Abstract
Background
Peripheral artery disease (PAD) is associated with heightened risk for major adverse cardiovascular and limb events, but data on the burden of risk for total (first and potentially subsequent) events, and the association with polyvascular disease, are limited. This post hoc analysis of the EUCLID (Examining Use of Ticagrelor in Peripheral Artery Disease) trial evaluated total cardiovascular and limb events among patients with symptomatic PAD, overall and by number of symptomatic vascular territories.
Methods and Results
In the EUCLID trial, patients with symptomatic PAD (lower extremity revascularization >30 days before randomization or ankle‐brachial index ≤0.80) were randomized to treatment with ticagrelor or clopidogrel. Relative effects on total events (cardiovascular death; nonfatal myocardial infarction and ischemic stroke; acute limb ischemia, unstable angina, and transient ischemic attack requiring hospitalization; coronary, carotid, and peripheral revascularization procedures; and amputation for symptomatic PAD) were summarized by hazard ratios (HRs), whereas absolute risks were estimated by incidence rates and mean cumulative functions. Among 13 885 randomized patients, 7600 total cardiovascular and limb events occurred during a median 2.7 years of follow‐up, translating to 60.0 and 62.5 events per 100 patients through 3 years for the ticagrelor and clopidogrel groups, respectively (HR, 0.96; 95% CI, 0.89–1.03; P=0.27). Among 1393 patients with disease in 3 vascular territories, event accrual rates through 3 years for the ticagrelor and clopidogrel groups were 87.3 and 97.7 events per 100 patients, respectively. Absolute risk reductions for ticagrelor relative to clopidogrel at 3 years were −0.2, 6.7, and 10.3 events per 100 patients for 1, 2, and 3 affected vascular territories, respectively (P interaction=0.09).
Conclusions
Patients with symptomatic PAD have nearly double the number of total events than first events, with rates reflecting the number of affected vascular territories. These findings highlight the clinical relevance of quantifying disease burden in terms of total events and the need for long‐term preventive treatments in high‐risk patient populations.
Registration
URL: https://clinicaltrials.gov/; Unique identifier: NCT01732822.
Keywords: clopidogrel, peripheral artery disease, polyvascular disease, ticagrelor, total events
Subject Categories: Peripheral Vascular Disease, Vascular Disease, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- EUCLID
Examining Use of Ticagrelor in Peripheral Artery Disease
- LER
lower extremity revascularization
- MACE
major adverse cardiovascular event
- MALE
major adverse limb event
Clinical Perspective
What Is New?
In the EUCLID (Examining Use of Ticagrelor in Peripheral Artery Disease) trial, the rate of total (first and potentially subsequent) cardiovascular and limb events was nearly double the number of first events over a median 2.7 years of follow‐up.
Event rates were higher among patients with symptomatic disease in multiple vascular territories (ie, a history of symptomatic coronary and/or cerebrovascular disease in addition to peripheral artery disease).
What Are the Clinical Implications?
These findings reinforce the clinical importance of quantifying disease burden in terms of total events and the need for long‐term preventive strategies in high‐risk patient populations, including patients with polyvascular disease.
Peripheral artery disease (PAD) is associated with increased risk for major adverse limb events (MALEs), including acute limb ischemia and amputation, and major adverse cardiovascular events (MACEs), including cardiovascular death, myocardial infarction, and stroke. 1 This increased risk when evaluated in clinical trials is usually described in terms of first events, although it may be inferred that this would also translate to increased risk for total (ie, first and subsequent) events.
A high rate of total cardiovascular and limb events and recurrent peripheral revascularization has been documented among patients who underwent recent lower extremity revascularization (LER), 2 but data on the burden of risk for total events in chronic stable symptomatic PAD, including those who have never had LER, and the possible association with polyvascular disease are limited. Furthermore, although analyses of total events have been reported for patients treated with ticagrelor 3 and clopidogrel 4 in the setting of acute coronary syndrome, the relative and absolute impacts of these treatments on total events among patients with PAD are unknown.
In the EUCLID (Examining Use of Ticagrelor in Peripheral Artery Disease) trial, patients with symptomatic PAD treated with ticagrelor or clopidogrel had similar rates of first cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. 5 The current post hoc analysis compares the treatment groups in the EUCLID trial in terms of total disease burden, quantified by total cardiovascular and limb events observed during study follow‐up, overall and in subgroups, including those defined by number of symptomatic affected vascular territories.
Methods
The data, analytic methods, and study materials are not available to other researchers for purposes of reproducing the results or replicating the procedures.
Population
Details of the study design 6 and primary efficacy and safety results 5 have been published. The study was conducted at 821 sites in 28 countries; each site obtained relevant institutional review board and ethics approvals. Eligible patients provided written informed consent and were aged ≥50 years and had symptomatic PAD defined by either LER >30 days before randomization or ankle‐brachial index (ABI) ≤0.80. Key exclusion criteria included planned long‐term dual‐antiplatelet therapy, requirement for aspirin, increased risk of bleeding, long‐term anticoagulation treatment, or poor clopidogrel metabolizer status. Double‐blind, double‐dummy randomization in a 1:1 ratio to treatment with ticagrelor, 90 mg twice daily, or clopidogrel, 75 mg once daily, was performed, with 13 885 patients meeting study entry criteria. Follow‐up visits were scheduled 2 and 6 months after randomization and every 6 months thereafter until a minimum of 1364 participants experienced a first primary end point event, after which surviving participants had a final end‐of‐study visit.
End Points
The primary efficacy outcome of the EUCLID trial was time to occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal ischemic stroke. Additional cardiovascular and limb events recorded in the trial were acute limb ischemia requiring hospitalization, unstable angina requiring hospitalization, and transient ischemic attack (TIA) requiring hospitalization; coronary, carotid, and peripheral revascularization procedures; amputation for symptomatic PAD; and noncardiovascular death. Definitions for each event have been published 5 ; events in the primary efficacy outcome, acute limb ischemia, unstable angina, TIA, and noncardiovascular death were confirmed by central adjudication, whereas procedures and amputations were investigator reported and not subject to adjudication. Events included in the primary analysis of the present report were total (ie, first and potentially subsequent) primary end point events (MACEs) and total other cardiovascular and limb events. Additional analyses of total events were by subcategory of event, as denoted in Table 1.
Table 1.
Categories of Total Events
| Event |
Ticagrelor (n=6930) |
Clopidogrel (n=6955) |
Total (n=13 885) |
|---|---|---|---|
| Total cardiovascular and limb events | 3710 | 3890 | 7600 |
| Acute cardiovascular and limb events | 1546 | 1544 | 3090 |
| Cardiovascular death* , † | 390 | 378 | 768 |
| Sudden cardiac, myocardial infarction, or ischemic stroke | 182 | 190 | 372 |
| Other cardiovascular | 57 | 64 | 121 |
| Undetermined | 151 | 124 | 275 |
| Nonfatal myocardial infarction † | 430 | 422 | 852 |
| Nonfatal ischemic stroke † , ‡ | 151 | 177 | 328 |
| Acute limb ischemia § | 151 | 139 | 290 |
| Unstable angina | 372 | 374 | 746 |
| Transient ischemic attack | 52 | 54 | 106 |
| Procedures | 2164 | 2346 | 4510 |
| Coronary revascularization || | 489 | 491 | 980 |
| Carotid revascularization || | 109 | 123 | 232 |
| Peripheral revascularization || | 1323 | 1480 | 2803 |
| Amputation for symptomatic PAD § , || | 243 | 252 | 495 |
| Noncardiovascular death | 266 | 283 | 549 |
Values are given as number. PAD indicates peripheral artery disease.
Includes deaths adjudicated to have an undetermined cause.
Included in major adverse cardiovascular events.
Includes strokes adjudicated to not be primarily hemorrhagic (ie, primarily ischemic or uncertain type).
Included in major adverse limb events.
Investigator reported; not subject to adjudication by independent committee.
Statistical Analysis
We applied a marginal proportional hazards model that allows for the possibility of multiple cardiovascular and limb events within a given participant while treating noncardiovascular death as a competing terminal event. For model convergence purposes, for a given patient, a maximum of 1 nonfatal event could occur on a given day. A robust sandwich variance estimate for the estimated SE of the log hazard ratio (HR) was applied to account for the dependence of event times within individual patients. Treatment effects on total cardiovascular and limb events are summarized by ticagrelor/clopidogrel HRs, corresponding 95% CIs, and P values. Treatment effects on additional categories of total events were estimated in separate models; cardiovascular deaths not included in a given category were also treated as competing terminal events. “On‐treatment” sensitivity analyses excluded follow‐up and events >2 days after permanent discontinuation of study treatment, consistent with conventions in other PAD studies. 7 Possible heterogeneity of relative treatment effects on total events for the PAD inclusion criterion (LER >30 days before randomization or ABI ≤0.80), by quartile of ABI, and for subgroups previously reported to have high event rates on the study primary efficacy outcome, including polyvascular disease, was assessed by the significance of interaction terms in marginal proportional hazards models. For polyvascular disease, an affected vascular territory was defined by the presence of PAD (based on the PAD inclusion criterion), coronary artery disease (history of myocardial infarction or coronary revascularization), or cerebrovascular disease (history of stroke, transient ischemic attack, carotid‐artery stenosis, or carotid revascularization).
Mean cumulative functions were used to estimate, by treatment group, accrual of total cardiovascular events, total acute cardiovascular and limb events, and total procedures over time in the presence of competing deaths. 8 Possible heterogeneity of absolute risk reductions for selected subgroups was assessed by quantitative interactions based on mean cumulative function estimates at 3 years after randomization. 9
Continuous variables are expressed as median (quartile 1–quartile 3), whereas categorical variables are expressed as counts and percentages. Incidence rates were calculated as number of events per 100 patient‐years of follow‐up. For all analyses, P<0.05, 2 tailed, was considered statistically significant, with no adjustment for multiple testing.
All analyses were conducted according to intention to treat, with the exception of the on‐treatment analyses described above. Although the primary analysis included events through the primary analysis censoring date (May 19, 2016), to maximize the number of events available for analysis, the current report included all events through the last patient’s last visit (September 26, 2016). The first author had full access to all the data in the study and takes responsibility for their integrity and the data analysis. Analyses were performed in SAS 9.4 and R 3.5.
Results
Duration of Follow‐Up and Total Events by Type and Ordinal Number
Patients were followed up for a median of 2.7 (2.5–2.9) years; selected baseline characteristics of the study population by treatment group are presented in Table S1. The types and counts of total cardiovascular and limb events and noncardiovascular deaths after randomization by treatment assignment are shown in Table 1. There were over twice as many total MACEs (cardiovascular death, nonfatal myocardial infarction, and ischemic stroke; 1948 events) than total MALEs (acute limb ischemia and amputation for symptomatic PAD; 785 events). Total revascularization procedures, however, were the most frequent event (4510 events) with the largest component peripheral revascularizations (2803) followed by coronary revascularizations (980).
Table 2 summarizes the distributions of cardiovascular and limb events and noncardiovascular deaths by event number. The total number of cardiovascular and limb events (7600, consisting of 3090 total acute cardiovascular and limb events and 4510 total procedures) was nearly double the number of first events (3878, consisting of 1858 first acute cardiovascular and limb events and 2020 first procedures), and 13.2% of the total study population (1829 patients) experienced >1 event. A similar number of total acute cardiovascular and limb events occurred in each treatment group (1546 for ticagrelor, and 1544 for clopidogrel), whereas there were numerically fewer total procedures in the ticagrelor group (2164) than in the clopidogrel group (2346). In addition, competing noncardiovascular death was relatively infrequent in both groups (266 for ticagrelor, and 283 for clopidogrel). Among 3090 total acute cardiovascular and limb events and 4510 total procedures, 1147 (37.1%) and 1454 (32.2%), respectively, occurred >2 days after the patients’ last dose of ticagrelor or clopidogrel study treatment.
Table 2.
Distribution of First and Subsequent Acute Cardiovascular Events, Procedures, and Noncardiovascular Death
| Event |
Ticagrelor (n=6930) |
Clopidogrel (n=6955) |
Total (n=13 885) |
|---|---|---|---|
| First cardiovascular and limb event | 1924 | 1954 | 3378 |
| Acute cardiovascular and limb event | 943 | 915 | 1858 |
| Cardiovascular death | 252 | 238 | 490 |
| Nonfatal* | 691 | 677 | 1368 |
| Procedure † | 981 | 1039 | 2020 |
| Noncardiovascular death | 183 | 200 | 383 |
| Second cardiovascular and limb event | 891 | 938 | 1829 ‡ |
| Acute cardiovascular and limb event | 284 | 269 | 553 |
| Cardiovascular death | 72 | 59 | 131 |
| Nonfatal* | 212 | 210 | 422 |
| Procedure † | 607 | 669 | 1276 |
| Noncardiovascular death | 47 | 39 | 86 |
| Third cardiovascular and limb event | 408 | 453 | 861 |
| Acute cardiovascular and limb event | 166 | 161 | 327 |
| Cardiovascular death | 32 | 37 | 69 |
| Nonfatal* | 134 | 124 | 258 |
| Procedure † | 242 | 292 | 534 |
| Noncardiovascular death | 14 | 28 | 42 |
| Fourth and subsequent cardiovascular and limb events | 487 | 545 | 1032 |
| Acute cardiovascular and limb events | 153 | 199 | 352 |
| Cardiovascular death | 34 | 44 | 78 |
| Nonfatal* | 119 | 155 | 274 |
| Procedures † | 334 | 346 | 680 |
| Noncardiovascular death | 22 | 16 | 38 |
| Total cardiovascular and limb events | 3710 | 3890 | 7600 |
| Acute cardiovascular and limb events | 1546 | 1544 | 3090 |
| Cardiovascular death | 390 | 378 | 768 |
| Nonfatal* | 1156 | 1166 | 2322 |
| Procedures † | 2164 | 2346 | 4510 |
| Noncardiovascular death | 266 | 283 | 549 |
Values are given as number.
Includes nonfatal myocardial infarction, nonfatal ischemic stroke, acute limb ischemia, unstable angina, and transient ischemic attack.
Includes coronary revascularization, carotid revascularization, peripheral revascularization, and amputation for symptomatic peripheral artery disease.
Represents the number of patients with >1 event during the study.
Figure 1 displays the rates of second events by type of first nonfatal event. Patients who experienced a first nonfatal acute cardiovascular or limb event were at higher risk of subsequent events relative to patients who experienced a first procedure, regardless of randomized treatment. Similar to the overall results, there was a higher rate of procedures as a second event than acute cardiovascular and limb events. Figure S1 shows these rates, where first and second events restricted to MACEs (nonfatal for first) or MALEs. Overall, patients with a first nonfatal MACE had a higher second event rate than patients with a first MALE. In addition, most second events among patients with a first nonfatal MACE were also MACEs, whereas most second events among patients with a first MALE were also MALEs.
Figure 1. Rates of second events by type of first nonfatal event.
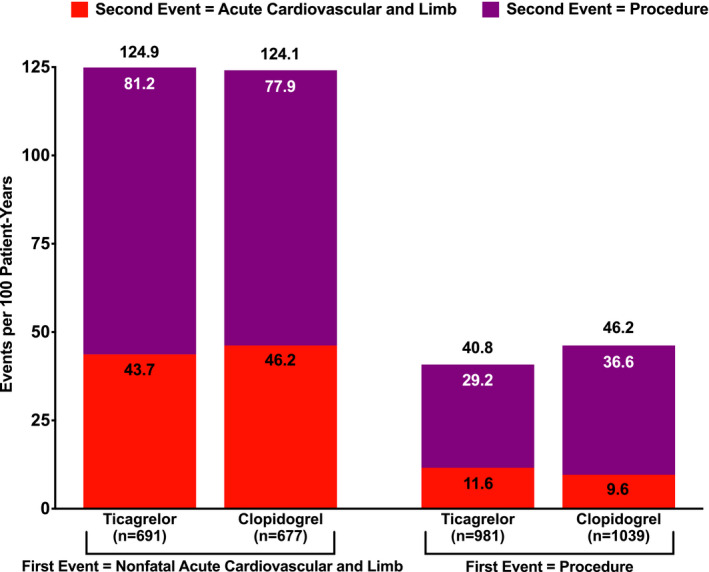
Values above bars are the sum of the rates of acute cardiovascular and limb events and procedures as a second event. Values within bars are the rates of acute cardiovascular and limb events or the rates of procedures as a second event.
Treatment Effects on Total Cardiovascular and Limb Events and Procedures
Figure 2 displays ticagrelor/clopidogrel treatment HRs and 95% CIs and number of events per 100 patient‐years by treatment group for total cardiovascular and limb events, overall and by event type. There was no significant difference between the treatment groups in the risk of total cardiovascular and limb events, with event rates per 100 patient‐years of 20.5 and 21.3 for the ticagrelor and clopidogrel groups, respectively (HR, 0.96; 95% CI, 0.89–1.03; P=0.27). There were also no differences when acute cardiovascular or limb events (HR, 1.01; 95% CI, 0.92–1.11; P=0.88) or procedures (HR, 0.93; 95% CI, 0.85–1.01; P=0.08) were analyzed separately, although ticagrelor treatment was associated with a nominally lower risk of total peripheral revascularizations (HR, 0.90; 95% CI, 0.81–1.00; P=0.0484). Similarly, there were no differences in total MACEs (event rates per 100 patient‐years of 5.4 and 5.4 for the ticagrelor and clopidogrel groups, respectively; HR, 1.00; 95% CI, 0.90–1.11; P=1.00) or total MALEs (event rates per 100 patient‐years of 2.2 and 2.1 for the ticagrelor and clopidogrel groups, respectively; HR, 1.01; 95% CI, 0.84–1.22; P=0.91). Figure S2 contains the mean cumulative functions for total cardiovascular and limb events by treatment group, with separate panels for total acute cardiovascular and limb events and for total procedures. The accrual of events was at a relatively constant rate over time, with an estimated 60.0 and 62.5 events per 100 patients after 3 years of follow‐up in the ticagrelor and clopidogrel groups, respectively.
Figure 2. Treatment effects on total cardiovascular and limb events and procedures by event type.
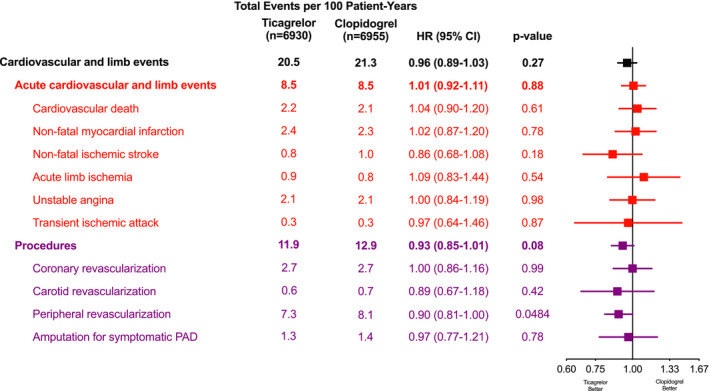
Hazard ratios (HRs), 95% CIs, and P values from marginal proportional hazards models with death not included in a given end point treated as a competing terminal event. PAD indicates peripheral artery disease.
Table S2 summarizes treatment HRs and 95% CIs and incidence rates by treatment group for on‐treatment sensitivity analyses of total events. Results were generally similar to the intention‐to‐treat results, although there was a nominally lower risk of total procedures with ticagrelor treatment (HR, 0.90; 95% CI, 0.81–0.99; P=0.0260) primarily because of lower risk of total peripheral revascularizations (HR, 0.87; 95% CI, 0.78–0.98; P=0.0192).
Subgroup analyses for total cardiovascular and limb events are shown in Figure S3; none of the relative tests of heterogeneity was significant. Patients with prior peripheral revascularization, a history of coronary artery disease (including stent implantation), or disease in multiple vascular territories had especially high event rates, regardless of randomized treatment assignment. Subgroup results for total MACEs and total MALEs are summarized in Tables S3 and S4. Across treatment groups, patients with a history of coronary artery disease or multiple affected vascular territories were at markedly higher risk of MACEs, whereas patients with prior peripheral revascularization were at elevated risk of MALEs. In addition, risk of MACEs increased with increasing numbers of affected vascular territories, whereas this was not the case for MALEs. There was significant heterogeneity in the relative treatment effects on MALEs across quartiles of ABI, where risk was numerically lower for ticagrelor among patients with ABI in the lowest 2 quartiles, whereas the risk for clopidogrel was numerically lower among patients with ABI in the highest 2 quartiles (P interaction=0.0472).
Mean cumulative functions for total cardiovascular and limb events by number of affected vascular territories and treatment group are presented in Figure 3. Absolute risk was associated with the number of affected vascular territories, with estimated rates of 87.3 and 97.7 events per 100 patients at 3 years for the ticagrelor and clopidogrel groups, respectively, among 1393 patients with disease in 3 vascular territories. The absolute risk reductions for ticagrelor relative to clopidogrel at 3 years were −0.2, 6.7, and 10.3 events per 100 patients for 1, 2, and 3 affected vascular territories, respectively (P interaction=0.09).
Figure 3. Mean cumulative functions for total cardiovascular and limb events and procedures by number of affected vascular territories.
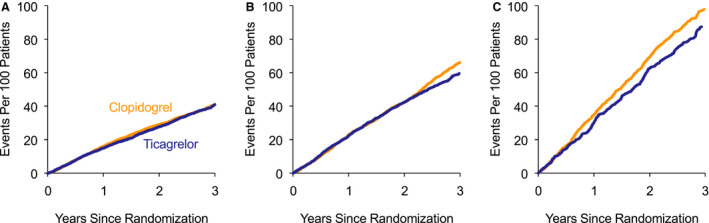
A, One affected vascular territory. B, Two affected vascular territories. C, Three affected vascular territories. The estimated numbers of events per 100 patients in the ticagrelor and clopidogrel groups at 3 years were 40.9 and 40.7 among the subgroup with 1 affected vascular territory, 59.6 and 66.3 among the subgroup with 2 affected vascular territories, and 87.3 and 97.7 for among the subgroup with 3 affected vascular territories, respectively. Absolute risk reductions for ticagrelor relative to clopidogrel at 3 years were −0.2, 6.7, and 10.3 events per 100 patients for 1, 2, and 3 affected vascular territories, respectively (P interaction=0.09).
Discussion
The current analyses reveal several novel aspects on the accrual of events in patients with chronic symptomatic PAD that, to our knowledge, have not been previously reported. The total event rates were high overall, driven by a high rate of peripheral revascularization and acute coronary events and procedures (nonfatal myocardial infarction, unstable angina, and coronary revascularization). The risk of a second event was exceptionally high among patients with a first nonfatal acute cardiovascular or limb event, with a second event rate of >100 events per 100 patient‐years of follow‐up that was essentially 3 times higher than that for patients who had a procedure as a first event. In addition, patients with a first nonfatal MACE were more likely to have a MACE than a MALE as a second event, whereas the converse was true for patients with a first MALE. Finally, the present report highlights the relationship of total events and risk according to symptomatic vascular territories, demonstrating the importance of polyvascular disease as a marker of atherosclerosis burden and atherothrombotic risk among patients with chronic symptomatic PAD.
Extending on previous findings from the EUCLID trial, the current analyses found no significant differences between treatment groups in total MACEs, which was similar to the primary findings of the trial. 5 More important, a substantial fraction of total events, 2601 of 7600, occurred in patients >2 days after their last dose of ticagrelor or clopidogrel study treatment. This likely reduced the ability to find differences between treatment groups in the risk of total events, although on‐treatment analyses revealed a nominal difference in total procedures.
The risk of events in both treatment groups was related to the extent of the patients’ cardiovascular disease at baseline, as defined by number of affected vascular territories. Patients with established coronary disease at baseline had total event rates that were markedly higher than patients without this history, consistent with prior reports from the study for first primary events 10 and other studies that included subsets of patients with coronary and peripheral disease. 11 , 12 , 13 , 14 Furthermore, relative to patients with only peripheral disease (ie, 1 affected vascular territory), patients with 2 affected territories had an ≈50% higher event rate, whereas patients with 3 affected territories had an event rate that was more than double. The total event rate among patients with only peripheral disease appeared to be considerably higher, however, than previous findings among patients with only documented cerebrovascular disease at baseline. 15 In terms of treatment differences, based on cumulative event rates at 3 years, patients with a greater extent of disease had nominally lower event rates with ticagrelor therapy relative to clopidogrel. Additional research is therefore needed to confirm if patients with PAD and more extensive cardiovascular disease might benefit from more potent antithrombotic therapy.
In the VOYAGER PAD (Vascular Outcomes Study of ASA [acetylsalicylic acid] Along with Rivaroxaban in Endovascular or Surgical Limb Revascularization for PAD) study, patients with symptomatic PAD who underwent recent LER had annual total MACE and MALE rates of ≈5 and 4 per 100 patient‐years of follow‐up, respectively. 2 The current analyses found a similar rate for MACE but a lower rate for MALE, although the MALE rate was higher among the subset of patients with prior peripheral revascularization, mirroring previous findings on first acute limb ischemia event. 16 This difference in MALE rates between the 2 studies may be explained by the fact that the patients in the EUCLID trial had more “stable” PAD, in that they either had hemodynamic evidence of PAD or a previous revascularization >30 days before randomization, whereas all patients in VOYAGER PAD had a revascularization procedure only days before randomization.
The high proportion of total cardiovascular and limb events that were attributable to MALEs and peripheral revascularization in the EUCLID trial, 47.2% of the total, was consistent with other studies where patients were enrolled on the basis of established PAD and the majority did not have known disease in other vascular territories. 2 Furthermore, among procedures, there were nearly 3 times as many peripheral revascularizations than coronary revascularizations. In contrast, acute limb events and procedures have been reported to be a relatively small fraction of total events in patients with established coronary or cerebrovascular disease and either no or small fractions with disease in additional territories. 15 , 17 , 18 , 19 Therefore, although it has been previously documented that total event risk in the coronary or cerebrovascular territory is elevated if the given territory has established disease at baseline, the current results support extending this observation to the peripheral territory.
In the EUCLID trial, the number of TIA events (106) was relatively low relative to the number of nonfatal ischemic stroke events (328). This may be an artifact of the fact that TIA was primarily collected for the purposes of cardiovascular‐related hospitalizations, rather than acute cerebrovascular events (along with ischemic stroke), and therefore hospitalizations may have been attributed to reasons other than TIA.
Limitations
A limitation of the analysis method is that although noncardiovascular deaths were treated as a competing event, cardiovascular deaths also removed 768 participants from the risk set for subsequent events. However, only 3.5% of participants (n=490) died because of cardiovascular causes before a first nonfatal cardiovascular event or procedure, so that the vast majority of participants were at risk for multiple events. In addition, the current analysis did not assign weights corresponding to severity to individual events, although results were also presented for MACEs and MALEs, which may be considered more severe than other outcomes. Although the presented on‐treatment results accounted for discontinuation of randomized study treatment during follow‐up, changes in other treatments that may modify risk were not accounted for in the analyses. Finally, other aspects of disease burden were not a part of the analysis, including increased risk of bleeding associated with antiplatelet therapy.
Conclusions
The observation that patients with PAD are at high risk for cardiovascular and limb events is largely based on a focus on first events, whereas considering total events (ie, first and potentially subsequent) may better quantify total disease burden. Over a median of 2.7 years of follow‐up in the EUCLID trial, total event risk among patients with symptomatic PAD was considerable, with >60 total cardiovascular and limb events occurring per 100 patients through 3 years of follow‐up. There were no statistically significant differences between the ticagrelor and clopidogrel groups in this risk, overall or by selected subgroups, although the analyses revealed patient subgroups, including those with disease in 3 vascular territories, who were at exceptionally high risk for events and may benefit from more potent antithrombotic therapy. These findings reinforce the clinical importance of quantifying total disease burden and the need for long‐term preventive strategies in high‐risk patient populations.
Sources of Funding
The EUCLID (Examining Use of Ticagrelor in Peripheral Artery Disease) trial was funded by AstraZeneca.
Disclosures
Dr Szarek: received fees for performing analyses, steering committee fees, and travel support from Sanofi and Regeneron; received consulting fees from CiVi and Esperion; received Data Safety and Monitoring Board membership fees from Resverlogix and Janssen; and member of the Journal of the American College of Cardiology (JACC) editorial board. Dr Hess: research grants to CPC Clinical Research from Amgen, Bayer, and Janssen. Dr Patel: institutional research grants from AstraZeneca, CSL, HeartFlow, and Janssen Research; Bayer, honoraria/advisory board: Janssen and Bayer. Dr Jones: research grants from Boehringer Ingelheim, Bristol‐Myers Squibb, Doris Duke Charitable Foundation, Merck, National Institutes of Health, and Patient‐Centered Outcomes Research Institute; honoraria/advisory committees from Bayer, Bristol‐Myers Squibb, and Janssen Pharmaceuticals. Dr Berger: institutional research grants from Astra Zeneca, National Heart, Lung, and Blood Institute, and American Heart Association; consulting fees from Janssen, Merck, and Takeda. Dr Baumgartner: educational grant from Abbott Vascular and research grant from Boston Scientific. Dr Katona: employee of AstraZeneca and AstraZeneca shareholder. Dr Mahaffey: available at http://med.stanford.edu/profiles/kenneth‐mahaffey. Dr Norgren: research grants from AnGes; consulting fees from AstraZeneca, Bayer, AnGes, and Pluristem. Dr Blomster: employed by AstraZeneca at the time of the study; consulting fees from AstraZeneca. Dr Rockhold: research funding from the National Institutes of Health, Patient‐Centered Outcomes Research Institute (PCORI), Duke Clinical Research Institute, Alzheimers Drug Discovery Foundation, AstraZeneca, ReNeuron, Luitpold, BMS, and Janssen; consulting/honoraria from California Institute for Regenerative Medicine, PCORI, BARDA, Merck Serono, Janssen, resTORbio, Eidos Therapeutics, FuturaMedical, AbbVie, Amgen, Complexa, Adverum Biotechnologies, AstraZeneca, Aldeyra, KLSMC, Merck Research Laboratories, and Chimerix; equity interest in GlaxoSmithKline, DataVant, and M3 Biotechnology. Dr Hsia: owns stock in AstraZeneca; received grant support from Amgen, AstraZeneca, and Janssen. Dr Fowkes: advisory boards for AstraZeneca, Bayer, and Merck. Dr Bonaca: reports grant support to CPC Clinical Research from Amgen, AstraZeneca, Bayer, JanOne, Janssen, Merck, Novo Nordisk, Regio, Pfizer, and Sanofi. Supported by the American Heart Association Strategically Focused Research Network in Vascular Disease under award numbers 18SFRN3390085 (Brigham and Women's Hospital Dartmouth‐Hitchcock Strategically Focused Research Network [BWH‐DH SFRN] Center) and 18SFRN33960262 (BWH‐DH Clinical Project). The article content is solely the responsibility of the authors and does not necessarily represent the official views of the American Heart Association.
Supporting information
Tables S1–S4
Figures S1–S3
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.025504
For Sources of Funding and Disclosures, see page 8 and 9.
References
- 1. Steg PG, Bhatt DL, Wilson PWF, D’Agostino R, Ohman EM, Röther J, Liau C‐S, Hirsch AT, Mas J‐L, Ikeda Y, et al. One‐year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. doi: 10.1001/jama.297.11.1197 [DOI] [PubMed] [Google Scholar]
- 2. Bauersachs RM, Szarek M, Brodmann M, Gudz I, Debus ES, Nehler MR, Anand SS, Patel MR, Hess CN, Capell WH, et al. Total ischemic event reduction with rivaroxaban after peripheral arterial revascularization in the voyager pad trial. J Am Coll Cardiol. 2021;78:317–326. doi: 10.1016/j.jacc.2021.05.003 [DOI] [PubMed] [Google Scholar]
- 3. Kohli P, Wallentin L, Reyes E, Horrow J, Husted S, Angiolillo DJ, Ardissino D, Maurer G, Morais J, Nicolau JC, et al. Reduction in first and recurrent cardiovascular events with ticagrelor compared with clopidogrel in the PLATO study. Circulation. 2013;127:673–680. doi: 10.1161/CIRCULATIONAHA.112.124248 [DOI] [PubMed] [Google Scholar]
- 4. Murphy SA, Antman EM, Wiviott SD, Weerakkody G, Morocutti G, Huber K, Lopez‐Sendon J, McCabe CH, Braunwald E. Reduction in recurrent cardiovascular events with prasugrel compared with clopidogrel in patients with acute coronary syndromes from the TRITON‐TIMI 38 trial. Eur Heart J. 2008;29:2473–2479. doi: 10.1093/eurheartj/ehn362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hiatt WR, Fowkes FG, Heizer G, Berger JS, Baumgartner I, Held P, Katona BG, Mahaffey KW, Norgren L, Jones WS, et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 2017;376:32–40. doi: 10.1056/NEJMoa1611688 [DOI] [PubMed] [Google Scholar]
- 6. Berger JS, Katona BG, Jones WS, Patel MR, Norgren L, Baumgartner I, Blomster J, Mahaffey KW, Held P, Millegard M, et al. Design and rationale for the Effects of Ticagrelor and Clopidogrel in Patients with Peripheral Artery Disease (EUCLID) trial. Am Heart J. 2016;175:86–93. doi: 10.1016/j.ahj.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 7. Bonaca MP, Bauersachs RM, Anand SS, Debus ES, Nehler MR, Patel MR, Fanelli F, Capell WH, Diao L, Jaeger N, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382:1994–2004. doi: 10.1056/NEJMoa2000052 [DOI] [PubMed] [Google Scholar]
- 8. Andersen PK, Angst J, Ravn H. Modeling marginal features in studies of recurrent events in the presence of a terminal event. Lifetime Data Anal. 2019;25:681–695. doi: 10.1007/s10985-019-09462-4 [DOI] [PubMed] [Google Scholar]
- 9. Gail M, Simon R. Testing for qualitative interactions between treatment effects and patient subsets. Biometrics. 1985;41:361–372. doi: 10.2307/2530862 [DOI] [PubMed] [Google Scholar]
- 10. Berger JS, Abramson BL, Lopes RD, Heizer G, Rockhold FW, Baumgartner I, Fowkes FGR, Held P, Katona BG, Norgren L, et al. Ticagrelor versus clopidogrel in patients with symptomatic peripheral artery disease and prior coronary artery disease: insights from the EUCLID trial. Vasc Med. 2018;23:523–530. doi: 10.1177/1358863X18775594 [DOI] [PubMed] [Google Scholar]
- 11. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 12. Bonaca MP, Gutierrez JA, Cannon C, Giugliano R, Blazing M, Park JG, White J, Tershakovec A, Braunwald E. Polyvascular disease, type 2 diabetes, and long‐term vascular risk: a secondary analysis of the improve‐it trial. Lancet Diabetes Endocrinol. 2018;6:934–943. doi: 10.1016/S2213-8587(18)30290-0 [DOI] [PubMed] [Google Scholar]
- 13. Bonaca MP, Bhatt DL, Storey RF, Steg PG, Cohen M, Kuder J, Goodrich E, Nicolau JC, Parkhomenko A, Lopez‐Sendon J, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol. 2016;67:2719–2728. doi: 10.1016/j.jacc.2016.03.524 [DOI] [PubMed] [Google Scholar]
- 14. Jukema JW, Szarek M, Zijlstra LE, de Silva HA, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, et al. Alirocumab in patients with polyvascular disease and recent acute coronary syndrome: ODYSSEY OUTCOMES trial. J Am Coll Cardiol. 2019;74:1167–1176. doi: 10.1016/j.jacc.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 15. Szarek M, Amarenco P, Callahan A, DeMicco D, Fayyad R, Goldstein LB, Laskey R, Sillesen H, Welch KM. Atorvastatin reduces first and subsequent vascular events across vascular territories: the SPARCL trial. J Am Coll Cardiol. 2020;75:2110–2118. doi: 10.1016/j.jacc.2020.03.015 [DOI] [PubMed] [Google Scholar]
- 16. Jones WS, Baumgartner I, Hiatt WR, Heizer G, Conte MS, White CJ, Berger JS, Held P, Katona BG, Mahaffey KW, et al. Ticagrelor compared with clopidogrel in patients with prior lower extremity revascularization for peripheral artery disease. Circulation. 2017;135:241–250. doi: 10.1161/CIRCULATIONAHA.116.025880 [DOI] [PubMed] [Google Scholar]
- 17. Szarek M, Bittner VA, Aylward P, Baccara‐Dinet M, Bhatt DL, Diaz R, Fras Z, Goodman SG, Halvorsen S, Harrington RA, et al. Lipoprotein(a) lowering by alirocumab reduces the total burden of cardiovascular events independent of low‐density lipoprotein cholesterol lowering: ODYSSEY OUTCOMES trial. Eur Heart J. 2020;41:4245–4255. doi: 10.1093/eurheartj/ehaa649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tikkanen MJ, Szarek M, Fayyad R, Holme I, Cater NB, Faergeman O, Kastelein JJP, Olsson AG, Larsen ML, Lindahl C, et al. Total cardiovascular disease burden: comparing intensive with moderate statin therapy insights from the ideal (incremental decrease in end points through aggressive lipid lowering) trial. J Am Coll Cardiol. 2009;54:2353–2357. doi: 10.1016/j.jacc.2009.08.035 [DOI] [PubMed] [Google Scholar]
- 19. LaRosa JC, Deedwania PC, Shepherd J, Wenger NK, Greten H, DeMicco DA, Breazna A. Comparison of 80 versus 10 mg of atorvastatin on occurrence of cardiovascular events after the first event (from the treating to new targets [TNT] trial). Am J Cardiol. 2010;105:283–287. doi: 10.1016/j.amjcard.2009.09.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S3


