Abstract
Background
Some myocardial diseases, such as cardiac sarcoidosis, predispose to complete atrioventricular block. The European Society of Cardiology Guidelines on cardiac pacing in 2021 recommend myocardial disease screening in patients with conduction disorder requiring pacemaker with multimodality imaging, including cardiac magnetic resonance (CMR) imaging. The ability of CMR imaging to detect myocardial disease in patients with a temporary pacing wire is not well documented.
Methods and Results
Our myocardial disease screening protocol is based on using an active fixation pacing lead connected to a reusable extracorporeal pacing generator (temporary permanent pacemaker) as a bridge to a permanent pacemaker. From 2011 to 2019, we identified 17 patients from our CMR database who underwent CMR imaging with a temporary permanent pacemaker for atrioventricular block. We analyzed their clinical presentations, CMR data, and pacemaker therapy. All CMRs were performed without adverse events. Pacing leads induced minor artifacts to the septal myocardial segments. The extent of late gadolinium enhancement in CMR imaging was used to screen patients for the presence of myocardial disease. Patients with evidence of late gadolinium enhancement underwent endomyocardial biopsy. If considered clinically indicated, also 18‐F‐fluorodeoxyglucose positron emission tomography and extracardiac tissue biopsy were performed if sarcoidosis was suspected. Eventually, 8 of 17 patients (47.1%) were diagnosed with histologically confirmed granulomatous inflammatory cardiac disease. Importantly, only 1 had a previously diagnosed extracardiac sarcoidosis at the time of presentation with high‐degree atrioventricular block.
Conclusions
CMR imaging with temporary permanent pacemaker protocol is an effective and safe early screening tool for myocardial disease in patients presenting with atrioventricular block requiring immediate, continuous pacing for bradycardia.
Keywords: cardiac sarcoidosis, high‐degree atrioventricular block, MRI safety, pacemaker, temporary pacing
Subject Categories: Magnetic Resonance Imaging (MRI), Pacemaker, Inflammatory Heart Disease
Nonstandard Abbreviations and Acronyms
- AVB
atrioventricular block
- CS
cardiac sarcoidosis
- EMB
endomyocardial biopsy
- FDG‐PET
18‐F‐fluorodeoxyglucose positron emission tomography
- GCM
giant cell myocarditis
- IQ
image quality
- LGE
late gadolinium enhancement
- TPPM
temporary permanent pacemaker
Clinical Perspective
What Is New?
Bradycardic atrioventricular block (AVB) requiring urgent pacing can be temporarily treated with active fixation pacing lead connected to an extracorporeal pacing generator located on the neck.
Cardiac magnetic resonance imaging of patients with a temporary active fixation pacing lead is safe, and image quality is sufficient for diagnostic purposes.
Imaging‐guided diagnostic protocol of AVB requiring urgent temporary pacing detected granulomatous inflammatory cardiac disease in 47.1%, and all patients except 1 did not have a history of extracardiac sarcoidosis.
What Are the Clinical Implications?
Cardiac magnetic resonance imaging–based myocardial disease screening is a useful method in the assessment of patients with AVB requiring urgent pacing.
Cardiac sarcoidosis is not an uncommon cause of AVB in patients with high‐degree bradycardic AVB.
Determining the etiology of AVB before the implantation of a permanent pacemaker is important, as cardiac sarcoidosis is an arrhythmogenic cardiomyopathy, and early implantation of an implantable cardioverter‐defibrillator is likely to improve the prognosis.
Early‐onset, progressive, high‐degree atrioventricular block (AVB) can be an early manifestation of myocardial disease caused by acute or chronic myocarditis or genetic disorder. 1 , 2 Early‐onset, high‐degree AVB, either isolated or with decreased left ventricular function, should prompt consideration for advanced tissue imaging of the heart according to current European Society of Cardiology guidelines on cardiac pacing. 2
Cardiac magnetic resonance (CMR) imaging can be performed safely in patients with a permanent cardiac implantable electronic device (CIED) when a dedicated safety protocol is followed. 3 , 4 , 5 However, CMR image quality (IQ) may be compromised in patients with CIED because of artifacts induced by the pacing device generator or pacing leads. 6 , 7 Traditional temporary pacemakers with floating pacing leads are considered an absolute contraindication because of safety hazards concerning especially excessive heating of the lead tip in ex vivo models. 8 , 9 Floating pacing leads can be implanted at the bedside, but the disadvantage is that the patient must stay on bed rest because of the risk for displacement and failure. In contrast, in the temporary permanent pacemaker (TPPM) approach, actively fixated leads, dedicated for permanent pacing, connected to a reusable extracorporeal pacing generator, have a lower risk for lead displacement (Figure 1). 9 , 10
Figure 1. Schematic representation of a temporary permanent pacemaker with an active fixation lead.
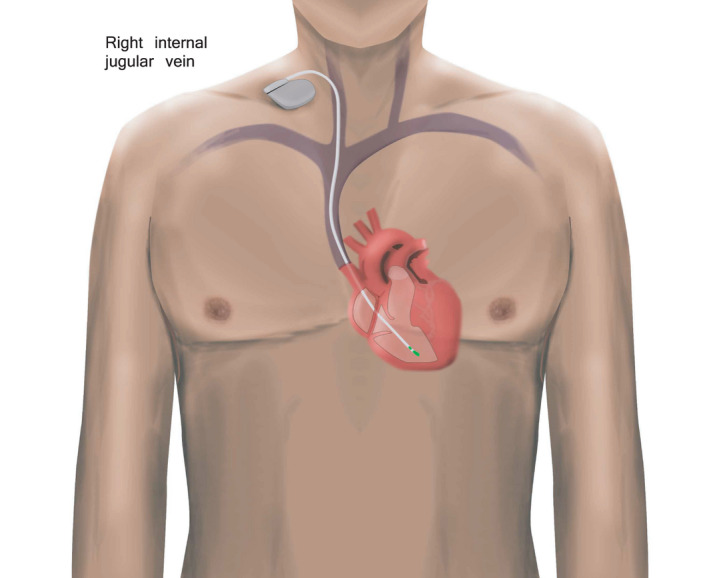
Here, we report our clinical protocol for CMR‐based testing for myocardial disease in the setting of bradycardic AVB requiring urgent temporary pacing for severe bradycardia. We also evaluated the safety and IQ of CMR in this setting.
Methods
The authors declare that all supporting data are available within the article. This retrospective study on clinical magnetic resonance imaging (MRI) examinations on adult patients with CIED performed at Meilahti Helsinki University Hospital between November 2011 and October 2019 was approved by the Helsinki University Hospital Medical Imaging Center review board. No informed consent was required. The data cannot be made available to other researchers for purposes of reproducing the results because of restrictions imposed by the research permit. Individual‐level data cannot be shared openly.
Study Design
All clinically indicated CMR imaging conducted on patients with TPPM for high‐degree AVB and performed at Meilahti Helsinki University Hospital between November 2011 and October 2019 were included in this observational retrospective study. All CMR examinations were performed with a 1.5T system (Siemens MAGNETOM Avanto that was updated to a Siemens MAGNETOM Avantofit in summer 2013 [both from Siemens Healthcare, Erlangen, Germany]).
The following information was collected from the electronic medical record: patients’ date of birth, sex, pacing device generator model, pacing lead model, date of the pacing device implantation, site of the pacing device generator, indication for TPPM treatment, permanent CIED model, date of the CMR scan, information of the pacing device interrogation before and after the CMR scan, findings of diagnostic tests of cardiac inflammatory disease (CMR, endomyocardial biopsy [EMB], and 18‐F‐fluorodeoxyglucose positron emission tomography, [FDG‐PET]), and clinical diagnosis underlying AVB. The electronic medical record was searched in particular for any pacing device–related safety hazards or adverse outcomes during or after the CMR scan, such as generator failure; power‐on reset; clinically relevant changes in the pacing threshold or sensing that required system revision or programming changes, unexpected battery depletion, and inhibition of pacing; and patient‐reported events such as discomfort, pain, a warm sensation in the location of the device, and palpitation.
MRI Safety Protocol
CMR imaging in patients with TPPM was performed according to our institutional MRI with CIED safety protocol. The MRI examinations of patients with a CIED were started at Helsinki University Hospital in November 2011 according to the safety protocol presented in detail earlier. 3 According to the institutional safety protocol, if MRI is considered to be the imaging method of choice, the MRI examination time is scheduled at least 6 weeks after the pacing device installation unless there is an urgent clinical need for an earlier MRI examination. Patients with an urgent clinical need for an earlier MRI examination were scanned according to the same safety protocol as other patients. The CIED was programmed before the MRI scan and reprogrammed immediately after the MRI scan by cardiologist. During the MRI scan, patients were monitored with ECG, pulse oximeter and with visual and audio communication. The radiographers performed the MRI acquisition. Radiologist, cardiologist, and physicist were available upon request, but they did not supervise the MRI scan.
CMR Protocol
The CMR study protocol typically consisted of localizer imaging; cine imaging in 2‐chamber, 3‐chamber, 4‐chamber, short‐axis and right ventricular outflow tract directions; T2‐weighted turbo spin echo imaging; rest first‐pass perfusion imaging; and late gadolinium enhancement (LGE) imaging. For the cine and LGE imaging, balanced steady‐state free precession sequences were used.
Image Analysis
The volumetric analysis was performed using QMass MR software version 7.6 (Medis Medical Imaging Systems, Leiden, The Netherlands). In patients with visually detectable LGE, the quantitative analysis of LGE sequences was performed computationally with the QMass MR software, using the 5 SDs method. 11
IQ was evaluated by 1 experienced radiologist, specialized in CMR (>10 years of experience). A 4‐point grading scale was used (1=very good IQ, no artifacts affecting cardiac anatomy; 2=good/average IQ, artifacts slightly interfering with cardiac anatomy; 3=below‐average IQ, artifacts moderately affecting cardiac anatomy; 4=poor IQ, artifacts severely affecting cardiac anatomy) to visually evaluate the effects of TPPM‐related artifacts on cine and LGE images. The evaluations were performed in short‐axis views of the myocardium. The 17‐segment model (American Heart Association) was used to detect regional differences in artifacts. The area of the susceptibility artifact induced by the pacing lead tip was measured. Also, the TPPM generator distance from the heart was measured from the coronal localizer images of the CMR: The shortest distance from the middle of the signal‐void area artifact induced by the TPPM generator to the heart border was measured.
Data Analysis
The numerical results are given as mean±SD, as appropriate. Data were analyzed with SPSS version 25.0 (IBM SPSS Statistics for Windows, Armonk, NY).
Results
Between November 2011 and October 2019, a total of 2338 MRI examinations, including 260 CMR examinations, were conducted on adult patients with CIED at Helsinki University Hospital. Of CMR examinations, 9.2% (24/260) were performed on patients with a TPPM. Seventeen CMR examinations were completed on adult patients with a TPPM for bradycardic AVB. Of these patients, 58.8% (n=10) were women. The mean age of patients at the time of CMR was 47.8±14.0 years (range, 28–71 years) (Table 1). One patient had previously diagnosed extracardiac sarcoidosis at the time of presentation.
Table 1.
Selected Characteristics of the Patient Cohort
| N=17 | Mean | SD | Range |
|---|---|---|---|
| LVEDV, mL | 183.8 | 75.3 | 76–412 |
| LVEDV, mL/m2 | 91.8 | 28.4 | 47–166 |
| LVEF, % | 46.3 | 13.3 | 22–66 |
| RVEDV, mL | 151.9 | 52.0 | 78–305 |
| RVEDV, mL/m2 | 74.0 | 19.9 | 48–123 |
| RVEF, % | 52.0 | 11.5 | 26–65 |
LVEDV indicates left ventricular end‐ diastolic volume; LVEF, left ventricular ejection fraction; RVEDV, right ventricular end‐diastolic volume; and RVEF, right ventricular ejection fraction.
All CMR examinations in patients with TPPM were performed safely without any adverse events. No clinically significant pacing lead parameter changes were detected after the CMR examinations. On average, the time from TPPM placement to the CMR scan was 2.6±1.9 days (range, 0–6 days). The data on TPPM generators, active fixation pacing leads, and venous access used on TPPM implantation are provided in Table 2. One patient had a TPPM generator placed on the left side of the neck, while the rest had the generator placed on the right side on the neck or pectoral or shoulder area. One CMR study was performed without contrast agent because the patient was pregnant. The artifacts caused by the TPPM generator did not interfere with the myocardium in cine or LGE sequences (Figures 2 and 3). Only the pacing leads induced mainly minor artifacts to the septal segments of the myocardium (segments 2, 3, 8, 9, and 14, as described in the American Heart Association 17‐segment model) in both cine and LGE sequences (Table 3, Figures 2 and 3).
Table 2.
Generator Model, Pacing Lead Modes, and Venous Access Used in TPPM Implantation
| TPPM generator model | n |
|---|---|
| Biotronik Effecta SR | 1 |
| Biotronik Enticos 4 DR | 1 |
| Biotronik Enticos 4 SR | 1 |
| Boston Scientific Altrua | 1 |
| Boston Scientific Essentio | 1 |
| Medtronic Adapta | 1 |
| St. Jude Medical (specific model not available) | 2 |
| St. Jude Medical Accent DR RF | 1 |
| St. Jude Medical Accent MRI 1224 | 2 |
| St. Jude Medical Assurity | 1 |
| St. Jude Medical Assurity MRI | 1 |
| Vitatron G20A1 SR | 1 |
| Vitatron 620 SR | 1 |
| N/A | 2 |
| Total | 17 |
| TPPM pacing lead model | |
| St. Jude Medical Tendril STS2088 TC 58 cm | 10 |
| N/A | 7 |
| Total | 17 |
| TPPM venous access | |
| Right internal jugular vein | 13 |
| Right axillary vein | 3 |
| Left internal jugular vein | 1 |
| Total | 17 |
N/A indicates not available; and TPPM, temporary permanent pacemaker.
Figure 2. Presentation of cardiac magnetic resonance image quality (IQ) in patients with temporary permanent pacemaker.
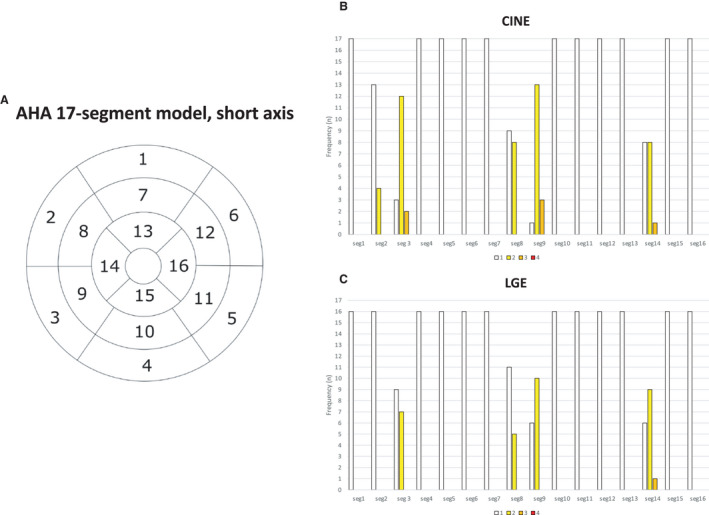
IQ was evaluated by segments according to the 17‐segment model (AHA, American Heart Association, A) IQ in the short axis plane in cine (B) and late‐gadolinium enhancement (LGE; C) sequences are presented. The frequency of each IQ grade per segment is presented on the bar charts. 1=very good IQ, no artifacts affecting cardiac anatomy; 2=good/average IQ, artifacts slightly interfering with cardiac anatomy; 3=below‐average IQ, artifacts moderately affecting cardiac anatomy; 4=poor IQ, artifacts severely affecting cardiac anatomy.
Figure 3. Example of cardiac magnetic resonance images with temporary permanent pacemaker.
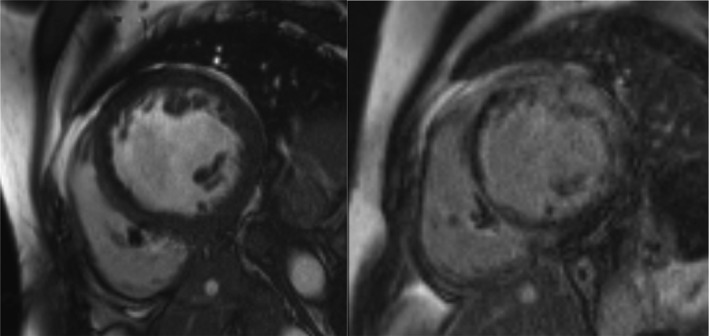
Cine sequence on the left and late gadolinium enhancement (LGE) sequence on the right. In both images, scarce artifacts are visible in septal segment 9 caused by pacing lead (image quality category 2=good/average in segment 9). In LGE images, extensive subepicardial and intramyocardial high signal intensity is present in left ventricular wall with mostly sparing of the subendocardium and direct continuity to right ventricle free wall. These LGE findings are suggestive of cardiac sarcoidosis.
Table 3.
Selected Image Quality Statistics of the Cardiac Magnetic Resonance Images
| N | Mean | SD | Range | |
|---|---|---|---|---|
| TPPM generator distance from myocardium (cm) | 17 | 12.5 | 2.9 | 6.1–17.8 |
| SA cine pacing lead tip artifact area (cm2) | 17 | 2.0 | 0.7 | 1.0–3.3 |
| SA LGE pacing lead tip artifact area (cm2) | 16 | 1.8 | 0.7 | 0.7–3.4 |
LGE indicates late gadolinium enhancement; SA, short axis; and TPPM, temporary permanent pacemaker.
Reduced left ventricular ejection fraction with LGE or LGE alone in CMR was used to decide whether to perform EMB. EMB based on CMR findings was performed on 11 patients. One EMB was performed on a patient with ventricular arrhythmias and AVB. This patient did not have visually detectable LGE in CMR. In this case, FDG‐PET was highly suggestive of cardiac inflammation (Table 4). Of these 12 patients, 4 (33.3%) had histology that was positive for cardiac sarcoidosis (CS) and 1 for giant cell myocarditis (GCM). If EMB was negative, we performed FDG‐PET to detect any extracardiac sign of inflammatory disease. Thus, 3 cases were diagnosed with CS from FDG‐PET–positive mediastinal lymph node histologic samples. Altogether, 47.1% of the patients were diagnosed with histologically confirmed granulomatous inflammatory cardiac disease: 7 patients with CS and 1 patient with GCM. Additionally, 1 patient was highly suspected of having CS on the basis of LGE in CMR and FDG‐PET–positive mediastinal lymph nodes, but EMB from the right ventricle was nondiagnostic and the patient refused mediastinoscopy and remained without histologic confirmation of sarcoidosis.
Table 4.
Results of Cardiac Sarcoidosis Screening Tests (CMR, EMB, FDG‐PET), Clinical Diagnosis, and Permanent Pacing Device Type
| N | Age, y | Sex | LGE 5SD % | EMB histology | FDG‐PET suggestive of inflammation cardiac/extra‐cardiac | AVB DIAGNOSIS | CS/GCM histology cardiac/extra‐cardiac | Permanent CIED type |
|---|---|---|---|---|---|---|---|---|
| 1 | 53 | F | 19 | Granulomatous inflammation | YES/YES | CS | Cardiac | CRT‐D |
| 2 | 47 | F | 18 | Granulomatous inflammation | … | CS | Cardiac | CRT‐D |
| 3 | 39 | M | no LGE | No diagnostic findings | YES/NO | Idiopathic AVB | Pacemaker | |
| 4 | 31 | M | no LGE | … | NO/NO | Lyme myocarditis | Pacemaker | |
| 5 | 65 | M | 13 | Giant cell myocarditis | … | GCM | Cardiac | ICD |
| 6 | 71 | F | 36 | Granulomatous inflammation | … | CS | Cardiac | CRT‐D |
| 7 | 27 | M | 30 | Granulomatous inflammation | YES/YES | CS | Cardiac | CRT‐D |
| 8 | 47 | M | 11 | No diagnostic findings | NO/NO | DCM | ICD | |
| 9 | 60 | F | 9 | No diagnostic findings | YES/YES | CS | Extra‐cardiac (mediastinal lymph node, granulomatous inflammation) | ICD |
| 10 | 63 | F | 9 | … | … | Sjögren syndrome–related AVB | Pacemaker | |
| 11 | 31 | F | 35 | Findings suggestive of myocarditis | YES/YES | CS | Extra‐cardiac (mediastinal lymph node, granulomatous inflammation) | ICD |
| 12 | 49 | F | 31 | No diagnostic findings | YES/YES | Suspicion of CS | No histologic confirmation (axillary lymph node insufficient sample) | ICD |
| 13 | 32 | F | N/A | … | NO/NO | Pregnancy‐related AVB | Pacemaker | |
| 14 | 37 | M | no LGE | … | NO/NO | Reflex AVB | Pacemaker | |
| 15 | 47 | F | no LGE | … | … | Reflex AVB | Pacemaker | |
| 16 | 45 | M | 7 | No diagnostic findings | YES/NO | Undefinied cardiac disease | CRT‐D | |
| 17 | 69 | F | 7 | Reactive findings | YES/YES | CS | Extra‐cardiac (mediastinal lymph node, granulomatous inflammation) | CRT‐D |
| Frequency | F=10 | EMB taken=12 (70.6%) | YES=8/6 | CS+GCM=8 (47.1%) | Cardiac=5 | Pacemaker=6 (35.3%) | ||
| M=7 | Extra‐cardiac=3 | ICD=5 (29.4%) | ||||||
| CRT‐D=6 (35.3%) | ||||||||
| Mean | 47.8 | 18.7 | ||||||
| SD | ±14.0 | ±11.4 |
AVB indicates atrioventricular block; CIED, cardiac implantable electronic device; CMR, cardiac magnetic resonance imaging; CRT‐D, cardiac resynchronization therapy defibrillator; CS, cardiac sarcoidosis; DCM, dilated cardiomyopathy; EMB, endomyocardial biopsy; FDG‐PET, 18‐F‐fluorodeoxyglucose positron emission tomography; GCM, giant cell myocarditis; ICD, implantable cardiac device; LGE, late gadolinium enhancement; N/A, not available; and TPPM, temporary permanent pacemaker.
In patients with CS/GCM, 3 of 8 had an indication for secondary prevention of ventricular arrhythmias and 5 of 8 had a CIED with defibrillation function (implantable cardioverter‐defibrillator [ICD] or cardiac resynchronization therapy defibrillator) installed for primary prevention. In the patient group with CS/GCM who were followed up for 325 months after permanent CIED implantation, 2 patients with CS and 1 patient with GCM had a pacing device–treated ventricular tachycardia or ventricular fibrillation. No sustained ventricular tachycardia or ventricular fibrillation was noticed during follow‐up time 288 months after permanent CIED implantation in the patients not diagnosed with CS or GCM (n=9).
Discussion
Temporary floating pacing wires are not MRI safe; consequently, CMR in patients requiring urgent pacing for severe bradycardia has not been possible. 6 , 8 According to European Society of Cardiology guidelines on cardiac pacing, patients with early‐onset AVB requiring a pacemaker should be investigated with advanced cardiac imaging to detect underlying myocardial disease. 2 In early‐onset AVB, CS is probably the most common myocardial disease. 12 We describe a CMR with TPPM‐based screening protocol for myocardial disease in patients with bradycardic AVB requiring urgent continuous pacing. We observed no adverse effect on the heart by CMR. Pacing leads caused minor artifacts in the septal segments in both cine and LGE sequences. Lack of LGE, normal wall structure, and normal left ventricular ejection fraction excluded the possibility of cardiac inflammatory disease in all but 1 patient. If CS was suspected, EMB was performed for histologic verification. If the EMB was negative for granulomatous inflammation, FDG‐PET was performed, and extracardiac histologic diagnosis was searched as CS extracardiac disease by imaging is seen in >70% of the patients. 13 With this diagnostic protocol, we identified granulomatous inflammatory cardiac disease in 47.1% of patients: 7 cases with CS and 1 with GCM. In all but in 1 case, CS presented with cardiac symptoms without any prior history of sarcoidosis. Thus, histologic confirmation of granulomatous inflammation was necessary. Treatment for CS, an arrhythmogenic inflammatory cardiomyopathy, comprises an ICD and immunosuppressive therapy. 14 Patients with CS presenting with AVB are at risk for life‐threatening ventricular arrhythmias and sudden cardiac death in addition to being at risk for heart failure. The risk of sudden cardiac death is 9% to 14% if AVB is initially accompanied by no arrhythmias and no or mild to moderate left ventricular dysfunction only. 1 As supported by the Heart Rhythm Society’s recommendation, we installed prophylactic ICD for all patients with CS requiring pacemaker therapy. 15
All CMR examinations conducted on patients with TPPM were performed safely without any adverse events, and the IQ remained diagnostic in all CMR examinations despite the presence of the TPPM. A few studies have reported successfully performing MRI examinations on patients with TPPM. 3 , 16 , 17 , 18 To our knowledge, no studies of CMR safety and IQ in patients with TPPM have been published. Recently, several studies have shown that MRI in patients with permanent CIED and endocardial pacing leads is safe, and possible adverse events, such as alteration in CIED function, heating of the pacing lead, or abnormal sensations during the scanning, are rare if the dedicated safety protocol is used. 3 , 4 , 5 The heating effect on active fixated pacing leads caused by MRI has been shown to be minor, whereas traditional temporary pacemakers with floating pacing leads are still considered an absolute contraindication for MRI because of safety hazards concerning especially heating of the lead tip in ex vivo models. 8 , 19 With recently implanted CIEDs (<6 weeks), an elevated risk for lead displacement unrelated to MRI exists. 20 Thus, published safety protocols and guidelines for MRI in patients with a CIED include a limitation on CIED system implantation duration before MRI. 3 , 18 A few reports of MRI scans conducted on patients with a newly implanted CIED have been published, and no safety hazards have been noted. 3 , 21 In this study, all CMRs were performed on patients with newly implanted TPPM systems, and no signs of possible deleterious effects, such as clinically significant pacing parameter changes, pacing lead displacement, or CIED malfunction, were detected. Our findings are in line with the limited previously published data in this particular setting. 3 , 16 , 17 , 18
We observed scarce susceptibility artifacts attributable to the pacing leads in septal segments of the myocardium in both cine and LGE sequences, whereas the TPPM generator had no effect on IQ. In our experience, these small local artifacts on the septum did not significantly affect the diagnostic interpretation of CMR. Earlier reports have shown that especially ICDs and cardiac resynchronization therapy defibrillators placed on the left side are likely to cause significant artifacts on CMR images, reducing the diagnostic value of the CMR. 6 , 7 , 22 Patients with a right‐sided pacemaker generator have fewer artifacts on CMR than patients with a left‐sided pacemaker generator because of greater distance between the generator and the heart. 7 A TPPM active fixation pacing lead is usually inserted through the right internal jugular vein, and the generator is located on the right side of the neck or shoulder area. 9 Also, the left internal jugular vein and right axillary vein are possible venous access routes for TPPM implantation, when the right internal jugular vein is not accessible, for example, because of the presence of a central vein catheter. However, with all venous access routes, the TPPM generator locates extracorporeally and in a greater distance to the heart than permanently implanted generators. Thus, the TPPM approach expectedly provides fewer artifacts interfering with the myocardium on CMR than permanent CIEDs.
This study is limited by the retrospective observational study design. The data from CIED interrogation values were not retrospectively available. According to the institutional safety protocol, only clinically significant changes in CIED parameters were entered in the electronic medical record. Thus, statistical analysis of CIED parameter changes was not possible. The patient cohort is limited in size, but to our knowledge this is the largest published patient cohort of CMR safety and IQ in patients with a TPPM. Additionally, the study population was selected from patients who underwent CMR with a TPPM, and this population does not necessarily represent the full variability of patients presenting with high‐degree AVB. Our study did not contain a comparative arm without CMR. These factors limit the large‐scale generalizability of the results.
We described a CMR‐based screening protocol for myocardial disease in patients who require emergency pacing for their AVB. We found CMR in patients with a TPPM to be safe and to provide images of diagnostic value. Diagnostic procedures revealed histologic evidence for granulomatous inflammation in 8 of 17 patients (47.1%). AVB attributable to CS can appear deceptively harmless, but it is associated with a significant risk for sudden cardiac death and heart failure.
Sources of Funding
This study was supported by a HUS Medical Imaging Center research grant (A.V.) and by University of Helsinki (Open Access Article Publication Charge payment).
Disclosures
None.
Acknowledgments
The authors thank the colleagues and staff at Meilahti Helsinki University Hospital for their contributions to this study. The authors especially thank Pauli Hyvönen for assistance with figures.
For Sources of Funding and Disclosures, see page 8.
See Editorial by Wald et al.
References
- 1. Nordenswan H‐K, Lehtonen J, Ekström K, Kandolin R, Simonen P, Mäyränpää M, Vihinen T, Miettinen H, Kaikkonen K, Haataja P, et al. Outcome of cardiac sarcoidosis presenting with high‐grade atrioventricular block. Circ Arrhythm Electrophysiol. 2018;11:e006145. doi: 10.1161/CIRCEP.117.006145 [DOI] [PubMed] [Google Scholar]
- 2. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, Barrabés JA, Boriani G, Braunschweig F, Brignole M, et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:3427–3520. doi: 10.1093/eurheartj/ehab364 [DOI] [PubMed] [Google Scholar]
- 3. Vuorinen A‐M, Pakarinen S, Jaakkola I, Holmström M, Kivistö S, Kaasalainen T. Clinical experience of magnetic resonance imaging in patients with cardiac pacing devices: unrestricted patient population. Acta Radiol. 2019;60:1414–1421. doi: 10.1177/0284185119830288 [DOI] [PubMed] [Google Scholar]
- 4. Russo RJ, Costa HS, Silva PD, Anderson JL, Arshad A, Biederman RW, Boyle NG, Frabizzio JV, Birgersdotter‐Green U, Higgins SL, et al. Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N Engl J Med. 2017;376:755–764. doi: 10.1056/NEJMoa1603265 [DOI] [PubMed] [Google Scholar]
- 5. Nazarian S, Hansford R, Rahsepar AA, Weltin V, McVeigh D, Gucuk Ipek E, Kwan A, Berger RD, Calkins H, Lardo AC, et al. Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med. 2017;377:2555–2564. doi: 10.1056/NEJMoa1604267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Symons R, Zimmerman SL, Bluemke DA. CMR and CT of the patient with cardiac devices. JACC Cardiovasc Imaging. 2019;12:890–903. [DOI] [PubMed] [Google Scholar]
- 7. Kaasalainen T, Kivisto S, Holmstrom M, Peltonen J, Pakarinen S, Hanninen H, Sipila O. Cardiac MRI in patients with cardiac pacemakers: practical methods for reducing susceptibility artifacts and optimizing image quality. Acta Radiol. 2016;57:178–187. doi: 10.1177/0284185115574873 [DOI] [PubMed] [Google Scholar]
- 8. Achenbach S, Moshage W, Diem B, Bieberlea T, Schibgilla V, Bachmann K. Effects of magnetic resonance imaging on cardiac pacemakers and electrodes. Am Heart J. 1997;134:467–473. doi: 10.1016/S0002-8703(97)70083-8 [DOI] [PubMed] [Google Scholar]
- 9. Suarez K, Banchs JE. A review of temporary permanent pacemakers and a comparison with conventional temporary pacemakers. J Innov Card Rhythm Manag. 2019;10:3652–3661. doi: 10.19102/icrm.2019.100506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kovach C, Swirka M, Mcguinn E, Honce JM, Groves DW, Tumolo AZ. Magnetic resonance imaging in a patient with temporary external pacemaker. HeartRhythm Case Rep. 2020;6:637–640. doi: 10.1016/j.hrcr.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schulz‐Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff‐Brenkenhoff F, Kramer CM, Pennell DJ, et al. Standardized image interpretation and post‐processing in cardiovascular magnetic resonance ‐ 2020 update. J Cardiovasc Magn Reson. 2020;22. doi: 10.1186/s12968-020-00610-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kandolin R, Lehtonen J, Kupari M. Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle‐aged adults. Circ Arrhythm Electrophysiol. 2011;4:303–309. doi: 10.1161/CIRCEP.110.959254 [DOI] [PubMed] [Google Scholar]
- 13. Simonen P, Lehtonen J, Kandolin R, Schildt J, Marjasuo S, Miettinen H, Airaksinen J, Vihinen T, Tuohinen S, Haataja P, et al. F‐18‐fluorodeoxyglucose positron emission tomography‐guided sampling of mediastinal lymph nodes in the diagnosis of cardiac sarcoidosis. Am J Cardiol. 2015;116:1581–1585. doi: 10.1016/j.amjcard.2015.08.025 [DOI] [PubMed] [Google Scholar]
- 14. Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, Kaikkonen K, Tuohinen S, Haataja P, Kerola T, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–632. doi: 10.1161/CIRCULATIONAHA.114.011522 [DOI] [PubMed] [Google Scholar]
- 15. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043 [DOI] [PubMed] [Google Scholar]
- 16. Chaudhry U, Svensson J, Mosen H, Mortsell D. Cardiac magnetic resonance imaging in a patient with temporary external pacemaker: a case report. Eur Heart J Case Rep. 2019;3:1–4. doi: 10.1093/ehjcr/ytz228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGuinn EM, Bhatia N, O'Leary JM, Crossley GH, Rottman JN. Emergent use of an MRI‐conditional external pacemaker in a patient with sinus arrest facilitating diagnosis of a temporal lobe neoplasm. HeartRhythm Case Rep. 2016;2:296–299. doi: 10.1016/j.hrcr.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller JD, Nazarian S, Halperin HR. Implantable electronic cardiac devices and compatibility with magnetic resonance imaging. J Am Coll Cardiol. 2016;68:1590–1598. [DOI] [PubMed] [Google Scholar]
- 19. Muthalaly RG, Nerlekar N, Ge Y, Kwong RY, Nasis A. MRI in patients with cardiac implantable electronic devices. Radiology. 2018;289:281–292. doi: 10.1148/radiol.2018180285 [DOI] [PubMed] [Google Scholar]
- 20. Fuertes B, Toquero J, Arroyo‐Espliguero R, Lozano IF. Pacemaker lead displacement: mechanisms and management. Indian Pacing Electrophysiol J. 2003;3:231–238. [PMC free article] [PubMed] [Google Scholar]
- 21. Friedman HL, Acker N, Dalzell C, Shen WK, Asirvatham SJ, Cha Y‐M, Hodge D, Felmlee J, Watson R, Friedman PA. Magnetic resonance imaging in patients with recently implanted pacemakers. Pacing Clin Electrophysiol. 2013;36:1090–1095. doi: 10.1111/pace.12213 [DOI] [PubMed] [Google Scholar]
- 22. Mesubi O, Ahmad G, Jeudy J, Jimenez A, Kuk R, Saliaris A, See V, Shorofsky S, Dickfeld T. Impact of ICD artifact burden on late gadolinium enhancement cardiac MR imaging in patients undergoing ventricular tachycardia ablation. Pacing Clin Electrophysiol. 2014;37:1274–1283. doi: 10.1111/pace.12405 [DOI] [PubMed] [Google Scholar]


