Abstract
Background
Lipoprotein(a) (Lp(a)) is a potent causal risk factor for cardiovascular events and mortality. However, its relationship with subclinical atherosclerosis, as defined by arterial calcification, remains unclear. This study uses the ARIC (Atherosclerosis Risk in Communities Study) to evaluate the relationship between Lp(a) in middle age and measures of vascular and valvular calcification in older age.
Methods and Results
Lp(a) was measured at ARIC visit 4 (1996–1998), and coronary artery calcium (CAC), together with extracoronary calcification (including aortic valve calcium, aortic valve ring calcium, mitral valve calcification, and thoracic aortic calcification), was measured at visit 7 (2018–2019). Lp(a) was defined as elevated if >50 mg/dL and CAC/extracoronary calcification were defined as elevated if >100. Logistic and linear regression models were used to evaluate the association between Lp(a) and CAC/extracoronary calcification, with further stratification by race. The mean age of participants at visit 4 was 59.2 (SD 4.3) years, with 62.2% women. In multivariable adjusted analyses, elevated Lp(a) was associated with higher odds of elevated aortic valve calcium (adjusted odds ratio [aOR], 1.82; 95% CI, 1.34–2.47), CAC (aOR, 1.40; 95% CI, 1.08–1.81), aortic valve ring calcium (aOR, 1.36; 95% CI, 1.07–1.73), mitral valve calcification (aOR, 1.37; 95% CI, 1.06–1.78), and thoracic aortic calcification (aOR, 1.36; 95% CI, 1.05–1.77). Similar results were obtained when Lp(a) and CAC/extracoronary calcification were examined on continuous logarithmic scales. There was no significant difference in the association between Lp(a) and each measure of calcification by race or sex.
Conclusions
Elevated Lp(a) at middle age is significantly associated with vascular and valvular calcification in older age, represented by elevated CAC, aortic valve calcium, aortic valve ring calcium, mitral valve calcification, thoracic aortic calcification. Our findings encourage assessing Lp(a) levels in individuals with increased cardiovascular disease risk, with subsequent comprehensive vascular and valvular assessment where elevated.
Keywords: aortic valve calcium, coronary artery calcium, extra‐coronary calcification, lipoprotein(a), subclinical atherosclerosis
Subject Categories: Lipids and Cholesterol, Cardiovascular Disease, Primary Prevention, Atherosclerosis, Computerized Tomography (CT)
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities Study
- AVC
aortic valve calcium
- AVRC
aortic valve ring calcium
- ECC
extracoronary calcification
- Lp(a)
lipoprotein(a)
- MVC
mitral valve calcification
- TAC
thoracic aortic calcification
Clinical Perspective
What Is New?
The relationship between lipoprotein(a) and subclinical atherosclerosis remains unclear.
In this prospective cohort study of 2083 older adults in the ARIC (Atherosclerosis Risk in Communities) study, we found that elevated lipoprotein(a) at middle age is significantly associated with vascular and valvular calcification in older age, represented by elevated calcification in the coronary artery, aortic valve ring, mitral valve, thoracic aorta, and most especially, the aortic valve.
What Are the Clinical Implications?
Our findings encourage assessing lipoprotein(a) levels in individuals with increased cardiovascular disease risk, with subsequent comprehensive vascular and valvular assessment where elevated.
Lipoprotein(a) (Lp(a)) is a plasma lipoprotein that is a potent causal risk factor for cardiovascular events and mortality. 1 , 2 , 3 Elevated Lp(a) levels have been independently associated with increased atherosclerotic cardiovascular disease risk, even among individuals on statin therapy and among those with low‐density lipoprotein cholesterol (LDL‐C) <70 mg/dL. 1 , 4 Additionally, elevated Lp(a) has been associated with increased risk for calcific valvular disease. 5 Lp(a) has a unique structure that comprises apolipoprotein B‐100, an LDL‐like moiety that is thought to promote atherosclerosis, and an apolipoprotein A moiety, similar to plasminogen that may mediate increased risk of thrombosis. 3 , 6 The mechanisms mediating its effects on the cardiovascular system are, however, incompletely understood. 1
Although Lp(a) has generated increased interest over the past several years and is recommended by some for inclusion in management guidelines, 3 more information concerning its role in the development of cardiovascular disease is needed. For example, the relationship between Lp(a) and coronary artery calcium (CAC), a robust marker of overall atherosclerotic burden and an individualizable integrator of cardiovascular risk, 7 , 8 is inconsistent in the literature, although it appears to be influenced by race, 9 , 10 , 11 sex, 12 , 13 and certain comorbidities. 13 , 14
Similarly, Lp(a) has also been directly implicated in the development and progression of some valvular diseases including aortic valve calcification (AVC), a precursor to calcific aortic valve stenosis, the most common valvular disease in North America. 5 , 15 , 16 , 17 Lp(a) has also been associated with increased severity and increased risk of mortality from calcific aortic valve stenosis. 5 , 15
To elucidate the relationship between Lp(a) and measures of vascular and valvular calcification, we used the ARIC (Atherosclerosis Risk in Communities) study to evaluate the association between Lp(a) in middle age and the presence of CAC, AVC, and other computed tomography‐defined measures of atherosclerosis including mitral valve calcification (MVC), aortic valve ring calcification (AVRC), and thoracic aortic calcification (TAC) in older age. Further, we sought to study whether the association between Lp(a) and CAC or extracoronary calcification (ECC) differed by race or sex.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the ARIC data coordinating center.
Study Population
The ARIC study is an ongoing prospective cohort of 15 792 individuals enrolled between 1987 and 1989 from 4 communities in the United States—Forsyth County, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland. Participants were aged 45 to 64 years at baseline and have subsequently undergone additional extensive examination at 6 other visits (1990–1992, 1993–1995, 1996–1998, 2011–2013, 2016–2017, 2018–2019). Participants provided informed consent at each visit and institutional review boards at each field center approved the protocol. More details about study design and sampling methodology have been published elsewhere. 18
Cardiac computed tomography was done for the first time in ARIC at visit 7 (February 2018–December 2019). Of the 3589 participants at visit 7, we excluded participants absent from visit 4 (1996–1998: when Lp(a) was measured; n=187), participants missing Lp(a) measurements from visit 4 (n=45), participants with race other than Black or White (n=9), participants with history of coronary heart disease (CHD) as of December 2015 per ARIC CAC scanning eligibility protocol (n=485), participants missing CAC measurements at visit 7 (n=770), and participants with missing information on covariates of interest (n=10). A final sample size of 2083 individuals were included in our analyses (Figure S1).
Lipoprotein Measurements
Lp(a) in EDTA‐plasma samples (stored at −70 °C before analysis) was measured at visit 4 (1996–1998), using a commercially sourced automated assay developed by Denka Seiken Co. Ltd, Tokyo, Japan, that is relatively insensitive to apolipoprotein A kringle IV type 2 repeats. 19 Total cholesterol, high‐density lipoprotein cholesterol, and triglycerides were measured using enzymatic procedures. LDL‐C was calculated using Martins‐Hopkins equation, and corrected LDL‐C was calculated as [Total LDL‐0.3*Lp(a)]. 20
CAC/ECC Measurements
CAC/ECC scans were performed using multidetector 64‐slice computed tomography scanners by trained technicians supervised by a physician at in each center. Calcification was defined as lesions with attenuation >130 Hounsfield units and area ≥1 mm2 in each slice level. CAC/ECC scores were quantified by the Agatston score. 21 ECC sites include AVC, AVRC, MVC, and TAC.
CAC was defined as calcification in any of the coronary arteries, AVC was defined as calcification on the aortic valve extending to but not including the aortic root, AVRC was defined as calcification involving the aortic root only, MVC was defined as calcification on the mitral valve annulus, and TAC was defined as the presence of calcification in the ascending or descending aorta within the field of view on the CAC scan.
Other Covariates
Age, sex, race, education, alcohol use, and smoking status at visit 4 were based on self‐report. Education level was evaluated, and participants were categorized into less than a high school education, higher education other than college, or at least some college. Cigarette smoking and alcohol use status were classified into 3 categories: current, former, and never user. Body mass index was calculated as weight (kg)/height2 (m2). Blood pressures were measured in duplicate after a 5‐minute rest in a seated position, and the mean of the 2 measurements was recorded. Systolic blood pressure was considered elevated if ≥140 mm Hg and use of antihypertensive medication was self‐reported. Diabetes was defined as fasting glucose level ≥126 mg/dL, nonfasting glucose level ≥200 mg/dL, self‐reported physician diagnosis, or use of antidiabetic medications. Medication use over the prior 2 weeks was verified by reviewing medication containers that participants brought to the visit.
Statistical Analysis
We first summarized baseline characteristics of participants by elevated versus nonelevated Lp(a) (defined as per prior literature as ≤versus>50 mg/dL). 22 Means (SD), median (interquartile interval), and proportions (%) were reported as appropriate.
We analyzed Lp(a) both continuously and categorically. Lp(a) was log‐transformed due to its skewed distribution. We primarily dichotomized Lp(a) at a clinical cut‐point of 50 mg/dL, as noted previously, and secondarily categorized Lp(a) using cut‐points of ≤10, 11 to 20, 21 to 30, 31 to 50, and >50 mg/dL. 23 , 24 Consistent with prior literature, we analyzed CAC/ECC primarily as “elevated versus not elevated” based on a cut‐point of 100 7 , 25 and secondarily as continuous variables on a logarithmic scale.
The association between Lp(a) and CAC/ECC was first assessed using logistic regression models with incremental adjustment for potential confounders. Model 1 was crude; Model 2 adjusted for age, race, sex, education level, smoking status, alcohol drinking status, and cardiovascular risk factors (body mass index, systolic blood pressure, diabetes, antihypertensive medication use, high‐density lipoprotein cholesterol, and triglycerides) and is referred to as the fully adjusted‐model. Total cholesterol and LDL‐C were not included in the adjusted models as Lp(a) is typically measured as a component of both. However, we performed sensitivity analyses including corrected LDL‐C. Additionally, to account for its importance in modifying lipid parameters, lipid‐lowering medication use was included separately in Model 3 (in addition to the variables in Model 2).
We also evaluated the association between continuous Lp(a) and CAC/ECC using restricted cubic splines with knots at values corresponding to the 25th, 50th, and 75th centiles (4.9, 12.4, and 37.9 mg/dL respectively). The reference point was set at a value corresponding to the 20th centile (3.9 mg/dL).
To assess the incremental value of Lp(a) over conventional risk factors for predicting CAC/ECC, a receiver operating characteristic curve was constructed with a base model including cardiovascular risk factors (age, sex, race, high‐density lipoprotein cholesterol, elevated systolic blood pressure/use of antihypertensive medication, diabetes, and smoking status). Another receiver operating characteristic curve was constructed with Lp(a) (in continuous log‐transformed form) added to the base model. The area under the curve for both models was then compared using chi‐square test.
For sensitivity analyses, the association between Lp(a) (modeled continuously as a log‐transformed variable, and with prespecified cut‐points) and CAC/ECC (modeled continuously as ln(CAC+1) or ln(ECC+1)) was assessed using linear regression, with the same models outlined previously.
Subgroup analyses by race (White versus Black) and sex were conducted to assess the association between Lp(a) (modeled as ≤50 versus >50 mg/dL) and CAC/ECC. To maximize power, CAC/ECC was modeled as score 0 versus >0. The P value for interaction was tested using the likelihood ratio test.
All analyses were conducted using Stata 15 software (Stata Corp, College Station, TX). Statistical significance was set as a 2‐sided P value of <0.05.
Results
Overall, the participants in our study had a mean age of 59.2 (SD 4.3) years at visit 4, with 62.2% women, 21.4% Black race, and 18.8% with Lp(a) >50 mg/dL. Compared with individuals with Lp(a) ≤50 mg/dL, a larger proportion of individuals with Lp(a) values >50 mg/dL were women, Black people, had hypertension or reported use of antihypertensive medication, and used lipid‐lowering medication (Table 1). Other characteristics were similarly distributed between the groups.
Table 1.
Baseline Characteristics of Participants by Lipoprotein(a) Category
| Characteristic | Total | Lp(a) ≤50 mg/dL | Lp(a) >50 mg/dL |
|---|---|---|---|
| N=2083 | N=1691 | N=392 | |
| Age, y | 59.2 (4.3) | 59.3 (4.2) | 58.9 (4.3) |
| Female sex | 1296 (62.2%) | 1018 (60.2%) | 278 (70.9%) |
| Black race | 446 (21.4%) | 319 (18.9%) | 127 (32.4%) |
| Education level | |||
| <High school | 200 (9.6%) | 169 (10.0%) | 31 (7.9%) |
| Higher education other than college | 860 (41.3%) | 697 (41.2%) | 163 (41.6%) |
| At least some college | 1023 (49.1%) | 825 (48.8%) | 198 (50.5%) |
| Body mass index, kg/m2 | 28.4 (5.2) | 28.4 (5.1) | 28.6 (5.5) |
| Systolic blood pressure, mm Hg | 121.7 (16.5) | 121.7 (16.5) | 121.4 (16.7) |
| Diastolic blood pressure, mm Hg | 71.4 (9.7) | 71.3 (9.7) | 71.8 (10.0) |
| Antihypertensive medication use | 585 (28.1%) | 466 (27.6%) | 119 (30.4%) |
| Smoking status | |||
| Never smoker | 992 (47.6%) | 797 (47.1%) | 195 (49.7%) |
| Former smoker | 885 (42.5%) | 722 (42.7%) | 163 (41.6%) |
| Current smoker | 206 (9.9%) | 172 (10.2%) | 34 (8.7%) |
| Alcohol use status | |||
| Never drinker | 388 (18.6%) | 303 (17.9%) | 85 (21.7%) |
| Former drinker | 478 (22.9%) | 385 (22.8%) | 93 (23.7%) |
| Current drinker | 1217 (58.4%) | 1003 (59.3%) | 214 (54.6%) |
| Diabetes | 154 (7.4%) | 123 (7.3%) | 31 (7.9%) |
| Lipid lowering medication use | 173 (8.3%) | 127 (7.5%) | 46 (11.7%) |
| Total cholesterol, mg/dL | 198.0 (177.0–222.0) | 196.0 (174.0–219.0) | 211.0 (190.0–232.0) |
| High‐density lipoprotein cholesterol, mg/dL | 50.0 (41.0–62.0) | 49.0 (40.0–62.0) | 53.0 (43.0–65.0) |
| Triglycerides, mg/dL | 114.0 (83.0–159.0) | 115.0 (84.0–163.0) | 106.0 (81.0–147.0) |
| Thoracic aorta >100 | 1430 (68.7%) | 1157 (68.4%) | 273 (69.6%) |
| Aortic valve ring >100 | 988 (47.4%) | 789 (46.7%) | 199 (50.8%) |
| Aortic valve >100 | 366 (17.6%) | 282 (16.7%) | 84 (21.4%) |
| Mitral valve >100 | 537 (25.8%) | 423 (25.0%) | 114 (29.1%) |
| Total coronary artery calcium >100 | 1327 (63.7%) | 1079 (63.8%) | 248 (63.3%) |
Values are mean (SD), median (interquartile interval), or n (%).Lp(a) indicates lipoprotein(a).
Coronary Artery Calcium
In the fully adjusted model, elevated Lp(a) was significantly associated with elevated CAC, with individuals having Lp(a) >50 mg/dL more likely to have CAC>100 (adjusted odds ratio [aOR], 1.40; 95% CI, 1.08–1.81). After additionally adjusting for lipid lowering medication, the association remained significant (aOR, 1.35; 95% CI, 1.04–1.75) (Table 2). Further sensitivity analyses adjusting for corrected‐LDL‐C showed similar results (Table S1). The odds of having elevated CAC (>100) across increasing levels of Lp(a) is depicted using restricted cubic splines in Figure 1A.
Table 2.
Adjusted Odds Ratio of CAC or ECC>100 Comparing Elevated Lp(a) to Normal Lp(a)
| Lp(a) ≤50 mg/dL | Lp(a) >50 mg/dL | |
|---|---|---|
| CAC | ||
| Model 1 | Reference | 0.98 (0.78–1.23) |
| Model 2 | Reference | 1.40 (1.08–1.81) |
| Model 3 | Reference | 1.35 (1.04–1.75) |
| Thoracic Aorta | ||
| Model 1 | Reference | 1.06 (0.83–1.34) |
| Model 2 | Reference | 1.36 (1.05–1.77) |
| Model 3 | Reference | 1.35 (1.04–1.76) |
| Aortic valve | ||
| Model 1 | Reference | 1.36 (1.04–1.79) |
| Model 2 | Reference | 1.82 (1.34–2.47) |
| Model 3 | Reference | 1.79 (1.32–2.43) |
| Aortic valve ring | ||
| Model 1 | Reference | 1.18 (0.95–1.47) |
| Model 2 | Reference | 1.36 (1.07–1.73) |
| Model 3 | Reference | 1.32 (1.04–1.67) |
| Mitral valve | ||
| Model 1 | Reference | 1.23 (0.96–1.57) |
| Model 2 | Reference | 1.37 (1.06–1.78) |
| Model 3 | Reference | 1.35 (1.04–1.76) |
Model 1 is unadjusted.
Model 2 is adjusted for age, race, sex, education level, smoking status, alcohol drinking status, and cardiometabolic risk factors (body‐mass index, systolic blood pressure, diabetes, antihypertensive medication use, high‐density lipoprotein cholesterol, and triglycerides).
Model 3 is Model 2 + lipid lowering therapy. CAC indicates coronary artery calcium; ECC, extracoronary calcification; and Lp(a), lipoprotein(a).
Figure 1. Multivariable adjusted restricted cubic splines for the association between Lp(a) and CAC/ECC.
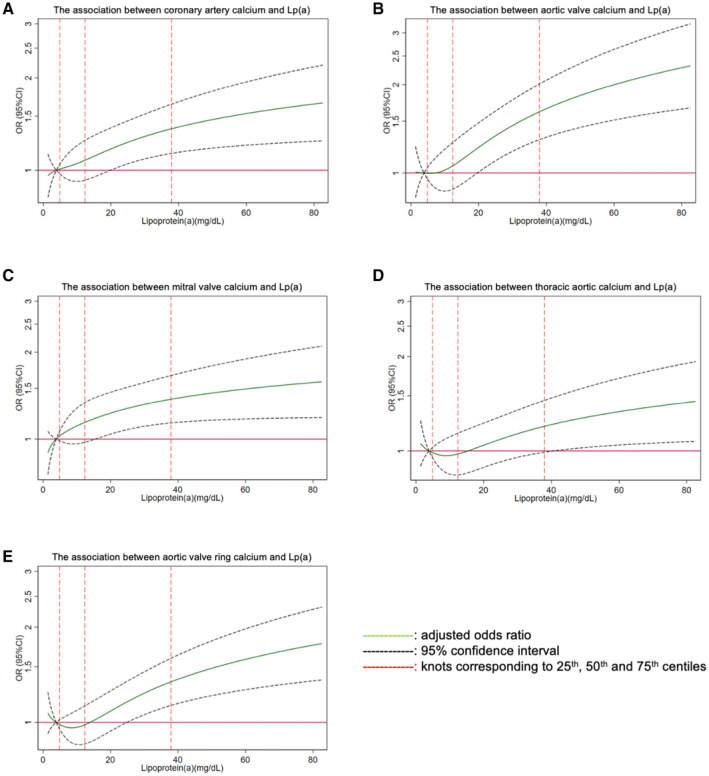
(A) The association between coronary artery calcium and Lp(a); (B) The association between aortic valve calcium and Lp(a); (C) The association between mitral valve calcium and Lp(a); (D) The association between thoracic aortic calcium and Lp(a); (E) The association between aortic valve ring calcium and Lp(a). CAC indicates coronary artery calcium; ECC, extracoronary calcification; Lp(a), lipoprotein(a); and OR, odds ratio.
In sensitivity analyses, using a multivariable‐adjusted linear regression model, there was a significant association between log‐transformed Lp(a), and log‐transformed CAC (adjusted coefficient, 0.11; 95% CI, 0.04–0.18) (Table S2). Additionally, in logistic regression models comparing categories of Lp(a) (11–20, 21–30, 31–50, and >50 mg/dL) to the lowest category of ≤10 mg/dL, only the highest group with Lp(a) >50 mg/dL had significantly higher odds of having elevated CAC >100 (aOR, 1.46; 95% CI, 1.10–1.94) (Table S3).
The addition of Lp(a) to the base model of cardiovascular risk factors nominally increased the area under the curve for prediction of CAC>100 from 0.7447 to 0.7450; however, this change was not significant (P=0.76) (Table S4).
Aortic Valve Calcium
Elevated Lp(a) (>50 mg/dL) was significantly associated with higher odds of AVC>100 across all models (fully adjusted aOR, 1.82; 95% CI, 1.34–2.47) (Table 2), as well as in sensitivity analyses (Table S1). The relationship between Lp(a) and AVC is graphically depicted using restricted cubic splines in Figure 1B.
In sensitivity analyses, using multivariable‐adjusted linear regression, we confirmed the significant association between log‐transformed Lp(a), and log‐transformed AVC (adjusted coefficient, 0.20; 95% CI, 0.12–0.28). In logistic regression models, compared with the lowest category of ≤10 mg/dL, the odds of elevated AVC among those with Lp(a) 21 to 30 mg/dL, 31 to 50 mg/dL, and >50 mg/dL were significantly elevated.
Lp(a) significantly improved the area under the curve for predicting the presence of AVC beyond cardiovascular risk factors from 0.7101 to 0.7211 (P=0.02) (Table S4).
Other Extracoronary Calcification
Elevated Lp(a) (>50 mg/dL) was also associated with elevated AVRC (aOR, 1.36; 95% CI, 1.07–1.73), MVC (aOR, 1.37; 95% CI, 1.06–1.78), and TAC (aOR, 1.36; 95% CI, 1.05–1.77) (Table 2), with similar findings in sensitivity analyses (Table S1). The relationship between Lp(a) and these other ECC sites is graphically depicted using restricted cubic splines in Figure 1C through 1E.
In sensitivity analyses, using multivariable‐adjusted linear regression models, we also confirmed the significant associations between log‐transformed Lp(a) and log‐transformed AVRC (adjusted coefficient, 0.12; 95% CI, 0.05–0.20) and MVC (adjusted coefficient, 0.13; 95% CI, 0.04–0.23) (Table S2). In logistic regression models, compared with the lowest category of ≤10 mg/dL, the odds of elevated MVC and TAC were significantly elevated only among those with Lp(a)>50 mg/dL (Table S3).
Lp(a) did not significantly improve the area under the curve for predicting AVRC, MAC, or TAC beyond cardiovascular risk factors (Table S4).
Subgroup Analyses by Race and Sex
There was no significant difference in the association between Lp(a) and measures of calcification by race or sex. However, looking within each race subgroup, among Black people, those with Lp(a) >50 mg/dL versus ≤50 mg/dL had 1.73 (95% CI, 1.10–2.72) times higher odds of having AVC present, 2.05 (95% CI, 1.11–3.80) times of having AVRC, and 2.44 (95% CI, 1.18–5.05) times of having CAC than those with Lp(a) (Figure 2). Among White people, those with Lp(a) >50 mg/dL had 2.25 (95% CI, 1.69–2.99) times higher odds of having AVC than those with Lp(a) ≤50 mg/dL (Figure 2).
Figure 2. Adjusted odds ratio of CAC/ECC presence comparing elevated Lp(a) to normal Lp(a) by race.
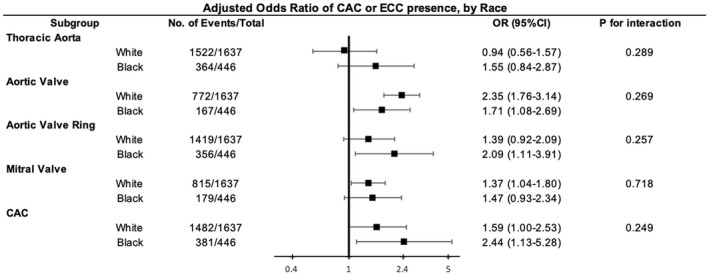
Model adjusted for age, race, sex, education level, smoking status, alcohol drinking status and cardiometabolic risk factors (body mass index, systolic blood pressure, diabetes, antihypertensive medication use, high‐density lipoprotein cholesterol, and triglycerides). CAC indicates coronary artery calcium; ECC, extracoronary calcification; Lp(a), lipoprotein(a); and OR, odds ratio.
Compared with women with Lp(a) ≤50 mg/dL, women with Lp(a) >50 mg/dL had 1.93 (95% CI, 1.45–2.57) times higher odds of having AVC present, 1.45 (95% CI, 1.10–1.92) times of having MVC and 1.83 (95% CI, 1.19–2.82) times of having CAC. Men with Lp(a) >50 mg/dL had 2.57 (95% CI, 1.60–4.12) times higher odds of having AVC than men with Lp(a) ≤50 mg/dL (Figure 3).
Figure 3. Adjusted odds ratio of CAC/ECC presence comparing elevated Lp(a) to normal Lp(a) by sex.

Model adjusted for age, race, sex, education level, smoking status, alcohol drinking status and cardiometabolic risk factors (body mass index, systolic blood pressure, diabetes, antihypertensive medication use, high‐density lipoprotein cholesterol, and triglycerides). CAC indicates coronary artery calcium; ECC, extracoronary calcification; Lp(a), lipoprotein(a); and OR, odds ratio.
Discussion
In this analysis of 2083 White and Black participants in the ARIC study, elevated baseline Lp(a) was consistently associated with all measures of subclinical vascular and valvular calcification, although results were strongest for AVC. No significant differences were observed for each measure of calcification by race or sex; however, within subgroups, elevated Lp(a) values were associated with AVRC, AVC, and CAC presence among Black people and with AVC, MVC, and CAC among women.
Lp(a) mediates vascular calcification through different mechanisms including its lysine binding sites, which facilitate its accumulation on the endothelium, and its oxidized phospholipids, which cause inflammation in the vascular wall. 26 Additionally, its inhibition of plasminogen activation and fibrin degradation further promulgates its effects in the vasculature. 26 In prior literature, however, the association between Lp(a) and CAC has been inconsistent. 10 , 27 , 28 Genetic polymorphisms in the genes that encode Lp(a) are associated with circulating Lp(a) levels, which could explain the variation in association between Lp(a) and CAC observed in different study populations. 28 , 29 However, our findings of a significant association between Lp(a) and CAC reinforce studies that observed significant associations between Lp(a) and CAC, extending their findings to older adults. 27 , 28 The association between Lp(a) and TAC also differs through the literature 30 , 31 , 32 ; however, Lp(a) was also associated with TAC in our study, supporting that Lp(a) mediates its effect on atherosclerosis in different vascular beds. Our findings have additional validity considering that the association between Lp(a) and CAC as well as TAC persists in a study population of older adults who have remained free of CHD, suggesting that Lp(a) has a role in the lifetime accumulation of disease burden in the vasculature even in seemingly healthy individuals. As elevated vascular calcification has been repeatedly associated with increased risk of CVD and all‐cause mortality, 7 , 33 , 34 , 35 incorporating the testing for Lp(a) among individuals at increased risk of CHD (with subsequent assessment of vascular calcification if elevated) in prevention guidelines would improve cardiovascular risk stratification, further refining the initiation of aggressive therapy.
The strong statistically significant association between the burden of Lp(a) and AVC among older adults in our study extends the rich body of literature with similar results to this subdemographic. 5 , 15 , 17 Lp(a) has been postulated to cause AVC via several mechanisms including the binding of its apolipoprotein A moiety to valvular endothelium damaged by mechanical stress in the left side of the heart, inflammation induced by its apolipoprotein B moiety and the oxidized phospholipids attached to it, as well as Lp(a) mediated deposition of cholesterol on the valvular leaflets, which act as calcification nodes. 36 Unlike vascular atherosclerosis, valvular calcification appears to be independent of the atherosclerotic pathway, and statins have thus far not been shown to have any effect on it. 36 Our findings encourage the testing of Lp(a) lowering therapies in deterring the progression of calcification among individuals with AVC, especially because younger individuals with elevated Lp(a) have been shown to progress quickly from mild aortic stenosis to severe stenosis requiring aortic valve replacement. 26 , 36 , 37 Our findings are even more important as the progression from subclinical calcification to clinically observable stenosis of the aortic valve typically occurs over a decade or more, providing ample opportunity for preventive therapy. 36
Although the prevalence of MVC in the general population is relatively low, our findings of a significant association between Lp(a) and elevated MVC are important as MVC, typically observed at the mitral annulus, has also been associated with fatal cardiovascular events and all‐cause mortality, as well as worsened postprocedure outcomes among individuals who have undergone transcatheter aortic valve replacement. 38 Our findings extend the results of Garg et al. in the younger Multi‐Ethnic Study of Atherosclerosis cohort to an older population. 32 These findings are important because they support the development of Lp(a) targeted therapy, which might provide a potential therapeutic entry point for the prevention of the disease.
Despite elevated Lp(a) levels being directly linked with CHD, prior research has shown that among Black individuals the risk of CHD does not increase proportionately to their burden of elevated Lp(a). 23 , 39 Our findings of significant associations between Lp(a), AVC, AVRC, and CAC among Black people are remarkable considering that Black people made up only about 20% of our sample size. It is possible that genetic factors, in addition to determining the circulating levels of Lp(a), 3 might also mediate the degree to which Lp(a) affects vascular and valvular calcification by race. Furthermore, among women in our study, elevated Lp(a) was associated with AVC, MVC, and CAC. This has additional face validity considering that other studies have shown that women with elevated Lp(a) have a much higher risk of CHD events than men. 40 Although there was no significant difference observed in the association between Lp(a) and measures of calcification by race or sex, our findings emphasize the need for assessment of potential differential impact of proposed Lp(a) therapies by race and sex in larger cohorts.
Our study leverages the ARIC study to examine the association between Lp(a) and calcification in different vascular and valvular beds using data collected over a long follow‐up period, allowing for the longitudinal assessment of the effect of lifelong exposure to Lp(a) on atherogenic markers. However, our findings should be interpreted considering certain limitations. Individuals with prior CHD were not included in our study. However, this is likely to have weakened our observed estimates, shifting them toward null, making our consistent positive associations even more notable. Also, a substantial proportion of participants alive at visit 7 were either unwilling or unable to attend the visit; thus, visit participants were likely to have been healthier than those who did not attend. However, this would likely have further shifted our estimates towards the null. Only Black and White participants were included in this study, which might limit the generalizability of our results. Also, the abdominal aorta and aortic arch were not scanned in our study.
Conclusions
In conclusion, elevated Lp(a) levels at middle age are significantly associated with vascular and valvular calcification at older age, represented by elevated CAC, AVRC, TAC, MVC, and most notably AVC. Our results support existing guidelines that recommend the measurement of Lp(a) at least once in certain intermediate to high‐risk individuals, and they encourage the assessment of subclinical vascular and valvular calcification among individuals with elevated Lp(a).
Sources of Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, HHSN268201700005I). This specific study was supported by R01HL136592 (Co‐PIs: Drs. Matsushita and Blaha).
Disclosures
M. J. Blaha is on advisory boards for both Amgen and Novartis, which make Lp(a) lowering therapies, and for Roche, which makes a Lp(a) assay. R. C. Hoogeveen received a research grant (to my institution) for the Lp(a) measurements performed in this study from Denka Seiken and is a consultant for Denka Seiken. All other authors have no disclosures.
Supporting information
Tables S1–S4
Figure S1
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Wu MF, Xu KZ, Guo YG, Yu J, Wu Y, Lin LM. Lipoprotein(a) and atherosclerotic cardiovascular disease: current understanding and future perspectives. Cardiovasc Drugs Ther. 2019;33:739–748. doi: 10.1007/s10557-019-06906-9 [DOI] [PubMed] [Google Scholar]
- 2. Willeit P, Kiechl S, Kronenberg F, Witztum JL, Santer P, Mayr M, Xu Q, Mayr A, Willeit J, Tsimikas S. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15‐year outcomes in the Bruneck study. J Am Coll Cardiol. 2014;64:851–860. doi: 10.1016/j.jacc.2014.03.061 [DOI] [PubMed] [Google Scholar]
- 3. Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albers JJ, Slee A, O’Brien KD, Robinson JG, Kashyap ML, Kwiterovich PO, Xu P, Marcovina SM. Relationship of apolipoproteins A‐1 and b, and lipoprotein(a) to cardiovascular outcomes: the aim‐high trial (atherothrombosis intervention in metabolic syndrome with low HDL/high triglyceride and impact on global health outcomes). J Am Coll Cardiol. 2013;62:1575–1579. doi: 10.1016/j.jacc.2013.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng KH, Tsimikas S, Pawade T, Kroon J, Jenkins WSA, Doris MK, White AC, Timmers NKLM, Hjortnaes J, Rogers MA, et al. Lipoprotein(a) and oxidized phospholipids promote valve calcification in patients with aortic stenosis. J Am Coll Cardiol. 2019;73:2150–2162. doi: 10.1016/j.jacc.2019.01.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boffa MB, Koschinsky ML. Thematic review series: Lipoprotein (a): coming of age at last: Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease? J Lipid Res. 2016;57:745–757. doi: 10.1194/jlr.R060582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hecht HS, Cronin P, Blaha MJ, Budoff MJ, Kazerooni EA, Narula J, Yankelevitz D, Abbara S. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Cardiovasc Comput Tomogr. 2017;11:74–84. doi: 10.1016/j.jcct.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 8. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678/FORMAT/EPUB [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huffman MD, Kandula NR, Baldridge AS, Tsai MY, Prabhakaran D, Kanaya AM. Evaluating the potential association between Lipoprotein(a) and atherosclerosis (from the Mediators of Atherosclerosis Among South Asians Living in America Cohort). Am J Cardiol. 2019;123:919–921;. doi: 10.1016/j.amjcard.2018.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guerra R, Yu Z, Marcovina S, Peshock R, Cohen JC, Hobbs HH. Lipoprotein(a) and apolipoprotein(a) isoforms: no association with coronary artery calcification in the Dallas heart study. Circulation. 2005;111:1471–1479. doi: 10.1161/01.CIR.0000159263.50305.BD [DOI] [PubMed] [Google Scholar]
- 11. Sharma A, Kasim M, Joshi PH, Qian Z, Krivitsky E, Akram K, Rinehart S, Vazquez G, Miller J, Rohman MS, et al. Abnormal Lipoprotein(a) levels predict coronary artery calcification in southeast Asians but not in Caucasians: use of noninvasive imaging for evaluation of an emerging risk factor. J Cardiovasc Transl Res. 2011;4:470–476. doi: 10.1007/s12265-011-9273-3 [DOI] [PubMed] [Google Scholar]
- 12. Erbel R, Lehmann N, Churzidse S, Möhlenkamp S, Moebus S, Mahabadi AA, Schmermund A, Stang A, Dragano N, Grönemeyer D, et al. Gender‐specific association of coronary artery calcium and lipoprotein parameters: the Heinz Nixdorf Recall Study. Atherosclerosis. 2013;229:531–540. doi: 10.1016/j.atherosclerosis.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 13. Qasim AN, Martin SS, Mehta NN, Wolfe ML, Park J, Schwartz S, Schutta M, Iqbal N, Reilly MP. Lipoprotein(a) is strongly associated with coronary artery calcification in type‐2 diabetic women. Int J Cardiol. 2011;150:17–21. doi: 10.1016/j.ijcard.2010.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jug B, Papazian J, Lee R, Budoff MJ. Association of lipoprotein subfractions and coronary artery calcium in patient at intermediate cardiovascular risk. Am J Cardiol. 2013;111:213–218. doi: 10.1016/j.amjcard.2012.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schnitzler JG, Ali L, Groenen AG, Kaiser Y, Kroon J. Lipoprotein(A) as orchestrator of calcific aortic valve stenosis. Biomolecules. 2019;9:760. doi: 10.3390/biom9120760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Makshood M, Joshi PH, Kanaya AM, Ayers C, Budoff M, Tsai MY, Blaha M, Michos ED, Post WS. Lipoprotein (a) and aortic valve calcium in South Asians compared to other race/ethnic groups. Atherosclerosis. 2020;313:14–19. doi: 10.1016/j.atherosclerosis.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, et al. Genetic associations with valvular calcification and aortic stenosis. Cardiol Rev. 2013;29:503–515. doi: 10.1056/nejmoa1109034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The ARIC Investigators . The Atherosclerosis Risk In Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 19. Marcovina SM, Albers JJ. Lipoprotein (a) measurements for clinical application. J Lipid Res. 2016;57:526–537. doi: 10.1194/jlr.R061648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. New Information on Accuracy of LDL‐C Estimation ‐ American College of Cardiology. Available at: https://www.acc.org/latest‐in‐cardiology/articles/2020/03/19/16/00/new‐information‐on‐accuracy‐of‐ldl‐c‐estimation. Accessed November 13, 2021.
- 21. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-T [DOI] [PubMed] [Google Scholar]
- 22. Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, Mcneal CJ, Nordestgaard BG, Orringer CE. Scientific statement use of Lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13:374–392. doi: 10.1016/j.jacl.2019.04.010 [DOI] [PubMed] [Google Scholar]
- 23. Virani SS, Brautbar A, Davis BC, Nambi V, Hoogeveen RC, Sharrett AR, Coresh J, Mosley TH, Morrisett JD, Catellier DJ, et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2012;125:241–249. doi: 10.1161/CIRCULATIONAHA.111.045120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aronis KN, Zhao DI, Hoogeveen RC, Alonso A, Ballantyne CM, Guallar E, Jones SR, Martin SS, Nazarian S, Steffen BT, et al. Associations of Lipoprotein(a) levels with incident atrial fibrillation and Ischemic Stroke: the ARIC (Atherosclerosis Risk in Communities) study. J Am Heart Assoc. 2017;6. doi: 10.1161/JAHA.117.007372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peng AW, Mirbolouk M, Orimoloye OA, Osei AD, Dardari Z, Dzaye O, Budoff MJ, Shaw L, Miedema MD, Rumberger J, et al. Long‐term all‐cause and cause‐specific mortality in asymptomatic patients with CAC ≥1,000: results from the CAC Consortium. JACC Cardiovasc Imaging. 2020. doi: 10.1016/j.jcmg.2019.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsimikas S. A test in context: Lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017. doi: 10.1016/j.jacc.2016.11.042 [DOI] [PubMed] [Google Scholar]
- 27. Greif M, Arnoldt T, Von Ziegler F, Ruemmler J, Becker C, Wakili R, D’Anastasi M, Schenzle J, Leber AW, Becker A. Lipoprotein (a) is independently correlated with coronary artery calcification. Eur J Intern Med. 2013;24:75–79. doi: 10.1016/j.ejim.2012.08.014 [DOI] [PubMed] [Google Scholar]
- 28. Pechlivanis S, Mahabadi AA, Hoffmann P, Nöthen MM, Broecker‐Preuss M, Erbel R, Moebus S, Stang A, Jöckel KH. Association between lipoprotein(a) (Lp(a)) levels and Lp(a) genetic variants with coronary artery calcification. BMC Med Genet. 2020;21(1). doi: 10.1186/s12881-020-01003-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Song ZK, Cao HY, Di WUH, Zhou LT, Qin L. LPA gene polymorphisms and gene expression associated with coronary artery disease. Biomed Res Int. 2017;2017. doi: 10.1155/2017/4138376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peltier M, Peltier MCI, Sarano ME, Lesbre JPM, Colas JL, Tribouilloy CM. Elevated serum lipoprotein(a) level is an independent marker of severity of thoracic aortic atherosclerosis. Chest. 2002;121:1589–1594. doi: 10.1378/chest.121.5.1589 [DOI] [PubMed] [Google Scholar]
- 31. Momiyama Y, Ohmori R, Fayad ZA, Tanaka N, Kato R, Taniguchi H, Nagata M, Ohsuzu F. Associations between serum lipoprotein(a) levels and the severity of coronary and aortic atherosclerosis. Atherosclerosis. 2012;222:241–244. doi: 10.1016/j.atherosclerosis.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 32. Garg PK, Guan W, Karger AB, Steffen BT, Budoff M, Tsai MY. Lipoprotein (a) and risk for calcification of the coronary arteries, mitral valve, and thoracic aorta: the Multi‐Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. 2021;15:154–160. doi: 10.1016/j.jcct.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. ACC/AHA guideline on the primary prevention of cardiovascular disease. J Am Coll Cardiol. 2019;2019. doi: 10.1016/j.jacc.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Budoff MJ, Young R, Burke G, Jeffrey Carr J, Detrano RC, Folsom AR, Kronmal R, Lima JAC, Liu KJ, McClelland RL, et al. Ten‐year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi‐ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39:2401–2408. doi: 10.1093/eurheartj/ehy217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santos RD, Rumberger JA, Budoff MJ, Shaw LJ, Orakzai SH, Berman D, Raggi P, Blumenthal RS, Nasir K. Thoracic aorta calcification detected by electron beam tomography predicts all‐cause mortality. Atherosclerosis. 2010;209:131–135. doi: 10.1016/j.atherosclerosis.2009.08.025 [DOI] [PubMed] [Google Scholar]
- 36. Thanassoulis G. Lipoprotein (a) in calcific aortic valve disease: from genomics to novel drug target for aortic stenosis. J Lipid Res. 2016;57:917–924. doi: 10.1194/jlr.R051870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kamstrup PR, Tybjærg‐Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–477. doi: 10.1016/j.jacc.2013.09.038 [DOI] [PubMed] [Google Scholar]
- 38. Chen HY, Engert JC, Thanassoulis G. Risk factors for valvular calcification. Curr Opin Endocrinol Diabetes Obes. 2019;26:96–102. doi: 10.1097/MED.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guan W, Cao J, Steffen BT, Post WS, Stein JH, Tattersall MC, Kaufman JD, McConnell JP, Hoefner DM, Warnick R, et al. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the multi‐ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:996–1001. doi: 10.1161/ATVBAHA.114.304785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A‐I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104:1108–1113. doi: 10.1161/hc3501.095214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figure S1


