Unlike conduction disease in elderly patients, advanced atrioventricular block in young and middle‐aged patients is predominantly attributable to causes other than degenerative conduction disease and coronary artery disease. 1 Frequent causes of advanced atrioventricular block in young people include cardiac sarcoidosis, 2 , 3 , 4 giant cell myocarditis, 2 genetic cardiomyopathies 5 caused by mutations in LMNA, 6 SCN5A, 7 and EMD, 8 and Lyme carditis in places where Lyme disease is endemic. 9
Identifying the cause of advanced atrioventricular block is critical because it guides clinical management, the cause‐specific treatments are often distinct, which in turn influences long‐term outcomes. 10 Unfortunately, the cause of advanced atrioventricular block in young people is often unrecognized, particularly in the absence of extracardiac manifestations of a systemic disease, such as sarcoidosis. In a large Danish study of 1027 patients aged <50 years who received their first permanent pacemaker between 1996 and 2015, the cause of the advanced atrioventricular block was unknown in 50%. 11 The consequences of not knowing the cause of the advanced atrioventricular block were described in a follow‐up study: these patients had a 3‐ to 4‐fold increase in the risk of the composite end point of death, hospitalization for heart failure, ventricular tachyarrhythmia, or aborted cardiac arrest at a median follow‐up of 9.8 years when compared with a cohort of matched controls without pacemakers. 12
The underlying cause of advanced atrioventricular block can frequently be identified with the help of cardiovascular magnetic resonance imaging (CMR) 13 , 14 ; in the study by Baritussio et al, CMR provided incremental diagnostic value in 65% of young and middle‐aged patients with advanced atrioventricular block. 13 The presence of subepicardial, septal‐predominant, multifocal late gadolinium enhancement on CMR would indicate cardiac sarcoidosis 15 as the underlying cause, whereas the absence of late gadolinium enhancement would definitively exclude cardiac sarcoidosis and giant cell myocarditis, allowing the clinician to look for causes of advanced atrioventricular block that could present without late gadolinium enhancement, such as Lyme carditis and degenerative conduction disease (Figure).
Figure 1. Example cardiovascular magnetic resonance imaging of patients with advanced atrioventricular block from 4 causes with distinct treatments.
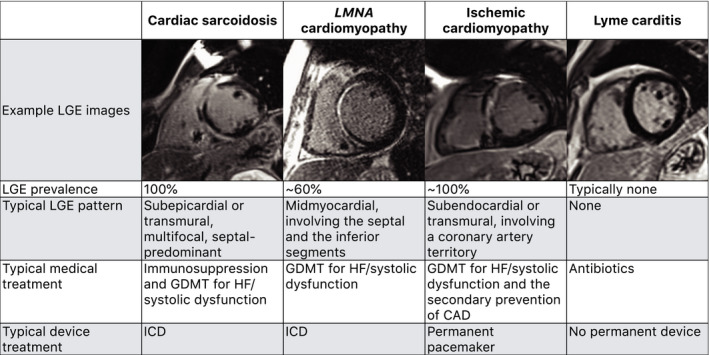
CAD indicates coronary artery disease; GDMT, guideline‐directed medical therapy; HF, heart failure; ICD, implantable cardioverter‐defibrillator; and LGE, late gadolinium enhancement.
The main challenge in doing CMR to diagnose the cause of advanced atrioventricular block is that patients may need continuous pacing while they get evaluated, and conventional temporary transvenous pacemakers may not be safe for magnetic resonance imaging. In this context, Vuorinen et al describe, in this issue of the Journal of the American Heart Association (JAHA), the use of temporary permanent pacemakers (TPPMs) to allow CMR in patients with advanced atrioventricular block requiring temporary pacing. 16 TPPM, which involves the use of a temporary active‐fixation lead with an externalized permanent generator taped to the skin, was originally devised as a solution for temporary pacing in pacemaker‐dependent patients requiring total removal of infected devices 17 and is an established method of temporary pacing in patients with a contraindication for immediate permanent pacemaker implantation. 18 Vuorinen et al show that TPPMs could allow CMR to be performed safely and effectively. No patient in the study had CMR‐related adverse events, and the TPPM generator did not lead to any significant artifacts on the CMR images. Among 17 patients, CMR led to a diagnosis of histologically confirmed granulomatous inflammatory cardiomyopathy in 8, including cardiac sarcoidosis in 7 and giant cell myocarditis in 1. Another patient was suspected of having cardiac sarcoidosis based on CMR, but histological confirmation could not be obtained.
Over the past several years, a wealth of data have demonstrated the safety of magnetic resonance imaging in patients with cardiac implantable electronic devices, including those considered to be non–magnetic resonance conditional. 19 Because TPPM involves the use of leads and generators that are designed to be used permanently, the patients can be scanned with the same safety precautions as those with permanent pacemakers. Although susceptibility artifacts are a consideration when doing CMR on patients with devices, they are less of a concern with permanent pacemakers compared with implantable cardioverters‐defibrillators; with right‐sided pacemakers, all conventional CMR sequences can be performed without any impact on image quality. 20 As described by Vuorinen et al, TPPMs on the right side should not result in any cardiac artifacts related to the generator. Thus, TPPMs should preferentially be placed on the right side to allow high‐quality CMR, including in patients in whom permanent devices would be implanted on the left side.
Although their experience was limited to only 17 patients, Vuorinen et al have indubitably demonstrated that CMR with TPPM is feasible, is safe, and can help diagnose cardiac pathologies with important clinical implications. Timely and accurate diagnosis of the underlying cause of advanced atrioventricular block by the routine use of CMR, particularly in young and middle‐aged patients, will enable cause‐specific treatments, and improve outcomes. Using TPPM in patients who need continuous pacing will make this clinical practice easier.
Sources of Funding
This work was supported by National Institutes of Health grant K23HL132011, National Institutes of Health grant R03HL157011, a University of Minnesota Clinical and Translational Science Institute K‐R01 Transition to Independence Grant (supported by the National Institutes of Health grant UL1TR002494), and a Lillehei Heart Institute Red Heart Soiree Seed Grant, all awarded to Dr Shenoy.
Disclosures
None.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Sources of Funding and Disclosures, see page 3.
See Article by Vuorinen et al.
REFERENCES
- 1. Barra SNC, Providência R, Paiva L, Nascimento J, Marques AL. A review on advanced atrioventricular block in young or middle‐aged adults. Pacing Clin Electrophysiol. 2012;35:1395–1405. doi: 10.1111/j.1540-8159.2012.03489.x [DOI] [PubMed] [Google Scholar]
- 2. Kandolin R, Lehtonen J, Kupari M. Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle‐aged adults. Circ Arrhythm Electrophysiol. 2011;4:303–309. doi: 10.1161/CIRCEP.110.959254 [DOI] [PubMed] [Google Scholar]
- 3. Nery PB, Beanlands RS, Nair GM, Green M, Yang J, Mcardle BA, Davis D, Ohira H, Gollob MH, Leung E, et al. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle‐aged adults. J Cardiovasc Electrophysiol. 2014;25:875–881. doi: 10.1111/jce.12401 [DOI] [PubMed] [Google Scholar]
- 4. Kazmirczak F, Chen K‐H, Adabag S, von Wald L, Roukoz H, Benditt DG, Okasha O, Farzaneh‐Far A, Markowitz J, Nijjar PS, et al. Assessment of the 2017 AHA/ACC/HRS guideline recommendations for implantable cardioverter‐defibrillator implantation in cardiac sarcoidosis. Circ Arrhythm Electrophysiol. 2019;12:e007488. doi: 10.1161/CIRCEP.119.007488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dyssekilde JR, Christiansen MK, Johansen JB, Nielsen JC, Bundgaard H, Jensen HK. Familial risk of atrioventricular block in first‐degree relatives. Heart. 2022:heartjnl‐2021‐320411. doi: 10.1136/heartjnl-2021-320411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hasselberg NE, Haland TF, Saberniak J, Brekke PH, Berge KE, Leren TP, Edvardsen T, Haugaa KH. Lamin A/C cardiomyopathy: young onset, high penetrance, and frequent need for heart transplantation. Eur Heart J. 2018;39:853–860. doi: 10.1093/eurheartj/ehx596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baruteau A‐E, Kyndt F, Behr ER, Vink AS, Lachaud M, Joong A, Schott J‐J, Horie M, Denjoy I, Crotti L, et al. SCN5A mutations in 442 neonates and children: genotype‐phenotype correlation and identification of higher‐risk subgroups. Eur Heart J. 2018;39:2879–2887. doi: 10.1093/eurheartj/ehy412 [DOI] [PubMed] [Google Scholar]
- 8. Ishikawa T, Mishima H, Barc J, Takahashi MP, Hirono K, Terada S, Kowase S, Sato T, Mukai Y, Yui Y, et al. Cardiac emerinopathy: a nonsyndromic nuclear envelopathy with increased risk of thromboembolic stroke due to progressive atrial standstill and left ventricular noncompaction. Circ Arrhythm Electrophysiol. 2020;13:e008712. doi: 10.1161/CIRCEP.120.008712 [DOI] [PubMed] [Google Scholar]
- 9. Yeung C, Baranchuk A. Diagnosis and treatment of Lyme carditis: JACC review topic of the week. J Am Coll Cardiol. 2019;73:717–726. doi: 10.1016/j.jacc.2018.11.035 [DOI] [PubMed] [Google Scholar]
- 10. Martins RP, Baruteau A‐E, Daubert J‐C. Poor prognosis in young patients with atrioventricular block of unknown aetiology: who is to blame? The physician or the pacemaker? Eur Heart J. 2021;42:2069–2071. doi: 10.1093/eurheartj/ehab130 [DOI] [PubMed] [Google Scholar]
- 11. Rudbeck‐Resdal J, Christiansen MK, Johansen JB, Nielsen JC, Bundgaard H, Jensen HK. Aetiologies and temporal trends of atrioventricular block in young patients: a 20‐year nationwide study. Europace. 2019;21:1710–1716. doi: 10.1093/europace/euz206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dideriksen JR, Christiansen MK, Johansen JB, Nielsen JC, Bundgaard H, Jensen HK. Long‐term outcomes in young patients with atrioventricular block of unknown aetiology. Eur Heart J. 2021;42:2060–2068. doi: 10.1093/eurheartj/ehab060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baritussio A, Ghosh Dastidar A, Frontera A, Ahmed N, De Garate E, Harries I, Diab I, Duncan E, Thomas G, Nisbet A, et al. Diagnostic yield of cardiovascular magnetic resonance in young‐middle aged patients with high‐grade atrio‐ventricular block. Int J Cardiol. 2017;244:335–339. doi: 10.1016/j.ijcard.2017.06.080 [DOI] [PubMed] [Google Scholar]
- 14. Situ Y, Lin L, Emmanuel S, Subbiah R, Watson B, Namasivayam M, McCrohon J, Jabbour A, Holloway CJ. The usefulness of cardiovascular magnetic resonance in the assessment of patients before pacemaker implantation. JACC Cardiovasc Imaging. 2021;14:305–306. doi: 10.1016/j.jcmg.2020.07.031 [DOI] [PubMed] [Google Scholar]
- 15. Okasha O, Kazmirczak F, Chen KA, Farzaneh‐Far A, Shenoy C. Myocardial involvement in patients with histologically diagnosed cardiac sarcoidosis: a systematic review and meta‐analysis of gross pathological images from autopsy or cardiac transplantation cases. J Am Heart Assoc. 2019;8:e011253. doi: 10.1161/JAHA.118.011253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vuorinen A‐M, Lehtonen J, Pakarinen S, Holmstrom M, Kivisto S, Kaasalainen T. Cardiac magnetic resonance imaging‐based screening for cardiac sarcoidosis in patients with atrioventricular block requiring temporary pacing. J Am Heart Assoc. 2022;11:e024257. doi: 10.1161/JAHA.121.024257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rastan AJ, Doll N, Walther T, Mohr FW. Pacemaker dependent patients with device infection–a modified approach. Eur J Cardiothorac Surg. 2005;27:1116–1118. doi: 10.1016/j.ejcts.2005.02.040 [DOI] [PubMed] [Google Scholar]
- 18. Shah NH, Shah P, Elsayed H, O’Callaghan P, Leong FT, Yousef Z. The indications and safety of prolonged temporary pacing using active‐fixation leads and externalized pulse generator. Pacing Clin Electrophysiol. 2021;44:506–512. doi: 10.1111/pace.14187 [DOI] [PubMed] [Google Scholar]
- 19. Bhuva AN, Moralee R, Brunker T, Lascelles K, Cash L, Patel KP, Lowe M, Sekhri N, Alpendurada F, Pennell DJ, et al. Evidence to support magnetic resonance conditional labelling of all pacemaker and defibrillator leads in patients with cardiac implantable electronic devices. Eur Heart J. 2021:ehab350. [published online August 26, 2021]. doi: 10.1093/eurheartj/ehab350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hilbert S, Jahnke C, Loebe S, Oebel S, Weber A, Spampinato R, Richter S, Doering M, Bollmann A, Sommer P, et al. Cardiovascular magnetic resonance imaging in patients with cardiac implantable electronic devices: a device‐dependent imaging strategy for improved image quality. Eur Heart J Cardiovasc Imaging. 2018;19:1051–1061. doi: 10.1093/ehjci/jex243 [DOI] [PubMed] [Google Scholar]


