Abstract
Background
Atrial and ventricular arrhythmias are commonly encountered in patients with advanced heart failure, with amiodarone being the most commonly used antiarrhythmic drug in continuous‐flow left ventricular assist device (CF‐LVAD) recipients. The purpose of this study was to assess the impact of amiodarone use on long‐term all‐cause mortality in ptients with a CF‐LVAD.
Methods and Results
A retrospective multicenter study of CF‐LVAD was conducted at 5 centers including all CF‐LVAD implants from 2007 to 2015. Patients were stratified based on pre–CF‐LVAD implant amiodarone use. Additional use of amiodarone after CF‐LVAD implantation was also evaluated. Primary outcome was all‐cause mortality during long‐term follow‐up. Kaplan‐Meier curves were used to assess survival outcomes. Multivariable Cox regression was used to identify predictors of outcomes. Propensity matching was done to address baseline differences. A total of 480 patients with a CF‐LVAD (aged 58±13 years, 81% men) were included. Of these, 170 (35.4%) were on chronic amiodarone therapy at the time of CF‐LVAD implant, and 310 (64.6%) were not on amiodarone. Rate of all‐cause mortality over the follow‐up period was 32.9% in the amiodarone group compared with 29.6% in those not on amiodarone (P=0.008). Similar results were noted in the propensity‐matched group (log‐rank, P=0.04). On multivariable Cox regression analysis, amiodarone use at baseline was independently associated with all‐cause mortality (hazard ratio, 1.68 [95% CI, 1.1–2.5]; P=0.01).
Conclusions
Amiodarone use was associated with significantly increased rates of all‐cause mortality in CF‐LVAD recipients. Earlier interventions for arrhythmias to avoid long‐term amiodarone exposure may improve long‐term outcomes in CF‐LVAD recipients and needs further study.
Keywords: amiodarone, arrhythmias, left ventricular assist device, mortality
Subject Categories: Arrhythmias, Cardiomyopathy, Pharmacology
Nonstandard Abbreviations and Acronyms
- AA
atrial arrhythmia
- CF‐LVAD
continuous‐flow left ventricular assist device
- LVAD
left ventricular assist device
- VA
ventricular arrhythmia
Clinical Perspective
What Is New?
Amiodarone use for atrial and ventricular arrhythmias in patients with advanced heart failure with continuous‐flow left ventricular assist devices is associated with increased all‐cause mortality.
Use of amiodarone before a continuous‐flow left ventricular assist device is associated with higher rates of death compared with initiation of amiodarone after a continuous‐flow left ventricular assist device.
What Are the Clinical Implications?
These results should prompt a careful evaluation of the risks and benefits of amiodarone use and reconsideration of the management strategies for atrial and ventricular arrhythmias in continuous‐flow left ventricular assist device recipients.
Amiodarone is a commonly used antiarrhythmic drug for suppressing atrial arrhythmias (AAs) and ventricular arrhythmias (VAs) in patients with advanced heart failure (HF), including those with continuous‐flow left ventricular assist devices (CF‐LVADs). Although effective, amiodarone use is associated with a high incidence (10% in 1 year to 50% with long‐term use) of both cardiac and extracardiac adverse effects. 1 In patients with advanced HF, antiarrhythmic drug options are limited, and amiodarone remains the most frequently used drug despite prior studies showing no significant improvement in long‐term outcomes. 2 In a recent analysis of the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) database of LVAD recipients, amiodarone use was associated with significantly reduced long‐term survival, but further details on indications and timing of use was not available. 3 In addition, limited data are available on the impact of amiodarone use before LVAD implantation on long‐term clinical outcomes in patients with a CF‐LVAD. The purpose of the current study was to assess the impact of amiodarone use at baseline as well as following LVAD implantation on clinical outcomes in a multicenter CF‐LVAD cohort.
Methods
We conducted a multicenter, retrospective study including patients who underwent CF‐LVAD implantation at 5 centers from 2007 to 2015. The study protocol, including complete waiver of informed consent, was approved by the institutional review boards at all the centers. All patients had CF‐LVADs implanted either as a bridge‐to‐transplantation or as destination therapy. Implanted CF‐LVADs included HeartMate II (Abbott Medical, Chicago, IL) and HVAD (Medtronic, Minneapolis, MN). The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Patients were divided into 2 groups based on pre‐LVAD implant chronic amiodarone use. Impact of pre‐LVAD amiodarone status on survival, hospitalizations, and incidence of post‐LVAD AA and VA were evaluated. In patients who were on amiodarone before LVAD implant, we also evaluated whether continued post‐LVAD use of amiodarone was associated with adverse outcomes. Primary outcome of the study was all‐cause mortality. The day of the CF‐LVAD implant marked the start date for follow‐up. The last day of follow‐up was the date of heart transplantation, CF‐LVAD explantation, or date of death, whichever came first.
Statistical Analysis
Kaplan‐Meier curves were used to assess survival outcomes, and the log‐rank test was used to compare survival estimates. Multivariable Cox regression modeling was used to identify predictors of outcomes. For the multivariable Cox model, all baseline demographic and clinical variables (reported in Table 1) were considered. Those baseline variables with significant difference between groups, defined as P value <0.1, were included in the multivariable Cox model. To further address the difference between the groups at baseline, we performed propensity matching between the groups. We initially generated a logistic regression model to identify propensity scores for individual patients based on their study group. We then used the individual propensity scores to perform a 1:1 matching of the patients. The final matched cohort had 244 patients (122 in each group).
Table 1.
Baseline Characteristics of the Study Population Stratified by Baseline Use of Amiodarone
| Characteristic | No‐amiodarone group, N=310 | Amiodarone group, N=170 | P value |
|---|---|---|---|
| Age, y | 56.7±14.1 | 60.3±11.9 | 0.01 |
| Male sex, % | 79% | 86% | 0.06 |
| White race, % | 63% | 67% | 0.7 |
| BMI, median, kg/m2 | 28.4 | 29.2 | 0.6 |
| Nonischemic cardiomyopathy, % | 50% | 43% | 0.06 |
| Diabetes, % | 42% | 47% | 0.27 |
| Hypertension, % | 65% | 68% | 0.4 |
| CAD, % | 56% | 62% | 0.2 |
| CKD, % | 39% | 52% | 0.009 |
| Destination therapy, % | 50% | 53% | 0.5 |
| CRTD, % | 50% | 62% | 0.009 |
| Other antiarrhythmic drugs, % | 5% | 11% | 0.02 |
| β‐blockers, % | 85% | 86% | 0.6 |
| PR, ms | 159.6±43.2 | 157.5±49.2 | 0.8 |
| QRS, ms | 137.1±34.5 | 155.9±34.3 | <0.0001 |
| QTC, ms | 513.7±60.9 | 532.2±67.5 | 0.002 |
| PreVAD LVEF, % | 16±6 | 16.4±6.7 | 0.7 |
| PreVAD LVEDD, cm | 7.1±1.0 | 7.1±1.0 | 0.9 |
| PreVAD LVESD, cm | 6.4±1.1 | 6.5+/−1.1 | 0.8 |
| AA, % | 57% | 59% | 0.6 |
| VA, % | 30% | 48% | <0.0001 |
| LVAD support, median, d | 469 | 489 | 0.4 |
AA indicates atrial arrhythmia; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; CRTD, cardiac resynchronization therapy defibrillator; LVAD, left ventricular assist device; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; VA, ventricular arrhythmias, and VAD, ventricular assist device.
Results
A total of 480 patients with a CF‐LVAD (aged 58±13 years, 81% men) were included. Two‐thirds of the patients were White, and about half had ischemic cardiomyopathy. Median duration of LVAD support was 479 days (interquartile range, 224–965 days). Of these 480 patients, 170 (35.4%) were on chronic amiodarone therapy at the time of LVAD implant, and 310 (64.6%) were not on amiodarone. Baseline (before LVAD implant) characteristics of the patients in the amiodarone and no‐amiodarone groups are presented in Table 1. Patients who were on chronic amiodarone therapy were older (aged 60±12 years versus 57±14 years, P=0.01) and had a higher prevalence of renal insufficiency (52% versus 39%, P=0.009). Rates of AA were similar between the groups, but patients in the amiodarone group had a higher prevalence of VA (48% versus 30%, P=0.0001).
All‐Cause Mortality
The rate of all‐cause mortality over the follow‐up period was 32.9% (n=56) in the amiodarone group compared with 29.6% (n=92) in those not on amiodarone. Kaplan‐Meir survival analyses showed a significantly higher rate of all‐cause mortality (P=0.008) in patients who were on amiodarone at the time of LVAD implantation (Figure 1).
Figure 1. Kaplan‐Meier analysis showing increased all‐cause mortality during follow‐up in patients with a left ventricular assist device stratified by use of amiodarone at baseline (amiodarone group [1] vs no‐amiodarone group [0]; log‐rank, P=0.008).
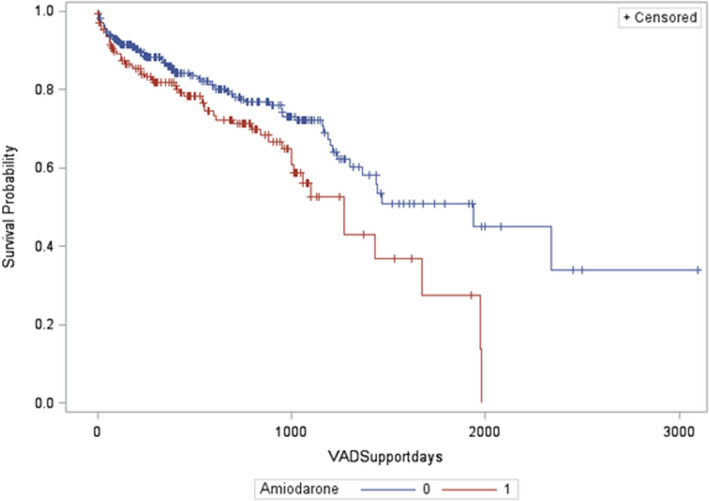
VAD indicates ventricular assist device.
Amiodarone Use After LVAD Implant
Among patients in the amiodarone group, the drug was continued after LVAD implantation in 70%. In patients not on amiodarone, it was started after LVAD implantation in 36%. All‐cause mortality based on before and after LVAD implantation amiodarone status is depicted in Figure 2. Patients in the amiodarone group who were continued on it after LVAD implantation had higher rates of all‐cause mortality compared with those in whom it was discontinued (log‐rank P=0.03; Figure 2). Interestingly, patients with amiodarone use before LVAD implantation had higher mortality rates compared with those in whom amiodarone was started after LVAD implantation. Following LVAD implantation, patients in the amiodarone group continued to have a higher incidence of VA (51% versus 37%, P=0.003; Table 2), but all‐cause and cardiac hospitalizations and incidence of AA were comparable between the groups.
Figure 2. Kaplan‐Meier analysis of all‐cause mortality in patients with a left ventricular assist device (LVAD) stratified by before and after left ventricular assist device use of amiodarone (log‐rank, P=0.03).
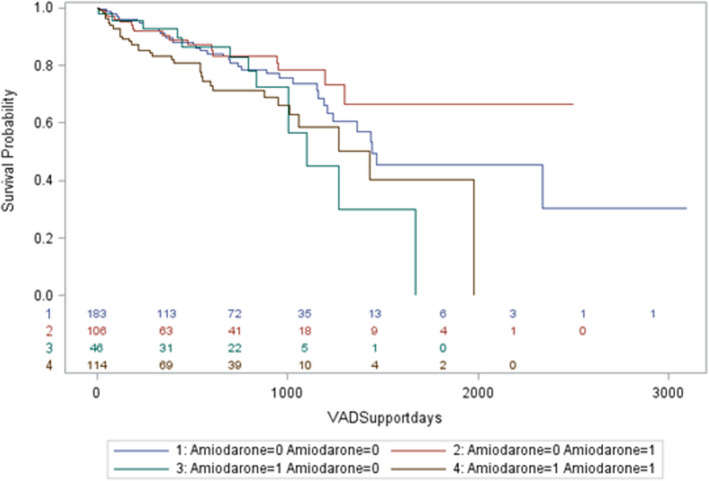
(1) No amiodarone use before or after LVAD. (2) No amiodarone use before LVAD, was on amiodarone after LVAD. (3) Amiodarone use at baseline (before LVAD); no amiodarone use after LVAD. (4) Amiodarone use before and after LVAD. VAD indicates ventricular assist device.
Table 2.
Incidence of Arrhythmias and Hospitalizations After Left Ventricular Assist Device Implantation in the Study Population
| Variable | No‐amiodarone group, N=310 | Amiodarone group, N=170 | P value |
|---|---|---|---|
| Post‐VAD AA | 55% | 54% | 0.9 |
| Post‐VAD VA | 37% | 51% | 0.003 |
| Total hospitalizations, median | 3 | 2 | 0.65 |
| No. of cardiac hospitalizations, median | 1 | 1 | 0.61 |
| Total hospitalization per 100 d of VAD support | 0.52 (0.22–0.99) | 0.62 (0.30–1.23) | 0.08 |
| Cardiac hospitalization per 100 d of VAD support | 0.17 (0–0.44) | 0.17 (0–0.45) | 0.87 |
AA indicates atrial arrhythmia; VA, ventricular arrhythmia; and VAD, ventricular assist device.
Predictors of Mortality
On multivariable Cox regression analysis accounting for age, baseline comorbidities, type of cardiac implantable electronic device, before LVAD VAs, and use of other antiarrhythmic medications, amiodarone use at baseline was the only variable that was independently associated with all‐cause mortality (hazard ratio, 1.68 [95% CI, 1.1–2.5]; P=0.01; Table 3).
Table 3.
Multivariable Cox Regression Analysis Evaluating Predictors of Mortality
| Parameter | Hazard ratio | Hazard ratio confidence limits | P value | |
|---|---|---|---|---|
| Age at implant | 1.015 | 0.999 | 1.031 | 0.06 |
| Sex, men=1, women=2 | 1.297 | 0.813 | 2.069 | 0.27 |
| CKD | 1.243 | 0.855 | 1.807 | 0.25 |
| ICD vs CRT | 1.478 | 0.968 | 2.256 | 0.07 |
| Other antiarrhythmic | 0.844 | 0.380 | 1.871 | 0.68 |
| QRS | 0.994 | 0.987 | 1.000 | 0.06 |
| QTc | 0.999 | 0.996 | 1.003 | 0.77 |
| Amiodarone | 1.683 | 1.129 | 2.508 | 0.01 |
| β‐blocker use | 0.792 | 0.478 | 1.310 | 0.36 |
| Pre‐LVAD VA | 1.196 | 0.793 | 1.804 | 0.39 |
CKD indicates chronic kidney disease; CRTD, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter‐defibrillator; LVAD, left ventricular assist device; and VA, ventricular arrhythmias.
Propensity‐Matched Cohort
A propensity‐matched analysis was performed, matching 22 baseline demographic and clinical variables. The matched cohort included 244 patients, 122 each in the amiodarone and no‐amiodarone group. There were no baseline differences between the groups (Table 4). Kaplan‐Meier analysis showed that patients in the amiodarone group had significantly lower survival when compared with the no‐amiodarone group (log‐rank, P=0.04; Figure 3).
Table 4.
Baseline Characteristics of the Propensity‐Matched Cohort (n=244) Stratified by Baseline Use of Amiodarone
| Characteristic | No‐amiodarone group, N=122 | Amiodarone group, N=122 | P value |
|---|---|---|---|
| Age, y | 63 (55–69) | 62 (52–70) | 0.71 |
| Male sex, % | 81% | 86% | 0.29 |
| White race, % | 60% | 73% | 0.07 |
| BMI, median, kg/m2 | 28 (25–32) | 28 (24–32) | 0.28 |
| Nonischemic cardiomyopathy, % | 56% | 55% | 0.79 |
| Diabetes, % | 50% | 45% | 0.44 |
| Hypertension, % | 74% | 68% | 0.32 |
| CAD, % | 61% | 60% | 0.79 |
| CKD, % | 48% | 46% | 0.79 |
| Destination therapy, % | 48% | 50% | 0.84 |
| CRTD, % | 65% | 63% | 0.78 |
| Other antiarrhythmic drugs, % | 8% | 9% | 0.81 |
| β‐blockers, % | 84% | 84% | 0.86 |
| PR, ms | 160 (120–190) | 160 (128–180) | 0.85 |
| QRS, ms | 158 (126–178) | 156 (125–177) | 0.95 |
| QTC, ms | 521 (490–568) | 539 (483–579) | 0.38 |
| Pre‐VAD LVEF, % | 15 (11–20) | 15 (12–18) | 0.48 |
| Pre‐VAD LVEDD, cm | 7.0 (6.4–7.7) | 7.0 (6.3–7.7) | 0.66 |
| Pre‐VAD LVESD, cm | 6.3 (5.7–7.0) | 6.4 (5.6–7.2) | 0.86 |
| AA, % | 55% | 54% | 0.89 |
| VA, % | 65% | 65% | 1 |
| LVAD support, median, d | 526 (219–956) | 494 (183–881) | 0.4 |
AA indicates atrial arrhythmias; BMI indicates body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; CRTD, cardiac resynchronization therapy defibrillator; LVAD, left ventricular assist device; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; VA, Ventricular arrhythmias, and VAD, ventricular assist device.
Figure 3. Kaplan‐Meier analysis showing increased all‐cause mortality during follow‐up in the propensity‐matched left ventricular assist device cohort stratified by use of amiodarone at baseline (amiodarone group [1] vs no‐amiodarone group [0]; log‐rank, P=0.04).
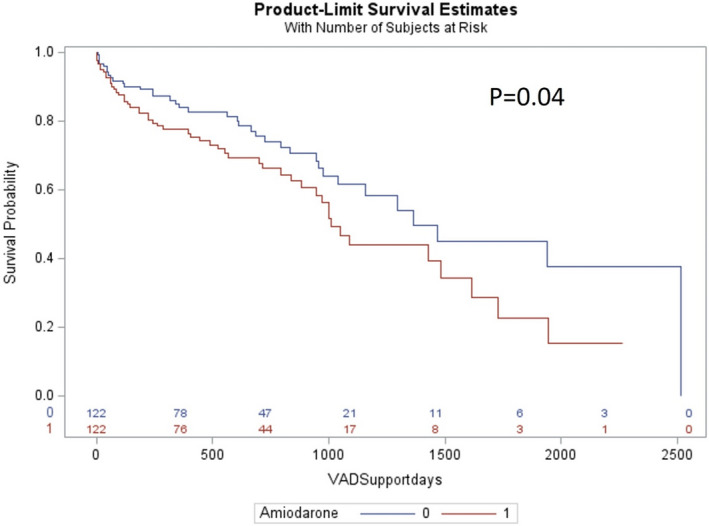
VAD indicates ventricular assist device.
Discussion
In this large multicenter cohort of patients with a CF‐LVAD, we report a significantly higher all‐cause mortality among patients on chronic amiodarone therapy before LVAD implantation. After adjusting for multiple comorbidities and performing a propensity‐matched analysis, amiodarone use before LVAD implantation was an independent predictor of death following CF‐LVAD implantation. Interestingly, amiodarone initiation after LVAD implantation for new arrhythmias did not affect long‐term mortality.
Despite a high incidence of VAs in patients with an LVAD, data are limited in terms of optimal management strategies and are limited to small observational studies. Most patients undergoing evaluation for durable long‐term mechanical support devices have long‐standing HF with significant adverse ventricular remodeling that acts as an arrhythmic substrate. Both AAs and VAs are commonly encountered in this population, with significant practice variation in management. Pharmacological approaches to management of VAs in this population with advanced HF is limited by concomitant comorbidities, drug interactions, and proarrhythmic and systemic side effects. 4 , 5 Amiodarone remains the most commonly used drug in this patient population because of all these factors and relatively benign short‐term side effects. 6 , 7 , 8 , 9 Consistent with this general practice, 48% of patients in the amiodarone group had VA compared with 30% in the no‐amiodarone group in our cohort. Patients with pre‐LVAD VAs have also been shown to have a higher incidence of post‐LVAD VAs in prior studies, advocating for continued use of implantable cardioverter‐defibrillator therapy in this population. 10 , 11 , 12 Even in our cohort, patients with pre‐LVAD VAs who were on amiodarone at baseline had a higher incidence of post‐LVAD VAs compared with those without VAs at baseline.
Findings of our current study showing that amiodarone use before LVAD implantation being an independent risk factor for post‐LVAD mortality should provide pause to address optimal management of VAs in patients with advanced HF progressing toward an LVAD implant. These findings are in accordance with other studies evaluating the effect of amiodarone in end‐stage HF populations undergoing LVAD implantation or heart transplantation. 3 , 13 , 14 Catheter ablation of VAs has emerged as a successful management strategy for VAs, with multiple recent trials demonstrating safety and efficacy. 15 However, existing data on efficacy of catheter ablation in patients with CF‐LVADs are limited to observational studies with variable outcomes. 16 Pooled analysis of data from individual cohorts of LVAD VA ablation have shown that only a quarter of the post‐LVAD VAs are related to the surgical cannula site, whereas the rest are secondary to preexisting cardiomyopathy substrate. 16 Catheter ablation in LVAD recipients is technically challenging. Approaches to the left ventricle are often limited to transseptal access, with careful attention to avoid the cannula during catheter manipulation. Artifacts produced by the LVAD also interfere with optimal electrocardiogram interpretation. Given these limitations and the adverse effects of long‐term amiodarone therapy, whether early catheter ablation of VAs in patients with advanced HF being considered for potential LVAD therapy improves outcomes needs to be studied.
Early primary prevention trials in patients with HF have shown a reduction in arrhythmic mortality with use of amiodarone, primarily in the era before the implantable cardioverter‐defibrillator. This benefit was seen predominantly in patients with nonischemic cardiomyopathy but did not translate to a reduction in all‐cause mortality, likely because of nonarrhythmic deaths from drug toxicity. 17 , 18 Even in these trials, there was a hint toward worse outcomes with the use of amiodarone in patients with advanced HF. For instance, in the SCD‐HeFT (Sudden Cardiac Death in Heart Failure Trial), patients with HF were allocated to amiodarone versus implantable cardioverter‐defibrillator versus placebo and stratified by functional class as a marker of HF severity. In functional class III patients, amiodarone use was associated with a significant increase in mortality. 19 Results of our study are in alignment with these findings, with chronic amiodarone use linked to higher mortality rates overall in patients with advanced HF.
Another important finding in our study is that a quarter (27%) of patients in the amiodarone group had pre‐LVAD AAs only with no documented VAs. Management of AAs, such as atrial fibrillation, in patients with HF is limited by antiarrhythmic medication options, often necessitating use of amiodarone. Catheter ablation outcomes is an appealing alternative in these patients to avoid long‐term amiodarone use. 20 Over the past few years, multiple studies, including large randomized trials, have demonstrated a significant beneficial effect of rhythm control with catheter ablation compared with antiarrhythmic drugs, especially in patients with HF. In the CASTLE‐AF (Catheter Ablation versus Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation) trial, patients with HF and left ventricular ejection fraction <35% were randomized to catheter ablation versus antiarrhythmic drugs for management of atrial fibrillation. Patients randomized to catheter ablation had a 38% overall reduction in the primary outcome of death or HF hospitalization. 21 In a subanalysis, this benefit did not meet statistical significance in patients on amiodarone. Similar results of improved overall outcomes with catheter ablation for atrial fibrillation in patients with HF have also been shown in the CABANA (Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation) trial. 22 Given these data and our current findings of worse long‐term outcomes with amiodarone use, early catheter ablation for AF in patients with advanced HF being considered for an LVAD implant could be a reasonable strategy and needs further study.
A common theme from most HF studies is that any antiarrhythmic benefit of amiodarone is offset by long‐term drug toxicity. In a summary of trials studying amiodarone for the primary prevention of sudden death, pulmonary toxicity was seen in 2.9%, thyroid toxicity in 3.6%, and hepatic toxicity in 1.8%. 23 A second issue could be significant drug interactions with medications such as digoxin and warfarin. A third factor to consider is the effect of amiodarone on cardiac repolarization. In our study, the amiodarone group had prolonged QTc and QRS compared with the no‐amiodarone group. Whether additional amiodarone‐induced QT prolongation increases further risk of VAs in this group is unclear. Finally, chronic use of amiodarone has been shown to increase defibrillation thresholds. 4 Thus, any potential benefits of amiodarone in patients with advanced HF must be tempered against these potential risks.
Limitations
We acknowledge several limitations inherent to the retrospective nature of this study. Adjudication of cause of death as arrhythmic versus nonarrhythmic was not available. Data on other management strategies for VAs, such as use of catheter ablation, were not available. Our findings need confirmation in other populations. It is possible that patients in the amiodarone group represented a sicker substrate to begin with. However, no significant baseline difference between groups in cardiomyopathy type, left ventricular dimensions, and LVAD indication was noted, and multivariable Cox regression analysis identified baseline amiodarone use as an independent predictor of mortality. Moreover, the results of the propensity‐matched cohort of 244 patients, which matched 22 baseline characteristics potentially contributing to increased sickness and frailty, also showed significantly reduced survival in the amiodarone group. Lastly, unrecognized systemic toxicity as well as drug interactions, in particular warfarin, in the post‐LVAD population may have played a role.
Conclusions
In this large, multicenter CF‐LVAD cohort, baseline use of amiodarone was associated with reduced survival after LVAD implantation. These findings should prompt a reconsideration of the management strategy for AAs and VAs in patients undergoing CF‐LVAD implantation.
Sources of Funding
None.
Disclosures
Dr Gopinathannair: consultant/honoraria, Abbott Medical, American Heart Association, Biotronik, Boston Scientific, and Zoll Medical. Dr Roukoz: consultant, Medtronic and Boston Scientific. Dr Al Ahmad: honoraria, Medtronic, Boston Scientific, and Abbott Medical. Dr Natale: consultant/honoraria, Medtronic, Boston Scientific, Abbott Medical, Biosense Webster, Biotronik, and Baylis. Dr Di Biase: consultant/honoraria/travel, Medtronic, Boston Scientific, Abbott Medical, Biosense Webster, Biotronik, Atricure, Pfizer, and Bristol Myers Squibb. Dr Slaughter: education/training grant, Heartware Inc. Dr Lakkireddy: consultant/honoraria, Abiomed, Biosense Webster, Boston Scientific, Biotronik, Janssen, and Abbott Medical. The other authors have no disclosures to report.
Presented in part at the Heart Rhythm 2017 Annual Scientific Sessions in Chicago, Illinois, from May 10 to 13, 2017, and published in abstract form [Heart Rhythm. 2017; 14:661–667. doi: 10.1016/j.hrthm.2017.01.003].
For Sources of Funding and Disclosures, see page 8.
References
- 1. Goldschlager N, Epstein AE, Naccarelli GV, Olshansky B, Singh B, Collard HR, Murphy E. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm. 2007;4:1250–1259. doi: 10.1016/j.hrthm.2007.07.020 [DOI] [PubMed] [Google Scholar]
- 2. Piccini JP, Berger JS, O'Connor CM. Amiodarone for the prevention of sudden cardiac death: a meta‐analysis of randomized controlled trials. Eur Heart J. 2009;30:1245–1253. doi: 10.1093/eurheartj/ehp100 [DOI] [PubMed] [Google Scholar]
- 3. Xia Y, Forest S, Friedmann P, Chou LC, Patel S, Jorde U, Goldstein D. Factors associated with prolonged survival in left ventricular assist device recipients. Ann Thorac Surg. 2019;107:519–526. doi: 10.1016/j.athoracsur.2018.08.054 [DOI] [PubMed] [Google Scholar]
- 4. Hohnloser SH, Dorian P, Roberts R, Gent M, Israel CW, Fain E, Champagne J, Connolly SJ. Effect of amiodarone and sotalol on ventricular defibrillation threshold: the optimal pharmacological therapy in cardioverter defibrillator patients (OPTIC) trial. Circulation. 2006;114:104–109. doi: 10.1161/CIRCULATIONAHA.106.618421 [DOI] [PubMed] [Google Scholar]
- 5. Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, Greene HL, Boczor S, Domanski M, Follmann D, et al. Meta‐analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21:2071–2078. doi: 10.1053/euhj.2000.2476 [DOI] [PubMed] [Google Scholar]
- 6. Raasch H, Jensen BC, Chang PP, Mounsey JP, Gehi AK, Chung EH, Sheridan BC, Bowen A, Katz JN. Epidemiology, management, and outcomes of sustained ventricular arrhythmias after continuous‐flow left ventricular assist device implantation. Am Heart J. 2012;164:373–378. doi: 10.1016/j.ahj.2012.06.018 [DOI] [PubMed] [Google Scholar]
- 7. Refaat M, Chemaly E, Lebeche D, Gwathmey JK, Hajjar RJ. Ventricular arrhythmias after left ventricular assist device implantation. Pacing Clin Electrophysiol. 2008;31:1246–1252. doi: 10.1111/j.1540-8159.2008.01173.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andersen M, Videbaek R, Boesgaard S, Sander K, Hansen PB, Gustafsson F. Incidence of ventricular arrhythmias in patients on long‐term support with a continuous‐flow assist device (HeartMate II). J Heart Lung Transplant. 2009;28:733–735. doi: 10.1016/j.healun.2009.03.011 [DOI] [PubMed] [Google Scholar]
- 9. Cantillon DJ, Tarakji KG, Kumbhani DJ, Smedira NG, Starling RC, Wilkoff BL. Improved survival among ventricular assist device recipients with a concomitant implantable cardioverter‐defibrillator. Heart Rhythm. 2010;7:466–471. doi: 10.1016/j.hrthm.2009.12.022 [DOI] [PubMed] [Google Scholar]
- 10. Oswald H, Schultz‐Wildelau C, Gardiwal A, Lusebrink U, Konig T, Meyer A, Duncker D, Pichlmaier MA, Klein G, Struber M. Implantable defibrillator therapy for ventricular tachyarrhythmia in left ventricular assist device patients. Eur J Heart Fail. 2010;12:593–599. doi: 10.1093/eurjhf/hfq048 [DOI] [PubMed] [Google Scholar]
- 11. Nakahara S, Chien C, Gelow J, Dalouk K, Henrikson CA, Mudd J, Stecker EC. Ventricular arrhythmias after left ventricular assist device. Circ Arrhythm Electrophysiol. 2013;6:648–654. doi: 10.1161/CIRCEP.113.000113 [DOI] [PubMed] [Google Scholar]
- 12. Kadado AJ, Akar JG, Hummel JP. Arrhythmias after left ventricular assist device implantation: incidence and management. Trends Cardiovasc Med. 2018;28:41–50. doi: 10.1016/j.tcm.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 13. Cooper LB, Mentz RJ, Edwards LB, Wilk AR, Rogers JG, Patel CB, Milano CA, Hernandez AF, Stehlik J, Lund LH. Amiodarone use in patients listed for heart transplant is associated with increased 1‐year post‐transplant mortality. J Heart Lung Transplant. 2017;36:202–210. doi: 10.1016/j.healun.2016.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tzou WS, Tung R, Frankel DS, Vaseghi M, Bunch TJ, Di Biase L, Tholakanahalli VN, Lakkireddy D, Dickfeld T, Saliaris A, et al. Ventricular tachycardia ablation in severe heart failure: an international ventricular tachycardia ablation center collaboration analysis. Circ Arrhythm Electrophysiol. 2017;10. doi: 10.1161/CIRCEP.116.004494 [DOI] [PubMed] [Google Scholar]
- 15. Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin J‐F, Thibault B, Rivard L, Gula L, Leong‐Sit P, et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375:111–121. doi: 10.1056/NEJMoa1513614 [DOI] [PubMed] [Google Scholar]
- 16. Gopinathannair R, Cornwell WK, Dukes JW, Ellis CR, Hickey KT, Joglar JA, Pagani FD, Roukoz H, Slaughter MS, Patton KK. Device therapy and arrhythmia management in left ventricular assist device recipients: a scientific statement from the American Heart Association. Circulation. 2019;139:e967–e989. doi: 10.1056/NEJM199507133330201 [DOI] [PubMed] [Google Scholar]
- 17. Singh SN, Fletcher RD, Fisher SG, Singh BN, Lewis HD, Deedwania PC, Massie BM, Colling C, Lazzeri D. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival trial of antiarrhythmic therapy in congestive heart failure. N Engl J Med. 1995;333:77–82. doi: 10.1056/NEJM199507133330201 [DOI] [PubMed] [Google Scholar]
- 18. Julian DG, Camm AJ, Frangin G, Janse MJ, Munoz A, Schwartz PJ, Simon P. Randomised trial of effect of amiodarone on mortality in patients with left‐ventricular dysfunction after recent myocardial infarction: EMIAT. European Myocardial Infarct Amiodarone Trial Investigators. Lancet. 1997;349:667–674. doi: 10.1016/S0140-6736(96)09145-3 [DOI] [PubMed] [Google Scholar]
- 19. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, et al. Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399 [DOI] [PubMed] [Google Scholar]
- 20. Gopinathannair R, Chen LY, Chung MK, Cornwell WK, Furie KL, Lakkireddy DR, Marrouche NF, Natale A, Olshansky B, Joglar JA, et al. Managing atrial fibrillation in patients with heart failure and reduced ejection fraction: a scientific statement from the American Heart Association. Circ Arrhythm Electrophysiol. 2021;14:HAE0000000000000078. doi: 10.1161/HAE.0000000000000078 [DOI] [PubMed] [Google Scholar]
- 21. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, et al. Bansch D and Investigators C‐A . Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855 [DOI] [PubMed] [Google Scholar]
- 22. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. doi: 10.1001/jama.2019.0693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Santangeli P, Di Biase L, Burkhardt JD, Bai R, Mohanty P, Pump A, Natale A. Examining the safety of amiodarone. Expert Opin Drug Saf. 2012;11:191–214. doi: 10.1517/14740338.2012.660915 [DOI] [PubMed] [Google Scholar]


