Abstract
Background
Despite successful recanalization, up to half of patients with acute ischemic stroke caused by large‐vessel occlusion treated with endovascular treatment (EVT) do not recover to functional independence. We aim to evaluate the role of cerebral circulation time (CCT) as outcome predictor after EVT.
Methods and Results
We retrospectively enrolled consecutive patients with acute ischemic stroke–large‐vessel occlusion undergoing EVT. Three categories of CCT based on digital subtraction angiography were studied: CCT of the stroke side, CCT of the healthy side), and change of CCT of the stroke side versus CCT of the healthy side. Dramatic clinical recovery was defined as a 24‐hour National Institutes of Health Stroke Scale score ≤2 or ≥8 points drop. A modified Rankin Scale score ≤2 at 3 months was considered a favorable outcome. Logistic regression analysis was performed to evaluate the prediction of CCT on prognosis. One hundred patients were enrolled, of which 38 (38.0%) experienced a dramatic clinical recovery and 43 (43.0%) achieved a favorable outcome. Logistic regression analysis found that shorter change of CCT of the stroke side versus CCT of the healthy side and CCT of the stroke side were independent positive prognostic factors for dramatic clinical recovery (odds ratio [OR], 0.189; P=0.033; OR, 0.581; P=0.035) and favorable outcomes (OR, 0.142; P=0.020; OR, 0.581; P=0.046) after adjustment for potential confounders. A model including the change of CCT of the stroke side versus CCT of the healthy side also had significantly higher area under the curve values compared with the baseline model in patients with dramatic clinical recovery (0.780 versus 0.742) or favorable outcome (0.759 versus 0.713).
Conclusions
To our knowledge, this is the first report that CCT based on digital subtraction angiography data exhibits an independent predictive performance for clinical outcome in patients with acute ischemic stroke–large‐vessel occlusion after EVT. Given that this readily available CCT can provide alternative perfusion information during EVT, a prospective, multicenter trial is warranted.
Keywords: cerebral circulation time, digital subtraction angiography, endovascular treatment, large‐vessel occlusion, outcome
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- AIS
acute ischemic stroke
- cCCT
the change of sCCT versus hCCT
- CCT
cerebral circulation time
- CTP
computed tomography perfusion
- DCR
dramatic clinical recovery
- DSA
digital subtraction angiography
- EVT
endovascular treatment
- hCCT
cerebral circulation time of the contralateral healthy side
- LVO
large‐vessel occlusion
- mRS
modified Rankin Scale
- mTICI
modified Thrombolysis in Cerebral Infarction
- NIHSS
National Institutes of Health Stroke Scale
- ORT
onset to reperfusion time
- sCCT
cerebral circulation time of the stroke side
Clinical Perspective
What Is New?
Cerebral circulation time based on digital subtraction angiography during endovascular treatment was found to be associated with clinical outcome in patients with acute ischemic stroke caused by large‐vessel occlusion.
What Are the Clinical Implications?
Our findings suggest that the readily available cerebral circulation time can provide alternative perfusion information during endovascular treatment to predict clinical outcomes in patients with stroke.
Acute ischemic stroke (AIS) leads to serious disability and high mortality rates, especially in patients with large‐vessel occlusion (LVO). 1 The focus of the treatment is to reperfuse the occluded vessel as early as possible, restore blood flow, and salvage the ischemic penumbra tissue. 2 , 3 , 4 Several randomized controlled trials demonstrated the efficacy of endovascular treatment (EVT) in patients with AIS‐LVO. 5 , 6 , 7 , 8 , 9 Despite successful reperfusion, up to half of patients have a poor functional outcome. 10 , 11 , 12 , 13 , 14 It is important to understand the clinical or neuroimaging features that may predict outcomes in the periprocedural care of the patient.
As a significant physiological parameter, cerebral circulation time (CCT) could reflect cerebral blood flow reserve. CCT based on ultrasound has been reported to be substantially prolonged in patients with multiple sclerosis or dementia, compared with controls. 15 , 16 CCT based on quantitative digital subtraction angiography (DSA) was found to be associated with the occurrence of disabling ischemic cerebrovascular events in patients who are not disabled, with symptomatic middle cerebral artery stenosis 17 and cerebral vasospasm in patients with subarachnoid hemorrhage. 18 Furthermore, cerebrovascular reserve as an independent risk factor for cerebral infarction 19 , 20 , 21 was found to be related to CCT derived from DSA. 22 , 23 , 24 , 25 , 26 , 27 In this context, we hypothesize that CCT can inform clinical outcome in patients with AIS undergoing EVT. To the best of our knowledge, there are no known studies about the role of CCT in AIS‐LVO, which deserve to be investigated given the availability of CCT during EVT.
In this study, we used CCT based on quantitative DSA to investigate the value of CCT after EVT in predicting early‐term and long‐term functional outcomes in patients with AIS‐LVO.
Methods
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics and Study Population
This retrospective study was approved by our institutional review board (IRB: Y(2020)087). Patient informed consent was waived given the retrospective nature of the analysis and minimal risk to subjects. We reviewed the clinical database of patients with AIS who received EVT from May 2019 and May 2021. Patients with anterior circulation LVO stroke presenting within 24 hours of onset were judged by the neurointerventionist as eligible for mechanical thrombectomy. Patients with stenosis (extra‐ or intracranial) were not excluded. We identified and enrolled consecutive patients who met the following criteria: (1) age ≥ 18 years; (2) modified Rankin Scale (mRS) score before onset ≤ 1; (3) Evidence of unilateral LVO of the internal carotid artery or middle cerebral artery M1 or proximal M2 segment; (4) endovascular reperfusion via mechanical thrombectomy, and achieved a successful reperfusion (modified Thrombolysis in Cerebral Infarction [mTICI] grade 2B‐3); and (5) complete DSA images were available. Exclusion criteria were as follows: (1) posterior circulation infarction; (2) prestroke disability (mRS score ≥2); (3) intra‐arterial thrombolysis; (4) unqualified DSA including poor quality or incomplete DSA imaging or symptomatic vasospasm as a result of thrombectomy maneuvers; and (5) incomplete clinical data.
Endovascular Thrombectomy, DSA Protocol, and Data Analysis
In our stroke center, once a patient with stroke with a highly suspected LVO arrives at the emergency department, noncontrast head computed tomography (CT) was performed immediately to exclude cerebral hemorrhage, followed by rapid transfer to the angiographic suite without additional CT angiography or computed tomography perfusion (CTP). DSA was performed on the normal side before proceeding to thrombectomy to evaluate the arterial occlusion site and collateral compensation of the circle of Willis as a basis for endovascular treatment. DSA was performed with a uniform and standard protocol in the General Hospital of the Northern Theater Command, Shenyang, Liaoning, China. All subjects were examined by a biplane angiography unit (PHILIPS. UNIQ Clarity FD20/20, Holland) with a power injector (8‐mL ioversol injection with 6 mL/s speed, 200 psi/kg pressure). The catheters were placed at the end of common carotid artery during angiography of the healthy side. The location of the catheter during angiography on the stroke side was determined by the interventionalist. The collateral grading system was determined on the basis of DSA according to American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology. The demographic characteristics, stroke risk factors, neurological function scores (National Institutes of National Institutes of Health Stroke Scale [NIHSS] and mRS), and clinical metrics such as onset to reperfusion time (ORT) were reviewed and analyzed. Hemorrhagic transformation was categorized according to the ECASS‐3 (European Cooperative Acute Stroke Study 3). 28 In the neuroimaging data, nonresponsible vessel stenosis of >50% was analyzed as a confounding factor in our study.
Cerebral Circulation Time Measurement
In concordance with prior studies, 29 CCT was defined as the time from the appearance of contrast at the siphon segment of the internal carotid to the end of the arterial phase during DSA on the anteroposterior and lateral image (Figure 1). The CCT was measured immediately after successful reperfusion (mTICI grade 2B‐3) was achieved. All images were evaluated by 2 experienced individuals who were blinded to the clinical data. A third adjudicator (an interventional neuroradiologist) who was unaware of the clinical information participated in the additional evaluation in case of disagreement. Specially, CCT of the same patient was measured 3 times to reduce error in data acquisition, and the final CCT was determined by the average of the 3 sets of data. In our center, angiography on the healthy side was conducted after thrombectomy if the patients had CT angiography or CTP. Otherwise, DSA series of the healthy side were acquired before proceeding to thrombectomy. In the present study, 3 components of CCT were used: (1) CCT of the stroke side (sCCT); (2) CCT of the contralateral healthy side (hCCT); (3) the change of sCCT versus hCCT (cCCT), which was calculated with the following formula: cCCT=(sCCT–hCCT) / hCCT.
Figure 1. Schematic diagram of the measurement of cerebral circulation time (CCT).
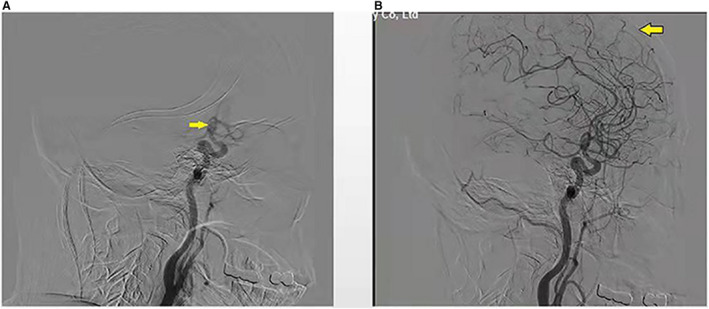
(A) Appearance of the siphon segment of internal carotid (yellow arrow); (B) end of the arterial phase (yellow arrow). The CCT was defined as the time from the appearance of the siphon segment of internal carotid (yellow arrow) to the end of the arterial phase during digital subtraction angiography.
Outcome Measures
Two clinical outcomes were used in the current study: (1) dramatic clinical recovery (DCR), which was defined as a decrease of ≥8 points from baseline NIHSS or a 24‐hour NIHSS score ≤2 30 ; and (2) good outcome, defined as an mRS score ≤2 at 3 months by 2 evaluators blinded to endovascular treatment and CCT results. Based on the 2 outcomes, the patients were divided into DCR (+) versus DCR (−) groups, and good versus poor outcome groups.
Statistical Analysis
Categorical variables are presented as absolute values and percentages, and continuous variables as mean with SD if normally distributed or median with interquartile range if not normally distributed. Statistical significance for intergroup differences was assessed by Fisher’s exact test for categorical variables and by Student’s t test or the Mann‐Whitney U test for continuous variables. Clinically relevant variables and those showing P<0.1 in univariate analysis were included in the multivariate model. For DCR, the multivariate model included admission NIHSS, number of passes, collateral score, and intracranial hemorrhage (ICH). Other than the above variables, Alberta Stroke Program Early Computed Tomographic Score and diabetes were not equally distributed for good outcome at 3 months and may have confounded the clinical outcome, while mTICI was demonstrated to be associated with good clinical outcome. Therefore, these variables were also added to the regression model for good outcome. Multivariable logistic regression analyses were performed for each group to determine factors that could be considered as independent predictors of early‐term and long‐term clinical outcome. Interrater variability of CCT was tested with the intraclass correlation coefficient. A 2‐sided P value <0.05 was considered significant for all tests. Receiver operating characteristic curve analysis of CCT was performed to evaluate the predictive accuracy of outcome in patients.
Results
During the study period, 230 consecutive patients with AIS‐LVO received EVT in our center. We excluded patients with posterior circulation (n=58), final mTICI grade 0‐2A (n=45), intra‐arterial thrombolysis (n=18), unqualified DSA including poor quality or incomplete DSA imaging or symptomatic vasospasm as a result of thrombectomy maneuvers (n=4), incomplete clinical data (n=3), and prestroke disability (mRS ≥2) (n=2). Finally, 100 patients were enrolled in the current study (Figure 2). Of 100 patients, 20 (20.0%) were women, with a mean age of 63.0±10.7 years, a mean NIHSS score of 15.7±4.5, a median Alberta Stroke Program Early Computed Tomographic Score of 9 (7–10), a median hCCT of 2.25 (1.67–2.83) seconds, a median sCCT of 2.17 (1.83–3.00) seconds, and a median cCCT of 0.00 (−0.15 to 0.33). After a median time of 410 (320–616) minutes from symptom onset to reperfusion, 43 (43.0%) achieved mTICI grade 2B, and 57 (57.0%) mTICI grade 3. As to the catheter location, 35 cases were uncertain, 20 at the distal aspect of the common carotid artery, 23 at the cervical segment of the internal carotid artery, 18 at the petrous segment of internal carotid artery, 4 at the lacerum segment of the internal carotid artery (Table 1). To minimize the effect of missing data, the catheter position as a confounding factor was adjusted in 65 patients with definite catheter position, and we found that lower cCCT was remained associated with good outcome at 3 months (P=0.047). The demographic and clinical data of the patients are shown in Table 2.
Figure 2. A flowchart of subject selection.
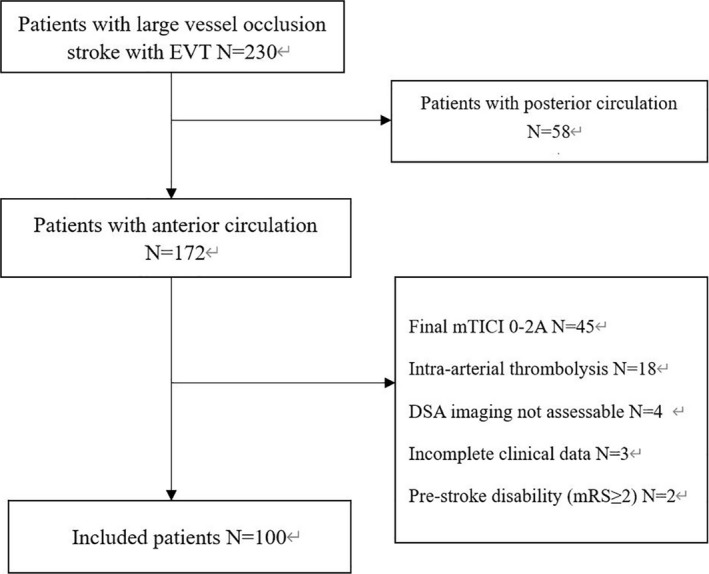
EVT indicates endovascular treatment.
Table 1.
Baseline Characteristics and Outcomes of 100 Patients
| Demographics | Values |
|---|---|
| Age, y, mean (SD) | 63.0±10.7 |
| Sex (female), n (%) | 20 (20.0) |
| Hypertension, n (%) | 65 (65.0) |
| Diabetes, n (%) | 28 (28.0) |
| Smoking story, n (%) | 58 (58.0) |
| Atrial fibrillation, n (%) | 32 (32.0) |
| Stroke, n (%) | 27 (27.0) |
| Clinical and imaging features | |
| Admission NIHSS, mean (SD) | 15.7±4.5 |
| ASPECTS, median (IQR) | 9 (7 to 10) |
| SBP after EVT, mean (SD) | 139.7±23.5 |
| DBP after EVT, mean (SD) | 82.6±14.7 |
| sCCT, s, median (IQR) | 2.17 (1.83 to 3.00) |
| hCCT, s, median (IQR) | 2.25 (1.67 to 2.83) |
| cCCT, median (IQR) | 0.00 (−0.15 to 0.33) |
| Collateral score, median (IQR) | 1 (0 to 3) |
| Number of passes, median (IQR) | 1 (1 to 2) |
| NRV stenosis, n (%) | 52 (52.0) |
| OPT, min, median (IQR) | 338 (232 to 536) |
| DPT, min, median (IQR) | 50 (35 to 89) |
| ORT, min, median (IQR) | 410 (320 to 616) |
| Final mTICI 2B, n (%) | 43 (43.0) |
| Final mTICI 3, n (%) | 57 (57.0) |
| TOAST, n (%) | |
| LAA | 60 (60.0) |
| Cardioembolism | 26 (26.0) |
| Undetermined | 14 (14.0) |
| ICH | 41 (41.0) |
| Catheter location, n (%) | |
| End of common carotid artery | 20 (20.0) |
| Cervical segment of internal carotid artery | 23 (23.0) |
| Petrous segment of internal carotid artery | 18 (18.0) |
| Lacerum segment of internal carotid artery | 4 (4.0) |
| Undetermined | 35 (35.0) |
| ICH type, n (%) | |
| HI‐1 | 4 (4.0) |
| HI‐2 | 9 (9.0) |
| PH‐1 | 7 (7.0) |
| PH‐2 | 21 (21.0) |
| Outcomes, n (%) | |
| DCR at 24 h | 38 (38.0) |
| mRS score ≤2 at 90 d | 43 (43.0) |
ASPECTS indicates Alberta Stroke Program Early CT Score; cCCT, the change of sCCT versus hCCT; CCT, cerebral circulation time; DBP, diastolic blood pressure; DCR, dramatic clinical recovery; DPT, door to puncture time; EVT, endovascular treatment; hCCT, CCT of the contralateral healthy side; HI‐1, hemorrhagic infarction‐1; HI‐2, hemorrhagic infarction‐2; ICH, intracranial hemorrhage; IQR, interquartile range; LAA, large artery atherosclerosis; mTICI, modified Thrombolysis in Cerebral Ischemia; NIHSS, National Institutes of Health Stroke Scale; NRV stenosis, nonresponsible vessel stenosis; OPT, onset to puncture time; ORT, onset‐to‐reperfusion time; PH‐1, parenchymal hematona‐1; PH‐2, parenchymal hematona‐2; SBP, systolic blood pressure; sCCT, CCT of the stroke ipsilateral to the side; and TOAST, Trial of Org 10172 in Acute Stroke Treatment.
Table 2.
Clinical Data and Outcomes for Patients With Anterior Circulation Large‐Vessel Occlusion Undergoing EVT
| DCR (−) (N=62) | DCR (+) (N=38) | P value | Poor outcome (N=57) | Good outcome (N=43) | P value | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Sex (female), n (%) | 11 (17.74) | 9 (23.68) | 0.607 | 8 (14.04) | 12 (31.58) | 0.129 |
| Age, y, mean (SD) | 62.85±10.80 | 63.29±10.73 | 0.845 | 62.98±11.18 | 63.07±10.22 | 0.968 |
| Hypertension, n (%) | 38 (61.29) | 26 (68.42) | 0.666 | 35 (61.40) | 29 (67.44) | 0.675 |
| Diabetes, n (%) | 20 (32.26) | 8 (21.05) | 0.259 | 22 (38.60) | 6 (13.95) | 0.007 |
| Atrial fibrillation, n (%) | 20 (32.26) | 12 (31.58) | 0.562 | 15 (26.32) | 17 (39.53) | 0.196 |
| Stroke, n (%) | 16 (25.81) | 11 (28.95) | 0.818 | 15 (26.32) | 12 (27.90) | 0.859 |
| Smoking story, n (%) | 34 (54.84) | 24 (63.16) | 0.532 | 35 (61.40) | 23 (53.49) | 0.540 |
| Clinical and imaging features | ||||||
| Admission NIHSS, mean (SD) | 15.56±4.75 | 15.89±4.05 | 0.722 | 16.65±4.66 | 14.42±3.03 | 0.011 |
| ASPECTS, median (IQR) | 9 (7–10) | 9 (8–10) | 0.636 | 8 (6.5–10) | 9 (8–10) | 0.062 |
| SBP after EVT, mean (SD) | 138.03±22.48 | 142.47±25.81 | 0.362 | 141.77±23.18 | 137.00±23.95 | 0.318 |
| DBP after EVT, mean (SD) | 81.13±13.60 | 84.87±16.31 | 0.219 | 83.52±14.48 | 81.55±13.74 | 0.834 |
| sCCT, s, median (IQR) |
2.33 (1.83–3.33) |
2.00 (1.67–2.33) |
0.009 |
2.33 (1.83–3.42) |
2.00 (1.67–2.33) |
0.015 |
| hCCT, s, median (IQR) |
2.17 (1.79–2.83) |
2.33 (1.67–2.71) |
0.946 |
2.17 (1.75–2.83) |
2.00 (1.67–2.67) |
0.859 |
| cCCT, median (IQR) |
0.13 (−0.08 to 0.38) |
−0.12 (−0.31 to 0.16) |
0.005 |
0.15 (−0.07 to 0.42) |
−0.10 (−0.17 to 0.14) |
0.003 |
| Collateral score, median (IQR) | 1 (0–2) | 2 (1–3) | 0.008 | 1 (0–2) | 2 (1–3) | 0.005 |
| Number of passes, median (IQR) | 2 (1–2) | 1 (1–2) | 0.014 | 2 (1–2) | 1 (1–2) | 0.041 |
| NRV stenosis, n (%) | 33 (53.23) | 19 (50.00) | 0.838 | 29 (50.88) | 23 (53.49) | 0.842 |
| OPT, min, median (IQR) |
317 (238–477) |
407 (225–751) |
0.369 |
374 (250–532) |
329 (229–451) |
0.347 |
| DPT, min, median (IQR) | 49 (36–79) | 53 (33–106) | 0.572 | 55 (41–98) | 46 (29–82) | 0.077 |
| ORT, min, median (IQR) |
436 (318–593) |
397 (319–655) |
0.915 |
487 (322–629) |
372 (311–576) |
0.204 |
| Final mTICI | 0.407 | 0.102 | ||||
| mTICI 2B, n (%) | 29 (46.77) | 14 (36.84) | 29 (50.88) | 14 (32.56) | ||
| mTICI 3, n (%) | 33 (53.23) | 24 (63.16) | 28 (49.12) | 29 (67.44) | ||
| TOAST, n (%) | 0.428 | 0.709 | ||||
| LAA | 35 (56.45) | 25 (65.79) | 36 (63.18) | 24 (55.81) | ||
| Cardioembolism | 19 (30.65) | 7 (18.42) | 13 (22.81) | 13 (30.23) | ||
| Undetermined | 8 (12.90) | 6 (15.79) | 8 (14.04) | 6 (13.95) | ||
| ICH | 30 (48.39) | 11 (28.95) | 0.063 | 29 (50.88) | 12 (27.91) | 0.025 |
| ICH type, n (%) | 0.408 | 0.046 | ||||
| HI‐1 | 3 (4.84) | 1 (2.63) | 1 (1.75) | 3 (6.98) | ||
| HI‐2 | 6 (9.68) | 3 (7.89) | 7 (12.28) | 2 (4.65) | ||
| PH‐1 | 6 (9.68) | 1 (2.63) | 6 (10.53) | 1 (2.32) | ||
| PH‐2 | 15 (24.19) | 6 (15.79) | 15 (26.32) | 6 (13.95) | ||
ASPECTS indicates Alberta Stroke Program Early CT Score; cCCT, the change of sCCT versus hCCT; CCT, cerebral circulation time; DBP, diastolic blood pressure; DCR, dramatic clinical recovery; DPT, door‐to‐puncture time; EVT, endovascular treatment; HI‐1, hemorrhagic infarction‐1; HI‐2, hemorrhagic infarction‐2; hCCT, CCT of the contralateral healthy side; ICH, intracranial hemorrhage; IQR, interquartile range; LAA, large artery atherosclerosis; mTICI, modified Thrombolysis in Cerebral Ischemia; NIHSS, National Institutes of Health Stroke Scale; NRV stenosis, non‐responsible vessel stenosis; OPT, onset‐to‐puncture time; ORT, onset‐to‐reperfusion time; PH‐1, parenchymal hematona‐1; PH‐2, parenchymal hematona‐2; SBP, systolic blood pressure; sCCT, CCT of the stroke ipsilateral to the side; and TOAST, Trial of Org 10172 in Acute Stroke Treatment.
At 24 hours after EVT, 38 (38.0%) patients experienced a DCR. Patients with DCR presented with lower sCCT (2.00 [1.67–2.33] versus 2.33 [1.83–3.33]; P=0.009) and lower cCCT ( −0.12 [−0.31 to 0.16] versus 0.13 [−0.08–0.38]; P=0.005) (Table 2, Figure 3). At 3 months after EVT, 43 (43.0%) patients achieved good outcome. Patients with good outcome had lower sCCT (2.00 [1.67–2.33] versus 2.33 [1.83–3.42]; P=0.015) and lower cCCT (−0.10 [−0.17 to 0.14] versus 0.15 [−0.07 to 0.42]; P=0.003).
Figure 3. Relationship of cerebral circulation time (CCT) to clinical outcome.
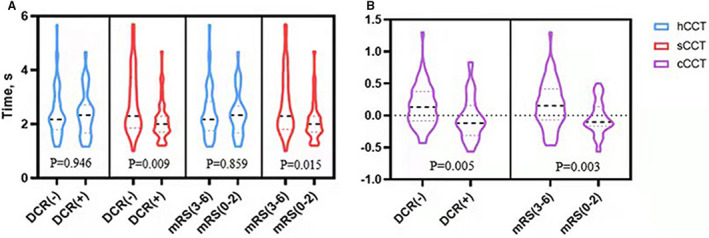
(A) No difference is seen in hCCT between DCR (−) and DCR (+) groups and between the mRS 3‐6 and mRS 0‐2 groups, but a significant difference in sCCT between DCR (−) and DCR (+) groups and between the mRS 3‐6 and mRS 0‐2 groups; (B) a significant difference is seen in cCCT between DCR (−) and DCR (+) groups and between the mRS 3‐6 and mRS 0‐2 groups. DCR, dramatic clinical recovery; hCCT indicates CCT of the healthy side; mRS, modified Rankin Scale; and sCCT, CCT of the stroke side.
Conversely, hCCT was not associated with early‐ or long‐term outcomes (P=0.946 and P=0.859, respectively; Table 2, Figure 3). Furthermore, lower initial NIHSS was not correlated with DCR (odds ratio [OR], 1.017; P=0.719; Table 3), while it was positively associated with good outcome at 3 months (OR, 0.882; P=0.017; Table 4). Higher collateral score was significantly associated with DCR (OR, 1.514; P=0.012; Table 3) and good clinical outcome (OR, 1.562; P=0.007; Table 4). Presence of ICH was negatively associated with good outcome (OR, 0.374; P=0.022; Table 4).
Table 3.
Univariable and Multivariable Logistic Regression Analyses for DCR at 24 Hour
| Parameter | DCR at 24 h | |
|---|---|---|
| OR (95% CI) | P value | |
| Univariable logistic regression | ||
| Admission NIHSS | 1.017 (0.929–1.113) | 0.719 |
| Number of passes | 0.381 (0.188–0.769) | 0.007 |
| Collateral score | 1.514 (1.094–2.096) | 0.012 |
| ICH | 0.435 (0.184–1.027) | 0.058 |
| sCCT | 0.509 (0.300–0.865) | 0.013 |
| cCCT | 0.166 (0.041–0.668) | 0.011 |
| Multivariable logistic regression | ||
| sCCT* | 0.581 (0.353–0.957) | 0.035 |
| cCCT* | 0.189 (0.040–0.892) | 0.033 |
cCCT indicates the change of sCCT versus hCCT; CCT, cerebral circulation time; DCR, dramatic clinical recovery; ICH, intracranial hemorrhage; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; and sCCT, CCT of the stroke ipsilateral to the side.
Adjusted for admission NIHSS, number of passes, collateral score, and ICH.
Table 4.
Univariable and Multivariable Logistic Regression Analyses for mRS≤2 at 3 Months
| Parameter | mRS≤2 at 3 months | |
|---|---|---|
| OR (95% CI) | P value | |
| Univariable logistic regression | ||
| Admission NIHSS | 0.882 (0.797–0.978) | 0.017 |
| Number of passes | 0.991 (0.974–1.009) | 0.315 |
| Collateral score | 1.562 (1.129–2.160) | 0.007 |
| ICH | 0.374 (0.161–0.870) | 0.022 |
| ASPECTS | 0.804 (0.627–1.032) | 0.086 |
| Diabetes | 0.258 (0.094–0.711) | 0.009 |
| Final mTICI | 0.848 (0.391–1.841) | 0.677 |
| sCCT | 0.456 (0.267–0.778) | 0.004 |
| cCCT | 0.124 (0.030–0.504) | 0.004 |
| Multivariable logistic regression | ||
| sCCT* | 0.581 (0.341–0.991) | 0.046 |
| cCCT* | 0.142 (0.028–0.734) | 0.020 |
ASPECTS indicates Alberta Stroke Program Early CT Score; cCCT, the change of sCCT versus hCCT; CCT, cerebral circulation time; ICH, intracranial hemorrhage; mRS, modified Rankin Scale; mTICI, modified Thrombolysis in Cerebral Ischemia; NIHSS, National Institutes of Health Stroke Scale; and sCCT, CCT of the stroke ipsilateral to the side.
Adjusted for admission NIHSS, number of passes, collateral score, ICH, ASPECTS, diabetes and final mTICI.
Logistic regression analysis revealed that shorter cCCT and sCCT were independent positive prognostic factors for early‐term functional (OR, 0.189; P=0.033; OR, 0.581; P=0.035; Table 3) and long‐term functional outcomes (OR, 0.142; P=0.020; OR, 0.581; P=0.046; Table 4) after adjustment for potential confounders.
The predictive models were constructed with baseline correlation variables and CCT. For the prediction of DCR, the area under the curve of model 1 (admission NIHSS, number of passes, collateral score, ICH, final mTICI) was 0.742 (95% CI, 0.642–0.843). When sCCT and cCCT were added to prediction model 1 separately, the area under the curve increased to 0.776 (95% CI, 0.681–0.872) in model 2 (model 1+sCCT), and 0.780 (95% CI, 0.682–0.879) in model 3 (model 1+cCCT). For favorable long‐term outcomes, the area under the curve was 0.713 (95% CI, 0.612–0.814) in model 1, 0.753 (95% CI, 0.660–0.847) in model 2, 0.759 (95% CI, 0.664–0.854) in model 3, respectively (Figure 4).
Figure 4. Receiver operating characteristic curve analysis of different cerebral circulation time (CCT) models for predicting DCR (A) and good outcome (B).
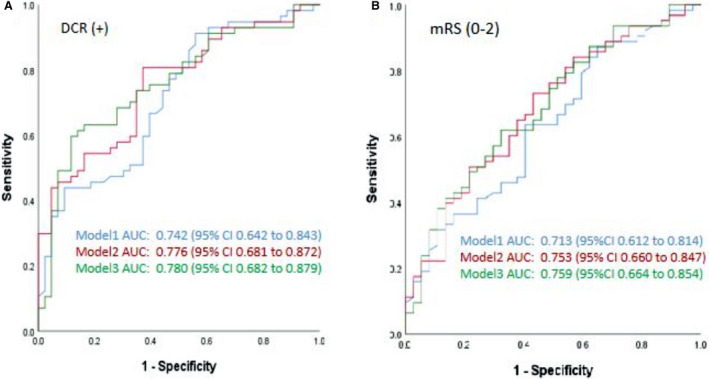
Model 1 adjusted by admission NIHSS, number of passes, collateral score, ICH, and final mTICI. Model 2 adjusted by admission NIHSS, number of passes, collateral score, ICH, final mTICI, and sCCT. Model 3 adjusted by admission NIHSS, number of passes, collateral score, ICH, final mTICI, and cCCT. AUC indicates area under the curve; cCCT, the change of sCCT versus hCCT; CCT, cerebral circulation time; DCR, dramatic clinical recovery; hCCT, CCT of the healthy side; ICH, intracranial hemorrhage; mRS, modified Rankin Scale; mTICI, modified Thrombolysis in Cerebral Infarction; NIHSS, National Institutes of Health Stroke Scale; and sCCT, CCT of the stroke side.
The optimal cutoff point of cCCT (−0.062) predicted good outcome at 3 months with 75% sensitivity and 58% specificity. In contrast, the optimal cutoff point of sCCT (2.19) predicted good outcome at 3 months with 70% sensitivity and 68% specificity. As shown in Figure 5, the probability for favorable outcome increased as cCCT or sCCT decreased.
Figure 5. Probability curves for good outcome (mRS 0‐2 at 3 months), stratified according to mTICI versus sCCT (A) or cCCT (B).
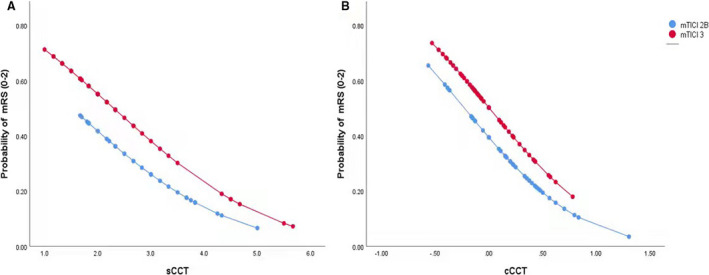
cCCT indicates the change of sCCT versus hCCT; CCT, cerebral circulation time; hCCT, cerebral circulation time of the healthy side; mRS, modified Rankin Scale; mTICI, modified Thrombolysis in Cerebral Infarction; and sCCT, cerebral circulation time of the stroke side.
Discussion
Despite successful reperfusion, patients with AIS‐LVO treated with EVT frequently do not achieve a favorable outcome. In our series, this phenomenon occurred in more than half (57.0%) of treated patients, which is in agreement with a prior report. 9 Previous studies suggested the NIHSS, ORT, and collateral status were associated with clinical outcomes after successful revascularization. 11 , 12 , 13 , 31 , 32 , 33 , 34 , 35 We used CCT based on DSA during the angiography procedure to assess its predictive value for outcome of patients with LVO and found that sCCT or cCCT after EVT was an independent and strong predictor of clinical functional outcome. To the best of our knowledge, this is the first report to describe these findings.
Cerebrovascular reserve is an important indicator of the cerebrovascular hemodynamic state, which is generally estimated by perfusion magnetic resonance imaging, perfusion CT, or single photon emission CT. As an endogenous neuroprotective mechanism, cerebrovascular reserve has been found to be significantly impaired in patients with vascular occlusion, even in the absence of obvious neurological damage. 36 , 37 It has been demonstrated that patients with impaired cerebrovascular reserve were ≈4 times more likely to develop stroke or transient ischemic attack. 38 Furthermore, cerebrovascular reserve was found to be a more accurate predictor of stroke than the degree of internal carotid artery or middle cerebral artery stenosis. 39
CCT was found to be related to cerebrovascular reserve, 23 , 24 , 25 and provide cerebrovascular reserve information during surgery without the need for SPECT or CTP. For example, (1) CCT was found to be correlated with prognosis for patients with subarachnoid hemorrhage: longer CCT predicted poorer outcome 24 ; (2) prolonged CCT is an independent prognostic factor for the occurrence of disabling ischemic cerebrovascular events in patients with symptomatic nondisabling middle cerebral artery stenosis. 17 Furthermore, Cronqvist et al found that CCT was more sensitive than the diffusible isotope technique for detecting the decreased flow associated with occlusive vascular disease. 40
In this context, we argue that CCT after EVT can offer perfusion information and reflect the intracranial hemodynamic change of reflow or no‐reflow after recanalization of an artery. The current results showed that sCCT or cCCT after EVT can predict clinical outcome in patients with AIS‐LVO. In the current study, we used hCCT as a self‐control, as it may be representative of the normal CCT of the patient’s stroke side before stroke onset.
Collectively, we argue that prolonged sCCT may reflect the poor pathophysiology of the ischemic penumbra after EVT, which may include decreased perfusion pressure with an evolving infarct and hemodynamic changes in the cerebral vessels with impairment of autoregulation and cerebral vessel disruption. Interestingly, we found that cCCT exhibited a more powerful prediction for clinical outcome than sCCT. The results are plausible because cCCT excluded the difference in CCT among individuals. In addition, short CCT and good outcome were more likely to be seen in patients who achieved mTICI grade 3, but shorter CCT will get higher probability of good outcome in patients with mTICI grade 3 (Figure 5), which is in line with higher area under the curve values in the model including sCCT and cCCT compared with the baseline model including mTICI (Figure 4). In our study, good outcome was found in 50.88% patients with TICI grade 3, which will increase to 73.91% in patients with mTICI3 and cCCT ≤ −0.062. Thus, CCT further improves the predictive performance. Given that CCT can be immediately obtained after EVT without additional analysis and equipment, a prospective study may be warranted.
It is worthwhile to note that the optimal cutoff point of cCCT was −0.062 in the present study. This seems to indicate that sCCT close to or slightly faster than hCCT could have a better prognosis. Importantly, for the same mTICI, the likelihood of good outcome increases significantly with a decrease in sCCT or cCCT; namely, prolonged sCCT or increased cCCT will result in worse outcome. Our findings suggest that patients with cCCT >−0.062 may be a target population for closer monitoring or neuroprotection even if successful reperfusion was achieved during EVT.
In our center, most patients with AIS‐LVO will directly transfer to angiography treatment after noncontrast CT without further CT angiography or CTP. We reason that the paradigm will significantly reduce the time from onset to EVT. In this context, we conduct angiography on the healthy side before proceeding to thrombectomy to evaluate the collateral compensation of the circle of Willis to inform the treatment strategy. This procedure usually takes about 1 minute, which is less than the time of CT angiography or CTP acquisition. We argue it is worthy given that the paradigm will reduce the onset‐to‐groin‐puncture time, which is further supported by a recent study demonstrating that a direct angiography treatment paradigm without repeated imaging for transferred patients with AIS‐LVO is reasonable. 41 In addition, we did not find the association of ORT with outcome, which has been noted in previous studies. 2 , 42 The small sample and longer ORT in the current study (410 minutes [320–616]) versus previous studies (286 minutes [215–363]) 2 may explain the discrepancy because the long ORT will weaken the effect of mechanical thrombectomy on outcome, resulting in the neutral association in the current study. Another possible explanation is that longer ORT will lead to a higher likelihood of cerebral autoregulation failure despite successful recanalization, 43 which may contribute to the differences in ipsilateral CCT.
Limitation
The main limitation of our study relates to the retrospective nature of our study with the relatively small sample of a single‐center, which was inevitably susceptible to selection bias and potential confounders. In the present study, CCT of the hCCT was used as a control to exclude potential confounding factors. Second, the measurement of CCT may be affected by multiple factors such as manual measurement methods, injection volume settings, and catheter position. The manual measurement was assured by good interobserver reproducibility. In addition, our patient images were routinely obtained with a power injector with a standardized rate to attenuate for this variation. We did not find the catheter position affected CCT on the basis of 3 results: (1) no effect was identified on the stroke side (P=0.406; Table 5); (2) the good consistency between interobservers for both hCCT (intraclass correlation coefficient, =0.992; 95% CI, 0.988–0.994; P<0.001) and sCCT (intraclass correlation coefficient, =0.977; 95% CI, 0.966–0.985; P<0.001); (3) CCT measured from 2 angiographs in the same patient showed excellent consistency between different catheter positions (intraclass correlation coefficient, =0.912; 95% CI, 0.826–0.957; P<0.001), based on our carotid artery stenting cohort. Third, in the current study, the EVT strategy was at the discretion of the interventionalist. No techniques were excluded, which may be a confounding factor. Fourth, proximal arterial stenosis may influence the CCT, which may represent a bias. In our cohort, there was a low rate of extra/intracranial carotid and middle cerebral artery stenosis on the ipsilateral side of the patient’s stroke (18% and 13%, respectively). Furthermore, there was no significant difference in sCCT between patients with stenosis and without stenosis (P=0.125). Based on these findings, it is likely that a proximal arterial stenosis had little effect on CCT in the current study. Other limitations in our analysis are the small number of cases, underrepresentation of female patients, and the imbalance in the number of cases between groups. Finally, the relationship between CCT and collateral circulation was not included in the analysis of this study, which will be investigated in another article.
Table 5.
Cerebral Circulation Time of Different Catheter Positions
| End of common carotid artery | Cervical segment of internal carotid artery | Petrous segment of internal carotid artery | Lacerum segment of internal carotid artery | P value | |
|---|---|---|---|---|---|
| sCCT, s, median | 2.085 (1.711–2.833) | 2.333 (1.670–3.330) | 2.500 (1.830–4.330) | 2.165 (1.873–3.080) | 0.406 |
sCCT indicates cerebral circulation time of the stroke ipsilateral to the side.
Conclusions
To the best of our knowledge, this is the first study to report that shorter sCCT and lower cCCT after EVT were independent positive prognostic factors for clinical outcome in patients with AIS‐LVO. Given that CCT is readily available and can provide alternative information during EVT, a prospective, multicenter trial is warranted.
Sources of Funding
The work was supported by grants from the Science and Technology Plan of Shen Yang (20‐205‐4‐007) and the Science and Technology Project Plan of Liao Ning Province (2018225023, 2019JH2/10300027).
Disclosures
None.
Acknowledgments
Author contributions: Drs J.‐Q. Wang, Y.‐J. Wang, Qiu, Li, Sun, Y.‐G. Zhao, X. Liu, and Z.‐A. Zhao contributed to the acquisition of data; Drs Li, Qiu, and Sun analyzed imaging data. Drs Y.‐J. Wang, and J.‐Q. Wang conducted data analysis; Dr J.‐Q. Wang drafted the manuscript; Dr Nguyen critically revised the manuscript. Dr Chen contributed to the study design and critically edited the manuscript. All authors approved the content of the manuscript.
For Sources of Funding and Disclosures, see page 10.
References
- 1. Smith WS, Lev MH, English JD, Camargo EC, Chou M, Johnston SC, Gonzalez G, Schaefer PW, Dillon WP, Koroshetz WJ, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke. 2009;40:3834–3840. doi: 10.1161/STROKEAHA.109.561787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CBLM, Dippel DW, Campbell BC, Nogueira RG, Demchuk AM, Tomasello A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta‐analysis. JAMA. 2016;316:1279–1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 3. Keigher KM. Large vessel occlusion in the acute stroke patient: identification, treatment, and management. Crit Care Nurs Clin North Am. 2020;32:21–36. doi: 10.1016/j.cnc.2019.11.007 [DOI] [PubMed] [Google Scholar]
- 4. Truelsen T, Hansen K, Andersen G, Sørensen L, Madsen C, Diaz A, Stavngaard T, Hundborg HH, Højgaard J, Hjort N, et al. Acute endovascular reperfusion treatment in patients with ischaemic stroke and large‐vessel occlusion (Denmark 2011–2017). Eur J Neurol. 2019;26:1044–1050. doi: 10.1111/ene.13931 [DOI] [PubMed] [Google Scholar]
- 5. Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, Guillemin F, THRACE investigators . Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–1147. doi: 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 6. Berkhemer OA, Majoie CB, Dippel DW, MR CLEAN Investigators . Endovascular therapy for ischemic stroke. N Engl J Med. 2015;372:2363. doi: 10.1056/NEJMc1504715 [DOI] [PubMed] [Google Scholar]
- 7. Bishop BM. Endovascular therapy for ischemic stroke. N Engl J Med. 2015;372:2364. doi: 10.1056/NEJMc1504715 [DOI] [PubMed] [Google Scholar]
- 8. Goyal M, Demchuk AM, Hill MD. Endovascular therapy for ischemic stroke. N Engl J Med. 2015;372:2366. doi: 10.1056/NEJMc1504715 [DOI] [PubMed] [Google Scholar]
- 9. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 10. Weyland CS, Mokli Y, Vey JA, Kieser M, Herweh C, Schönenberger S, Bendszus M, Möhlenbruch MA, Ringleb PA, Nagel S. Predictors for failure of early neurological improvement after successful thrombectomy in the anterior circulation. Stroke. 2021;52:1291–1298. doi: 10.1161/STROKEAHA.120.030519 [DOI] [PubMed] [Google Scholar]
- 11. Shi Z‐S, Liebeskind DS, Xiang B, Ge SG, Feng L, Albers GW, Budzik R, Devlin T, Gupta R, Jansen O, et al. Predictors of functional dependence despite successful revascularization in large‐vessel occlusion strokes. Stroke. 2014;45:1977–1984. doi: 10.1161/STROKEAHA.114.005603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kennedy SA, Baerlocher MO, Baerlocher F, Socko D, Sacks D, Nikolic B, Wojak JC, Haskal ZJ. Meta‐analysis of local endovascular therapy for acute ischemic stroke. J Vasc Interv Radiol. 2016;27(3):307–321.e2. doi: 10.1016/j.jvir.2015.11.053. doi: 10.1016/j.jvir.2015.11.053 [DOI] [PubMed] [Google Scholar]
- 13. Zhang YH, Shi MC, Wang ZX, Li C, Sun MY, Zhou J, Zhang WB, Huo LW, Wang SC. Factors associated with poor outcomes in patients undergoing endovascular therapy for acute ischemic stroke due to large‐vessel occlusion in acute anterior circulation: a retrospective study. World Neurosurg. 2021;149:e128–e134. doi: 10.1016/j.wneu.2021.02.064 [DOI] [PubMed] [Google Scholar]
- 14. Riou‐Comte N, Guillemin F, Gory B, Lapergue B, Zhu F, Soudant M, Piotin M, Humbertjean L, Mione G, Lacour J‐C, et al. Predictive factors of functional independence after optimal reperfusion in anterior circulation ischaemic stroke with indication for intravenous thrombolysis plus mechanical thrombectomy. Eur J Neurol. 2021;28:141–151. doi: 10.1111/ene.14509 [DOI] [PubMed] [Google Scholar]
- 15. Mancini M, Morra VB, Di Donato O, Maglio V, Lanzillo R, Liuzzi R, Salvatore E, Brunetti A, Iaccarino V, Salvatore M, et al. Multiple sclerosis: cerebral circulation time. Radiology. 2012;262:947–955. doi: 10.1148/radiol.11111239 [DOI] [PubMed] [Google Scholar]
- 16. Schreiber SJ, Doepp F, Spruth E, Kopp UA, Valdueza JM. Ultrasonographic measurement of cerebral blood flow, cerebral circulation time and cerebral blood volume in vascular and Alzheimer's dementia. J Neurol. 2005;252:1171–1177. doi: 10.1007/s00415-005-0826-8 [DOI] [PubMed] [Google Scholar]
- 17. Chen Z, Li M, Wu Z, Zhang M, Weng G, Li M, Liao R, Zhao P, Wu J, Zhu S, et al. Cerebral circulation time is a potential predictor of disabling ischemic cerebrovascular events in patients with non‐disabling middle cerebral artery stenosis. Front Neurol. 2021;12: 653752. doi: 10.3389/fneur.2021.653752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin CF, Hsu SP, Lin CJ, Guo WY, Liao CH, Chu WF, Hung SC, Shih YS, Lin YT. Prolonged cerebral circulation time is the best parameter for predicting vasospasm during Initial CT perfusion in subarachnoid hemorrhagic patients. PLoS One. 2016;11:e0151772. doi: 10.1371/journal.pone.0151772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bashir A, Abebe ZA, McInnes KA, Button EB, Tatarnikov I, Cheng WH, Haber M, Wilkinson A, Barron C, Diaz‐Arrastia R, et al. Increased severity of the CHIMERA model induces acute vascular injury, sub‐acute deficits in memory recall, and chronic white matter gliosis. Exp Neurol. 2020;324: 113116. doi: 10.1016/j.expneurol.2019.113116 [DOI] [PubMed] [Google Scholar]
- 20. Cramer NP, Korotcov A, Bosomtwi A, Xu X, Holman DR, Whiting K, Jones S, Hoy A, Dardzinski BJ, Galdzicki Z. Neuronal and vascular deficits following chronic adaptation to high altitude. Exp Neurol. 2019;311:293–304. doi: 10.1016/j.expneurol.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 21. Guenego A, Fahed R, Albers GW, Kuraitis G, Sussman ES, Martin BW, Marcellus DG, Olivot J‐M, Marks MP, Lansberg MG, et al. Hypoperfusion intensity ratio correlates with angiographic collaterals in acute ischaemic stroke with M1 occlusion. Eur J Neurol. 2020;27:864–870. doi: 10.1111/ene.14181 [DOI] [PubMed] [Google Scholar]
- 22. Aikawa H, Kazekawa K, Tsutsumi M, Onizuka M, Iko M, Kodama T, Nii K, Hamaguchi S, Etou H, Sakamoto K. Intraprocedural changes in angiographic cerebral circulation time predict cerebral blood flow after carotid artery stenting. Neurol Med Chir (Tokyo). 2010;50:269–274. doi: 10.2176/nmc.50.269 [DOI] [PubMed] [Google Scholar]
- 23. Yamauchi K, Enomoto Y, Otani K, Egashira Y, Iwama T. Prediction of hyperperfusion phenomenon after carotid artery stenting and carotid angioplasty using quantitative DSA with cerebral circulation time imaging. J Neurointerv Surg. 2018;10:576–579. doi: 10.1136/neurintsurg-2017-013259 [DOI] [PubMed] [Google Scholar]
- 24. Yoshimoto Y, Tanaka Y, Sanada T. Angiographic assessment of cerebral circulation time for outcome prediction in patients with subarachnoid hemorrhage. Surg Neurol. 2004;62:115–120. doi: 10.1016/j.surneu.2003.08.035 [DOI] [PubMed] [Google Scholar]
- 25. Yamamoto S, Watanabe M, Uematsu T, Takasawa K, Nukata M, Kinoshita N. Correlation of angiographic circulation time and cerebrovascular reserve by acetazolamide‐challenged single photon emission CT. AJNR Am J Neuroradiol. 2004;25:242–247. [PMC free article] [PubMed] [Google Scholar]
- 26. Lin CJ, Chang FC, Tsai FY, Guo WY, Hung SC, Chen DY, Lin CH, Chang CY. Stenotic transverse sinus predisposes to poststenting hyperperfusion syndrome as evidenced by quantitative analysis of peritherapeutic cerebral circulation time. AJNR Am J Neuroradiol. 2014;35:1132–1136. doi: 10.3174/ajnr.A3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin CJ, Hung SC, Guo WY, Chang FC, Luo CB, Beilner J, Kowarschik M, Chu WF, Chang CY. Monitoring peri‐therapeutic cerebral circulation time: a feasibility study using color‐coded quantitative DSA in patients with steno‐occlusive arterial disease. AJNR Am J Neuroradiol. 2012;33:1685–1690. doi: 10.3174/ajnr.A3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 29. Kwan ES, Hall A, Enzmann DR. Quantitative analysis of intracranial circulation using rapid‐sequence DSA. AJR Am J Roentgenol. 1986;146:1239–1245. doi: 10.2214/ajr.146.6.1239 [DOI] [PubMed] [Google Scholar]
- 30. Rubiera M, Garcia‐Tornel A, Olivé‐Gadea M, Campos D, Requena M, Vert C, Pagola J, Rodriguez‐Luna D, Muchada M, Boned S, et al. Computed tomography perfusion after thrombectomy: an immediate surrogate marker of outcome after recanalization in acute stroke. Stroke. 2020;51:1736–1742. doi: 10.1161/STROKEAHA.120.029212 [DOI] [PubMed] [Google Scholar]
- 31. Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CBLM, van der Lugt A, de Miquel MA, et al. Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 32. Gauberti M, Lapergue B, Martinez de Lizarrondo S, Vivien D, Richard S, Bracard S, Piotin M, Gory B. Ischemia‐reperfusion injury after endovascular thrombectomy for ischemic stroke. Stroke. 2018;49:3071–3074. doi: 10.1161/STROKEAHA.118.022015 [DOI] [PubMed] [Google Scholar]
- 33. Al‐Dasuqi K, Payabvash S, Torres‐Flores GA, Strander SM, Nguyen CK, Peshwe KU, Kodali S, Silverman A, Malhotra A, Johnson MH, et al. Effects of collateral status on infarct distribution following endovascular therapy in large vessel occlusion stroke. Stroke. 2020;51:e193–e202. doi: 10.1161/STROKEAHA.120.029892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berkhemer OA, Jansen IGH, Beumer D, Fransen PSS, van den Berg LA, Yoo AJ, Lingsma HF, Sprengers MES, Jenniskens SFM, Lycklama à Nijeholt GJ, et al. Collateral status on baseline computed tomographic angiography and intra‐arterial treatment effect in patients with proximal anterior circulation stroke. Stroke. 2016;47:768–776. doi: 10.1161/STROKEAHA.115.011788 [DOI] [PubMed] [Google Scholar]
- 35. Seker F, Pereira‐Zimmermann B, Pfaff J, Purrucker J, Gumbinger C, Schönenberger S, Bendszus M, Möhlenbruch MA. Collateral scores in acute ischemic stroke: a retrospective study assessing the suitability of collateral scores as standalone predictors of clinical outcome. Clin Neuroradiol. 2020;30:789–793. doi: 10.1007/s00062-019-00858-1 [DOI] [PubMed] [Google Scholar]
- 36. Gupta A, Chazen JL, Hartman M, Delgado D, Anumula N, Shao H, Mazumdar M, Segal AZ, Kamel H, Leifer D, et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta‐analysis. Stroke. 2012;43:2884–2891. doi: 10.1161/STROKEAHA.112.663716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Douvas I, Moris D, Karaolanis G, Bakoyiannis C, Georgopoulos S. Evaluation of cerebrovascular reserve capacity in symptomatic and asymptomatic internal carotid stenosis with transcranial Doppler. Physiol Res. 2016;65:917–925. doi: 10.33549/physiolres.933303 [DOI] [PubMed] [Google Scholar]
- 38. Petersen NH, Ortega‐Gutierrez S, Reccius A, Masurkar A, Huang A, Marshall RS. Dynamic cerebral autoregulation is transiently impaired for one week after large‐vessel acute ischemic stroke. Cerebrovasc Dis. 2015;39:144–150. doi: 10.1159/000368595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hu YS, Guo WY, Lee IH, Chang FC, Lin CJ, Lin CJ, Luo CB, Wu CC, Lee HJ. Prolonged cerebral circulation time is more associated with symptomatic carotid stenosis than stenosis degree or collateral circulation. J Neurointerv Surg. 2018;10:476–480. doi: 10.1136/neurintsurg-2017-013293 [DOI] [PubMed] [Google Scholar]
- 40. Cronqvist S, Greitz T. Cerebral circulation time and cerebral blood flow. Acta Radiologica Diagnosis. 1969;8:296–304. doi: 10.1177/028418516900800402 [DOI] [PubMed] [Google Scholar]
- 41. Sarraj A, Goyal N, Chen M, Grotta JC, Blackburn S, Requena M, Kamal H, Abraham MG, Elijovich L, Dannenbaum M, et al. Dannenbaum M. direct to angiography vs repeated imaging approaches in transferred patients undergoing endovascular thrombectomy. JAMA Neurol. 2021;78:916–926. doi: 10.1001/jamaneurol.2021.1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim JT, Goyal M, Levy EI, Liebeskind D, Jahan R, Pereira VM, Gralla J, Bonafe A, Saver JL. Onset to reperfusion time as a determinant of outcomes across a wide range of ASPECTS in endovascular thrombectomy: pooled analysis of the SWIFT, SWIFT PRIME, and STAR studies. J Neurointerv Surg. 2020;12:240–245. doi: 10.1136/neurintsurg-2019-014906 [DOI] [PubMed] [Google Scholar]
- 43. Xiong LI, Liu X, Shang TY, Smielewski P, Donnelly J, Guo Z‐N, Yang YI, Leung T, Czosnyka M, Zhang R, et al. Impaired cerebral autoregulation: measurement and application to stroke. J Neurol Neurosurg Psychiatry. 2017;88:520–531. doi: 10.1136/jnnp-2016-314385 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


