 0.00000 |
| Yes | 693 325 (33.7) | 693 325 (33.7) | |
| Chronic respiratory condition | |||
| No | 1 872 316 (91.0) | 1 872 316 (91.0) | 0.00000 |
| Yes | 185 933 (9.0) | 185 933 (9.0) | |
| Covariates | |||
| Social deprivation index (quintiles) | |||
| 1 (least deprived) | 343 795 (16.7) | 330 208 (16.0) | 0.05887 |
| 2 | 366 832 (17.8) | 364 376 (17.7) | |
| 3 | 393 467 (19.1) | 393 311 (19.1) | |
| 4 | 422 536 (20.5) | 428 084 (20.8) | |
| 5 (most deprived) | 449 430 (21.8) | 459 712 (22.3) | |
| Unknown | 82 189 (4.0) | 82 558 (4.0) | |
| Smoking‐related condition | |||
| No | 2 001 677 (97.3) | 1 975 967 (96.0) | 0.06923 |
| Yes | 56 572 (2.7) | 82 282 (4.0) | |
| Alcohol‐related condition | |||
| No | 2 025 242 (98.4) | 2 027 375 (98.5) | –0.00838 |
| Yes | 33 007 (1.6) | 30 874 (1.5) | |
| Obesity‐related condition | |||
| No | 2 015 058 (97.9) | 2 015 593 (97.9) | –0.00182 |
| Yes | 43 191 (2.1) | 42 656 (2.1) | |
| Liver failure | |||
| No | 2 030 710 (98.7) | 2 042 408 (99.2) | –0.05568 |
| Yes | 27 539 (1.3) | 15 841 (0.8) | |
| NSAID | |||
| No | 1 732 982 (84.2) | 1 719 946 (83.6) | 0.01722 |
| Yes | 325 267 (15.8) | 338 303 (16.4) | |
| Low‐dose aspirin | |||
| No | 1 827 030 (88.8) | 1 514 305 (73.6) | 0.39618 |
| Yes | 231 219 (11.2) | 543 944 (26.4) | |
| Antiplatelet agent | |||
| No | 2 042 260 (99.2) | 2 005 808 (97.5) | 0.13885 |
| Yes | 15 989 (0.8) | 52 441 (2.5) | |
| Heparin | |||
| No | 2 044 349 (99.3) | 2 045 508 (99.4) | –0.00702 |
| Yes | 13 900 (0.7) | 12 741 (0.6) | |
| Anticoagulant | |||
| No | 2 010 491 (97.7) | 1 999 837 (97.2) | 0.03266 |
| Yes | 47 758 (2.3) | 58 412 (2.8) | |
| Oral corticosteroid | |||
| No | 1 944 371 (94.5) | 1 948 469 (94.7) | –0.00878 |
| Yes | 113 878 (5.5) | 109 780 (5.3) | |
| Anxiolytic | |||
| No | 1 872 500 (91.0) | 1 823 368 (88.6) | 0.07887 |
| Yes | 185 749 (9.0) | 234 881 (11.4) | |
| Hypnotic | |||
| No | 1 975 317 (96.0) | 1 952 287 (94.9) | 0.05349 |
| Yes | 82 932 (4.0) | 105 962 (5.1) | |
| Antidepressant | |||
| No | 1 902 683 (92.4) | 1 847 672 (89.8) | 0.09399 |
| Yes | 155 566 (7.6) | 210 577 (10.2) | |
| Antipsychotic | |||
| No | 2 044 795 (99.3) | 2 040 905 (99.2) | 0.02193 |
| Yes | 13 454 (0.7) | 17 344 (0.8) | |
| Statin description | |||
| Type of statin | |||
| Atorvastatin | 827 752 (40.2) | ||
| Fluvastatin | 55 585 (2.7) | ||
| Pravastatin | 375 936 (18.3) | ||
| Rosuvastatin | 384 904 (18.7) | ||
| Simvastatin | 414 072 (20.1) | ||
| Statin intensity | |||
| Low | 431 167 (20.9) | ||
| Moderate | 1 496 809 (72.7) | ||
| High | 130 273 (6.3) | ||
| Statin intensity and its type | |||
| Low | |||
| Fluvastatin 20/40 | 34 713 (1.7) | ||
| Pravastatin 10/20 | 288 465 (14.0) | ||
| Simvastatin 10 | 107 989 (5.2) | ||
| Moderate | |||
| Atorvastatin 10/20 | 718 121 (34.9) | ||
| Fluvastatin 80 | 20 872 (1.0) | ||
| Pravastatin 40 | 87 471 (4.2) | ||
| Rosuvastatin 5/10 | 364 262 (17.7) | ||
| Simvastatin 20/40 | 306 083 (14.9) | ||
| High | |||
| Atorvastatin 40/80 | 109 631 (5.3) | ||
| Rosuvastatin 20 | 20 642 (1.0) | ||
Statin users and nonusers were matched for residence area defined at departmental level (101 French departments). For the purpose of the presentation, these departments were aggregated into 15 regions.
Statin users and nonusers were comparable regarding the most extensively studied covariates (ASD <0.10), except for low‐dose aspirin (ASD, 0.40): statin users were more likely to use low‐dose aspirin than nonusers (26.4% versus 11.2%, respectively). For antiplatelet agents, the difference was marginal in terms of ASD (2.5% versus 0.8%; ASD, 0.14) (Table 1). ASDs were close to 0 for these variables after IPTW (Figure S1).
Among statin users, atorvastin was the most frequently used (40.2%), followed by simvastatin (20.1%), rosuvastatin (18.7%), pravastatin (18.3%), and fluvastatin (2.7%). When statins were categorized according to their intensity of activity on low‐density lipoprotein cholesterol reduction, moderate‐intensity statins were primarily used (72.7%), followed by low‐intensity (20.9%) and high‐intensity (6.3%) statins. The statin group was also described according to intensity and type. The results are reported in Table 1.
Table 2 shows the association between statin use––with its 4 definitions (statin exposure [no/yes], type of statin, statin intensity, and statin intensity and its type)––and the risk of hospitalization for COVID‐19. Of the total number of study participants, 9396 were hospitalized for COVID‐19: 4372 statin users and 5024 nonusers. Overall, the results from crude and adjusted models show a lower risk of hospitalization among statin users compared with nonusers. The fully adjusted (model 2) and IPTW further adjusted models (model 4) provided similar results. The results from the model with IPTW are presented in Table S6. Statin users had a 16% lower risk of hospitalization for COVID‐19 than nonusers (adjusted hazard ratio [HR], 0.84; 95% CI, 0.81–0.88 [P <0.0001]).
Table 2.
Association Between Statin Exposure and Hospitalization for COVID‐19
|
Hospitalization N=9396 |
Unadjusted model* | Fully adjusted model † | IPTW further adjusted model ‡ | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Statin exposure | |||||||
| No | 5024 (0.24) | 1 | 1 | 1 | |||
| Yes | 4372 (0.21) | 0.87 (0.83–0.90) | <0.0001 | 0.84 (0.81–0.88) | <0.0001 | 0.84 (0.80–0.87) | <0.0001 |
| Type of statin | |||||||
| No exposure | 5024 (0.24) | 1 | 1 | 1 | |||
| Atorvastatin | 1944 (0.23) | 0.93 (0.87–0.99) | 0.0152 | 0.89 (0.84–0.95) | 0.0006 | 0.88 (0.83–0.94) | 0.0002 |
| Fluvastatin | 92 (0.17) | 0.74 (0.56–0.97) | 0.0293 | 0.75 (0.57–0.99) | 0.0401 | 0.71 (0.53–0.95) | 0.0212 |
| Pravastatin | 730 (0.19) | 0.86 (0.78–0.95) | 0.0027 | 0.84 (0.76–0.93) | 0.0006 | 0.84 (0.76–0.93) | 0.0012 |
| Rosuvastatin | 794 (0.21) | 0.83 (0.75–0.91) | <0.0001 | 0.80 (0.72–0.88) | <0.0001 | 0.80 (0.72–0.88) | <0.0001 |
| Simvastatin | 812 (0.20) | 0.80 (0.73–0.88) | <0.0001 | 0.79 (0.72–0.87) | <0.0001 | 0.78 (0.71–0.87) | <0.0001 |
| Statin intensity | |||||||
| No exposure | 5024 (0.24) | 1 | 1 | 1 | |||
| Low | 778 (0.18) | 0.79 (0.72–0.87) | <0.0001 | 0.78 (0.71–0.86) | <0.0001 | 0.78 (0.71–0.87) | <0.0001 |
| Moderate | 3231 (0.22) | 0.87 (0.83–0.91) | <0.0001 | 0.84 (0.80–0.89) | <0.0001 | 0.83 (0.79–0.88) | <0.0001 |
| High | 363 (0.28) | 1.10 (0.95–1.28) | 0.1957 | 1.01 (0.86–1.18) | 0.9090 | 1.04 (0.88–1.23) | 0.6193 |
| Statin intensity and its type | |||||||
| No exposure | 5024 (0.24) | 1 | 1 | 1 | |||
| Low | |||||||
| Fluvastatin 20/40 | 58 (0.17) | 0.75 (0.54–1.06) | 0.1031 | 0.77 (0.54–1.08) | 0.1331 | 0.74 (0.51–1.06) | 0.0973 |
| Pravastatin 10/20 | 537 (0.19) | 0.83 (0.74–0.93) | 0.0015 | 0.81 (0.72–0.91) | 0.0005 | 0.81 (0.72–0.92) | 0.0007 |
| Moderate | |||||||
| Simvastatin 10 | 183 (0.17) | 0.72 (0.59–0.87) | 0.0006 | 0.72 (0.59–0.87) | 0.0008 | 0.72 (0.59–0.89) | 0.0018 |
| Atorvastatin 10/20 | 1638 (0.23) | 0.90 (0.84–0.96) | 0.0026 | 0.88 (0.82–0.94) | 0.0002 | 0.86 (0.80–0.93) | <0.0001 |
| Fluvastatin 80 | 34 (0.16) | 0.72 (0.46–1.12) | 0.1454 | 0.72 (0.46–1.14) | 0.1599 | 0.67 (0.42–1.09) | 0.1048 |
| Pravastatin 40 | 193 (0.22) | 0.95 (0.78–1.16) | 0.6144 | 0.92 (0.75–1.13) | 0.4427 | 0.94 (0.76–1.17) | 0.5806 |
| Rosuvastatin 5/10 | 737 (0.20) | 0.81 (0.73–0.89) | <0.0001 | 0.78 (0.71–0.87) | <0.0001 | 0.78 (0.71–0.87) | <0.0001 |
| Simvastatin 20/40 | 629 (0.21) | 0.83 (0.75–0.93) | 0.0007 | 0.82 (0.73–0.91) | 0.0003 | 0.81 (0.72–0.90) | 0.0001 |
| High | |||||||
| Atorvastatin 40/80 | 306 (0.28) | 1.08 (0.92–1.27) | 0.3426 | 0.99 (0.84–1.18) | 0.9442 | 1.02 (0.85–1.22) | 0.8238 |
| Rosuvastatin 20 | 57 (0.28) | 1.24 (0.84–1.83) | 0.2793 | 1.11 (0.74–1.65) | 0.6174 | 1.18 (0.78–1.80) | 0.4359 |
HR indicates hazard ratio.
Conditional Cox proportional hazards model.
Conditional Cox proportional hazards model adjusted for the following covariates: social deprivation index; smoking‐, alcohol‐, and obesity‐related conditions; liver failure; and concomitant medications (NSAID, low‐dose aspirin, antiplatelet agent, heparin, anticoagulant, oral corticosteroid, anxiolytic, hypnotic, antidepressant, antipsychotic).
Conditional Cox proportional hazards model with inverse probability of treatment weighting (IPTW) and further adjustment with the same variables as those in the full adjusted model.
The strength of the association remained unchanged after participants taking low‐dose aspirin were excluded (Table S7).
All types of statins were significantly associated with a lower risk of hospitalization, with the adjusted HR ranging from 0.75 (95% CI, 0.57–0.99) for fluvastatin to 0.89 (95% CI, 0.84–0.95) for atorvastatin. Low‐ and moderate‐intensity statins showed a lower adjusted risk compared with nonusers (adjusted HR, 0.78 [95% CI, 0.71–0.86] and 0.84 [95% CI, 0.80–0.89], respectively); whereas high‐intensity statins were not associated (adjusted HR, 1.01; 95% CI, 0.86–1.18). This subgroup, representing 6.3% of the statin group, had a different profile from those with low and moderate intensity: individuals with high‐intensity statins were younger and more likely to have cardiovascular disease risks (male, diabetes, smoking, obesity) and to be treated for cardiovascular conditions other than those listed in the noninclusion criteria, necessitating a higher use of low‐dose aspirin and antiplatelet agents (Table S8). The absence of a lower risk of hospitalization among high‐intensity statin users persisted after participants taking low‐dose aspirin were excluded (Table S7).
Similar results were observed when the exposure was categorized according to statin intensity and type. For certain groups, the strength of the association did not reach statistical significance because of the small number of events in each group. The results of the association between all covariates and hospitalization, examined in a fully adjusted model, are displayed in Table S9.
The E‐values (relative risk) for the point estimate and upper confidence bound for hospitalization for COVID‐19 were 1.70 and 1.56, respectively.
Similar observations can be made when the association between statin use and in‐hospital deaths from COVID‐19 was examined. However, the reduction of risk with statin use (adjusted HR, 0.77; 95% CI, 0.69–0.86) was higher with this outcome (Table 3 and Table S10). A subanalysis conducted only in patients hospitalized for COVID‐19 also showed a lower risk of in‐hospital death for COVID‐19 (Table S11).
Table 3.
Association Between Statin Exposure and In‐Hospital Death for COVID‐19
|
Death N=1648 |
Unadjusted model* | Fully adjusted model † | IPTW further adjusted model ‡ | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Statin exposure | |||||||
| No exposure | 914 (0.044) | 1 | 1 | 1 | |||
| Statin exposure | 734 (0.036) | 0.80 (0.73–0.88) | <0.0001 | 0.77 (0.69–0.86) | <0.0001 | 0.76 (0.68–0.85) | <0.0001 |
| Type of statin | |||||||
| No exposure | 914 (0.044) | 1 | 1 | 1 | |||
| Atorvastatin | 329 (0.040) | 0.93 (0.80–1.08) | 0.3375 | 0.87 (0.74–1.02) | 0.0849 | 0.83 (0.70–0.98) | 0.0280 |
| Fluvastatin | 22 (0.040) | 0.88 (0.50–1.56) | 0.6619 | 0.84 (0.46–1.54) | 0.5755 | 0.88 (0.47–1.65) | 0.6970 |
| Pravastatin | 118 (0.031) | 0.70 (0.55–0.88) | 0.0027 | 0.68 (0.53–0.87) | 0.0023 | 0.66 (0.51–0.85) | 0.0014 |
| Rosuvastatinv | 126 (0.033) | 0.71 (0.57–0.89) | 0.0035 | 0.69 (0.54‐0.88) | 0.0023 | 0.72 (0.56–0.92) | 0.0084 |
| Simvastatin | 139 (0.034) | 0.73 (0.58–0.91) | 0.0048 | 0.75 (0.59–0.94) | 0.0142 | 0.75 (0.59–0.96) | 0.0212 |
| Statin intensity | |||||||
| No exposure | 914 (0.044) | 1 | 1 | 1 | |||
| Low | 142 (0.033) | 0.76 (0.61–0.94) | 0.0134 | 0.76 (0.60–0.96) | 0.0190 | 0.74 (0.59–0.94) | 0.0116 |
| Moderate | 527 (0.035) | 0.78 (0.70–0.88) | <0.0001 | 0.75 (0.66–0.86) | <0.0001 | 0.75 (0.66–0.85) | <0.0001 |
| High | 65 (0.050) | 1.18 (0.83–1.69) | 0.3619 | 1.06 (0.72–1.55) | 0.7586 | 1.00 (0.66–1.51) | 0.9977 |
| Statin intensity and its type | |||||||
| No exposure | 914 (0.044) | 1 | 1 | 1 | |||
| Fluvastatin 20/40 | 14 (0.040) | 0.74 (0.37–1.47) | 0.3859 | 0.76 (0.37–1.55) | 0.4461 | 0.82 (0.39–1.72) | 0.6016 |
| Pravastatin 10/20 | 91 (0.032) | 0.69 (0.53–0.91) | 0.0076 | 0.68 (0.52–0.91) | 0.0078 | 0.66 (0.50–0.88) | 0.0041 |
| Simvastatin 10 | 37 (0.034) | 1.00 (0.63–1.58) | 1.0000 | 1.04 (0.65–1.69) | 0.8569 | 1.01 (0.62–1.64) | 0.9769 |
| Atorvastatin 10/20 | 273 (0.038) | 0.88 (0.75–1.03) | 0.1149 | 0.83 (0.70–0.99) | 0.0368 | 0.80 (0.67–0.96) | 0.0169 |
| Fluvastatin 80 | 8 (0.038) | 1.33 (0.46–3.84) | 0.5943 | 1.10 (0.37–3.31) | 0.8655 | 1.08 (0.33–3.55) | 0.9052 |
| Pravastatin 40 | 27 (0.031) | 0.70 (0.43–1.16) | 0.1680 | 0.67 (0.39–1.13) | 0.1351 | 0.67 (0.38–1.17) | 0.1628 |
| Rosuvastatin 5/10 | 117 (0.032) | 0.71 (0.56–0.90) | 0.0045 | 0.68 (0.53–0.87) | 0.0025 | 0.71 (0.55–0.91) | 0.0072 |
| Simvastatin 20/40 | 102 (0.033) | 0.66 (0.51–0.85) | 0.0014 | 0.67 (0.52–0.88) | 0.0038 | 0.68 (0.52–0.91) | 0.0079 |
| Atorvastatin 40/80 | 56 (0.051) | 1.30 (0.88–1.94) | 0.1927 | 1.13 (0.74–1.73) | 0.5633 | 1.02 (0.65–1.61) | 0.9380 |
| Rosuvastatin 20 | 9 (0.044) | 0.75 (0.32–1.78) | 0.5141 | 0.79 (0.32–1.94) | 0.6052 | 0.93 (0.37–2.34) | 0.8697 |
HR indicates hazard ratio.
Conditional Cox proportional hazards model.
Conditional Cox proportional hazards model adjusted for the following covariates: social deprivation index; smoking‐, alcohol‐, and obesity‐related conditions; liver failure; and concomitant medications (NSAID, low‐dose aspirin, antiplatelet agent, heparin, anticoagulant, oral corticosteroid, anxiolytic, hypnotic, antidepressant, antipsychotic).
Conditional Cox proportional hazards model with inverse probability of treatment weighting (IPTW) and further adjustment with the same variables as those in the full adjusted model.
Subgroup analyses conducted using the fully adjusted model showed a lower risk with statin use in all age classes, men and women, regardless of whether the participants had comorbidities (hypertension, diabetes, and chronic respiratory condition) (Table 4).
Table 4.
Association Between Statin Exposure and COVID‐19 Outcomes: Subgroup Analyses
| Hospitalization among nonusers n=5024 | Hospitalization among statin users n=4372 | Fully adjusted model* | Death among nonusers n=914 | Death among statin users n=734 | Fully adjusted model* | |||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||||
| Age categories, y | ||||||||
| 40–59 | 882/395 018 (0.22) | 805/395 018 (0.20) | 0.90 (0.81–1.00) | 0.0494 | 62/395 018 (0.016) | 42/395 018 (0.011) | 0.64 (0.39–1.05) | 0.0746 |
| 60–69 | 1437/683 378 (0.21) | 1237/683 378 (0.18) | 0.85 (0.78–0.92) | 0.0001 | 193/683 378 (0.028) | 129/683 378 (0.019) | 0.71 (0.55–0.92) | 0.0107 |
| 70–79 | 1499/660 264 (0.23) | 1329/660 264 (0.20) | 0.84 (0.78–0.91) | <0.0001 | 268/660 264 (0.041) | 242/660 264 (0.037) | 0.87 (0.71–1.06) | 0.1625 |
| ≥80 | 1206/319 589 (0.38) | 1001/319 589 (0.31) | 0.79 (0.72–0.86) | <0.0001 | 391/319 589 (0.122) | 321/319,589 (0.100) | 0.75 (0.63–0.89) | 0.0011 |
| Sex | ||||||||
| Men | 2,841/958,989 (0.30) | 2,414/958,989 (0.25) | 0.82 (0.77–0.87) | <0.0001 | 567/958,989 (0.059) | 454/958,989 (0.047) | 0.77 (0.67–0.89) | 0.0003 |
| Women | 2,183/1,099,260 (0.20) | 1,958/1,099,260 (0.18) | 0.87 (0.81–0.93) | <0.0001 | 347/1,099,260 (0.032) | 280/1,099,260 (0.025) | 0.77 (0.64–0.92) | 0.0034 |
| Hypertension | ||||||||
| No | 3,249/1,198,186 (0.27) | 2,853/1,198,186 (0.24) | 0.84 (0.80–0.89) | <0.0001 | 614/1,198,186 (0.051) | 471/1,198 186 (0.039) | 0.74 (0.65–0.85) | <0.0001 |
| Yes | 1775/860 063 (0.21) | 1519/860 063 (0.18) | 0.84 (0.78–0.90) | <0.0001 | 300/860 063 (0.035) | 263/860 063 (0.031) | 0.85 (0.71–1.03) | 0.0985 |
| Diabetes | ||||||||
| No | 2522/1 364 924 (0.18) | 2152/1 364 924 (0.16) | 0.83 (0.78–0.88) | <0.0001 | 416/1 364 924 (0.030) | 343/1 364 924 (0.025) | 0.79 (0.67–0.92) | 0.0034 |
| Yes | 2502/693 325 (0.36) | 2220/693 325 (0.32) | 0.85 (0.80–0.91) | <0.0001 | 498/693 325 (0.072) | 391/693 325 (0.056) | 0.77 (0.66–0.90) | 0.0007 |
| Chronic respiratory condition | ||||||||
| No | 4279/1 872 316 (0.23) | 3746/1 872 316 (0.20) | 0.84 (0.80–0.88) | <0.0001 | 765/1 872 316 (0.041) | 620/1 872 316 (0.033) | 0.77 (0.69–0.87) | <0.0001 |
| Yes | 745/185 933 (0.40) | 626/185 933 (0.34) | 0.83 (0.74–.94) | 0.0024 | 149/185 933 (0.080) | 114/185 933 (0.061) | 0.77 (0.58–1.03) | 0.0754 |
HR indicates hazard ratio.
Conditional Cox proportional hazards model adjusted for the following covariates: social deprivation index; smoking‐, alcohol‐, and obesity‐related conditions; liver failure; and concomitant medications (NSAID, low‐dose aspirin, antiplatelet agent, heparin, anticoagulant, oral corticosteroid, anxiolytic, hypnotic, antidepressant, antipsychotic).
Discussion
This population‐based matched cohort study was conducted in >2 million adults aged ≥40 years who used statins for the primary prevention of cardiovascular diseases compared with 2 million of those who did not use statins. Our results show that statins were associated with a lower risk of hospitalization attributable to COVID‐19: statin users had a 16% lower risk than nonusers. This lower risk was observed in all age classes, men and women, regardless of whether the participants had comorbidities (hypertension, diabetes, and chronic respiratory condition). All types of statins showed a lower risk of COVID‐19 outcomes. When we examined statin users according to statin intensity on low‐density lipoprotein cholesterol–lowering reduction, we did not observe an association between high‐intensity statin use and the risk of hospitalization. We observed similar results with in‐hospital deaths from COVID‐19.
Possible Underlying Mechanisms
COVID‐19 is primarily a respiratory viral illness; however, it has widespread effects on the body including hypercoagulability, a hyperinflammatory state, and endothelial dysfunction. An autopsy study of COVID‐19–positive patients showed that the lung was injured with diffuse alveolar damage (90%), while other effects include pulmonary emboli and microthrombi in multiple organ systems including the brain, as well as hemophagocytosis and cardiac enlargement 82 ; results that are consistent with the clinical presentation of symptomatic patients with COVID‐19. 83
The lower risk of hospitalization among statin users compared with nonusers that we found in this study, if causal, would likely be attributable to the pleiotropic beneficial effects of statins as anti‐inflammatory, immune‐modulatory, and anticoagulant agents. 84 Indeed, several in vitro studies have supported the argument that statins may prevent individuals from being infected or having a serious COVID‐19 outcome. 85 , 86 , 87 , 88 SARS‐CoV‐2 infects type II pneumocytes present in the oral mucosa and lungs of the host by docking its spike protein onto ACE‐2 85 on the plasma membrane. 86 Lipid rafts––plasma membrane microdomains mainly composed of cholesterol, glycosphingolipids, and phospholipids––including ACE‐2 are the sites of the initial binding, activation, internalization, and cell‐to‐cell transmission of SARS‐CoV‐2. 87 They also are key regulators of immune and inflammatory responses following the infection. Depletion of cholesterol by statins is shown to disrupt lipid rafts, which, in turn, disturbs viral binding to ACE‐2 cells and leads to a significant reduction in viral replication. 88
Comparison With Other Studies
We found one study 32 that examined the association between statin use and the risk of hospitalization for COVID‐19. Oh et al concluded that the risk of developing COVID‐19 was 35% lower in statin users compared with nonusers (odds ratio, 0.65; 95% CI, 0.60–0.71). However, the level of evidence was not sufficient given its design: first, the authors selected eligible participants based on a case‐control design–COVID‐19 patients matched with the general population for age, sex, and place of residence––and performed a second matching based on propensity score between statin users and nonusers. We also identified studies that focused on risk factors and drugs associated with SARS‐CoV‐2 infection, conducted on patients with varied conditions (history of diabetes, 89 hypertension, 90 undergoing transcatheter aortic valve implantation, 91 or pancreas, biliary, or liver conditions 92 ) or in the general population. 93 , 94 , 95 , 96 , 97 Results of association with statin use in these studies were heterogeneous: a significantly lower risk, 89 , 93 , 97 a lower risk but not statistically significant, 90 , 91 , 95 and an increased risk of COVID‐19 diagnosis. 92 , 94 , 96 Our cohort study, specifically planned using a matched exposed/nonexposed design to examine the relationship between statin use and hospitalization for COVID‐19, showed strong evidence of lower risk of COVID‐19 outcomes associated with statins.
We also present other original findings. Our study, which was sufficiently powered to examine the risk of hospitalization for COVID‐19 according to types of statins, showed that all types of statins were significantly associated with a lower risk. When we examined the exposed group according to the intensity of statins, 75 we identified a small percentage of high‐intensity statin users (6.3%). This subgroup had a different profile from those with low‐ and moderate‐intensity statin subgroups, with more risk factors for cardiovascular diseases. The absence of lower risk of COVID‐19 outcomes in the high‐intensity statin group compared with the unexposed group may be attributable to: (1) the lack of statistical power because of the low frequency of this group, (2) the inability to control for unmeasured confounders, or (3) a lower risk associated with statins potentially being hindered by an increased risk of hospitalization associated with cardiovascular disease risk factors.
Regarding the secondary outcome, namely in‐hospital deaths from COVID‐19, we found a lower risk among participants treated with statins compared with those without this treatment. This finding is consistent with that observed in numerous studies. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46
Limitations and Strengths
This study has some limitations. First, our study could not assess any association between statin use and SARS‐CoV‐2 infection. Because databases containing this information were not available, we used a surrogate outcome: hospitalization attributable to COVID‐19. In doing so, we did not include participants with asymptomatic or mild symptoms that did not lead to hospitalization.
Second, our study may have been impacted by selection bias as individuals who take statins might generally be more health conscious than nonusers and, therefore, manage their comorbidities better and seek care earlier in the course of COVID‐19. To evaluate this bias, we used an indicator that may reflect health‐conscious behavior such as the history of influenza vaccination within 2 years before the index date. Indeed, statin users were more likely to receive this vaccination than nonusers: 48.6% versus 40.0%, respectively (ASD, 0.17). The strength of the association between statin exposure and severe COVID‐19 outcomes remained unchanged (Table S12).
Third, as in all observational studies, we cannot rule out a residual confounding effect from unmeasured covariates, in particular those of socioeconomic status such as education. However, the sensitivity analysis using E‐value methodology 81 indicated that the observed HR of 0.84 for COVID‐19–related hospitalization could only be explained by an unmeasured confounder that was associated with both statin use and COVID‐19–related hospitalization by a relative risk association at least as large as 1.70, conditional on the measured covariates in this study (upper confidence bound, 1.56). In our study, the HRs for some of the known COVID‐19–related hospitalization risk factors were 1.49 (95% CI, 1.34–2.12) for obesity‐related conditions, 1.69 (95% CI, 1.34–2.12) for liver failure, and 1.55 (95% CI, 1.37–1.75) for oral corticosteroids (Table S9). It is not likely that an unmeasured or unknown confounder would have a substantially greater effect on COVID‐19–related hospitalization than these known risk factors by having a relative risk exceeding 1.70.
Last, to limit selection or collider bias, 60 our matched cohort was set up from the general population––unlike other studies where hospitalized or COVID‐19–positive patients were included––with the exposed group taking statins for the primary prevention of cardiovascular disease.
The SNDS, a claims database comprising the entire population of France, has allowed us to comprehensively examine the association between statins and severe COVID‐19 outcomes. To avoid confounding bias as much as possible, we limited the study of the effect of statins to the context of primary prevention of cardiovascular diseases as these comorbidities are known to be strongly associated with an increased risk of hospitalization for COVID‐19. 24 , 98 After matching for age, sex, residence area, hypertension, diabetes, and chronic respiratory condition, statin users and nonusers were comparable for 14 of 15 covariates. The only imbalanced variable was low‐dose aspirin. This imbalance was taken into account by including this variable in multivariable analyses and in the calculation of IPTW, which rendered comparison groups similar among all covariates. In observational studies, adjustment for adequate covariates is the most important step. This is particularly crucial in studies examining the association between statins and COVID‐19 outcomes. To illustrate this, we observed unadjusted and adjusted ratio measures (OR or HR) in published studies investigating the role of statins in in‐hospital mortality by COVID‐19 (Figure S2): in propensity score–matched cohort studies, unadjusted odds ratios or HRs were very close to those with adjustment. 7 , 12 , 27 In other studies where this design was not applied, adjustment systematically decreased odds ratios or HRs.† In certain cases, the direction of odds ratios or HRs changed drastically after adjustment: statin use was significantly associated with a higher risk in unadjusted analysis while it was associated with lower risk in adjusted analysis. 7 , 18 In addition, not including adjusted ratio measures in meta‐analyses, which is recommended by the Cochrane group, 99 leads to spurious results, notably the absence of association between statin use and COVID‐19 outcomes. 100 Meta‐analyses that did include adjusted ratio measures showed a lower risk of COVID‐19 outcomes with the statin use. 101 , 102
Our findings indicate that the lower risk of statins on hospitalization for COVID‐19, although modest, is robust. Statins are now known to be beneficial in primary prevention, decreasing all‐cause mortality, cardiovascular disease, coronary heart disease, and stroke. Furthermore, there is no evidence of any serious harm caused by their use. 103 Our study found an additional lower risk of statins against serious COVID‐19 symptoms that lead to hospitalization. Since the beginning of the COVID‐19 pandemic, many clinicians have suggested that statins could be used as an adjunctive treatment for the SARS‐CoV‐2 infection. This population‐based matched cohort study conducted in 2 million adults aged ≥40 years who used statins for the primary prevention of cardiovascular diseases compared with 2 million nonusers supports the hypothesis that statin use is associated with a lower risk of hospitalization for COVID‐19. All types of statins showed a similar effect.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1‐S12
Figures S1–S2
Acknowledgments
We thank Peter Hancock for the English revision of the article. We are grateful to the Technical Agency for Information on Hospital Care (ATIH) team for providing us with hospital discharge data (PMSI) through a fast‐track procedure.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023357
For Sources of Funding and Disclosures, see page 13.
Footnotes
References
- 1. Rodrigues‐Diez RR, Tejera‐Muñoz A, Marquez‐Exposito L, Rayego‐Mateos S, Santos Sanchez L, Marchant V, Tejedor Santamaria L, Ramos AM, Ortiz A, Egido J, et al. Statins: could an old friend help in the fight against COVID‐19? Br J Pharmacol. 2020;177:4873–4886. doi: 10.1111/bph.15166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417–435. doi: 10.1016/j.antiviral.2013.06.018 [DOI] [PubMed] [Google Scholar]
- 3. Fedson DS. Treating the host response to emerging virus diseases: lessons learned from sepsis, pneumonia, influenza and Ebola. Ann Transl Med. 2016;4:421. doi: 10.21037/atm.2016.11.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shrivastava‐Ranjan P, Flint M, Bergeron É, McElroy AK, Chatterjee P, Albariño CG, Nichol ST, Spiropoulou CF. Statins suppress Ebola virus infectivity by interfering with glycoprotein processing. MBio. 2018;9. doi: 10.1128/mBio.00660-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yuan S. Statins may decrease the fatality rate of Middle East respiratory syndrome infection. MBio. 2015;6. doi: 10.1128/mBio.01120-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ho P, Zheng JQ, Wu CC, Hou YC, Liu WC, Lu CL, Zheng CM, Lu KC, Chao YC. Perspective adjunctive therapies for COVID‐19: beyond antiviral therapy. Int J Med Sci. 2021;18:314–324. doi: 10.7150/ijms.51935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee KC, Sewa DW, Phua GC. Potential role of statins in COVID‐19. Int J Infect Dis. 2020;96:615–617. doi: 10.1016/j.ijid.2020.05.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fedson DS, Opal SM, Rordam OM. Hiding in plain sight: an approach to treating patients with severe COVID‐19 infection. MBio. 2020;11. doi: 10.1128/mBio.00398-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tikoo K, Patel G, Kumar S, Karpe PA, Sanghavi M, Malek V, Srinivasan K. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem Pharmacol. 2015;93:343–351. doi: 10.1016/j.bcp.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 10. Dong B, Zhang C, Feng JB, Zhao YX, Li SY, Yang YP, Dong QL, Deng BP, Zhu L, Yu QT, et al. Overexpression of ACE2 enhances plaque stability in a rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1270–1276. doi: 10.1161/ATVBAHA.108.164715 [DOI] [PubMed] [Google Scholar]
- 11. Chacko SR, DeJoy R, Lo KB, Albano J, Peterson E, Bhargav R, Gu F, Salacup G, Pelayo J, Azmaiparashvili Z, et al. Association of pre‐admission statin use with reduced in‐hospital mortality in COVID‐19. Am J Med Sci. 2021;361:725–730. doi: 10.1016/j.amjms.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan Y, Guo T, Yan F, Gong M, Zhang XA, Li C, He T, Luo H, Zhang L, Chen M, et al. Association of statin use with the in‐hospital outcomes of 2019‐Coronavirus disease patients: a retrospective study. Front Med. 2020;7. doi: 10.3389/fmed.2020.584870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daniels LB, Sitapati AM, Zhang J, Zou J, Bui QM, Ren J, Longhurst CA, Criqui MH, Messer K. Relation of statin use prior to admission to severity and recovery among COVID‐19 inpatients. Am J Cardiol. 2020;136:149–155. doi: 10.1016/j.amjcard.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid‐19. N Engl J Med. 2020;382:2582. doi: 10.1056/NEJMc2021225 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Rodriguez‐Nava G, Trelles‐Garcia DP, Yanez‐Bello MA, Chung CW, Trelles‐Garcia VP, Friedman HJ. Atorvastatin associated with decreased hazard for death in COVID‐19 patients admitted to an ICU: a retrospective cohort study. Crit Care Lond Engl. 2020;24:429. doi: 10.1186/s13054-020-03154-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greco S, D’Amuri A, Giorgini E, Luciani F, Lopreiato M, Fortunato V, Scopa A, Vestita G, Capatti E, Passaro A. Role of statins in coronavirus‐related disease (COVID‐19): a retrospective cohort study in Northern Italy. High Blood Press Cardiovasc Prev. 2021;28:355–364. doi: 10.1007/s40292-021-00452-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta A, Madhavan MV, Poterucha TJ, DeFilippis EM, Hennessey JA, Redfors B, Eckhardt C, Bikdeli B, Platt J, Nalbandian A, et al. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID‐19. Res Sq. 2020. doi: 10.21203/rs.3.rs-56210/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nicholson CJ, Wooster L, Sigurslid HH, Li RF, Jiang W, Tian W, Cardenas CL, Malhotra R. Estimating risk of mechanical ventilation and mortality among adult COVID‐19 patients admitted to mass general Brigham: the VICE and DICE scores. MedRxiv Prepr Serv Health Sci. 2020. doi: 10.1101/2020.09.14.20194670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aparisi Á, Amat‐Santos IJ, Otero DL, Marcos‐Mangas M, González‐Juanatey JR, San Román JA. Impact of statins in patients with COVID‐19. Rev Esp Cardiol. 2021. doi: 10.1016/j.recesp.2021.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saeed O, Castagna F, Agalliu I, Xue X, Patel SR, Rochlani Y, Kataria R, Vukelic S, Sims DB, Alvarez C, et al. Statin use and in‐hospital mortality in patients with diabetes mellitus and COVID‐19. J Am Heart Assoc. 2020;9:e018475. doi: 10.1161/JAHA.120.018475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mallow PJ, Belk KW, Topmiller M, Hooker EA. Outcomes of hospitalized COVID‐19 patients by risk factors: results from a United States hospital claims database. J Health Econ Outcomes Res. 2020;7:165–174. doi: 10.36469/jheor.2020.17331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee HY, Ahn J, Park J, Kyung Kang C, Won SH, Wook Kim D, Park JH, Chung KH, Joh JS, Bang JH et al. Beneficial effect of statins in COVID‐19–related outcomes. Arterioscler Thromb Vasc Biol. 2021;41:e175–e182. doi: 10.1161/ATVBAHA.120.315551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lohia P, Kapur S, Benjaram S, Mir T. Association between antecedent statin use and severe disease outcomes in COVID‐19: a retrospective study with propensity score matching. J Clin Lipidol. 2021;15:451–459. doi: 10.1016/j.jacl.2021.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID‐19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Memel ZN, Lee JJ, Foulkes AS, Chung RT, Thaweethai T, Bloom PP. Association of statins and 28‐day mortality rates in patients hospitalized with severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2022;225:19–29. doi: 10.1093/infdis/jiab539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Masana L, Correig E, Rodríguez‐Borjabad C, Anoro E, Arroyo JA, Jericó C, Pedragosa A, Miret M, Näf S, Pardo A, et al. Effect of statin therapy on SARS‐CoV‐2 infection‐related mortality in hospitalized patients. Eur Heart J — Cardiovasc Pharmacother. 2020. doi: 10.1093/ehjcvp/pvaa128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang XJ, Qin JJ, Cheng XU, Shen L, Zhao YC, Yuan Y, Lei F, Chen MM, Yang H, Bai L, et al. In‐hospital use of statins is associated with a reduced risk of mortality among individuals with COVID‐19. Cell Metab. 2020;32:176–187.e4. doi: 10.1016/j.cmet.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in‐hospital mortality in a US national sample of patients with COVID‐19. JAMA Netw Open. 2020;3:e2029058. doi: 10.1001/jamanetworkopen.2020.29058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Torres‐Peña JD, Pérez‐Belmonte LM, Fuentes‐Jiménez F, López Carmona MD, Pérez‐Martinez P, López‐Miranda J, Carrasco Sánchez FJ, Vargas Núñez JA, del Corral Beamonte E, Magallanes Gamboa JO, et al. Prior treatment with statins is associated with improved outcomes of patients with COVID‐19: data from the SEMI‐COVID‐19 registry. Drugs. 2021;81:685–695. doi: 10.1007/s40265-021-01498-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahlström B, Frithiof R, Hultström M, Larsson IM, Strandberg G, Lipcsey M. The Swedish covid‐19 intensive care cohort: risk factors of ICU admission and ICU mortality. Acta Anaesthesiol Scand. 2021;65:525–533. doi: 10.1111/aas.13781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oddy C, McCaul J, Keeling P, Allington J, Senn D, Soni N, Morrison H, Mawella R, Samuel T, Dixon J. Pharmacological predictors of morbidity and mortality in COVID‐19. J Clin Pharmacol. 2021;61:1286–1300. doi: 10.1002/jcph.1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oh TK, Song IA, Jeon YT. Statin therapy and the risk of COVID‐19: a cohort study of the national health insurance service in South Korea. J Pers Med. 2021;11:116. doi: 10.3390/jpm11020116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bifulco M, Ciccarelli M, Bruzzese D, Dipasquale A, Lania AG, Mazziotti G, Gazzerro P. The benefit of statins in SARS‐CoV‐2 patients: further metabolic and prospective clinical studies are needed. Endocrine. 2020. doi: 10.1007/s12020-020-02550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Spiegeleer A, Bronselaer A, Teo JT, Byttebier G, De Tré G, Belmans L, Dobson R, Wynendaele E, Van De Wiele C, Vandaele F, et al. The effects of ARBs, ACEis, and statins on clinical outcomes of COVID‐19 infection among nursing home residents. J Am Med Dir Assoc. 2020;21:909–914.e2. doi: 10.1016/j.jamda.2020.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ikari Y, Matsue Y, Torii S, Hasegawa M, Aihara K, Kuroda S, Sano T, Kitai T, Yonetsu T, Kohsaka S, et al. Association between statin use prior to admission and lower coronavirus disease 2019 (COVID‐19) severity in patients with cardiovascular disease or risk factors. Circ J. 2021;85:939–943. doi: 10.1253/circj.CJ-21-0087 [DOI] [PubMed] [Google Scholar]
- 36. Song SL, Hays SB, Panton CE, Mylona EK, Kalligeros M, Shehadeh F, Mylonakis E. Statin use is associated with decreased risk of invasive mechanical ventilation in COVID‐19 patients: a preliminary study. Pathog Basel Switz. 2020;9:759. doi: 10.3390/pathogens9090759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Byttebier G, Belmans L, Alexander M, Saxberg BE, De Spiegeleer B, De Spiegeleer A, Devreker N, Van Praet JT, Vanhove K, Reybrouck R, et al. Hospital mortality in COVID‐19 patients in Belgium treated with statins, ACE inhibitors and/or ARBs. Hum Vaccines Immunother. 2021;1–10. doi: 10.1080/21645515.2021.1920271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wander PL, Lowy E, Beste LA, Tulloch‐Palomino L, Korpak A, Peterson AC, Young BA, Boyko EJ. Risk factors for adverse outcomes among 35 879 veterans with and without diabetes after diagnosis with COVID‐19. BMJ Open Diabetes Res Care. 2021;9. doi: 10.1136/bmjdrc-2021-002252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bergqvist R, Ahlqvist VH, Lundberg M, Hergens MP, Sundström J, Bell M, Magnusson C. HMG‐CoA reductase inhibitors and COVID‐19 mortality in Stockholm, Sweden: a registry‐based cohort study. PLoS Medicine. 2021;18:e1003820. doi: 10.1371/journal.pmed.1003820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choi D, Chen Q, Goonewardena SN, Pacheco H, Mejia P, Smith RL, Rosenson RS. Efficacy of statin therapy in patients with hospital admission for COVID‐19. Cardiovasc Drugs Ther. 2021;1–9. doi: 10.1007/s10557-021-07263-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Daniels LB, Ren J, Kumar K, Bui QM, Zhang J, Zhang X, Sawan MA, Eisen H, Longhurst CA, Messer K. Relation of prior statin and anti‐hypertensive use to severity of disease among patients hospitalized with COVID‐19: findings from the American Heart Association’s COVID‐19 cardiovascular disease registry. PLoS One. 2021;16:e0254635. doi: 10.1371/journal.pone.0254635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haji Aghajani M, Moradi O, Azhdari Tehrani H, Amini H, Pourheidar E, Hatami F, Rabiei MM, Sistanizad M. Promising effects of atorvastatin on mortality and need for mechanical ventilation in patients with severe COVID‐19; a retrospective cohort study. Int J Clin Pract. 2021;75:e14434. doi: 10.1111/ijcp.14434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee SW, Kim SY, Moon SY, Yoo IK, Yoo EG, Eom GH, Kim JM, Shin JI, Jeong MH, Yang JM, et al. Statin use and COVID‐19 infectivity and severity in South Korea: two population‐based nationwide cohort studies. JMIR Public Health Surveill. 2021;7:e29379. doi: 10.2196/29379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lohia P, Kapur S, Benjaram S, Cantor Z, Mahabadi N, Mir T, Badr MS. Statins and clinical outcomes in hospitalized COVID‐19 patients with and without Diabetes Mellitus: a retrospective cohort study with propensity score matching. Cardiovasc Diabetol. 2021;20:140. doi: 10.1186/s12933-021-01336-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McAlister FA, Wang T, Wang X, Chu A, Goodman SG, van Diepen S, Jackevicius CA, Kaul P, Udell J, Ko DT, et al. Statins and SARS‐CoV‐2 Infection: results of a population‐based prospective cohort study of 469 749 adults from 2 Canadian provinces. J Am Heart Assoc. 2021;10:e022330. doi: 10.1161/JAHA.121.022330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Umakanthan S, Senthil S, John S, Madhavan MK, Das J, Patil S, Rameshwaram R, Cintham A, Subramaniam V, Yogi M, et al. The protective role of statins in COVID‐19 patients: a retrospective observational study. Transl Med Commun. 2021;6:22. doi: 10.1186/s41231-021-00102-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Butt JH, Gerds TA, Schou M, Kragholm K, Phelps M, Havers‐Borgersen E, Yafasova A, Gislason GH, Torp‐Pedersen C, Køber L, et al. Association between statin use and outcomes in patients with coronavirus disease 2019 (COVID‐19): a nationwide cohort study. BMJ Open. 2020;10:e044421. doi: 10.1136/bmjopen-2020-044421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tan WYT, Young BE, Lye DC, Chew DEK, Dalan R. Statin use is associated with lower disease severity in COVID‐19 infection. Sci Rep. 2020;10:17458. doi: 10.1038/s41598-020-74492-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, Bonanomi E, Cabrini L, Carlesso E, Castelli G, et al. Risk factors associated with mortality among patients with COVID‐19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yetmar ZA, Challener DW, Tleyjeh IM, Sohail MR, Cerhan JR, Badley AD, O’Horo JC. Association between chronic statin use and 30‐day mortality in hospitalized patients with COVID‐19. Mayo Clin Proc Innov Qual Outcomes. 2021. doi: 10.1016/j.mayocpiqo.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ramos‐Rincón JM, Pérez‐Belmonte LM, Carrasco‐Sánchez FJ, Jansen‐Chaparro S, De‐Sousa‐Baena M, Bueno‐Fonseca J, Pérez‐Aguilar M, Arévalo‐Cañas C, Bacete Cebrian M, Méndez‐Bailón M, et al. Cardiometabolic therapy and mortality in very old patients with diabetes hospitalized due to COVID‐19. J Gerontol A Biol Sci Med Sci. 2021;76:e102–e109. doi: 10.1093/gerona/glab124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ayeh SK, Abbey EJ, Khalifa BAA, Nudotor RD, Osei AD, Chidambaram V, Osuji N, Khan S, Salia EL, Oduwole MO, et al. Statins use and COVID‐19 outcomes in hospitalized patients. PLoS One. 2021;16:e0256899. doi: 10.1371/journal.pone.0256899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. El‐Solh AA, Lawson Y, El‐Solh DA. All‐cause mortality in COVID‐19 patients receiving statin therapy: analysis of veterans affairs database cohort study. Intern Emerg Med. 2021. doi: 10.1007/s11739-021-02848-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kuno T, So M, Iwagami M, Takahashi M, Egorova NN. The association of statins use with survival of patients with COVID‐19. J Cardiol. 2022;79:494–500. doi: 10.1016/j.jjcc.2021.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nateghi S, Gomari MM, Hosamirudsari H, Behnoush B, Razmjoofard A, Azimi G, Ordookhani S, Jafarpour A, Faraji N. A historical cohort study to investigation of statins safety in COVID‐19 hospitalized patients. Therapie. 2021. doi: 10.1016/j.therap.2021.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Russo V, Silverio A, Scudiero F, Attena E, D'Andrea A, Nunziata L, Parodi G, Celentani D, Varbella F, Albani S, et al. Preadmission statin therapy and clinical outcome in hospitalized patients with COVID‐19: an Italian multicenter observational study. J Cardiovasc Pharmacol. 2021;78:e94–e100. doi: 10.1097/FJC.0000000000001041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vila‐Corcoles A, Satue‐Gracia E, Vila‐Rovira A, de Diego‐Cabanes C, Forcadell‐Peris MJ, Hospital‐Guardiola I, Ochoa‐Gondar O, Basora‐Gallisa J. COVID19‐related and all‐cause mortality among middle‐aged and older adults across the first epidemic wave of SARS‐COV‐2 infection in the region of Tarragona, Spain: results from the COVID19 TARRACO Cohort Study, March‐June 2020. medRxiv. 2021;21:1795. doi: 10.1186/s12889-021-11879-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wargny M, Potier L, Gourdy P, Pichelin M, Amadou C, Benhamou P‐Y, Bonnet JB, Bordier L, Bourron O, Chaumeil C, et al. Predictors of hospital discharge and mortality in patients with diabetes and COVID‐19: updated results from the nationwide CORONADO study. Diabetologia. 2021;64:778–794. doi: 10.1007/s00125-020-05351-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cariou B, Goronflot T, Rimbert A, Boullu S, Le May C, Moulin P, Pichelin M, Potier L, Smati S, Sultan A, et al. Routine use of statins and increased COVID‐19 related mortality in inpatients with type 2 diabetes: results from the CORONADO study. Diabetes Metab. 2020;47:101202. doi: 10.1016/j.diabet.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Griffith GJ, Morris TT, Tudball MJ, Herbert A, Mancano G, Pike L, Sharp GC, Sterne J, Palmer TM, Davey Smith G, et al. Collider bias undermines our understanding of COVID‐19 disease risk and severity. Nat Commun. 2020;11:5749. doi: 10.1038/s41467-020-19478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tuppin P, Rudant J, Constantinou P, Gastaldi‐Ménager C, Rachas A, de Roquefeuil L, Maura G, Caillol H, Tajahmady A, Coste J, et al. Value of a national administrative database to guide public decisions: from the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65:S149–S167. doi: 10.1016/j.respe.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 62. Bouillon K, Bertrand M, Maura G, Blotière PO, Ricordeau P, Zureik M. Risk of bleeding and arterial thromboembolism in patients with non‐valvular atrial fibrillation either maintained on a vitamin K antagonist or switched to a non‐vitamin K‐antagonist oral anticoagulant: a retrospective, matched‐cohort study. Lancet Haematol. 2015;2:e150–e159. doi: 10.1016/S2352-3026(15)00027-7 [DOI] [PubMed] [Google Scholar]
- 63. Bouillon K, Bertrand M, Boudali L, Ducimetière P, Dray‐Spira R, Zureik M. Short‐term risk of bleeding during heparin bridging at initiation of vitamin K antagonist therapy in more than 90 000 Patients with nonvalvular atrial fibrillation managed in outpatient care. J Am Heart Assoc Cardiovasc Cerebrovasc Dis. 2016;5. doi: 10.1161/JAHA.116.004065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bouillon K, Bertrand M, Bader G, Lucot JP, Dray‐Spira R, Zureik M. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018;319:375–387. doi: 10.1001/jama.2017.21269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lemaitre M, Kirchgesner J, Rudnichi A, Carrat F, Zureik M, Carbonnel F, Dray‐Spira R. Association between use of Thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318:1679–1686. doi: 10.1001/jama.2017.16071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weill A, Nguyen P, Labidi M, Cadier B, Passeri T, Duranteau L, Bernat A‐L, Yoldjian I, Fontanel S, Froelich S, et al. Use of high dose cyproterone acetate and risk of intracranial meningioma in women: cohort study. BMJ. 2021;372. doi: 10.1136/bmj.n37 [DOI] [PubMed] [Google Scholar]
- 67. Zureik M, Baricault B, Vabre C, Semenzato L, Drouin J, Cuenot F, Penso L, Herlemont P, Sbidian E, Weill A, et al. Nicotine‐replacement therapy, as a surrogate of smoking, and the risk of hospitalization with Covid‐19 and all‐cause mortality: a nationwide, observational cohort study in France. MedRxiv. 2020. doi: 10.1101/2020.07.28.20160630 [DOI] [Google Scholar]
- 68. Semenzato L, Botton J, Drouin J, Baricault B, Vabre C, Cuenot F, Penso L, Herlemont P, Sbidian E, Weill A, et al. Antihypertensive drugs and COVID‐19 risk: a cohort study of 2 million hypertensive patients. Hypertens Dallas Tex. 2021;77:833–842. doi: 10.1161/HYPERTENSIONAHA.120.16314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Roland N, Drouin J, Desplas D, Cuenot F, Dray‐Spira R, Weill A, Zureik M. Effects of the Coronavirus Disease 2019 (COVID‐19) lockdown on the use of contraceptives and ovulation inductors in France. Obstet Gynecol. 2021;137:415–417. doi: 10.1097/AOG.0000000000004281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Billioti de Gage S, Drouin J, Desplas D, Cuenot F, Dray‐Spira R, Weill A, Zureik M. Intravitreal anti–vascular endothelial growth factor use in France during the Coronavirus disease 2019 pandemic. JAMA Ophthalmol. 2021;139:240–242. doi: 10.1001/jamaophthalmol.2020.5594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Meyer A, Drouin J, Zureik M, Weill A, Dray‐Spira R. Colonoscopy in France during the COVID‐19 pandemic. Int J Colorectal Dis. 2021;36:1073–1075. doi: 10.1007/s00384-020-03816-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Meyer A, Semenzato L, Zureik M, Weill A, Carbonnel F, Dray‐Spira R. Risk of severe COVID‐19 in patients treated with inflammatory bowel disease medications: a French nationwide study. Aliment Pharmacol Ther. 2021. doi: 10.1111/apt.16410 [DOI] [PubMed] [Google Scholar]
- 73. Semenzato L, Botton J, Drouin J, Cuenot F, Dray‐Spira Y, Weill A, Zureik M. Chronic diseases, health conditions and risk of COVID‐19‐related hospitalization and in‐hospital mortality during the first wave of the epidemic in France: a cohort study of 66 million people. Lancet Reg Health Eur. 2021;8:100158. doi: 10.1016/j.lanepe.2021.100158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rachas A, Gastaldi‐Menager C, Denis P, Lesuffleur T, Nicolas M, Pestel L, Mette C, Drouin J, Riviere S, Tajahmady A, et al. Prevalences and healthcare expenditures related to 58 health conditions from 2012 to 2017 in France: diseases and healthcare expenditure mapping, a national population‐based study. MedRxiv. 2020. doi: 10.1101/2020.09.21.20198853 [DOI] [Google Scholar]
- 75. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cohen J. The statistical power of abnormal‐social psychological research: a review. J Abnorm Soc Psychol. 1962;65:145–153. doi: 10.1037/h0045186 [DOI] [PubMed] [Google Scholar]
- 77. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat ‐ Simul Comput. 2009;38:1228–1234. doi: 10.1080/03610910902859574 [DOI] [Google Scholar]
- 78. Yang D, Dalton J. A unified approach to measuring the effect size between two groups using SAS 2012.
- 79. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐Value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 82. Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley MB, Albrecht R, Hernandez T, Stock A, Zhao Z, AlRasheed MR, et al. Pathophysiology of SARS‐CoV‐2: the Mount Sinai COVID‐19 autopsy experience. Mod Pathol. 2021;1–12. doi: 10.1038/s41379-021-00793-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Coronavirus disease 2019 (COVID‐19) ‐ Symptoms, diagnosis and treatment . BMJ Best Pract 2021. https://bestpractice.bmj.com/topics/en‐gb/3000201 (Accessed June 28, 2021).
- 84. Castiglione V, Chiriacò M, Emdin M, Taddei S, Vergaro G. Statin therapy in COVID‐19 infection. Eur Heart J Cardiovasc Pharmacother. 2020;6:258–259. doi: 10.1093/ehjcvp/pvaa042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xudong X, Junzhu C, Xingxiang W, Furong Z, Yanrong L. Age‐ and gender‐related difference of ACE2 expression in rat lung. Life Sci. 2006;78:2166–2171. doi: 10.1016/j.lfs.2005.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan YI, Yang P, Zhang Y, Deng W, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gordon D. Statins may be a key therapeutic for Covid‐19. Med Hypotheses. 2020;144: doi: 10.1016/j.mehy.2020.110001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Maddaloni E, D'Onofrio L, Alessandri F, Mignogna C, Leto G, Coraggio L, Sterpetti S, Pascarella G, Mezzaroma I, Lichtner M, et al. Clinical features of patients with type 2 diabetes with and without Covid‐19: a case control study (CoViDiab I). Diabetes Res Clin Pract. 2020;169: doi: 10.1016/j.diabres.2020.108454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vila‐Corcoles A, Satue‐Gracia E, Ochoa‐Gondar O, Torrente‐Fraga C, Gomez‐Bertomeu F, Vila‐Rovira A, Hospital‐Guardiola I, Diego‐Cabanes C, Bejarano‐Romero F, Rovira‐Veciana D, et al. Use of distinct anti‐hypertensive drugs and risk for COVID‐19 among hypertensive people: a population‐based cohort study in Southern Catalonia. Spain. J Clin Hypertens. 2020;22:1379–1388. doi: 10.1111/jch.13948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kibler M, Dietrich L, Kanso M, Carmona A, Marchandot B, Matsushita K, Trimaille A, How‐Choong C, Odier A, Gennesseaux G, et al. Risk and severity of COVID‐19 and ABO blood group in transcatheter aortic valve patients. J Clin Med. 2020;9:3769. doi: 10.3390/jcm9113769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dayem Ullah AZM, Sivapalan L, Kocher HM, Chelala C. COVID‐19 in patients with hepatobiliary and pancreatic diseases: a single‐centre cross‐sectional study in East London. BMJ Open. 2021;11. doi: 10.1136/bmjopen-2020-045077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hippisley‐Cox J, Young D, Coupland C, Channon KM, Tan PS, Harrison DA, Rowan K, Aveyard P, Pavord ID, Watkinson PJ. Risk of severe COVID‐19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106:1503–1511. doi: 10.1136/heartjnl-2020-317393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ho FK, Celis‐Morales CA, Gray SR, Katikireddi SV, Niedzwiedz CL, Hastie C, Ferguson LD, Berry C, Mackay DF, Gill JMR, et al. Modifiable and non‐modifiable risk factors for COVID‐19, and comparison to risk factors for influenza and pneumonia: results from a UK Biobank prospective cohort study. BMJ Open. 2020;10:e040402. doi: 10.1136/bmjopen-2020-040402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Huh K, Ji W, Kang M, Hong J, Bae GH, Lee R, Na Y, Choi H, Gong SY, Jung J. Association of previous medications with the risk of COVID‐19: a nationwide claims‐based study from South Korea. MedRxiv. 2020. doi: 10.1101/2020.05.04.20089904 [DOI] [Google Scholar]
- 96. Yan H, Valdes AM, Vijay A, Wang S, Liang L, Yang S, Wang H, Tan X, Du J, Jin S, et al. Role of drugs used for chronic disease management on susceptibility and severity of COVID‐19: a large case‐control study. Clin Pharmacol Ther. 2020;108:1185–1194. doi: 10.1002/cpt.2047 [DOI] [PubMed] [Google Scholar]
- 97. Satué‐Gracia EM, Vila‐Córcoles A, de Diego‐Cabanes C, Vila‐Rovira A, Torrente‐Fraga C, Gómez‐Bertomeu F, Hospital‐Guardiola I, Ochoa‐Gondar O, Martín‐Luján F. Susceptibility and risk of SARS‐COV‐2 infection among middle‐aged and older adults in Tarragona area, Spain. Med Clin (Barc). 2021. doi: 10.1016/j.medcli.2021.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ganjali S, Bianconi V, Penson PE, Pirro M, Banach M, Watts GF, Sahebkar A. Commentary: Statins, COVID‐19, and coronary artery disease: killing two birds with one stone. Metabolism. 2020;113:154375. doi: 10.1016/j.metabol.2020.154375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Higgins J, Li T, Deeks J. Chapter 6: choosing effect measures and computing estimates of effect. Cochrane Handb Syst Rev Interv. Version 62, Cochrane; 2021. [Google Scholar]
- 100. Hariyanto TI, Kurniawan A. Statin and outcomes of coronavirus disease 2019 (COVID‐19): a systematic review, meta‐analysis, and meta‐regression. Nutr Metab Cardiovasc Dis. 2021. doi: 10.1016/j.numecd.2021.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kollias A, Kyriakoulis KG, Kyriakoulis IG, Nitsotolis T, Poulakou G, Stergiou GS, Syrigos K. Statin use and mortality in COVID‐19 patients: updated systematic review and meta‐analysis. Atherosclerosis. 2021;330:114–121. doi: 10.1016/j.atherosclerosis.2021.06.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Diaz‐Arocutipa C, Melgar‐Talavera B, Alvarado‐Yarasca Á, Saravia‐Bartra MM, Cazorla P, Belzusarri I, Hernandez AV. Statins reduce mortality in patients with COVID‐19: an updated meta‐analysis of 147 824 patients. Int J Infect Dis. 2021;110:374–381. doi: 10.1016/j.ijid.2021.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, Ward K, Ebrahim S, Gay HC. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2021;2021. doi: 10.1002/14651858.CD004816.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S12
Figures S1–S2


