Abstract
Background
The REHAB‐HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial showed that a novel, early, transitional, tailored, progressive, multidomain physical rehabilitation intervention improved physical function and quality of life in older, frail patients hospitalized for acute decompensated heart failure. This analysis examined the relationship between intervention adherence and outcomes.
Methods and Results
Adherence was defined as percent of sessions attended and percent of sessions attended adjusted for missed sessions for medical reasons. Baseline characteristics were examined to identify predictors of session attendance. Associations of session attendance with change in physical function (Short Physical Performance Battery [primary outcome], 6‐minute walk distance, quality of life [Kansas City Cardiomyopathy Questionnaire], depression, and clinical events [landmarked postintervention]) were examined in multivariate analyses. Adherence was 67%±34%, and adherence adjusted for missed sessions for medical reasons was 78%±34%. Independent predictors of higher session attendance were the following: nonsmoking, absence of myocardial infarction history and depression, and higher baseline Short Physical Performance Battery. After adjustment for predictors, adherence was significantly associated with larger increases in Short Physical Performance Battery (parameter estimate: β=0.06[0.03–0.10], P=0.001), 6‐minute walk distance (β=1.8[0.2–3.5], P=0.032), and Kansas City Cardiomyopathy Questionnaire score (β=0.62[0.26–0.98], P=0.001), and reduction in depression (β=−0.08[−0.12 to 0.04], P<0.001). Additionally, higher adherence was significantly associated with reduced 6‐month all‐cause rehospitalization (rate ratio: 0.97 [0.95–0.99], P=0.020), combined all‐cause rehospitalization and death (0.97 [0.95–0.99], P=0.017), and all‐cause rehospitalization days (0.96 [0.94–0.99], P=0.004) postintervention.
Conclusions
In older, frail patients with acute decompensated heart failure, higher adherence was significantly associated with improved patient‐centered and clinical event outcomes. These data support the efficacy of the comprehensive adherence plan and the subsequent intervention‐related benefits observed in REHAB‐HF.
Registration
URL: https://clinicaltrials.gov/; Unique identifier: NCT02196038.
Keywords: adherence, heart failure, physical function, quality of life, rehabilitation
Subject Categories: Heart Failure, Rehabilitation, Compliance/Adherence
Nonstandard Abbreviations and Acronyms
- 6MWD
6‐minute walk distance
- ADHF
Acute decompensated heart failure
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- SPPB
Short Physical Performance Battery
Clinical Perspective
What Is New?
High adherence was achieved during a physical rehabilitation intervention using tailored adherence strategies designed to address specific functional and social determinant of health needs of older, frail patients with acute decompensated heart failure and with multiple comorbidities.
High adherence was associated with greater improvements in physical function, quality of life, reduction in depression, and improved clinical event outcomes.
What Are the Clinical Implications?
The effectiveness of the physical rehabilitation intervention was attributable in large part to the high adherence accomplished in the intervention program.
Patient factors traditionally viewed as potential barriers to intervention adherence can be addressed with a priori strategic planning to ensure intervention fidelity, which is key to maximizing the benefits from a physical rehabilitation intervention, especially for older patients with serious illnesses like HF.
Patients with acute decompensated heart failure (ADHF) have severely impaired physical function, poor quality of life (QOL), and persistently high rates of rehospitalization and death. 1 The recently completed REHAB‐HF trial showed that an innovative, early, transitional, tailored, progressive physical rehabilitation intervention that addressed 4 physical function domains (strength, balance, mobility, and endurance) was feasible, safe, and effective in improving physical function, frailty, quality of life, and depression in a diverse population of older patients hospitalized for ADHF. 2
Adherence is critical to maximizing the plethora of potential benefits associated with exercise interventions. 3 , 4 In the HF‐ACTION (A Controlled Trial Investigating Outcomes of Exercise Training) trial of aerobic exercise in relatively young (mean age 59 years), stable outpatients with chronic heart failure with reduced ejection fraction, adherence rates were relatively low (only 40% self‐reported weekly training volumes at or above recommended threshold) and may have impacted overall study results. 5 Post hoc analyses showed that greater adherence was associated with improved exercise capacity and reduced risk for cardiovascular death and heart failure (HF) hospitalization. 6 Low adherence rates were associated with demographic factors, such as younger age, Black race, and male sex, as well as baseline measures of depression, low QOL, and low functional capacity. 5
In contrast, the REHAB‐HF population included those with ADHF, heart failure with preserved ejection fraction, 7 advanced age (mean 73 years), multiple comorbidities, 8 high rates of frailty, 9 severely impaired physical function, cognitive impairment, 10 and low QOL. 11 The intervention differed as well, addressing multiple domains of physical function, and being tailored to individuals’ specific deficits. The REHAB‐HF trial designed comprehensive adherence strategies that were consistent with the National Institutes of Health Behavior Change Consortium Treatment Fidelity Workgroup recommendations in order to specifically address potential adherence risks. 12 , 13 Although the mean adherence rate was relatively high, there was substantial variation, and the relationships between adherence to the intervention and outcomes in this high‐risk population are unknown.
The goal of this analysis was to examine the relationship between adherence to the REHAB‐HF intervention and key trial outcomes, as well as to identify specific baseline factors that were associated with adherence. We hypothesized that the greater adherence to the REHAB‐HF intervention, defined in terms of session attendance, would be associated with improved outcomes.
Methods
Trial Design
The data that support the findings of this study are available from the first author upon reasonable request. Details of trial design and the intervention have been previously described and primary trial results have been previously published. 2 , 13 , 14 Briefly, REHAB‐HF was a multisite study of a 12‐week, tailored, progressive, multidomain physical rehabilitation intervention. Participants were 349 patients aged ≥60 years, hospitalized ≥24 hours for ADHF, regardless of ejection fraction (EF). Participants were required to be independent and ambulatory before admission and be expected to be discharged home. Key exclusion criteria included acute myocardial infarction, end‐stage HF, estimated glomerular filtration rate ≤20 mL/min per 1.73 m2 or requiring dialysis, current participation in formal cardiac rehabilitation, and inability to participate because of dementia, stroke, or other disorder. The protocol was approved by the Institutional Review Board at each of the 7 clinical sites. All patients provided informed consent. After stabilization of ADHF and obtaining informed consent, participants underwent baseline testing and were randomized to the Rehabilitation Intervention or usual care Attention Control. Randomization was stratified by EF category and clinical site.
Intervention
The intervention began as inpatient, 1 session daily when possible, and continued as outpatient for 12 weeks, 3 d/wk, for a total of 36 sessions. The intervention was multidomain, targeting strength, balance, mobility, and endurance. Sessions were one‐on‐one (participant:interventionist), tailored to individuals’ deficits, and designed to progress through specific exercises to improve each of the 4 domains. At each session, participants were assessed across the 4 domains to systematically guide interventionists in the exercise progression.
After initial discharge from the hospital, each participant received a home visit from an interventionist to perform a home and built environment assessment. This was designed to reestablish the goals of the intervention; identify and address potential barriers to participating in the intervention, such as lack of transportation or social support from informal or formal caregivers; identify safe and accessible areas for exercise, including sturdy surfaces, quality roads and sidewalks, and community‐based resources such as exercise facilities and walking paths; and establish a home exercise regimen that could be safely implemented. The home exercise program consisted of light‐intensity walking and strengthening exercises, gradually increasing toward a goal of 30 minutes on nonfacility days.
After the 3‐month outpatient intervention and outcomes assessments, participants transitioned to a self‐guided maintenance phase. Interventionists prepared participants by developing a maintenance exercise prescription, inclusive of potential resources for continued exercise options in their home and/or built environment, such as local parks or fitness clubs, senior centers, Silver Sneakers, or Phase III cardiac rehabilitation (if indicated). Participants were followed with monthly telephone calls to assess exercise adherence until month 6 post index hospital discharge.
Adherence Strategies
As extensively described in several prior publications, an a priori comprehensive adherence plan with multiple strategies was implemented to maximize participant retention and adherence. 2 , 7 , 13 , 14 The plan, consistent with the National Institutes of Health Behavior Change Consortium Treatment Fidelity Workgroup recommendations, 12 was designed to address specific functional and social determinant of health needs of older, frail patients and are described in Table 1. 15 The Sustaining Participant Engagement Committee, which is a team of experts in physical therapy, exercise physiology, behavioral modification, and clinical trial management, provided oversight for intervention progression, retention, and adherence.
Table 1.
REHAB‐HF Adherence Plan
| Strategy | Implementation examples |
|---|---|
| Identify and address medical and social barriers |
|
| Engage social support |
|
| Communicate study expectations |
|
| Manage interruptions to the intervention |
|
| Monitor and report participant progress |
|
| Develop participant self‐efficacy |
|
SPEC indicates Sustaining Participant Engagement Committee.
Intervention Adherence Metrics
The prespecified measure of adherence to the intervention was intervention session attendance. The raw rate of adherence was defined as a percent of the 36 sessions attended by participants. Because of the high comorbidity burden, frequent rehospitalizations, and frequent medical appointments necessary for these participants, adherence was also calculated as a percent of scheduled sessions, where the numerator is the number of sessions attended and the denominator is 36 less the number of sessions missed for medical reasons. Missed sessions were tracked for all participants. Those that were missed because of rehospitalizations, acute illness, or conflicting medical appointments constituted missed sessions for medical reasons. Precedent for adjusting attendance for scheduled medical‐related visits in National Institute of Aging‐funded trials of older, frail participants was established in the LIFE‐Pilot and LIFE trials, the largest multisite physical function intervention in older adults that used Short Physical Performance Battery (SPPB) as primary outcome. 16 , 17 Participant retention was defined as the percentage of participants who did not prematurely discontinue the intervention (excluding death). Participants who died during the intervention were excluded from analysis.
Outcomes
Outcomes of physical function, QOL, depression, and cognition were assessed at baseline in the hospital after initial treatment and stabilization for ADHF, and at 3 months following discharge from the index hospitalization discharge. All assessments were conducted by study personnel blinded to randomized group assignment.
The primary outcome was the SPPB. The SPPB is a standardized, reproducible, global physical function measure that has been validated in older and frail populations and is predictive of a range of clinical outcomes. 18 , 19 , 20 It consists of 3 components: a standing balance test, a 4‐meter walk test, and a repeated chair‐stand test. Each component is scored on a scale of 0–4 with a total score of 0–12, with higher scores indicating better physical function.
Physical function was also assessed using gait speed (from the 4‐meter walk), and 6‐minute walk distance (6MWD). QOL was assessed using the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall score, on a scale of 0–100, with a higher score indicating better health status. 21 Depressive symptoms were assessed using the Geriatric Depression Scale‐15. 22 Cognition was assessed using the Montreal Cognitive Assessment. 23 Frailty was assessed using the Fried frailty phenotype, as previously described. 9 , 24 For this article, change in 3‐month outcomes from baseline was used for analysis.
Clinical events of rehospitalization, rehospitalization days, and death were collected through participant interview and electronic medical record through 6 months following discharge from index hospitalization. Since clinical events occurring during the intervention period could potentially affect adherence, overall adherence was calculated at the end of the intervention period. Therefore, only clinical events occurring in the postintervention period were included in the association analyses described below.
Statistical Analysis
These analyses were conducted in the intervention arm of the trial. Continuous data are presented as means±SD or frequency (percent). In all analyses, the number of sessions attended was chosen as the metric of adherence in order for associations to be described per session. General linear models were constructed to examine the bivariate relationships between number of sessions attended and baseline participant characteristics. Characteristics that were significant in bivariate analysis at a level of P<0.10 as well as number of missed sessions for medical reasons were entered into a regression model with backwards selection with number of sessions attended as the dependent variable to test for independent predictors of adherence. Spearman correlations were calculated to assess the relationship between adherence and change in 3‐month outcomes. To examine the adjusted relationship between session attendance and change in 3‐month outcomes, multiple linear regression models were constructed with change in 3‐month outcomes as the dependent variable, adjusting for age, sex, clinical site, EF category, baseline outcome measure, number of missed sessions for medical reasons, and significant independent predictors of adherence identified in the prior analyses. Results were reported as regression parameter estimates (βs) and 95% CIs. Model and partial R 2 were also presented to represent percent of variance explained. The relationship between session attendance and the total number of all‐cause rehospitalization, death, and combined all‐cause rehospitalization and death occurring in the postintervention period were assessed using generalized linear models with a Poisson distribution, adjusted for age, sex, EF category, clinical site, and significant independent predictors of adherence identified in the prior analyses. The relationship of session attendance with rehospitalization days occurring in the postintervention period were assessed using generalized linear models with a negative binomial distribution to account for overdispersion, adjusted for age, sex, EF category, clinical site, and significant independent predictors of adherence identified in the prior analysis. Results were reported as rate ratios and 95% CIs. A 2‐tailed P value of <0.05 was used to determine significance.
Results
Of the 349 participants enrolled in the REHAB‐HF trial, 175 were randomized to intervention. Intervention participants were 73.1±8.5 years, 49% female, 46% non‐White, and 53% heart failure with preserved ejection fraction, had high rates of previous hospitalizations (43%) and comorbidities (mean: 5.3±2.0), and 53% were frail. Physical function and QOL were severely impaired, with an average SPPB score of 6.0±2.8, 6MWD of 194±104 meters, and a KCCQ score of 40±21 (Table 2). The median time from index hospital discharge to initiation of outpatient exercise sessions was 10 days (interquartile range: 6–13).
Table 2.
Baseline Characteristics of Rehabilitation Intervention Participants and Bivariate Associations with Session Attendance
| Characteristics | N=175 | Parameter estimate (95% CI) | P value |
|---|---|---|---|
| Age (y) | 73.1±8.5 | 0.8 (−0.1 to 0.3) | 0.50 |
| Women | 85 (49%) | −2.5 (−6.2 to 1.3) | 0.19 |
| Non‐White | 81 (46%) | 2.1 (−1.6 to 5.8) | 0.27 |
| BMI (kg/m2) | 32.9±8.2 | 0.0 (−0.2 to 0.2) | 0.94 |
| Preserved ejection fraction (≥45%) | 93 (53%) | 2.1 (−1.6 to 5.9) | 0.26 |
| Days hospitalized at index hospitalization, median (IQR) | 4 (3–7) | −0.1 (−0.6 to 0.4) | 0.82 |
| Patients with previous hospitalizations | 76 (43%) | −2.5 (6.3 to 1.2) | 0.19 |
| Smoking | 17 (10%) | −6.7 (−12.9 to −0.4) | 0.036 |
| Alcohol abuse | 7 (4%) | 6.1 (−3.4 to 15.6) | 0.21 |
| ≥ High school education | 140 (80%) | 1.9 (−2.8 to 6.5) | 0.43 |
| Live with spouse or partner | 67 (38%) | 2.0 (−1.9 to 5.8) | 0.32 |
| Comorbidities (N) | 5.4±2.0 | −0.4 (−1.3 to 0.5) | 0.38 |
| Hypertension | 159 (91%) | 0.8 (−5.7 to 7.3) | 0.80 |
| History of myocardial infarction | 31 (18%) | −5.9 (−10.8 to −1.1) | 0.015 |
| History of coronary revascularization | 55 (31%) | 0.6 (−3.4 to 4.6) | 0.77 |
| Atrial fibrillation | 89 (51%) | 2.0 (−1.8 to 5.7) | 0.30 |
| Diabetes | 103 (59%) | 0.4 (−3.4 to 4.2) | 0.82 |
| Hyperlipidemia | 110 (63%) | −3.1 (−6.9 to 0.7) | 0.11 |
| Depression | 29 (17%) | −4.6 (−9.6 to 0.4) | 0.071 |
| Dementia or cognitive impairment | 6 (3%) | 1.7 (−8.5 to 12.0) | 0.74 |
| Urinary incontinence* | 19 (13%) | −1.2 (−7.0 to 4.6) | 0.68 |
| Patients with falls in last 3 months † | 24 (17%) | −1.6 (−6.9 to 3.8) | 0.57 |
| Baseline assessments | |||
| SPPB score | 6.0±2.8 | 0.62 (−0.04 to 1.28) | 0.067 |
| 6MWD (m) | 194±104 | 0.19 (0.01 to 0.37) ‡ | 0.041 |
| KCCQ overall score | 40±21 | 0.08 (−0.01 to 0.17) | 0.070 |
| GDS‐15 score | 4.7±3.3 | −0.3 (−0.9 to 0.3) | 0.30 |
| MoCA score | 21.9±4.2 | 0.1 (−0.4 to 0.5) | 0.71 |
| Frail (≥3 frailty criteria) | 92 (53%) | 2.2 (−1.6 to 5.9) | 0.26 |
| Number of frailty criteria | 2.5±1.1 | −1.4 (−3.0 to 0.3) | 0.11 |
Data presented as N (%) or mean±SD, unless otherwise indicated. Parameter estimates shown as association with number of intervention sessions attended. 6MWD indicates 6‐minute walk distance; BMI, body mass index; GDS‐15, Geriatric Depression Scale; IQR, interquartile range; KCCQ, Kansas City Cardiomyopathy Questionnaire; MoCA, Montreal Cognitive Assessment; and SPPB, Short Physical Performance Battery.
N assessed=144.
N assessed=143.
Per 10‐meter difference.
Of the participants randomized to intervention, 163 were alive at 3‐month follow‐up (N=12 died before 3‐month follow‐up, completing an average of 11 sessions, and were not included in analyses). 2 Average sessions attended were 24±12, for an adherence rate of 67%±34% (Table 3). Participants missed an average of 15.4±12.2 sessions during the 3‐month intervention, with 30% (N=829) being because of medical reasons, including frequent rehospitalizations during the intervention period (total of 97 rehospitalizations among 58 participants, Table S1). When accounting for missed sessions because of illness, medical appointments, and rehospitalizations, the adherence rate was 78%±34%. Furthermore, adherence rates were even higher for participants who did not discontinue the intervention (N=133) to an average of 81%±22% (or 88%±18% when adjusted for missed sessions for medical reasons).
Table 3.
Adherence to the Rehabilitation Intervention
| Intervention participants | Number of patients | Average sessions attended | Adherence rate (% of 36 sessions) | Medically adjusted adherence (% of scheduled sessions) |
|---|---|---|---|---|
| Alive at 3‐mo follow‐up | 163 | 24.3±12.4 | 67.4±34.4 | 75.0±33.9 |
| Alive with primary outcome | 149 | 26.1±11.0 | 72.6±30.7 | 79.8±29.6 |
| Alive and completing intervention (did not prematurely discontinue) | 133 | 29.0±7.8 | 80.5±21.6 | 88.2±17.7 |
Data presented as N or mean±SD.
In general linear models, nonsmoking, absence of history of myocardial infarction, absence of depression, and higher baseline SPPB, 6MWD, and KCCQ score were positively associated with session attendance (Table 2). Additionally, there were nonsignificant trends for associations between hyperlipidemia, depression, and low QOL, and greater number of frailty criteria with lower adherence. Number of missed sessions for medical reasons was also associated with adherence (β=−0.68[−0.93, −0.44], P<0.001). In multivariate regression analyses, number of missed sessions for medical reasons had the biggest impact on session attendance (β [95% CI]=−0.77 [−1.00 to −0.55], P<0.001), partial R 2=0.18, representing 18% of the variance in adherence not explained by other predictors. Other independent predictors of session attendance included history of myocardial infarction (−7.2 [−11.3, −3.1], P<0.001) and depression (−4.2 [−8.6,0.06], P=0.053), smoking (−6.9 [−12.4, −1.4], P=0.014), and baseline SPPB (0.57 [0.01,1.14], P=0.046) (Table 4).
Table 4.
Independent Predictors of Session Attendance
| Predictors | Parameter estimate (95%CI) | Partial R 2 | P value |
|---|---|---|---|
| Missed sessions for medical reasons | −0.77 (−1.00 to −0.55) | 0.18 | <0.001 |
| Myocardial infarction | −7.2 (−11.3 to −3.1) | 0.07 | <0.001 |
| Depression | −4.2 (−8.6, to 0.1) | 0.03 | 0.053 |
| Smoking | −6.9 (−12.4 to −1.4) | 0.03 | 0.014 |
| Baseline SPPB score | 0.57 (0.01 to 1.14) | 0.02 | 0.046 |
| Baseline 6MWD | (removed from model) | ||
| Baseline KCCQ overall score | (removed from model) |
Variables entered into model included all variables with bivariate association with sessions attended at a P<0.1 level of significance. Variables removed from model did not achieve statistical significance after backwards selection. 6MWD indicates 6‐minute walk distance; KCCQ, Kansas City Cardiomyopathy Questionnaire; and SPPB, Short Physical Performance Battery.
Higher session attendance was significantly associated with larger improvements in SPPB, gait speed, 6MWD, KCCQ, and depressive symptoms by the Geriatric Depression Scale (Table 5). These associations remained significant after adjustments for potential confounders and covariates of baseline outcome measure, age, sex, EF category, clinical site, and number of missed sessions for medical reasons, myocardial infarction, depression, smoking, and baseline SPPB.
Table 5.
Associations of Session Attendance with Change in 3‐Month Outcomes
| 3‐Month outcome | Correlations | Multivariate associations* | ||||
|---|---|---|---|---|---|---|
| r | P value | Model R 2 |
Parameter estimate (95% CI) |
Partial R 2 | P value | |
| Δ SPPB score | 0.22 | 0.008 | 0.35 | 0.06 (0.03 to0.10) | 0.16 | 0.001 |
| Δ Gait speed (m/s) | 0.23 | 0.004 | 0.27 | 0.004 (0.001 to0.008) | 0.08 | 0.012 |
| Δ 6MWD (m) | 0.24 | 0.007 | 0.25 | 1.8 (0.2 to3.5) | 0.06 | 0.032 |
| Δ KCCQ overall score | 0.24 | 0.004 | 0.42 | 0.62 (0.26 to0.98) | 0.07 | 0.001 |
| Δ MoCA score | 0.03 | 0.76 | 0.01 | 0.01 (−0.05 to −0.09) | 0.00 | 0.63 |
| Δ GDS‐15 score | −0.20 | 0.018 | 0.41 | −0.08 (−0.12 to −0.04) | 0.07 | <0.001 |
Data presented for all participants with follow‐up measure. 6MWD indicates 6‐minute walk distance; GDS‐15, Geriatric Depression Scale; KCCQ, Kansas City Cardiomyopathy Questionnaire; MoCA, Montreal Cognitive Assessment; and SPPB, Short Physical Performance Battery.
Adjusted for age, sex, clinical site, ejection fraction category, baseline measure, number of missed medical sessions, myocardial infarction, depression, smoking, and baseline SPPB score.
Session attendance was also significantly associated with a decreased rate of 6‐month all‐cause rehospitalization in the 3 months following intervention (0.97 [0.96–0.99], P=0.020), combined all‐cause rehospitalization and death (0.98 [0.96–1.00], P=0.026), and all‐cause rehospitalization days (rate ratio, 0.96 [0.94–0.99], P=0.002) during the postintervention (Table 6). There was no association between session attendance and all‐cause death (N=9).
Table 6.
Multivariable Associations of Session Attendance with 6‐Month Clinical Event Outcomes Postintervention Period
| Clinical event outcome |
Parameter estimate (95% CI) |
P value |
|---|---|---|
| All‐cause rehospitalizations after intervention | 0.97 (0.95–0.99) | 0.020 |
| All‐cause death after intervention | 0.95 (0.88–1.39) | 0.27 |
| Combined all‐cause rehospitalization and death after intervention | 0.97 (0.95–0.99) | 0.017 |
| All‐cause rehospitalization days after intervention | 0.96 (0.94–0.99) | 0.004 |
Adjusted for age, sex, clinical site, ejection fraction category, number of missed medical visits, myocardial infarction, depression, smoking, and baseline Short Physical Performance Battery score.
Although we have previously reported trends toward evidence of heterogeneity by EF subgroup, 7 there was no significant interaction by EF subgroup for relation between adherence and outcomes.
Discussion
This report examined the relationship between adherence to an innovative, transitional, tailored, progressive, multidomain physical rehabilitation intervention and physical function, QOL, and clinical event outcomes in an older, frail, high‐risk population of patients with ADHF. Despite frailty, multimorbidity, and barriers to exercise, REHAB‐HF attained a high rate adherence of 78% adjusted for missed sessions for medical reasons. Independent predictors of higher adherence included nonsmoking, absence of history of myocardial infarction, absence of depression, higher baseline SPPB, and fewer missed sessions for medical reasons. Higher adherence to the intervention was significantly associated with greater improvements in multiple patient‐important measures including physical function, HF‐specific QOL, and depressive symptoms. Notably, higher adherence was also associated with lower rates of clinical event outcomes, including all‐cause hospitalizations and combined rehospitalizations and death following the intervention period. These associations remained even after adjustment for participant characteristics that independently predicted adherence. These findings support the efficacy of the adherence strategies implemented, the intervention‐related benefits demonstrated in the REHAB‐HF trial, and the importance of maintaining high adherence in exercise interventions for older patients with ADHF.
Although several factors had bivariate associations with session attendance, relatively few patient characteristics appeared to be independently associated with session attendance in multivariate analyses. Notably, factors such as age, sex, race, as well as cognitive dysfunction, living alone, and alcohol abuse were not associated with session attendance. Unsurprisingly, the strongest factor influencing session attendance was missed visits for medical reasons, explaining 18% of the variance in adherence not explained by other factors, while only a small percent of attendance was explained by patient‐specific baseline factors. The association between a medical history of myocardial infarction, and the nonsignificant trend of hyperlipidemia suggests a potential atherosclerotic heart disease link. Other independent predictors of adherence such as nonsmoking (which is itself a health behavior), higher physical function, and absence of depression are in agreement with prior studies. For example, in HF‐ACTION, patients with poor adherence were more likely to be young, female, Black, have higher body mass index, more severe baseline New York Heart Association class, depression, and lower baseline peak oxygen capacity and KCCQ. 5 While our findings suggest some overlap of factors influencing adherence, including physical function and depression, our study did not identify demographic factors as predictors of adherence. This is in agreement with the LIFE‐Pilot (Lifestyle Interventions and Independence for Elders Pilot Study) study, which also found that a small amount of the variance in adherence was explained by demographic factors, and that proactive strategies for addressing nonadherence are essential for successful interventions. 25 These results suggest that relatively strong adherence observed in the REHAB‐HF trial may have been because of the comprehensive and targeted strategies designed to promote adherence.
These results also highlight the importance of adherence and its relation to efficacy in exercise interventions, particularly in HF populations and older adults. In the HF‐ACTION study of stable patients with heart failure with reduced ejection fraction, adherence rates were low with only ≈40% of patients reporting targeted training volumes despite strenuous adherence and retention efforts. 6 Despite low adherence to the HF‐ACTION intervention in an overall positive trial, adherence to the exercise program yielded significantly greater benefit in peak oxygen consumption in post hoc analyses. 6 In the EJECTION‐HF (Exercise Joins Education: Combined Therapy to Improve Outcomes in Newly‐Discharged Heart Failure) study of patients with recent ADHF, adherence was quite low: only 43% of participants attended at least 50% of scheduled sessions, and may have contributed to a neutral trial result of reducing death and rehospitalization. 26 In contrast, adherence rates in the LIFE (Lifestyle Interventions and Independence for Elders) trial and its pilot study of older adults at risk of mobility disability were higher, at 63% of scheduled sessions adjusting for missed sessions for medical reasons, 16 and ranged 50%–76% in the LIFE‐Pilot study. 17 In post hoc analyses, the LIFE‐Pilot study demonstrated associations between higher adherence to a physical activity intervention and greater improvement in SPPB score of 1.4 units in participants achieving ≥150 min/wk of moderate activity. 17
In REHAB‐HF, high adherence was associated with multiple important outcomes. These included larger improvements in multiple patient‐centered outcome measures including physical function by the SPPB and 6MWD, QOL as assessed by the KCCQ, and reduced depressive symptoms by the Geriatric Depression Scale. Notably, all‐cause rehospitalization, combined all‐cause rehospitalization and death, and days rehospitalized were reduced by rates of 3%, 3%, and 4% per additional session attended, respectively. Collectively, these findings provide strong support for a dose–response relationship between adherence to the REHAB‐HF intervention and improved outcomes and support the benefits observed in the intervention participants. Our findings also significantly extend prior studies in multiple ways, by demonstrating the effectiveness of a comprehensive adherence plan in a cohort that was older and frail, with broad functional impairments, and burdened by multiple comorbid conditions, resulting in high adherence rates during a robust and effective physical rehabilitation intervention.
What may have produced these high rates of adherence demonstrated in the REHAB‐HF trial? First, by design, and with recognition that adherence is crucial to the impact of a behavioral intervention, the REHAB‐HF trial was equipped with several mechanisms and strategies (in accord with the National Institutes of Health Behavior Change Consortium Treatment Fidelity Workgroup recommendations) to enhance adherence both before participant enrollment and in real‐time after participant enrollment (Figure). 12 , 13 , 14 As demonstrated by the high numbers of rehospitalizations during the intervention (33% of participants) and missed sessions for medical reasons, a comprehensive a priori plan was essential in promoting adherence in patients with ADHF. Second, the individualized, tailored intervention was designed specifically for patients with ADHF being discharged from the hospital with severely impaired physical function, frailty, cognitive dysfunction, and multiple comorbidities, who have been typically excluded from traditional exercise programs and studies. 27 , 28 , 29 By addressing the needs of older patients with ADHF, targeting deficits across strength, balance, mobility, and endurance domains, the intervention was effective at improving multiple outcomes. 2 Third, it is likely that for these frail, sick patients, being aware of their deficits and their progression in the intervention may have enhanced their motivation and adherence. This is supported by the vast improvements in HF‐related QOL and physical function.
Figure . Increased adherence rate in REHAB‐HF was the combined effect of a comprehensive adherence plan, a robust and effective intervention designed to target specific deficits in ADHF, and subjective patient awareness of improvement.
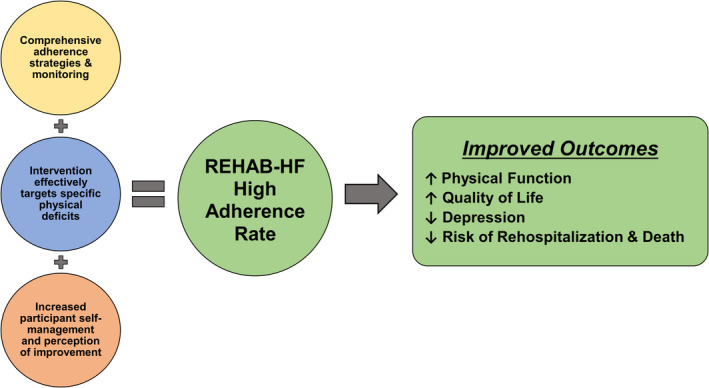
Higher adherence was related to greater improvements in physical function, quality of life, reduced depression, and reduced clinical events. ADHF indicates acute decompensated heart failure.
There are several clinical implications based on these findings. First, the effectiveness of this intervention was attributable in large part to the high adherence accomplished in the intervention program. Second, our data demonstrate that patient factors traditionally viewed as potential barriers to intervention adherence can be addressed with a priori strategic planning and implementation. Further individualization of those strategies in real‐time to address participant‐specific functional deficits and intervention barriers is a key to maximizing the benefits from a physical rehabilitation intervention, especially for patients with HF.
This study has several strengths. Participants were well‐phenotyped, included both heart failure with preserved ejection fraction and heart failure with reduced ejection fraction, were older, frail, and diverse with ≈50% female and minority representation. The multisite design, which included both community and tertiary care sites, increases the generalizability of our findings. We also implemented a highly novel intervention with comprehensive, research‐based strategies to enhance adherence. Finally, we prospectively collected detailed adherence data, allowing for analyses between adherence and outcomes.
However, there are several limitations. The sample size may be insufficient for a complete analysis of independent predictors of adherence. While we took care to systematically identify characteristics that affected adherence and other potential confounders, it is possible that our small sample size could have missed factors that are explanatory of adherence. There may be unmeasured variables that were confounders to these analyses. Because of heterogeneity in design and implementation of the interventions and how adherence is assessed in trials, direct comparisons of adherence numbers must be interpreted with caution. Additionally, clinical event outcome data are limited by relatively short follow‐up time of only 6 months posthospitalization. Finally, while it may be desirable to examine adherence in low versus high groups, our sample size was insufficient to do this with adequate power. While we cannot establish an adherence threshold for benefit, our data strongly support that greater adherence is associated with better outcomes across multiple patient‐centered and clinical event outcomes.
Conclusions
The comprehensive adherence plan with multiple strategies implemented in the REHAB‐HF trial resulted in high rates of retention and adherence in the intervention arm, despite high frailty rates, multiple comorbidities, and barriers to exercise. Higher session attendance was significantly associated with greater gains in physical function, improvements in QOL and depression, and reduced clinical events, including all‐cause death and rehospitalization following the intervention. These data support the efficacy of the comprehensive adherence plan and the subsequent intervention‐related benefits observed in REHAB‐HF.
Sources of Funding
This study was supported in part by the following research grants from the National Institutes of Health: R01AG045551; R01AG18915; P30AG021332; P30AG028716; U24AG059624, UL1RT001420. Also supported in part by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine and by the Oristano Family Fund at Wake Forest School of Medicine.
Disclosures
Dr Kitzman received honoraria outside the present study as a consultant for Bayer, Merck, Medtronic, Relypsa, Merck, Corvia Medical, Boehringer‐Ingelheim, NovoNordisk, AstraZeneca, Rivus, Pfizer, and Novartis; grant funding outside the present study from Novartis, Bayer, NovoNordisk, and AstraZeneca; and has stock ownership in Gilead Sciences. Dr Whellan received research support and consulting fees from Amgen, CVRx, Cytokinetics, Fibrogen, Novartis, and NovoNordisk. Dr Mentz received research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Medtronic, Merck, Novartis, Roche, Sanofi, and Vifor. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
Supporting information
Table S1
For Sources of Funding and Disclosures, see page 9.
References
- 1. Cheng RK, Cox M, Neely ML, Heidenreich PA, Bhatt DL, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J. 2014;168:721–730. doi: 10.1016/j.ahj.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 2. Kitzman D, Whellan D, Duncan P, Pastva A, Mentz R, Reeves G, Nelson B, Chen H, Upadhya B, Reed S, et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. 2021;385:203–216. doi: 10.1056/NEJMoa2026141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barbour KA, Miller NH. Adherence to exercise training in heart failure: a review. Heart Fail Rev. 2008;13:81–89. doi: 10.1007/s10741-007-9054-x [DOI] [PubMed] [Google Scholar]
- 4. Conraads VM, Deaton C, Piotrowicz E, Santaularia N, Tierney S, Piepoli MF, Pieske B, Schmid J‐P, Dickstein K, Ponikowski PP, et al. Adherence of heart failure patients to exercise: barriers and possible solutions: a position statement of the Study Group on Exercise Training in Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2012;14:451–458. doi: 10.1093/eurjhf/hfs048 [DOI] [PubMed] [Google Scholar]
- 5. Cooper LB, Mentz RJ, Sun J‐L, Schulte PJ, Fleg JL, Cooper LS, Piña IL, Leifer ES, Kraus WE, Whellan DJ, et al. Psychosocial factors, exercise adherence, and outcomes in heart failure patients: insights from heart failure: a controlled trial investigating outcomes of exercise training (HF‐ACTION). Circ Heart Fail. 2015;8:1044–1051. doi: 10.1161/CIRCHEARTFAILURE.115.002327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keteyian SJ, Leifer ES, Houston‐Miller N, Kraus WE, Brawner CA, O’Connor CM, Whellan DJ, Cooper LS, Fleg JL, Kitzman DW, et al. Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol. 2012;60:1899–1905. doi: 10.1016/j.jacc.2012.08.958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mentz RJ, Whellan DJ, Reeves GR, Pastva AM, Duncan P, Upadhya B, Nelson MB, Chen H, Reed SD, Rosenberg PB, et al. Rehabilitation intervention in older patients with acute heart failure with preserved versus reduced ejection fraction. JACC: Heart Failure. 2021;9:747–757. doi: 10.1016/j.jchf.2021.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reeves G, Whellan D, Patel M, O'Connor C, Duncan P, Eggebeen J, Morgan T, Hewston L, Pastva A, Kitzman D. Comparison of frequency of frailty and severely impaired physical function in patients >/=60 years hospitalized with acute decompensated heart failure vs chronic stable heart failure with reduced and preserved left ventricular ejection fraction. Am J Cardiol. 2016;117:1953–1958. doi: 10.1016/j.amjcard.2016.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pandey A, Kitzman D, Whellan D, Duncan P, Mentz R, Pastva A, Nelson M, Upadhya B, Chen H, Reeves G. Frailty among older decompensated heart failure patients: prevalence, association with patient‐centered outcomes, and efficient detection methods. J Am Coll Cardiol. 2019;7:1079–1088. doi: 10.1016/j.jchf.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pastva AM, Hugenschmidt CE, Kitzman DW, Nelson MB, Brenes GA, Reeves GR, Mentz RJ, Whellan DJ, Chen H, Duncan PW. Cognition, physical function, and quality of life in older patients with acute decompensated heart failure. J Card Fail. 2021;27:286–294. doi: 10.1016/j.cardfail.2020.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aladin AI, Whellan D, Mentz RJ, Pastva AM, Nelson MB, Brubaker P, Duncan P, Reeves G, Rosenberg P, Kitzman DW. Relationship of physical function with quality of life in older patients with acute heart failure. J Am Geriatr Soc. 2021;69:1836–1845. doi: 10.1111/jgs.17156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, Ogedegbe G, Orwig D, Ernst D, Czajkowski S. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH behavior change consortium. Health Psychol. 2004;23:443–451. doi: 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- 13. Pastva AM, Duncan PW, Reeves GR, Nelson MB, Whellan DJ, O'Connor CM, Eggebeen JD, Hewston LA, Taylor KM, Mentz RJ, et al. Strategies for supporting intervention fidelity in the rehabilitation therapy in older acute heart failure patients (REHAB‐HF) trial. Contemp Clin Trials. 2018;64:118–127. doi: 10.1016/j.cct.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reeves GR, Whellan DJ, Duncan P, O'Connor CM, Pastva AM, Eggebeen JD, Hewston LA, Morgan TM, Reed SD, Rejeski WJ, et al. Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB‐HF) trial: design and rationale. Am Heart J. 2017;185:130–139. doi: 10.1016/j.ahj.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White‐Williams C, Rossi LP, Bittner VA, Driscoll A, Durant RW, Granger BB, Graven LJ, Kitko L, Newlin K, Shirey M. Addressing social determinants of health in the care of patients with heart failure: a scientific statement from the American Heart Association. Circulation. 2020;141:e841–e863. doi: 10.1161/CIR.0000000000000767 [DOI] [PubMed] [Google Scholar]
- 16. Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, Espeland MA, Fielding RA, Gill TM, Groessl EJ, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fielding RA, Katula J, Miller ME, Abbott‐Pillola K, Jordan A, Glynn NW, Goodpaster B, Walkup MP, King AC, Rejeski WJ, et al. Activity adherence and physical function in older adults with functional limitations. Med Sci Sports Exerc. 2007;39:1997–2004. doi: 10.1249/mss.0b013e318145348d [DOI] [PubMed] [Google Scholar]
- 18. Pavasini R, Guralnik J, Brown JC, di Bari M, Cesari M, Landi F, Vaes B, Legrand D, Verghese J, Wang C, et al. Short physical performance battery and all‐cause mortality: systematic review and meta‐analysis. BMC Med. 2016;14:215. doi: 10.1186/s12916-016-0763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soubra R, Aly C, Novella J. A systematic review of thirty‐one assessment tests to evaluate mobility in older adults. Biomed Res Int. 2019;2019:1–17. doi: 10.1155/2019/1354362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Volpato S, Cavalieri M, Sioulis F, Guerra G, Maraldi C, Zuliani G, Fellin R, Guralnik J. Predictive value of the short physical performance battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66A:89–96. doi: 10.1093/gerona/glq167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/S0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 22. Wancata J, Alexandrowicz R, Marquart B, Weiss M, Friedrich F. The criterion validity of the geriatric depression scale: a systematic review. Acta Psychiatr Scand. 2006;114:398–410. doi: 10.1111/j.1600-0447.2006.00888.x [DOI] [PubMed] [Google Scholar]
- 23. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 24. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 25. Rejeski WJ, Miller ME, Studenski SA, Katula JA, Fielding RA, Glynn NW, Walkup MP, Ashmore JA, and Investigators for the LIFE study . Predictors of adherence to physical activity in the Lifestyle Interventions and Independence for Elders pilot study (LIFE‐P). Clin Interv Aging. 2012;2:485–494. [PMC free article] [PubMed] [Google Scholar]
- 26. Mudge A, Denaro C, Scott A, Meyers D, Adsett J, Mullins R, Suna J, Atherton J, Marwick T, Scuffham P, et al. Addition of supervised exercise training to a post‐hospital disease management program for patients recently hospitalized with acute heart failure. JACC Heart Failure. 2018;6:143–152. doi: 10.1016/j.jchf.2017.11.016 [DOI] [PubMed] [Google Scholar]
- 27. Fleg J. Preventing readmission after hospitalization for acute heart failure: a quest incompletely fulfilled. JACC: Heart Failure. 2018;6:153–155. doi: 10.1016/j.jchf.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 28. Fleg JL, Cooper LS, Borlaug BA, Haykowsky MJ, Kraus WE, Levine BD, Pfeffer MA, Piña IL, Poole DC, Reeves GR, et al. Exercise training as therapy for heart failure: current status and future directions. Circ Heart Fail. 2015;8:209–220. doi: 10.1161/CIRCHEARTFAILURE.113.001420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. CMS. Centers for Medicare and Medicaid Services . Decision Memo for Cardiac Rehabilitation (CR) Programs ‐ Chronic Heart Failure (CAG‐00437N). February 18, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


