Abstract
Background
Current evidence might support the use of omega‐3 fatty acids (preferably docosahexaenoic acid and eicosapentaenoic acid) for lowering blood pressure (BP), but the strength and shape of the dose‐response relationship remains unclear.
Methods and Results
This study included randomized controlled trials published before May 7, 2021, that involved participants aged ≥18 years, and examined an association between omega‐3 fatty acids (docosahexaenoic acid, eicosapentaenoic acid, or both) and BP. A random‐effects 1‐stage cubic spline regression model was used to predict the average dose‐response association between daily omega‐3 fatty acid intake and changes in BP. We also conducted stratified analyses to examine differences by prespecified subgroups. Seventy‐one trials were included, involving 4973 individuals with a combined docosahexaenoic acid+eicosapentaenoic acid dose of 2.8 g/d (interquartile range, 1.3 g/d to 3.6 g/d). A nonlinear association was found overall or in most subgroups, depicted as J‐shaped dose‐response curves. The optimal intake in both systolic BP and diastolic BP reductions (mm Hg) were obtained by moderate doses between 2 g/d (systolic BP, −2.61 [95% CI, −3.57 to −1.65]; diastolic BP, −1.64 [95% CI, −2.29 to −0.99]) and 3 g/d (systolic BP, −2.61 [95% CI, −3.52 to −1.69]; diastolic BP, −1.80 [95% CI, −2.38 to −1.23]). Subgroup studies revealed stronger and approximately linear dose‐response relations among hypertensive, hyperlipidemic, and older populations.
Conclusions
This dose‐response meta‐analysis demonstrates that the optimal combined intake of omega‐3 fatty acids for BP lowering is likely between 2 g/d and 3 g/d. Doses of omega‐3 fatty acid intake above the recommended 3 g/d may be associated with additional benefits in lowering BP among groups at high risk for cardiovascular diseases.
Keywords: docosahexaenoic acid, eicosapentaenoic acid, hypertension, long‐chain fatty acids, 1‐stage regression
Subject Categories: High Blood Pressure, Hypertension
Nonstandard Abbreviations and Acronyms
- DBP
diastolic blood pressure
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- JELIS
Japan Eicosapentaenoic Acid Lipid Intervention Study
- REDUCE‐IT
Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial
- SBP
systolic blood pressure
- ω3 PUFA
omega‐3 polyunsaturated fatty acid
Clinical Perspective
What Is New?
Intake of omega‐3 fatty acids has a nonlinear association with reductions in blood pressure.
The optimal daily intake of omega‐3 fatty acid for blood pressure control appears to be 3 g.
What Are the Clinical Implications?
An optimal dose of omega‐3 fatty acids is potentially needed for blood pressure control in the general population, but individuals who are at high risk of developing cardiovascular diseases may benefit from higher doses.
Epidemiologic and experimental studies indicate that omega‐3 polyunsaturated fatty acids (ω3 PUFAs), preferably including docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), may have cardiovascular health benefits by reducing modifiable risk factors. For example, intake of EPA was associated with reduced risks of major vascular events in JELIS (Japan Eicosapentaenoic Acid Lipid Intervention Study) 1 and REDUCE‐IT (Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial). 2 However, recently completed clinical studies and meta‐analyses 5 , 6 showed that supplementation of ω3 PUFAs did not offer significant favorable impacts on cardiovascular events, such as the risk of cardiovascular disease, myocardial infarction, or stroke. Previous meta‐analyses have also examined the association between ω3 PUFA intake and blood pressure (BP), 7 , 8 , 9 , 10 , 11 but have been unable to reveal a significant dose‐response relationship 8 , 10 , 12 or have shown conflicting trends. 7 , 11 These past meta‐analyses examined the dose‐response relationship using pooled meta‐regression 8 , 10 or, by grouping categories of exposure into separate meta‐analyses, 7 , 11 approaches that are prone to biases and do not take into account the correlations among effects at different dose levels. 13
These limitations warrant further examination of the effects of ω3 PUFAs on changes in BP among randomized controlled trials (RCTs). To fully capture the dose‐response effect and reflect heterogeneity among the studies, we utilized a 1‐stage cubic spline regression model, recently developed 13 and used for dose‐response meta‐analyses in 2 BP systematic reviews. 14 , 15 The 1‐stage spline mixed model is advantageous since it allows estimation of nonlinear dose‐response curves, including J or L shape, and allows for the inclusion of studies with <3 exposure levels, in comparison to 2‐stage methods. 13 Following a comprehensive literature review for RCTs, this study aimed to more precisely characterize the dose‐response effect of ω3 PUFAs (DHA, EPA, or both) on BP in the general population and relevant subgroups.
Methods
The study followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines for the conduct of meta‐analysis of randomized trials and a checklist was attached (Table S1). The data that support the findings of this study are available from the corresponding author on reasonable request. This meta‐analysis was performed with the previously published trials. Therefore, ethical review or institutional review board approval was not applicable.
Literature Review
A systematic literature search was conducted for articles published before May 7, 2021, using PubMed and Embase databases (Table S2). Manual searches were undertaken to screen the reference lists of relevant studies, reviews, and meta‐analyses for additional studies. Two reviewers (X.Z. and X.L.) screened each study independently and discrepancies were resolved through discussion. The prespecified eligibility criteria were parallel or crossover RCTs that examined the association between intake of DHA/EPA (combined or individual) and systolic BP (SBP) and/or diastolic BP (DBP) in adults (aged ≥18 years). Studies were eligible if they examined intake of DHA/EPA through diet or fatty oil supplementation. We excluded trials in which: (1) concurrent inactive placebo controls were lacking; (2) intervention duration was <4 weeks; (3) a washout period of <4 weeks was applied between treatments in crossover trials; (4) patients with hypertension received concurrent BP‐lowering medications 11 , 12 ; and (5) studies were conducted in pregnant and nursing women, or individuals with preexisting cardiovascular events (eg, those with myocardial infarction or heart failure), renal diseases, or secondary hypertension. Assessment of the methodological quality was performed independently using the Cochrane risk‐of‐bias tool 2. 16
Data Extraction
For each eligible study, information was extracted independently by 2 of the authors (N.Z. and X.Z.) and confirmed by a third author (X.L.) using a standardized form. The effects of each dose of exposure were extracted individually in our study. In experiments with multiple follow‐up time points, only changes in SBP and DBP levels at the end of the treatment versus pretreatment were extracted, avoiding multiple measurements from the same trial. If an SD was not provided directly, we calculated it from the SE, interquartile range, or CI. 17
Exposure and Outcome Assessment
Most studies that examined the effects of omega‐3 fatty acids used a combined supplementation of EPA and DHA. The exposure levels were expressed by DHA+EPA combined or DHA/EPA alone. For intake of DHA/EPA through diet, the exposure level was determined by the fraction of pure DHA/EPA amount over the food consumed daily. For fatty oil supplementation trials, the exposure level was determined by the pure DHA/EPA content as claimed by the researchers or the manufacturers. We determined the net mean difference in BP (ΔBPbetween) between the exposure levels of each RCT as the difference at the end of the intervention minus the corresponding pretreatment value (ΔBPintragroup).
Publication Bias Assessment
Publication bias was examined visually using funnel plots to assess the SE as a function of effect size, and performing Egger regression test to examine small‐study bias using R metafor functions. 18 We also used the trim‐and‐fill method to estimate the number of potential missing studies caused by publication bias. A leave‐one‐out strategy was applied for sensitivity analyses, where we repeatedly ran the dose‐response analysis to assess the missing study's influence on overall mean BP change.
Dose‐Response Analysis
The placebo dose (0 g/d) was used as the reference for all analyses. A 1‐stage random‐effects dose‐response model 13 was performed to predict the average dose‐response relationship between administration of DHA+EPA and changes in SBP and DBP levels. We tested the linearity assumption underlying the dose‐response relationship by fitting a restricted cubic spline model with 3 knots (10th, 50th, and 90th percentiles) of the doses. 19 Included studies were pooled into a continuous dose‐response curve, and then the predicted effect of omega‐3 on BP was estimated from the curve at given doses (ie, 1 g/d, 2 g/d, 3 g/d, 4 g/d, and 5 g/d). Additionally, subgroup analyses were conducted by stratifying studies according to study design (crossover versus parallel), hypertension (SBP ≥140 mm Hg or DBP ≥90 mm Hg), or hyperlipidemia (total cholesterol ≥200 mg/dL or triglycerides ≥150 mg/dL) status, intervention (supplementation versus diet), exposure composition (fish oil versus purified ethyl ester), duration of treatment (≥12 weeks or not), sex, and average age (≥45 years or <45 years). We also conducted subgroup analyses by baseline SBP (≥130 mm Hg versus <130 mm Hg), according to the new cut point suggested in a recent American Heart Association hypertension guideline. 20 The 1‐stage cubic spline regression model was conducted using the dosresmeta R packages (https://github.com/alecri/dosresmeta). 13 , 21 , 22
Results
Study Characteristics
After removing duplicates, the systematic search retrieved 3066 relevant articles. The title and abstract review further excluded 2897 articles. Full‐text examination of 169 articles yielded 71 eligible RCTs (references 23 and 24 and references 36 to 104 in the Supplemental Material) that were included in the analyses. A PRISMA flow diagram of the literature screening is shown in Figure 1. Study characteristics of the included trials are shown in Table S3. These trials, published between 1987 and 2020, reported an overall sample size of 4973 participants with an average age between 22 to 86 years. A parallel design was adopted predominantly in 60 trials, and only 11 trials used a crossover design. These trials were conducted in Europe (n=27), North America (n=25), Oceania (n=16), and Asia (n=3). More than a half of the trials (43 of 71) included both men and women, whereas 25 included only men and 3 included only women. Most trials were restricted to participants without hypertension (n=56 [79%], average baseline SBP <140 mm Hg) and without hyperlipidemia (n=57 [80%], average total cholesterol<200 mg/dL [5.2 mmol/L] and triglycerides <150 mg/dL [1.7 mmol/L]). In terms of outcome measurement, BP was measured either manually (n=13), automatically (n=44), or not reported (n=14), in ambulatory (n=5), rest (n=8), seated (n=32), supine (n=12), or unknown (n=14) modalities. The average intervention duration was 10 weeks (interquartile range, 6–12 weeks) (Figure S1A), and the duration was longer than 12 weeks (ranging from 12 to 52 weeks) in 29 trials and <12 weeks in 42 trials. In the majority of studies (n=64), interventions of supplementation were accomplished by capsuled fish oil, algal oil, or purified fish oil ethyl esters. The remainder of studies (n=7) used a dietary intervention that included intake of fish meals (eg, mackerel, salmon, trout, and tuna) and other fish oil–fortified foods, either cooked at home or by a dietitian. The most commonly used placebo was olive oil, along with the remainder consisting of types of vegetable oils, such as safflower, sunflower, corn, soybean, and palm oils. Fifty‐three of 71 trials reported the combined effects of DHA and EPA, with an average combined dose of 2.8 g/d (interquartile range, 1.3–3.6; range 0.2–15 g/d) (Figure S1B), DHA dose of 1.4 g/d (range, 0 to 6 g/d), and EPA dose of 1.8 g/d (range, 0 to 9 g/d); only 11 and 6 trials observed the effects of individual DHA or EPA, respectively.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram of systematic literature search and screening for randomized controlled trials published through May 2021 that met the study inclusion and exclusion criteria.
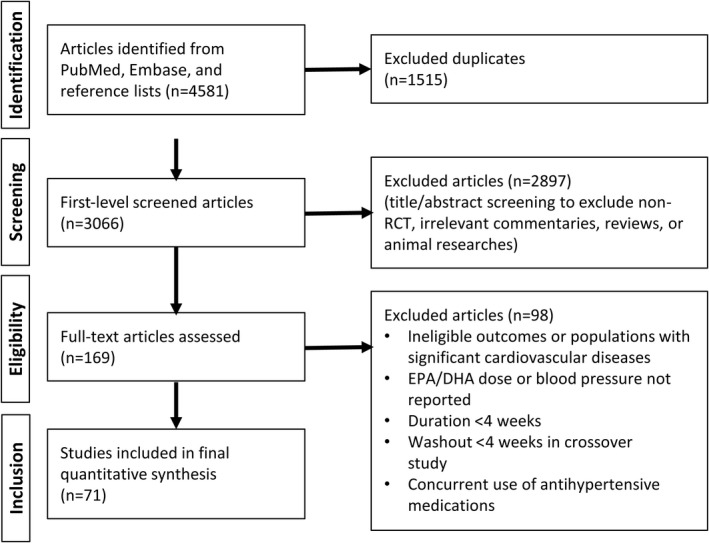
DHA indicates docosahexaenoic acid; and EPA, eicosapentaenoic acid.
Overall Dose‐Response Analysis
The Table summarizes the impact of combined doses of DHA+EPA at 1 g/d, 2 g/d, 3 g/d, 4 g/d, and 5 g/d on average changes in BP, compared with the placebo or control group (combined dose=0 g/d). We found a significant nonlinear dose‐response relationship for both SBP and DBP models (Figure 2) (=3.87 [P=0.0001] and z=2.68 [P=0.0073], respectively). The J‐shaped curves suggest that dosages of DHA+EPA at 2 g/d to 3 g/d are associated with the strongest changes in both SBP and DBP relative to the reference dose (0 g/d). The estimated average dose‐response curves and corresponding CIs also indicate that the dose region of apparent improvement for SBP and DBP is from 0 g/d to 5 g/d. When compared with the reference (0 g/d), the average mean changes in SBP were −2.61 mm Hg (95% CI, −3.57 to −1.65) for 2 g/d of DHA+EPA, and −2.61 mm Hg (95% CI, −3.52 to −1.69) for 3 g/d of DHA+EPA. The average mean changes in DBP were −1.64 mm Hg (95% CI, −2.29 to −0.99) for 2 g/d of DHA+EPA, and −1.80 mm Hg (95% CI, −2.38 to −1.23) for 3 g/d of DHA+EPA (Table). In both SBP and DBP models, combined doses >3 g/d were associated with weaker or null changes in BP (Table). The width of the CIs was wider at exposure levels >6 g/d for both SBP and DBP. Only 2 trials 23 , 24 examined a dose >7 g/d (specifically, at 15 g/d). Removal of these 2 trials did not change the shape of the dose‐response curve, despite the narrower CIs (Figure S2).
Table .
Estimated Average Dose‐Response Relationship Between DHA+EPA Consumption and BP Reduction
| BP | Participants | No* | 1.0 g/d | 2.0 g/d | 3.0 g/d | 4.0 g/d | 5.0 g/d | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MD | (95% CI) | MD | (95% CI) | MD | (95% CI) | MD | (95% CI) | MD | (95% CI) | |||
| SBP | All | 70 | −1.81 | (−2.52 to −1.10) | −2.61 | (−3.57 to −1.65) | −2.61 | (−3.52 to −1.69) | −2.15 | (−3.08 to −1.22) | −1.57 | (−2.79 to −0.34) |
| DBP | All | 69 | −1.07 | (−1.57 to −0.57) | −1.64 | (−2.29 to −0.99) | −1.80 | (−2.38 to −1.23) | −1.73 | (−2.27 to −1.19) | −1.59 | (−2.34 to −0.84) |
| Baseline SBP, mm Hg | ||||||||||||
| SBP | ≥130 | 19 | −1.53 | (−2.67 to −0.40) | −2.57 | (−4.32 to −0.81) | −3.22 | (−5.21 to −1.23) | −3.62 | (−5.64 to −1.59) | −3.88 | (−5.88 to −1.88) |
| <130 | 44 | −1.73 | (−2.72 to −0.75) | −2.38 | (−3.62 to −1.13) | −2.20 | (−3.29 to −1.10) | −1.65 | (−2.79 to −0.51) | −1.07 | (−2.65 to 0.51) | |
| DBP | ≥80 | 19 | −1.46 | (−2.14 to −0.78) | −2.49 | (−3.58 to −1.40) | −3.18 | (−4.48 to −1.87) | −3.64 | (−5.03 to −2.25) | −3.99 | (−5.41 to −2.58) |
| <80 | 45 | −0.83 | (−1.50 to −0.16) | −1.31 | (−2.13 to −0.49) | −1.54 | (−2.21 to −0.87) | −1.66 | (−2.36 to −0.95) | −1.76 | (−2.83 to −0.69) | |
| Hypertension status, SBP ≥140 mm Hg or DBP ≥90 mm Hg | ||||||||||||
| SBP | Hypertension | 16 | −2.56 | (−3.46 to −1.65) | −3.99 | (−5.29 to −2.70) | −4.54 | (−6.02 to −3.05) | −4.42 | (−6.33 to −2.52) | −3.89 | (−6.62 to −1.16) |
| No hypertension | 55 | −1.66 | (−2.52 to −0.80) | −2.22 | (−3.30 to −1.14) | −1.97 | (−2.90 to −1.03) | −1.35 | (−2.32 to −0.39) | −0.70 | (−2.06 to 0.66) | |
| DBP | Hypertension | 16 | −1.23 | (−1.90 to −0.55) | −2.14 | (−3.25 to −1.03) | −2.81 | (−4.18 to −1.45) | −3.30 | (−4.80 to −1.81) | −3.68 | (−5.25 to −2.10) |
| No hypertension | 55 | −0.94 | (−1.55 to −0.33) | −1.42 | (−2.18 to −0.67) | −1.57 | (−2.18 to −0.95) | −1.55 | (−2.15 to −0.96) | −1.53 | (−2.41 to −0.64) | |
| Hyperlipidemia status, total cholesterol ≥200 mg/dL or triglycerides ≥150 mg/dL | ||||||||||||
| SBP | Hyperlipidemia | 14 | −1.84 | (−3.00 to −0.69) | −3.17 | (−4.82 to −1.52) | −3.78 | (−5.21 to −2.35) | −4.03 | (−5.65 to −2.41) | −4.24 | (−6.75 to −1.73) |
| No hyperlipidemia | 56 | −1.68 | (−2.52 to −0.84) | −2.36 | (−3.50 to −1.21) | −2.26 | (−3.35 to −1.17) | −1.72 | (−2.73 to −0.71) | −1.06 | (−2.26 to 0.13) | |
| DBP | Hyperlipidemia | 14 | −1.55 | (−2.71 to −0.39) | −2.42 | (−4.03 to −0.80) | −2.34 | (−3.61 to −1.07) | −1.80 | (−3.18 to −0.43) | −1.21 | (−3.54 to 1.13) |
| No hyperlipidemia | 55 | −0.94 | (−1.50 to −0.39) | −1.48 | (−2.22 to −0.75) | −1.69 | (−2.34 to −1.03) | −1.70 | (−2.32 to −1.09) | −1.65 | (−2.50 to −0.81) | |
| Study duration, wk | ||||||||||||
| SBP | ≥12 | 29 | −0.76 | (−2.09 to 0.57) | −1.28 | (−2.42 to −0.15) | −1.66 | (−3.10 to −0.23) | −2.02 | (−4.96 to 0.91) | −2.38 | (−6.99 to 2.24) |
| <12 | 41 | −2.46 | (−3.52 to −1.39) | −3.50 | (−4.87 to −2.12) | −3.39 | (−4.56 to −2.22) | −2.52 | (−3.53 to −1.51) | −1.28 | (−2.85 to 0.30) | |
| DBP | ≥12 | 29 | −0.91 | (−1.93 to 0.11) | −1.51 | (−2.51 to −0.51) | −1.91 | (−2.63 to −1.20) | −2.29 | (−3.51 to −1.06) | −2.66 | (−4.70 to −0.62) |
| <12 | 40 | −0.99 | (−1.60 to −0.38) | −1.56 | (−2.42 to −0.70) | −1.76 | (−2.56 to −0.95) | −1.70 | (−2.35 to −1.05) | −1.52 | (−2.24 to −0.79) | |
| Study design | ||||||||||||
| SBP | Crossover | 11 | −1.35 | (−3.21 to 0.50) | −1.80 | (−4.39 to 0.78) | −1.52 | (−4.19 to 1.14) | −0.71 | (−3.62 to 2.20) | 0.44 | (−3.54 to 4.41) |
| Parallel | 59 | −1.95 | (−2.75 to −1.16) | −2.75 | (−3.78 to −1.71) | −2.67 | (−3.61 to −1.73) | −2.20 | (−3.14 to −1.25) | −1.68 | (−2.90 to −0.46) | |
| DBP | Crossover | 11 | −1.67 | (−3.30 to −0.05) | −2.43 | (−4.72 to −0.14) | −2.44 | (−4.66 to −0.22) | −1.91 | (−3.69 to −0.12) | −1.03 | (−2.71 to 0.65) |
| Parallel | 58 | −0.91 | (−1.46 to −0.36) | −1.45 | (−2.14 to −0.77) | −1.70 | (−2.27 to −1.12) | −1.81 | (−2.41 to −1.20) | −1.90 | (−2.79 to −1.01) | |
| Mean age, y | ||||||||||||
| SBP | ≥45 | 35 | −1.76 | (−2.82 to −0.71) | −2.58 | (−3.79 to −1.37) | −2.82 | (−3.91 to −1.73) | −2.87 | (−4.28 to −1.45) | −2.91 | (−5.00 to −0.81) |
| <45 | 21 | −1.10 | (−2.48 to 0.29) | −1.50 | (−3.50 to 0.51) | −1.29 | (−3.27 to 0.69) | −0.67 | (−2.26 to 0.91) | 0.14 | (−1.04 to 1.33) | |
| DBP | ≥45 | 33 | −0.61 | (−1.26 to 0.04) | −1.17 | (−1.93 to −0.40) | −1.68 | (−2.31 to −1.05) | −2.18 | (−2.85 to −1.51) | −2.68 | (−3.66 to −1.70) |
| <45 | 22 | −1.22 | (−2.03 to −0.41) | −1.75 | (−2.92 to −0.58) | −1.68 | (−2.85 to −0.51) | −1.21 | (−2.17 to −0.24) | −0.53 | (−1.34 to 0.27) | |
| Fish oil composition | ||||||||||||
| SBP | Ethyl ester | 12 | −0.57 | (−1.68 to 0.53) | −1.36 | (−3.03 to 0.31) | −2.41 | (−4.22 to −0.60) | −3.57 | (−5.69 to −1.45) | −4.75 | (−7.50 to −2.00) |
| Fish oil | 58 | −1.97 | (−2.76 to −1.18) | −2.71 | (−3.74 to −1.68) | −2.49 | (−3.43 to −1.55) | −1.70 | (−2.65 to −0.74) | −0.72 | (−2.06 to 0.63) | |
| DBP | Ethyl ester | 12 | −1.11 | (−1.64 to −0.58) | −1.69 | (−2.40 to −0.98) | −1.84 | (−2.49 to −1.19) | −1.73 | (−2.36 to −1.10) | −1.53 | (−2.39 to −0.66) |
| Fish oil | 57 | −1.12 | (−1.66 to −0.58) | −1.69 | (−2.4 to −0.99) | −1.84 | (−2.49 to −1.20) | −1.74 | (−2.37 to −1.10) | −1.54 | (−2.42 to −0.67) | |
| Intervention type | ||||||||||||
| SBP | Diet | 8 | −2.05 | (−4.13 to 0.04) | −2.54 | (−4.99 to −0.09) | −2.02 | (−4.07 to 0.04) | −1.07 | (−3.40 to 1.25) | −0.10 | (−3.59 to 3.39) |
| Supplementation | 64 | −1.78 | (−2.53 to −1.03) | −2.58 | (−3.59 to −1.57) | −2.62 | (−3.59 to −1.65) | −2.24 | (−3.22 to −1.25) | −1.75 | (−3.02 to −0.47) | |
| DBP | Diet | 7 | 0.34 | (−0.37 to 1.05) | −0.05 | (−1.07 to 0.97) | −0.94 | (−2.75 to 0.88) | −2.08 | (−5.19 to 1.03) | −3.27 | (−7.83 to 1.29) |
| Supplementation | 64 | −1.16 | (−1.69 to −0.63) | −1.76 | (−2.45 to −1.06) | −1.90 | (−2.51 to −1.29) | −1.78 | (−2.35 to −1.22) | −1.60 | (−2.39 to −0.82) | |
| Sex | ||||||||||||
| SBP | Men | 24 | −1.28 | (−2.32 to −0.23) | −2.12 | (−3.89 to −0.36) | −2.19 | (−4.10 to −0.28) | −1.59 | (−3.18 to 0.00) | −0.54 | (−1.70 to 0.62) |
| Women | 3 | 1.37 | (−6.26 to 9.00) | 1.00 | (−4.77 to 6.76) | −0.31 | (−2.61 to 2.00) | −1.74 | (−11.28 to 7.80) | −3.17 | (−20.45 to 14.11) | |
| DBP | Men | 25 | −1.13 | (−1.71 to −0.55) | −1.89 | (−2.85 to −0.93) | −2.01 | (−3.03 to −1.00) | −1.60 | (−2.46 to −0.75) | −0.85 | (−1.63 to −0.07) |
| Women | 3 | 3.86 | (−2.99 to 10.70) | 2.39 | (−3.04 to 7.82) | −1.92 | (−5.90 to 2.05) | −6.62 | (−16.60 to 3.36) | −11.32 | (−28.27 to 5.63) | |
| Individual effect of DHA or EPA | ||||||||||||
| SBP | DHA only | 11 | −1.95 | (−3.52 to −0.38) | −2.37 | (−3.86 to −0.88) | −2.03 | (−4.08 to 0.03) | −1.56 | (−5.21 to 2.09) | −1.10 | (−6.56 to 4.37) |
| EPA only | 6 | 1.42 | (−2.52 to 5.35) | 1.02 | (−3.30 to 5.33) | −0.58 | (−3.24 to 2.08) | −2.68 | (−5.14 to −0.21) | −4.82 | (−9.89 to 0.25) | |
| DBP | DHA only | 11 | −1.10 | (−3.06 to 0.86) | −1.04 | (−2.66 to 0.57) | −0.40 | (−2.03 to 1.23) | 0.34 | (−3.04 to 3.71) | 1.07 | (−4.35 to 6.50) |
| EPA only | 6 | 2.73 | (1.72 to 3.74) | 2.48 | (1.27 to 3.68) | 0.26 | (−1.18 to 1.70) | −2.78 | (−5.06 to −0.49) | −5.89 | (−9.28 to −2.49) | |
BP indicates blood pressure; DHA, docosahexaenoic acid; DBP, diastolic blood pressure; EPA, eicosapentaenoic acid; MD, mean difference, mm Hg; and SBP, systolic blood pressure. Note: *Numbers may not sum to group totals because of missing data or unspecified subgroups in the trials. The total number is >71 because of the multiple intervention types in 1 trial.
Figure 2. Dose‐response relationship between changes in blood pressure and combined docosahexaenoic acid (DHA)+eicosapentaenoic acid (EPA) intake.
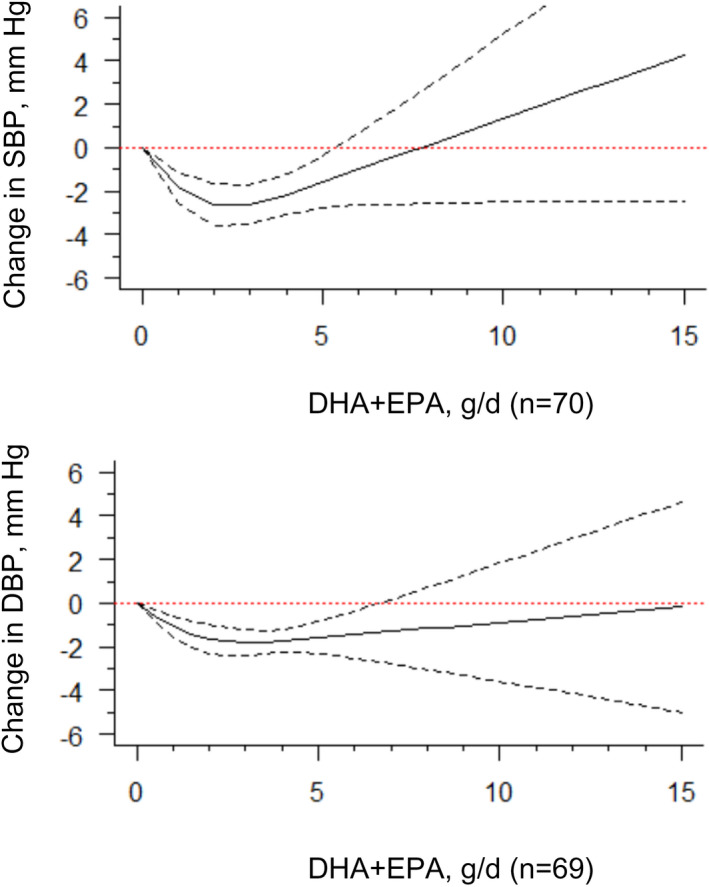
Marginal average dose‐response curve (solid line) with 95% point‐wise CIs (dashed lines) estimated by a 1‐stage random‐effects restricted cubic spline model, using 0 g/d as the referent. Studies included n=70 for systolic blood pressure (SBP) and n=69 for diastolic blood pressure (DBP).
Subgroup Analyses
For studies including an average baseline SBP of 130 mm Hg, we found evidence that DHA+EPA supplementation had an approximately linear trend with BP, where increasing supplementation resulted in stronger reductions in SBP and DBP (Figure 3, Table). This trend was not evident among those with a baseline SBP of <130 mm Hg, although a similar optimal intake of 2 g/d to 3 g/d as our original findings was found. Similar findings were also seen when stratified by hypertension status (SBP ≥140 mm Hg, as defined in most included trials), where patients with hypertension showed greater reductions in SBP and DBP, compared with those without hypertension (Table, Figure S3). When stratifying by the presence of hyperlipidemia, we found an approximately linear relationship among those with hyperlipidemia for SBP, suggesting that increasing supplementation was associated with greater reductions in SBP. Again, this trend was not evident among those without hyperlipidemia for SBP, but an optimal intake of 2 g/d to 3 g/d could be seen. For DBP, there was also some indication that patients with hyperlipidemia may have greater reductions in DBP at 2 g/d to 3 g/d, compared with those without hyperlipidemia (Table, Figure 4).
Figure 3. Dose‐response relationship between changes in blood pressure and combined docosahexaenoic acid (DHA)+eicosapentaenoic acid (EPA) intake of the studies stratified by the baseline systolic blood pressure (SBP) level.
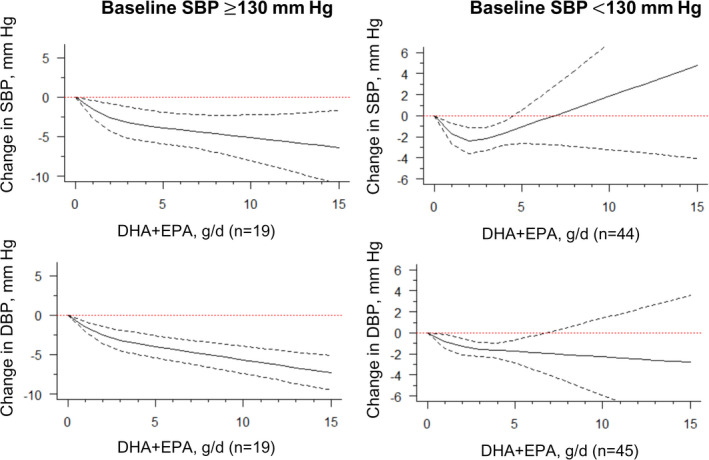
Marginal average dose‐response curve (solid line) with 95% point‐wise CIs (dashed lines) estimated by a 1‐stage random‐effects restricted cubic spline model, using 0 g/d as the referent, in participants with baseline SBP ≥130 mm Hg or <130 mm Hg. DBP indicates diastolic blood pressure; and n, number of the included study.
Figure 4. Dose‐response relationship between changes in blood pressure and combined docosahexaenoic acid (DHA)+eicosapentaenoic acid (EPA) intake of the studies stratified by the status of hyperlipidemia.
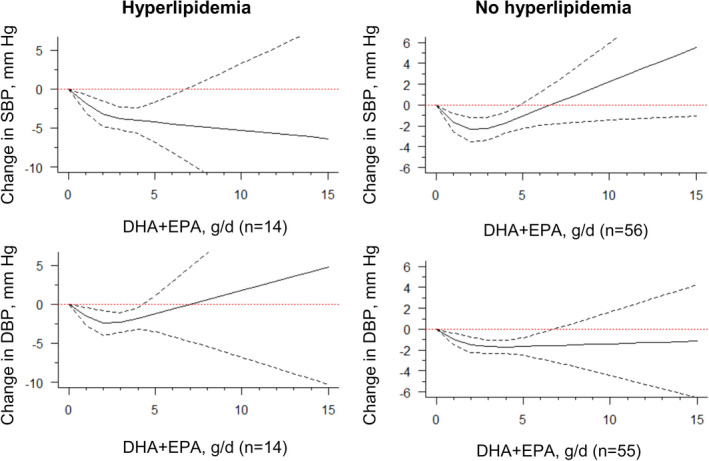
Marginal average dose‐response curve (solid line) with 95% point‐wise CIs (dashed lines) estimated by a 1‐stage random‐effects restricted cubic spline model, using 0 g/d as the referent, in participants with or without hyperlipidemia. n indicates the number of the included study.
We also found stronger effects among studies examining study participants with an average age of ≥45 years (Table, Figure 5). The negligible departure from linearity between DHA+EPA and reductions in BP appeared to be limited to ≥45 years in both SBP and DBP models, while studies in patients with a mean age of <45 years showed null effects. When examining by study duration, studies conducted <12 weeks tended to show stronger findings for SBP at 2 g/d to 3 g/d. However, in studies with a duration of ≥12 weeks, DHA+EPA intake was found to lower BP in a fashion with a minor departure from linearity across the entire range of doses (Table, Figure S4). In a subgroup analysis stratified by study design (crossover versus parallel), we found slightly stronger effects among studies with a parallel design, in which relatively narrower CIs were estimated (Table, Figure S5).
Figure 5. Dose‐response relationship between changes in blood pressure and combined docosahexaenoic acid (DHA)+eicosapentaenoic acid (EPA) intake of the studies stratified by the mean of age.
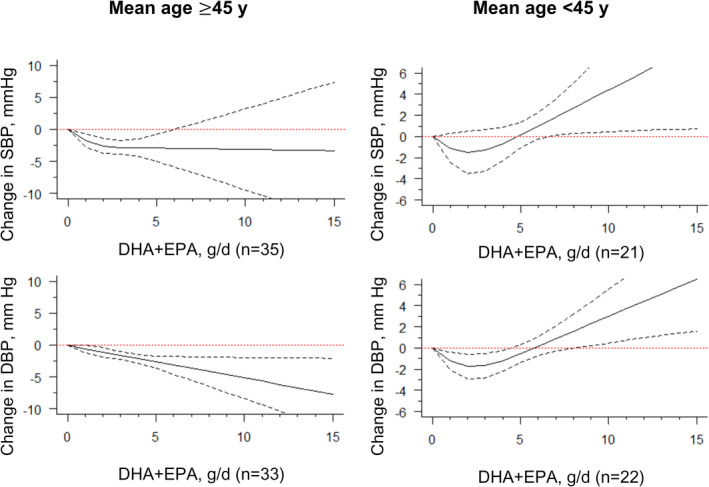
Marginal average dose‐response curve (solid line) with 95% point‐wise CIs (dashed lines) estimated by a 1‐stage random‐effects restricted cubic spline model, using 0 g/d as the referent, among participants with a mean age ≥45 years or <45 years. n indicates the number of the included study.
We found no strong differences when stratifying by intervention type (diet versus supplementation), sex, and fish oil consumption (natural fish oil versus purified ethyl ester), possibly attributable to few studies that reported relationships for diet, women, and use of ethyl esters (Table, Figures S6 through S8). We retrieved few trials that evaluated DHA (n=11) or EPA (n=6) as individual fatty acids. There was insufficient statistical power to detect a meaningful difference between individual EPA and DHA on lowering either SBP or DBP (Table, Figure S9).
Risk of Study Bias and Publication Bias
One and 5 trials were ranked as high and moderate risk of bias, respectively, while the remainder of trials were ranked as low risk of bias (Table S4). Exclusion of moderate and high risk‐of‐bias trials did not appreciably change the shape of the dose‐response curve (results not shown). The funnel plot and Egger regression test indicated asymmetry in the overall SBP model (z=−3.05, P=0.002). There was no evidence of plot asymmetry in pooled DBP and stratified models (Figures S10 and S11). This suggests that publication bias, if present because of small‐study effects, did not strongly impact our overall findings. The leave‐one‐out sensitivity analyses in 1‐stage regression models proved that overall effects were not driven by a small number of specific trials, but reflected the global effect of the included trials (Figures S12 and S13).
Discussion
Using a new 1‐stage strategy, we examined the strength and shape of the dose‐response association between DHA+EPA intake and BP with up‐to‐date literature and multiple subgroup analyses. We found evidence of a J‐shaped dose‐response curve, where the greatest reductions of SBP and DBP occurred at moderate DHA+EPA doses between 2 g/d and 3 g/d. These findings were slightly stronger in studies where the average participant age was ≥45 years for SBP. We also found evidence of a stronger, approximately linear dose‐response relationship among hyperlipidemic and hypertensive populations, suggesting that this is a population that could be more responsive to the beneficial impacts of ω3 PUFA intake on reductions in BP. Moreover, our data also demonstrated that ω3 PUFA intake above the recommended intake of 3 g/d was not associated with additional benefits, particularly in normotensive subgroups.
Our findings are different from other meta‐analyses that examined the relationship between ω3 PUFA intake and changes in BP among RCT studies. Previous meta‐analyses assumed a linear function. 8 , 12 These studies found that BP reductions were not associated with DHA+EPA intake within a dose range of 0.2 g/d to 15 g/d. Morris et al 7 attempted to test a dose‐response effect with a meta‐regression model with varying doses from 2 g/d to 6 g/d. They proposed a linear dose‐response effect among the hypertensive studies, but the absence of doses between 7 g/d and 15 g/d seemed to put disproportionately more weight on the trial that used a dosage of 15 g/d. Similar to our study, Campbell et al 10 later demonstrated that the BP‐lowering effect was diminished with the increasing dose between 1 g/d and 6 g/d. Another effort was made a decade later by categorizing the ω3 PUFA intake. 11 The stratum of 3 g/d to 4 g/d exerted the strongest effect of −3.85 mm Hg on SBP and −1.86 mm Hg on DBP, respectively, suggesting the existence of a dose threshold. 11 Overall, although they have been unable to smoothly shape the relationship between fish oil intake and BP over the entire range of exposure, these studies suggested a nonlinear association and sparked further investigations. Our study builds on past evidence by examining the relationship using up‐to‐date literature, and novel methods that allow for the estimation of a nonlinear trend that accounts for the correlation between studies.
In our study, using overall and subgroup analyses we found a consistent J‐shaped curve in our models. The optimal or threshold doses were estimated to fall between 2 g/d and 3 g/d in our models, which coincided with the range of EPA and DHA dose exhibiting maximal effects on BP. 8 , 10 , 11 We also observed a minor departure from linearity of BP decline in participants with baseline SBP ≥130 mm Hg and a wider beneficial range in participants with hypertension compared with normotensive populations. 8 , 11 Moreover, our findings are consistent with previous synthesized results in which DBP reductions were significantly greater in older populations (mean age ≥45 years) compared with younger populations. 8 Considering cardiometabolic comorbidities, we further compared the effects of fish oil between participants with and those without hyperlipidemia. Our data suggested that ω3 PUFA intake had larger reductions in SBP in populations with hyperlipidemia, which made our models more applicable given the increasing prevalence of metabolic syndromes.
Our analyses showed a positive and approximately linear (or L‐shaped) dose‐response association in respective subgroups of hypertensive, hyperlipidemic, and older participants. The approximately linear association could be interpreted as there is no dose threshold, particularly in the hypertensive subgroup. It is unclear why approximately linear associations were evident for these subgroups, in comparison to the J‐shaped curves seen in the main analyses. It could be that high‐risk population, such as those with hypertension and hyperlipidemia, could benefit differently from ω3 PUFA intake supplementation in comparison to younger and healthier populations, particularly since ω3 PUFA is hypothesized to interact with many pathways, such as triglycerides, inflammation, and heart rate. 25 , 26 Additionally, there could be mechanistic differences in bioavailability and efficacy of ω3 PUFA intake in these populations. 25 , 26 However, given that few studies have investigated the relationship at higher doses (ie >7 g/d), more research is needed to elucidate this relationship, including biological mechanisms.
We are not the first to propose a nonlinear model for the dose‐response of fish oil intake on the BP effect. The J‐shaped dose‐response effects have been tentatively proposed in prospective cohort studies and clinical trial meta‐analyses. For example, summarized data of 6 selected independent prospective cohort studies indicated that there was also a J‐shaped association between the increment of ω3 PUFA intake and risk of hypertension within the low dose range of 0 g/d to 2 g/d. 27 A nonlinear negative and L‐shaped association between ω3 PUFA intake and the risk of hypertension was later proposed, with a dose at ≈3.4 g/d reaching the maximal BP risk‐lowering effect in a cross‐sectional study. 28 In these 2 reports, an apparent J‐shaped relationship between ω3 PUFA intake and hypertension risk was indicated with restricted cubic splines, a finding that is supported in our dose‐response analysis examining changes in BP.
Our findings of a curvilinear relationship between BP effects and fish oil intake may have considerable implications in the cardioprotection of ω3 PUFAs. Given the moderate dose at 3 g/d, as shown in our dose‐response relationship, both a fish oil diet or supplementation resulted in a decrease in BP ≈2 mm Hg to 3 mm Hg in overall and most stratified effects. In 2009, the European Food Safety Authority recommended that an intake of EPA and DHA of ≈3 g/d was required to bring out the claimed hypotensive effects. 29 Our findings seem to support this suggested daily dosage. Moreover, we found associations among both hypertensive and nonhypertensive groups, suggesting that ω3 PUFAs intake could be beneficial for controlling BP even before the onset of hypertension. This means that the intake of ω3 PUFAs could have implications on a person’s future risk of stroke, ischemic heart disease, 30 , 31 and all‐cause mortality. 32
We recognize that there are some potential limitations to the conclusion that can be drawn from the current studies. The intrinsically significant variations among original trials, such as the device of BP measurement (automatic versus manual), the year of study (conducted 1987–2020), and the type of intervention (diet versus supplementation) are likely to bring some uncertainty to our results and potentially weaken the conclusion. Although we attempted to examine the influence of these factors on our overall findings in subgroup analyses, we acknowledge it is not possible to account for this heterogeneity directly in our analyses. Future research could benefit from examining a more biologically relevant exposure, such as the use of the absorbed DHA/EPA amount as the active exposure levels, use of standardized BP methods to ensure strict quality control, and further examination of how intervention type may influence the relationship. There are several other limitations. First, the absence of doses between 7 g/d and 15 g/d increases the uncertainty in the effect estimates at higher doses. However, the removal of these extreme data points did not strongly change our trends in overall and stratified effects. Second, we did not perform analyses based on the binary outcomes to predict the risk ratio because of the limited studies retrieved. Third, the mechanism of these J‐shaped relationships is not clear. The appearance of the response plateau might reflect a saturating status of fatty acid incorporation into the cell membrane. 33 The change point towards possibly increasing BP may indicate the enhanced α‐adrenergic vasoconstriction 34 or disrupted ion exchanges. 35 Nevertheless, attention should be focused on the selection of optimal fish oil intake in the management of hypertension. Finally, because of the few available studies, we could not assess the impact of DHA+EPA on changes in BP by sex, DHA‐ or EPA‐only, or diet‐only effects. Future studies should further investigate these issues.
Conclusions
We conducted a dose‐response meta‐analysis to characterize the effects of DHA+EPA supplementation and dietary enrichment on BP levels using updated literature. This research helps to improve our understanding of the moderate effects of omega‐3 fatty acids on BP reduction. The use of the new model suggests that an optimal dose of 3 g/d in overall and subgroup analyses may yield the greatest BP‐lowering performance. The seemingly J‐shaped associations between DHA+EPA dose and BP reduction in many subgroups might help reform preventive strategies for reducing cardiovascular risks in the general adult population. However, individuals who are at high risk for developing cardiovascular diseases, such as those with hypertension, may be more responsive to the beneficial impacts of ω3 PUFA intake on reductions in BP.
Sources of Funding
This work was supported by the Macau Science and Technology Development Fund (FDCT) (0123/2020/A and 0053/2021/A1) and the Faculty Research Grants of Macau University of Science and Technology (FRG‐21‐038‐SP).
Disclosures
None.
Supporting information
Tables S1–S4
Figures S1–S13
References 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104
For Sources of Funding and Disclosures, see page 11.
See Editorial by George et al.
Contributor Information
Bingshu E. Chen, Email: bechen@ctg.queensu.ca.
Xinzhi Li, Email: xizli@must.edu.mo.
References
- 1. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open‐label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3 [DOI] [PubMed] [Google Scholar]
- 2. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Juliano RA, Jiao L, Granowitz C, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 3. Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJ, Koenig W, McGuire DK, et al. Effect of high‐dose omega‐3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268–2280. doi: 10.1001/jama.2020.22258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalstad AA, Myhre PL, Laake K, Tveit SH, Schmidt EB, Smith P, Nilsen DW, Tveit A, Fagerland MW, Solheim S, et al. Effects of n‐3 fatty acid supplements in elderly patients after myocardial infarction: a randomized, controlled trial. Circulation. 2021;143:528–539. doi: 10.1161/CIRCULATIONAHA.120.052209 [DOI] [PubMed] [Google Scholar]
- 5. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega‐3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta‐analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374 [DOI] [PubMed] [Google Scholar]
- 6. Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P, et al. Associations of omega‐3 fatty acid supplement use with cardiovascular disease risks: meta‐analysis of 10 trials involving 77917 individuals. JAMA Cardiol. 2018;3:225–234. doi: 10.1001/jamacardio.2017.5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta‐analysis of controlled trials. Circulation. 1993;88:523–533. doi: 10.1161/01.cir.88.2.523 [DOI] [PubMed] [Google Scholar]
- 8. Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–1499. doi: 10.1097/00004872-200208000-00010 [DOI] [PubMed] [Google Scholar]
- 9. Cabo J, Alonso R, Mata P. Omega‐3 fatty acids and blood pressure. Br J Nutr. 2012;107:S195–S200. doi: 10.1017/S0007114512001584 [DOI] [PubMed] [Google Scholar]
- 10. Campbell F, Dickinson HO, Critchley JA, Ford GA, Bradburn M. A systematic review of fish‐oil supplements for the prevention and treatment of hypertension. Eur J Prev Cardiol. 2013;20:107–120. doi: 10.1177/2047487312437056 [DOI] [PubMed] [Google Scholar]
- 11. Miller PE, Van Elswyk M, Alexander DD. Long‐chain omega‐3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta‐analysis of randomized controlled trials. Am J Hypertens. 2014;27:885–896. doi: 10.1093/ajh/hpu024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Appel LJ, Miller ER III, Seidler AJ, Whelton PK. Does supplementation of diet with 'fish oil' reduce blood pressure? A meta‐analysis of controlled clinical trials. Arch Intern Med. 1993;153:1429–1438. doi: 10.1001/archinte.1993.00410120017003 [DOI] [PubMed] [Google Scholar]
- 13. Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One‐stage dose‐response meta‐analysis for aggregated data. Stat Methods Med Res. 2019;28:1579–1596. doi: 10.1177/0962280218773122 [DOI] [PubMed] [Google Scholar]
- 14. Filippini T, Naska A, Kasdagli MI, Torres D, Lopes C, Carvalho C, Moreira P, Malavolti M, Orsini N, Whelton PK, et al. Potassium intake and blood pressure: a dose‐response meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2020;9:e015719. doi: 10.1161/JAHA.119.015719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Filippini T, Malavolti M, Whelton PK, Naska A, Orsini N, Vinceti M. Blood pressure effects of sodium reduction: dose‐response meta‐analysis of experimental studies. Circulation. 2021;143:1542–1567. doi: 10.1161/CIRCULATIONAHA.120.050371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane Database Syst Rev. 2022. Available from www.training.cochrane.org/handbook [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 19. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta‐analysis for linear and nonlinear dose‐response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426–e483. doi: 10.1161/CIR.0000000000000597 [DOI] [PubMed] [Google Scholar]
- 21. Crippa A, Orsini N. Multivariate dose‐response meta‐analysis: the dosresmeta R package. J Stat Softw. 2016;72:1–15. doi: 10.18637/jss.v072.c01 [DOI] [Google Scholar]
- 22. Orsini N. Weighted mixed‐effects dose–response models for tables of correlated contrasts. The Stata Journal: Promoting communications on statistics and Stata. 2021;21:320–347. doi: 10.1177/1536867x211025798 [DOI] [Google Scholar]
- 23. Knapp HR, FitzGerald GA. The antihypertensive effects of fish oil. A controlled study of polyunsaturated fatty acid supplements in essential hypertension. N Engl J Med. 1989;320:1037–1043. doi: 10.1056/nejm198904203201603 [DOI] [PubMed] [Google Scholar]
- 24. Levinson PD, Iosiphidis AH, Saritelli AL, Herbert PN, Steiner M. Effects of n‐3 fatty acids in essential hypertension. Am J Hypertens. 1990;3:754–760. doi: 10.1093/ajh/3.10.754 [DOI] [PubMed] [Google Scholar]
- 25. Stanton AV, James K, Brennan MM, O’Donovan F, Buskandar F, Shortall K, El‐Sayed T, Kennedy J, Hayes H, Fahey AG, et al. Omega‐3 index and blood pressure responses to eating foods naturally enriched with omega‐3 polyunsaturated fatty acids: a randomized controlled trial. Sci Rep. 2020;10:15444. doi: 10.1038/s41598-020-71801-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maki KC. Long‐chain omega‐3 fatty acid bioavailability: implications for understanding the effects of supplementation on heart disease risk. J Nutr. 2018;148:1701–1703. doi: 10.1093/jn/nxy205 [DOI] [PubMed] [Google Scholar]
- 27. Yang B, Shi MQ, Li ZH, Yang JJ, Fish LD. Long‐chain n‐3 PUFA and Incidence of elevated blood pressure: a meta‐analysis of prospective cohort studies. Nutrients. 2016;8:58. doi: 10.3390/nu8010058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen J, Sun B, Zhang D. Association of dietary n3 and n6 fatty acids intake with hypertension: NHANES 2007–2014. Nutrients. 2019;11:1232. doi: 10.3390/nu11061232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grynberg A. Hypertension prevention: from nutrients to (fortified) foods to dietary patterns. Focus on fatty acids. J Hum Hypertens. 2005;19:S25–S33. doi: 10.1038/sj.jhh.1001957 [DOI] [PubMed] [Google Scholar]
- 30. Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, Roccella EJ, Stout R, Vallbona C, Winston MC, et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882–1888. doi: 10.1001/jama.288.15.1882 [DOI] [PubMed] [Google Scholar]
- 31. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective SC. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 32. Li ZH, Zhong WF, Liu S, Kraus VB, Zhang YJ, Gao X, Lv YB, Shen D, Zhang XR, Zhang PD, et al. Associations of habitual fish oil supplementation with cardiovascular outcomes and all cause mortality: evidence from a large population based cohort study. BMJ. 2020;368:m456. doi: 10.1136/bmj.m456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schuchardt JP, Hahn A. Bioavailability of long‐chain omega‐3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2013;89:1–8. doi: 10.1016/j.plefa.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 34. Kenny D, Brooks HL, Warltier DC. Enhanced alpha‐adrenergic vasoconstriction by n‐3 fatty acids in conscious dogs. Am J Physiol. 1990;258:H1660–H1667. doi: 10.1152/ajpheart.1990.258.6.H1660 [DOI] [PubMed] [Google Scholar]
- 35. Singh TU, Garg SK, Mishra SK. Evaluation of effects of eicosapentaenoic acid on Na(+)‐K(+)‐ATPase in sheep pulmonary artery. Hum Exp Toxicol. 2012;31:579–587. doi: 10.1177/0960327111417909 [DOI] [PubMed] [Google Scholar]
- 36. Albert BB, Derraik JG, Brennan CM, Biggs JB, Garg ML, Cameron‐Smith D, Hofman PL, Cutfield WS. Supplementation with a blend of krill and salmon oil is associated with increased metabolic risk in overweight men. Am J Clin Nutr. 2015;102:49–57. doi: 10.3945/ajcn.114.103028 [DOI] [PubMed] [Google Scholar]
- 37. Armstrong P, Kelley DS, Newman JW, Staggers FE Sr, Hartiala J, Allayee H, Stephensen CB. Arachidonate 5‐lipoxygenase gene variants affect response to fish oil supplementation by healthy African Americans. J Nutr. 2012;142:1417–1428. doi: 10.3945/jn.112.159814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bach R, Schmidt U, Jung F, Kiesewetter H, Hennen B, Wenzel E, Schieffer H, Bette L, Heyden S. Effects of fish oil capsules in two dosages on blood pressure, platelet functions, haemorheological and clinical chemistry parameters in apparently healthy subjects. Ann Nutr Metab. 1989;33:359–367. doi: 10.1159/000177559 [DOI] [PubMed] [Google Scholar]
- 39. Barcelo‐Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK. Flaxseed oil and fish‐oil capsule consumption alters human red blood cell n‐3 fatty acid composition: a multiple‐ dosing trial comparing 2 sources of n‐3 fatty acid. Am J Clin Nutr. 2008;88:801–809. doi: 10.1093/ajcn/88.3.801 [DOI] [PubMed] [Google Scholar]
- 40. Blonk MC, Bilo HJ, Nauta JJ, Popp‐Snijders C, Mulder C, Donker AJ. Dose‐response effects of fish‐oil supplementation in healthy volunteers. Am J Clin Nutr. 1990;52:120–127. doi: 10.1093/ajcn/52.1.120 [DOI] [PubMed] [Google Scholar]
- 41. Bonaa KH, Bjerve KS, Straume B, Gram IT, Thelle D. Effect of eicosapentaenoic and docosahexaenoic acids on blood pressure in hypertension. A population‐based intervention trial from the Tromso study. N Engl J Med. 1990;322:795–801. doi: 10.1056/NEJM199003223221202 [DOI] [PubMed] [Google Scholar]
- 42. Buckley JD, Burgess S, Murphy KJ, Howe PR. DHA‐rich fish oil lowers heart rate during submaximal exercise in elite Australian Rules footballers. J Sci Med Sport. 2009;12:503–507. doi: 10.1016/j.jsams.2008.01.011 [DOI] [PubMed] [Google Scholar]
- 43. Burgin‐Maunder CS, Brooks PR, Hitchen‐Holmes D, Russell FD. Moderate dietary supplementation with omega‐3 fatty acids does not impact plasma von Willebrand factor profile in mildly hypertensive subjects. Biomed Res Int. 2015;2015:1–8. doi: 10.1155/2015/394871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carter JR, Schwartz CE, Yang H, Joyner MJ. Fish oil and neurovascular control in humans. Am J Physiol Heart Circ Physiol. 2012;303:H450–H456. doi: 10.1152/ajpheart.00353.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chin JP, Gust AP, Nestel PJ, Dart AM. Marine oils dose‐dependently inhibit vasoconstriction of forearm resistance vessels in humans. Hypertension. 1993;21:22–28. doi: 10.1161/01.hyp.21.1.22 [DOI] [PubMed] [Google Scholar]
- 46. Cobiac L, Clifton PM, Abbey M, Belling GB, Nestel PJ. Lipid, lipoprotein, and hemostatic effects of fish vs fish‐oil n‐3 fatty acids in mildly hyperlipidemic males. Am J Clin Nutr. 1991;53:1210–1216. doi: 10.1093/ajcn/53.5.1210 [DOI] [PubMed] [Google Scholar]
- 47. Cobiac L, Nestel PJ, Wing LM, Howe PR. A low‐sodium diet supplemented with fish oil lowers blood pressure in the elderly. J Hypertens. 1992;10:87–92. doi: 10.1097/00004872-199201000-00014 [DOI] [PubMed] [Google Scholar]
- 48. Conquer JA, Cheryk LA, Chan E, Gentry PA, Holub BJ. Effect of supplementation with dietary seal oil on selected cardiovascular risk factors and hemostatic variables in healthy male subjects. Thromb Res. 1999;96:239–250. doi: 10.1016/s0049-3848(99)00106-1 [DOI] [PubMed] [Google Scholar]
- 49. Dart AM, Riemersma RA, Oliver MF. Effects of Maxepa on serum lipids in hypercholesterolaemic subjects. Atherosclerosis. 1989;80:119–124. doi: 10.1016/0021-9150(89)90019-1 [DOI] [PubMed] [Google Scholar]
- 50. Demke DM, Peters GR, Linet OI, Metzler CM, Klott KA. Effects of a fish oil concentrate in patients with hypercholesterolemia. Atherosclerosis. 1988;70:73–80. doi: 10.1016/0021-9150(88)90101-3 [DOI] [PubMed] [Google Scholar]
- 51. Derosa G, Maffioli P, D'Angelo A, Salvadeo SA, Ferrari I, Fogari E, Gravina A, Mereu R, Randazzo S, Cicero AF. Effects of long chain omega‐3 fatty acids on metalloproteinases and their inhibitors in combined dyslipidemia patients. Expert Opin Pharmacother. 2009;10:1239–1247. doi: 10.1517/14656560902865601 [DOI] [PubMed] [Google Scholar]
- 52. Derosa G, Cicero AF, Fogari E, D'Angelo A, Bonaventura A, Romano D, Maffioli P. Effects of n‐3 PUFAs on postprandial variation of metalloproteinases, and inflammatory and insulin resistance parameters in dyslipidemic patients: evaluation with euglycemic clamp and oral fat load. J Clin Lipidol. 2012;6:553–564. doi: 10.1016/j.jacl.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 53. Dewell A, Marvasti FF, Harris WS, Tsao P, Gardner CD. Low‐ and high‐dose plant and marine (n‐3) fatty acids do not affect plasma inflammatory markers in adults with metabolic syndrome. J Nutr. 2011;141:2166–2171. doi: 10.3945/jn.111.142240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dyerberg J, Eskesen DC, Andersen PW, Astrup A, Buemann B, Christensen JH, Clausen P, Rasmussen BF, Schmidt EB, Tholstrup T, et al. Effects of trans‐ and n‐3 unsaturated fatty acids on cardiovascular risk markers in healthy males. An 8 weeks dietary intervention study. Eur J Clin Nutr. 2004;58:1062–1070. doi: 10.1038/sj.ejcn.1601934 [DOI] [PubMed] [Google Scholar]
- 55. Flaten H, Hostmark AT, Kierulf P, Lystad E, Trygg K, Bjerkedal T, Osland A. Fish‐oil concentrate: effects on variables related to cardiovascular disease. Am J Clin Nutr. 1990;52:300–306. doi: 10.1093/ajcn/52.2.300 [DOI] [PubMed] [Google Scholar]
- 56. Geelen A, Zock PL, Swenne CA, Brouwer IA, Schouten EG, Katan MB. Effect of n‐3 fatty acids on heart rate variability and baroreflex sensitivity in middle‐aged subjects. Am Heart J. 2003;146:E4. doi: 10.1016/S0002-8703(03)00441-1 [DOI] [PubMed] [Google Scholar]
- 57. Grieger JA, Miller MD, Cobiac L. Investigation of the effects of a high fish diet on inflammatory cytokines, blood pressure, and lipids in healthy older Australians. Food Nutr Res. 2014;58: doi: 10.3402/fnr.v58.20369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Grimsgaard S, Bonaa KH, Hansen JB, Myhre ES. Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on hemodynamics in humans. Am J Clin Nutr. 1998;68:52–59. doi: 10.1093/ajcn/68.1.52 [DOI] [PubMed] [Google Scholar]
- 59. Grundt H, Nilsen DW, Hetland O, Aarsland T, Baksaas I, Grande T, Woie L. Improvement of serum lipids and blood pressure during intervention with n‐3 fatty acids was not associated with changes in insulin levels in subjects with combined hyperlipidaemia. J Intern Med. 1995;237:249–259. doi: 10.1111/j.1365-2796.1995.tb01173.x [DOI] [PubMed] [Google Scholar]
- 60. Hallund J, Madsen BO, Bugel SH, Jacobsen C, Jakobsen J, Krarup H, Holm J, Nielsen HH, Lauritzen L. The effect of farmed trout on cardiovascular risk markers in healthy men. Br J Nutr. 2010;104:1528–1536. doi: 10.1017/S0007114510002527 [DOI] [PubMed] [Google Scholar]
- 61. Harris WS, Lemke SL, Hansen SN, Goldstein DA, DiRienzo MA, Su H, Nemeth MA, Taylor ML, Ahmed G, George C. Stearidonic acid‐enriched soybean oil increased the omega‐3 index, an emerging cardiovascular risk marker. Lipids. 2008;43:805–811. doi: 10.1007/s11745-008-3215-0 [DOI] [PubMed] [Google Scholar]
- 62. Hellsten G, Boman K, Saarem K, Hallmans G, Nilsson TK. Effects on fibrinolytic‐activity of corn‐ oil and a fish‐oil preparation enriched with omega‐3‐polyunsaturated fatty‐acids in a long‐ term study. Curr Med Res Opin. 1993;13:133–139. doi: 10.1185/03007999309111542 [DOI] [PubMed] [Google Scholar]
- 63. Hill AM, Buckley JD, Murphy KJ, Howe PR. Combining fish‐oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. Am J Clin Nutr. 2007;85:1267–1274. doi: 10.1093/ajcn/85.5.1267 [DOI] [PubMed] [Google Scholar]
- 64. Howe PR, Evans HM, Kuszewski JC, Wong RH. Effects of long chain omega‐3 polyunsaturated fatty acids on brain function in mildly hypertensive older adults. Nutrients. 2018;10:1413. doi: 10.3390/nu10101413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huerta AE, Navas‐Carretero S, Prieto‐Hontoria PL, Martinez JA, Moreno‐Aliaga MJ. Effects of alpha‐lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity (Silver Spring). 2015;23:313–321. doi: 10.1002/oby.20966 [DOI] [PubMed] [Google Scholar]
- 66. Hughes GS, Ringer TV, Watts KC, DeLoof MJ, Francom SF, Spillers CR. Fish oil produces an atherogenic lipid profile in hypertensive men. Atherosclerosis. 1990;84:229–237. doi: 10.1016/0021-9150(90)90095-z [DOI] [PubMed] [Google Scholar]
- 67. Jones PJ, Senanayake VK, Pu S, Jenkins DJ, Connelly PW, Lamarche B, Couture P, Charest A, Baril‐Gravel L, West SG, et al. DHA‐enriched high‐oleic acid canola oil improves lipid profile and lowers predicted cardiovascular disease risk in the canola oil multicenter randomized controlled trial. Am J Clin Nutr. 2014;100:88–97. doi: 10.3945/ajcn.113.081133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kelley DS, Siegel D, Vemuri M, Mackey BE. Docosahexaenoic acid supplementation improves fasting and postprandial lipid profiles in hypertriglyceridemic men. Am J Clin Nutr. 2007;86:324–333. doi: 10.1093/ajcn/86.2.324 [DOI] [PubMed] [Google Scholar]
- 69. Kestin M, Clifton P, Belling GB, Nestel PJ. n‐3 fatty acids of marine origin lower systolic blood pressure and triglycerides but raise LDL cholesterol compared with n‐3 and n‐6 fatty acids from plants. Am J Clin Nutr. 1990;51:1028–1034. doi: 10.1093/ajcn/51.6.1028 [DOI] [PubMed] [Google Scholar]
- 70. Kristensen S, Schmidt EB, Schlemmer A, Rasmussen C, Lindgreen E, Johansen MB, Christensen JH. The effect of marine n‐3 polyunsaturated fatty acids on cardiac autonomic and hemodynamic function in patients with psoriatic arthritis: a randomised, double‐blind, placebo‐ controlled trial. Lipids Health Dis. 2016;15:216. doi: 10.1186/s12944-016-0382-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lee JB, Notay K, Klingel SL, Chabowski A, Mutch DM, Millar PJ. Docosahexaenoic acid reduces resting blood pressure but increases muscle sympathetic outflow compared with eicosapentaenoic acid in healthy men and women. Am J Physiol Heart Circ Physiol. 2019;316:H873–H881. doi: 10.1152/ajpheart.00677.2018 [DOI] [PubMed] [Google Scholar]
- 72. Lindqvist HM, Langkilde AM, Undeland I, Sandberg AS. Herring (Clupea harengus) intake influences lipoproteins but not inflammatory and oxidation markers in overweight men. Br J Nutr. 2009;101:383–390. doi: 10.1017/S0007114508003073 [DOI] [PubMed] [Google Scholar]
- 73. Lofgren RP, Wilt TJ, Nichol KL, Crespin L, Pluhar R, Eckfeldt J. The effect of fish oil supplements on blood pressure. Am J Public Health. 1993;83:267–269. doi: 10.2105/ajph.83.2.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Logan SL, Spriet LL. Omega‐3 fatty acid supplementation for 12 weeks increases resting and exercise metabolic rate in healthy community‐dwelling older females. PLoS One. 2015;10:e0144828. doi: 10.1371/journal.pone.0144828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Maki KC, Reeves MS, Farmer M, Griinari M, Berge K, Vik H, Hubacher R, Rains TM. Krill oil supplementation increases plasma concentrations of eicosapentaenoic and docosahexaenoic acids in overweight and obese men and women. Nutr Res. 2009;29:609–615. doi: 10.1016/j.nutres.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 76. Meland E, Fugelli P, Laerum E, Rønneberg R, Sandvik L. Effect of fish oil on blood pressure and blood lipids in men with mild to moderate hypertension. Scand J Prim Health Care. 1989;7:131–135. doi: 10.3109/02813438909087229 [DOI] [PubMed] [Google Scholar]
- 77. Mills DE, Mah M, Ward RP, Morris BL, Floras JS. Alteration of baroreflex control of forearm vascular resistance by dietary fatty acids. Am J Physiol. 1990;259:R1164–1171. doi: 10.1152/ajpregu.1990.259.6.R1164 [DOI] [PubMed] [Google Scholar]
- 78. Monahan KD, Wilson TE, Ray CA. Omega‐3 fatty acid supplementation augments sympathetic nerve activity responses to physiological stressors in humans. Hypertension. 2004;44:732–738. doi: 10.1161/01.HYP.0000145292.38579.f4 [DOI] [PubMed] [Google Scholar]
- 79. Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34:253–260. doi: 10.1161/01.hyp.34.2.253 [DOI] [PubMed] [Google Scholar]
- 80. Murphy KJ, Meyer BJ, Mori TA, Burke V, Mansour J, Patch CS, Tapsell LC, Noakes M, Clifton PA, Barden A, et al. Impact of foods enriched with n‐3 long‐chain polyunsaturated fatty acids on erythrocyte n‐3 levels and cardiovascular risk factors. Br J Nutr. 2007;97:749–757. doi: 10.1017/S000711450747252X [DOI] [PubMed] [Google Scholar]
- 81. Neff LM, Culiner J, Cunningham‐Rundles S, Seidman C, Meehan D, Maturi J, Wittkowski KM, Levine B, Breslow JL. Algal docosahexaenoic acid affects plasma lipoprotein particle size distribution in overweight and obese adults. J Nutr. 2011;141:207–213. doi: 10.3945/jn.110.130021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nestel P, Shige H, Pomeroy S, Cehun M, Abbey M, Raederstorff D. The n‐3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76:326–330. doi: 10.1093/ajcn/76.2.326 [DOI] [PubMed] [Google Scholar]
- 83. Noreen EE, Brandauer J. The effects of supplemental fish oil on blood pressure and morning cortisol in normotensive adults: a pilot study. J Complement Integr Med. 2012;9. doi: 10.1515/1553-3840.1467 [DOI] [PubMed] [Google Scholar]
- 84. Pase MP, Grima N, Cockerell R, Stough C, Scholey A, Sali A, Pipingas A. The effects of long‐chain omega‐3 fish oils and multivitamins on cognitive and cardiovascular function: a randomized, controlled clinical trial. J Am Coll Nutr. 2015;34:21–31. doi: 10.1080/07315724.2014.880660 [DOI] [PubMed] [Google Scholar]
- 85. Passfall J, Philipp T, Woermann F, Quass P, Thiede M, Haller H. Different effects of eicosapentaenoic acid and olive oil on blood pressure, intracellular free platelet calcium, and plasma lipids in patients with essential hypertension. Clin Investig. 1993;71:628–633. doi: 10.1007/BF00184490 [DOI] [PubMed] [Google Scholar]
- 86. Prisco D, Paniccia R, Bandinelli B, Filippini M, Francalanci I, Giusti B, Giurlani L, Gensini GF, Abbate R, Neri Serneri GG. Effect of medium‐term supplementation with a moderate dose of n‐3 polyunsaturated fatty acids on blood pressure in mild hypertensive patients. Thromb Res. 1998;91:105–112. doi: 10.1016/s0049-3848(98)00046-2 [DOI] [PubMed] [Google Scholar]
- 87. Radack K, Deck C, Huster G. The effects of low doses of n‐3 fatty acid supplementation on blood pressure in hypertensive subjects. A randomized controlled trial. Arch Intern Med. 1991;151:1173–1180. doi: 10.1001/archinte.1991.00400060097017 [DOI] [PubMed] [Google Scholar]
- 88. Ryu J, Lerner J, Sullivan JM. Unresponsiveness of forearm hemodynamics to omega‐3 polyunsaturated fatty acids and aspirin. Prostaglandins. 1990;39:339–347. doi: 10.1016/0090-6980(90)90051-v [DOI] [PubMed] [Google Scholar]
- 89. Sagara M, Njelekela M, Teramoto T, Taguchi T, Mori M, Armitage L, Birt N, Birt C, Yamori Y. Effects of docosahexaenoic Acid supplementation on blood pressure, heart rate, and serum lipids in Scottish men with hypertension and hypercholesterolemia. Int J Hypertens. 2011;2011:1–7. doi: 10.4061/2011/809198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sanders TA, Gleason K, Griffin B, Miller GJ. Influence of an algal triacylglycerol containing docosahexaenoic acid (22: 6n–3) and docosapentaenoic acid (22: 5n–6) on cardiovascular risk factors in healthy men and women. Br J Nutr. 2006;95:525–531. doi: 10.1079/bjn20051658 [DOI] [PubMed] [Google Scholar]
- 91. Sanders TA, Hall WL, Maniou Z, Lewis F, Seed PT, Chowienczyk PJ. Effect of low doses of long‐ chain n‐3 PUFAs on endothelial function and arterial stiffness: a randomized controlled trial. Am J Clin Nutr. 2011;94:973–980. doi: 10.3945/ajcn.111.018036 [DOI] [PubMed] [Google Scholar]
- 92. Shabrina A, Tung TH, Nguyen NT, Lee HC, Wu HT, Wang W, Huang SY. n‐3 PUFA and caloric restriction diet alters lipidomic profiles in obese men with metabolic syndrome: a preliminary open study. Eur J Nutr. 2020;59:3103–3112. doi: 10.1007/s00394-019-02149-4 [DOI] [PubMed] [Google Scholar]
- 93. Shen T, Xing G, Zhu J, Zhang S, Cai Y, Li D, Xu G, Xing E, Rao J, Shi R. Effects of 12‐week supplementation of marine omega‐3 PUFA‐based formulation omega3q10 in older adults with prehypertension and/or elevated blood cholesterol. Lipids Health Dis. 2017;16:253. doi: 10.1186/s12944-017-0617-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sjoberg NJ, Milte CM, Buckley JD, Howe PR, Coates AM, Saint DA. Dose‐dependent increases in heart rate variability and arterial compliance in overweight and obese adults with DHA‐rich fish oil supplementation. Br J Nutr. 2010;103:243–248. doi: 10.1017/S000711450999153X [DOI] [PubMed] [Google Scholar]
- 95. Stark KD, Holub BJ. Differential eicosapentaenoic acid elevations and altered cardiovascular disease risk factor responses after supplementation with docosahexaenoic acid in postmenopausal women receiving and not receiving hormone replacement therapy. Am J Clin Nutr. 2004;79:765–773. doi: 10.1093/ajcn/79.5.765 [DOI] [PubMed] [Google Scholar]
- 96. Sveinsdottir K, Martinsdottir E, Ramel A. Blood pressure‐lowering effects of long chain n‐3 fatty acids from meals enriched with liquid fish oil and from microencapsulated powder. Int J Food Sci Nutr. 2016;67:1017–1023. doi: 10.1080/09637486.2016.1208733 [DOI] [PubMed] [Google Scholar]
- 97. Theobald HE, Goodall AH, Sattar N, Talbot DC, Chowienczyk PJ, Sanders TA. Low‐dose docosahexaenoic acid lowers diastolic blood pressure in middle‐aged men and women. J Nutr. 2007;137:973–978. doi: 10.1093/jn/137.4.973 [DOI] [PubMed] [Google Scholar]
- 98. Toft I, Bonaa KH, Ingebretsen OC, Nordoy A, Jenssen T. Effects of n‐3 polyunsaturated fatty acids on glucose homeostasis and blood pressure in essential hypertension. A randomized, controlled trial. Ann Intern Med. 1995;123:911–918. doi: 10.7326/0003-4819-123-12-199512150-00003 [DOI] [PubMed] [Google Scholar]
- 99. (THPCRG) ToHPCRG . The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the trials of hypertension prevention, phase I. JAMA. 1992;267:1213–1220. doi: 10.1001/jama.1992.03480090061028 [DOI] [PubMed] [Google Scholar]
- 100. Vandongen R, Mori TA, Burke V, Beilin LJ, Morris J, Ritchie J. Effects on blood pressure of omega 3 fats in subjects at increased risk of cardiovascular disease. Hypertension. 1993;22:371–379. doi: 10.1161/01.hyp.22.3.371 [DOI] [PubMed] [Google Scholar]
- 101. Vericel E, Calzada C, Chapuy P, Lagarde M. The influence of low intake of n‐3 fatty acids on platelets in elderly people. Atherosclerosis. 1999;147:187–192. doi: 10.1016/s0021-9150(99)00171-9 [DOI] [PubMed] [Google Scholar]
- 102. von Houwelingen R, Nordoy A, van der Beek E, Houtsmuller U, de Metz M, Hornstra G. Effect of a moderate fish intake on blood pressure, bleeding time, hematology, and clinical chemistry in healthy males. Am J Clin Nutr. 1987;46:424–436. doi: 10.1093/ajcn/46.3.424 [DOI] [PubMed] [Google Scholar]
- 103. Wang S, Ma AQ, Song SW, Quan QH, Zhao XF, Zheng XH. Fish oil supplementation improves large arterial elasticity in overweight hypertensive patients. Eur J Clin Nutr. 2008;62:1426–1431. doi: 10.1038/sj.ejcn.1602886 [DOI] [PubMed] [Google Scholar]
- 104. Wu SY, Mayneris‐Perxachs J, Lovegrove JA, Todd S, Yaqoob P. Fish‐oil supplementation alters numbers of circulating endothelial progenitor cells and microparticles independently of eNOS genotype. Am J Clin Nutr. 2014;100:1232–1243. doi: 10.3945/ajcn.114.088880 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S13
References 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104


