Abstract
Background
Determine the prevalence and predictors associated with underdiagnosis and undertreatment of modifiable cardiovascular disease (CVD) risk factors (hypertension, dyslipidemia, glucose intolerance/diabetes) among adult survivors of childhood cancer at high risk of premature CVD.
Methods and Results
This was a cross‐sectional study of adult‐aged survivors of childhood cancer treated with anthracyclines or chest radiotherapy, recruited across 9 US metropolitan regions. Survivors completed questionnaires and in‐home clinical assessments. The comparator group was a matched sample from the National Health and Nutrition Examination Survey. Multivariable logistic regression estimated the risk (odds ratios) of CVD risk factor underdiagnosis and undertreatment among survivors compared with the National Health and Nutrition Examination Survey. Survivors (n=571; median age, 37.7 years and 28.5 years from cancer diagnosis) were more likely to have a preexisting CVD risk factor than the National Health and Nutrition Examination Survey (n=345; P<0.05 for all factors). While rates of CVD risk factor underdiagnosis were similar (27.1% survivors versus 26.1% National Health and Nutrition Examination Survey; P=0.73), survivors were more likely undertreated (21.0% versus 13.9%, P=0.007; odds ratio, 1.8, 95% CI, 1.2–2.7). Among survivors, the most underdiagnosed and undertreated risk factors were hypertension (18.9%) and dyslipidemia (16.3%), respectively. Men and survivors who were overweight/obese were more likely to be underdiagnosed and undertreated. Those with multiple adverse lifestyle factors were also more likely undertreated (odds ratio, 2.2, 95% CI, 1.1–4.5). Greater health‐related self‐efficacy was associated with reduced undertreatment (odds ratio, 0.5; 95% CI, 0.3–0.8).
Conclusions
Greater awareness of among primary care providers and cardiologists, combined with improving self‐efficacy among survivors, may mitigate the risk of underdiagnosed and undertreated CVD risk factors among adult‐aged survivors of childhood cancer.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03104543.
Keywords: cancer survivors, cardiovascular risk factor, undertreatment
Subject Categories: Risk Factors, Epidemiology, Pediatrics, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- CCSS
Childhood Cancer Survivor Study
- MHLC
Multidimensional Health Locus of Control
- NHANES
National Health and Nutrition Examination Survey
Clinical Perspective
What Is New?
Although the prevalence of underdiagnosed cardiovascular disease risk factors (ie, hypertension, dyslipidemia, glucose intolerance/diabetes) was similar between adult‐aged survivors of childhood cancer previously exposed to cardiotoxic cancer therapies and the general population, cancer survivors were nearly twice as likely to be undertreated for these conditions.
What Are the Clinical Implications?
Greater awareness of the substantially increased risk of cardiovascular disease among cancer survivors and their health care providers, combined with increased health‐related self‐efficacy among survivors and more aggressive control of cardiovascular risk factors by providers, may mitigate this risk.
Five‐year survival after a diagnosis of childhood cancer now exceeds 85% with an estimated half‐million survivors of childhood cancer living in the United States. 1 , 2 However, the excess risk of mortality after 5‐year survival remains considerable, 3 with cardiovascular disease (CVD) the leading noncancer cause of premature mortality in this population. 4 , 5 Attributable to cancer treatment–related exposures, survivors of childhood cancer have a >5‐fold increased risk of serious CVD and death, and a greater burden of modifiable CVD risk factors such as hypertension, dyslipidemia, and diabetes compared with the general population. 4 , 5 These modifiable risk factors appear to be synergistic with the cardiotoxic effects of cancer treatments and potentiate survivors’ risk of ischemic heart disease and heart failure more than they do in the general population. 5 , 6 Survivors also tend to develop CVD risk factors at younger ages compared with siblings or the general population. 7 , 8
Given this high risk of CVD and associated mortality among childhood cancer survivors, early diagnosis and control of CVD risk factors are critical. Most adult‐aged survivors of childhood cancer report only receiving general medical care not specific to their history of cancer. 9 With the earlier onset of CVD risk factors compounded by limited knowledge of cancer survivor–specific screening guidelines among primary care providers, 10 , 11 most high‐risk survivors are not receiving recommended cardiovascular screening in a timely fashion. 12 , 13 Therefore, underdiagnosis (ie, survivors with risk factors but unaware of them) and undertreatment (ie, previously diagnosed risk factors but inadequately treated) are significant concerns for this growing high‐risk population. Prior work in single institutional settings suggests that CVD risk factor underdiagnosis is common. 8 , 14 To our knowledge, the degree of CVD risk factor undertreatment in this population has not been examined.
To address this gap in knowledge, we used a standardized clinical protocol to prospectively measure the prevalence of hypertension, dyslipidemia, and diabetes underdiagnosis and undertreatment among a national sample of adult‐aged, long‐term survivors of childhood cancer at high risk for premature CVD being recruited for a randomized clinical trial. We hypothesized that rates of underdiagnosis and undertreatment would be greater among survivors compared with a matched comparison group of adults without a history of cancer. We then examined predictors associated with a differential risk of underdiagnosis and undertreatment as potential targets for future intervention.
Methods
Participants
The CCSS (Childhood Cancer Survivor Study) consists of 25 665 individuals diagnosed with cancer before age 21 years at 31 institutions in the United States and Canada between 1970 and 1999, and who survived at least 5 years following diagnosis. 15 Detailed information about the original cancer diagnosis and treatment (chemotherapy agents and doses, radiotherapy fields and doses, surgeries) were abstracted from medical records. For this analysis, US‐based CCSS participants who were aged ≥18 years, free of known ischemic heart disease or heart failure, and living within 50 miles of 9 major metropolitan areas were recruited as part of a randomized clinical trial testing whether a survivorship care plan telehealth intervention can improve cardiovascular outcomes among long‐term survivors of childhood cancer (NCT03104543). 16 Eligibility included being classified as having an increased risk of ischemic heart disease or heart failure (≈10% cumulative incidence or greater by age 50 years) per previously validated CCSS risk prediction models based on demographic information and cancer treatment exposures (ie, anthracycline or chest radiation exposure). 17 , 18 This analysis includes participants recruited beginning September 2017 until April 2020, and with data available as of May 2020. Study procedures were approved by the institutional review boards at the Fred Hutchinson Cancer Research Center and St. Jude Children’s Research Hospital. All participants provided informed consent before undergoing study procedures. Data used in this analysis, as with all CCSS data, can be requested through the CCSS resource (ccss.stjude.org).
Measurements
Participants completed a questionnaire assessing past medical history, including cardiovascular health and treatment, Morisky medication adherence scale, 19 lifestyle habits per the Behavioral Risk Factor Surveillance System (ie, weekly minutes spent engaged in moderate and vigorous physical activity, average daily fruit/vegetable intake, and smoking/tobacco use), 20 health‐related self‐efficacy, 21 and the Multidimensional Health Locus of Control (MHLC) scale, Form B. 22 Adverse lifestyle behaviors were categorized as (1) not meeting national guidelines for moderate (≥150 min/wk) to vigorous physical activity (≥75 min/wk) 23 ; (2) eating <5 servings of daily fruit/vegetable 24 ; and (3) currently smoking/using tobacco. Self‐efficacy and MHLC were scored according to their respective published recommendations. Self‐efficacy scores were converted to standardized T‐scores using the developer’s US adult reference population.
Participants then underwent a standardized clinical assessment at home. A trained examiner measured participants’ standing height, weight, waist circumference, and resting blood pressure (seated, 3 measurements, each ≥3 minutes apart). For blood pressure, the highest systolic and diastolic values were then dropped, and the remaining values averaged. At the same visit, the examiner drew blood for a lipid profile, random glucose, and hemoglobin A1c. To minimize barriers to participation, the study did not require participants to fast before the blood draw, but the duration of any fasting was recorded. The elimination of routine fasting before lipid testing is consistent with current clinical practice and recommendations. 25 National guidelines provided guidance on interpretation of nonfasting lipid and random glucose testing. 25 , 26 , 27 , 28 , 29 Samples were shipped overnight to a central clinical laboratory (St. Jude Children’s Research Hospital) and results provided within 1 business day of receipt.
Finally, to better quantify the degree of possible “white coat” or “masked” hypertension, 30 a random subset of participants also received a Bluetooth‐enabled home blood pressure monitor (Omron 10 Series, Omron Healthcare, Lake Forest, IL) following their home visit and were asked to record 3 measurements per week over a 1‐month period. Participants used the Eureka Research Platform, a National Institutes of Health–funded mobile health platform, to automatically and securely transmit these measurements to the study team for analysis.
Comparison Group
Individuals from the 2015 to 2016 National Health and Nutrition Examination Survey (NHANES) who underwent both the examination and standardized laboratory study components (n=9544) served as a contemporaneous, noncancer population for comparison purposes. 31 After excluding individuals with a history of cancer, heart failure, or ischemic heart disease, the remaining individuals (n=8650) were further limited to those who had >95% completion of the selected examination and laboratory assays of interest in this analysis and frequency‐matched with the CCSS study population by 5‐year age group, sex, and race and ethnicity (0.6:1 ratio; n=345).
Outcomes Definitions
Underdiagnosis among both CCSS and NHANES participants was defined by (1) lack of any self‐reported history of the target condition (hypertension, dyslipidemia, or diabetes); and (2) study measurements consistent with the condition based on established clinical guidelines. Specifically, the presence of possible undiagnosed hypertension was suspected based on cutoffs from the 2017 revised US guidelines if average systolic values were ≥130 mm Hg or average diastolic values ≥80 mm Hg. 30 These cutoffs were chosen given that study participants were predicted to have an ≈10% or greater risk of ischemic heart disease or heart failure by age 50 years because of their treatment for childhood cancer. Dyslipidemia was suspected if low‐density lipoprotein was ≥160 mg/dL or fasting triglyceride was ≥150 mg/dL (or if <10 hours fasting, ≥200 mg/dL). 27 , 28 , 29 Diabetes was suspected if fasting glucose was ≥126 mg/dL (or if <8 hours fasting, ≥200 mg/dL) or hemoglobin A1c ≥6.5%. 26 We also separately examined prediabetes if fasting glucose was 100 to 125 mg/dL (or if <8 hours fasting, glucose 140–199 mg/dL) or hemoglobin A1c was 5.7% to 6.4%.
While any participant who was previously undiagnosed is also technically undertreated, we specifically defined undertreatment in this study as occurring among those who self‐reported being previously diagnosed with hypertension, dyslipidemia, or diabetes managed by either lifestyle modifications or medication therapy, but whose home visit measurement fell outside the commonly recommended therapeutic range: blood pressure ≥140/≥90 mm Hg, low‐density lipoprotein ≥160 mg/dL, fasting triglyceride ≥150 mg/dL (or ≥200 mg/dL if not fasting), or hemoglobin A1c ≥7.0%. As a secondary outcome, among participants aged 20 to 60 years, we also estimated the 30‐year risk of hard CVD events (coronary death, myocardial infarction, and stroke) on the basis of models developed for the general population. 32
Statistical Analysis
Univariate comparisons were assessed using t tests and chi‐square tests. Comparisons of mean physiologic values between CCSS and NHANES used linear regression adjusted for the frequency‐matched variables (current age, sex, race or ethnicity). To examine the potential influence of participation bias, we also performed a sensitivity analysis in which we estimated underdiagnosis and undertreatment prevalence rates with inverse probability weights accounting for the distribution of sex, racial or ethnic status, history of hypertension requiring medications, and history of dyslipidemia requiring medications from the entire CCSS sample approached for this study (n=1591). Multivariable logistic regression, adjusting for current age, sex, and race or ethnicity, estimated the odds ratios (ORs) and the 95% CIs of any CVD risk factor underdiagnosis or undertreatment in CCSS compared with NHANES, and those associated with demographic and clinical characteristics within the CCSS population. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). Reported P values were all 2‐sided, with values <0.05 considered statistically significant. One of the authors (E.J.C.) had full access to all study data and takes responsibility for its integrity and the data analysis.
Results
We approached 1591 survivors thought to be eligible on the basis of their most recent CCSS survey. Of these, 766 consented (48.1%), and after excluding those subsequently found to be ineligible (n=29), having missing questionnaires (n=30), not completing the home visit (n=130), having nonanalyzable blood samples (n=1), or withdrawing consent for the randomized clinical trial (n=5), 571 participants were available for analysis (Figure 1). Participants (56.9% women; 85.1% non‐Hispanic White) had a median age of 37.7 years (range, 20.1–65.0) and were 28.5 years (range, 18.3–49.0) from their original cancer diagnosis (Table 1; survivor characteristics by individual cancer types shown in Table S1). Compared with NHANES, survivors were more likely to have a history of CVD risk factors (hypertension, 18.0% versus 11.0%; dyslipidemia, 14.0% versus 4.9%; diabetes, 6.5% versus 3.2%; all P<0.05). Although 68.3% of survivors (n=390) reported meeting national recommendations for physical activity, 67.6% (n=386) had at least 1 adverse lifestyle factor, with nearly one‐quarter (n=131) having ≥2 adverse lifestyle factors. The NHANES sample was much less physically active and more likely to be current smokers compared with survivors. Finally, compared with participating cancer survivors, nonparticipating survivors were more likely to be men but did not otherwise appear to differ greatly by other demographic or clinical characteristics (Table S2).
Figure 1. Consolidated Standards of Reporting Trials diagram.
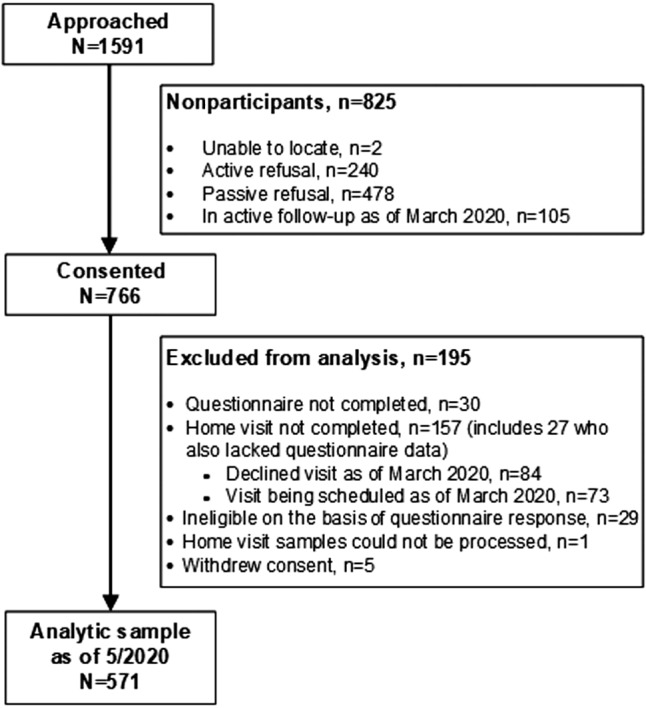
Childhood cancer survivors were recruited between September 2017 and April 2020, when study procedures were halted because of COVID‐19 pandemic–related restrictions in the United States.
Table 1.
Characteristics of Childhood Cancer Survivors (CCSS) at High Cardiovascular Disease Risk and a Matched General Population Comparison Group (NHANES)
| Characteristic, n (%) |
CCSS N=571 |
NHANES N=345 |
P value |
|---|---|---|---|
| Female sex | 325 (56.9) | 187 (54.2) | 0.42 |
| Non‐Hispanic White race and ethnicity | 486 (85.1) | 288 (83.5) | 0.51 |
| Median current age, y (range) | 37.7 (20.1–65.0) | 38.0 (20.0–64.0) | 0.84 |
| Median age at cancer diagnosis, y (range) | 8.3 (0.01–20.8) | … | |
| Cancer diagnosis | |||
| Bone cancer/sarcoma | 86 (15.1) | … | |
| Central nervous system | 59 (10.3) | … | |
| Kidney | 55 (9.6) | … | |
| Leukemia | 196 (34.3) | … | |
| Lymphoma | 142 (24.9) | … | |
| Neuroblastoma | 33 (5.8) | … | |
| Anthracycline exposure | 441 (77.4) | … | |
| Chest radiation exposure | 245 (43.1) | … | |
| History of hypertension | 153 (26.8) | 73 (21.2) | 0.06 |
| Currently on medications | 103 (18.0) | 38 (11.0) | 0.004 |
| History of dyslipidemia | 191 (33.5) | 75 (21.7) | <0.001 |
| Currently on medications | 80 (14.0) | 17 (4.9) | <0.001 |
| History of diabetes | 52 (9.1) | 13 (3.8) | 0.002 |
| Currently on medications | 37 (6.5) | 11 (3.2) | 0.03 |
| Lifestyle factors | |||
| Physically active* | 390 (68.3) | 124 (35.9) | <0.001 |
| ≥5 cups daily fruits/vegetables | 120 (21.0) | … † | |
| Current smoking/tobacco use | 68 (12.5) | 104 (30.1) | <0.001 |
| Health care utilization | |||
| Health insurance coverage | 533 (93.3) | … | |
| Routine checkup within 1–2 years ‡ | 517 (90.5) | … | |
| History of survivorship clinic attendance | 262 (45.9) | … | |
CCSS indicates Childhood Cancer Survivor Study; and NHANES, National Health and Nutrition Examination Survey.
Reporting ≥150 minutes of moderate activity/week or ≥75 minutes of vigorous activity/week, or equivalent mixture of both.
Not assessed by NHANES.
Defined as having had a checkup by a physician, physician assistant, or nurse practitioner within 1–2 years.
Compared with NHANES, cancer survivors had lower mean body mass indices (BMIs) and measures of central adiposity (Table 2). However, mean blood pressures, lipid values, and measures of glucose tolerance were not consistently more favorable in one group versus the other. Based on models developed in the general population, the mean predicted 30‐year risk of having a hard CVD event was lower among cancer survivors versus NHANES. Differences between cancer survivors and NHANES when stratified by underdiagnosis or undertreated status generally showed less favorable physiologic profiles among NHANES (Table S3). When physiologic parameters were compared within survivors alone, with and without any underdiagnosed or undertreated CVD risk factor, those with a possible underdiagnosed condition had greater BMI and waist circumference, along with worse mean blood pressures, cholesterol/triglyceride, blood glucose, and predicted hard CVD event risk. These differences were even greater among those with undertreated risk factors.
Table 2.
Physiologic Measurements of Childhood Cancer Survivors (CCSS) at High Cardiovascular Disease Risk and the General Population (NHANES)
| Outcomes,* n (%) | CCSS vs NHANES overall | CCSS only | ||||||
|---|---|---|---|---|---|---|---|---|
|
CCSS N=571 |
NHANES N=345 |
P value † |
No underdiagnosis or undertreatment N=323 ‡ |
Any underdiagnosis N=155 ‡ |
P value § |
Any undertreatment N=120 ‡ |
P value § | |
| BMI, kg/m2 | 27.4 (6.4) | 29.2 (7.2) | 0.001 | 26.2 (6.0) | 28.8 (6.9) | <0.001 | 29.4 (6.4) | <0.001 |
| No. overweight 25–29 kg/m2 (%) | 178 (31.2) | 94 (27.3) | 0.001 | 91 (28.2) | 61 (39.4) | <0.001 | 36 (30.0) | <0.001 |
| No. obese ≥30 kg/m2 (%) | 160 (28.0) | 136 (39.4) | 72 (22.3) | 49 (31.6) | 50 (41.7) | |||
| Waist circumference, cm | 89.1 (15.6) | 99.1 (17.0) | <0.001 | 85.7 (14.8) | 93.1 (15.3) | <0.001 | 94.6 (15.4) | <0.001 |
| No. with central adiposity (%) ║ | 182 (31.9) | 190 (55.1) | <0.001 | 90 (27.9) | 56 (36.1) | 0.07 | 50 (41.7) | 0.006 |
| Systolic blood pressure, mm Hg | 113.5 (12.6) | 117.7 (13.7) | <0.001 | 109.7 (10.2) | 118.7 (11.9) | <0.001 | 118.9 (15.2) | <0.001 |
| Diastolic blood pressure, mm Hg | 73.6 (8.9) | 70.0 (11.0) | 0.002 | 70.6 (7.0) | 79.4 (8.4) | <0.001 | 75.9 (10.3) | <0.001 |
| Total cholesterol, mg/dL | 187.6 (40.2) | 191.6 (42.0) | 0.11 | 177.5 (30.4) | 198.7 (46.7) | <0.001 | 211.0 (50.7) | <0.001 |
| High density lipoprotein, mg/dL | 52.3 (17.1) | 54.3 (16.7) | 0.04 | 56.9 (16.5) | 48.9 (18.1) | <0.001 | 42.3 (10.5) | <0.001 |
| Low density lipoprotein, mg/dL | 105.4 (33.0) | 114.3 (36.8) | <0.001 | 99.6 (25.7) | 112.2 (36.7) | <0.001 | 119.1 (46.0) | <0.001 |
| Triglyceride, mg/dL | 155.5 (126.8) | 111.2 (71.0) | <0.001 | 106.0 (50.7) | 201.0 (156.1) | <0.001 | 265.1 (174.7) | <0.001 |
| Blood glucose, mg/dL | 90.7 (29.4) | 103.2 (27.7) | <0.001 | 85.4 (16.7) | 91.7 (36.7) | 0.045 | 108.2 (45.1) | <0.001 |
| Hemoglobin A1c, % | 5.6 (0.9) | 5.4 (0.8) | 0.004 | 5.4 (0.4) | 5.6 (0.81) | 0.005 | 6.3 (1.50) | <0.001 |
| 30‐y hard CVD event risk, % ¶ | ||||||||
| Lipid model | 9.9 (10.3) | 11.7 (11.6) | 0.02 | 6.5 (6.5) | 10.9 (9.0) | <0.001 | 19.8 (14.5) | <0.001 |
| BMI model | 12.3 (12.8) | 14.5 (14.3) | 0.02 | 9.1 (10.0) | 12.4 (10.9) | 0.02 | 21.7 (16.8) | <0.001 |
BMI indicates body mass index; CCSS, Childhood Cancer Survivor Study; CVD, cardiovascular disease; and NHANES, National Health and Nutrition Examination Survey.
Mean value (standard deviation) unless otherwise noted.
Adjusted for sex, current age, and race and ethnicity, plus fasting duration (triglyceride and blood glucose only).
Numbers sum to >571 for CCSS, as some individuals had both at least 1 underdiagnosed condition and at least 1 undertreated condition (ie, hypertension, dyslipidemia, or diabetes/prediabetes); among NHANES, n=90 with underdiagnosis and n=48 with undertreatment.
Compared against those with no underdiagnosed or undertreated hypertension, dyslipidemia, or diabetes.
Men ≥102 cm or women ≥88 cm.
Pencina et al, Circulation 2009 32 ; values shown are the mean risks (SD).
Overall, the proportion with potential CVD risk factor underdiagnosis was similar between cancer survivors (n=155; 27.1%) and NHANES (n=90; 26.1%; P=0.73). However, undertreatment was more common among survivors (n=120 [21.0%]) versus NHANES (n=48 [13.9%], P=0.007; OR, 1.8; 95% CI, 1.2–2.7). After weighting for potential characteristics associated with study nonparticipation, the prevalence rates of underdiagnosis and undertreatment among survivors were minimally altered: 27.4% and 20.1%, respectively. Survivors also remained at increased risk of undertreatment compared with NHANES even after adjusting for BMI and smoking status (OR, 2.0; 95% CI, 1.3–3.1).
In sensitivity analyses, examiner‐measured blood pressure values were compared with self‐measured values from home blood pressure monitoring (n=32 survivors; 50.0% female; median age, 38.0 years; median 14.5 measurements [range, 3–120] over 5 weeks). Overall, the average examiner‐measured values tended to be lower compared with self‐measured home monitoring (112/73 mm Hg versus 118/78 mm Hg) with fewer survivors (25.0% versus 31.3%) classified as hypertensive (ie, average value ≥130/≥80 mm Hg).
When the proportions of underdiagnosis and undertreatment among cancer survivors were examined for each CVD risk factor, potentially underdiagnosed conditions were identified in 18.9% for hypertension, 13.1% for dyslipidemia, and 0.7% for diabetes (but an additional 22.9% with potential prediabetes; Figure 2). Potential undertreatment was identified in 4.9% for both hypertension and diabetes, and 16.3% for dyslipidemia. Among survivors without a known history of a given CVD risk factor, 20% to 26% had examiner‐measured values consistent with a possible new diagnosis of hypertension, dyslipidemia, or impaired glucose tolerance. Among survivors with a history of a given risk factor, the proportion with potential undertreatment ranged from 18.3% for those with hypertension to 48.7% and 53.8% for dyslipidemia and diabetes, respectively. Relatively few survivors had multiple underdiagnosed or undertreated risk factors (Table S4).
Figure 2. Sunburst charts showing the distribution of childhood cancer survivors (n=571) with normal and abnormal measurements corresponding to potential (A) hypertension, (B) dyslipidemia, and (C) glucose intolerance (diabetes and prediabetes).
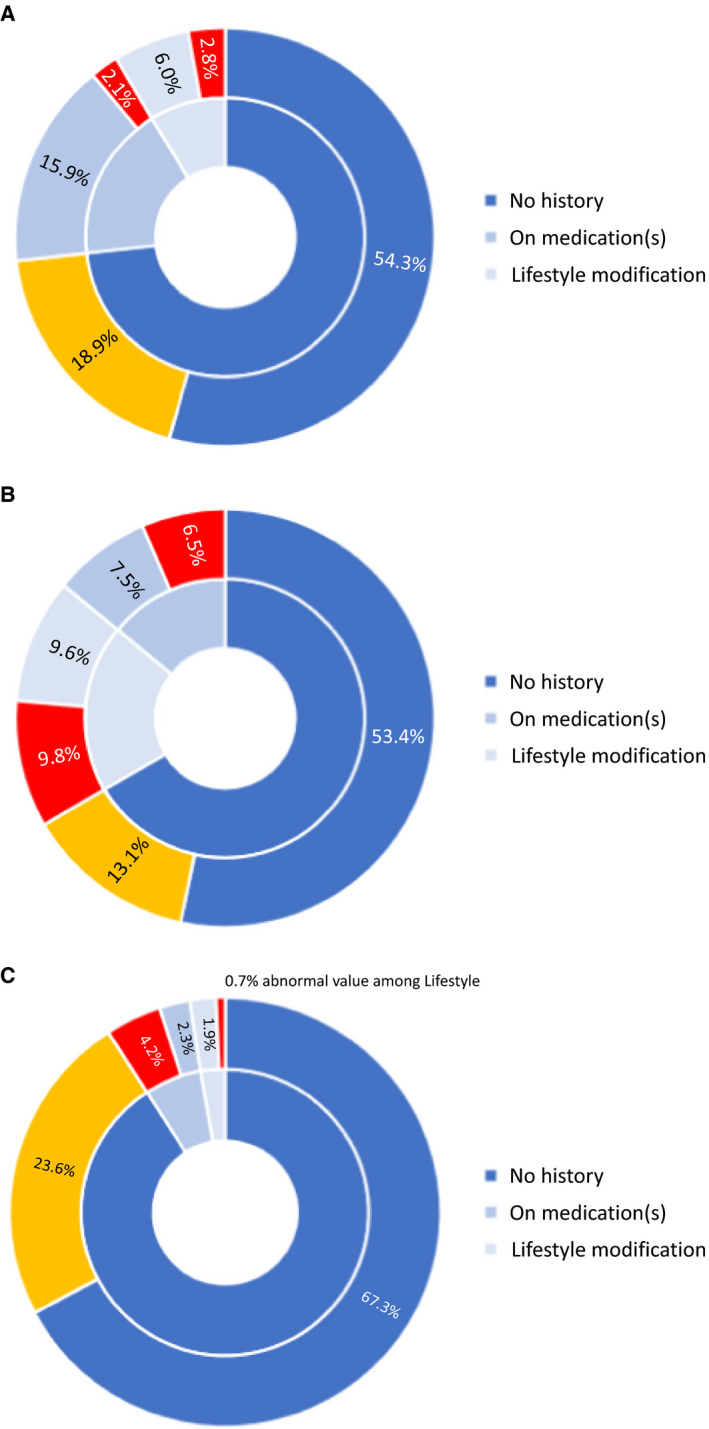
The inner rings correspond to the proportions of survivors who reported no known history of the target cardiovascular risk factor (dark blue), survivors currently on medications (medium blue), and survivors with known risk factor but managed on lifestyle modification alone (light blue). The outer rings denote the proportion of survivors with a potentially underdiagnosed (gold) and undertreated (red) risk factor based on study measurements.
Characteristics associated with both underdiagnosis and undertreatment in cancer survivors included male sex, increased BMI, and the presence of ≥2 adverse lifestyle factors (Table 3). Additional characteristics associated with an increased risk of undertreatment included older current age (OR, 2.3–2.9) and a history of chest radiotherapy exposure (OR, 2.0; 95% CI, 1.3–3.1). Furthermore, survivors who reported greater health‐related self‐efficacy (OR, 0.5; 95% CI, 0.3–0.8) and those who scored higher on internal locus of control beliefs (OR, 0.7; 95% CI, 0.5–0.9) were less likely to be undertreated. In contrast, survivors who scored higher on external locus of control (powerful others) were more likely to be undertreated (OR, 1.5; 95% CI, 1.1–1.9). Secondary analyses that included these additional covariates concurrently (chest radiotherapy, adverse lifestyle factors, BMI, self‐efficacy, and MHLC scales) showed similar results (Table S5). Finally, prior attendance at a survivorship (ie, long‐term follow‐up) clinic or having a recent routine checkup were not associated with a differential risk of underdiagnosis or undertreatment. Medication adherence was also not associated with a differential risk of undertreatment (data not shown).
Table 3.
Odds of Cardiovascular Disease Risk Factor Underdiagnosis or Undertreatment Among Childhood Cancer Survivors, Adjusted for Sex, Race/Ethnicity, and Current Age
| Characteristic |
Underdiagnosis N=155* |
Undertreatment N=120* |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Male vs female (ref) | 1.8 (1.2–2.6) | 0.003 | 1.9 (1.2–2.9) | 0.004 |
| Other races and ethnicities vs non‐Hispanic White (ref) | 0.7 (0.4–1.3) | 0.30 | 0.9 (0.5–1.7) | 0.72 |
| Current age, y | ||||
| 20–34 | 1.0 (ref) | 1.0 (ref) | ||
| 35–39 | 1.0 (0.6–1.6) | 0.94 | 2.3 (1.2–4.2) | 0.009 |
| ≥40 | 0.9 (0.6–1.5) | 0.78 | 2.9 (1.7–4.9) | <0.001 |
| Anthracycline exposure vs none (ref) | 1.5 (0.9–2.5) | 0.12 | 0.7 (0.5–1.2) | 0.22 |
| Chest radiotherapy exposure vs none (ref) | 1.0 (0.7–1.5) | 0.88 | 2.0 (1.3–3.1) | 0.002 |
| Survivorship clinic attendance vs none (ref) | 0.8 (0.5–1.2) | 0.23 | 0.9 (0.6–1.4) | 0.65 |
| No routine vs routine checkup (ref) | 1.3 (0.6–2.5) | 0.50 | 0.6 (0.2–1.7) | 0.37 |
| No. adverse lifestyle factors † | ||||
| 0 | 1.0 (ref) | 1.0 (ref) | ||
| 1 | 1.1 (0.6–1.9) | 0.72 | 1.4 (0.7–2.6) | 0.34 |
| ≥2 | 1.8 (1.0–3.3) | 0.06 | 2.2 (1.1–4.5) | 0.03 |
| Body mass index, kg/m2 | ||||
| <25 | 1.0 (ref) | 1.0 (ref) | ||
| 25–29 | 2.3 (1.5–3.6) ‡ | <0.001 | 1.7 (1.0–2.9) | 0.07 |
| ≥30 | 2.9 (1.7–4.9) | <0.001 | ||
| High vs low health‐related self‐efficacy (ref) § | 0.7 (0.5–1.1) | 0.13 | 0.5 (0.3–0.8) | 0.003 |
| MHLC scale ║ | ||||
| Internal | 0.9 (0.7–1.2) | 0.49 | 0.7 (0.5–0.9) | 0.01 |
| Chance | 1.0 (0.8–1.3) | 0.70 | 1.1 (0.8–1.4) | 0.53 |
| Powerful others | 1.0 (0.8–1.3) | 0.95 | 1.5 (1.1–1.9) | 0.009 |
MHLC indicates Multidimensional Health Locus of Control; OR, odds ratio; and ref, referent.
Compared with survivors without any cardiovascular disease risk factor underdiagnosis and undertreatment (n=323; referent).
Consisting of physical inactivity, low fruit/vegetable consumption, and current smoking/tobacco use.
25–29 and ≥30 kg/m2 categories combined as univariate analyses showed ORs 2.4 for both categories when compared with <25 kg/m2.
Defined as T‐score ≥50 vs <50 (ref).
Modeled as a linear term with risk estimates reflecting the association with a 1‐unit increase in the scale value.
Discussion
Prior research from CCSS and other groups have shown that childhood cancer survivors are at increased risk of CVD, particularly among those with cardiotoxic cancer treatment exposures and those who develop subsequent CVD risk factors. 4 , 5 The presence of these risk factors in conjunction with prior anthracycline or radiotherapy has been shown to have a synergistic effect on subsequent CVD risk, further emphasizing the need to mitigate potentially modifiable conditions in this high‐risk population. 5 , 6 , 33 , 34 , 35 In this analysis, we found that survivors at exceedingly high risk of premature CVD because of prior cancer treatment exposures had similar rates of CVD risk factor underdiagnosis as a matched general population sample. More than 90% of survivors in our study reported having health insurance and having had a routine checkup within the past 2 years, similar to rates reflective of the overall CCSS cohort. 36 , 37 This is not dissimilar to recent national estimates showing that <10% of US adults aged <65 years are uninsured. 38 However, survivors with a known CVD risk factor were nearly twice as likely to be undertreated compared with the general population.
Characteristics associated with undertreatment included male sex, older age, history of chest radiotherapy, greater number of adverse lifestyle factors, and higher BMI. Notably, we found that survivors of childhood cancer predicted to be at high risk of future ischemic heart disease or heart failure were more likely to have a history of CVD risk factors compared with a matched general population sample despite reporting greater physical activity, less current smoking/tobacco use, and being less obese. These results highlight the need for clinicians caring for these young and middle‐aged adult survivors to routinely screen for modifiable CVD risk factors at younger ages than in adults without cancer treatment risks, and to consider intervening more aggressively when any are detected. Prior surveys of CCSS participants showed that nearly 60% of these adult‐aged patients (mean age of 32 years at time of survey) received only general medical care, with <20% receiving risk‐based, cancer survivor–focused care. 9 The overwhelming site of care was community based, with <15% of participants still receiving care at a cancer center. 9
For survivors of childhood cancer living in the United States, the primary evidence‐based guidelines for long‐term follow‐up are generated and maintained by the Children’s Oncology Group, a clinical trial network with >200 institutions, predominantly in North America. Current evidence‐based recommendations from these guidelines include annual measurement of blood pressure for virtually all cancer survivors, and periodic (every 2 year) screening of lipid profiles and glucose metabolism among those exposed to abdominal radiation, or if comorbid CVD risk factors are present, including hypertension and being overweight/obese. 12 For survivors who received anthracyclines or chest radiation (stage A heart failure), routine echocardiography to monitor for early‐onset cardiomyopathy also is recommended. 13 , 39
These recommendations differ from those for the general population. For example, the current US Preventive Services Task Force recommendation for young adults (aged <40 years) who are normotensive and without other apparent risk factors is to repeat blood pressure screening every 3 to 5 years. 40 While the US Preventive Services Task Force recommends periodic screening for diabetes and dyslipidemia in older adults (aged ≥40 years), particularly if overweight or obese, no recommendations have been made for younger adults. 40 , 41 , 42 Interventions for those aged ≥40 years are typically based on 10‐year cardiovascular disease risks as estimated by general population risk calculators. 43 Notably, 10‐year risk estimates are typically not available for those aged <40 years. Furthermore, as was seen in this analysis, general population risk calculators also do not account for cardiotoxic cancer treatments and therefore likely underestimate CVD risk among cancer survivors. 6 , 44
Increasing primary care and cardiology provider awareness of cancer survivors’ risks may help further reduce the likelihood of underdiagnosis and undertreatment. Surveys of family physicians and internists reported low knowledge of survivor‐specific follow‐up guidelines and low provider efficacy regarding needs of childhood cancer survivors. 10 , 11 For childhood cancer survivors, a shared care model between primary care and survivorship specialists was endorsed by most primary care‐based survey respondents. This included the provision of patient‐specific follow‐up recommendations (ie, survivorship care plans), which has been recommended by the National Academy of Medicine. 45
In this study, CVD risk factor undertreatment was also more common among those with lower self‐efficacy, external locus of control, and a belief that powerful others control health outcomes. Our findings in cancer survivors are consistent with associations reported in the general population for conditions such as diabetes, heart disease, and health‐related lifestyle behaviors. 22 , 46 , 47 , 48 For example, among patients with diabetes, higher self‐efficacy was strongly associated with improved diet and physical activity as key components of improved diabetes self‐management. 47 Similarly, internal locus of control has been associated with lifestyle behaviors that influence cardiovascular health. 48 These results suggest that interventions that increase survivors’ health‐related self‐efficacy and improve their internal sense of control over health outcomes may also reduce undertreatment. Participants with an undertreated condition have since been randomized in an ongoing 1‐year health promotion trial that tests a telehealth‐delivered self‐management intervention beginning with the review of a personalized survivorship care plan. 16 This intervention will also attempt to improve primary care providers’ self‐efficacy toward survivorship care.
Adverse lifestyle factors remain important modifiers of subsequent CVD risk and mortality among childhood and adult cancer survivors. 49 , 50 , 51 , 52 Even though cancer survivors in this and other studies may report healthier lifestyle profiles compared with the general population, 53 , 54 a significant proportion of survivors remain inactive, use tobacco, and may benefit from a healthier diet. Prior research also suggests that cancer survivors, including survivors of childhood and adolescent cancers, are interested in and may be more motivated to pursue lifestyle changes following their cancer experience. 55
Our results should be interpreted with several limitations in mind. As our study participation was linked to an ongoing randomized clinical trial with a 1‐year time commitment, our overall consent rate was 48%. This rate may have been higher without the COVID‐19 pandemic, as some potentially eligible participants were still being actively approached when study visits had to cease. Aside from sex, nonresponders did not differ substantially from responders, and we did not find our estimates to be markedly altered when we weighted our sample for characteristics reflective of the entire study population. Although the participation rate may raise concerns about the generalizability of the cancer survivors in our sample, our use of an age, sex, race and ethnicity‐matched general population comparison group strengthens the validity of our findings. Related to generalizability, we also acknowledge that the US population has become much more diverse in the past 20 years, especially children and young adults. However, CCSS’s overall racial and ethnic composition is reflective of the US population when study participants were diagnosed with cancer (1970–1999). 56
Another limitation is the potential for measurement error and misclassification of undertreatment status based on 1‐time measurements. Clinically, the diagnosis of hypertension, dyslipidemia, and diabetes typically require repeat testing for confirmation. However, any misclassification should affect survivors and NHANES participants nondifferentially, and these methods, when applied to NHANES, have remained invaluable in providing estimates of health for the US population at large. For blood pressure, which may be subject to the greatest amount of moment‐to‐moment variability, our examiner‐measured values did not appear to be higher than self‐measured values.
In conclusion, CVD risk factor underdiagnosis and undertreatment among childhood cancer survivors at increased risk of heart disease was common. Greater awareness among survivors, primary care providers, and cardiologists, combined with increased self‐efficacy among survivors and more aggressive control of these risk factors by providers, may mitigate this risk. However, whether survivors experience the same degree of risk reduction with tighter control of potentially modifiable risk factors as seen in general population clinical trials remains to be determined.
Sources of Funding
NCT03104546 is directly funded by the US National Institutes of Health (CA204378; Dr Chow), while CCSS is funded by CA55727 (Dr Armstrong), with additional support to St. Jude Children’s Research Hospital from CA21765 and the American Lebanese Syrian Associated Charities. The Eureka Research Platform is funded by NIH grant U2CEB021881 (Dr Olgin).
Disclosures
None.
Supporting information
Tables S1–S5
Acknowledgments
The authors thank Mr David Doody for his assistance selecting and creating the NHANES comparison group.
Results from this work were presented in part at the American Society of Clinical Oncology Annual Meeting, May 29–31, 2020.
For Sources of Funding and Disclosures, see page 10.
References
- 1. Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Cancer SEER, et al. Statistics Review, 1975‐2018. Bethesda, MD: National Cancer Institute. Available at: https://seer.cancer.gov/csr/1975_2018/, based on November 2020 SEER data submission, posted to the SEER web site, April 2021. Accessed March 18, 2022. [Google Scholar]
- 2. Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life‐long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70. doi: 10.1038/nrc3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, Stovall M, Oeffinger KC, Bhatia S, Krull KR, et al. Reduction in late mortality among 5‐year survivors of childhood cancer. N Engl J Med. 2016;374:833–842. doi: 10.1056/NEJMoa1510795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, Hudson MM, Kremer LC, Landy DC, Miller TL, et al. Long‐term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128:1927–1995. doi: 10.1161/CIR.0b013e3182a88099 [DOI] [PubMed] [Google Scholar]
- 5. Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, Stovall M, Chow EJ, Sklar CA, Mulrooney DA, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Y, Chow EJ, Oeffinger KC, Border WL, Leisenring WM, Meacham LR, Mulrooney DA, Sklar CA, Stovall M, Robison LL, et al. Traditional cardiovascular risk factors and individual prediction of cardiovascular events in childhood cancer survivors. J Natl Cancer Inst. 2020;112:256–265. doi: 10.1093/jnci/djz108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oeffinger KC, Adams‐Huet B, Victor RG, Church TS, Snell PG, Dunn AL, Eshelman‐Kent DA, Ross R, Janiszewski PM, Turoff AJ, et al. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27:3698–3704. doi: 10.1200/JCO.2008.19.7251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steinberger J, Sinaiko AR, Kelly AS, Leisenring WM, Steffen LM, Goodman P, Mulrooney DA, Dietz AC, Moran A, Perkins JL, et al. Cardiovascular risk and insulin resistance in childhood cancer survivors. J Pediatr. 2012;160:494–499. doi: 10.1016/j.jpeds.2011.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nathan PC, Greenberg ML, Ness KK, Hudson MM, Mertens AC, Mahoney MC, Gurney JG, Donaldson SS, Leisenring WM, Robison LL, et al. Medical care in long‐term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26:4401–4409. doi: 10.1200/JCO.2008.16.9607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nathan PC, Daugherty CK, Wroblewski KE, Kigin ML, Stewart TV, Hlubocky FJ, Grunfeld E, Del Giudice ME, Ward L‐A, Galliher JM, et al. Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. J Cancer Surviv. 2013;7:275–282. doi: 10.1007/s11764-013-0271-0 [DOI] [PubMed] [Google Scholar]
- 11. Suh E, Daugherty CK, Wroblewski K, Lee H, Kigin ML, Rasinski KA, Ford JS, Tonorezos ES, Nathan PC, Oeffinger KC, et al. General internists’ preferences and knowledge about the care of adult survivors of childhood cancer: a cross‐sectional survey. Ann Intern Med. 2014;160:11–17. doi: 10.7326/M13-1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Children's Oncology Group . Long‐term follow‐up guidelines for survivors of childhood, adolescent and young adult cancers, Version 5.0. Available at: http://www.survivorshipguidelines.org/. Accessed March 18, 2022.
- 13. Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, Nathan PC, Tissing WJE, Shankar S, Sieswerda E, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16:e123–e136. doi: 10.1016/S1470-2045(14)70409-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, Green DM, Armstrong GT, Nottage KA, Jones KE, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, Green DM, Hammond S, Meadows AT, Mertens AC, et al. The childhood cancer survivor study: a National Cancer Institute‐supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chow EJ, Baldwin L‐M, Hagen AM, Hudson MM, Gibson TM, Kochar K, McDonald A, Nathan PC, Syrjala KL, Taylor SL, et al. Communicating health information and improving coordination with primary care (CHIIP): rationale and design of a randomized cardiovascular health promotion trial for adult survivors of childhood cancer. Contemp Clin Trials. 2020;89:105915. doi: 10.1016/j.cct.2019.105915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chow EJ, Chen Y, Kremer LC, Breslow NE, Hudson MM, Armstrong GT, Border WL, Feijen EAM, Green DM, Meacham LR, et al. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol. 2015;33:394–402. doi: 10.1200/JCO.2014.56.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chow EJ, Chen Y, Hudson MM, Feijen EAM, Kremer LC, Border WL, Green DM, Meacham LR, Mulrooney DA, Ness KK, et al. Prediction of ischemic heart disease and stroke in survivors of childhood cancer. J Clin Oncol. 2018;36:44–52. doi: 10.1200/JCO.2017.74.8673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007 [DOI] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention . Behavioral Risk Factor Surveillance System. Available at: http://www.cdc.gov/brfss/. Accessed March 18, 2022.
- 21. Schwarzer R, Jerusalem M. Generalized self‐efficacy scale. In: Weinman J, Wright S, Johnston M, eds. Measures in Health Psychology: a User’s Portfolio. Casual and Control Beliefs. Windsor, UK: NFER‐NELSON; 1995:35–37. [Google Scholar]
- 22. Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) Scales. Health Educ Monogr. 1978;6:160–170. doi: 10.1177/109019817800600107 [DOI] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention . Physical activity. Available at: https://www.cdc.gov/physicalactivity/index.html. Accessed March 18, 2022.
- 24. Blanck HM, Gillespie C, Kimmons JE, Seymour JD, Serdula MK. Trends in fruit and vegetable consumption among U.S. men and women, 1994–2005. Prev Chronic Dis. 2008;5:A35. [PMC free article] [PubMed] [Google Scholar]
- 25. Nordestgaard BG. A test in context: lipid profile, fasting versus nonfasting. J Am Coll Cardiol. 2017;70:1637–1646. doi: 10.1016/j.jacc.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 26. Professional Practice Committee . Standards of medical care in diabetes‐2018. Diabetes Care. 2018;41:S3. [DOI] [PubMed] [Google Scholar]
- 27. Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J, American Heart Association Expert Panel on Population and Prevention Science, American Heart Association Council on Cardiovascular Disease in the Young, American Heart Association Council on Epidemiology and Prevention, American Heart Association Council on Nutrition, Physical Activity and Metabolism, American Heart Association Council on High Blood Pressure Research, American Heart Association Council on Cardiovascular Nursing, American Heart Association Council on the Kidney in Heart Disease, Interdisciplinary Working Group on Quality of Care and Outcomes Research . Cardiovascular risk reduction in high‐risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–2738. doi: 10.1161/CIRCULATIONAHA.106.179568 [DOI] [PubMed] [Google Scholar]
- 28. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128:S213–S256. doi: 10.1542/peds.2009-2107C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 30. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 31. Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey. Available from: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. Accessed March 18, 2022.
- 32. Pencina MJ, D'Agostino RB Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30‐year risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Armenian SH, Sun C‐L, Vase T, Ness KK, Blum E, Francisco L, Venkataraman K, Samoa R, Wong FL, Forman SJ, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120:4505–4512. doi: 10.1182/blood-2012-06-437178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chow EJ, Wong K, Lee SJ, Cushing‐Haugen KL, Flowers ME, Friedman DL, Leisenring WM, Martin PJ, Mueller BA, Baker KS. Late cardiovascular complications after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:794–800. doi: 10.1016/j.bbmt.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Nimwegen FA, Schaapveld M, Cutter DJ, Janus CPM, Krol ADG, Hauptmann M, Kooijman K, Roesink J, van der Maazen R, Darby SC, et al. Radiation dose‐response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016;34:235–243. doi: 10.1200/JCO.2015.63.4444 [DOI] [PubMed] [Google Scholar]
- 36. Park ER, Kirchhoff AC, Nipp RD, Donelan K, Leisenring WM, Armstrong GT, Kuhlthau KA. Assessing health insurance coverage characteristics and impact on health care cost, worry, and access: a report from the childhood cancer survivor study. JAMA Intern Med. 2017;177:1855–1858. doi: 10.1001/jamainternmed.2017.5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mueller EL, Park ER, Kirchhoff AC, Kuhlthau K, Nathan PC, Perez GK, Rabin J, Hutchinson R, Oeffinger KC, Robison LL, et al. Insurance, chronic health conditions, and utilization of primary and specialty outpatient services: a Childhood Cancer Survivor Study report. J Cancer Surviv. 2018;12:639–646. doi: 10.1007/s11764-018-0700-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berchick ED, Barnett JC, Upton RD. Current Population Reports, P60–267(RV): Health insurance coverage in the United States: 2018. US Government Printing Office; 2019. [Google Scholar]
- 39. Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 Appropriate use criteria for multimodality imaging in the assessment of cardiac structure and function in nonvalvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2019;73:488–516. doi: 10.1016/j.jacc.2018.10.038 [DOI] [PubMed] [Google Scholar]
- 40. Siu AL. US Preventive Services Task Force . Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778–786. doi: 10.7326/M15-2223 [DOI] [PubMed] [Google Scholar]
- 41. Siu AL. US Preventive Services Task Force . Screening for abnormal blood glucose and type 2 diabetes mellitus: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:861–868. doi: 10.7326/M15-2345 [DOI] [PubMed] [Google Scholar]
- 42. US Preventive Services Task Force , Bibbins‐Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, García FAR, Gillman MW, Kemper AR, Krist AH, Kurth AE, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997–2007. doi: 10.1001/jama.2016.15450 [DOI] [PubMed] [Google Scholar]
- 43. Goff DC, Lloyd‐Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 44. Lipshultz ER, Chow EJ, Doody DR, Armenian SH, Asselin BL, Baker KS, Bhatia S, Constine LS, Freyer DR, Kopp LM, et al. Cardiometabolic risk in childhood cancer survivors: a report from the Children’s Oncology Group. Cancer Epidemiol Biomarkers Prev. 2022;31:536–542. doi: 10.1158/1055-9965.EPI-21-0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hewitt M, Greenfield S, Stovall E, eds. Committee on Cancer Survivorship: Improving Care and Quality of Life, National Cancer Policy Board. From Cancer Patient to Cancer Survivor: Lost in Transition. Institute of Medicine and National Research Council, National Academies Press; 2006. [Google Scholar]
- 46. AbuSabha R, Achterberg C. Review of self‐efficacy and locus of control for nutrition‐ and health‐related behavior. J Am Diet Assoc. 1997;97:1122–1132. [DOI] [PubMed] [Google Scholar]
- 47. King DK, Glasgow RE, Toobert DJ, Strycker LA, Estabrooks PA, Osuna D, Faber AJ. Self‐efficacy, problem solving, and social‐environmental support are associated with diabetes self‐management behaviors. Diabetes Care. 2010;33:751–753. doi: 10.2337/dc09-1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mercer DA, Ditto B, Lavoie KL, Campbell T, Arsenault A, Bacon SL. Health locus of control is associated with physical activity and other health behaviors in cardiac patients. J Cardiopulm Rehabil Prev. 2018;38:394–399. doi: 10.1097/HCR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 49. Tonorezos ES, Robien K, Eshelman‐Kent D, Moskowitz CS, Church TS, Ross R, Oeffinger KC. Contribution of diet and physical activity to metabolic parameters among survivors of childhood leukemia. Cancer Causes Control. 2013;24:313–321. doi: 10.1007/s10552-012-0116-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fung C, Sesso HD, Williams AM, Kerns SL, Monahan P, Abu Zaid M, Feldman DR, Hamilton RJ, Vaughn DJ, Beard CJ, et al. Multi‐institutional assessment of adverse health outcomes among North American testicular cancer survivors after modern cisplatin‐based chemotherapy. J Clin Oncol. 2017;35:1211–1222. doi: 10.1200/JCO.2016.70.3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leger KJ, Baker KS, Cushing‐Haugen KL, Flowers MED, Leisenring WM, Martin PJ, Mendoza JA, Reding KW, Syrjala KL, Lee SJ, et al. Lifestyle factors and subsequent ischemic heart disease risk after hematopoietic cell transplantation. Cancer. 2018;124:1507–1515. doi: 10.1002/cncr.31227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scott JM, Li N, Liu QI, Yasui Y, Leisenring W, Nathan PC, Gibson T, Armenian SH, Nilsen TS, Oeffinger KC, et al. Association of exercise with mortality in adult survivors of childhood cancer. JAMA Oncol. 2018;4:1352–1358. doi: 10.1001/jamaoncol.2018.2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chow EJ, Baker KS, Lee SJ, Flowers ME, Cushing‐Haugen KL, Inamoto Y, Khera N, Leisenring WM, Syrjala KL, Martin PJ. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol. 2014;32:191–198. doi: 10.1200/JCO.2013.52.6582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gibson TM, Liu W, Armstrong GT, Srivastava DK, Hudson MM, Leisenring WM, Mertens AC, Klesges RC, Oeffinger KC, Nathan PC, et al. Longitudinal smoking patterns in survivors of childhood cancer: an update from the Childhood Cancer Survivor Study. Cancer. 2015;121:4035–4043. doi: 10.1002/cncr.29609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Demark‐Wahnefried W, Rogers LQ, Alfano CM, Thomson CA, Courneya KS, Meyerhardt JA, Stout NL, Kvale E, Ganzer H, Ligibel JA. Practical clinical interventions for diet, physical activity, and weight control in cancer survivors. CA Cancer J Clin. 2015;65:167–189. doi: 10.3322/caac.21265 [DOI] [PubMed] [Google Scholar]
- 56. Bhatia S, Gibson TM, Ness KK, Liu QI, Oeffinger KC, Krull KR, Nathan PC, Neglia JP, Leisenring W, Yasui Y, et al. Childhood cancer survivorship research in minority populations: a position paper from the Childhood Cancer Survivor Study. Cancer. 2016;122:2426–2439. doi: 10.1002/cncr.30072 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5


