Abstract
In use for over a century, the Wood’s lamp is a time-tested tool to aid in the diagnosis of certain superficial infections, pigmentary disorders, and metabolic diseases. To achieve its high utility, the Wood’s lamp projects ultraviolet light onto the skin which in turn reflects a visible light that a trained eye can use to diagnose and monitor multiple dermatological ailments. Although new alternatives to Wood’s lamp have been considered, it still remains a favored method of diagnosis because it is safe, cost-effective, and reliable. In this review, the authors explore the myriad applications of Wood’s lamp in the field of dermatology.
Keywords: Wood’s lamp, ultraviolet light, fluorescence, phosphor, porphyria, dermatophyte
In 1903, Robert Williams Wood, a well-known American physicist, developed an instrument that produced an apparent paradox: invisible light.1 This light source, the Wood’s lamp, had a special filter comprised of barium silicate with 9% nickel oxide that blocked much of the visible electromagnetic spectrum and allowed transmission of ultraviolet (UV) light.1-2 Wood primarily used his invention in UV photography. Later, the Wood’s lamp found applications in other scientific fields, including criminal forensics, emergency medicine, ophthalmology, gynecology, and veterinary medicine.3-9 However, Wood’s lamp has arguably gained greatest recognition for its role in dermatology, where it is used to diagnose and monitor an array of fungal and bacterial infections, pigmentary conditions, and metabolic disorders.
A brief discussion of electromagnetic radiation is warranted to contextualize the role of the Wood’s lamp. The electromagnetic spectrum comprises a range of energy that radiates, or travels in waves. Arranged within this spectrum are seven energies: radio, microwave, infrared, visible, ultraviolet, X-ray, and gamma. Radio waves have the lowest energy and longest wavelengths, whereas gamma rays have the highest energy and shortest wavelengths. Perceptible by the human retina, visible light has a narrow range from 700nm (red) to 400nm (violet). Slightly more energetic, and invisible, is ultraviolet light. It is in this part of the electromagnetic spectrum that Wood’s lamp emits light, from 320nm to 400nm, with a peak wavelength of 365nm.
PHYSICS OF FLUORESCENCE
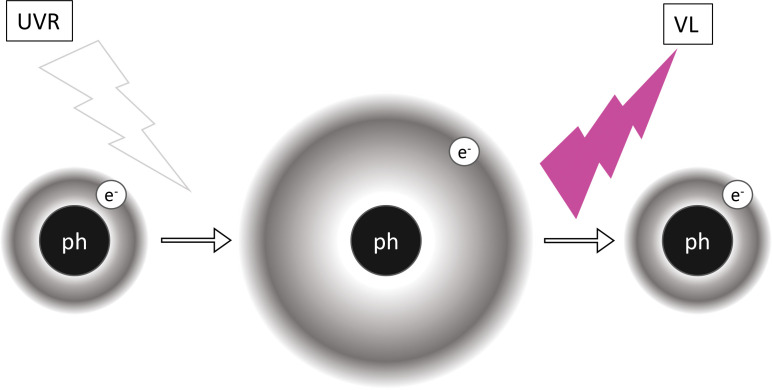
To understand how Wood’s lamp functions, one must take a closer look at what is occurring at a molecular level. When the relatively energetic and short waves of UV light shine on certain substances, known as phosphors, visible light of lower energy and longer wavelength is produced (Figure 1). This is known as fluorescence. Thus, phosphors essentially convert invisible UV light to visible light. The skin contains natural phosphors, such as collagen and elastin.2 To excite other electrons, photons need to have a certain amount of energy. Elastin contains several phosphors, one of which is a crosslinking tricarboxylic amino acid with a pyridinium ring, and collagen’s main phosphor is tyrosine, an amino acid.10-11 Both of these phosphors contain electrons that can be excited by UV light. Once excited, almost immediately the electrons become unstable and seek their lower energy ground state.12 This electronic transition from high to low energy states occurs through vibrational relaxation.13 This energy decay process releases photons of visible light.13
FIGURE 1.
Schematic of electronic transitions during fluorescence. When the relatively more energetic and shorter wavelengths of ultraviolet radiation (UVR) shine on a phosphor (ph), its electrons (e-) are moved to excited states. Since such excited states are unstable, electrons soon revert to their ground states. Energy decays in the system via vibrational relaxation and other means, and the remainder is emitted in relatively less energetic and longer wavelengths of visible light (VL).
ALTERNATIVES
A traditional Wood’s lamp contains Wood’s glass, which is a mixture of barium-sodium-silicate glass and 9% nickel oxide.1-2 This glass coats the inside of tubes through which the UV light is transmitted. However, Wood’s glass is unique in that it blocks much of the visible light passing through the filter. A recent study has suggested that ordinary blacklights are a less expensive and comparable alternative to Wood’s lamp.14 All blacklights, including light emitting diodes, fluorescent light bulbs, and blacklight blue bulbs, emit UV light, similar to Wood’s lamp.15 However, not all blacklights emit the same intensity and peak wavelength of UV light; each blacklight produces slightly different fluorescence.16 Wood’s lamp produces a peak wavelength of 365nm, whereas commercially available blacklight sources can have peak wavelengths of 375nm, 385nm, or 395nm.17 The longer the peak UV wavelength, the more visible light will be produced, which will result in less fluorescence. Blacklights that produce less fluorescence make diagnosing dermatological conditions more difficult. Another difference between a conventional blacklight and Wood’s light is that Wood’s light can magnify objects by 1.5 times.18
Interestingly, a second alternative to Wood’s lamp can be created by simply generating a blue screen background on a smartphone.19,20 Blue light is absorbed well by melanin, however, the luminescence is much harder to see, given the increased levels of visible light.2 Digital screens produce short-wavelength visible light, not UV light.21 A blue screen on a smartphone is a practical option when in a resource-poor setting where the provider may not have access to Wood’s lamp. Clinicians can use an internet search engine to search for “blue image” and download it. Then, the clinician should increase the smartphone screen brightness to the maximum level and turn off the screen timeout setting.19 The rest of the procedure is identical to using a Wood’s lamp; simply darken the room and hold the phone 4 to 5 inches away from the skin. While Wood’s lamp is always the superior option, conventional blacklights and even a blue-lit smartphone screen are acceptable alternatives in resource-poor settings.
PERFORMING AN EXAMINATION
To conduct a Wood’s lamp examination, the provider will need a light source and a dark room.1 As per the manual, it is ideal to allow the lamp to warm up for about one minute.22 Other sources say only 20 seconds is needed.1 It is recommended to hold the Wood’s lamp 4 to 5 inches away from the surface of the skin.1 Many extraneous artifacts could influence the lamp’s fluorescence. For example, if the patient has showered or bathed recently, it will decrease the fluorescence.2 There are also certain medications, detergents, and fibers that will cause inappropriate fluorescence.2 Many substances produce false positives from Wood’s lamp, especially in pediatric dermatology. These include colored markers (highlighters), dried soap and laundry detergents with optical brighteners, hyperkeratotic scale, invisible ink, lemon juice, lint, semen, serum, saliva, milk, cosmetics and hair dyes, select sunscreens and ointments, and wet ear wax.23 When there is unusual fluorescence observed in atypical locations, it is important to consider exposure to these contaminants. Ophthalmologists have indicated that Wood’s lamp has no harmful effects on the superficial structures of the eye.8 However, in pediatric dermatology, the use of UV light protective goggles is recommended to shield the retina, given that children may impulsively look directly into the light.24 Chronic exposure to Wood’s lamp may cause cataract formation and ocular aging.24
APPLICATIONS IN DERMATOLOGY
Literature on Wood’s lamp use in dermatology is discussed in detail below and summarized in Table 1.
TABLE 1.
A summary of Wood’s lamp findings and associations relevant to dermatology
| CONDITION OR USE | ASSOCIATION | WOOD’S LAMP FINDING | SOURCE OF FLUORESCENCE | REFERENCE |
|---|---|---|---|---|
| Bacterial infections | ||||
| Cellulitis after burns, subungual infection, and interdigital infection | Pseudomonas aeruginosa | Green | Pyoverdine | 18, 19 |
| Erythrasma | Corynebacterium minutissimum | Coral-red | Coproporphyrin III | 20, 21 |
| Trichomycosis axillaris | Corynebacterium tenuis and other diphtheroids | Pale yellow | Unknown, possibly gluelike concretions | 23-25 |
| Progressive macular hypomelanosis | Cutibacterium acnes | Orange-red color in pilosebaceous follicles | Coproporphyrin III | 26, 27 |
| Fungal infections | ||||
| Dermatophytosis* | Microsporum audouinii | Blue-green | Pteridine | 2, 28 |
| Microsporum canis | Yellow-green | Tryptophan metabolites | 9 | |
| Microsporum ferrugineum | Yellow-green | Tryptophan metabolites | 32 | |
| Trichophyton schoenleinii | Dull blue | – | 28 | |
| Pityriasis versicolor | Malassezia globosa | Yellow-orange | Pityrialactone | 33, 34 |
| Hypopigmented conditions | ||||
| Vitiligo | - | Blue-white | Lack of melanin | 2, 41 |
| Nevus depigmentosus | - | Dull, off-white | Decrease in melanin | 36 |
| Tuberous sclerosis | - | White ash-leaf macule | Decrease in melanin | 37 |
| Linear scleroderma/ morphea | - | Blue-white | Decrease in melanin | 38-40 |
| Melasma | - | Epidermal: Brown or black, Dermal: Grey-blue | Increase in melanin | 43, 44 |
| Metabolic disorders | ||||
| Congenital erythropoietic porphyria | Uroporphyrinogen III synthase defect | Pink to red | Uroporphyrin I and coproporphyrin I in urine, blood, and enamel | 2, 47-48 |
| Porphyria cutanea tarda | Autosomal dominant or acquired uroporphyrinogen decarboxylase defect | Pink to red | Uroporphyrin I and III in urine; few case reports cite fluorescence of skin or enamel | 2, 47-48 |
| Hepatoerythropoietic porphyria | Autosomal recessive uroporphyrinogen decarboxylase defect | Pink to red | Uroporphyrin I and III in urine, blood, and ename | 46 |
| Erythropoietic protoporphyria | Ferrochelatase defect | Pink to red | Protoporphyrin IX in blood | 49 |
| Miscellaneous conditions | ||||
| Solar urticaria | – | Erythema and urticaria | – | 50 |
| Milia | – | Bright yellow | Keratin in dermis | 51 |
| Annular dermatoses/ neoplasms | Granuloma annulare | Blue-black at the center, red on the edges | Keratin | 13 |
| Lichen planus annulari | Blue-black without a ring structure | |||
| Porokeratosis | Blue-brown at the center and white on the edge | |||
| Skin cancer margins | ||||
| Lentigo maligna melanoma | – | Dark brown and black; Blue-white | Melanin, Loss of melanin in areas of regression | 53 |
| Basal cell carcinoma with in-vivo photodiagnosis | – | Coral-red | Methyl aminolevulinate (MAL) cream | 54 |
| Cosmetic applications | ||||
| Administering chemical peels | Salicylic acid Fluorescein sodium |
Green Yellow/orange |
Salicylic acid Fluorescein sodium |
55 |
| Sunscreen application | - | Blue-white | Sunscreen | 56, 57 |
| Tetracycline compliance | - | Yellow | Tetracycline | 58 |
| Dihydroxyacetone application | - | Salmon | DHA | 59 |
*Most dermatophytoses do not fluoresce.
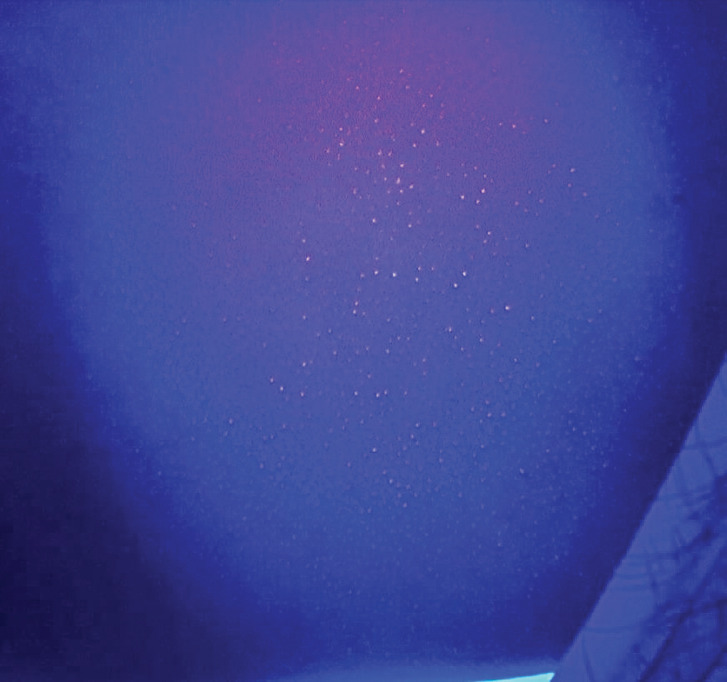
Certain pathogenic bacteria of the skin fluoresce with the use of the Wood’s lamp. Pseudomonas aeruginosa infections are commonly associated with burns, onycholysis, and interdigital spaces. Pseudomonas secretes pyoverdine, a phosphor, which will fluoresce green under Wood’s lamp.25 Thus, green fluorescence, in the proper clinical context, insinuates infection with Pseudomonas (Figure 2).26-28 Similarly, erythrasma is a superficial bacterial infection caused by Corynebacterium minutissimum.29 Erythrasma can mimic other intertriginous dermatoses, such as inverse psoriasis or candidiasis, sometimes making diagnosis difficult.30 However, C. minutissimum produces coproporphyrin III and fluoresces a coral-red color, which elucidates its diagnosis.31 Other causes of intertrigo will not fluoresce similarly.31 Trichomycosis axillaris is a superficial bacterial infection in which small concretions form on axillary hair. Trichomycosis is usually associated with Corynebacterium tenuis and other diphtheroid species.32 Instead of a coral-red fluorescence like erythrasma, trichomycosis axillaris displays a pale-yellow fluorescence under Wood’s lamp.32 Although the precise phosphor in the case of trichomycosis axillaris is unknown, it is postulated to be the actual gluelike concretions, whether bacteriogenic or from apocrine sweat.33-34 Lastly, Cutibacterium acnes causes progressive macular hypomelanosis (PMH).35 This hypopigmentation disorder mimics tinea versicolor and post-inflammatory hypopigmentation.30 Under Wood’s lamp, Cutibacterium acnes fluoresces an orange-red color in pilosebaceous follicles, clinching the diagnosis of PMH (Figure 3).35-36
FIGURE 2.
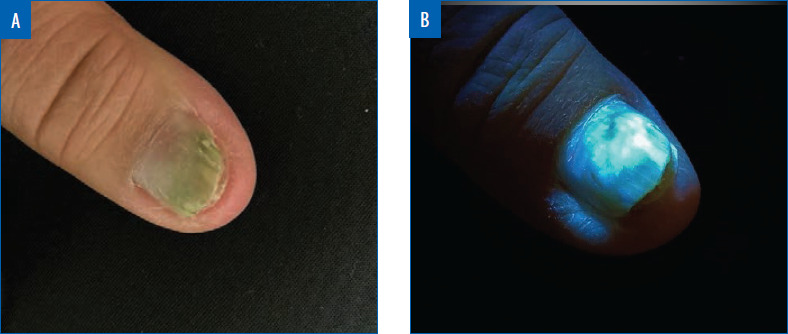
Pseudomonal green nail in normal light (A) and under Wood’s lamp (B). Green fluorescence can be seen under Wood’s lamp.
FIGURE 3.
Cutibacterium acnes on the trunk under Wood’s lamp. Orange-red fluorescence can be seen localized to pilosebaceous units. Photo courtesy of Daniel Chang, MD, FAAD.
In addition to bacterial infections, Wood’s lamp allows for rapid diagnosis of certain fungal infections, especially when compared to the delay associated with fungal cultures. Of the three genera of dermatophytes—Trichophyton, Epidermophyton, and Microsporum—only Microsporum and a few Trichophyton species will produce fluorescence.37 Dermatophytes in the genus Microsporum will produce a blue-green fluorescence from the porphyrin pteridine; most in genus Trichophyton will not produce any fluorescence, although T. schoenleinii fluoresces dull blue and T. verrucosum fluoresces in cattle (not humans).2,38,39 Historically, most cases of tinea capitis in the United States were caused by M. audouinii, and Wood’s lamp provided a reliable and quick diagnosis.40 Currently, T. tonsurans is the leading cause of tinea capitis in the United States and does not fluoresce.40 Therefore, when tinea capitis is suspected, a negative Wood’s lamp test does not rule out this infection, and a fungal culture should be performed. M. ferrugineum is a prominent cause of juvenile tinea capitis in Russia, Asia, Africa, and Eastern Europe.41 This dermatophyte also creates a green-yellow fluorescence due to tryptophan metabolite accumulation.41 Another common superficial fungal skin infection is pityriasis versicolor, also known as tinea versicolor, caused primarily by Malassezia globosa. Wood’s lamp displays a yellow-orange fluorescence with tinea versicolor infections due to the porphyrin pityrialactone.42,43 Wood’s lamp has limited utility with diagnosing onychomycosis since dermatophytes, yeasts, and nondermatophyte molds are all causative agents, and the most common culprits do not fluoresce.44
Pigmentary disorders are a standard purview for Wood’s lamp diagnosis. Skin devoid of melanin fluoresces brightly. In particular, the depigmentation of vitiligo appears strikingly white and sharply delineated under a Wood’s lamp.2 Nevus depigmentosus, a misleading appellation, is often hypopigmented, and thus shows only a dull, off-white glow with Wood’s lamp.45 Similarly, the hypopigmented macules of tuberous sclerosis complex (TSC) can also be accentuated by Wood’s lamp. These elliptical or lancinate macules, termed ash-leaf spots, are more apparent under a Wood’s lamp.46 Whenever there is a history of TSC in a family, a newborn should receive a complete skin examination with a Wood’s lamp to screen for ash-leaf macules.46 Wood’s lamp effectively magnifies hypopigmentation that the unaided eye can miss otherwise.
A Wood’s lamp aids in the evaluation of linear scleroderma and morphea, considering active areas contain loss of pigment.47, 48 Under Wood’s lamp, a new plaque of morphea can be detected before induration is appreciable. Similarly, expanding scleroderma might be detected earlier with Wood’s lamp.49
Apart from diagnosing and delineating disorders of hypopigmentation, Wood’s lamp has further particular use in vitiligo: to assess disease stability, to aid in skin graft harvesting, and to gauge success of surgical grafting itself. A stable disease state is a prerequisite for successful vitiligo surgery and is characterized by sharply demarcated borders.50 Repigmentation after skin grafting procedures may be easily and inexpensively assessed using Wood’s lamp.50 One pilot study utilized Wood’s lamp as an ultraviolet source to improve the speed and quality of suction blister harvesting for use in vitiligo; however, its authors did not enumerate technical parameters, including fluence, of the Wood’s lamp device used. Further, they noted that induction of blisters might have been associated with heat, not UV light, produced by the Wood’s lamp.51
Wood’s lamp assists in diagnosing hyperpigmentation disorders, such as melasma. There is an increase in melanin in the epidermis and/or the dermis with melasma.52 When using Wood’s lamp, epidermal melasma will appear as a sharply circumscribed brown or black patch, and dermal melasma will appear unaccentuated grey-blue.53 Knowing the depth of melanin deposition may help tailor an appropriate treatment regimen.
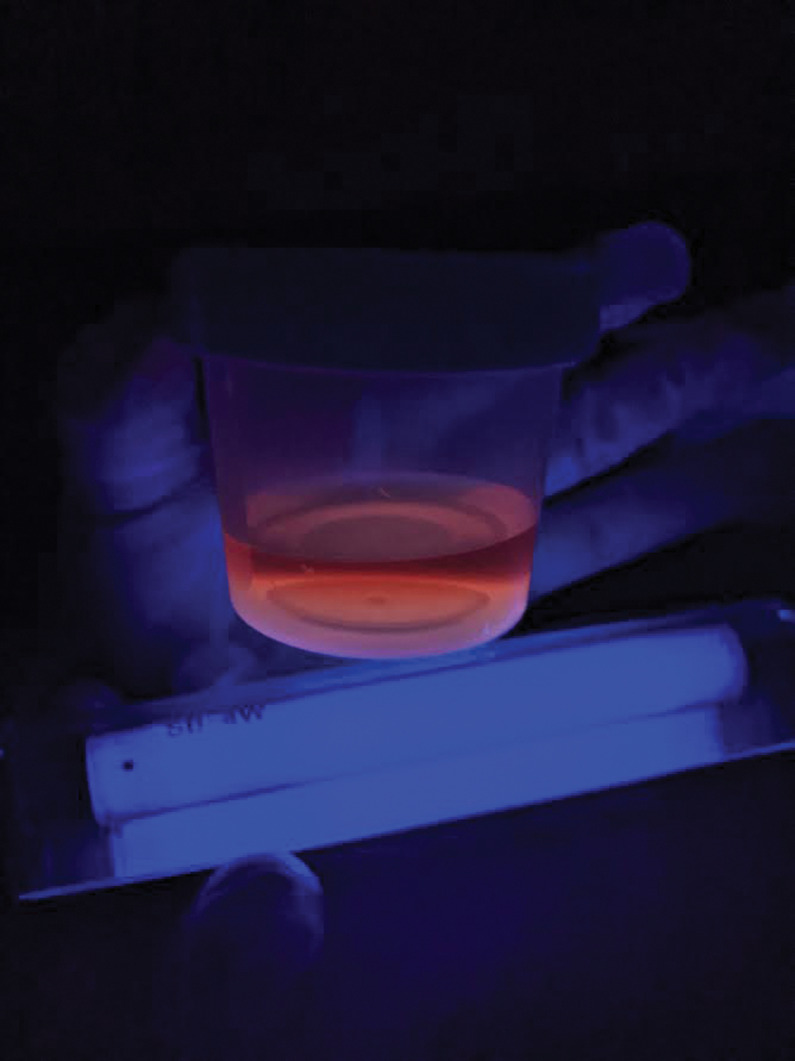
Wood’s lamp has distinctive findings in some cutaneous porphyrias, which arise from enzymatic defects in heme synthesis and subsequent accumulation of photoreactive precursors called porphyrins in tissue, blood, urine, and stool.2 Diagnosis of a specific porphyria in modern medicine may utilize biochemical analysis to identify a specific porphyrin and its ratio in blood, urine, or feces, as well as genetic analysis to identify the specific mutation underlying an enzyme defect. Historically, however, Wood’s lamp highlighted the presence of porphyrins as a classical screening test. Specifically, the porphyrins produced with porphyria cutanea tarda (PCT) fluoresce pink or orange-red in urine and feces under Wood’s lamp (Figure 4). Urine specimens should be shielded from light, and acidification with undiluted acetic acid may enhance fluorescence.2,54-55 Hepatoerythropoietic porphyria, as a biallelic form of PCT, has a more severe phenotype and striking Wood’s lamp findings, including fluorescent red blood cells and erythrodontia.5,6 Congenital erythropoietic porphyria can present with red or violet wet diapers, intense photosensitivity, and similarly fluorescent erythrodontia; urine, blood, and teeth fluoresce bright coral, due to massive amounts of uroporphyrin I and coproporphyrin I.2, 57-58 Skin, teeth, nails, and urine in patients with erythropoietic protoporphyria fail to fluoresce, though affected erythrocytes will.59
FIGURE 4.
Urine in porphyria cutanea tarda emits a pink to red fluorescence. This specimen was not acidified prior to Wood’s lamp examination. Photo courtesy of Brittany Smirnov, DO, FAAD.
Lastly, Wood’s lamp is utilized in many miscellaneous cutaneous conditions. In particular, the diagnosis of solar urticaria includes exposure to UVA light to produce a reaction, so a Wood’s lamp is commonly used.60 A common condition seen in dermatology is milia; which are small cysts caused by retention of keratin in the dermis.61 The keratin produces a bright yellow fluorescence under Wood’s lamp; thus, milia can be differentiated easily from other minute facial papules.61 Finally, one study asserts that a Wood’s lamp can delineate among porokeratosis, granuloma annulare, and lichen planus annularis. Granuloma annulare fluoresces blue-black at the center and red on the edges; lichen planus annularis fluoresces blue-black without a ring structure; porokeratosis fluoresce blue-brown at the center and white on the edge, resembling a diamond.18
SURGICAL APPLICATIONS
Determination of appropriate surgical margins can be aided by Wood’s lamp.62 To answer this question when removing lentigo maligna melanomas, Wood’s lamp is used to outline the borders and excise the full lesion.62 This method will accentuate the hyperpigmentation in the epidermis of the lesion. When trying to take the narrowest margin, the Wood’s lamp is an excellent additional step. In a study, Wood’s lamp was used to delineate the margins before surgical resection of lentigo maligna. In this particular study, only one of the 16 patients had a recurrence of melanoma eight years after surgery and most lesions were resected with a margin of 0.6 to 1.0 cm.63 Along with using Wood’s lamp to delineate neoplastic lesional margins, there is a new pre-operative technique that allows surgeons to only take damaged tissue and keep as much healthy skin as possible. Photodiagnosis uses methyl aminolevulinate (MAL) cream as a phosphor when applied to patients with basal cell carcinoma (BCC) and then this is fluoresced with Wood’s lamp.64 The bright red fluorescence is the main area of cancerous tissue, but there is also a fainter fluorescence termed the “gray zone.”64 Photodiagnosis has allowed surgeons to achieve radical excision in 90 percent of patients by taking the gray zone, without taking too much tissue.64 Wood’s lamp is valuable in surgical procedures when visualizing cancerous margins.
MEDICAL APPLICATIONS
Superficial chemical peels are used frequently in dermatology to address lentigines, rhytides, keratoses, comedonal acne, and dyschromia.65 Wood’s lamp can be used after applying a chemical peel to avoid missing or over-treating areas on the skin.65 Salicylic acid and fluorescein sodium both can be added to chemical peels to create fluorescence. These two peels produce two different fluorescence; salicylic acid creates a green fluorescence, and the fluorescein sodium peel is a yellow-orange color.65 Wood’s lamp can also be used before and after applying the peel to measure its effectiveness based on the depth of pigment.65 Similarly, Wood’s lamp is also able to assess the proper application of sunscreen, especially in relatively inaccessible areas such as the back.66,67 Wood’s lamp is advantageous when evaluating patient adherence to tetracycline by examining the toenails for yellow fluorescence.68 Dihydroxyacetone (DHA) is a chemical in sunless tanning cosmetics and is often used to cover vitiligo lesions.69 Wood’s lamp can determine the actual size of vitiligo lesions, as DHA fluoresces a salmon color and vitiligo lesions are bright blue-white.69
CONCLUSION
Wood’s lamp is an inexpensive yet invaluable asset in a dermatologist’s arsenal. It is easy to use, safe, cost effective, and yields rapid results. Its utility in dermatology encompasses superficial infections, pigmentary disorders, and metabolic diseases. It also has many practical applications in cutaneous surgery and cosmetics. Although it has been over a century since its invention, the Wood’s lamp is a valuable tool in dermatology, helping to reveal the unseen.
Contributor Information
Joseph M. Dyer, Dr. Dyer is Clinical Assistant Professor at the Philadelphia College of Osteopathic Medicine in Suwanee, Georgia..
Valerie M. Foy, Ms. Foy is a medical student at the Philadelphia College of Osteopathic Medicine in Philadelphia, Pennsylvania..
REFERENCES
- Sharma S, Sharma A. Robert Williams Wood: pioneer of invisible light. Photodermatol Photoimmunol Photomed. 2016;32(2):60–65. doi: 10.1111/phpp.12235. [DOI] [PubMed] [Google Scholar]
- Klatte JL, van der Beek N, Kemperman PM. 100 years of Wood’s lamp revised. J Eur Acad Dermatol Venereol. 2015;29(5):842–847. doi: 10.1111/jdv.12860. [DOI] [PubMed] [Google Scholar]
- Pasquetti M, Min ARM, Scacchetti S et al. Infection by Microsporum canis in Paediatric Patients: A Veterinary Perspective. Vet Sci. 2017;4(3):E46. doi: 10.3390/vetsci4030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci KA, Nelson DG, McQuillen KK, Duffy SJ, Linakis JG. Wood’s lamp utility in the identification of semen. Pediatrics. 1999;104(6):1342–1344. doi: 10.1542/peds.104.6.1342. [DOI] [PubMed] [Google Scholar]
- Casavant MJ, Shah MN, Battels R. Does fluorescent urine indicate antifreeze ingestion by children. Pediatrics. 2001;107(1):113–114. doi: 10.1542/peds.107.1.113. [DOI] [PubMed] [Google Scholar]
- Hooker EA, Faulkner WJ, Kelly LD et al. Prospective study of the sensitivity of the Wood’s lamp for common eye abnormalities. Emerg Med J. 2019;36(3):159–162. doi: 10.1136/emermed-2018-208235. [DOI] [PubMed] [Google Scholar]
- Ulubay M, Ozturk M, Fidan U et al. Using Wood’s Light as a Diagnostic Tool for Vaginal Atrophy. J Clin Diagn Res. 2015;9(1):QC05–8. doi: 10.7860/JCDR/2015/11287.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappano DA, Bass ES. Wood’s lamp employed as a provocative technique to identify pseudoseizures. Pediatr Emerg Care. 2004;20(11):773–774. doi: 10.1097/01.pec.0000144922.93371.7a. [DOI] [PubMed] [Google Scholar]
- Zawar VP, Zawar SV, Pawar MK, Gogate P. Wood’s lamp examination: a novel diagnostic approach to detect Bitot’s spots in vitamin A deficiency. Clin Exp Ophthalmol. 2018;46(6):705–706. doi: 10.1111/ceo.13144. [DOI] [PubMed] [Google Scholar]
- Georgakoudi I, Jacobson BC, Müller MG et al. NAD(P)H and collagen as in vivo quantitative fluorescent biomarkers of epithelial precancerous changes. Cancer Res. 2002;62(3):682–687. [PubMed] [Google Scholar]
- Shen Y, Zhu D, Lu W et al. The Characteristics of Intrinsic Fluorescence of Type I Collagen Influenced by Collagenase I. Appl Sci. 2018;8(10):1947. [Google Scholar]
- Atkins P. New York, NY: Oxford University Press; 2017. Molecular spectroscopy 2: electronic transitions. In: Atkins’ physical chemistry. 8th ed. pp. p. 481–510. [Google Scholar]
- So PT, Dong CY. Fluorescence spectrophotometry. e LS. 2001. May 30.
- Cardillo H, Kohler J, Kriner E Applications of Wood’s Lamp technology to detect skin infections in resource-constrained settings. In: Global Humanitarian Technology Conference (GHTC), 2014. p. IEEE.
- Kaliyadan F, Kuruvilla J. Using a hand-held black-light source instead of a Wood’s lamp. J Am Acad Dermatol. 2015;72(6):e153–154. doi: 10.1016/j.jaad.2015.02.1096. [DOI] [PubMed] [Google Scholar]
- Forbes PD, Davies RE, D’Aloisio LC et al. Emission spectrum differences in fluorescent blacklight lamps. Photochem Photobiol. 1976;24(6):613–615. doi: 10.1111/j.1751-1097.1976.tb06883.x. [DOI] [PubMed] [Google Scholar]
- Rajapakse D. An Investigation of the Activation of Multi-Colour Changing Photochromic Textiles. Tekstil ve Mühendis. 2019;26(114):196–208. [Google Scholar]
- Sun R, Chen H, Zhu W et al. Wood’s lamp image of porokeratosis. Photodermatol Photoimmunol Photomed. 2017;33(2):114–116. doi: 10.1111/phpp.12285. [DOI] [PubMed] [Google Scholar]
- Agrawal S, Sharma A, Dhurat R Using the blue screen of a smartphone as an alternative to Wood’s lamp for examination of vitiligo. J Am Acad Dermatol. published online: April 20, 2019. [DOI] [PubMed]
- García-Gil MF, Monte Serrano J et al. Examination of vitiligo using the blue light from the screen of mobile devices as an alternative to a Wood’s lamp. Aten Primaria. 2020;52(9):649–650. doi: 10.1016/j.aprim.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri M, Darabyan M, Izadbakhsh E et al. Exposure to Visible Light Emitted from Smartphones and Tablets Increases the Proliferation of Staphylococcus aureus: Can this be Linked to Acne. J Biomed Phys Eng. 2017;7(2):163–168. [PMC free article] [PubMed] [Google Scholar]
- 2011. pp. p.1–6. Operation. In: SilverFox-1004; UV Wood’s Lamp User Guide. Cosmetronic.
- Silverberg JI, Silverberg NB. False "highlighting" with Wood’s lamp. Pediatr Dermatol. 2014;31(1):109–110. doi: 10.1111/j.1525-1470.2012.01787.x. [DOI] [PubMed] [Google Scholar]
- Herro EM, Cosan T, Jacob SE. Ultraviolet protective eyewear for Wood’s light use. Pediatr Dermatol. 2011;28(3):351–352. doi: 10.1111/j.1525-1470.2011.01438.x. [DOI] [PubMed] [Google Scholar]
- Reis J, Cunha Velho G. Visual Dermatology: Using Wood’s Lamp to Detect Early Infection by Pseudomonas aeruginosa. J Cutan Med Surg. 2020;24(3):308. doi: 10.1177/1203475420915442. [DOI] [PubMed] [Google Scholar]
- Dyer, J. Figure 1. Pseudomonal green nail – Normal
- Dyer, J. Figure 2. Pseudomonal green nail - UV Light
- Amichai B, Finkelstein E, Halevy S. Early detection of Pseudomonas infection using a Wood’s lamp. Clin Exp Dermatol. 1994;19(5):449. doi: 10.1111/j.1365-2230.1994.tb02713.x. [DOI] [PubMed] [Google Scholar]
- Janeczek M, Kozel Z, Bhasin R et al. High Prevalence of Erythrasma in Patients with Inverse Psoriasis: A Cross-sectional Study. J Clin Aesthet Dermatol. 2020;13(3):12–14. [PMC free article] [PubMed] [Google Scholar]
- Garcia-Souto F. Visual Dermatology: Erythrasma Fluorescence Under Wood’s Lamp. J Cutan Med Surg. 2020;24(1):94. doi: 10.1177/1203475419858935. [DOI] [PubMed] [Google Scholar]
- Polat M, İlhan MN. The prevalence of interdigital erythrasma: a prospective study from an outpatient clinic in Turkey. J Am Podiatr Med Assoc. 2015;105(2):121–124. doi: 10.7547/0003-0538-105.2.121. [DOI] [PubMed] [Google Scholar]
- Gupta V, Sharma VK. Four views of trichomycosis axillaris: Clinical, Wood’s lamp, dermoscopy and microscopy. Indian J Dermatol Venereol Leprol. 2018;84(6):748–749. doi: 10.4103/ijdvl.IJDVL_567_17. [DOI] [PubMed] [Google Scholar]
- Levit F. Trichomycosis axillaris: a different view. J Am Acad Dermatol. 1988;18(4 Pt 1):778–779. doi: 10.1016/s0190-9622(88)70097-3. [DOI] [PubMed] [Google Scholar]
- Shelley WB, Miller MA. Electron microscopy, histochemistry, and microbiology of bacterial adhesion in trichomycosis axillaris. J Am Acad Dermatol. 1984;10(6):1005–1014. doi: 10.1016/s0190-9622(84)80325-4. [DOI] [PubMed] [Google Scholar]
- Cavalcanti SM, de França ER, Lins AK et al. Investigation of Propionibacterium acnes in progressive macular hypomelanosis using real-time PCR and culture. Int J Dermatol. 2011;50(11):1347–1352. doi: 10.1111/j.1365-4632.2011.04978.x. [DOI] [PubMed] [Google Scholar]
- Chang, D. Figure 3. Cutibacterium acnes in truncal acne
- Pflederer RT, Wuennenberg JP, Foote C et al. Use of Wood’s lamp to diagnose progressive macular hypomelanosis. J Am Acad Dermatol. 2017;77(4):e99–e100. doi: 10.1016/j.jaad.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Andrews MD, Burns M. Common tinea infections in children. Am Fam Physician. 2008;77(10):1415–1420. [PubMed] [Google Scholar]
- Rippon JW. Philadelphia: Saunders; 1988. Medical mycology: the pathogenic fungi and the pathogenic actinomycetes. [Google Scholar]
- Prevost E. The rise and fall of fluorescent tinea capitis. Pediatr Dermatol. 1983;1(2):127–133. doi: 10.1111/j.1525-1470.1983.tb01103.x. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Summerbell RC. Tinea capitis. Med Mycol. 2000;38(4):255–287. doi: 10.1080/mmy.38.4.255.287. [DOI] [PubMed] [Google Scholar]
- Shah A, Koticha A, Ubale M et al. Identification and speciation of malassezia in patients clinically suspected of having pityriasis versicolor. Indian J Dermatol. 2013;58(3):239. doi: 10.4103/0019-5154.110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayser P, Stapelkamp H, Krämer HJ et al. Pityrialactone- a new fluorochrome from the tryptophan metabolism of Malassezia furfur. Antonie Van Leeuwenhoek. 2003;84(3):185–191. doi: 10.1023/a:1026042903354. [DOI] [PubMed] [Google Scholar]
- Sato T, Asahina Y, Toshima S et al. Usefulness of Wood’s Lamp for the Diagnosis and Treatment Follow-up of Onychomycosis. Med Mycol J. 2020;61(2):17–21. doi: 10.3314/mmj.20-00004. [DOI] [PubMed] [Google Scholar]
- Xu AE, Huang B, Li YW et al. Clinical, histopathological and ultrastructural characteristics of naevus depigmentosus. Clin Exp Dermatol. 2008;33(4):400–405. doi: 10.1111/j.1365-2230.2008.02714.x. [DOI] [PubMed] [Google Scholar]
- Ruggieri M, Carbonara C, Magro G et al. Tuberous sclerosis complex: neonatal deaths in three of four children of consanguineous, non-expressing parents. J Med Genet. 1997;34(3):256–260. doi: 10.1136/jmg.34.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss P, Singh G, Lo Sicco K et al. Wood’s lamp as a tool in the evaluation of morphea. J Am Acad Dermatol. 2018;78(2):e33–e34. doi: 10.1016/j.jaad.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Peña-Romero AG, García-Romero MT. Diagnosis and management of linear scleroderma in children. Curr Opin Pediatr. 2019;31(4):482–490. doi: 10.1097/MOP.0000000000000785. [DOI] [PubMed] [Google Scholar]
- Sánchez JL, Vázquez M, Sánchez NP. Vitiligo like macules in systemic scleroderma. Arch Dermatol. 1983;119(2):129–133. doi: 10.1001/archderm.1983.01650260037013. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Chang CC, Cheng KL. Wood’s lamp for vitiligo disease stability and early recognition of initiative pigmentation after epidermal grafting. Int Wound J. 2017;14(6):1391–1394. doi: 10.1111/iwj.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliyadan F, Venkitakrishnan S, Manoj J. Use of a wood’s lamp as a ultraviolet light source to improve the speed and quality of suction blister harvesting. Indian J Dermatol Venereol Leprol. 2010;76(4):429–431. doi: 10.4103/0378-6323.66603. [DOI] [PubMed] [Google Scholar]
- Sehgal VN, Verma P, Srivastava G et al. Melasma: treatment strategy. J Cosmet Laser Ther. 2011;13(6):265–279. doi: 10.3109/14764172.2011.630088. [DOI] [PubMed] [Google Scholar]
- Weismann K, Lorentzen HF. Dermoscopic color perspective. Arch Dermatol. 2006;142(9):1250. doi: 10.1001/archderm.142.9.1250. [DOI] [PubMed] [Google Scholar]
- Harber LC, Bickers DR. Photosensitivity Disease. Principles of Diagnosis and Treatment, 2nd ed. Philadelphia, B.C. Decker, Inc. 1989. pp. pp 421–433.
- Smimov, B. Figure 4. PCT Urine.
- Paller AS, Mancini AJ. Chapter 19- Photosensitivity and Photoreactions. Hurwitz Clinical Pediatric Dermatology. 4th ed. London: Elsevier Health Sciences. 2011. pp. 436–453.
- Hogeling M, Nakano T, Dvorak CC et al. Severe neonatal congenital erythropoietic porphyria. Pediatr Dermatol. 2011;28(4):416–420. doi: 10.1111/j.1525-1470.2010.01376.x. [DOI] [PubMed] [Google Scholar]
- Blasco Morente G, Martínez Peinado C, Martínez García E, Tercedor Sánchez J. Wood’s lamp in congenital erythropoietic porphyria. An Pediatr (Barc). 2014;81(6):403–404. doi: 10.1016/j.anpedi.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Michaels BD, Del Rosso JQ, Mobini N et al. Erythropoietic protoporphyria: a case report and literature review. J Clin Aesthet Dermatol. 2010;3(7):44–48. [PMC free article] [PubMed] [Google Scholar]
- Choudhary S, Srivastava A. Wood’s Lamp as an Inexpensive, Handy Tool to Diagnose Solar Urticaria. Indian Dermatol Online J. 2020;11(5):833–834. doi: 10.4103/idoj.IDOJ_625_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kwon HS, Jung HM et al. Wood’s lamp-induced fluorescence of milia. J Am Acad Dermatol. 2018;78(5):e99–e100. doi: 10.1016/j.jaad.2017.12.046. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Robins P. Wood’s lamp and surgical margins in malignant melanoma in situ. J Dermatol Surg Oncol. 1988;14(1):22. doi: 10.1111/j.1524-4725.1988.tb03336.x. [DOI] [PubMed] [Google Scholar]
- Robinson JK. Margin control for lentigo maligna. J Am Acad Dermatol. 1994;31(1):79–85. doi: 10.1016/s0190-9622(94)70140-7. [DOI] [PubMed] [Google Scholar]
- Borroni RG, Barruscotti S, Carugno A et al. Usefulness of in vivo photodiagnosis for the identification of tumor margins in recurrent basal cell carcinoma of the face. Photodermatol Photoimmunol Photomed. 2015;31(4):195–201. doi: 10.1111/phpp.12166. [DOI] [PubMed] [Google Scholar]
- Matarasso SL, Glogau RG, Markey AC. Wood’s lamp for superficial chemical peels. J Am Acad Dermatol. 1994;30(6):988–992. doi: 10.1016/s0190-9622(94)70124-5. [DOI] [PubMed] [Google Scholar]
- Light JG, Frantz T, McNamara K et al. Efficacy of Applicator Devices for Self-Application of Topicals to the Back. J Cutan Med Surg. 2020;24(3):249–252. doi: 10.1177/1203475420906081. [DOI] [PubMed] [Google Scholar]
- Jeanmougin M, Bouloc A, Schmutz JL. A new sunscreen application technique to protect more efficiently from ultraviolet radiation. Photodermatol Photoimmunol Photomed. 2014;30(6):323–331. doi: 10.1111/phpp.12138. [DOI] [PubMed] [Google Scholar]
- Hendricks AA. Yellow lunulae with fluorescence after tetracycline therapy. Arch Dermatol. 1980;116(4):438–440. [PubMed] [Google Scholar]
- Jankowski M, Nowowiejska L, Czajkowski R. Wood’s lamp fluorescence of dihydroxyacetone treated skin. J Eur Acad Dermatol Venereol. 2016;30(11):e12–e126. doi: 10.1111/jdv.13401. [DOI] [PubMed] [Google Scholar]