Abstract
Background
Several studies have shown that obesity is associated with better outcomes in patients with cardiogenic shock (CS). Although this phenomenon, the “obesity paradox,” reportedly manifests differently based on sex in other disease entities, it has not yet been investigated in patients with CS.
Methods and Results
A total of 1227 patients with CS from the RESCUE (Retrospective and Prospective Observational Study to Investigate Clinical Outcomes and Efficacy of Left Ventricular Assist Device for Korean Patients With Cardiogenic Shock) registry in Korea were analyzed. The study population was classified into obese and nonobese groups according to Asian Pacific criteria (BMI ≥25.0 kg/m2 for obese). The clinical impact of obesity on in‐hospital mortality according to sex was analyzed using logistic regression analysis and restricted cubic spline curves. The in‐hospital mortality rate was significantly lower in obese men than nonobese men (34.2% versus 24.1%, respectively; P=0.004), while the difference was not significant in women (37.3% versus 35.8%, respectively; P=0.884). As a continuous variable, higher BMI showed a protective effect in men; conversely, BMI was not associated with clinical outcomes in women. Compared with patients with normal weight, obesity was associated with a decreased risk of in‐hospital death in men (multivariable‐adjusted odds ratio [OR], 0.63; CI, 0.43–0.92 [P=0.016]), but not in women (multivariable‐adjusted OR, 0.94; 95% CI, 0.55–1.61 [P=0.828]). The interaction P value for the association between BMI and sex was 0.023.
Conclusions
The obesity paradox exists and apparently occurs in men among patients with CS. The differential effect of BMI on in‐hospital mortality was observed according to sex.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02985008.
Keywords: body mass index, cardiogenic shock, critical care, mortality, obesity, prognosis
Subject Categories: Cardiopulmonary Resuscitation and Emergency Cardiac Care, Heart Failure, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- CS
cardiogenic shock
- RESCUE
Retrospective and Prospective Observational Study to Investigate Clinical Outcomes and Efficacy of Left Ventricular Assist Device for Korean Patients With Cardiogenic Shock
Clinical Perspective
What Is New?
In our cardiogenic shock–dedicated registry, obesity was associated with less in‐hospital mortality, a phenomenon known as the “obesity paradox.”
This was observed differently between sexes, with the paradoxical pattern displayed only in men and not women.
What Are the Clinical Implications?
The current result confirms that the obesity paradox, a well‐known phenomenon in the field of cardiology, can also be identified in the setting of cardiogenic shock, providing more information on the prediction of the disease’s prognosis.
The sex difference seen in the obesity paradox may give further insights into the exact mechanism, with more studies needed in the future.
Cardiogenic shock (CS) is a life‐threatening condition with high morbidity and mortality, and no definite management has shown clear clinical favorable outcome despite advances in therapeutic options. 1 , 2 In many studies, factors that determine the clinical course of CS were evaluated and various risk factors were shown to contribute to negative outcomes. 3 Obesity, one of the risk factors traditionally known to be associated with worse outcomes in patients with heart failure (HF), 4 was also reported to contribute to negative outcomes in CS. 5 , 6
Some researchers, however, have suggested that obesity is associated with better outcomes among patients with various cardiovascular diseases. The “obesity paradox” phenomenon has been confirmed in HF, myocardial infarction (MI), valve diseases, and arrhythmia. 7 , 8 , 9 , 10 In several studies, unequal presentation of the obesity paradox was shown between sexes, and, specifically, in patients with HF with or without acute coronary syndrome, studies show that the obesity paradox occurred only in men and was not observed in women. 11 , 12 However, in the setting of CS, only a few studies were conducted on the association of obesity with clinical outcomes. 13 , 14 Although positive results were found, all studies involved only CS originating from MI and no other causes. Furthermore, analyzing the association between obesity and sex in CS has not yet been investigated. Therefore, we investigated whether the prognostic impact of obesity differs based on sex, and whether an interaction between sex and body mass index (BMI) exists in patients with CS using a multicenter, dedicated CS registry.
Methods
Study Population
The RESCUE (Retrospective and Prospective Observational Study to Investigate Clinical Outcomes and Efficacy of Left Ventricular Assist Device for Korean Patients With Cardiogenic Shock) (NCT02985008 at https://www.clinicaltrials.gov/) registry is a nationwide, prospective and retrospective, multicenter registry investigating clinical characteristics, treatments, and outcomes of CS in Korea. From January 2014 to December 2018, the 12 regional representative hospitals in Korea participated in this study and enrolled consecutive patients with CS. Inclusion criteria were the following: systolic blood pressure <90 mm Hg for 30 minutes or need for inotrope or vasopressor support to achieve a systolic blood pressure >90 mm Hg, and presence of pulmonary congestion and signs of impaired organ perfusion (altered mental status, cold periphery, oliguria <0.5 mL/kg per hour for the previous 6 hours, or blood lactate >2 mmol/L). Exclusion criteria were out‐of‐hospital cardiac arrest and evidence of septic or hypovolemic shock. Patients whose BMI was unavailable (n=20) were also excluded from the study.
Recruited patients were then divided into men and women, and then further divided into 4 BMI subgroups: underweight, normal weight, overweight, and obese. When categorizing patients based on BMI, the classification for the Asian population was used 15 ; accordingly, patients were categorized into underweight (BMI <18.5 kg/m2), normal weight (18.5 ≤BMI<23.0 kg/m2), overweight (23.0 ≤BMI<25.0 kg/m2), and obese (BMI ≥25.0 kg/m2). Categorizing patients into 5 groups including extremely obese patients (>30 kg/m2) was considered; however, because of the limited number of extremely obese patients to have statistical significance (n=50, 4.1%), the classification was not included, and these patients were included in the obese group. The data that support the findings of this study are available from the corresponding author on reasonable request.
Data Collection and Measurement
Various data regarding the conditions, management, and outcomes of patients were collected using a web‐based record form. All baseline data were measured on admission of patients. BMI was calculated from measured weight and height at admission. Body weight was measured with a weight scale or calibrated stretcher scale installed in the patient’s bed, while height was measured with a ruler as the distance between the soles of the feet and the highest point on the head. The “ischemic” cause of CS was defined as acute MI or worsening of ischemic cardiomyopathy as a cause of shock. Acute MI was defined according to the universal definition of MI. 16 Ischemic cardiomyopathy was defined as HF with the presence of any epicardial coronary vessels with >75% stenosis or any history of MI or coronary revascularization, excluding those with single‐vessel disease without left main or proximal left anterior descending arterial lesion, or history of MI or revascularization in concordance with previous studies. 17 Additional information was obtained from medical records or by telephone contact if necessary. The primary outcome was in‐hospital mortality. The RESCUE registry provided and gained informed consent for all prospectively enrolled participants, while the need for informed consent was waived for the retrospectively enrolled participants. No additional consent was required for this study. The study was approved by the institutional review board of Samsung Medical Center (approval number 2016‐03‐130).
Statistical Analysis
Categorical or discrete variables were compared using chi‐square or Fisher exact test. Continuous variables were presented as the mean±SD and analyzed using unpaired t test or Mann–Whitney rank sum test depending on their distribution. To evaluate the differential impact of obesity on in‐hospital mortality according to sex, univariable and multivariable logistic regression analyses were performed. The multivariable model was constructed using all variables with a significance of P<0.1 in the univariable analysis and all clinically relevant variables. The final multivariate model was constructed using backward elimination to identify the best Akaike information criterion, and odds ratios (ORs) and 95% CIs were identified. The final model included the variables of age, sex, acute cause of shock, hypertension, diabetes, dyslipidemia, current smoker, and prior MI. Restricted cubic spline curves with 3 knots were used to evaluate the continuous effects of BMI on the outcome based on men and women, with 25 kg/m2 as the reference value of BMI for relative ORs. A P value for interaction between sex and BMI was calculated.
Statistical analyses were performed using SPSS version 25 for Windows (SPSS Inc), and R version 3.6.2 (R Foundation for Statistical Computing). All tests were 2‐tailed, and P<0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 1227 patients were finally included in the present study (Figure S1). Overall, 848 patients (69.1%) were men and 379 (30.9%) were women (Table S1). Figure 1 shows the distribution of BMI subgroups, including obesity, overweight, normal weight, and underweight, in men and women, respectively.
Figure 1. Distribution of body mass index (BMI) subgroups in men and women.
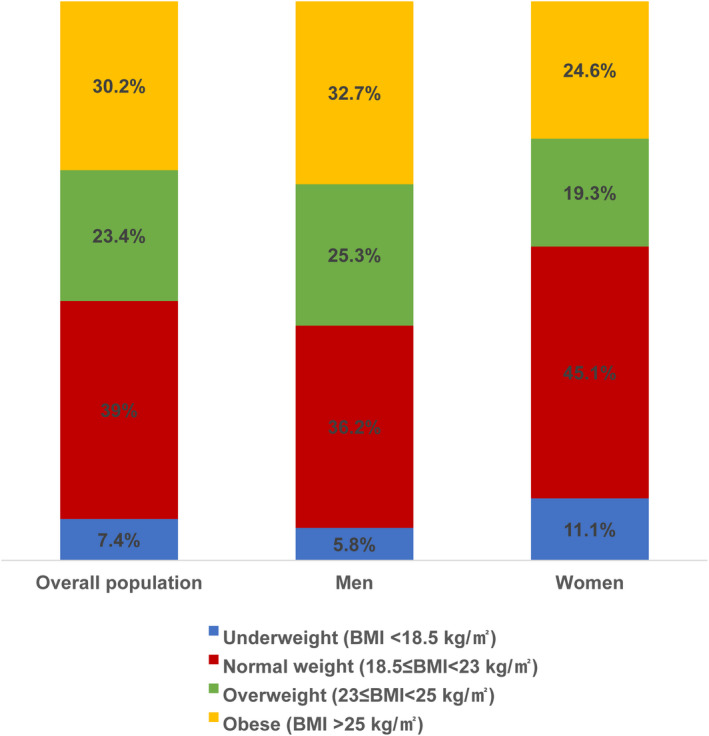
Table 1 summarizes the baseline characteristics of the study population based on obesity and sex difference. Among men, the obese patients were significantly younger than nonobese patients; however, a significant difference was not observed in age among women. The incidence of hypertension was significantly higher in obese men than in nonobese men; however, this difference was not observed between obese and nonobese women. Left ventricular ejection fraction was significantly higher in obese patients than in nonobese patients in both men and women. Although the majority of CS causes were ischemic in the total population (987 of 1227, 80.4%), the incidence of the ischemic cause was more frequent in obese than nonobese men (88.8% versus 82.8%, respectively; P=0.021).
Table 1.
Baseline Characteristics
| Men (n=848) | Women (n=379) | |||||
|---|---|---|---|---|---|---|
|
Nonobese (n=570) |
Obese (n=278) |
P value |
Nonobese (n=284) |
Obese (n=95) |
P value | |
| Age, y | 65.7±12.7 | 60.4±13.2 | <0.001 | 69.3±15.3 | 69.0±13.0 | 0.844 |
| Mean blood pressure, mm Hg | 54.7±21.5 | 58.7±23.5 | 0.014 | 54.4±21.0 | 56.2±24.5 | 0.497 |
| Body mass index, kg/m2 | 21.9±2.2 | 27.4±2.1 | <0.001 | 21.1±2.4 | 28.0±2.5 | <0.001 |
| Left ventricular ejection fraction, % | 34.5±15.7 | 38.2±16.8 | 0.003 | 35.2±16.4 | 40.2±16.6 | 0.021 |
| Cardiac arrest as presentation | 113 (19.8) | 52 (18.7) | 0.769 | 48 (16.9) | 18 (18.9) | 0.765 |
| Cause of shock | 0.032 | 0.986 | ||||
| Acute cause* | 440 (77.2) | 233 (83.8) | 207 (72.9) | 70 (73.7) | ||
| Chronic cause † | 130 (22.8) | 45 (16.2) | 77 (27.1) | 25 (26.3) | ||
| Ischemic cause | 472 (82.8) | 247 (88.8) | 0.021 | 203 (71.5) | 65 (68.4) | 0.571 |
| Comorbidities | ||||||
| Hypertension | 268 (47.0) | 156 (56.1) | 0.016 | 163 (57.4) | 63 (66.3) | 0.158 |
| Diabetes | 206 (36.1) | 98 (35.3) | 0.860 | 98 (34.5) | 36 (37.9) | 0.636 |
| Dyslipidemia | 149 (26.1) | 88 (31.7) | 0.110 | 65 (22.9) | 25 (26.3) | 0.589 |
| Current smoker | 215 (37.7) | 112 (40.3) | 0.518 | 15 (5.3) | 7 (7.4) | 0.617 |
| Chronic kidney disease | 58 (10.2) | 20 (7.2) | 0.199 | 30 (10.6) | 12 (12.6) | 0.714 |
| Peripheral arterial occlusive disease | 29 (5.1) | 10 (3.6) | 0.425 | 8 (2.8) | 5 (5.3) | 0.419 |
| Prior myocardial infarction | 77 (13.5) | 44 (15.8) | 0.423 | 25 (8.8) | 9 (9.5) | 1.000 |
| Prior cerebrovascular accident | 56 (9.8) | 21 (7.6) | 0.341 | 29 (10.2) | 11 (11.6) | 0.855 |
| Laboratories | ||||||
| Hemoglobin, mg/dL | 12.9±2.5 | 14.0±2.3 | <0.001 | 11.2±2.0 | 11.4±2.6 | 0.662 |
| Lactic acid, mmol/L | 7.1±8.2 | 6.7±4.8 | 0.427 | 6.5±4.3 | 7.1±4.9 | 0.371 |
| NT‐proBNP , pg/dL |
3244.0 [326.0–9701.5] |
1318.0 [108.0–3890.0] |
<0.001 |
6074.0 [994.0–20073.0] |
4659.0 [1167.5–10939.0] |
0.117 |
Values are presented as mean±SD, number (percentage), or median [25th percentiles–75th percentile]. Obese and nonobese were defined as body mass index ≥25 and <25 kg/m2, respectively. NT‐proBNP indicates N‐terminal pro‐B‐type natriuretic peptide.
Acute cause included acute myocardial infarction, myocarditis, stress‐induced cardiomyopathy, and pulmonary embolism.
Chronic cause included ischemic cardiomyopathy, dilated cardiomyopathy, valvular heart disease, arrhythmia, heart transplant rejection, and unspecified cardiomyopathy.
In‐Hospital Management and Outcomes
Table 2 shows the medical and mechanical management performed during clinical course and outcomes including in‐hospital mortality. Application of mechanical ventilation was significantly lower in obese men compared with nonobese men. This was followed by significantly shorter stays in both the intensive care unit and the hospital for obese and nonobese men (intensive care unit stay: 7.0 versus 13.6 days, respectively [P<0.001]; hospital stay: 12.7 versus 21.1 days, respectively [P<0.001]). The in‐hospital mortality rate was significantly lower in obese men compared with nonobese men (24.1% versus 34.2%, respectively; P=0.004). In women, a significant difference was not observed in the management or clinical course of CS. In addition, a significant difference was not found in in‐hospital mortality based on obesity among women with CS (P=0.884). Analysis of in‐hospital mortality with regards only to the presence of obesity confirmed that obese patients showed more favorable outcome than nonobese patients. Based on chi‐square test, in‐hospital mortality rates of obese and nonobese patients were 27.1% and 35.2%, respectively (P=0.005), and based on logistic regression with BMI as a continuous variable, BMI was a significant factor for lower in‐hospital mortality (OR, 0.97; 95% CI, 0.93–0.99 [P=0.038]). Furthermore, when we stratified patients into groups according to BMI, the obese group showed favorable outcomes compared with those in the normal‐weight group (multivariable‐adjusted OR, 0.72; 95% CI, 0.53–0.98, respectively [P=0.036]) (Table S2).
Table 2.
In‐Hospital Management and Outcomes
| Men (n=848) | Women (N=379) | |||||
|---|---|---|---|---|---|---|
|
Nonobese (n=570) |
Obese (n=278) |
P value |
Nonobese (n=284) |
Obese (n=95) |
P value | |
| Intra‐aortic balloon pump | 146 (25.6) | 77 (27.7) | 0.573 | 63 (22.2) | 26 (27.4%) | 0.372 |
| Extracorporeal membrane oxygenator | 238 (41.8) | 100 (36) | 0.124 | 110 (38.7) | 38 (40%) | 0.922 |
| Shock to ECMO time, min | 420.5±883.3 | 407.0±915.4 | 0.900 | 391.1±739.3 | 388.7±771.7 | 0.987 |
| ECMO duration, d | 5.9±6.6 | 4.8±4.3 | 0.130 | 5.8±5.5 | 4.9±4.5 | 0.477 |
| Continuous renal replacement therapy | 142 (24.9) | 56 (20.1) | 0.146 | 59 (20.8) | 26 (27.4%) | 0.233 |
| Mechanical ventilation | 343 (60.2) | 136 (48.9) | 0.002 | 155 (54.6) | 62 (65.3%) | 0.089 |
| ICU stay, d | 13.6±27.5 | 7.0±9.0 | <0.001 | 13.4±27.8 | 9.1±13.6 | 0.049 |
| Hospital stay, d | 21.1±33.7 | 12.7±14.8 | <0.001 | 23.1±33.0 | 17.7±22.4 | 0.078 |
| In‐hospital death | 195 (34.2) | 67 (24.1) | 0.004 | 106 (37.3) | 34 (35.8%) | 0.884 |
Values are presented as mean±SD or number (percentage). Obese and nonobese were defined as body mass index ≥25 and <25 kg/m2, respectively. ECMO indicates extracorporeal membranous oxygenation; and ICU, intensive care unit.
Different Prognostic Effects of BMI Based on Sex Difference
When in‐hospital mortality was analyzed based on the BMI subgroups in men and women, different effects of BMI on outcomes were observed (Table 3). Based on univariable analysis, underweight and overweight men showed no significant difference in in‐hospital mortality compared with normal‐weight men (P=0.543 and P=0.737, respectively). Conversely, obese men showed significantly lower in‐hospital mortality than normal‐weight patients (OR, 0.59; 95% CI, 0.41–0.85 [P=0.004]). This result was consistent even after adjusting for age (OR, 0.65; 95% CI, 0.45–0.94 [P=0.022]) and other confounders (OR, 0.63; 95% CI, 0.43–0.92 [P=0.016]). However, a significant difference was not observed in clinical outcomes based on the BMI subgroups in women. Figure 2 summarizes the continuous ORs for in‐hospital mortality, with 25 kg/m2 as the reference value. In men, a protective effect from obesity was observed; however, obesity showed neither protective nor harmful effects in women (P for interaction, 0.023 between BMI and sex).
Table 3.
Risk of In‐Hospital Mortality According to BMI and Sex
| Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Event rate, % | Unadjusted OR | 95% CI | P value | Event rate, % | Unadjusted OR | 95% CI | P value | Interaction P value (sex*BMI) | |
| Underweight | 30.6 | 0.82 | 0.43–1.57 | 0.543 | 28.6 | 0.70 | 0.34–1.47 | 0.350 | 0.023 |
| Normal weight | 35.1 | Reference | 36.3 | Reference | |||||
| Overweight | 33.6 | 0.94 | 0.65–1.36 | 0.737 | 45.2 | 1.45 | 0.83–2.53 | 0.190 | |
| Obese | 24.1 | 0.59 | 0.41–0.85 | 0.004 | 35.5 | 0.97 | 0.57–1.64 | 0.901 | |
| Age‐adjusted OR | 95% CI | P value | Age‐adjusted OR | 95% CI | P value | Interaction P value (sex*BMI) | |
|---|---|---|---|---|---|---|---|
| Underweight | 0.75 | 0.38–1.44 | 0.383 | 0.68 | 0.32–1.44 | 0.311 | 0.049 |
| Normal weight | Reference | Reference | |||||
| Overweight | 0.92 | 0.64–1.34 | 0.679 | 1.48 | 0.84–2.60 | 0.176 | |
| Obese | 0.65 | 0.45–0.94 | 0.022 | 0.97 | 0.57–1.66 | 0.925 | |
|
Multivariable‐adjusted* OR |
95% CI | P value |
Multivariable‐adjusted* OR |
95% CI | P value | Interaction P value (sex*BMI) | |
|---|---|---|---|---|---|---|---|
| Underweight | 0.85 | 0.43–1.65 | 0.626 | 0.69 | 0.32–1.48 | 0.341 | 0.032 |
| Normal weight | Reference | Reference | |||||
| Overweight | 0.91 | 0.62–1.32 | 0.605 | 1.40 | 0.79–2.48 | 0.256 | |
| Obese | 0.63 | 0.43–0.92 | 0.016 | 0.94 | 0.55–1.61 | 0.828 | |
Based on body mass index (BMI), patients were stratified into underweight (BMI <18.5 kg/m2), normal weight (18.5≤BMI<23.0 kg/m2), overweight (23.0≤BMI<25.0 kg/m2), and obese (BMI ≥25.0 kg/m2). OR indicates odds ratio.
Multivariable logistic regression analysis was performed with the variables of age, acute cause of shock, hypertension, diabetes, dyslipidemia, current smoker, and prior myocardial infarction.
Figure 2. Association between body mass index (BMI) and in‐hospital mortality according to the sexes.
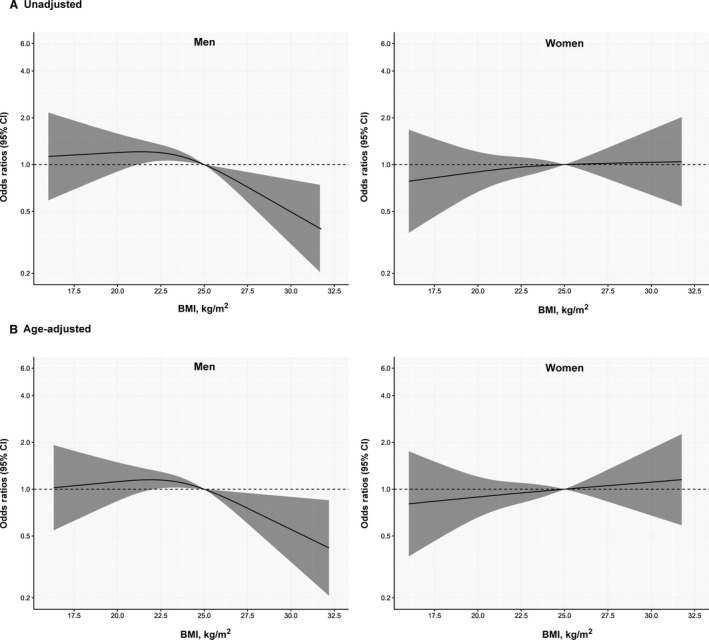
Reference value was 25 kg/m2 of BMI. (A) Unadjusted and (B) age‐adjusted odds ratios for in‐hospital mortality according to the sex difference.
Discussion
In the present study, clinical characteristics and outcomes between obese and nonobese men and women were compared. In addition, whether an association existed between BMI and sex was analyzed using a relatively large, CS‐dedicated registry. Major findings showed that obesity exerts a protective effect against in‐hospital mortality in men, in accordance with the obesity paradox; however, the same result was not observed in women. The interaction between sex and BMI was significant.
The obesity paradox is not a new discovery. The description dates back to the early 2000s and has since been associated with various diseases, 18 , 19 , 20 , 21 , 22 especially those in the cardiovascular field where obesity has traditionally been regarded as a risk factor for negative outcomes. Similarly, the present study’s analysis results showed that better in‐hospital survival occurred in the obese group. In numerous studies, causes for the obesity paradox have been investigated. Some researchers have mentioned various neurohormonal statuses of obese people, 23 or “fat but fit” concepts, that higher BMI may not always represent pathological obesity that acts as a risk factor for cardiovascular disease. 22 , 23 , 24 Others suggest possible selection bias 25 , 26 attributable to obese people receiving more frequent health checkups or dying earlier.
Whether different patterns of the obesity paradox exist between the sexes has been investigated in a small number of studies in the field of HF. Clark et al 7 used both BMI and waist circumference to investigate the effects of obesity on systolic HF and found that a survival benefit of high BMI and waist circumference were only observed in men; however, their study was limited by the small number of women (n=94). Furthermore, their later study showed both women and men were affected by the obesity paradox. 27 Hong et al 11 studied 3145 patients with systolic HF and found that those in the high BMI group were associated with lower 1‐year mortality in men but not in women; the study results did not confirm that the difference of association between BMI and in‐hospital mortality was statistically significant. The data in the present study show that this type of sex‐related difference also exists for the obesity paradox in CS, with the obesity paradox observed in men but not women. This was identified with BMI both as a continuous variable and categorical variable and the P value for interaction was statistically significant, providing evidence for an interaction between BMI and sex.
The cause of discrepancy between the sexes regarding the obesity paradox is not clearly understood. For HF, Hong et al argued that because proinflammatory biomarkers and extracellular matrix remodeling were found to be significantly lower and obesity has a slight anti‐inflammatory effect, protective effects of obesity might be more prominent in men. 11 , 28 Some investigators have approached this as an unresolved bias. 25 , 26 Because cardiovascular diseases tend to occur more in men, studies have generally included fewer women than men; thus, selection bias has been suggested as a possible cause. 23 The women in the present study accounted for less than a third of total participants, therefore statistical power may have been limited. Some studies included younger obese patients compared with their normal‐weight counterparts, 10 which could provide prognostic effects of age to improve prognoses in the former. In the present study, the patients in the obese group were also younger than in the nonobese group, particularly for men. However, the result did not change after age‐ or multivariable‐adjusted analysis.
The present study has several limitations. Perhaps most importantly, a cutline between obese and nonobese status of BMI 25.0 kg/m2 applies to the Asian population 29 and not to the population of other regions. Therefore, extrapolation of the results to a Western population, where a different definition for obesity is used, may be limited. Because the extremely obese in our study had the same BMI as obese individuals in Western countries, and worse outcomes were reported in the extremely obese group in previous studies, 14 , 30 , 31 , 32 further analysis with this group would have helped to better understand the effects of obesity; however, the number of patients in the extremely obese group was too small to have any statistical power (n=50). However, in a cubic spline curve drawn with BMI as a continuous variable, men with BMIs >30.0 kg/m2 still showed significant lower ORs for mortality, so this might at least give a clue of what might have occurred had our population had a sufficient number of patients with higher BMI––although this is mere speculation and should be investigated in future larger studies. In addition, chronicity of HF can lead to cachexia and lower BMI. Unfortunately, in this analysis, we could not assess the chronicity of HF and its management including guideline‐directed medical therapy or device therapy. When we evaluated the impact of acute HF according to sex and obesity, the final results were consistent.
The obesity paradox exists in patients with CS and its prognostic effect apparently extends only to men. A significant association between sex and BMI in patients with CS was confirmed for the first time using a large‐scale dedicated CS registry. Further studies in a shock setting are required to confirm this finding.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S2
Figure S1
See Editorial by Lavie et al.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024143
For Sources of Funding and Disclosures, see page 7.
References
- 1. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 2. Heo JH. Do ECMO first even in the desperate situation. Korean Circ J. 2021;51:545–546. doi: 10.4070/kcj.2021.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harjola VP, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, Parissis J, Banaszewski M, Silva‐Cardoso J, Carubelli V, et al. Clinical picture and risk prediction of short‐term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17:501–509. doi: 10.1002/ejhf.260 [DOI] [PubMed] [Google Scholar]
- 4. daSilva‐deAbreu A, Alhafez BA, Lavie CJ, Milani RV, Ventura HO. Interactions of hypertension, obesity, left ventricular hypertrophy, and heart failure. Curr Opin Cardiol. 2021;36:453–460. doi: 10.1097/HCO.0000000000000868 [DOI] [PubMed] [Google Scholar]
- 5. Swaminathan PD, Stancu M, Venkatesh P, Khosla S, Arora RR. Obesity is associated with higher mortality in patients with cardiogenic shock. Int J Cardiol. 2007;117:278–279. doi: 10.1016/j.ijcard.2006.05.039 [DOI] [PubMed] [Google Scholar]
- 6. Hashmi KA, Abbas K, Hashmi AA, Irfan M, Edhi MM, Ali N, Khan A. In‐hospital mortality of patients with cardiogenic shock after acute myocardial infarction; impact of early revascularization. BMC Res Notes. 2018;11:721. doi: 10.1186/s13104-018-3830-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail. 2011;17:374–380. doi: 10.1016/j.cardfail.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 8. Kanic V, Vollrath M, Frank B, Kanic Z. An obesity paradox in patients with myocardial infarction undergoing percutaneous intervention. Nutr Metab Cardiovasc Dis. 2021;31:127–136. doi: 10.1016/j.numecd.2020.08.024 [DOI] [PubMed] [Google Scholar]
- 9. Rossi A, Gaibazzi N, Bellelli G, Nistri S, Cicoira M, Cioffi G, Faden G, Temporelli PL, Faggiano P. Obesity paradox in patients with aortic valve stenosis. Protective effect of body mass index independently of age, disease severity, treatment modality and non‐cardiac comorbidities. Int J Cardiol. 2014;176:1441–1443. doi: 10.1016/j.ijcard.2014.08.037 [DOI] [PubMed] [Google Scholar]
- 10. Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. 2017;70:2022–2035. doi: 10.1016/j.jacc.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 11. Hong S, Lee JH, Kim KM, Lee JW, Youn YJ, Ahn MS, Ahn SG, Lee SH, Yoon J, Choe KH, et al. Is there a sex‐related difference in the obesity paradox in systolic heart failure? Sex‐related difference in the obesity paradox. Yonsei Med J. 2018;59:57–62. doi: 10.3349/ymj.2018.59.1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee SY, Kim HL, Kim MA, Park JJ, Choi DJ, Kim JJ, Jeon ES, Cho MC. Obesity paradox in Korean male and female patients with heart failure: a report from the Korean Heart Failure Registry. Int J Cardiol. 2021;325:82–88. doi: 10.1016/j.ijcard.2020.10.013 [DOI] [PubMed] [Google Scholar]
- 13. Chatterjee K, Gupta T, Goyal A, Kolte D, Khera S, Shanbhag A, Patel K, Villablanca P, Agarwal N, Aronow WS, et al. Association of obesity with in‐hospital mortality of cardiogenic shock complicating acute myocardial infarction. Am J Cardiol. 2017;119:1548–1554. doi: 10.1016/j.amjcard.2017.02.030 [DOI] [PubMed] [Google Scholar]
- 14. Shah M, Patil S, Patnaik S, Agrawal M, Patel B, Tripathi B, Jorde U, Lavie C. Outcomes in cardiogenic shock from acute coronary syndrome depending on severity of obesity. Am J Cardiol. 2019;123:1267–1272. doi: 10.1016/j.amjcard.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 15. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 16. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial Infarction . Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 17. Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210–218. doi: 10.1016/s0735-1097(01)01738-7 [DOI] [PubMed] [Google Scholar]
- 18. Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, Ahmed LM, Kent KM, Pichard AD, Suddath WO, et al. The impact of obesity on the short‐term and long‐term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39:578–584. doi: 10.1016/s0735-1097(01)01802-2 [DOI] [PubMed] [Google Scholar]
- 19. Vemmos K, Ntaios G, Spengos K, Savvari P, Vemmou A, Pappa T, Manios E, Georgiopoulos G, Alevizaki M. Association between obesity and mortality after acute first‐ever stroke: the obesity‐stroke paradox. Stroke. 2011;42:30–36. doi: 10.1161/STROKEAHA.110.593434 [DOI] [PubMed] [Google Scholar]
- 20. Karagozian R, Bhardwaj G, Wakefield DB, Baffy G. Obesity paradox in advanced liver disease: obesity is associated with lower mortality in hospitalized patients with cirrhosis. Liver Int. 2016;36:1450–1456. doi: 10.1111/liv.13137 [DOI] [PubMed] [Google Scholar]
- 21. Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18:56. doi: 10.1007/s11912-016-0539-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega FB, Milani RV. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61:142–150. doi: 10.1016/j.pcad.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 23. Horwich TB, Fonarow GC, Clark AL. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2018;61:151–156. doi: 10.1016/j.pcad.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 24. Oreopoulos A, Kalantar‐Zadeh K, McAlister FA, Ezekowitz JA, Fonarow GC, Johnson JA, Norris CM, Padwal RS. Comparison of direct body composition assessment methods in patients with chronic heart failure. J Card Fail. 2010;16:867–872. doi: 10.1016/j.cardfail.2010.06.416 [DOI] [PubMed] [Google Scholar]
- 25. Charnigo R, Guglin M. Obesity paradox in heart failure: statistical artifact, or impetus to rethink clinical practice? Heart Fail Rev. 2017;22:13–23. doi: 10.1007/s10741-016-9577-0 [DOI] [PubMed] [Google Scholar]
- 26. Donini LM, Pinto A, Giusti AM, Lenzi A, Poggiogalle E. Obesity or BMI paradox? Beneath the tip of the Iceberg. Front Nutr. 2020;7:53. doi: 10.3389/fnut.2020.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clark AL, Chyu J, Horwich TB. The obesity paradox in men versus women with systolic heart failure. Am J Cardiol. 2012;110:77–82. doi: 10.1016/j.amjcard.2012.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meyer S, van der Meer P, van Deursen VM, Jaarsma T, van Veldhuisen DJ, van der Wal MH, Hillege HL, Voors AA. Neurohormonal and clinical sex differences in heart failure. Eur Heart J. 2013;34:2538–2547. doi: 10.1093/eurheartj/eht152 [DOI] [PubMed] [Google Scholar]
- 29. Arafa A, Lee HH, Eshak ES, Shirai K, Liu K, Li J, Anni NS, Shim SY, Kim HC, Iso H. Modifiable risk factors for cardiovascular disease in Korea and Japan. Korean Circ J. 2021;51:643. doi: 10.4070/kcj.2021.0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sreenivasan J, Khan MS, Sharedalal P, Hooda U, Fudim M, Demmer RT, Yuzefpolskaya M, Ahmad H, Khan SS, Lanier GM, et al. Obesity and outcomes following cardiogenic shock requiring acute mechanical circulatory support. Circ Heart Fail. 2021;14:e007937. doi: 10.1161/CIRCHEARTFAILURE.120.007937 [DOI] [PubMed] [Google Scholar]
- 31. Ventura HO, daSilva‐deAbreu A, Lavie CJ. Obesity is a heavy load in cardiogenic shock and mechanical circulation. Circ Heart Fail. 2021;14:e008300. doi: 10.1161/circheartfailure.121.008300 [DOI] [PubMed] [Google Scholar]
- 32. Elagizi A, Carbone S, Lavie CJ, Mehra MR, Ventura HO. Implications of obesity across the heart failure continuum. Prog Cardiovasc Dis. 2020;63:561–569. doi: 10.1016/j.pcad.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figure S1


