Abstract
Background
Catheter ablation (CA) is a safe, effective, cost‐effective technique and may be considered a first‐line strategy for the treatment of symptomatic supraventricular tachycardias (SVT). Despite the high prospect of cure and the recommendations of international guidelines in considering CA as a first‐line treatment strategy, the average time between diagnosis and the procedure may be long. The present study aims to evaluate predictors related to non‐referral for CA as first‐line treatment in patients with SVT.
Methods and Results
The model was derived from a retrospective cohort of patients with SVT or ventricular pre‐excitation referred for CA in a tertiary center. Clinical and demographical features were used as independent variables and non‐referral for CA as first‐line treatment the dependent variable in a stepwise logistic regression analysis. Among 20 clinical‐demographic variables from 350 patients, 10 were included in initial logistic regression analysis: age, women, presence of pre‐excitation on ECG, palpitation, dyspnea and chest discomfort, number of antiarrhythmic drugs before ablation, number of concomitant symptoms, symptoms’ duration and evaluations in the emergency room due to SVT. After multivariable adjusted analysis, age (odds ratio [OR], 1.2; 95% CI 1.01–1.32; P=0.04), chest discomfort during supraventricular tachycardia (OR, 2.7; CI 1.6–4.7; P<0.001) and number of antiarrhythmic drugs before ablation (OR, 1.8; CI 1.4–2.3; P<0.001) showed a positive independent association for non‐referral for CA as SVT first‐line treatment.
Conclusions
The independent predictors of non‐referral for CA as first‐line treatment in our logistic regression analysis indicate the existence of biases in the decision‐making process in the referral process of patients who would benefit the most from catheter ablation. They very likely suggest a skewed medical decision‐making process leading to catheter ablation underuse.
Keywords: catheter ablation, drug therapy, logistic models, quality of life, supraventricular tachycardia
Subject Categories: Catheter Ablation and Implantable Cardioverter-Defibrillator, Arrhythmias, Electrophysiology
Nonstandard Abbreviations and Acronyms
- AVRT
atrioventricular reentrant tachycardia
- CA
catheter ablation
- SVT
supraventricular tachycardias
Clinical Perspective
What Is New?
This multivariable adjusted analysis of a retrospective cohort of patients with supraventricular tachycardia or ventricular pre‐excitation referred for catheter ablation indicates that advanced age, chest discomfort during supraventricular tachycardia, and a lower number of antiarrhythmic drugs to prevent supraventricular tachycardia episodes have been independent predictors of nonreferral for catheter ablation as first‐line treatment, and they do not seem to be rational.
These results indicate the existence of biases in the decision‐making process when referring patients for catheter ablation as supraventricular first‐line treatment.
What Are the Clinical Implications?
More information relative to catheter ablation benefits and risks would improve the medical decision‐making process in supraventricular tachycardia treatment.
Lack of education on the part of the referring doctor may explain, at least in part, underuse of catheter ablation as first‐line therapy for supraventricular tachycardia.
Catheter ablation (CA) is a safe, effective, cost‐effective technique and may be considered a first‐line strategy for the treatment of symptomatic supraventricular tachycardias (SVT). 12 In a randomized clinical trial, CA was shown to be safe and superior to drug treatment in reducing hospitalization for interruption of SVT over a 5‐year follow‐up. 3 As if the least efficacy was not enough, drug treatment is still related to worsening quality of life, whether due to a greater probability of recurrence of symptoms or due to side effects. 4 , 5 Catheter ablation is associated with improvement in the quality of life and a higher degree of satisfaction in controlling patients’ symptoms. 6 From the economic point of view, CA proved cost‐effective in the treatment of highly symptomatic patients and, despite the lack of analysis in less symptomatic individuals has not been performed, a small improvement in the quality of life would be enough to justify CA in SVT patients. 7
Despite the high prospect of cure and the recommendations of international guidelines in considering CA as a first‐line treatment strategy for SVT, 2 , 8 the average time between diagnosis and the procedure may be as long as 14 years, 9 , 10 suggesting a delay in CA indication. The present study aims to evaluate predictors related to non‐referral for catheter ablation as first‐line treatment in patients with SVT. For this purpose, a multivariate analysis was performed on variables extracted from a group of patients undergoing CA.
Methods
Study Design
The data that support the findings of this study is available from the corresponding author upon reasonable request. Retrospective and observational cohort derived from a prospective registry of patients with STV referred for catheter ablation.
Patient Population
Four hundred and fifty‐six patients referred for SVT CA in a tertiary center specialized in cardiac electrophysiology were assessed for eligibility. ECGs performed during SVT episodes and/or in the absence of symptoms were reviewed by 3 cardiologists and one experienced electrophysiologist. Only patients in which SVT or pre‐excitation were confirmed in a consensual meeting were eligible. Patients whose ECGs were diagnosed as atrial fibrillation or flutter, atrial tachycardia, junctional tachycardia, pregnant women, and individuals with any contraindications to electrophysiological study or CA were excluded (Figure 1). Patients with complaints suggestive of SVT but without an ECG with pre‐excitation or an ECG with SVT were also excluded. In a medical appointment by a cardiologist prior to CA, clinical features and information related to SVT symptoms were evaluated prospectively through the application of a standardized questionnaire. The main purpose of the study was to evaluate whether catheter ablation had been considered a first‐line treatment at the time SVT was diagnosed.
Figure 1. Screening and inclusion of patients.
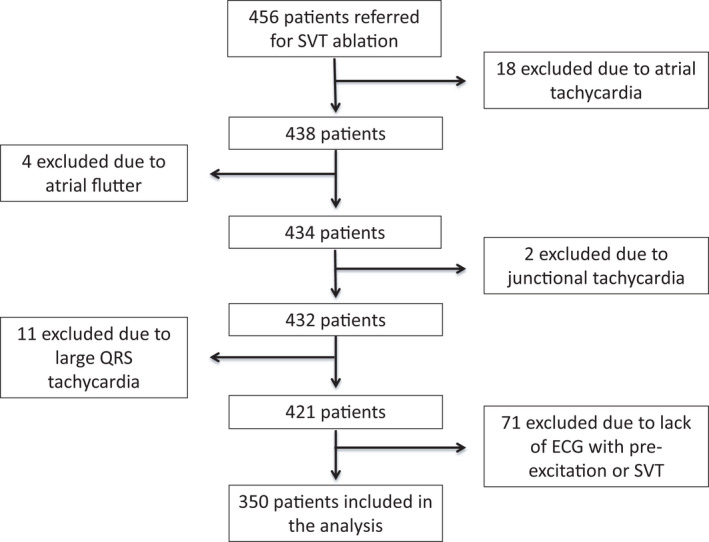
Among 456 patients referred for CA of SVT 106 were excluded: 35 due to other arrhythmias and 71 because, despite presenting symptoms compatible with SVT, they did not have an electrocardiographic record of the arrhythmia and did not present pre‐excitation on the resting ECG. CA indicates catheter ablation; and SVT, supraventricular tachycardias.
The protocol follows the Declaration of Helsinki, 11 which was submitted and approved by the ethics and research committee of the Federal University of São Paulo. All participants signed a free and informed consent form. Data were collected anonymously to guarantee confidentiality.
Study Outcome
The study outcome was defined as the non‐proposition of CA as an SVT first‐line treatment at the time of diagnosis among patients referred for CA therapy.
Statistical Analysis
Only patients with atrioventricular reentrant nodal tachycardia, atrioventricular reentrant tachycardia (AVRT), or ventricular pre‐excitation were included in the study. Firstly, population characteristics were described using measures of central tendency or dispersion, according to normal distribution. Students’ t‐tests and Mann‐Whitney tests were used for numerical variables and Chi‐Square for categorical variables. Values of P<0.05 were considered statistically significant.
Twenty variables were submitted to a bivariate statistical analysis and the ones that reached the threshold of statistical significance lower than 5% were subjected to a stepwise logistic regression analysis to identify independent predictors for the non‐proposition of CA as an SVT first‐line treatment at the time of diagnosis. Values of P<0.05 were considered statistically significant for maintaining a variable in the model. The definition of independent variables of the regression model was based on clinical data considered relevant in the follow‐up of patients with SVT and were obtained from a database created for the purpose of monitoring these patients. Missing data was considered negligible and, therefore, no further statistical treatment was considered necessary.
Finally, we estimated the Receiver Operating Characteristic (ROC) curve using the elements of the final model to describe its accuracy and the Hosmer‐Lemeshow test revealed the model was well‐calibrated.
Statistical analysis was performed using SPSS software (IBM SPSS Statistics 23).
For the analytical approach, in terms of logistic regression analysis, although 5 to 9 events per variable are considered acceptable (depending on the situation), we chose to use the cutoff of a minimum of 10 events per covariate presented in the initial etiological model. 12 , 13
Results
Three hundred and fifty patients were included between March 2007 and February 2009. The average age was 40±17 years, 62% were women, 29%, hypertensive, 7% diabetic, and 0.6% had heart failure. Palpitation alone or associated with some other symptom was the most commonly reported complaint and the median time between symptom onset and catheter ablation was 60 (IQR 24–168) months. The median of antiarrhythmic drugs before catheter ablation was 1 (IQR 0–2) and 90% of patients have never undergone electrical cardioversion due to SVT. The median of evaluations in the emergency room motivated by SVT was 3 (IQR 1–8) and, on average, only 1 event occurred in the month before ablation (IQR 0–2). Other clinical characteristics are described in Table 1. Although women had a higher duration of symptoms until CA than men, this difference was not statistically significant (48 months IQR 17–144 versus 72 months IQR 24–180; P=0.18). Similarly, patients with diagnosed AVNRT and AVRT were equally not referred for CA as first‐line therapy (72% versus 62%; P=0.3). In other words, the median time (months) between the onset of symptoms and catheter ablation was similar between patients with AVNRT and AVRT (72 months IQR 24–184 versus 62 months IQR 36–144; P=0.68, respectively).
Table 1.
Demographic and Clinical Data for Sample
| Patient characteristics |
N (350) |
Ablation as first Therapy (n=126) |
Ablation not first therapy (n=224) |
P value |
|---|---|---|---|---|
| Age, y | 41±17 | 38±18 | 42±17 | 0.01 |
| Women, n (%) | 216 (62) | 68 (54) | 148 (66) | 0.03 |
| Medical history | ||||
| Hypertension, n (%) | 103 (29) | 31 (25) | 72 (32) | 0.14 |
| Diabetes, n (%) | 25 (7) | 9 (7) | 16 (7) | 1.0 |
| Heart failure, n (%) | 2 (1) | 1 (0.8) | 1 (0.4) | 0.8 |
| Coronary artery disease, n (%) | 4 (1) | 2 (1.6) | 2 (0.9) | 0.56 |
| Obesity, n (%) | 57 (16) | 19 (15) | 38 (17) | 0.63 |
| Left ventricular ejection fraction, n (SD) | 67±10 | 66±14 | 68±7 | 0.43 |
| Arrhtythmia history | ||||
| ECG pre‐excitation, n (%) | 108 (31) | 49 (39) | 59 (27) | 0.02 |
| Palpitation, n (%) | 322 (92) | 110 (87) | 212 (95) | 0.03 |
| Syncope, n (%) | 17 (5) | 7 (6) | 10 (5) | 0.65 |
| Dyspnea, n (%) | 120 (34) | 31 (25) | 89 (40) | 0.005 |
| Chest disconfort, n (%) | 112 (32) | 23 (18) | 89 (40) | <0.001 |
| Medications before ablation, n (IQR) | 1.0 (0–2) | 1.0 (0–1) | 1.0 (1–2) | <0.001 |
| Unsatisfactory symptom control with medication, n (%) | 150 (43) | 48 (38) | 102 (46) | 0.18 |
| Average number of concomitant symptoms, n (IQR) | 2 (1–2) | 1 (1–2) | 2 (1–2) | <0.001 |
| Time between symptoms and ablation (mo), n (IQR) | 60 (24–168) | 36 (6–108) | 72 (35–183) | <0.001 |
| Life‐long emergency room evaluations, n (IQR) | 3 (1–8) | 2 (0–4) | 3 (1–10) | <0.001 |
| Number of symptoms in the month prior ablation, n (IQR) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.37 |
| Electrical cardioversions, n (SD) | 0.12±0.4 | 0.13±0.38 | 0.12±0.41 | 0.87 |
These variables were included in the univariate model and those that reached statistical significance (P<0.10) were included in the multivariate model. ECV indicates electrical cardioversion. Unsatisfactory symptom control with medication: patients who remained symptomatic despite antiarrhythmic treatment. Values are expressed in absolute numbers (n), percentages (%), mean±SD, median, and interquartile range (IQR).
Ventricular Pre‐Excitation Subpopulation
Among the 108 patients presenting ventricular pre‐excitation on surface ECG, only 45% were referred for CA as first line therapy. When compared with individuals without ventricular pre‐excitation, those with ventricular pre‐excitation were younger (33±17 versus 44±17 years; P<0.01), used, on average, fewer medications (0.83 versus 1.42; P=0.001), required fewer evaluations in the emergency room (2.7 versus 7.8 evaluations; P<0.01), were referred to CA earlier (36 versus 73 months; P=0.003) and were less likely to be women (41% versus 71%; P<0.01) Table 2.
Table 2.
Comparison Between Symptomatic Individuals With Ventricular Pre‐Excitation in Resting ECG and No Electrocardiographic SVT Documentation With Symptomatic Individuals With SVT Documentation
| Patient characteristics | Pre‐excited ECG with symptoms ‐ 30% (108/350) |
Manifest SVT (AVNRT or AVRT) ‐ 70% (242/350) |
P value |
|---|---|---|---|
| Age | 33±16 | 44±17 | <0.001 |
| Women, n (%) | 41% | 71% | <0.001 |
| Arrhythmia characteristics | |||
| Time between symptoms and ablation (mo), n (IQR) | 36 (12–96) | 72 (24–192) | <0.001 |
| Medications before ablation, n (SD) | 0.8±0.9 | 1.4±1.1 | <0.001 |
| Electrical cardiovertions, n (SD) | 0.12±0.4 | 0.12±0.4 | 0.95 |
| ER evaluations, n (IQR) | 1 (0–3) | 4 (2–10) | <0.001 |
| SVT events in the month prior to ablation, n (IQR) | 0 (0–2) | 1 ( 0–2) | 0.16 |
ER indicates emergency department; and SVT, supraventricular tachycardia.
Despite the association between ventricular pre‐excitation and non‐referral for CA as SVT first‐line treatment in bivariate analysis, when included in the multivariable adjusted model, pre‐excitation did not remain an independent predictor for non‐referral to interventional treatment, being the first one to leave the etiological model (odds ratio [OR], 1,04; CI 0.59–1.82, P=0.89).
Sixty‐four percent of individuals reported CA was not proposed as an initial strategy (Figure 2) and among the 36% (126/350) to whom CA ablation was proposed as an initial therapy, 61% (77/126) underwent the procedure. Even so, 39% (49/126) of individuals referred to CA did not perform the procedure: 21% (26/126) did not have access to the electrophysiology laboratory, 14% (18/126) refused the procedure because of fear related to complications, and 4% (5/126) chose pharmacological treatment.
Figure 2. Comparison between individuals with and without indication for CA as first‐line treatment of SVT.
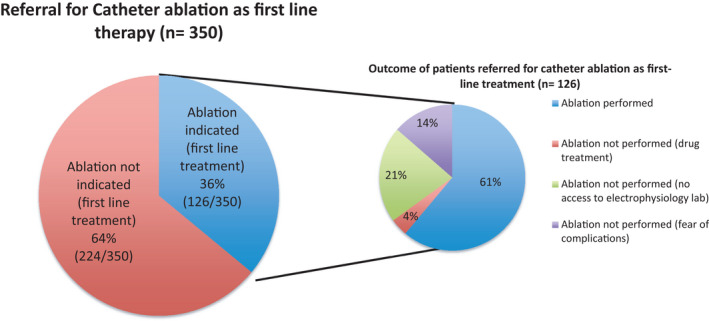
The largest pizza represents the percentage of patients referred for catheter ablation as the first treatment. The smaller pizza shows what happened to the population referred for ablation as the first‐line treatment. CA indicates catheter ablation; and SVT, supraventricular tachycardias.
Logistic Regression Model
Among 350 patients included, 224 were not referred for CA as an initial therapeutic strategy. Twenty clinical‐demographic variables were initially considered and 10 were excluded through the stepwise selection procedure: hypertension, diabetes, heart failure, coronary artery disease, obesity, left ventricular ejection fraction, syncope, unsatisfactory symptom control with medication, life‐long emergency room evaluations, electrical cardioversion. Ten variables were included in logistic regression analysis: older age, women, pre‐excitation on ECG, palpitation, dyspnea, chest discomfort during SVT, higher number of antiarrhythmic drugs before CA, number of concomitant symptoms, time since onset symptoms in months and number of evaluations in the emergency room due to SVT (Table 1).
After multivariable adjusted analysis, 3 variables revealed independent association with non‐referral of CA as an initial therapeutic strategy: age in decades (OR, 1.2; CI 1.01–1.32; P=0.04), chest discomfort during SVT (OR, 2.7; CI 1.6–4.7; P<0.001) and number of antiarrhythmic before ablation (OR, 1.8; CI 1.4–2.3; P<0.001 Table 3). Increased age, chest discomfort, and increased number of antiarrhythmic drugs were associated with decreased odds of CA as a first line treatment for SVT. The model demonstrated moderate discriminatory power, judging by the AUC of 0.71 (CI 0.66–0.77; P<0.001), and an adequate calibration (χ2 by the Hosmer‐Lemeshow test, 4.5; P=0.81).
Table 3.
Stepwise Logistic Regression Analysis
| Variable | Odds Ratio | CI 95% | P value |
|---|---|---|---|
| Age | 1.2 | 1.004–1.32 | 0.04 |
| Chest discomfort | 2.7 | 1.6–4.7 | <0.001 |
| Medications before ablation | 1.8 | 1.4–2.3 | <0.001 |
| ECG pre‐excitation | … | … | 0.89 |
| Average number of concomitant symptoms | … | … | 0.81 |
| Time between symptoms and ablation | … | … | 0.72 |
| Life long emergency room evaluations | … | … | 0.64 |
| Palpitation | … | … | 0.56 |
| Women | … | … | 0.43 |
| Dyspnea | … | … | 0.23 |
Result of stepwise logistic regression analysis showing the odds ratio of each independent predictor variable and the 10 variables retained in the model. Medications before the ablation were considered the use of antiarrhythmic medications prescribed to avoid new supraventricular tachycardias episodes.
Discussion
In a multivariable adjusted analysis, older individuals (OR, 1.2; CI 1.01–1.32; P=0.04), chest discomfort during SVT (OR, 2.7; CI 1, 6–4.7; P<0.001), and more trials of antiarrhythmic medications (OR, 1.8; CI 1.4–2.3; P<0.001) were associated with a greater chance for non‐referral for CA as SVT first‐line treatment. To our knowledge, this is the first study to identify clinical predictors associated with a lower chance of CA as an SVT first‐line treatment.
Demographic characteristics were similar to those presented in other studies, including the predominance of women and a similar prevalence of comorbidities. 6 The time between symptoms onset and CA was prolonged (Median 60 months, IQR 24–168), although intervals as long as 14 years have been described in the literature. 9 Putting in perspective the small number of emergency room evaluations due to SVT (Median of 3, IQR 1–8) lifelong, and the fact that the number of SVT episodes (Median of 1, IQR 0–2) in the month preceding ablation may be a crude indicator of the frequency of symptoms, patients or doctors may have underestimated the importance of SVT symptoms, despite the loss of quality of life infringed by arrhythmia. Some patients, during this time, may have undergone different trials, associations, or up‐titration of antiarrhythmic drugs. We also cannot exclude that symptoms’ unpredictability, as well as a prolonged interval between SVT episodes, may have created a false perception that symptoms were under control, leading to non‐purposeful therapeutic inertia.
Each additional decade of life was associated with 1.2 times the odds of CA not being a first‐line treatment for SVT. The reasons are speculative but may reflect the fear of complications related to the procedure in supposedly more fragile individuals, greater acceptance or adaptation of symptoms (self‐limitation) by the elderly, or even an option for drug treatment. Whether aging has generated a confirmatory bias or a “perceived safety” that drug treatment would be enough in controlling symptoms, mistakenly based on the paradigm of “less is more” is speculative. In this sense, older individuals would be deprived of choosing a treatment with a high rate of success, better efficacy than medical therapy, and low complication rates. 3 Besides, studies in older populations have shown that CA safety and efficacy profiles are similar to those in younger populations. 14 Regarding the participation of patients in the decision‐making process of the treatment strategy, only a small percentage of individuals for whom CA was proposed rejected the procedure for fear of complications, and an even smaller group of people actively chose drug treatment (4.9% and 1.4%, respectively).
Chest discomfort during STV was associated with a 2.7 greater chance for non‐referral for CA as a first‐line treatment. Despite the low prevalence of risk factors for coronary artery disease in SVT population, making the pre‐test probability of acute coronary syndrome low, and the fact that even specific characteristics of chest pain have shown little if any accuracy in diagnosing coronary artery disease, 15 , 16 , 17 there is still a great concern in diagnosing and treating coronary artery disease. Additionally, the most common mechanism of SVT relies on a reentry circuit, which does not correlate with coronary artery disease. These misconceptions deviate the flowchart towards coronary disease investigation, at the expense of not treating SVT properly. The prevalence of acute coronary syndrome in patients complaining of chest discomfort during SVT referred for coronary angiography is very low, even when elevations in troponin levels or ST segment depression are detected. 18 , 19 , 20 , 21 , 22 Predicting models may better estimate the pretest probability for coronary artery disease in individuals with acute chest discomfort during SVT and would help to conduct these scenarios more appropriately. 23 Although we claim that chest pain favors a coronary disease work up bias, we lack any objective data to support it and we recognize that the cognitive bias hypothesis is speculative and should be evaluated in future studies.
More trials of antiarrhythmic drugs were an independent predictor related to a 1.8 greater chance of non‐referral for CA as a first‐line treatment. This may reflect a physician's choice of trying different antiarrhythmic drug treatments before CA ablation. We cannot eliminate the possibility that drug treatment might have played a “perceived safety” effect too, having made doctors feel confident that prescribing antiarrhythmics, theoretically, would guarantee good symptom control, leading to CA postponement. This data is corroborated by the fact that 64% of individuals were not initially referred for catheter ablation and, only 4% of patients to whom CA was offered, actively opted for drug treatment (Figure 2). The health care system does not seem to play a significant role in limiting CA, since among those referred for ablation only 21% of individuals reported difficulties in having access to the electrophysiology lab. Therefore, in line with international guidelines recommendations, to improve quality of life and possibly reduce costs, 1 , 2 , 8 CA should be discussed as a treatment option with patients.
ECG pre‐excitation subgroup analysis is justified because this condition may be associated with an increased risk of sudden death, especially in younger individuals, which would justify a more aggressive approach. ECG pre‐excited individuals were referred for CA when they were younger, earlier after diagnosis (Table 2), and although it is a predictor of non‐referral for CA in univariate analysis, after correction of confounding factors, this variable did not prove to be an independent predictor. These findings can be explained by the fact that individuals at higher risk, usually younger, may have undergone catheter ablation even earlier.
Although advanced age, chest discomfort during STV, and a lower number of antiarrhythmic drugs to prevent SVT episodes have been showing independent predictors of non‐referral for CA as first‐line treatment, when they are contextualized together in a dichotomized fashion within the medical decision‐making process, they do not seem to be rational. Considering rationality as the process that helps people achieve their goals, 24 which in this case, it means improving quality of life at the least expenditure of resources, all independent predictors of non‐referral for CA do not point toward this direction. Based on rationality, we would expect that asymptomatic ventricular pre‐excitation, well‐controlled patients on antiarrhythmic drugs, individuals with a fewer associated number of symptoms, and those with less time since onset of symptoms would be the profile of patients not referred for CA. For these patients, therefore, catheter ablation would have no benefit. Our findings suggest a lack of rationality in the medical decision‐making process regarding SVT treatment leading to the underuse of catheter ablation.
In this sense, cognitive biases, a common failure in the medical rational thinking, influence medical decisions, and may explain our findings. We hypothesize that some “anchoring effect” on physicians’ decision‐making process by overestimating CA complications, may have happened making this lone piece of information the basis of a heuristic based on the misconception that CA is too dangerous to be offered. 25 The anticipated regret, should a bad result occurs, is greater if the undesired consequence seems to result more from the doctor’s action than from inaction and may explain to some extent a tendency for doctors to insist on drug treatment. To some extent, preempting possible regret is one way in which doctors make themselves feel better about their treatment decisions. 26
From a didactic point of view, underuse can occur due to failure of one among 4 steps in patient care: (1) inaccessibility to the therapeutic resource in the health system (absence of the structure or inability to purchase for the service); (2) unavailability of effective services, (3) failure of clinicians to deliver or prescribe medications or interventions; or (4) failure of patients to stick to or perform affordable treatments. In summary, underuse may be the result of failures related to the patients (in sticking to), to the system (in making available), or to clinicians (in discussing and indicating). 27 In our case, the most probable reason CA was underused in SVT patients is related to physicians, since just a few patients complained of difficulty in accessing electrophysiology labs and most of them revealed that CA was not discussed as a treatment option. In another study, in order to better understand medical thinking regarding treatment of SVTs, we asked 100 physicians from 3 different hospitals about cost‐effectiveness of CA of SVTs: 48% internists and 19% of cardiologists had no opinion about it. When compared with internists, cardiologists found it more difficult to refer a patient with SVT for CA (43% versus 53%, respectively), although more internists had no opinion about the effectiveness and safety of CA in SVT patients: 45% versus 10%, and 28% versus 0%, respectively (unpublished data). This data suggests that lack of education on the part of the referring doctor may explain, at least in part, underuse of CA as first‐line therapy for SVT.
Limitations
Since the study represents patients referred for CA, selection bias cannot be eliminated. A prospective study evaluating the initial treatment strategy at the time of SVT diagnosis would be necessary to better estimate results and improve external validity, although issues related to feasibility would make the study time consuming and difficult to be performed. Our findings, however, are robust and warn to the fact that, possibly, CA has not been discussed as a first‐line treatment option with patients. It must be kept in mind that patients’ conveniences and aspirations must be considered in the therapeutic decision, including in the elderly.
Sometimes diagnosing paroxysmal SVT in a patient with palpitations is challenging and the 60‐month median duration between symptom onset and catheter ablation may in fact represent the challenges in diagnosing paroxysmal SVT and capturing a rhythm on ECG, rather than delays to referral for catheter ablation. Due to the retrospective design of the study, we were unable to rule out this possibility. The assessment of time elapsed between the diagnosis of SVT and CA would help better understand the long time between the onset of symptoms and CA since the symptoms of certain psychiatric conditions are confused with those of SVT. In a retrospective survey of 107 consecutive patients with SVT Lessmeier et al found that 67% of patients fulfilled the criteria for panic disorder and 55% of patients with SVT were misdiagnosed as having psychiatric disorders. 28 In this same cohort, psychiatric misdiagnoses were more frequent in women with SVT than in men. In this sense, we cannot rule out the possibility that the delay in performing CA is secondary to a delay in the diagnosis of SVT, either because overlapping of arrhythmia with psychiatric illnesses, either because of misdiagnosis or even due to a delay in referral, even after a correct diagnosis of SVT. In relation to sex and SVT subtype (AVNRT or AVRT), our results differ from those presented in the literature 10 , 28 , 29 , 30 since, despite the numerical difference of the time elapsed between the onset of symptoms and CA, it was not statistically significant. The same goes for comparing AVNRT and AVRT. This may be attributed to differences in sample size, in the way physicians treat SVT in women in different regions or even by chance. Notwithstanding that, it is worthy to stress that in the paper of Carnlöf et al 29 patients with pre‐excited ECG were excluded, and this is an import recruitment bias favoring the entry of women in the study.
Someone may argue the fact that catheter ablation is safe and effective, does not mean it should be done right after diagnosis and may to favor waiting until quality of life is markedly affected. It must be considered, however, that SVT episodes impact the quality of life not only due to organic symptoms, but also due to psychological effects such as fear, anxiety, and feeling of loss of control that may be relevant in the decision‐making process.
Severity of symptoms is an important predictor of referral for catheter ablation and would be better assessed by direct measures of quality of life at that point in time. The study retrospective design, however, made it impossible to have it evaluated. In this sense, we indirectly quantified quality of life impairment assessing lifetime emergency room visits, number of drugs before ablation (average) and the time between the onset of symptoms and ablation. We assumed that the longer the duration of symptoms, the more frequent emergency room visits and the greater the number of medications the worse the quality of life.
Memory bias may have been a confounding factor and may have influenced patients’ responses to some degree since many patients have reported a long period since diagnosis and symptoms onset. It is possible that some individuals have not remembered the CA proposal as the first treatment strategy. This probability, however, is unlikely, since cardiological procedures usually stress people's memory due to a certain degree of esteem.
Conclusion
The independent predictors of non‐referral for CA as first‐line treatment in our logistic regression analysis indicate the existence of biases in the decision‐making process involved when referring patients who would benefit the most from CA. They very likely suggest a skewed medical decision‐making process leading to CA underuse. This is the largest study in the literature that attempts to understand, from a multivariable adjusted perspective, the factors associated with the non‐referral of patients who would potentially benefit from catheter ablation. More information relative to CA benefits and risks would improve the medical decision‐making process in SVT treatment.
Sources of Funding
None.
Disclosures
None.
For Sources of Funding and Disclosures, see page 8.
References
- 1. Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, Estes NAM III, Field ME, Goldberger ZD, Sc H, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;134:e506–e574. [DOI] [PubMed] [Google Scholar]
- 2. Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomstrom‐Lundqvist C, Calkins H, Corrado D, Defteros SG, DIller JC, et al. 2019 ESC Guidelines for themanagement of patients with supraventricular tachycardia. Eur Heart J. 2020;41:655–720. [DOI] [PubMed] [Google Scholar]
- 3. Katritsis DG, Zografos T, Katritsis GD, Giazitzoglou E, Vachliotis V, Paxinos G, Camm AJ, Josephson ME. Catheter ablation vs. antiarrhythmic drug therapy in patients with symptomatic atrioventricular nodal re‐entrant tachycardia: a randomized, controlled trial. Europace. 2017;19:602–606. [DOI] [PubMed] [Google Scholar]
- 4. Larson MS, McDonald K, Young C, Sung R, Hlatky MA. Quality of life before and after radiofrequency catheter ablation in patients with drug refractory atrioventricular nodal reentrant tachycardia. Am J Cardiol. 1999;84:471–473. doi: 10.1016/S0002-9149(99)00338-0 [DOI] [PubMed] [Google Scholar]
- 5. Goldberg AS, Bathina MN, Mickelsen S, Nawman R, West G, Kusumoto FM. Long‐term outcomes on quality‐of‐life and health care costs in patients with supraventricular tachycardia (radiofrequency catheter ablation versus medical therapy). Am J Cardiol. 2002;89:1120–1123. doi: 10.1016/S0002-9149(02)02285-3 [DOI] [PubMed] [Google Scholar]
- 6. Brachmann J, Lewalter T, Kuck K‐H, Andresen D, Willems S, Spitzer SG, Straube F, Schumacher B, Eckardt L, Danilovic D, et al. Long‐term symptom improvement and patient satisfaction following catheter ablation of supraventricular tachycardia: insights from the German ablation registry. Eur Heart J. 2017;38:1317–1326. doi: 10.1093/eurheartj/ehx101 [DOI] [PubMed] [Google Scholar]
- 7. Cheng CH, Sanders GD, Hlatky MA, Heidenreich P, McDonald KM, Lee BK, Larson MS, Owens DK. Cost‐effectiveness of radiofrequency ablation for supraventricular tachycardia. Ann Intern Med. 2000;133:864–876. doi: 10.7326/0003-4819-133-11-200012050-00010 [DOI] [PubMed] [Google Scholar]
- 8. Katritsis DG, Boriani G, Cosio FG, Hindricks G, Jäis P, Josephson ME, Keegan R, Kim YH, Knight KKH, et al. European heart rhythm association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia‐Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Elect. Europace. 2017;19:465–511. [DOI] [PubMed] [Google Scholar]
- 9. Farkowski MM, Pytkowski M, MacIag A, Golicki D, AliceWood K, Kowalik I, Kuteszko R, Szwed H. Gender‐related differences in outcomes and resource utilization in patients undergoing radiofrequency ablation of supraventricular tachycardia: results from patients’ perspective on radiofrequency catheter ablation of AVRT and AVNRT Study. Europace. 2014;16:1821–1827. doi: 10.1093/europace/euu130 [DOI] [PubMed] [Google Scholar]
- 10. Dagres N, Clague JR, Breithardt G, Borggrefe M. Significant gender‐related differences in radiofrequency catheter ablation therapy. J Am Coll Cardiol. 2003;42:1103–1107. doi: 10.1016/S0735-1097(03)00925-2 [DOI] [PubMed] [Google Scholar]
- 11. Review C, Communication S, Principles G. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81:14–18. [PubMed] [Google Scholar]
- 12. Demidenko E. Sample size and optimal design for logistic regression with binary interaction. Stat Med. 2008;27:36–46. doi: 10.1002/sim.2980 [DOI] [PubMed] [Google Scholar]
- 13. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052 [DOI] [PubMed] [Google Scholar]
- 14. Pedrinazzi C, Durin O, Agricola P, Romagnoli P, Inama G. Efficacy and safety of radiofrequency catheter ablation in the elderly. J Interv Card Electrophysiol. 2007;19:179–185. doi: 10.1007/s10840-007-9153-6 [DOI] [PubMed] [Google Scholar]
- 15. Body R, Cook G, Burrows G, Carley S, Lewis PS. Can emergency physicians “rule in” and “rule out” acute myocardial infarction with clinical judgement? Emerg Med J. 2014;31:872–876. doi: 10.1136/emermed-2014-203832 [DOI] [PubMed] [Google Scholar]
- 16. Goodacre SW, Locker T, Morris F, Campbell S. How useful are clinical features in the diagnosis of acute, undifferentiated chest pain? Acad Emerg Med. 2002;9:203–208. doi: 10.1197/aemj.9.3.203 [DOI] [PubMed] [Google Scholar]
- 17. Khan NA, Daskalopoulou SS, Karp I, Eisenberg MJ, Pelletier R, Tsadok MA, Dasgupta K, Norris CM, Pilote L. Sex differences in acute coronary syndrome symptom presentation in young patients. JAMA Intern Med. 2013;173:1863–1871. doi: 10.1001/jamainternmed.2013.10149 [DOI] [PubMed] [Google Scholar]
- 18. Dorenkamp M, Zabel M, Sticherling C. Role of coronary angiography before radiofrequency ablation in patients presenting with paroxysmal supraventricular tachycardia. J Cardiovasc Pharmacol Ther. 2007;12:137–144. doi: 10.1177/1074248407300775 [DOI] [PubMed] [Google Scholar]
- 19. Murer M, Cuculi F, Toggweiler S, Weberndoerfer V, Young M, Kobza R. Elevated high‐sensitivity troponin does not indicate the presence of coronary artery disease in patients presenting with supraventricular tachycardia. Cardiol J. 2017;24:642–648. doi: 10.5603/CJ.a2017.0058 [DOI] [PubMed] [Google Scholar]
- 20. Costabel JP, Urdapilleta M, Lambardi F, Campos R, Vergara JM, Ariznavarreta P, Trivi M. High‐sensitivity cardiac troponin levels in supraventricular tachyarrhythmias. Pacing Clin Electrophysiol. 2016;39:588–591. doi: 10.1111/pace.12851 [DOI] [PubMed] [Google Scholar]
- 21. Noorvash D, Ramos R, Hatch L, Muck A, Olson AS. Assessment of the utility of ordering a troponin in low‐ and intermediate‐risk patients presenting to the emergency department with supraventricular tachycardia: a retrospective chart review. J Emerg Med. 2018;55:1–6. doi: 10.1016/j.jemermed.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 22. Bukkapatnam RN, Robinson M, Turnipseed S, Tancredi D, Amsterdam E, Srivatsa UN. Relationship of myocardial ischemia and injury to coronary artery disease in patients with supraventricular tachycardia. Am J Cardiol. 2010;106:374–377. doi: 10.1016/j.amjcard.2010.03.035 [DOI] [PubMed] [Google Scholar]
- 23. Correia LCL, Cerqueira M, Carvalhal M, Ferreira F, Garcia G, da Silva AB, Sá N, Lopes F, Barcelos AC, Noya‐Rabelo M. A multivariate model for prediction of obstructive coronary disease in patients with acute chest pain: development and validation. Arq Bras Cardiol. 2017;108:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Djulbegovic B, Elqayam S, Dale W. Rational decision making in medicine: implications for overuse and underuse. J Eval Clin Pract. 2018;24:655–665. doi: 10.1111/jep.12851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:1–14. doi: 10.1186/s12911-016-0377-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bornstein BH, Emler AC. Rationality in medical decision making: a review of the literature on doctors’ decision‐making biases. J Eval Clin Pract. 2000;7:97–107. [DOI] [PubMed] [Google Scholar]
- 27. Glasziou P, Straus S, Brownlee S, Trevena L, Dans L, Guyatt G, Elshaug AG, Janett R, Saini V. Evidence for underuse of effective medical services around the world. Lancet. 2017;390:169–177. doi: 10.1016/S0140-6736(16)30946-1 [DOI] [PubMed] [Google Scholar]
- 28. Lessmeier TJ, Gamperling D, Johnson Liddon V, Fromm BS, Steinman RT, Meissner MDLM. Unrecognized paroxysmal supraventricular tachycardia‐ potential for misdiagnosis as panic disorder. Arch Intern Med. 1997;157:537–543. doi: 10.1001/archinte.1997.00440260085013 [DOI] [PubMed] [Google Scholar]
- 29. Carnlöf C, Iwarzon M, Jensen‐Urstad M, Gadler F, Insulander P. Women with PSVT are often misdiagnosed, referred later than men, and have more symptoms after ablation. Scand Cardiovasc J. 2017;51:299–307. doi: 10.1080/14017431.2017.1385837 [DOI] [PubMed] [Google Scholar]
- 30. Deneke T, Müller P, Lawo T, Lemke B, Horlitz M, Calcum B, Bösch LI, Mügge A, Grewe PH. Gender differences in onset of symptoms in AV nodal re‐entrant and accessory pathway‐mediated re‐entrant tachycardia. Herzschrittmacherther Elektrophysiol. 2009;20:33–38. doi: 10.1007/s00399-009-0036-7 [DOI] [PubMed] [Google Scholar]


