Abstract
Background
Cardiovascular diseases are the leading cause of death in the United States, yet a significant proportion of adults at high risk remain undetected by standard screening practices. Polygenic risk score for coronary artery disease (CAD‐PRS) improves precision in determining the 10‐year risk of atherosclerotic cardiovascular disease but health benefits and health care costs associated with CAD‐PRS are unknown. We examined the cost‐effectiveness of including CAD‐PRS as a risk‐enhancing factor in the pooled cohort equation (PCE)—the standard of care for determining the risk of atherosclerotic cardiovascular disease—versus PCE alone.
Methods and Results
We applied a Markov model on a cohort of 40‐year‐old individuals with borderline or intermediate 10‐year risk (5% to <20%) for atherosclerotic cardiovascular disease to identify those in the top quintile of the CAD‐PRS distribution who are at high risk and eligible for statin prevention therapy. Health outcomes examined included coronary artery disease (CAD; ie, myocardial infarction) and ischemic stroke. The model projected medical costs (2019 US$) of screening for CAD, statin prevention therapy, treatment, and monitoring patients living with CAD or ischemic stroke and quality‐adjusted life‐years for PCE+CAD‐PRS versus PCE alone. Deterministic and probabilistic sensitivity analyses and scenario analyses were performed to examine uncertainty in parameter inputs. PCE+CAD‐PRS was dominant compared with PCE alone in the 5‐ and 10‐year time horizons. We found that, respectively, PCE+CAD‐PRS had 0.003 and 0.011 higher mean quality‐adjusted life‐years and $40 and $181 lower mean costs per person screened, with 29 and 50 fewer events of CAD and ischemic stroke in a cohort of 10 000 individuals compared with PCE alone. The risk of developing CAD, the effectiveness of statin prevention therapy, and the cost of treating CAD had the largest impact on the cost per quality‐adjusted life‐year gained. However, this cost remained below the $50 000 willingness‐to‐pay threshold except when the annual risk of developing CAD was <0.006 in the 5‐year time horizon. Results from Monte Carlo simulation indicated that PCE+CAD‐PRS would be cost‐effective. with the probability of 94% and 99% at $50 000 willingness‐to‐pay threshold in the 5‐ and 10‐year time horizon, respectively.
Conclusions
Implementing CAD‐PRS as a risk‐enhancing factor in the PCE to determine the risk of atherosclerotic cardiovascular disease reduced the mean cost per individual, improved quality‐adjusted life‐years, and averted future events of CAD and ischemic stroke when compared with PCE alone.
Keywords: coronary artery disease, cost‐effectiveness, polygenic risk score
Subject Categories: Precision Medicine, Coronary Artery Disease, Cost-Effectiveness, Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- CAD‐PRS
polygenic risk score for coronary artery disease
- NMB
net monetary benefit
- PCE
pooled cohort equation
- PPP
primary prevention population
- PRS
polygenic risk score
Clinical Perspective
What Is New?
Using polygenic risk score for coronary artery disease as a risk‐enhancing factor in the pooled cohort equation to guide statin therapy intervention is both cost‐effective and cost saving among US adults, from a payer perspective.
What Are the Clinical Implications?
Using genetic tests to identify high‐risk individuals is sustainable, removing cost as a barrier to the widespread adoption of polygenic risk score in cardiovascular disease prevention.
Experts who develop guidelines and policy makers should consider integrating polygenic risk score in the pooled cohort equation to identify individuals at high risk for developing cardiovascular disease who remain invisible to the current risk assessment methods.
Atherosclerotic cardiovascular diseases (ASCVDs) are the leading cause of death in the United States and are highly preventable, but identifying all adults at high risk remains a challenge for clinicians. The pooled cohort equation (PCE) is used to determine an individual’s 10‐year risk of ASCVD, but it does not identify all individuals at high risk, leading to missed opportunities to intervene and prevent adverse health outcomes. 1 Strong evidence shows that a substantial proportion of coronary artery disease (CAD) is attributable to genetic factors, 2 which are not considered in the current PCE. The integration of such genetic risk factors into CAD primary prevention remains limited and the cost‐effectiveness is unknown.
To guide preventive therapy interventions, the PCE 10‐year risk for ASCVD stratifies individuals into 4 risk categories: low (<5%), borderline (5% to <7.5%), intermediate (≥7.5% to <20%), and high (≥20%). 3 Statin therapy is effective in preventing CAD and is recommended for individuals in the high‐risk category. 3 However, for those in the categories of borderline or intermediate risk, the presence of additional risk‐enhancing factors, which by definition increase ASCVD risk by at least 2‐fold, is needed to guide preventive therapy decisions. 3 Previous work has shown increased 30‐day all‐cause mortality and worse health outcomes in patients with ST‐segment–elevation myocardial infarction in the absence of standard clinical cardiovascular risk factors in the PCE (eg, hypercholesterolemia, diabetes, and smoking) compared with those with risk factors, 4 indicating an urgent need to improve the risk models used to determine ASCVD risk and to guide preventive therapy.
In a large‐scale multi‐ancestry US‐based study, polygenic risk score (PRS) for CAD (CAD‐PRS) has been shown to be a strong independent predictor of disease with individuals who fall in the top quintile of the CAD‐PRS distribution having an ≈2‐fold increased risk of CAD events compared with the remainder of the population (odds ratio [OR], 1.9; [95% CI, 1.8–2.0] 1 ). Comparable ORs (2.5; [95% CI, 2.4–2.6] 5 ) were reported from the UK Biobank. As such, the CAD‐PRS has been proposed as an additional risk‐enhancing factor to the PCE to improve precision in determining an individual’s risk, particularly among patients with borderline or intermediate PCE 10‐year risk (5% to <20%) 1 who require an additional risk factor to inform prevention therapy decisions. 3 The PRS was developed using large populations and clinical biobanks, and integrates the number of risk variant alleles for an individual weighted by the impact of each allele on disease risk. Applying PRS as a risk‐enhancing factor to individuals with borderline or intermediate PCE 10‐year risk identifies nearly 17% of additional individuals eligible for prevention therapy compared with only 5.6% with PCE alone based on other risk‐enhancing factors (eg, family history). 1
Establishing the cost‐effectiveness of CAD‐PRS in a clinical setting may encourage the implementation of PRS testing in standard clinical practices. The purpose of this study was to project health benefits and health care costs associated with including CAD‐PRS as a risk‐enhancing factor among individuals with borderline or intermediate risk of ASCVD derived from the PCE.
Methods
Overview
We developed a Markov model to project health care costs, health outcomes, and quality‐adjusted life‐years (QALYs) of integrating CAD‐PRS with PCE in a cohort of 40‐year‐old individuals in the United States with borderline or intermediate 10‐year risk of ASCVD, compared with PCE alone. The model had an annual cycle length with 18 health states (Figure 1) defined to reflect the initial PCE risk strata (high‐risk and nonhigh‐risk), health outcomes (CAD and ischemic stroke), statin side effects (diabetes, hemorrhagic stroke, and myopathy), and death. The analysis was performed in a 5‐ and 10‐year time horizon. Our study used published data from the literature and did not use any data that required institutional review board approval. All of the data and supporting materials are provided within the article and supplementary files.
Figure 1. Model structure.
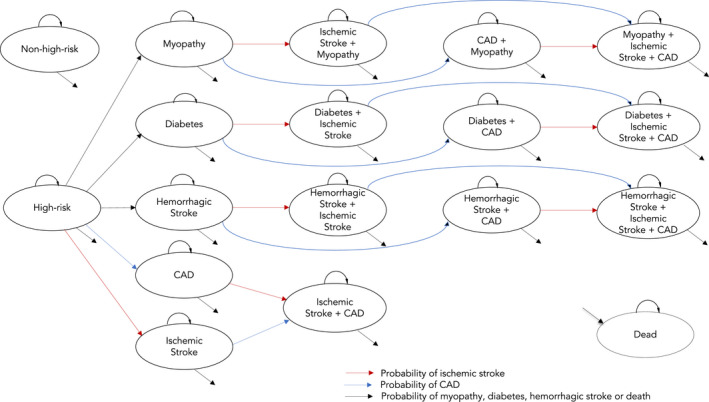
The Markov model structure used in this study is shown with a total of 18 health states. The initial cohort was distributed in 2 groups: high‐risk cohort and nonhigh‐risk cohort. We defined high risk as individuals in the top quintile of the polygenic risk score (PRS) for coronary artery disease (CAD‐PRS) distribution or having other risk‐enhancing factor (eg, family history), while the nonhigh‐risk group included individuals in the bottom 80% of the CAD‐PRS distribution without any risk‐enhancing factor. In the pooled cohort equation (PCE)+CAD‐PRS strategy, all of the high‐risk cohort was initiated on statin preventive therapy to reduce the risk of coronary artery disease (CAD) and stroke, while for the PCE‐alone strategy only a proportion of patients with other risk‐enhancing factors initiated statins. We accounted for statin side effects such as diabetes, myopathy, and hemorrhagic stroke and subsequent risk of ischemic stroke and CAD. In the PCE‐alone strategy, CAD‐PRS was not considered as a risk‐enhancing factor, so only those with other risk‐enhancing factors initiated statins. Health outcomes were not examined for the nonhigh‐risk cohort.
Strategies
We modeled 2 strategies (PCE alone and PCE+CAD‐PRS). PCE alone represented current clinical practice that uses conventional risk factors (sex, race, age, blood pressure, lipids, diabetes, and smoking status) to determine an individual’s 10‐year risk for a first ASCVD event, 3 while the PCE+CAD‐PRS strategy included the same risk factors as the PCE‐alone strategy with the addition of CAD‐PRS as a risk‐enhancing factor. In PCE+CAD‐PRS, more high‐risk individuals were identified and initiated on statin prevention therapy to prevent CAD and ischemic stroke events compared with PCE alone. We assumed that individuals in the top quintile of the CAD‐PRS distribution without any risk‐enhancing factors remained unidentified by the PCE‐alone strategy over the analytical time horizon. Since the risk of a first ASCVD event is estimated at a 10‐year period, the impact of age on the disease risk is limited within those 10 years, particularly for a younger cohort of 40 years. However, we performed a scenario analysis with an annual increase in the risk of CAD and ischemic stroke attributable to aging.
Study Population
The study population cohort consisted of individuals with borderline or intermediate PCE risk, defined as high‐risk if they were in the top quintile of the CAD‐PRS distribution, and the remainder of the cohort defined as nonhigh‐risk. Among the high‐risk proportion (0.168) of the initial cohort, 34% had other traditional risk‐enhancing factors (eg, family history) and were identified by the PCE‐alone strategy, but, with the addition of CAD‐PRS, the reminder (66%) of the high‐risk cohort without traditional risk‐enhancing factors was also identified under the PCE+CAD‐PRS strategy. Health outcomes were assessed only among the high‐risk proportion of the cohort because of data limitations on individuals with traditional risk‐enhancing factors in the nonhigh‐risk cohort. We assessed this assumption in the scenario analysis.
Model Structure
At the start of the model, the entire cohort was assumed to be disease free, with 0.168 and 0.832 proportions of the cohort in the high‐risk and nonhigh‐risk health state, respectively. Per model cycle, the proportion of the cohort in the high‐risk health state had a risk of death (age‐adjusted natural mortality), CAD, or ischemic stroke, or to remain disease free and high‐risk. Under the PCE‐alone strategy, only 34% of the cohort in the high‐risk health state were identified and initiated statin preventive therapy, while all patients (0.168) were identified under the PCE+CAD‐PRS strategy. The proportion of the high‐risk cohort who were adherent to the therapy had a reduced risk of CAD and ischemic stroke as a result of the effectiveness of statin therapy in reducing the risk of CAD and stroke but were also at risk for statin side effects (myopathy, diabetes, hemorrhagic stroke). As shown in Figure 1, fractions of the cohort with statin side effects were also at risk for CAD or/and ischemic stroke, and the risk varied by side effect. Although health outcomes were not assessed for the nonhigh‐risk proportion of the cohort, we accounted for the risk of death (age‐adjusted natural mortality), and those who did not die remained disease‐free and nonhigh‐risk.
Annual costs were applied to health states to represent the cost incurred in the respective health states, except for the death health state. The cost of screening for ASCVD was applied once at the beginning of the model, as well as the cost for a primary care visit among those with high CAD‐PRS (top quintile of the CAD‐PRS distribution). The cost of statins was applied only to those who were adherent to prevention therapy per model cycle.
Parameter inputs were derived from published sources with costs estimated from a payer perspective and inflation adjusted to 2019 US$ using the gross domestic product deflator. The relative performance of strategies was assessed using the incremental cost‐effectiveness ratio, expressed in US$ per QALY gained, and the cost‐effectiveness was determined according to the willingness‐to‐pay (WTP) threshold equivalent to $50 000. 6 Future costs and QALYs were discounted at an annual rate of 3%. Uncertainty in parameter inputs was assessed using deterministic and probabilistic sensitivity analyses. Analyses were performed at a 5‐ and 10‐year time horizon with half‐cycle correction using TreeAge Pro Software 2021 (TreeAge LLC). The CHEERS (Consolidated Health Economic Evaluation Reporting Standards) checklist was used to prepare the article (Table S2).
Model Parameters
Parameter inputs used in the model are listed in Table 1. The initial distribution of the cohort was based on a retrospective study that examined a large sample (N=47 108) of medical claims from a multi‐ancestry population in the United States. 1 Of those, 16 002 were classified as the primary prevention population (PPP) with 5890 having borderline or intermediate PCE 10‐year risk of ASCVD. Nearly 17% (987) of individuals with borderline or intermediate 10‐year risk were in the top quintile of the CAD‐PRS distribution, with 11.07% (652) not taking statin preventive therapy and therefore classified as high‐risk based on high CAD‐PRS and invisible to current clinical ASCVD risk assessment. 1 Only 5.69% (335) of individuals in the top quintile were taking statin preventive therapy. We assumed these individuals had other risk‐enhancing factors (eg, family history) and were therefore identified in both the PCE‐alone and PCE+CAD‐PRS strategy.
Table 1.
Annual Model Parameters
| Domain | Description |
Baseline (range)*/[95% CI] |
Distributions Beta ) Log normal ) Gamma () |
Source |
|---|---|---|---|---|
| Initial distribution | High risk | 0.168 (0.159–0.169) | Beta (3250.53, 16147.25) | 1 |
| Nonhigh risk | 1‐high risk | |||
| CAD † | Probability of CAD | 0.013 (0.005–0.022) | Beta (9.33, 697.44) | 3 |
| OR of CAD (high PRS) | 1.9 (1.8–2.0) | Log normal (0.64, 0.05) | 1 | |
| HR of CAD (with diabetes) | 2.00 [1.83–2.19] | Log normal (0.57, 0.22) | 7 | |
| Probability of CAD after ischemic stroke | 0.017 (0.014–0.019) | Beta (174.59, 10095.92) | 8 | |
| Ischemic stroke | Probability of ischemic stroke | 0.004 (0.003–0.005) | Beta (95.65, 23817.31) | 9 |
| Probability of ischemic stroke after CAD | x1.015 | 10 | ||
| HR of ischemic stroke with diabetes | 2.27 [1.95–2.65] | Log normal (0.37, 0.26) | 7 | |
| Probability of ischemic stroke post‐hemorrhagic stroke | 0.057 [0.048–0.068] | Beta (117.64, 1946.26) | 11 | |
| Statin effectiveness | HR of CAD risk reduction | 0.560 [0.400–0.780] | Log normal (−0.58, 0.09) | 12 |
| HR for ischemic stroke risk reduction | 0.770 [0.630–0.940] | Log normal (−0.26, 0.08) | 13 | |
| Adherence | Statin adherence | 0.500 (0.4–0.6) | Beta (47.52, 47.52) | 14, 15 |
| Statin side effects | Probability of myopathy | 0.0001 (0.0001–0.0002) | Beta (2397.88, 4793360.99) | 16 |
| Probability of diabetes | 0.0015 (0.0010–0.0020) | Beta (847.59, 112165.19) | 16 | |
| Probability of hemorrhagic stroke | 0.0002 (0.0001–0.0002) | Beta (862.67, 1149370.30) | 16 | |
| Mortality ‡ | Probability of death, acute CAD | 0.228 (0.182–0.274) | Beta (73.91, 250.27) | 17 |
| Probability of death, post‐acute CAD | 0.070 (0.067–0.072) | Beta (14100.39, 58209.33) | 18 | |
| HR (diabetes and CAD) | 1.81 [1.44–2.28] | Log normal (0.69, 0.09) | 19 | |
| Probability of death after ischemic stroke or hemorrhagic stroke and CAD | 0.075 (0.05–0.1) | Beta (88.72, 1094.73) | Assumption 20 | |
| Probability of death, acute ischemic stroke | 0.100 (0.080–0.120) | Beta (86.34, 777.02) | 21 | |
| Probability of death, post‐hemorrhagic, or post‐ischemic stroke | 0.069 (0.055–0.082) | Beta (89.39, 1215.65) | 22 | |
| RR (with diabetes and ischemic stroke) | 1.67 (1.58–1.76) | Log normal (0.80, 0.18) | 23 | |
| Probability, acute hemorrhagic stroke | 0.39 (0.33–0.45) | Beta (98.62, 154.25) | 21 | |
| HR (diabetes vs no diabetes) | 1.68 [1.52–1.87] | Log normal (0.51, 0.09) | 19 | |
| Utility weights | CAD | 0.790 (0.730–0.860) | Beta (118.38, 31.46) | 24 |
| Myopathy | 0.917 (0.896–0.938) | Beta (697.06, 54.95) | 25 | |
| Diabetes | 0.800 (0.620–0.980) | Beta (14.38, 3.59) | 26 | |
| Stroke | 0.630 (0.440–0.780) | Beta (18.89, 11.09) | 24 | |
| Disutility weights | Acute CAD | 0.041 (0.021–0.062) | Beta (14.69, 343.73) | 27 |
| Acute stroke | 0.220 [0.180–0.260] | Beta (90.42, 320.59) | 28 | |
| Age disutility | 0.004 (0.002–0.006) | Beta (15.30, 3809.93) | 29 | |
| Costs (2019, $) | PRS test § | 100 (80–120) | Gamma (96.04, 0.96) | Allelica, Inc |
| Primary care visit § | 114 (91–137) | Gamma (96.04, 0.84) | 30 | |
| Statin therapy | 132 (106–158) | Gamma (96.04, 0.73) | 31 | |
| Background health care costs | 4 941 (3953–5930) | Gamma (96.04, 0.02) | 32 | |
| Acute § | ||||
| Nonfatal CAD | 65 442 (43 818–100 531) | Gamma (20.46, 0.0003) | 33 | |
| Fatal CAD | 18 246 (14 597–21 896) | Gamma (96.04, 0.0053) | 34 | |
| Nonfatal ischemic stroke | 40 225 (11 539–100 184) | Gamma (3.16, 0.0001) | 33 | |
| Fatal ischemic stroke | 11 256 (9005–13 507) | Gamma (96.04, 0.0085) | 34 | |
| Nonfatal hemorrhagic stroke | 38 246 (30 596–45 895) | Gamma (96.04, 0.0025) | 35 | |
| Fatal hemorrhagic stroke | 18 246 (14 597–21 896) | Gamma (96.04, 0.0053) | 34 | |
| Follow‐up | ||||
| CAD | 11 815 (7865–16 186) | Gamma (30.99, 0.003) | 36 | |
| Stroke (hemorrhagic/ischemic) | 20 005 (16 004–24 006) | Gamma (96.04, 0.0048) | 37 | |
| Myopathy | 20 438 (16 351–24 536) | Gamma (96.04, 0.0047) | 38 | |
| Diabetes | 10 026 (8021–12 031) | Gamma (96.04, 0.0096) | 39 | |
HR indicates hazard ratio; OR, odds ratio; CAD, coronary artery disease; PRS polygenic risk score; and RR, relative risk.
Range (+/−20% of the baseline value). The range and 95% CI were used in sensitivity analysis.
Statin‐induced myopathy did not change the risk of coronary artery disease (CAD) and stroke or mortality. 40 , 41 , 42 , 43 Further, the risk of CAD did not change after hemorrhagic stroke. 11
Mortality after stroke and CAD is significantly higher compared with only CAD or stroke. In a Medicare study population, >50% of patients with stroke and CAD died in the first year. 20 Because of data limitations and our younger study population, we assumed 75% (50% to 100%) of patients will die in 10 years. We also assumed a higher mortality rate for stroke among individuals with diabetes compared with those without diabetes based on a non‐US study. Although no study has been performed in the US population, studies including a meta‐analysis showed increased mortality among patients with stroke who had diabetes. 23 , 44 , 45
Annual cost applied in the first year of the event.
The risk of developing CAD was calculated as the average 10‐year risk of ASCVD for individuals with borderline or intermediate risk, 3 with a 1.9‐fold increase in risk for being in the top quintile of the CAD‐PRS distribution. 1 The risk of ischemic stroke was assumed to be equal to that of the general population since stroke was not considered an outcome in the original study by Aragam et al in 2020, 1 which we used to identify individuals in the top quintile of the CAD‐PRS distribution. According the Centers for Disease Control and Prevention, the risk of stroke doubles every decade after the age of 55 years. 46 However, since our baseline cohort was aged 40 years and within the 10‐year time horizon, the cohort age will be 50 years, the risk of ischemic stroke was assumed to be constant in both the 5‐ and 10‐year time horizons.
We applied risk reduction on developing CAD 12 and ischemic stroke 13 among individuals taking statin preventive therapy in the top quintile of the CAD‐PRS distribution. We assumed 50% of the cohort consistently used statins. Although adherence to statins in primary prevention tends to be low (<50%) and decreases over time, 14 there is evidence of higher adherence to therapy among adults in the United States 15 and individuals who are aware of their high PRS. 47 Those adherent to statins had a risk of developing side effects including myopathy, diabetes, and hemorrhagic stroke. 16 The risk of ischemic stroke and CAD was higher among individuals with diabetes, 7 but only for ischemic stroke among those with hemorrhagic stroke, 11 and there was no increased risk for those with myopathy.
Mortality varied based on the health state. Data for risk of death in the event‐free cohort came from the social security life tables (Table S1). Acute stages of the disease (CAD, 17 , 18 ischemic stroke and hemorrhagic stroke 21 , 22 ) had higher mortality rates compared to chronic stages. Also, mortality was higher among individuals with diabetes, 19 diabetes and CAD, 19 and diabetes and ischemic stroke 44 compared to those without diabetes.
Costs
We considered only medical costs incurred by the payer (Table 1). Costs included PRS testing, statin therapy, in‐patient hospitalization for fatal and nonfatal acute events, and follow‐up costs after hospital discharge. The cost of genetic testing in the United States has decreased significantly over the years and, the cost varies based on the type of test performed. The one‐time cost for PRS testing was based on current prices of genotyping arrays and the required bioinformatic analysis (Source: Allelica, Inc). An additional primary care visit cost was included for the cohort with high CAD‐PRS for further genetic counseling. 30 The cost of statin therapy came from online pharmacy prices. 31 We derived costs for treating acute CAD and ischemic stroke from a systematic review of costs associated with major cardiovascular conditions. 33 The cost of acute fatal events included hospitalization and procedures performed for patients who did not survive the acute stage. 34 The cost of recurrent events was calculated as the product of the risk of occurrence and cost of treatment. We included background health care costs per patient‐year to account for health care expenditure per capita for a privately insured population. 32
Health State Utility Values
Utility weights were derived from the literature and assigned to health states to represent the severity of the disease (0=death, 1=perfect health). We assumed fractions of the cohort without any events had perfect health but experienced a disutility attributable to aging. 29 The utility weights for CAD and stroke health states came from a systematic review that examined the utility values for cardiovascular diseases. 24 We applied disutility for acute CAD 27 and stroke 28 events, and utility weights for statin side effects including diabetes 26 and myopathy. 25
Sensitivity Analysis
We varied parameter inputs over a range of plausible values to identify the main drivers of variation in the incremental net monetary benefit (NMB). Cost variables included PRS testing, statin therapy, and treatment for CAD, stroke, and statin side effects; and transition probabilities: percentage of the cohort adhering to statins, statin effectiveness in reducing CAD and stroke events, risk of death and developing CAD, ischemic stroke, or statin side effects. For probabilistic sensitivity analysis, we assumed beta, lognormal, and gamma distributions for probabilities, relative risk, hazard and ORs, and cost variables, respectively. The beta distribution bounds the probabilities between 0 and 1, and the gamma distribution restricts costs to >0. We performed 10 000 Monte Carlo simulations, and results are reported using cost‐effectiveness planes and cost‐effectiveness acceptability curves.
We assessed the value of getting additional information in parameter uncertainty using the population expected value of perfect information (EVPI) approach, which estimates the value of eliminating uncertainty in the model by assuming perfect information. This analysis informs decision makers whether they need to invest more resources to gain additional information. Using 10 000 Monte Carlo simulations, in each iteration, an NMB was calculated with the strategy that maximizes the NMB at a given WTP threshold being preferred to the alternative. EVPI was calculated as the difference between the NMB of the optimal strategy under current uncertainty and the maximum NMB possible per iteration. When the EVPI is equal to zero, this implies that the decision does not change regardless of the new additional information. In this study, the target population was adults between the ages of 40 and 75 years (recommended age for ASCVD screening) without ASCVD, diabetes, or severe hypercholesterolemia (low‐density lipoprotein >190 mg/dL) and categorized as at borderline or intermediate risk. To define this population, we estimated that 42% of the current US population fall between the ages of 40 and 75 years based on 2019 census data. 50 We excluded 21% of adults aged 40 to 75 years (13% prevalence of diabetes 48 and 8% prevalence of ASCVD 49 among adults in the United States) to get the PPP. We assumed 37% of the PPP had borderline or intermediate PCE risk based on Aragam et al. 1 We applied a 0.25% annual growth rate in adults aged 40 to 75 years 50 (Figure S1).
Scenario Analysis
Four scenario analyses were performed: (1) we assessed outcomes at different cohort start ages after accounting for the impact of aging on the risk of CAD and ischemic stroke. We applied a 3.5% annual increase in the baseline risk of CAD attributable to aging, 51 and the risk of ischemic stroke increased linearly and doubled every decade after age 55. 46 Results were reported for 5‐year, 10‐year, and lifetime time horizons. (2) We considered individuals in the bottom 80% of the CAD‐PRS distribution (nonhigh‐risk) who were eligible for statin prevention therapy since in the base case analysis we only considered high‐risk individuals in the top quintile. In the study by Aragam et al, 46.8% of individuals in the bottom 80% of the CAD‐PRS distribution were eligible for statin prevention therapy based on the American Heart Association statin eligibility guidelines, but only 23.8% received statin prescription. These estimates were only available for the overall sample but not for individuals with borderline or intermediate risk, so we assumed the same percentages applied to all PCE categories including borderline or intermediate. 1 (3) We applied PRS testing only to individuals without any other risk‐enhancing factors since the base case analysis tested the whole population of individuals with borderline or intermediate risk to be consistent with the Aragam et al study. 1 By first applying PCE alone and identifying high‐risk individuals with other risk‐enhancing factors, PRS was applied to fewer targeted individuals and was thus more efficient. (4) We assessed a scenario where PRS has improved predictive performance, being able to identify more individuals in the top quintile of the CAD‐PRS distribution with increased risk of developing CAD (expert communication from Allelica, Inc).
Model Validation
We validated the model using an external validation approach by corroborating the life expectancy for the event‐free cohort in the model to that of the general population in the United States, and the life expectancy for the cohort with CAD with estimates from the literature. Since no study has examined the cost‐effectiveness of PCE+CAD‐PRS versus PCE alone among populations at intermediate/borderline risk, primary outcomes (ie, events of CAD and ischemic stroke) were unavailable in the literature to validate the model. Model‐projected years of survival were comparable to the life expectancy of Americans in the general population 52 (Figure 2) and after acute CAD 53 , 54 (Figure 3).
Figure 2. Life expectancy in the US general population compared with the event‐free cohort in the model.
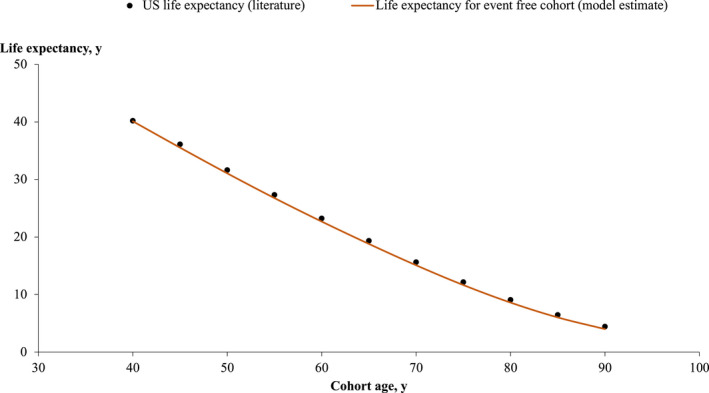
Projected life expectancy of the event‐free cohort in the model with the mean ages of 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, and 90 years. The projected years of survival were comparable to the life expectancy of Americans with the same age. 52
Figure 3. Life expectancy postmyocardial infarction.
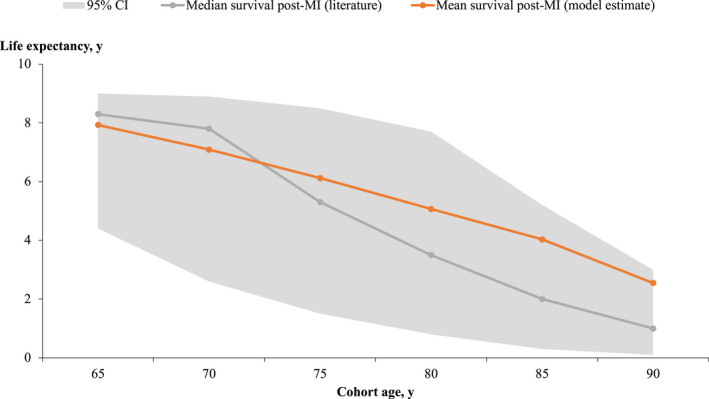
Life expectancy of the cohort after acute myocardial infarction (MI) estimated from the model compared with data from the literature. 53 , 54 We found the life expectancy generated by the model to be within 95% CIs of the life‐expectancy values from the literature.
Results
Base Case Analysis
Results for the base case scenario are reported in Table 2. PCE+CAD‐PRS had 0.003 and 0.011 more QALYs gained and $40 and $181 lower mean costs per person screened in the 5‐ and 10‐year time horizon, respectively, compared with PCE alone. The breakdown of total and incremental costs is provided in Table S3. In a cohort of 10 000 individuals with borderline or intermediate PCE 10‐year risk of ASCVD and not taking statin therapy, PCE+CAD‐PRS would prevent ≈29 and 50 events of CAD and ischemic stroke, with an average cost savings of $13 000 and $36 000 per event averted in 5‐ and 10‐year time horizon, respectively (Table 3).
Table 2.
Results for the Base Case Analysis (Cost: 2019 US$)
| Time Horizon | Strategy | Cost* | Incremental cost | QALYs* | QALYs gained | ICER |
|---|---|---|---|---|---|---|
| 5 y | PCE alone | 28 932 | 4.556 | |||
| (23 598–34 586) | (4.485–4.624) | |||||
| PCE+CAD‐PRS | 28 892 | (40) | 4.559 | 0.003 | Dominant | |
| (23 549–34 532) | (4.488–4.627) | |||||
| 10 y | PCE alone | 49 681 | 8.313 | |||
| (40 590–59 822) | (8.06–8.56) | |||||
| PCE+CAD‐PRS | 49 500 | (181) | 8.323 | 0.011 | Dominant | |
| (40 479–59 610) | (8.08–8.57) |
Base case cost‐effectiveness analysis results for 5‐ and 10‐year time horizons in a cohort of 40‐year‐old Americans with borderline or intermediate risk of atherosclerotic cardiovascular disease. Compared with pooled cohort equation (PCE) alone, PCE+polygenic risk score for coronary artery disease (CAD‐PRS) had higher mean quality‐adjusted life‐years (QALYs; 0.003–0.011) and lower mean costs ($40, $181) per person screened in 5‐ and 10‐year time horizons, respectively. CAD indicates coronary artery disease; and ICER, incremental cost‐effectiveness ratio.
Intervals represent 2.5th to 97.5th percentiles.
Table 3.
Events of CAD and Ischemic Stroke per 10 000 Individuals Screened
| Time Horizon | Strategy | CAD | Ischemic Stroke | Events Averted | Cost saved per event averted |
|---|---|---|---|---|---|
| 5 y | PCE alone | 184 | 31 | ||
| PCE+CAD‐PRS | 157 | 29 | 29 | 13 000 | |
| 10 y | PCE alone | 344 | 60 | ||
| PCE+CAD‐PRS | 297 | 57 | 50 | 36 000 |
Base case analysis events of coronary artery disease (CAD) and ischemic stroke when the model was applied in 10 000 individuals with borderline or intermediate risk. Pooled cohort equation (PCE)+polygenic risk score for coronary artery disease (CAD‐PRS) had ≈29 and 50 fewer events of CAD and ischemic stroke compared with PCE alone, resulting in a cost savings of $13 000 and $36 000 per event averted in 5‐ and 10‐year time horizons, respectively.
One‐Way Sensitivity Analysis
Results for 1‐way sensitivity analysis are shown in Figures 4 and 5. PCE+CAD‐PRS had an incremental NMB of $186 and $711 in the 5‐ and 10‐year time horizon, respectively. The risk of developing CAD, the effectiveness of statin prevention therapy, and the cost of treating CAD had the largest impact on the cost per QALY gained, but PCE+CAD‐PRS remained cost‐effective (incremental cost‐effectiveness ratio <$50 000 WTP threshold) across all parameters’ uncertainty in both time horizons except when the annual risk of developing CAD was <0.006 in the 5‐year time horizon.
Figure 4. The 5‐year incremental net monetary benefit of pooled cohort equation (PCE) alone vs PCE+polygenic risk score for coronary artery disease (CAD‐PRS), with PCE+CAD‐PRS preferred across all parameter value variations except when the annual risk of developing CAD was <0.006.
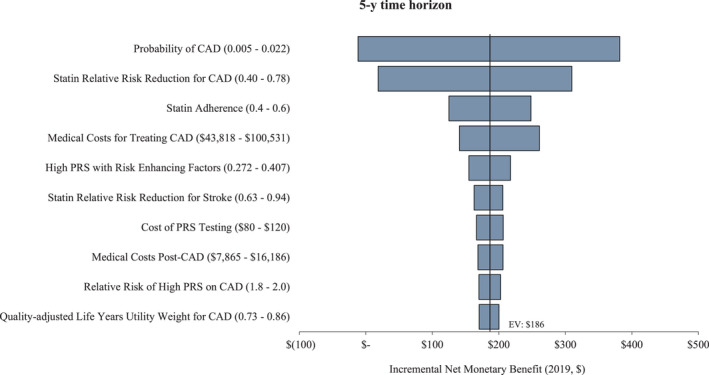
CAD indicates coronary artery disease; EV, expected value; and PRS, polygenic risk score.
Figure 5. The 10‐year incremental net monetary benefit of pooled cohort equation (PCE) alone vs PCE+polygenic risk score for coronary artery disease (CAD‐PRS), with PCE+CAD‐PRS preferred across all parameter value variations.
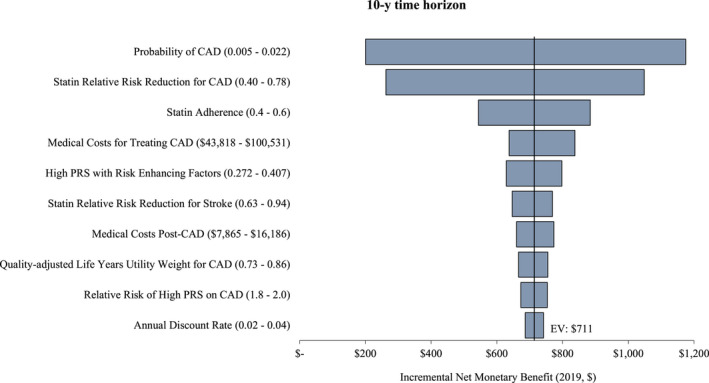
Figures 4 and 5 show the incremental net monetary benefit of pooled cohort equation (PCE) alone vs PCE+polygenic risk score for coronary artery disease (CAD‐PRS), with PCE+CAD‐PRS preferred across all parameter value variations except when the annual risk of developing CAD was <0.006 in the 5‐year time horizon.
Probabilistic Sensitivity and Value of Information Analysis
Results from Monte Carlo simulation indicated a significant proportion of the joint distribution of incremental effectiveness (QALY gained) and incremental costs on the cost‐effectiveness plane fell below the WTP threshold of $50 000 and in the southeast quadrant, indicating that PCE+CAD‐PRS was more likely to be effective and cost saving compared with PCE alone (Figure S2). The cost‐effectiveness acceptability curves (Figure 6) show that PCE+CAD‐PRS would be cost‐effective with a probability of 94% and 99% at $50 000 WTP threshold and 98% and 99% at $100 000 WTP threshold in the 5‐ and 10‐year time horizons, respectively. The value of information analysis showed an individual EVPI of $1.56 and $0.09 at $50 000 WTP threshold in the 5‐ and 10‐year time horizons. Figure 7 shows population EVPI values over a range of WTP thresholds. As shown in Figure 6, the probability of cost‐effectiveness increased with the increase in WTP, which implies increased decision certainty and decreased EVPI over the WTP thresholds.
Figure 6. Cost‐effectiveness acceptability curves.
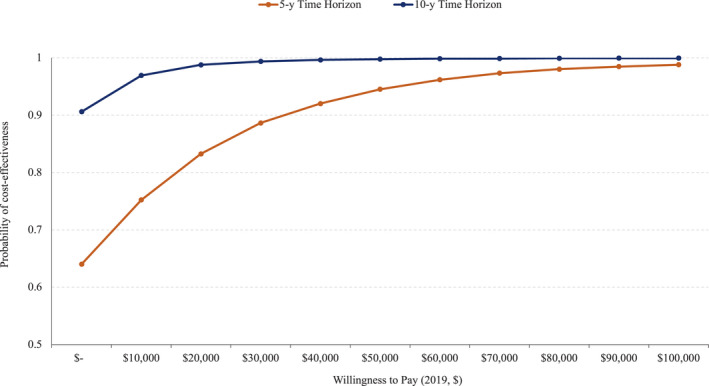
Results from the probabilistic sensitivity analysis indicating the probability of the pooled cohort equation (PCE)+polygenic risk score for coronary artery disease (CAD‐PRS) being cost‐effective at different willingness‐to‐pay (WTP) thresholds. Compared with PCE alone, PCE+CAD‐PRS is likely to be cost‐effective with a probability of 94% and 99% at $50 000 WTP threshold and 98% and 99% at $100 000 WTP threshold in 5‐ and 10‐year time horizons, respectively.
Figure 7. Expected value of perfect information (EVPI) at different willingness‐to‐pay (WTP) thresholds.
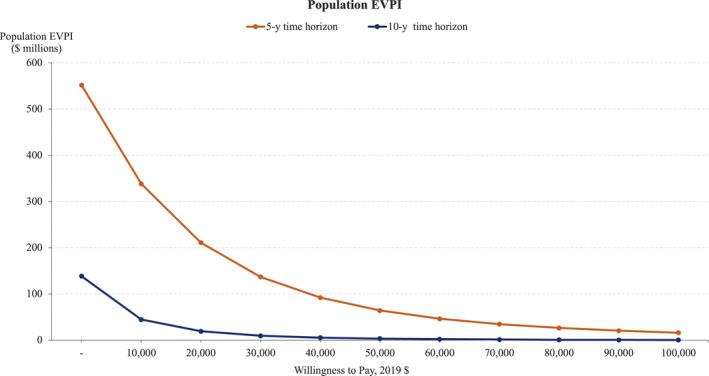
Population EVPI of ≈$64.3 M and $3.53 M at a $50 000 WTP threshold in the 5‐ and 10‐year time horizons. EVPI decreased with increase in WTP thresholds suggesting high certainty in the cost‐effectiveness analysis results.
Scenario Analysis
PCE+CAD‐PRS remained a dominant strategy compared with PCE alone after accounting for the impact of age on the risk of CAD and ischemic stroke. In a cohort of patients with a mean age of 40 years and a 3.5% annual increase in the risk of CAD, we found PCE+CAD‐PRS to be cost saving ($50.11, $217.79, and $38.75) in all time horizons (5‐year, 10‐year, and lifetime), respectively (Table S4). However, cost savings were lower in the lifetime time horizon since more individuals are identified by PCE alone in the long run, and, with longer survival in the PCE+CAD‐PRS strategy, the increased health care expenditure reduces the cost advantage of CAD‐PRS. In addition, considering the same cohort, at 60 years, PRS would no longer be effective but cost more since all high‐risk individuals would be identified by PCE alone. The findings were consistent with the base case analysis when we included high‐risk individuals in the bottom 80% of the CAD‐PRS distribution in the model (Table S6). PCE+CAD‐PRS was more cost saving compared with the base case ($90 versus $40 and $230 versus $181 for the 5‐ and 10‐year time horizon, respectively) when the cost of PRS testing was applied only to individuals without any PCE risk‐enhancing factors (Table S7). Finally, with more high‐risk individuals identified and the risk of developing CAD increased up to 3‐fold, PCE+CAD‐PRS was more cost saving and cost‐effective compared with PCE alone in a 5‐ and 10‐year time horizon, respectively (Table S8 and Figure S3). Compared with the base case analysis, PCE+CAD‐PRS was cost saving up to >$250 and $550 and improved QALYs by up to >0.007 and 0.023 per person screened in the 5‐ and 10‐year time horizon, respectively.
Discussion
We developed a Markov model to examine health benefits and health care costs associated with integrating CAD‐PRS as a risk‐enhancing factor in PCE to identify high‐risk individuals who are undetected by current clinical practice—PCE alone—and are eligible for statin prevention therapy. We found that PCE+CAD‐PRS was cost‐effective (incremental cost‐effectiveness ratio <$50 000 WTP threshold) and cost saving with higher mean QALYs and lower mean costs per person screened compared with PCE alone in all time horizons. The risk of developing CAD, the effectiveness of statin prevention therapy, and the cost of treating CAD had the largest impact on the cost per QALY gained, which is consistent with previous studies. 55 , 56
This study underscores both health and economic benefits of integrating CAD‐PRS into risk assessments for ASCVD. More than 29 and 50 CAD and ischemic stroke events were averted by PCE+CAD‐PRS compared with PCE alone per 10 000 individuals screened in 5‐ and 10‐year time horizons, respectively. As a result, an average of $13 000 to $36 000 per event averted could be saved between 5‐ to 10‐year time horizons, indicating that the longer the outlook, the more beneficial implementing PRS becomes. Furthermore, from a societal perspective, PCE+CAD‐PRS may be even more cost saving when loss of productivity from CAD or stroke events is also considered. 57
Findings were largely robust to parameter uncertainty, particularly in the longer time horizon of 10 years. The annual risk of developing CAD had the largest impact on the incremental cost‐effectiveness ratio in the 5‐year time horizon when the risk was <0.6%, which is substantially below the mean risk (1.32%) for the population with borderline and intermediate risk. However, since the larger proportion of the study population was in the group with intermediate risk, 1 this scenario is less likely to occur. In the Monte Carlo simulations, the probability of cost‐effectiveness was >94%, indicating that PCE+CAD‐PRS is highly cost‐effective compared with PCE alone. Further, the value of information analysis showed that the EVPI decreased with the increase in the WTP threshold suggesting that future research in parameter uncertainty may not be a good value to invest resources. However, since data were collected from the literature and multiple studies, it was not possible to account for potential correlation between parameter inputs, which could have an impact on the EVPI.
CAD‐PRS is cost‐effective for all ages including young adults given the limitations of traditional risk factors in identifying the risk of ASCVD at a younger age. Our results indicate higher QALYs gained when PCE+CAD‐PRS was implemented in a younger cohort (ie, aged 40 years) and followed over a lifetime, primarily attributable to the long‐term benefits from prevention of adverse health outcomes over their lifetime (Table S5). Previous work has found that individuals who receive their genetic results are more likely to report positive behavior changes especially in nutiriton. 58 Therefore, implementing CAD‐PRS in young adults could have the corollary of improving quality of life in the long run through lifestyle changes.
While our findings are broadly comparable with previous work, they also provide important novel insights on the efficiency of precision medicine, particularly in primary prevention for CAD. In one study, genetic testing was more beneficial when targeting individuals in whom traditional risk factors do not provide an accurate risk assessment. 55 Combining traditional risk factors with genetic testing for a segment of individuals with 17% to 22% 10‐year risk reduced the average cost of treating cardiovascular diseases per individual in a population of 100 000 adults by $3.04 in a 10‐year follow‐up compared with using traditional risk factors on their own. 55 In our study, PCE+CAD‐PRS saved >$181 with 0.011 QALYs gained per individual screened, compared with PCE alone after 10 years. This translated to an average of $67 saved per 40‐year‐old individual in the PPP. Our study identified a higher proportion of high‐risk individuals because genetic testing was performed in a larger percentage of patients (36%)—those with 5% to <20% 10‐year risk—of the PPP compared with the optimization approach used by Hynninen et al (2019) where genetic testing was performed in only 3% of the population—those with 17% to <22% 10‐year risk based on traditional risk factors—and excluding those with <10% 10‐year risk from any preventive therapy. 55 In a recent US‐based study, PRS testing on individuals with low to borderline (2.5% to 7.5%) 10‐year risk was found to not be cost‐effective in informing statin therapy decisions. 56 However, these authors only considered individuals with low and borderline 10‐year risk and applied genetic testing to classify high‐risk individuals for preventive care, whereas guidelines recommend that additional risk‐enhancing factors should be used in individuals at borderline or intermediate 10‐year risk (5% to <20% 10‐year risk). 3 Accordingly, in this study, we implemented CAD‐PRS as a risk‐enhancing factor in the PCE in individuals with borderline/intermediate risk and demonstrate improved health and economic outcomes when focusing on this subgroup.
Limitations
Our study has several limitations. First, alternative preventive therapies were not considered and individuals who experienced statin therapy side effects were assumed to not be taking any preventive therapy. Although alternative prevention strategies including exercising and plant‐based diet may reduce the risk of cardiovascular disease, their long‐term effectiveness is difficult to measure in a real‐world setting because of challenges with adherence, access, and affordability of a healthy diet for the majority of the people in the United States. Preventive therapies such as proprotein convertase subtilisin/kexin type 9 may mitigate the risk of CAD but statin therapy remains the recommended first‐line preventive care for cardiovascular diseases in the United States. 3 Second, we assumed that high‐risk individuals remained unidentified under the PCE‐alone strategy throughout the analytic time horizon and did not initiate any preventive therapy, although age is a strong risk factor for CAD and may inform prevention decisions in the future. However, this assumption did not substantially impact the results since the risk of CAD is usually estimated at 10 years and the risk of stroke is less likely to change before age 55. 46 Third, because of data limitations, our study cohort combined individuals with borderline and intermediate risk although guidelines recommend moderate‐intensity statin therapy for those with borderline risk and an additional risk‐enhancing factor and high‐intensity statin therapy for those with intermediate risk and additional risk‐enhancing factor. 3 However, our findings are conservative since we assumed moderate‐intensity statin therapy. Fourth, we assumed that patient behavior did not change over the analytical time horizon, while there is some evidence that genetic testing is associated with positive changes in patient behavior. 58 Finally, we did not account for correlation between model parameters, which may have an impact on the findings.
Conclusions
To inform preventive care decisions for cardiovascular diseases in clinical settings in the United States, we developed a Markov model to examine health benefits and health care costs associated with implementing CAD‐PRS as a risk‐enhancing factor in the PCE among adults with borderline or intermediate PCE 10‐year risk of ASCVD. We found PCE+CAD‐PRS to be a highly efficient use of health care resources, with lower costs, higher QALYs, and future events of CAD and ischemic stroke averted when compared with PCE alone. This study supports the growing evidence on the value of PRS in chronic disease prevention.
Sources of Funding
This work was supported by private funding from Allelica, Inc.
Disclosures
Dr Mujwara, P. Di Domenico, Dr Busby, and Dr Bottà are employees of Allelica, Inc. G. Henno is an employee of Pacific Biosciences of California, Inc., and a former employee of Illumina Inc. S. Peng and Dr Schroeder are employees of Illumina, Inc.
Supporting information
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.025236
For Sources of Funding and Disclosures, see page 13.
References
- 1. Aragam KG, Dobbyn A, Judy R, Chaffin M, Chaudhary K, Hindy G, Cagan A, Finneran P, Weng LC, Loos RJ, et al. Limitations of contemporary guidelines for managing patients at high genetic risk of coronary artery disease. J Am Coll Cardiol. 2020;75:2769–2780. doi: 10.1016/j.jacc.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bolli A, Di Domenico P, Pastorino R, Busby GB, Bottà G. Risk of coronary artery disease conferred by low‐density lipoprotein cholesterol depends on polygenic background. Circulation. 2021;143:1452–1454. doi: 10.1161/circulationaha.120.051843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1016/j.jacc.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Figtree GA, Vernon ST, Hadziosmanovic N, Sundström J, Alfredsson J, Arnott C, Delatour V, Leósdóttir M, Hagström E. Mortality in STEMI patients without standard modifiable risk factors: a sex‐disaggregated analysis of SWEDEHEART registry data. Lancet. 2021;397:1085–1094. doi: 10.1016/S0140-6736(21)00272-5 [DOI] [PubMed] [Google Scholar]
- 5. Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, Natarajan P, Lander ES, Lubitz SA, Ellinor PT, et al. Genome‐wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50:1219–1224. doi: 10.1038/s41588-018-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost‐Effectivness in Health and Medicine. 2nd ed. Oxford, UK: Oxford University Press; 2017. [Google Scholar]
- 7. Sarwar N, Gao P, Kondapally Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boulanger M, Béjot Y, Rothwell PM, Touzé E. Long‐term risk of myocardial infarction compared to recurrent stroke after transient ischemic attack and ischemic stroke: systematic review and meta‐analysis. J Am Heart Assoc. 2018;7. doi: 10.1161/JAHA.117.007267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stroke Facts|cdc.gov . Accessed August 27, 2021. https://www.cdc.gov/stroke/facts.htm
- 10. Loh E, Sutton MS, Wun CC, Rouleau JL, Flaker GC, Gottlieb SS, Lamas GA, Moyé LA, Goldhaber SZ, Pfeffer MA. Ventricular dysfunction and the risk of stroke after myocardial infarction. N Engl J Med. 1997;336:251–257. doi: 10.1056/NEJM199701233360403 [DOI] [PubMed] [Google Scholar]
- 11. Murthy SB, Diaz I, Wu X, Merkler AE, Iadecola C, Safford MM, Sheth KN, Navi BB, Kamel H. Risk of arterial ischemic events after intracerebral hemorrhage. Stroke. 2020;51:137–142. doi: 10.1161/STROKEAHA.119.026207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Natarajan P, Young R, Stitziel NO, Padmanabhan S, Baber U, Mehran R, Sartori S, Fuster V, Reilly DF, Butterworth A, et al. Polygenic risk score identifies subgroup with higher burden of atherosclerosis and greater relative benefit from statin therapy in the primary prevention setting. Circulation. 2017;135:2091–2101. doi: 10.1161/CIRCULATIONAHA.116.024436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Byington RP, Davis BR, Plehn JF, White HD, Baker J, Cobbe SM, Shepherd J. Reduction of stroke events with pravastatin: the Prospective Pravastin Pooling (PPP) project. Circulation. 2001;103:387–392. doi: 10.1161/01.CIR.103.3.387 [DOI] [PubMed] [Google Scholar]
- 14. Maningat P, Gordon BR, Breslow JL. How do we improve patient compliance and adherence to long‐term statin therapy? Curr Atheroscler Rep. 2013;15:1–12. doi: 10.1007/s11883-012-0291-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Colantonio LD, Rosenson RS, Deng L, Monda KL, Dai Y, Farkouh ME, Safford MM, Philip K, Mues KE, Muntner P. Adherence to statin therapy among US adults between 2007 and 2014. J Am Heart Assoc. 2019;8:1–20. doi: 10.1161/JAHA.118.010376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561. doi: 10.1016/S0140-6736(16)31357-5 [DOI] [PubMed] [Google Scholar]
- 17. Caughey MC, Arora S, Qamar A, Chunawala Z, Gupta MD, Gupta P, Vaduganathan M, Pandey A, Dai X, Smith SC, et al. Trends, management, and outcomes of acute myocardial infarction hospitalizations with in‐hospital‐onset versus out‐of‐hospital onset: the ARIC study. J Am Heart Assoc. 2021;10:1–12. doi: 10.1161/JAHA.120.018414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rapsomaniki E, Thuresson M, Yang E, Blin P, Hunt P, Chung SC, Stogiannis D, Pujades‐rodriguez M, Timmis A, Denaxas SC et al. Using big data from health records from four countries to evaluate chronic disease outcomes : a study in 114,364 survivors of myocardial infarction. Eur Heart J. Published online 2016:172–183. doi: 10.1093/ehjqcco/qcw004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li S, Wang J, Zhang B, Li X, Liu Y. Diabetes mellitus and cause‐specific mortality: a population‐based study. Diabetol Metab J. 2019;43:319–341. doi: 10.4093/dmj.2018.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merkler AE, Diaz I, Wu X, Murthy SB, Gialdini G, Navi BB, Yaghi S, Weinsaft JW, Okin PM, Safford MM, et al. Duration of heightened ischemic stroke risk after acute myocardial infarction. J Am Heart Assoc. 2018;7:1–6. doi: 10.1161/JAHA.118.010782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ovbiagele B, Nguyen‐Huynh MN. Stroke epidemiology: advancing our understanding of disease mechanism and therapy. Neurotherapeutics. 2011;8:319–329. doi: 10.1007/s13311-011-0053-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koton S, Schneider AL, Rosamond WD, Shahar E, Sang Y, Gottesman RF, Coresh J, IMPORTANCE . Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312:259–268. doi: 10.1001/jama.2014.7692. [DOI] [PubMed] [Google Scholar]
- 23. Eriksson M, Carlberg B, Eliasson M. The disparity in long‐term survival after a first stroke in patients with and without diabetes persists: the Northern Sweden MONICA study. Cerebrovasc Dis. 2012;34:153–160. doi: 10.1159/000339763 [DOI] [PubMed] [Google Scholar]
- 24. Betts MB, Rane P, Bergrath E, Chitnis M, Bhutani MK, Gulea C, Qian Y, Villa G. Utility value estimates in cardiovascular disease and the effect of changing elicitation methods: a systematic literature review. Health Qual Life Outcomes. 2020;18:1–12. doi: 10.1186/s12955-020-01407-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitchell D, Guertin JR, Iliza AC, Fanton‐aita F, Lelorier J. Economic Evaluation of a pharmacogenomics test for statin‐ induced myopathy in cardiovascular high‐risk patients initiating a statin. Mol Diagn Ther. 2017;21:95–105. doi: 10.1007/s40291-016-0238-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang P, Brown MB, Bilik D, Ackermann RT, Li R, Herman WH. Health utility scores for people with type 2 diabetes in U.S. managed care health plans. Diabetes Care. 2012;35:2250–2256. doi: 10.2337/dc11-2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sullivan PW, Ghushchyan V. Preference‐based EQ‐5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410–420. doi: 10.1177/0272989X06290495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luengo‐Fernandez R, Gray AM, Bull L, Welch S, Cuthbertson F, Rothwell PM. Quality of life after TIA and stroke: ten‐year results of the oxford vascular study. Neurology. 2013;81:1588–1595. doi: 10.1212/WNL.0b013e3182a9f45f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ara R, Brazier JE. Populating an economic model with health state utility values: Moving toward better practice. Value in Health. 2010;13:509–518. doi: 10.1111/j.1524-4733.2010.00700.x [DOI] [PubMed] [Google Scholar]
- 30. Machlin SR, Mitchell EM. Expenses for Office‐Based Physician Visits by Specialty and Insurance Type. Medical Expenditure Panel Survey. Published 2016. Accessed April 21, 2021. https://meps.ahrq.gov/data_files/publications/st517/stat517.shtml [Google Scholar]
- 31. Simvastatin Prices, Coupons & Patient Assistance Programs ‐ Drugs.com . Accessed May 27, 2021. https://www.drugs.com/price‐guide/simvastatin
- 32. Lassman D, Sisko AM, Catlin A, Barron MC, Benson J, Cuckler GA, Hartman M, Martin AB, Whittle L. Health spending by state 1991–2014: measuring per capita spending by payers and programs. Health Aff. 2017;36:1318–1327. doi: 10.1377/HLTHAFF.2017.0416 [DOI] [PubMed] [Google Scholar]
- 33. Nicholson G, Gandra SR, Halbert RJ, Richhariya A, Nordyke RJ. Patient‐level costs of major cardiovascular conditions: a review of the international literature. ClinicoEconomics and Outcomes Res. 2016;6:495–506. doi: 10.2147/CEOR.S89331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O’Sullivan AK, Rubin J, Nyambose J, Kuznik A, Cohen DJ, Thompson D. Cost estimation of cardiovascular disease events in the US. Pharmacoeconomics. 2011;29:693–704. doi: 10.2165/11584620-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 35. Wang G, Zhang Z, Ayala C, Dunet DO, George MG, Prevention S. Costs of hospitalization for stroke patients aged 18–64 years in the United States. J Stroke Cerebrovasc. 2015;23:861–868. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kern DM, Mellström C, Hunt PR, Tunceli O, Wu B, Westergaard M, Hammar N. Long‐term cardiovascular risk and costs for myocardial infarction survivors in a US commercially insured population. Curr Med Res Opin. 2016;32:703–711. doi: 10.1185/03007995.2015.1136607 [DOI] [PubMed] [Google Scholar]
- 37. Godwin KM, Wasserman J, Ostwald SK. Cost associated with stroke: outpatient rehabilitative services and medication. Top Stroke Rehabil. 2011;18:676–684. doi: 10.1310/tsr18s01-676 [DOI] [PubMed] [Google Scholar]
- 38. Larkindale J, Yang W, Hogan PF, Simon CJ, Zhang Y, Jain A, Habeeb‐Louks EM, Kennedy A, Cwik VA. Cost of illness for neuromuscular diseases in the United States. Muscle Nerve. 2014;49:431–438. doi: 10.1002/mus.23942 [DOI] [PubMed] [Google Scholar]
- 39. Yang W, Dall TM, Beronjia K, Lin J, Semilla AP, Chakrabarti R, Hogan PF, Petersen MP. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917–928. doi: 10.2337/dci18-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hansen KE, Hildebrand JP, Ferguson EE, Stein JH. Outcomes in 45 patients with statin‐associated myopathy. Arch Intern Med. 2005;165:2671–2676. doi: 10.1001/archinte.165.22.2671 [DOI] [PubMed] [Google Scholar]
- 41. Vinci P, Panizon E, Tosoni LM, Cerrato C, Pellicori F, Mearelli F, Biasinutto C, Fiotti N, Giorgio F, Girolamo D, et al. Statin‐associated myopathy: emphasis on mechanisms and targeted therapy. Int J Mol Sci. 2021;22:11687. doi: 10.3390/ijms222111687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abd TT, Jacobson TA. Statin‐induced myopathy: a review and update. Expert Opinion on Drug Safety. 2011;10:373–387. doi: 10.1517/14740338.2011.540568 [DOI] [PubMed] [Google Scholar]
- 43. Ballantyne CM, Corsini A, Davidson MH, Holdaas H, Jacobson TA, Leitersdorf E, März W, Reckless JPD, Stein EA. Risk for myopathy with statin therapy in high‐risk patients. Arch Intern Med. 2003;163:553–564. doi: 10.1001/archinte.163.5.553 [DOI] [PubMed] [Google Scholar]
- 44. Lau LH, Lew J, Borschmann K, Thijs V, Ekinci EI. Prevalence of diabetes and its effects on stroke outcomes: a meta‐analysis and literature review. J Diabetes Investig. 2019;10:780–792. doi: 10.1111/jdi.12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lei C, Wu B, Liu M, Chen Y. Association between hemoglobin A1C levels and clinical outcome in ischemic stroke patients with or without diabetes. J Clin Neurosci. 2015;22:498–503. doi: 10.1016/j.jocn.2014.08.030 [DOI] [PubMed] [Google Scholar]
- 46. Centers for Disease Control and Prevention . Family history and other characteristics that increase risk for high blood pressure. The CDC. Published 2016. Accessed November 10, 2021. https://www.cdc.gov/stroke/family_history.htm
- 47. Kim JO, Schaid DJ, Vachon CM, Cooke A, Couch FJ, Kim CA, Sinnwell JP, Hasadsri L, Stan DL, Goldenberg B, et al. Impact of personalized genetic breast cancer risk estimation with polygenic risk scores on preventive endocrine therapy intention and uptake. Cancer Prev Res. 2021;14:175–184. doi: 10.1158/1940-6207.CAPR-20-0154 [DOI] [PubMed] [Google Scholar]
- 48. Centers for Disease Control and Prevention (CDC). National Diabetes Statistics Report, 2020. 2020. [Google Scholar]
- 49. Klimchak AC, Patel MY, Iorga ŞR, Kulkarni N, Wong ND. Lipid treatment and goal attainment characteristics among persons with atherosclerotic cardiovascular disease in the United States. Am J Prev Cardiol. 2020;1:100010. doi: 10.1016/j.ajpc.2020.100010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. U.S. Census Bureau . United States Population by Age and Sex. Accessed February 19, 2022. https://www.census.gov/popclock/
- 51. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 52. Social Security . Actuarial Life Table: Social Security Area Population. 2017. Accessed March 10, 2021. https://www.ssa.gov/oact/STATS/table4c6.html
- 53. Bucholz EM, Normand SL, Wang Y, Ma S, Lin H, Krumholz HM. Life expectancy and years of potential life lost after acute myocardial infarction by sex and race: a cohort‐based study of medicare beneficiaries. J Am Coll Cardiol. 2015;66:645–655. doi: 10.1016/j.jacc.2015.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kochar A, Chen AY, Sharma PP, Pagidipati NJ, Fonarow GC, Cowper PA, Roe MT, Peterson ED, Wang TY. Long‐term mortality of older patients with acute myocardial infarction treated in US clinical practice. J Am Heart Assoc. 2018;7:e007230. doi: 10.1161/JAHA.117.007230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hynninen Y, Linna M, Vilkkumaa E. Value of genetic testing in the prevention of coronary heart disease events. PLoS One. 2019;14:1–16. doi: 10.1371/journal.pone.0210010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jarmul J, Pletcher MJ, Lich KH, Wheeler SB, Weinberger M, Avery CL, Jonas DE, Earnshaw S, Pignone M. Cardiovascular genetic risk testing for targeting statin therapy in the primary prevention of atherosclerotic cardiovascular disease. Circ Cardiovasc Qual Outcomes. 2018;11:1–12. doi: 10.1161/CIRCOUTCOMES.117.004171 [DOI] [PubMed] [Google Scholar]
- 57. Song X, Quek RG, Gandra SR, Cappell KA, Fowler R, Cong Z. Productivity loss and indirect costs associated with cardiovascular events and related clinical procedures. BMC Health Serv Res. 2015;15:245. doi: 10.1186/s12913-015-0925-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Horne J, Madill J, O’Connor C, Shelley J, Gilliland J. A systematic review of genetic testing and lifestyle behaviour change: are we using high‐quality genetic interventions and considering behaviour change theory? Lifestyle Genom. 2018;11:49–63. doi: 10.1159/000488086 [DOI] [PubMed] [Google Scholar]
- 59. Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, Kuntz KM. State‐transition modeling: a report of the ISPOR‐SMDM modeling good research practices task force‐3. Med Decis Making. 2012;32:690–700. doi: 10.1177/0272989X12455463 [DOI] [PubMed] [Google Scholar]
- 60. Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling transformation, translation and appropriate application. Pharmacoeconomics. 2007;25:1–4. papers2://publication/uuid/8850A6C3‐6F44‐4FA2‐B3D9‐F04ABC2769DE. doi: 10.2165/00019053-200725010-00002 [DOI] [PubMed] [Google Scholar]
- 61. Briggs AH. Handling uncertainty in combined endpoints. Pharmacoecnomics. 2000;17:479–500. doi: 10.2165/00019053-200017050-00006 [DOI] [PubMed] [Google Scholar]
- 62. Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel D. Model parameter estimation and uncertainty: a report of the ISPOR‐SMDM modeling good research practices task force‐6. Value in Health. 2012;15:835–842. doi: 10.1016/j.jval.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 63. Linos E, Fiorentino D, Lingala B, Krishnan E, Chung L. Atherosclerotic cardiovascular disease and dermatomyositis: an analysis of the Nationwide Inpatient Sample survey. Arthritis Res Ther. 2013;15:1–5. doi: 10.1186/ar4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li M, Wang X, Li X, Chen H, Hu Y, Zhang X, Tang X, Miao Y, Tian G, Shang H. Statins for the primary prevention of coronary heart disease. Biomed Res Int. 2019;2019:1–15. doi: 10.1155/2019/4870350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gu Q, Paulose‐Ram R, Burt VL, Kit BK. Prescription cholesterol‐lowering medication use in adults aged 40 and over: United States, 2003‐2012. NCHS data brief. 2014;1–8. [PubMed] [Google Scholar]
- 66. Zhang X, Xing LU, Jia X, Pang X, Xiang Q, Zhao X, Ma L, Liu Z, Hu K, Wang Z, et al. Comparative lipid‐lowering/increasing efficacy of 7 statins in patients with dyslipidemia, cardiovascular diseases, or diabetes mellitus: systematic review and network meta‐analyses of 50 randomized controlled trials. Cardiovasc Ther. 2020;2020:1–21. doi: 10.1155/2020/3987065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lambrecht LJ, Malini PL. Efficacy and tolerability of simvastatin 20 mg vs pravastatin 20 mg in patients with primary hypercholesterolemia. European Study Group. Acta Cardiologica. 1993;48:541–554. [PubMed] [Google Scholar]
- 68. Wetterstrand KA. DNA Sequencing Costs: Data from the NHGRI Genome Sequencing Program (GSP). Accessed September 4, 2021. https://www.genome.gov/about‐genomics/fact‐sheets/DNA‐Sequencing‐Costs‐Data
- 69. Venkataraman P, Kawakami H, Huynh Q, Mitchell G, Nicholls SJ, Stanton T, Tonkin A, Watts GF, Marwick TH. Cost‐effectiveness of coronary artery calcium scoring in people with a family history of coronary disease. JACC: Cardiovasc Imaging. Published Online. 2021. doi: 10.1016/j.jcmg.2020.11.008 [DOI] [PubMed] [Google Scholar]
- 70. Wang Y, Leifheit E, Normand SL, Krumholz HM. Association between subsequent hospitalizations and recurrent acute myocardial infarction within 1 year after acute myocardial infarction. J Am Heart Assoc. 2020;9:e014907. doi: 10.1161/JAHA.119.014907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MS. Recurrent stroke and cardiac risks after first ischemic stroke. The Northern Manhattan Study. Neurology. 2006;66. doi: 10.1212/01.wnl.0000201253.93811.f6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


