Abstract
Background
Torsade de pointes (TdP) is a potentially fatal cardiac arrhythmia that is often drug induced. Clinical decision support (CDS) may help minimize TdP risk by guiding decision making in patients at risk. CDS has been shown to decrease prescribing of high‐risk medications in patients at risk of TdP, but alerts are often ignored. Other risk‐management options can potentially be incorporated in TdP risk CDS. Our goal was to evaluate actions clinicians take in response to a CDS advisory that uses a modified Tisdale QT risk score and presents management options that are easily selected (eg, single click).
Methods and Results
We implemented an inpatient TdP risk advisory systemwide across a large health care system comprising 30 hospitals. This CDS was programmed to appear when prescribers attempted ordering medications with a known risk of TdP in a patient with a QT risk score ≥12. The CDS displayed patient‐specific information and offered relevant management options including canceling offending medications and ordering electrolyte replacement protocols or ECGs. We retrospectively studied the actions clinicians took within the advisory and separated by drug class. During an 8‐month period, 7794 TdP risk advisories were issued. Antibiotics were the most frequent trigger of the advisory (n=2578, 33.1%). At least 1 action was taken within the advisory window for 2700 (34.6%) of the advisories. The most frequent action taken was ordering an ECG (n=1584, 20.3%). Incoming medication orders were canceled in 793 (10.2%) of the advisories. The frequency of each action taken varied by drug class (P<0.05 for all actions).
Conclusions
A modified Tisdale QT risk score–based CDS that offered relevant single‐click management options yielded a high action/response rate. Actions taken by clinicians varied depending on the class of the medication that evoked the TdP risk advisory, but the most frequent was ordering an ECG.
Keywords: decision support systems, clinical; long QT syndrome; Torsades de Pointes
Subject Categories: Arrhythmias, Information Technology
Nonstandard Abbreviations and Acronyms
- CDS
clinical decision support
- DDI
drug–drug interaction
- QTc
corrected QT
- TdP
torsades de pointes
Clinical Perspective
What Is New?
A clinical decision support warning for medications associated with torsades de pointes in high‐risk patients was implemented and evaluated in a large multifacility health system.
What Are the Clinical Implications?
Clinical decision support can be effectively implemented to advise clinicians when ordering medications with a known risk of torsades de pointes.
Torsades de pointes (TdP) is a serious cardiac arrhythmia that may degenerate into ventricular fibrillation. Individuals who have corrected QT (QTc) interval prolongation are at an increased risk of TdP and sudden cardiac death. 1 , 2 CredibleMeds maintains a list of >200 medications that prolong the QTc interval and categorizes those drugs based on their risk of provoking TdP. 3 The highest risk medications on the CredibleMeds list are those on the “Known Risk of TdP” list, which includes drugs that prolong the QTc interval and are known to be associated risk with TdP, even when taken as recommended.
Patient‐specific risk assessment tools have been developed to help determine the risk of a patient developing QTc interval prolongation. The Tisdale QT risk score assigns weighted values to various risk factors and calculates the risk of QT interval prolongation. 4 Factors evaluated in this score include age, sex, hypokalemia, admission QTc interval, use of a loop diuretic, number of QT interval–prolonging drugs, sepsis, heart failure, and admission for acute myocardial infarction. Although this score was developed and validated in cardiac care units, there is a potential for the score to be applicable in a broader inpatient setting.
Risk of QTc prolongation is one of the most frequent triggers for drug–drug interaction (DDI) alerts generated by interaction surveillance programs; 1 study demonstrated that 64% of DDI alerts were for QTc prolongation risk. 5 However, low specificity of DDI alerts has been recognized as a cause of alert fatigue with most alerts being overridden. 6 More advanced QT‐related clinical decision support (CDS) that goes beyond potential DDIs with greater specificity of messages may be an important tool to help inform clinicians when patients are at high risk of TdP and to provide methods to manage the risk.
The Tisdale QT risk score has been incorporated into a CDS tool designed to alert clinicians when patients were being prescribed medications with a known risk of TdP and likely to result in a high risk of excessive QTc prolongation (defined as a QTc ≥500 ms or an increase of ≥60 ms). 7 This alert led to a reduction in the prescribing of noncardiac medications with a known risk of TdP in a cardiac care unit and a decrease in the occurrence of excessive QTc prolongation. Two other examples of QTc‐related CDS tools are alerts that appear when clinicians attempted to order a medication with a risk of QTc prolongation or TdP for patients who had a QTc ≥500 ms. 8 , 9 The Mayo Clinic tool triggers on orders for CredibleMeds known or possible risk drugs and effectively reduced the number of high‐risk medications administered to these patients by 13.9%. 3 , 8 The CDS tool implemented by Trinkley et al was triggered by 10 different QTc‐prolonging medications and resulted in the discontinuation of 37.8% of triggered medication orders. Although these 3 CDS tools significantly reduced the prescribing of high‐risk medications, there is a possibility for CDS to offer clinicians additional management options and be applicable in patient populations beyond a cardiac care unit or those who have a QTc ≥500 ms. In addition to discontinuing offending medications, other potential management include monitoring the ECG and correcting electrolytes. 10
We designed and implemented an advanced TdP risk advisory operating across all inpatient settings of a large health care system. The CDS tool used a modified Tisdale QT risk score, provided patient‐specific risk information, and allowed for single‐click management options to help mitigate the risk. The purpose of this study was to evaluate clinician actions that were taken in response to the advanced TdP risk advisory.
METHODS
The advanced CDS tool for TdP was implemented across a health system comprising 30 hospitals in 6 western states in the United States. This study was approved by the University of Arizona and University of Utah Institutional Review Boards, and a waiver of consent was granted. Implementation of the CDS was approved by the health system’s Pharmacy and Therapeutics Committee, the Hospital Medicine Clinical Consensus Group, and the Critical Care Clinical Consensus Group. The data that support the findings of this study are available from the corresponding author upon reasonable request.
The TdP risk CDS tool was programmed within the Cerner (Kansas City, MO) Millennium electronic health record and designed to appear when clinicians attempt to order a medication with a CredibleMeds.org “known risk of TdP” for inpatients who had modified Tisdale QT risk scores ≥12. 3 Purposefully, therefore, to selectively target high‐risk patients and minimize alert fatigue, the advisory was programmed to appear only for patients at high risk of a prolonged QTc interval. The advisory could potentially be preempted and not displayed if alternative DDI alerts on the electronic health record platform (Multum medications CDS incorporated in the Cerner electronic health record and provided by the vendor) led to discontinuing a medication order that would otherwise evoke the TdP advisory. The modified Tisdale QT risk score used in this CDS assigned 4 points if the most recent ECG recorded a Fridericia‐corrected QT interval >500 ms, whereas the standard Tisdale QT score assigns 2 points if the admission QTc >450 ms. 4 , 11 This scoring change was done because of the ability in this system to obtain QTc data from the most recent ECG and the greater risk established with having a QTc >500 ms. The advisory presents the clinician with the patient’s QT risk score, those factors contributing to the score, and relevant single‐click management options including (1) ordering a routine or STAT ECG, (2) ordering electrolyte replacement protocols conditionally appearing if a patient is deficient (magnesium <2 mEq/L, potassium <3.5 mmol/L, calcium <7.5 mg/dL, or ionized calcium <4.5 mg/dL), and (3) canceling incoming or existing medication orders for medications with a known risk of TdP. Incoming medications were those that evoked the TdP CDS, and existing medications were those that the patient had already been receiving.
Data were collected retrospectively for warnings that occurred between April 2020 and December 2020. We evaluated the actions taken by clinicians in response to the advisory, categorized by drug class evoking the advisory. Because ondansetron is frequently ordered in the inpatient setting as an “as needed” medication, it was placed in its own class for analysis. A χ2 statistic was calculated for the differences in rates of each action taken between each medication class. Logistic regression was performed to calculate odds ratios for canceling the incoming medication order comparing each medication class to the class with the highest frequency of evoking the CDS. The α value was set at 0.05 for all analyses.
RESULTS
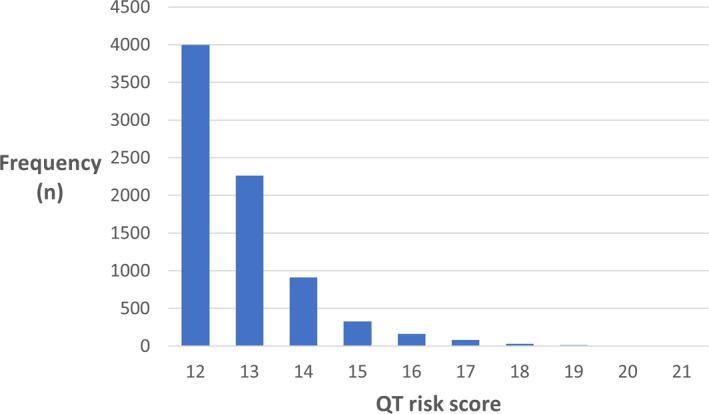
During a period of 9 months, there were 7794 TdP risk advisories displayed to clinicians and evaluated in this study. Basic demographics of patients for whom the advisory appeared are shown in Table 1. The mean age was 70 years (SD ±15), and 927 (12%) patients died in the hospital. The distribution of medications triggering the CDS are shown in Table 2. Antibiotics were the most frequent medications evoking the TdP risk advisory (n=2578, 33.1%), followed by ondansetron (n=2530, 32.5%). There was a skewed distribution of QT risk scores among patients for whom the advisory appeared, with most patients having a score of 12 (n=3997, 51%; Figure).
Table 1.
Characteristics of Patients With Corrected QT Risk Scores ≥12
| Characteristic | Value |
|---|---|
| Total number of patients | 7794 |
| Mean (SD) age, y | 70 (15) |
| Female patients, n (%) | 4647 (59.6) |
| Median (range) modified Tisdale QT risk score* | 12 (12–21) |
| COVID‐19 positive, n (%) | 963 (12.4) |
| Long QT syndrome diagnosis code (ICD‐10), n (%) | 252 (3.2) |
| Died in hospital, n (%) | 927 (11.9) |
ICD‐10 indicates International Classification of Diseases, Tenth Revision.
Clinical decision support advisory was not programmed to appear for scores <12.
Table 2.
Medications That Evoked a TdP Risk Clinical Decision Support Advisory
| Medication class | Frequency (percentage of all TdP risk advisories) | Known risk of TdP medications in class (in descending order of frequency within class) |
|---|---|---|
| Antibiotic | 2578 (33.1) | Azithromycin, levofloxacin, ciprofloxacin, erythromycin, moxifloxacin, clarithromycin |
| Antiemetic | 2530 (32.5) | Ondansetron |
| Antiarrhythmic | 980 (12.6) | Amiodarone, sotalol, flecainide, dofetilide, dronedarone, ibutilide, quinidine, procainamide |
| Other | 917 (11.8) | Propofol, escitalopram, citalopram, donepezil, methadone, hydroxychloroquine, cilostazol, oxaliplatin |
| Antipsychotic | 443 (5.7) | Haloperidol, chlorpromazine |
| Antifungal | 346 (4.4) | Fluconazole, voriconazole, posaconazole, pentamidine, itraconazole |
TdP indicates torsades de pointes.
Figure 1. Distribution of patients’ QT risk scores that triggered the torsades de pointes risk advisory.
At least 1 immediate action was taken within the advisory window for 2700 (34.6%) of the TdP risk advisories. Table 3 shows the actions taken in response to the advisory categorized by drug class. Among each possible action taken, there was a significant difference in the frequency of the action taken between the medication categories (P<0.05 for magnesium replacement protocol ordered, P<0.0001 for all other actions). The most frequent action taken was ordering a routine ECG (n=1584, 20.3%), and the rate of ECG ordering varied among all medication classes (range, 13.9%–24.2%). There were 1385 medication orders canceled through the advisory, with 793 (10.2%) cancellations of incoming medications and 592 of existing medications (1, 2, or 3 existing medications were canceled 570, 17, and 5 times, respectively). Incoming orders for ondansetron were the most likely to be canceled (n=458, 18.1%) compared with all other medication classes (P<0.05), with orders for other drug classes being canceled between 4.7% and 7.4% of the time (Table 3). Table 4 shows the odds ratios of canceling the incoming medication order, comparing each drug class to antibiotics. Canceling existing TdP risk medications was most common when the incoming medication was an antipsychotic (n=48, 11.2%) and least common when the incoming medication was ondansetron (n=118, 4.7%).
Table 3.
Actions Taken in Response to Torsades de Pointes Risk Advisories Categorized by Drug Class
| Action taken* | Antibiotic | Antipsychotic | Antifungal | Antiarrhythmic | Ondansetron | Other |
|---|---|---|---|---|---|---|
| Incoming drug order canceled | 173 (6.7) | 22 (5.0) | 25 (7.2) | 72 (7.4) | 458 (18.1) | 43 (4.7) |
| Existing drug order canceled | 230 (8.9) | 48 (11.2) | 24 (6.9) | 106 (10.8) | 118 (4.7) | 66 (7.2) |
| STAT ECG ordered | 37 (1.4) | 18 (4.1) | 11 (3.2) | 29 (3.0) | 19 (0.8) | 25 (2.7) |
| Routine ECG ordered | 470 (18.2) | 93 (21.0) | 79 (22.8) | 230 (23.5) | 351 (13.9) | 222 (24.2) |
| Potassium replacement protocol ordered | 48 (1.9) | 3 (0.7) | 3 (0.9) | 17 (1.7) | 107 (4.2) | 23 (2.5) |
| Magnesium replacement protocol ordered | 38 (1.5) | 3 (0.7) | 4 (1.2) | 22 (2.2) | 60 (2.4) | 20 (2.2) |
| Calcium replacement protocol ordered | 5 (0.2) | 1 (0.2) | 1 (0.3) | 4 (0.4) | 5 (0.2) | 13 (1.4) |
Data are provided as number (32.4).
For each action taken, there was a statistically significant difference in the action taken by medication class (P<0.05 for magnesium replacement protocol ordered, P<0.0001 for all other actions).
Table 4.
Comparison of Rates of Canceling Incoming Medication Orders Among Drug Classes
| Medication class | Odds ratios compared with antibiotics (95% Wald confidence limits) |
|---|---|
| Antibiotic | Reference group |
| Ondansetron | 3.07 (2.56–3.70) |
| Antipsychotic | 0.73 (0.46–1.15) |
| Antifungal | 1.08 (0.70–1.67) |
| Antiarrhythmic | 1.10 (0.83–1.47) |
| Other | 0.68 (0.49–0.96) |
Ordering electrolyte replacement protocols was the least frequent action taken. Among the electrolyte replacement protocol ordering, calcium replacement in patients with the incoming medication an antibiotic was the least frequent (n=5, 0.2%), and potassium replacement with the incoming medication ondansetron was the most frequent (n=107, 4.2%).
DISCUSSION
Implementing a CDS advisory for risk of TdP appeared to be effective in clinicians modifying medication therapy or monitoring patients by ordering an ECG. Differences in response to the advisory were observed depending on the medication that evoked the warning. Ordering a routine ECG occurred in response to ≈20% of warnings, although ECGs were only ordered 14% of the time with ondansetron orders. Antibiotics and ondansetron accounted for the majority of the medications that evoked the TdP risk advisory. Incoming orders for ondansetron were the most frequently canceled. This may be explained by ondansetron being frequently prescribed “as needed” and some alternative medications in the antiemetic class have a lower risk of causing TdP. The lower rate of ECG ordering observed with ondansetron may be explained by the high rate of canceling incoming ondansetron orders and thus potentially obviating the need for an ECG.
Ordering electrolyte replacement via protocols was generally less frequent than ordering ECGs or canceling orders for medications with a known risk of TdP. One explanation for the lower rate of this action is that the option to order these electrolyte replacement protocols was properly programmed to only appear if a patient’s most recent laboratory values fell below the low thresholds (magnesium <2.0 mg/dL, potassium <3.5 mEq/L, and ionized calcium <4.5 mg/dL or total calcium <7.0 mg/dL). Because most patients’ electrolytes are not below these thresholds, the option to order electrolyte replacement protocols was not provided in most cases. In addition, if the patient was already on an electrolyte replacement protocol (common and found on most admission order sets at our hospitals), this option did not appear.
Other studies examining the response to QT‐related CDS have shown a 21% reduction of ordering noncardiac QTc interval–prolonging medications in a cardiac care unit and a 13.9% to 37.8% reduction in administering QTc interval–prolonging medications to patients with a history of significant QTc prolongation (QTc ≥500 ms). 7 , 8 , 9 In comparison, this study yielded a cancellation rate of 10.2% of incoming known TdP risk medications, and existing medications were canceled in 7.6% of the advisories. There may have been some overlap with situations where both incoming and existing medications were canceled in a single advisory, and the total number of existing known risk medications is not known, but the overall cancellation rate in this study appears similar to those in other QT‐related CDS studies despite differences in the populations to whom the alerts applied. Other non–QT‐specific DDI alerts have demonstrated a low adherence rate, such as a DDI alert for hyperkalemia that yielded low adherence (16.8%) and no difference in the adherence rate when the alert was enhanced with laboratory values (15.3%) (P=0.71). 12
Although our CDS tool was based on the Tisdale QT risk score, this is not the only risk assessment tool that could be incorporated into CDS. Berger et al developed a model that includes age, sex, cardiac comorbidities, hypertension, diabetes, renal function, serum potassium concentrations, loop diuretics, and QTc‐prolonging drugs as risk factors, and this was applied across all inpatient settings. 13 Using their scoring system, a score ≥6 yielded a sensitivity of 76.6% and specificity of 28.5% in identifying patients with a QTc interval >450 for male patients and >470 for female patients. A comparison of the Tisdale QT risk score and the model by Berger et al has not yet been conducted.
This study has several limitations that should be considered when interpreting the findings. Not all medications could be appropriately grouped into a few categories, and thus an “other” category was created and contains medications used for a variety of indications. For this study, all described actions taken were a direct result of selecting options in the advisory, and the possibility of other actions taken after the advisory appeared was not assessed. There are many potential confounding issues with electrolyte replacement protocol ordering, including potential differences in the number of patients who were deficient for each electrolyte, and there may have already been existing replacement protocols ordered. Therefore, comparisons of the frequency of ordering electrolyte replacement protocols might be biased.
All actions taken by clinicians that are reported in this study are the direct actions taken while interacting with the TdP advisory. Although the overall observed action rate was 35%, the true action rate as a result of the advisory may be higher. For example, some clinicians might have reviewed the electronic health record and made decisions to cancel offending medications, order an ECG, or order electrolyte replacement protocols after closing the TdP risk advisory. These actions would not have been documented as consequential to the CDS. Limiting the advisory to high‐risk patients, having a well‐designed appearance of the advisory, and including convenient and helpful single‐click management options are likely factors contributing to the high observed action rate.
CONCLUSIONS
This TdP advisory with relevant single‐click management options yielded a high action rate among clinicians who received the advisory. The actions that clinicians take in response to a TdP warning vary depending on the class of medication that evokes the advisory, but the most common action taken by clinicians was ordering an ECG.
Sources of Funding
This work was supported by the Agency for Healthcare Research and Quality Grant R18HS026662. Part of the work reported here (creation of the torsades alert) was supported by the US Food and Drug Administration’s Safe Use Initiative Award HHSF223201400189C and the Flinn Foundation (Phoenix, AZ).
Disclosures
None.
Acknowledgments
We thank Nick Ernzen and Brenda Stoffer for their efforts in programming the torsade de pointes risk advisory.
For Sources of Funding and Disclosures, see page 5.
REFERENCES
- 1. Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12‐lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–1894. doi: 10.1161/01.CIR.83.6.1888 [DOI] [PubMed] [Google Scholar]
- 2. Straus SMJM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MCJM, Stricker BHC, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. doi: 10.1016/j.jacc.2005.08.067 [DOI] [PubMed] [Google Scholar]
- 3. Woosley RL, Heise CW, Gallo T, Tate J, Woosley D, Romero KA. www.CredibleMeds.org, QTdrugs List . August 12, 2020, AZCERT, Inc. 1822 Innovation Park Dr., 85755.
- 4. Tisdale JE, Jaynes HA, Kingery JR, Mourad NA, Trujillo TN, Overholser BR, Kovacs RJ. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479–487. doi: 10.1161/CIRCOUTCOMES.113.000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gatenby J, Blomqvist M, Burke R, Ritchie A, Gibson K, Patanwala AE. Adverse events targeted by drug‐drug interaction alerts in hospitalized patients. Int J Med Inform. 2020;143:104266. doi: 10.1016/j.ijmedinf.2020.104266 [DOI] [PubMed] [Google Scholar]
- 6. Payne TH, Hines LE, Chan RC, Hartman S, Kapusnik‐Uner J, Russ AL, Chaffee BW, Hartman C, Tamis V, Galbreth B, et al. Recommendations to improve the usability of drug‐drug interaction clinical decision support alerts. J Am Med Inform Assoc. 2015;22:1243–1250. doi: 10.1093/jamia/ocv011 [DOI] [PubMed] [Google Scholar]
- 7. Tisdale JE, Jaynes HA, Kingery JR, Overholser BR, Mourad NA, Trujillo TN, Kovacs RJ. Effectiveness of a clinical decision support system for reducing the risk of QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2014;7:381–390. doi: 10.1161/CIRCOUTCOMES.113.000651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sorita A, Bos JM, Morlan BW, Tarrell RF, Ackerman MJ, Caraballo PJ. Impact of clinical decision support preventing the use of QT‐prolonging medications for patients at risk for torsade de pointes. J Am Med Inform Assoc. 2015;22:e21–e27. doi: 10.1136/amiajnl-2014-002896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trinkley KE, Pell JM, Martinez DD, Maude NR, Hale G, Rosenberg MA. Assessing prescriber behavior with a clinical decision support tool to prevent drug‐induced long QT syndrome. Appl Clin Inform. 2021;12:190–197. doi: 10.1055/s-0041-1724043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trinkley KE, Page RL II, Lien H, Yamanouye K, Tisdale JE. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin. 2013;29:1719–1726. doi: 10.1185/03007995.2013.840568 [DOI] [PubMed] [Google Scholar]
- 11. Fridericia LS. Die Systolendauer im elektrokardiogramm bei normalen menschen und bei herzkranken. Acta Med Scand. 1920;53:489. doi: 10.1111/j.0954-6820.1920.tb18267.x [DOI] [Google Scholar]
- 12. Duke JD, Li X, Dexter P. Adherence to drug‐drug interaction alerts in high‐risk patients: a trial of context‐enhanced alerting. J Am Med Inform Assoc. 2013;20:494–498. doi: 10.1136/amiajnl-2012-001073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berger FA, van der Sijs H, Becker ML, van Gelder T, van den Bemt PMLA. Development and validation of a tool to assess the risk of QT drug‐drug interactions in clinical practice. BMC Med Inform Decis Mak. 2020;20:171. doi: 10.1186/s12911-020-01181-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


