Abstract
Background
Adenosine shortens action potential duration and refractoriness and provokes atrial fibrillation. This study aimed to evaluate the effect of adenosine on mechanisms of wavefront propagation during atrial fibrillation.
Methods and Results
The study included 22 patients undergoing catheter ablation for persistent atrial fibrillation. Left atrial mapping was performed using the AcQMap charge density system before and after administration of intravenous adenosine at 1 or more of 3 time points during the procedure (before pulmonary vein isolation, after pulmonary vein isolation, and after nonpulmonary vein isolation ablation). Wave‐front propagation patterns were evaluated allowing identification and quantification of localized rotational activation (LRA), localized irregular activation, and focal firing. Additional signal processing was performed to identify phase singularities and calculate global atrial fibrillation cycle length and dominant frequency. A total of 35 paired maps were analyzed. Adenosine shortened mean atrial fibrillation cycle length from 181.7±14.3 to 165.1±16.3, (mean difference 16.6 ms; 95% CI, 11.3–21.9, P<0.0005) and increased dominant frequency from 6.0±0.7 Hz to 6.6±0.8 Hz (95% CI, 0.4–0.9, P<0.0005). This was associated with a 50% increase in the number of LRA occurrences (16.1±7.6–24.2±8.1; mean difference 8.1, 95% CI, 4.1–12, P<0.0005) as well as a 20% increase in the number of phase singularities detected (30.1±7.8–36.6±9.3; mean difference 6.5; 95% CI, 2.6–10.0, P=0.002). The percentage of left atrial surface area with LRA increased with adenosine and 42 of 70 zones (60%) with highest density of LRA coincided with high density LRA zones at baseline with only 28% stable across multiple maps.
Conclusions
Adenosine accelerates atrial fibrillation and promotes rotational activation patterns with no impact on focal activation. There is little evidence that rotational activation seen with adenosine represents promising targets for ablation aimed at sites of stable arrhythmogenic sources in the left atrium.
Keywords: AcQMap, adenosine, atrial fibrillation, localized rotational activation
Subject Categories: Atrial Fibrillation, Electrophysiology, Mechanisms
Nonstandard Abbreviations and Acronyms
- AFCL
atrial fibrillation cycle length
- LIA
localized irregular activation
- LRA
localized rotational activation
Clinical Perspective
What Is New?
Adenosine appears to exert a functional effect on atrial electrophysiology during atrial fibrillation resulting in an increase in rotational activation patterns throughout the left atrium that coincides with an increase in dominant frequency and shortening of whole chamber fibrillatory cycle length.
Adenosine does not affect the number of focal firings.
What Are the Clinical Implications?
Rotational activation during atrial fibrillation may represent the functional electrophysiological properties of the chamber rather than local “drivers.”
There is little stability in sites of rotational activation after administration of adenosine suggesting these are poor targets for focal ablation of atrial fibrillation mechanisms.
Caution should be exercised when interpreting data obtained during noncontact or noninvasive mapping with far field ventricular signals excluded using adenosine to achieve atrioventricular conduction block.
Although pulmonary vein ectopy is widely recognized as the prevalent trigger for atrial fibrillation (AF), mechanisms responsible for maintaining AF and resulting in the progression from paroxysmal to persistent phenotypes are poorly understood. Adenosine is known to shorten atrial myocardial action potential duration and refractoriness, and its administration may provoke AF. 1 , 2 , 3 , 4 This effect is mediated by specific G‐protein coupled cell surface receptors and activation of outward potassium currents. 3 , 5 These are the same currents activated by cholinergic stimulation through the binding of acetylcholine to muscarinic receptors but despite the ionic basis for this effect being well recognized, the impact on the properties of dynamic wavefront propagation during AF are less clear. Spatially limited dominant frequency mapping studies showed adenosine (or acetylcholine) infusion resulted in increased dominant frequency with differential effects between atrial sites potentially due to the heterogenous distribution of adenosine receptors across the atrial myocardium. 6 , 7 , 8 This has been proposed as indirect evidence of high‐frequency reentry mechanisms maintaining AF and accelerated by adenosine. 6 , 9 Hansen et al recently examined the effect of adenosine on AF in explanted human hearts (with and without a history of AF) and 10 patients in whom ablation confirmed “drivers” had been identified. 10 However, the direct functional effect of adenosine on dynamic wavefront propagation in a broader population of patients undergoing ablation for persistent AF has not been evaluated.
The AcQMap (Acutus Medical, Carlsbad, CA, USA) noncontact mapping system allows visualization of high‐density whole chamber atrial propagation using a sampling frequency of 3125 Hz and spatial resolution of 2 mm on a 3‐dimensional anatomical mesh of ≈3500 vertices. 11 , 12 Localized patterns of AF propagation may be classified as localized rotational activation (LRA), localized irregular activation (LIA), and focal firing. 11 Ablation of these regions with repetitive activation patterns has produced favorable outcomes in patients with persistent AF. 13
Adenosine is frequently used during noncontact mapping to cause transient atrioventricular block to reduce the effect of far‐field ventricular signals. In this study we sought to evaluate the effect of adenosine on atrial propagation patterns in patients undergoing catheter ablation for symptomatic AF. The aim was to both further examine the mechanisms involved in AF maintenance and establish the extent to which atrial propagation is altered by adenosine thus potentially restricting the clinical utility of adenosine to aid AF mapping.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patients
Patients undergoing elective catheter ablation for persistent AF using the AcQMap system were included in the analysis. This included patients enrolled in parallel research studies (RECOVER‐AF [Utilizing Novel Dipole Density Capabilities to Objectively Visualize the Etiology of Recurrent Atrial Fibrillation Following a Failed AF Ablation], NCT03368781; and BiMap‐AF [Biatrial Global High‐Density Electroanatomical Mapping of Atrial Fibrillation], NCT03812601) and undergoing both first‐time and repeat procedures for recurrent AF. These studies were approved by the local ethics committee and conducted according to the principles of the Declaration of Helsinki. All patients gave written informed consent. Patients with a clinical contraindication to adenosine administration were excluded.
Activation Pattern Characterization
Activation patterns were classified using AcQTrack (Acutus Medical) to identify regions of rotational, irregular, and focal activation according to the definitions outlined next. This is an automated algorithm that tracks and evaluates conduction at every vertex of the anatomy (≈3500 in total) thereby removing any subjectivity from the classification and quantification of activation patterns. The distance between each vertex is ≈2 mm, which represents the spatial resolution.
Localized Rotational Activation
The LRA algorithm computes the degrees of conduction propagation around a central point by summing the angle differences of sequential conduction velocity vector directions around the central point. If the rotational angle of conduction exceeds 270 degrees, rotation is detected at the central point. An area of ≈300 mm2 around the central point is considered.
To ensure smooth propagation around the central point, an r 2 of a linear fit of activation time to position around the central vertex must exceed 0.7.
Conduction velocity vector directions changes cannot exceed 45 degrees per position change around the vertex
Activation time difference around the central obstacle must be >50 ms.
Localized Irregular Activation
The LIA algorithm computes the difference in angle between cardiac conduction entering and leaving a confined region. If the angle difference of conduction entering and leaving a confined region exceeds 90 degrees, LIA is detected.
An area of ≈200 mm2 is considered a confined region
Wavefronts are considered to be passing through the region if the activation time differences between the border of the confined region and the central vertex would result in a conduction velocity between 0.3 m/s to 3.0 m/s.
Activations are grouped into entering and leaving the region based on the activation time with comparison to the central vertex. A mean conduction vector entering the region and leaving the region are then computed. Angle difference between the vector entering and leaving the region are computed and if the difference exceeds 90 degrees, LIA is detected.
Focal Activation
The focal activation algorithm determines whether an activation at a vertex came from a previous cardiac wavefront, or whether activation spontaneously started from the current activation. Focal activation is detected at a vertex if an activation is earlier than its neighbors’ activation by at least 3 ms, and conduction spreads outward from the early activation.
Activations are connected as a wavefront if the time difference between the 2 activation times would produce a conduction velocity >0.05 m/s.
For each activation detected at a vertex of the chamber, a disc (as illustrated in the examples in Figure 1A through 1C) is shown corresponding to the confined region in which the pattern is detected. For quantification of these activation patterns, as described in detail later, where these discs overlap at the same point in time (for example in the case of a meandering LRA), then a single occurrence is counted.
Figure 1. AcQTrack identifies FF (A) characterized by radial activation from a central earliest point, LRA (B) where smooth rotational activation of >270 degrees is observed; and LIA (C through F). LIA includes a range of specific patterns of activation, all characterized by changing wavefront direction of >90 degrees.
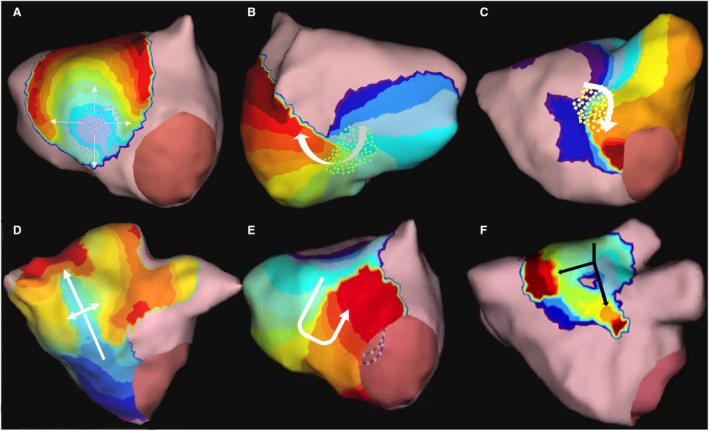
Pink, green, and yellow dots (A through C) are shown as examples of what can be seen during a procedure to illustrate the region in which FF, LRA, and LIA are detected using AcQTrack. FF indicates focal firing; LIA, localized irregular activation; and LRA, localized rotational activation.
Electrophysiological Mapping Procedure
Procedures were carried out under general anesthetic in line with standard institutional practice. With the exception of amiodarone, antiarrhythmic drugs were stopped a minimum of 5 days before the procedure. Heparin boluses were administered before transseptal puncture followed by continuous heparin infusion to maintain an active clotting time >350 s. A decapolar catheter (Inquiry, Abbott Medical) was inserted into the coronary sinus and the AcQMap mapping catheter was inserted into the left atrium. A circular mapping catheter (Inquiry Optima, Abbott Medical) was used to guide pulmonary vein isolation (PVI). In patients attending the procedure in sinus rhythm, AF was induced with burst pacing from the coronary sinus and only sustained AF was mapped for inclusion in the analysis (no patients demonstrated spontaneous termination after induction). A 3‐dimensional ultrasound generated anatomy of the left atrium was reconstructed and electrophysiological recordings from 48 noncontact electrodes obtained using the AcQMap system as previously described. 11 , 12 Recordings were taken at 1 or more of 3 time points during the procedure for each participant: before PVI (in those undergoing first‐time procedures), immediately after PVI, and after nonpulmonary vein left atrial ablation. At each time point, an additional recording was obtained during the administration of a bolus of 15 mg of adenosine with pharmacological effect confirmed by the observation of transient atrioventricular conduction block and/or hypotension. Radiofrequency ablation was delivered to achieve PVI (or reisolation). Following this, additional nonpulmonary vein ablation was delivered guided by baseline AcQMap propagation maps targeting regions with highly repetitive patterns of focal firing, LIA, or LRA over several map segments as determined by the operator.
Propagation Map Construction, Export, and Analysis
Electrophysiological recordings before and after adenosine administration were processed for construction of propagation history maps. For each time period, a 5‐second segment was taken, which in the case of adenosine corresponded with maximal atrioventricular conduction block. A 100 Hz low pass filter and 50 Hz notch filter were applied, outlier electrode signals were excluded after manual interrogation (to identify spurious signals as a result of electrode damage during catheter insertion/preparation), and the QRS‐T wave subtraction algorithm applied for all recordings (including those obtained after adenosine administration). Propagation maps were calculated and displayed using the default minimum amplitude sensitivity of 0.02 mV, time threshold of 70 ms (representing a conservative value for minimum atrial refractoriness) and window width of 80 ms (determining the duration of display of propagating wavefronts). Wavefront propagation patterns were evaluated using the AcQTrack system and data exported for analysis in custom designed software.
Propagation Pattern Quantification
For each 5‐second map, AcQTrack data for LRA, LIA, and focal firing were extracted and quantified. Example patterns are shown in Figure 1 and a detailed explanation of the AcQTrack algorithm was outlined previously. A predefined method of pattern quantification was used in order to allow statistical comparison of activation patterns observed and is outlined in further detail in the Supplementary Methods and Figures S1 through S3. All occurrences for the segment were initially included and displayed as a static density map on the reconstructed anatomy. LRA and LIA were then quantified according to the raw number of occurrences of these patterns, the percentage of time over the segment that these patterns were present, and the percentage of atrial surface area covered. For focal firing, only the number of occurrences during the recording period were counted.
In order to correct for false positive pattern detection and isolated occurrences and to identify those regions in which specified activation patterns are repeatedly observed, a method of applying detection cutoffs was devised for LRA and LIA. A dynamic threshold, individualized for each recording is required given significant variation in frequency and duration of patterns across the chamber, which could result in a fixed frequency threshold, at a point suitable for 1 patient/map segment, either excluding all activation occurrences, or not sufficiently excluding regions with low relative frequency when applied to another recording. In addition, the relationship between frequency and duration of pattern occurrences is not fixed meaning that a threshold considering only a set percentage of pattern occurrences without including the duration these are present may be inadequate. First, a static map was generated with all pattern occurrences included and the percentage of time the pattern was present anywhere in the chamber was calculated. Occurrences were then excluded using cutoff values according to the frequency of each pattern that represent a 5%, 10%, 20%, 30%, and 40% relative drop in the total time the pattern was present resulting in gradually greater exclusion of regions with infrequent pattern occurrences and leaving those with the greatest frequency of detection (Figure 2). As well as measuring the number of occurrences of each activation pattern, measuring the proportion of time these patterns are present over the fixed map duration (5 s) provides a measure of the duration that LIA or LRA persists and is therefore a marker of stability. Further detailed explanation is included in the supplementary methods.
Figure 2. Method of applying cutoffs to LIA detection to exclude infrequent pattern occurrences.
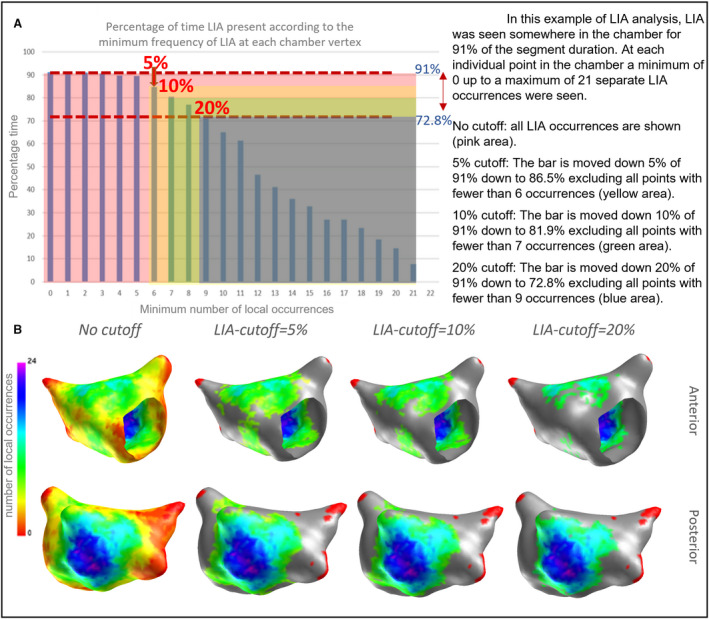
(A) With no cutoff applied, all occurrences are counted and the percentage of time occupied calculated (y axis). Cutoffs are then applied based on this analysis. Applying a 5%, 10%, and 20% relative reduction in the total time that patterns are present results in gradual exclusion of the least frequent occurrences below the minimum number of local occurrences (at each vertex) shown on the x axis. (B). In this example the 5% cutoff results in excluding regions with fewer than 6 LIA occurrences, with increasing exclusion with higher cutoffs. (Regions with excluded occurrences are shown in grey, red shows areas with no occurrences irrespective of cutoff). LIA indicates localized irregular activation.
Signal Processing and Dominant Frequency Analysis
Virtual electrograms from each vertex of the surface mesh (≈3500 in total) making up the reconstructed anatomy were also exported for analysis and calculation of atrial fibrillation cycle length (AFCL) and identification of phase singularities. Virtual electrograms were filtered and phase reconstruction achieved using a method of sinusoidal recomposition and the Hilbert transform as previously described. 14 Phase singularity detection and lifespan calculation were then completed in line with previously described methods. 15 For AFCL calculation, the cycle length at each nodal point was calculated for the 5 s recording from the phase signals and results for all signals combined to provide the mean global AFCL over the duration of the 5 s segment.
Virtual dipole signals were exported from each vertex of the chamber and dominant frequency (DF) identified as the maximum peak of the frequency‐power spectrum at values within a presumed physiological range between 4 and 10 Hz after Fast Fourier Transform performed in Matlab (Mathworks 2019a). The mean and SD for all signals across the chamber were calculated to give the chamber DF value for comparison. Regions of high DF, defined as a localized zone with DF higher than all surrounding regions, were identified and compared with regions of high‐frequency LRA.
Clinical Outcomes
Clinical outcomes were assessed according to acute procedural effects resulting in ablation terminating AF (either directly, or via an organized atrial tachycardia) or requiring direct current cardioversion to restore sinus rhythm. Differences in propagation patterns before and after adenosine were compared on the first map obtained in each patient (ie, at the earliest stage of the procedure) according to acute procedural outcome.
Statistical Analysis
Statistical analysis was performed using SPSS (version 25, IBM) or MatLab (Mathworks, R2019a). Continuous variables were assessed for normality of distribution using the Shapiro‐Wilk test and expressed as mean±SD. Data were compared using the paired samples t‐test comparing each map obtained with and without adenosine. Where differences were not normally distributed data were additionally transformed and analyzed using a 1‐sample t test. If the results are concordant then the paired sample t test is reported. Categorical data are expressed as a number and percentage.
Results
Patients
Twenty‐two patients with persistent AF were included in the analysis. Mean age of the population was 60±12 and 68% were male. Other characteristics are shown in Table 1. Maps with and without adenosine were created before PVI in 11 participants, immediately after PVI in 15 participants, and after additional left atrial ablation in 9 participants resulting in a total of 35 paired maps. In all cases, a clear effect of adenosine on atrioventricular conduction was observed with a mean longest RR interval of 5708±2933 ms.
Table 1.
Patient Characteristics for All Patients (n=22)
| Characteristic | All patients |
|---|---|
| Age, y, mean (SD) | 60±12 |
| Male, n (%) | 15 (68) |
| Body mass index , kg/m2, median (Q1, Q3) | 29 (25–30) |
| Amiodarone periprocedure, n (%) | 6 (27) |
| Ejection fraction, %, median (Q1, Q3) | 55 (54–60) |
| Left atrial diameter, mm, median (Q1, Q3) | 45 (39–50) |
| Time since diagnosis, y, median (Q1, Q3) | 3.5 (2–5) |
| First time procedure, n (%) | 8 (47) |
| In atrial fibrillation at procedure start, n (%) | 14 (82) |
Wavefront Propagation Patterns
At baseline, the number of LIA occurrences was 99±20 (within the 5 s of recorded segment), which increased to 109±26 with adenosine, a statistically significant difference of 10 (95% CI, 2–19, P=0.012). When a 5% cutoff was used, LIA occurrences with adenosine were 83±16 compared with 76±15, reflecting a statistically significant difference of 7 (95% CI, 2.5–11, P=0.003). At 10%, 20%, 30%, and 40% cutoffs there was no difference in the number of LIA occurrences with adenosine (71±13 from 67±11, difference 4, 95% CI, −1.1 to 7.7, P=0.133; 53±9 from 51±9, difference 2, 95% CI, −1.8 to 4.7, P=0.372; 41±8 from 41±7, difference 0, 95% CI, −2.8 to 3.1, P=0.906; and 32±5 from 32±5, difference 0, 95% CI, −2.8 to 2.3, P=0.840 for 20%, 30%, and 40% cutoffs respectively–see Table 2). When analyzed as a percentage of time over which LIA occurred, adenosine resulted in a small but statistically significant increase in time LIA was present at all but the 40% cutoffs. The percentage of left atrial surface area in which LIA occurred was not affected by adenosine except for a small decrease in the surface area at the 30% (19.2±5.6 to 16.4±3.5, difference 2.8, 95% CI, 0.5–5, P=0.019) and 40% (14.0±5.4 to 11.6±3.0, difference 2.4, 95% CI, 0.4–4.3, P=0.021) cutoffs.
Table 2.
Effect of Adenosine of LIA, LRA, and FF for All Maps, n=35 of 5‐S Duration
| Cutoff | Baseline (SD) | Adenosine (SD) | Difference (95% CI) | P value |
|---|---|---|---|---|
| LIA number of occurrences | ||||
| 0% | 99±20 | 109±26 | 10.0 (2 to 19) | 0.012 |
| 5% | 83±16 | 76±15 | 7 (2.5 to 11.0) | 0.003 |
| 10% | 67±11 | 71±13 | 3.3 (−1.1 to 7.7) | 0.133 |
| 20% | 51±9 | 53±9 | 1.5 (−1.8 to 4.7) | 0.372 |
| 30% | 41±7 | 41±8 | 0.2 (−2.8 to 3.1) | 0.906 |
| 40% | 32±5 | 32±5 | −0.3 (−2.8 to 2.3) | 0.840 |
| LIA % time | ||||
| 0% | 76.4±11.1 | 79.9±12.4 | 3.5 (0.5 to 6.7) | 0.025 |
| 5% | 69.9±12.6 | 74.2±12.3 | 4.3 (1.5 to 7.2) | 0.004 |
| 10% | 66.2±11.2 | 70.2±11.0 | 4.0 (1.1 to 6.8) | 0.007 |
| 20% | 57.7±10.2 | 61.1±9.1 | 3.4 (1.0 to 5.8) | 0.007 |
| 30% | 50.1±8.2 | 52.9±9.6 | 2.7 (0.2 to 5.3) | 0.035 |
| 40% | 41.6±7.4 | 44.0±7.4 | 2.4 (−0.2 to 5.1) | 0.072 |
| LIA % surface area | ||||
| 0% | 85.8±11.3 | 86.1±13.8 | 0.3 (−3.5 to 4.0) | 0.889 |
| 5% | 46.1±10.0 | 46.4±10.2 | 0.3 (−3.6 to 4.2) | 0.887 |
| 10% | 37.9±7.8 | 35.6±8.4 | −2.3 (−6.3 to 1.6) | 0.242 |
| 20% | 25.5±5.2 | 23.5±5.9 | −2.0 (−4.5 to 0.4) | 0.096 |
| 30% | 19.2±5.6 | 16.4±3.5 | −2.8 (−5.0 to −0.5) | 0.019 |
| 40% | 14.0±5.4 | 11.6±3.0 | −2.4 (−4.3 to −0.4) | 0.021 |
| LRA number of occurrences | ||||
| 0% | 16.1±7.6 | 24.2±9.8 | 8.1 (4.1 to 12) | <0.0005 |
| 5% | 11.3±6.5 | 18.6±8.1 | 7.3 (3.7 to 10.7) | <0.0005 |
| 10% | 10.7±6.3 | 17.9±7.9 | 7.2 (4.0 to 10.5) | <0.0005 |
| 20% | 8.4±5.2 | 14.1±6.4 | 5.7 (2.7 to 8.6) | <0.0005 |
| 30% | 6.8±4.1 | 11.6±4.9 | 4.8 (2.6 to 6.7) | <0.0005 |
| 40% | 5.5±3.3 | 10.2±4.3 | 4.7 (2.8 to 6.5) | <0.0005 |
| LRA % time | ||||
| 0% | 29.8±11.8 | 43.3±15.5 | 13.5 (7.1 to 19.9) | <0.0005 |
| 5% | 24.5±11.6 | 37.4±14.5 | 12.9 (6.8 to 19.0) | <0.0005 |
| 10% | 23.6±36.3 | 36.3±14.1 | 12.7 (6.9 to 18.5) | <0.0005 |
| 20% | 19.6±10.3 | 30.1±12.5 | 10.5 (4.9 to 16.3) | 0.001 |
| 30% | 17.0±9.0 | 25.5±10.3 | 8.5 (4.3 to 12.8) | <0.0005 |
| 40% | 13.9±7.6 | 23.0±9.5 | 9.1 (5.0 to 13.1) | <0.0005 |
| LRA % surface area | ||||
| 0% | 22.3±8.3 | 29.6±10.2 | 7.3 (3.3 to 11.3) | 0.001 |
| 5% | 12.7±6.5 | 16.0±6.7 | 3.3 (0.19 to 6.5) | 0.038 |
| 10% | 11.1±5.4 | 14.8±6.3 | 3.7 (1.1 to 6.2) | 0.007 |
| 20% | 7.7±3.3 | 10.1±4.1 | 2.4 (0.6 to 4.2) | 0.012 |
| 30% | 6.0±2.5 | 7.7±3.7 | 1.7 (0.6 to 3.0) | 0.005 |
| 40% | 4.7±2.2 | 6.3±2.8 | 1.6 (0.6 to 2.7) | 0.004 |
| FF numbers | ||||
| 24.5±8.2 | 22.0±7.33 | −2.5 (−6 to 1.2) | 0.18 | |
FF indicates focal firing; LIA, localized irregular activation; and LRA, localized rotational activation.
Irrespective of cutoff value, there was a significant increase in LRA with adenosine. The smallest increase in LRA occurrences was from 8.4±5.2 to 14.1±5.7 observed at the 20% cutoff (5.7, 95% CI, 2.7–8.6, P<0.0005), with the largest effect from 16.1±7.6 to 24.2±8.1 detected with no cutoff applied (8.1, 95% CI, 4.1–12, P<0.0005). The same pattern was observed when measured as a percentage of time LRA was present as illustrated in Figure 3. Adenosine administration resulted in a small increase in the proportion of the left atrium surface area in which LRA occurred. See Table 2 for full results. Examples of the effect of adenosine are shown in Videos S1 and S2 and in Figure 4.
Figure 3. Effect of adenosine on percentage time of LRA occurrences for all maps (n=35).
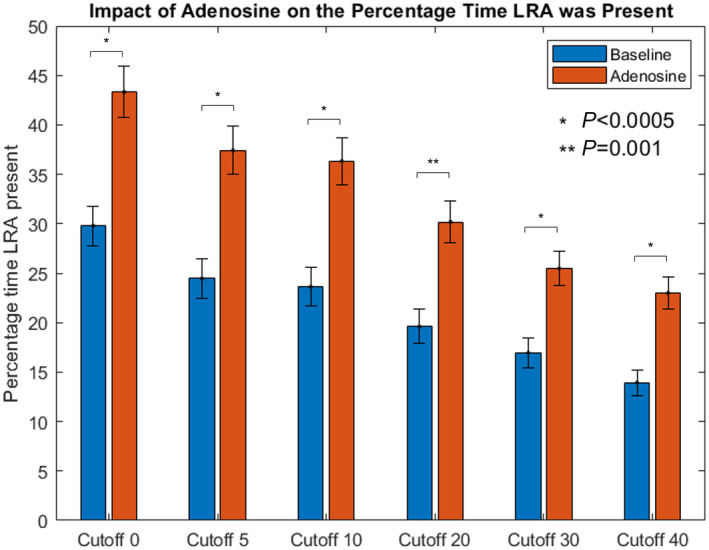
LRA indicates localized rotational activation. Bars represent SE.
Figure 4. Example activation map of a zone of LRA on the inferoposterior LA seen after adenosine injection (A).
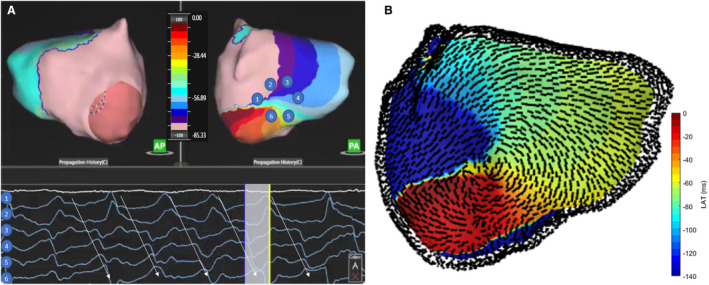
Dipole signals around this rotational pivot point demonstrate progressive activation. The same pattern of activation is shown in Videos S1 and S2 and the conduction velocity vector map (B) demonstrates the angles of propagation around the central point. AP indicates anterior posterior; LA, left atrium; LRA, localized rotational activation; and PA, posterior anterior.
Although differences were observed at all stages, this did not reach statistical significance at baseline, and was most pronounced post PVI and after nonpulmonary vein ablation (see Figure 5 and Table S1).
Figure 5. Effect of adenosine on LRA duration before and after pulmonary vein isolation, and after nonpulmonary vein ablation (bars represent standard error).
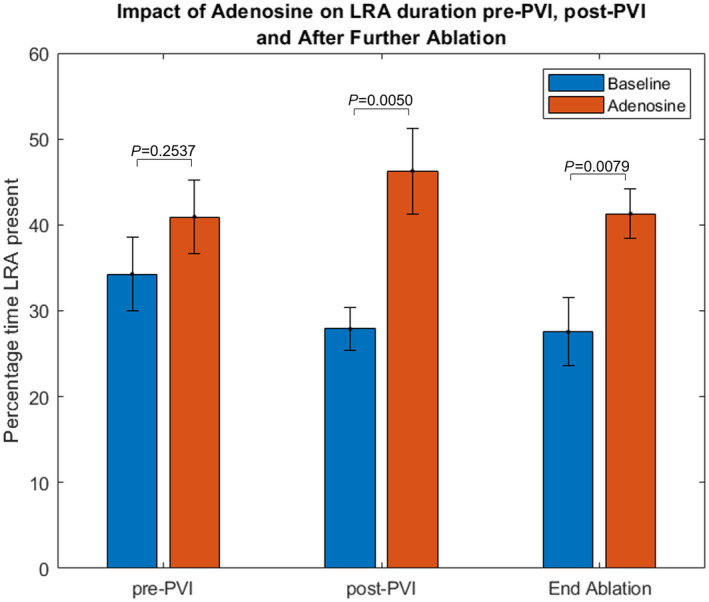
(n=11 pre‐PVI, n=15 post‐PVI, n=9 end ablation). LRA indicates localized rotational activation; and PVI, pulmonary vein isolation.
Adenosine had no significant effect on the number of focal firings observed (24.5±8.2 versus 22.0±7.3 at baseline, difference −2.5, 95% CI, −6 to 1.2, P=0.18).
AF Cycle Length
At baseline, AFCL was 181.7±14.3 ms, which reduced to 165.1±16.3 ms with adenosine, a mean shortening of 16.6 ms (95% CI, 11.3–21.9, P<0.0005). The value for the fifth percentile of AFCL in each map was also analyzed to reflect the fastest activation rates, proposed as a surrogate of refractoriness. Adenosine resulted in a similar shortening of the fifth percentile of AFCL from 135.0±11.1 ms to 127.5±12.2 ms, a mean reduction of 7.5 ms (95% CI, 3.8–11.2, P<0.0005). The coefficient of variance of AFCL (SD/mean) was 0.072±0.028 at baseline, and 0.052±0.016 with adenosine (difference 0.020, 95% CI, 0.01–0.03, P<0.0005).
There was a concomitant increase in global DF with adenosine from a mean of 6.0±0.7 Hz to 6.6±0.8 Hz, a statistically significant mean difference of 0.6Hz (95% CI, 0.4–0.9, P<0.0005). Coefficient of variance in DF reduced with adenosine from 0.08±0.05 to 0.06±0.02, but this was not statistically significant (mean difference 0.02, 95% CI, −0.003 to 0.03, P=0.0915).
Phase Singularities
Multiple short‐lasting phase singularities were found in all recordings. There were a greater number of phase singularities seen with adenosine (36.6±9.3) compared with baseline (30.1±7.8), which represented a statistically significant mean difference of 6.5 (95% CI, 2.6–10.0, P=0.002). There was a small but statistically significant increase in the lifespan of phase singularities with adenosine from 509±161 to 599±245 ms (90; 95% CI, 13–167; P=0.023).
Spatial distribution of LRA
In light of the observation of LRA occurring over a greater left atrium surface area with adenosine, each map was visually inspected at the highest cutoff to identify whether regions with repetitive LRA remained spatially consistent or were changed with adenosine use. At baseline, a region of repetitive LRA could be identified in 89% (31 of the 35) of maps, with 61 zones identified in total (1.7±1.1 per map). Following adenosine infusion, 70 zones were identified across all 35 maps (2±1 per map), with 42 of these zones (60%; 1.2±1.0 per map) localized to the same site as LRA in baseline maps. Figure 6 illustrates regions of repetitive LRA in 2 patients before and after adenosine infusion. Regions of high‐frequency LIA and LRA may overlap, given that multiple wavefronts over different AF cycles may or may not satisfy criteria for LRA within the same confined region (eg, partial rotation through 180 degrees) and be classified as LIA. A total of 81% of sites with high‐frequency LRA with adenosine coincided with sites where high‐frequency LIA was seen on baseline maps. There were 11 maps (in 9 patients) where the zones of LRA observed in adenosine maps were all in different sites from the baseline maps. In 4 of these, there were no regions of repetitive LRA seen at baseline.
Figure 6. Regions of highly repetitive LRA after adenosine infusion identified at 20% cutoff threshold (in pink/purple) occur at regions of LRA at baseline at lower frequency in patient 10 (top images) in contrast with patient 8 (lower panel) in whom adenosine promotes LRA in regions widely distributed throughout the chamber with little correlation to those zones seen at baseline.
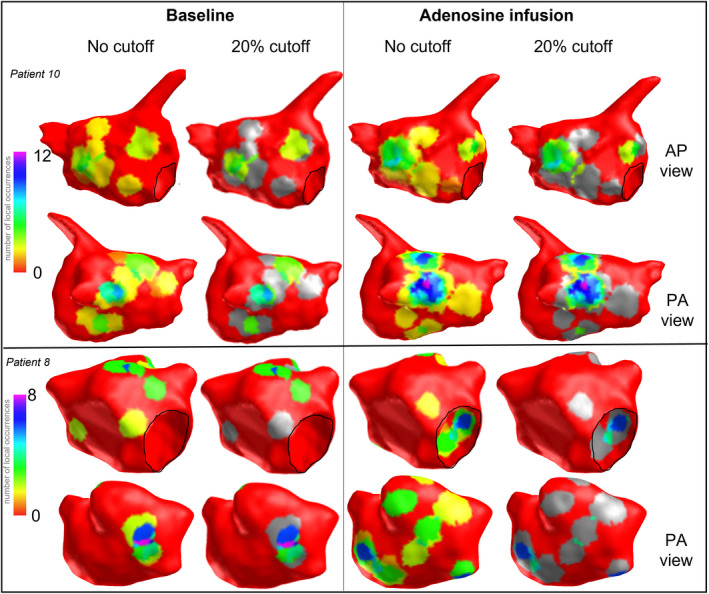
Mitral annulus is outlined in black, areas of excluded occurrences are shown in grey. AP indicates anterior posterior; LRA, localized rotational activation; and PA, posterior anterior.
In those patients where mapping was performed at more than 1 time point during the procedure (n=12; 1 patient had maps at all 3 time points), regions with the highest frequency of LRA seen on adenosine maps were compared. Across maps, 54 sites were identified, with 15 (28%) of these present at more than 1 time point during the procedure.
Across 26 maps at baseline, 40 sites of high DF were identified with 13 of these (33%) correlating with sites of high‐frequency LRA. After adenosine injection, 44 high DF sites were identified (in 30 maps), with 12 (27%) of these aligning with sites of high‐frequency LRA (see Figure 7).
Figure 7. Dominant frequency (DF) maps for the same patients included in Figure 6.
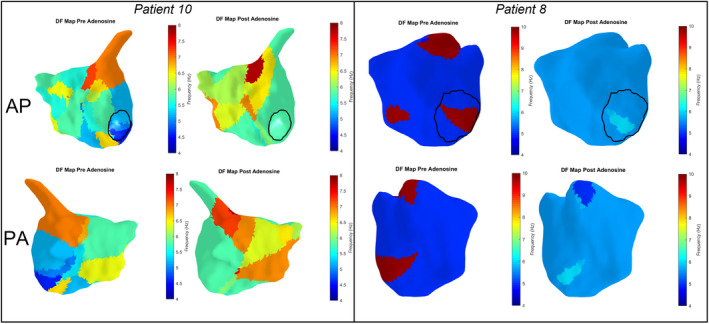
A zone of highest DF in patient 10 is seen at the base of the LAA in the AP view both before and after adenosine injection, which does not correlate with the region of high‐frequency LRA seen on the posterior wall (see Figure 6). Zones of high DF are also seen in patient 8 in the septum, roof, and mitral isthmus at baseline. A region of high‐frequency LRA is seen on the roof of the LA (Figure 6) but there is no correlation with other zones of high DF. A region of high DF in the posterolateral mitral isthmus after adenosine correlates with a site of LRA. Mitral annulus is outlined in black, color scale ranges from lowest to highest DF in each patient. AP indicates anterior posterior; LA, left atrium; LRA, localized rotational activation; and PA, posterior anterior.
Clinical Outcomes
Ablation resulted in acute termination to sinus rhythm in 6 (27%), with the remainder undergoing direct current cardioversion at the end of the procedure. Those in whom direct current cardioversion was required had more LRA on baseline maps compared with patients in whom ablation terminated AF. This was most prominent at 20% cutoff when measured as a number of LRA occurrences (9.0±5.2 versus 3.7±2.3, 95% CI, 0.7–10, P=0.0270), proportion of time LRA was present (20.8±10.8 versus 10.3±6.5, 95% CI, 0.6–20.5, P=0.0380) or the surface area affected (8.3±3.3 versus 3.9±2.3, 95% CI, 1.3–7.5, P=0.0080). There was no difference between the 2 groups on adenosine maps on any measure. See Tables S2 and S3 for full results.
Discussion
The main findings of our study are that intravenous adenosine injection has the following effects on AF: (1) AFCL shortening with increase in DF, (2) a modest increase in wave propagation irregularity (as defined by LIA parameter), (3) marked increase in the number of short‐lasting rotating waves (assessed both by activation time and phase singularity mapping), and (4) an increase in the lifespan of rotating waves. We found no effect of adenosine on focal firing frequency. Although the majority (60%) of regions found to have high LRA counts on adenosine maps were consistent with the zones identified at baseline, 40% of regions seen were revealed only with use of adenosine and only 28% were consistent across multiple mapping time points.
The mechanisms responsible for maintenance of persistent AF are poorly understood. Early studies in animal models led to the description of localized rotors, defined as organized and repetitive reentry circuits thought to represent organized drivers. 16 , 17 , 18 , 19 In recent years, efforts have focused on developing techniques to map these sources to guide additional ablation beyond the pulmonary veins in a bid to improve clinical outcomes. Although early results of this strategy were promising, 20 , 21 , 22 , 23 recent randomized studies evaluating this approach have been disappointing. 24 Furthermore, these methods have primarily relied on a phase mapping approach applied either to multielectrode basket catheters or noninvasive body surface electrodes, which aim to identify phase singularities representing the pivot point of localized rotors. 25 Further studies and evaluation of this technique have revealed contrasting results and highlighted the limitations of this approach. 26 , 27 , 28 , 29
The noncontact mapping employed in this study reveals patterns of propagation based on charge density reconstruction. 12 Uniquely, this system allows visualization of the dynamics of wavefront propagation during AF and reveals recurrent patterns of partial rotation, directional changes, conduction slowing, and focal firing with a high degree of spatial stability. 12 Although the ionic effects of adenosine have been well defined, the functional impact of these changes on wavefront propagation are less clear. The presumption that hyperpolarizing the atrial myocardial cell membrane, as well as shortening action potential duration and refractoriness, promotes reentry appears theoretically sound and is supported by our results with the predominant effect of adenosine being a promotion of LRA. 30 Hansen et al showed that adenosine promoted local reentry at sites of AF termination or cycle length slowing, proposed as evidence of adenosine unmasking sites of reentry driving AF propagation. 10 However, although 9 of the 15 sites identified (in the 10 patients studied) were more pronounced with adenosine, 6 were not. 10 We found that only 28% of sites identified with adenosine were stable between recordings. Moreover, the rotational activation promoted by adenosine was evident both in regions with repetitive patterns (as identified using the higher cutoffs) but also distributed more widely through the chamber (seen with no cutoff applied). Patients in whom ablation terminated AF had less rotational activation at baseline but the effect of adenosine was the same irrespective of acute procedural outcome, which is not in keeping with the theory that sites revealed with the use of adenosine represent arrhythmogenic sources responsible for AF propagation. The degree of rotational activation measured after adenosine appears to represent the pharmacological properties of the drug, independent of the individual substrate properties of the patient. The degree of rotational activation before adenosine administration was, however, lower in patients in whom ablation achieved sinus rhythm. It may be that the degree of rotational activation seen serves as a surrogate marker of atrial functional electrophysiological properties that affect the likelihood of acute termination, but these are concealed by use of adenosine rather than revealed and do not provide evidence that targeting ablation to areas identified with the use of adenosine are likely to yield improved procedural outcomes.
Sites of high‐frequency LRA and LIA may overlap as these both represent localized directional changes in propagation. A total of 81% of LRA zones identified using adenosine occurred in regions with high‐frequency LIA at baseline. Reentry mechanisms have been shown to anchor to structural heterogeneities, 31 , 32 whereas LIA zones are highly stable between recordings, perhaps reflective of local structural abnormalities or anatomical complexities, 33 which results in directional change in propagation at these sites. Shortening of atrial refractoriness with adenosine enables the directional change to complete >270 degrees of rotation and persist for longer therefore appearing as LRA. Importantly, these are generally short‐lived patterns of incomplete rotation rather than stable complete reentry. Zaman et al reviewed reconstructed isochronal maps from sites of acute AF termination during ablation of persistent AF guided by focal impulse and rotor modulation mapping. The predominant activation pattern seen was that of incomplete rotation (in 46%) with stable rotational sources identified in only 21%, in keeping with high‐density contact mapping studies. 34 , 35 We did not find stable rotating waves using both approaches (LRA and phase singularity mapping). It may be that regions of high‐frequency LIA best reflect anatomical properties that affect wavefront propagation, with LRA determined by functional atrial properties. Importantly, the patterns of LRA promoted by adenosine occur in a spatiotemporally diverse distribution and are therefore of limited clinical relevance when devising ablation strategies.
No effect on focal firing was observed. Hyperpolarization of the atrial myocardial cell membrane results in slowing in the rate of diastolic depolarization thus reducing automaticity. In addition, adenosine is known to inhibit catecholaminergic sensitive L‐type inward calcium currents within the atria. 30 Importantly, however, all the patients included in our analysis had persistent AF. Adenosine appears to have differential effects in patients with paroxysmal versus persistent arrhythmia possibly because of the relative difference in etiological contribution of the pulmonary veins and wider atrial myocardial substrate. 5 , 6 Increased pulmonary vein firing after adenosine infusion is well described but is likely to be of greater significance in paroxysmal AF and not observed during persistent arrhythmia. 36 , 37 Furthermore, apparent focal firing during persistent AF may be related to epicardial to endocardial breakthrough rather than automaticity (which is not affected by adenosine). 38 Reentry mechanisms may occur at the micro‐anatomic level, detectable only using high‐resolution optical mapping 32 and not revealed at the resolution of the clinical mapping system used in this study. However, if present and promoted by adenosine, then it would be anticipated that this would manifest as an increase in focal activations appearing on the endocardial surface, which was not observed.
These results suggest a global acceleration of AF within the left atrium as demonstrated by a reduction in mean AFCL with significant (albeit small) reduction in variation of AFCL from 7.2% to 5.2% (measured by coefficient of variance of AFCL), which is supported by the concomitant increase in DF. This suggests a global atrial pharmacological effect rather than limited focal changes that would be expected to cause greater heterogeneity in AFCL (or DF) across the chamber.
Limitations
These results are restricted to analysis of left atrial propagation. The distribution of adenosine receptors is heterogenous 7 and in persistent AF, an increase in dominant frequency with adenosine was demonstrated only in the high right atrium. 6 The right atrium is known to represent sites of mechanistic significance in a proportion of patients with AF and a differential effect of adenosine on right atrial propagation cannot be excluded. 21 , 39
Only short segments of maps (5 s duration) were analyzed. Spatiotemporal variation in AF propagation may mean that patterns of propagation change during different or longer map durations. The differences observed after adenosine administration were highly consistent suggesting this was not simply the result of a different time period of measurement. Although an increase in LIA was also observed, these changes were small, only significant (in terms of number of pattern occurrences) in raw analysis and 5% cutoff, and not likely to be clinically significant. Given the whole chamber acceleration in AFCL, a greater number of AF cycles would be expected over the same recording period and a small increase in the occurrences of infrequent spatially diverse irregular activation may therefore be observed. Furthermore, the proportion of time LIA patterns were present increased by only 3.2%–3.8%, which is unlikely to be significant and may also be driven by the reduction in refractoriness.
The technology used in this study is based on the resolution of local charge density derived through application of the inverse solution to multiple simultaneously recorded intracavitary unipolar electrograms. 12 Electrical activity on the endocardial surface (discretized as ≈3500 vertices) is obtained as an inverse solution based on measurements of 48 electrodes of the basket catheter. This is a unique approach aimed at achieving a more accurate local representation of cardiac activation and has been validated against contact unipolar recordings. 40 However, the precise implication of this technique on the visualized map of atrial activation compared with alternative techniques is difficult to discern and requires further extensive comparison given the lack of sufficient validation of activation patterns against contact recordings or optical mapping during AF.
It is difficult to establish the effect of ablation. The effects on LRA were consistent at all stages of the procedure but not statistically significant before any ablation. The primary analysis was intended to detect an effect across all maps and may have been underpowered to detect differences with subanalyses. The difference in statistical significance was driven in part by a reduction in LRA observed post PVI, which may be the result of pulmonary vein ablation incorporating sites of LRA. However, this difference was not statistically significant and if PVI eliminated rotational sites it would be anticipated that this would also reduce the impact of adenosine, which was not observed. Although the number of rotational activation patterns were lower in patients in whom ablation was acutely successful, it is difficult to draw further conclusions about the process of sinus rhythm restoration, which was not seen to occur instantaneously after ablation delivery. It cannot therefore be determined whether the first area targeted, or the last, or a combination of all of them was the critical mechanism maintaining AF. Furthermore, although we report acute procedural outcomes, these may not correspond to clinically meaningful long‐term outcomes, which would require further analyses beyond the scope of this study.
Conclusions
Our results suggest that rotational activation, in contrast with focal firing, and much less so LIA, is influenced by adenosine. Adenosine allows for a higher density of rotating wavefronts at spatially variable sites throughout the left atrium and shorter global cycle length with increase in DF. The degree of rotational activation may serve as a surrogate measure of individual atrial functional properties, with little evidence that rotational activation seen with adenosine represents promising targets for ablation aimed at arrhythmogenic sources of AF perpetuation within the left atrium particularly as there is little stability of LRA sites within patients. Caution should be exercised when interpreting maps obtained after adenosine administration to guide nonpulmonary vein ablation.
Sources of Funding
This study was supported by the Oxford Biomedical Research Centre and funded by internal departmental budgets.
Disclosures
Michael Pope has received honoraria and support for conference attendance from Acutus Medical. Pawel Kuklik provides consultancy services to Acutus Medical. Timothy Betts has received honoraria from Acutus Medical and is a member of the Medical Advisory Board.
Supporting information
Data S1
Tables S1–S3
Figures S1‐S4
Videos S1‐S2
For Sources of Funding and Disclosures, see page 12.
References
- 1. Belhassen B, Pelleg A, Shoshani D, Laniado S. Atrial fibrillation induced by adenosine triphosphate. Am J Cardiol. 1984;53:1405–1406. doi: 10.1016/0002-9149(84)90104-8 [DOI] [PubMed] [Google Scholar]
- 2. Nakai T, Watanabe I, Kunimoto S, Kojima T, Kondo K, Saito S, Ozawa Y, Kanmatsuse K. Electrophysiological effect of adenosine triphosphate and adenosine on atrial and ventricular action potential duration in humans. Jpn Circ J. 2000;64:430–435. doi: 10.1253/jcj.64.430 [DOI] [PubMed] [Google Scholar]
- 3. Belardinelli L, Isenberg G. Isolated atrial myocytes: adenosine and acetylcholine increase potassium conductance. Am J Physiol. 1983;244:H734–H737. doi: 10.1152/ajpheart.1983.244.5.H734 [DOI] [PubMed] [Google Scholar]
- 4. Drury AN, Szent‐Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68:213–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berenfeld O. Ionic and substrate mechanism of atrial fibrillation: rotors and the exitacion frequency approach. Arch Cardiol Mex. 2010;80:301–314. [PMC free article] [PubMed] [Google Scholar]
- 6. Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, Arenal A, Villacastín JP, Torrecilla EG, Sánchez A, et al. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation. 2006;114:2434–2442. doi: 10.1161/CIRCULATIONAHA.106.633735 [DOI] [PubMed] [Google Scholar]
- 7. Li N, Csepe TA, Hansen BJ, Sul LV, Kalyanasundaram A, Zakharkin SO, Zhao J, Guha A, Van Wagoner DR, Kilic A, et al. Adenosine‐induced atrial fibrillation: localized reentrant drivers in lateral right atria due to heterogeneous expression of adenosine A1 Receptors and GIRK4 subunits in the human heart. Circulation. 2016;134:486–498. doi: 10.1161/CIRCULATIONAHA.115.021165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sarmast F, Kolli A, Zaitsev A, Parisian K, Dhamoon AS, Guha PK, Warren M, Anumonwo JM, Taffet SM, Berenfeld O, et al. Cholinergic atrial fibrillation: I(K, ACh) gradients determine unequal left/right atrial frequencies and rotor dynamics. Cardiovasc Res. 2003;59:863–873. doi: 10.1016/S0008-6363(03)00540-6 [DOI] [PubMed] [Google Scholar]
- 9. Sanders P, Berenfeld O, Hocini M, Jaïs P, Vaidyanathan R, Hsu L‐F, Garrigue S, Takahashi Y, Rotter M, Sacher F, et al. Spectral analysis identifies sites of high‐frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011 [DOI] [PubMed] [Google Scholar]
- 10. Hansen BJ, Zhao J, Helfrich KM, Li N, Iancau A, Zolotarev AM, Zakharkin SO, Kalyanasundaram A, Subr M, Dastagir N, et al. Unmasking arrhythmogenic hubs of reentry driving persistent atrial fibrillation for patient‐specific treatment. J Am Heart Assoc. 2020;9:e017789. doi: 10.1161/JAHA.120.017789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi R, Norman M, Chen Z, Wong T. Individualized ablation strategy guided by live simultaneous global mapping to treat persistent atrial fibrillation. Future Cardiol. 2018;14:237–249. doi: 10.2217/fca-2017-0109 [DOI] [PubMed] [Google Scholar]
- 12. Grace A, Willems S, Meyer C, Verma A, Heck P, Zhu M, Shi X, Chou D, Dang L, Scharf C, et al. High‐resolution noncontact charge‐density mapping of endocardial activation. JCI Insight. 2019;4. doi: 10.1172/jci.insight.126422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Willems S, Verma A, Betts TR, Murray S, Neuzil P, Ince H, Steven D, Sultan A, Heck PM, Hall MC, et al. Targeting nonpulmonary vein sources in persistent atrial fibrillation identified by noncontact charge density mapping. Circ Arrhythm Electrophysiol. 2019;12:e007233. doi: 10.1161/CIRCEP.119.007233 [DOI] [PubMed] [Google Scholar]
- 14. Kuklik P, Zeemering S, Maesen B, Maessen J, Crijns HJ, Verheule S, Ganesan AN, Schotten U. Reconstruction of instantaneous phase of unipolar atrial contact electrogram using a concept of sinusoidal recomposition and Hilbert transform. IEEE Trans Biomed Eng. 2015;62:296–302. doi: 10.1109/TBME.2014.2350029 [DOI] [PubMed] [Google Scholar]
- 15. Kuklik P, Zeemering S, van Hunnik A, Maesen B, Pison L, Lau DH, Maessen J, Podziemski P, Meyer C, Schaffer B, et al. Identification of rotors during human atrial fibrillation using contact mapping and phase singularity detection: technical considerations. IEEE Trans Biomed Eng. 2017;64:310–318. [DOI] [PubMed] [Google Scholar]
- 16. Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–199. doi: 10.1161/01.CIR.101.2.194 [DOI] [PubMed] [Google Scholar]
- 17. Jalife J, Berenfeld O, Skanes A, Mandapati R. Mechanisms of atrial fibrillation: mother rotors or multiple daughter wavelets, or both? J Cardiovasc Electrophysiol. 1998;9:S2–S12. [PubMed] [Google Scholar]
- 18. Jalife J. Rotors and spiral waves in atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14:776–780. doi: 10.1046/j.1540-8167.2003.03136.x [DOI] [PubMed] [Google Scholar]
- 19. Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res. 2002;54:204–216. doi: 10.1016/S0008-6363(02)00223-7 [DOI] [PubMed] [Google Scholar]
- 20. Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller JM, Kalra V, Das MK, Jain R, Garlie JB, Brewster JA, Dandamudi G. Clinical benefit of ablating localized sources for human atrial fibrillation: the Indiana University FIRM Registry. J Am Coll Cardiol. 2017;69:1247–1256. doi: 10.1016/j.jacc.2016.11.079 [DOI] [PubMed] [Google Scholar]
- 22. Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD, Ellenbogen KA, Hummel JD, Baykaner T, Krummen DE, et al. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol. 2014;25:921–929. doi: 10.1111/jce.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat M‐Q, et al. Driver domains in persistent atrial fibrillation. Circulation. 2014;130:530–538. doi: 10.1161/CIRCULATIONAHA.113.005421 [DOI] [PubMed] [Google Scholar]
- 24. Brachmann JHJ, Wilber DJ, Sarver AE, Rapkin J, Shpun S, Szili‐Torok T. Prospective randomized comparison of rotor ablation vs conventional ablation for treatment of persistent atrial fibrillation ‐ The REAFFIRM trial. Heart Rhythm. 2019;16:963. [Google Scholar]
- 25. Umapathy K, Nair K, Masse S, Krishnan S, Rogers J, Nash MP, Nanthakumar K. Phase mapping of cardiac fibrillation. Circ Arrhythm Electrophysiol. 2010;3:105–114. doi: 10.1161/CIRCEP.110.853804 [DOI] [PubMed] [Google Scholar]
- 26. Child N, Clayton RH, Roney CR, Laughner JI, Shuros A, Neuzil P, Petru J, Jackson T, Porter B, Bostock J, et al. Unraveling the underlying arrhythmia mechanism in persistent atrial fibrillation: results from the STARLIGHT study. Circ Arrhythm Electrophysiol. 2018;11:e005897. doi: 10.1161/CIRCEP.117.005897 [DOI] [PubMed] [Google Scholar]
- 27. Laughner J, Shome S, Child N, Shuros A, Neuzil P, Gill J, Wright M. Practical considerations of mapping persistent atrial fibrillation with whole‐chamber basket catheters. JACC Clin Electrophysiol. 2016;2:55–65. doi: 10.1016/j.jacep.2015.09.017 [DOI] [PubMed] [Google Scholar]
- 28. Rodrigo M, Climent AM, Liberos A, Fernández‐Avilés F, Berenfeld O, Atienza F, Guillem MS. Technical considerations on phase mapping for identification of atrial reentrant activity in direct‐ and inverse‐computed electrograms. Circ Arrhythm Electrophysiol. 2017;10. doi: 10.1161/CIRCEP.117.005008 [DOI] [PubMed] [Google Scholar]
- 29. Roney CH, Cantwell CD, Bayer JD, Qureshi NA, Lim PB, Tweedy JH, Kanagaratnam P, Peters NS, Vigmond EJ, Ng FS. Spatial resolution requirements for accurate identification of drivers of atrial fibrillation. Circ Arrhythm Electrophysiol. 2017;10:e004899. doi: 10.1161/CIRCEP.116.004899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Belardinelli L, Shryock JC, Song Y, Wang D, Srinivas M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J. 1995;9:359–365. doi: 10.1096/fasebj.9.5.7896004 [DOI] [PubMed] [Google Scholar]
- 31. Haissaguerre M, Shah AJ, Cochet H, Hocini M, Dubois R, Efimov I, Vigmond E, Bernus O, Trayanova N. Intermittent drivers anchoring to structural heterogeneities as a major pathophysiological mechanism of human persistent atrial fibrillation. J Physiol. 2016;594:2387–2398. doi: 10.1113/JP270617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, Kalyanasundaram A, Lim P, Bratasz A, Powell KA, et al. Atrial fibrillation driven by micro‐anatomic intramural re‐entry revealed by simultaneous sub‐epicardial and sub‐endocardial optical mapping in explanted human hearts. Eur Heart J. 2015;36:2390–2401. doi: 10.1093/eurheartj/ehv233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pope MT, Kuklik P, Briosa EGA, Leo M, Mahmoudi M, Paisey J, Betts TR. Spatial and temporal variability of rotational, focal, and irregular activity: practical implications for mapping of atrial fibrillation. J Cardiovasc Electrophysiol. 2021;32:2393–2403. doi: 10.1111/jce.15170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zaman JAB, Sauer WH, Alhusseini MI, Baykaner T, Borne RT, Kowalewski CAB, Busch S, Zei PC, Park S, Viswanathan MN, et al. Identification and characterization of sites where persistent atrial fibrillation is terminated by localized ablation. Circ Arrhythm Electrophysiol. 2018;11:e005258. doi: 10.1161/CIRCEP.117.005258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee S, Sahadevan J, Khrestian CM, Cakulev I, Markowitz A, Waldo AL. Simultaneous Biatrial high‐density (510–512 Electrodes) epicardial mapping of persistent and long‐standing persistent atrial fibrillation in patients: new insights into the mechanism of its maintenance. Circulation. 2015;132:2108–2117. doi: 10.1161/CIRCULATIONAHA.115.017007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ip JE, Cheung JW, Chung JH, Liu CF, Thomas G, Markowitz SM, Lerman BB. Adenosine‐induced atrial fibrillation: insights into mechanism. Circ Arrhythm Electrophysiol. 2013;6:e34–37. doi: 10.1161/CIRCEP.113.000480 [DOI] [PubMed] [Google Scholar]
- 37. Cheung JW, Lin FS, Ip JE, Bender SR, Siddiqi FK, Liu CF, Thomas G, Markowitz SM, Lerman BB. Adenosine‐induced pulmonary vein ectopy as a predictor of recurrent atrial fibrillation after pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2013;6:1066–1073. doi: 10.1161/CIRCEP.113.000796 [DOI] [PubMed] [Google Scholar]
- 38. de Groot N, van der Does L, Yaksh A, Lanters E, Teuwen C, Knops P, van de Woestijne P, Bekkers J, Kik C, Bogers AD, et al. Direct proof of endo‐epicardial asynchrony of the atrial wall during atrial fibrillation in humans. Circ Arrhythm Electrophysiol. 2016;9. doi: 10.1161/CIRCEP.115.003648 [DOI] [PubMed] [Google Scholar]
- 39. Knecht S, Sohal M, Deisenhofer I, Albenque J‐P, Arentz T, Neumann T, Cauchemez B, Duytschaever M, Ramoul K, Verbeet T, et al. Multicentre evaluation of non‐invasive biatrial mapping for persistent atrial fibrillation ablation: the AFACART study. Europace. 2017;19:1302–1309. doi: 10.1093/europace/euw168 [DOI] [PubMed] [Google Scholar]
- 40. Shi R, Parikh P, Chen Z, Angel N, Norman M, Hussain W, Butcher C, Haldar S, Jones DG, Riad O, et al. Validation of dipole density mapping during atrial fibrillation and sinus rhythm in human left atrium. JACC Clin Electrophysiol. 2020;6:171–181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S3
Figures S1‐S4
Videos S1‐S2


