ABSTRACT
The ability of pathogenic fungi to obtain essential nutrients from the host is vital for virulence. In Candida albicans, acquisition of the macronutrient phosphate is regulated by the Pho4 transcription factor and is important for both virulence and resistance to host-encountered stresses. All cells store phosphate in the form of polyphosphate (polyP), a ubiquitous polymer comprising tens to hundreds of phosphate residues. Release of phosphate from polyP is one of the first responses evoked in response to phosphate starvation, and here, we sought to explore the importance of polyP mobilization in the pathobiology of C. albicans. We found that two polyphosphatases, Ppn1 and Ppx1, function redundantly to release phosphate from polyP in C. albicans. Strikingly, we reveal that blocking polyP mobilization prevents the activation of the Pho4 transcription factor: following Pi starvation, Pho4 fails to accumulate in the nucleus and induce Pi acquisition genes in ppn1Δ ppx1Δ cells. Consequently, ppn1Δ ppx1Δ cells display impaired resistance to the same range of stresses that require Pho4 for survival. In addition, cells lacking both polyphosphatases are exquisitely sensitive to DNA replication stress, indicating that polyP mobilization is needed to support the phosphate-demanding process of DNA replication. Blocking polyP mobilization also results in significant morphological defects, as ppn1Δ ppx1Δ cells form large pseudohypha-like cells that are resistant to serum-induced hypha formation. Thus, polyP mobilization impacts key processes important for the pathobiology of C. albicans, and consistent with this, we found that blocking this process attenuates the virulence of this important human fungal pathogen.
KEYWORDS: Candida albicans, morphogenesis, phosphate metabolism, stress response, virulence
INTRODUCTION
Candida albicans is a gut commensal and opportunistic fungal pathogen of humans. In immunocompromised hosts, this fungus can bypass diminished mucosal immune defenses and enter the bloodstream, resulting in systemic infections which are associated with an alarming mortality rate of over 40% (1). One notable characteristic of C. albicans is its ability to colonize multiple anatomical niches within the host, both as a commensal organism and during systemic infections. A key driver in promoting C. albicans colonization of diverse host environments is its metabolic flexibility, which allows the acquisition of essential nutrients in resource-poor settings and in complex microenvironments where competition for nutrients is high (2). Sophisticated mechanisms are in place to allow the acquisition of essential micronutrients, exemplified by secretion of a scavenger protein, Pra1, which sequesters zinc from host cells before reassociating with the fungal cell via the zinc transporter Zrt1 (3). Recent studies have shown that acquisition of the macronutrient phosphate (Pi) is also essential for C. albicans survival in host environments (4, 5). This is perhaps unsurprising, as Pi is an essential component of nucleic acids and phospholipids and, in the form of ATP, is a universal cellular energy source. Moreover, due to its negative charge at physiological pH, Pi is extensively used as a signaling molecule via the posttranslational modification of numerous proteins (6).
The systems involved in Pi acquisition have been extensively studied in the model yeast Saccharomyces cerevisiae. Following growth under Pi-limiting conditions, S. cerevisiae activates the PHO pathway, which culminates in the nuclear accumulation of the Pho4 transcription factor and the induction of a suite of genes involved in Pi acquisition and storage (7). This pathway is seemingly conserved in C. albicans, as Pi starvation similarly elicits the nuclear accumulation of a Pho4 orthologue and the Pho4-dependent expression of genes involved in Pi homeostasis (5). Proteins involved in Pi acquisition include secreted acid phosphatases such as Pho100 and the high-affinity Pi transporter Pho84, which collectively facilitate scavenging of Pi from the extracellular environment. Consequently, C. albicans cells lacking Pho4 struggle to grow in Pi-limiting environments and under alkaline pH conditions, as this also triggers a Pi starvation response (5, 8). Notably, Pho4, Pho100, and Pho84 have all been shown to contribute to C. albicans pathogenesis, illustrating that Pi acquisition is an important virulence trait in this fungal pathogen (5, 9, 10).
Despite the essential role of Pi, cytosolic levels of Pi are tightly controlled, as cells need to balance the biosynthetic and signaling requirements for Pi against elevated cytosolic Pi levels. This is because, as a product of all nucleotide-hydrolyzing reactions, Pi has the potential to stall metabolism if levels get too high (11). Thus, the PHO pathway also regulates genes involved in Pi storage, including components of the vacuole transporter chaperone (VTC) complex, which synthesizes the Pi storage molecule polyphosphate (polyP). Synthesis and translocation of polyP into the vacuole are tightly linked, possibly because polyP produced in the cytosol is toxic and this mechanism ensures buffering of cytosolic Pi levels (12). Indeed, the majority of polyP in fungal cells is stored in the vacuole, with only small pools located in the cytoplasm, mitochondria, and nucleus (13). The S. cerevisiae VTC complex is a heterotrimer composed of three structural units (Vtc1, Vtc2, and Vtc3) and the polyP synthetase Vtc4 (14). A fifth subunit, Vtc5, has recently been shown to physically associate with VTC complex to accelerate polyP synthesis (15). The VTC complex, located on the vacuolar membrane, synthesizes linear polymers ranging from 10 to several hundred Pi molecules linked by high-energy phosphoanhydride bonds (16). Release of Pi from polyP stores is regulated by several polyphosphatases, including Ppn1 and Ppx1 (reviewed in reference 17). Ppn1 is a vacuolar endopolyphosphatase that cleaves internal phosphoanhydride bonds (18) and depends on vacuolar proteases for activation (19). In contrast, Ppx1 is a cytoplasmic enzyme and exhibits potent exopolyphosphatase activity, releasing Pi from the ends of polyP chains (20), although it can also function as an endophosphatase under certain conditions (21). PPN1, but not PPX1, is induced in response to Pi starvation in S. cerevisiae (22). The same holds true in C. albicans, where Pho4 regulates the induction of PPN1 following Pi limitation (5). Intriguingly, it is not yet known how the opposing functions of polyP synthesis and mobilization are coordinated to maintain optimal cellular Pi levels.
PolyP is one of the most ancient and conserved molecules in biology, and although it was once dismissed as a “molecular fossil,” there has been a resurgence of interest in this enigmatic polymer due to its emergence as more than simply a Pi storage molecule (16). The pioneering work of Arthur Kornberg and colleagues revealed a number of diverse functions for polyP in bacteria, including, stress resistance, motility, quorum sensing, biofilm formation, and virulence (reviewed in reference 23). More recently, polyP has been shown to function as a potent chaperone that protects bacterial cells against stress-induced protein aggregation, including oxidative stress (24). Less is known about polyP function in eukaryotes, although recent studies in S. cerevisiae have uncovered a role for polyP in cell cycle progression and genome stability (25), and there is a significant body of work linking polyP with osmoregulation in trypanosomes (26) and blood coagulation in humans (27). Furthermore, the finding that in eukaryotes polyP can be added to proteins has revealed polyphosphorylation to be a novel regulatory posttranslational protein modification (28).
Notably, disruption of Pi homeostasis in C. albicans results in a myriad of phenotypes in addition to impaired growth under Pi-limiting conditions (5, 9, 29, 30). For example, loss of Pho4 results in cells that are exquisitely sensitive to superoxide stress and cationic stresses, and similar phenotypes are seen upon loss of the Pi transporter Pho84 (5, 9). As polyP has been implicated in stress resistance in several organisms, it was reasoned that the stress-sensitive phenotypes attributed to loss of Pho4 could be due to the lack of polyP in these cells. However, extensive phenotypic analysis of C. albicans mutants (vtc1Δ and vtc4Δ mutants) lacking polyP revealed few stress-protective roles for this polymer (5). Indeed, the only function attributed to the presence of polyP thus far is as a manganese storage reservoir. However, polyP is rapidly mobilized in response to a number of stresses in addition to Pi limitation in C. albicans (5). This suggests that the ability to mobilize Pi from polyP may be important for Pi homeostasis and stress resistance. Here, we set out to explore the cellular processes that require polyP mobilization in C. albicans and its importance in virulence.
RESULTS
The polyphosphatases Ppn1 and Ppx1 exhibit functional redundancy.
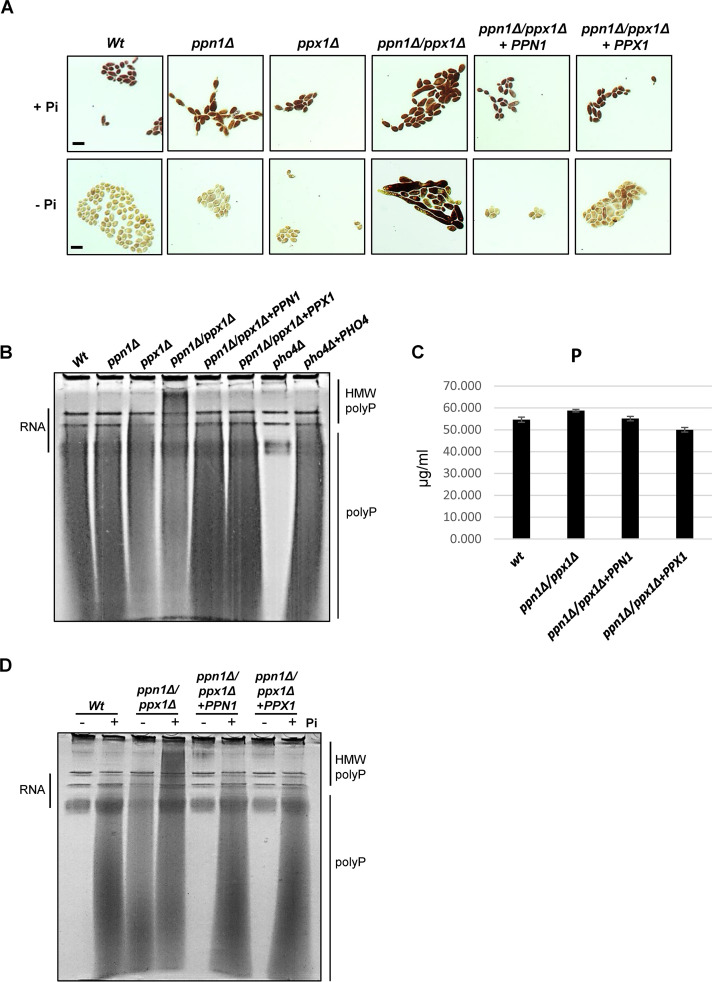
To identify polyphosphatases in C. albicans we used the amino acid sequences of the S. cerevisiae Ppx1 exopolyphosphatase and the Ppn1 endo/exopolyphosphatase to conduct a BLAST search against the C. albicans genome database. This identified both Ppx1 (C2_06110W_A) and Ppn1 (C7_00980W_A) homologues in C. albicans with 34% and 44% identity to the respective S. cerevisiae proteins (see Fig. S1 in the supplemental material). To explore functional conservation, we generated C. albicans strains lacking PPX1 and PPN1 homologues and examined polyP levels and the size of polyP chains in these mutants. PolyP can be detected in whole cells by Neisser staining (31) and in cell extracts by toluidine blue staining of polyP resolved on polyacrylamide gels (32). Interestingly, deletion of either PPN1 or PPX1 had no obvious impact on polyP levels (Fig. 1A) or on the size of the polyP chains (Fig. 1B). Hence, to explore potential functional redundancy between Ppn1 and Ppx1 in C. albicans, a double ppx1Δ ppn1Δ mutant was created. Increased Neisser staining of cells indicated higher levels of polyP in the ppx1Δ ppn1Δ mutant compared to wild-type cells (Fig. 1A), and resolution of polyP by PAGE revealed the presence of longer polyP chains upon deletion of both polyphosphatases (Fig. 1B). Despite the presence of longer polyP chains in the ppx1Δ ppn1Δ mutant, no significant differences in overall cellular Pi levels compared to wild-type cells were evident (Fig. 1C). However, reintegration of either PPN1 or PPX1 reduced the levels and size of polyP to those seen in wild-type cells (Fig. 1B). These results indicate that Ppx1 and Ppn1 have redundant functions in releasing Pi from polyP in C. albicans.
FIG 1.
Ppn1 and Ppx1 function redundantly to mobilize polyP stores. (A) Neisser staining of the indicated strains following growth in YPD+Pi or YPD-LPi for 16 h. Bars represent 10 μm, and the scale is the same across each row of images. (B) Toluidine blue staining of RNA/polyP extracts after electrophoresis on urea-polyacrylamide gels from cells grown in YPD. The high-molecular-weight (HMW) polyP in ppn1Δ ppx1Δ cells is indicated. (C) Impact of PPX1 and PPN1 loss on intracellular phosphate levels. Whole-cell nitric acid digests of WT, ppn1Δ ppx1Δ, ppn1Δ ppx1Δ+PPN1, and ppn1Δ ppx1Δ+PPX1 cells grown in YPD were analyzed by ICP-MS. Phosphate levels shown are means and SD for three independent cultures. (D) Toluidine blue staining of RNA/polyP extracts after electrophoresis on urea-polyacrylamide gels from cells grown in YPD+Pi or YPD-LPi for 16 h.
Sequence alignment of Ppn1 and Ppx1 orthologues in S. cerevisiae and C. albicans. Sequences were aligned using ClustalW. Identical amino acids are annotated with asterisks, conserved amino acids with colons, and semiconserved amino acids with dots. Gaps introduced to maximize alignment are indicated with dashes. (A) Sequences of C. albicans (Ca) Ppn1 and S. cerevisiae (Sc) Ppn1. The endopolyphosphatase domain is highlighted in yellow. (B) Sequences of C. albicans (Ca) Ppx1 and S. cerevisiae (Sc) Ppx1. The Ppx1 superfamily domain is highlighted in red and the DHHA2 domain in yellow. Download FIG S1, TIF file, 1.9 MB (2MB, tif) .
Copyright © 2022 Ahmed et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We next asked whether Ppn1 and Ppx1 are required for the active liberation of Pi from polyP in response to Pi starvation. Consistent with previous results (5), polyP stores are completely mobilized in wild-type cells following 16 h Pi starvation (Fig. 1A and D). In contrast, polyP mobilization is drastically impaired in ppn1Δ ppx1Δ cells, as Neisser staining revealed similar levels of polyP in this mutant irrespective of the level of Pi in the growth media (Fig. 1A). Such analysis also indicated that ppn1Δ ppx1Δ cells exhibit morphological defects, which is examined in more detail below. Following resolution and visualization of polyP on polyacrylamide gels, it was evident that some of the higher-molecular-weight polyP chains, seen in ppn1Δ ppx1Δ cells, are resolved to shorter chains following Pi starvation (Fig. 1D). Notably, reintegration of either PPX1 or PPN1 into ppn1Δ ppx1Δ cells completely restored the ability of cells to mobilize polyP following Pi starvation (Fig. 1D). Collectively, these results indicate that Ppn1 and Ppx1 function redundantly in C. albicans to mobilize polyP under Pi-limiting conditions and that both polyphosphatases need to be inactivated before longer polyP chains accumulate under Pi-replete conditions.
Prevention of polyP mobilization impacts the activation of the PHO pathway.
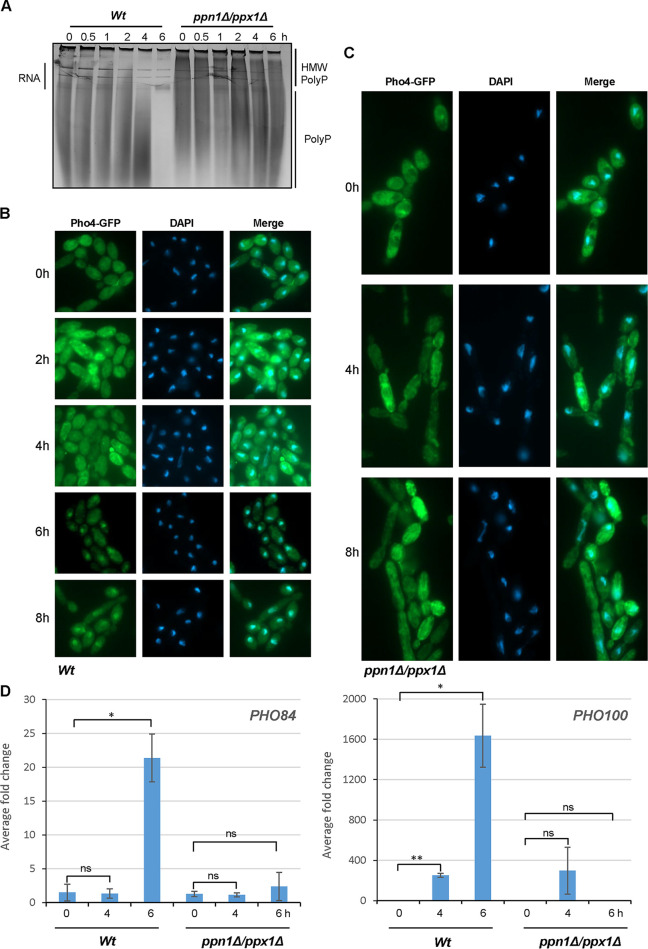
As cells respond to Pi limitation by mobilizing Pi stores from polyP, we asked whether preventing polyP mobilization would have an impact on activation of the PHO pathway, such as preventing Pho4 nuclear accumulation and the induction of Pho4-dependent genes. First, the kinetics of polyP mobilization and Pho4 nuclear accumulation were examined. Wild-type and ppn1Δ ppx1Δ cells expressing Pho4-GFP were grown under both Pi-replete and Pi-limiting conditions, and cell samples were dually processed to examine polyP levels and the cellular localization of Pho4. PolyP mobilization was visualized by Neisser staining of polyP following urea-PAGE (Fig. 2A) and fluorescence microscopy was employed to determine the cellular localization of Pho4 (Fig. 2B and 2C). In wild-type cells, some polyP mobilization was evident after 4 h growth in Pi-limiting medium, with complete mobilization occurring by 6 h (Fig. 2A). Such mobilization was severely impaired in ppn1Δ ppx1Δ cells (Fig. 2A), consistent with that observed previously (Fig. 1D). Examining the cellular localization of Pho4 revealed that nuclear accumulation of Pho4 was seen only in wild-type cells following 6 h growth in Pi-limiting medium (Fig. 2B). In contrast, nuclear accumulation of Pho4 was not observed in ppn1Δ ppx1Δ cells following 8 h (Fig. 2C) or even 16 h (Fig. S2A) growth in Pi-limiting medium. In some ppn1Δ ppx1Δ cells, a punctate staining pattern was seen, but this did not colocalize with the DAPI (4′,6-diamidino-2-phenylindole) nuclear stain (Fig. 2C). This deregulation of localization is not due to processing of the Pho4-GFP fusion, as Western blotting using a green fluorescent protein (GFP) antibody revealed that Pho4-GFP is intact in ppn1Δ ppx1Δ cells (Fig. S2B). These results, showing that Pho4 nuclear accumulation (i) coincides with the complete mobilization of polyP and (ii) is inhibited when polyP mobilization is impaired, suggest that polyP mobilization precedes Pho4 activation in C. albicans.
FIG 2.
Pho4 activation in ppn1Δ ppx1Δ cells. (A) Toluidine blue staining of RNA/polyP extracts after electrophoresis on urea-polyacrylamide gels from WT and ppn1Δ ppx1Δ cells expressing Pho4-GFP grown in YPD until mid-log phase (t = 0) and then moved to YPD-LPi medium for the indicated times. (B and C) Cells from the cultures described above were processed to examine Pho4-GFP localization using fluorescence microscopy. DAPI staining illustrates nuclear positioning. (D) RT-qPCR analysis showing fold induction of the Pho4 target genes PHO84 and PHO100 after growth for 4 and 6 h in YPD-LPi. Transcript levels were measured relative to the internal ACT1 mRNA control and normalized to the level of transcript in WT cells with Pi. Means and standard deviations for three biological replicates are shown. ns, not significant; *, P < 0.05; **, P < 0.01.
Analysis of PHO pathway activation after extended growth in Pi-limiting medium. (A) WT and ppn1Δ ppx1Δ cells expressing Pho4-GFP were grown in YPD until mid-log phase (t = 0), moved to YPD-LPi medium for 16 h, and processed to examine Pho4-GFP localization using fluorescence microscopy. DAPI staining illustrates nuclear positioning. (B) WT and ppn1Δ ppx1Δ cells expressing Pho4-GFP were grown in YPD-LPi medium for 16 h, and Pho4-GFP expression was examined by Western blotting of whole-cell extracts using an anti-GFP antibody. An antitubulin antibody was used as a loading control. (C) RT-qPCR analysis showing fold induction of Pho4 target genes PHO84 and PHO100 after growth of the indicated strains for 16 h in YPD-LPi. Transcript levels were measured relative to the internal ACT1 mRNA control and normalized to the level of transcript in WT cells with Pi. Means and standard deviations for three biological replicates are shown. ns, not significant; *, P < 0.05; **, P < 0.01. Download FIG S2, TIF file, 2.7 MB (2.8MB, tif) .
Copyright © 2022 Ahmed et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
As nuclear accumulation of Pho4 following Pi limitation was not evident in ppn1Δ ppx1Δ cells, we investigated whether this relayed to changes in the transcript profile of Pho4-dependent genes, including the high-affinity Pi symporter PHO84 and the secreted acid phosphatase PHO100. Both of these genes are induced in a Pho4-dependent manner in response to Pi starvation in C. albicans to facilitate Pi acquisition from external sources (5). Reverse transcription-quantitative PCR (RT-qPCR) revealed that both PHO84 and PHO100 were highly expressed in wild-type cells following 6 h growth under Pi-limiting conditions, with some induction of PHO100 also observed after 4 h (Fig. 2D). However, such induction was drastically reduced in ppn1Δ ppx1Δ cells (Fig. 2D), even following extended growth in Pi-limiting conditions (Fig. S2C), which is consistent with the lack of Pho4 nuclear accumulation. This impaired induction of Pi acquisition genes is not due to significantly slower growth of ppn1Δ ppx1Δ cells (Fig. S3A). Furthermore, reintegration of either PPN1 or PPX1 into ppn1Δ ppx1Δ cells fully restored PHO100 induction and partially restored PHO84 induction (Fig. S3B). To explore the links between polyP and Pho4 activation further, the induction of PHO100 and PHO84 in vtc4Δ cells lacking polyP was also determined. In contrast to that seen in ppn1Δ ppx1Δ cells, induction of these genes was not impaired in vtc4Δ cells, with faster activation of PHO100 occurring in cells lacking polyP (Fig. S3C). Taken together, these results show that polyP mobilization coincides with PHO pathway activation in C. albicans and that inhibiting Pi release from polyP via deletion of Ppn1 and Ppx1 impairs both the nuclear accumulation of Pho4 and the induction of Pho4-dependent genes.
(A) Analysis of growth of the indicated strains under the conditions used for cells in the RT-qPCR experiments. Cells numbers rather than OD were recorded due to morphological defects of ppn1Δ ppx1Δ cells. Means and standard deviations for three biological replicates are shown. (B) Reintegration of PPN1 or PPX1 restores Pho4-dependent gene expression. RT-qPCR analysis of Pho4 target genes PHO84 and PHO100 in the indicated strains. Means and standard deviations for three biological replicates are shown. ns, not significant; *, P < 0.05; **, P < 0.01. (C) Pho4-dependent gene expression occurs with faster kinetics in cells lacking polyP. RT-qPCR analysis of Pho4 target genes PHO84 and PHO100 in wild-type and vtc4Δ strains. Means and standard deviations for three biological replicates are shown. ns, not significant; *, P < 0.05; **, P < 0.01. Download FIG S3, TIF file, 1.6 MB (1.7MB, tif) .
Copyright © 2022 Ahmed et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
PolyP mobilization contributes to stress resistance.
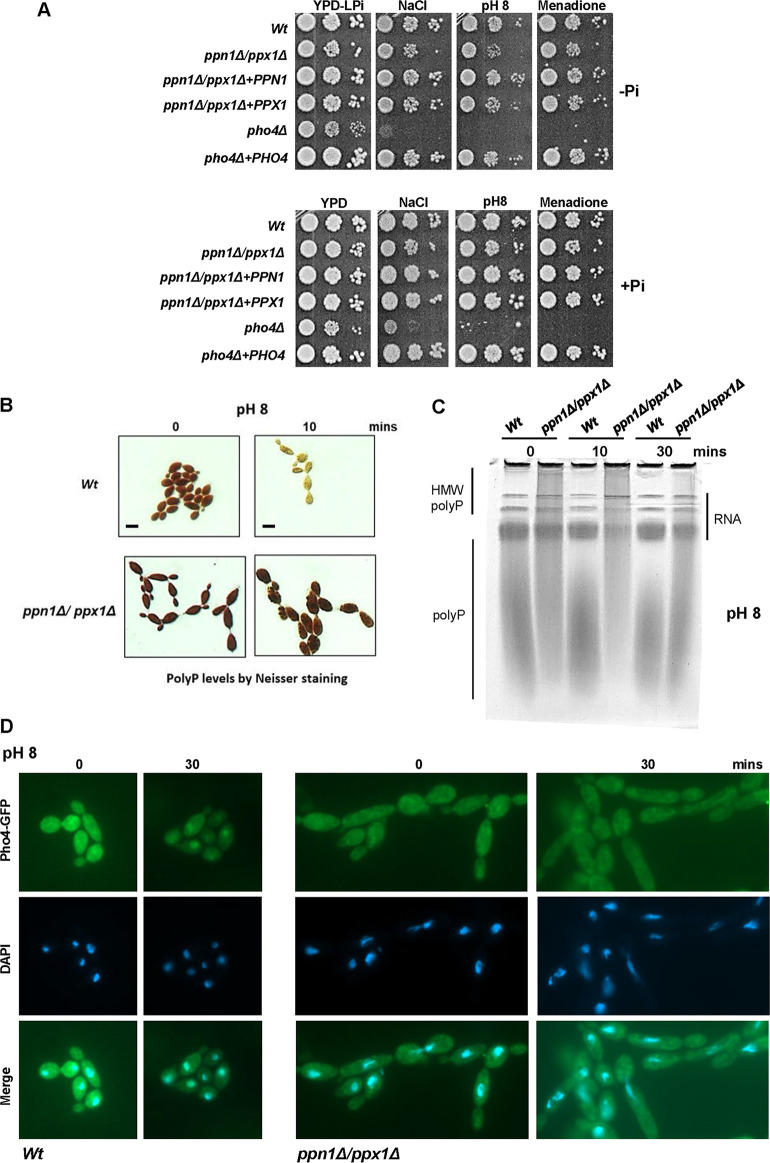
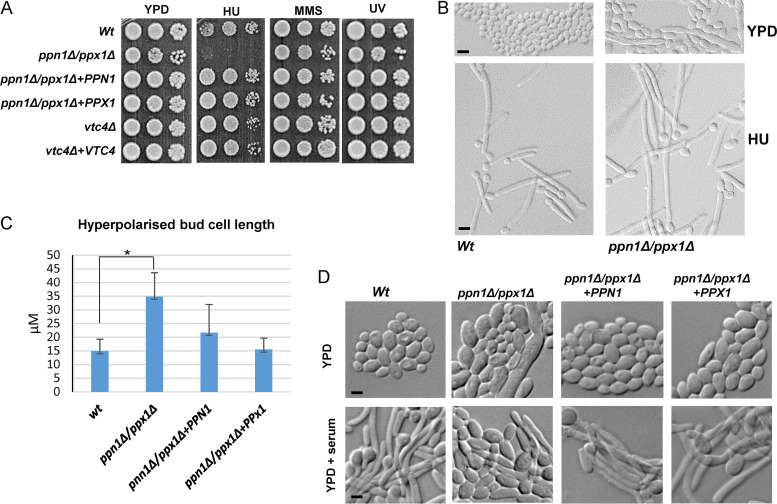
Previously we reported that cells lacking the Pho4 transcription factor display significantly impaired resistance to alkaline stress, cationic stress, and menadione-imposed superoxide stress (5). However, analysis of the Pho4-mediated transcriptome indicated that this transcription factor does not directly regulate stress-protective genes (5). Thus, an alternative possibility is that reduced intracellular Pi levels in pho4Δ cells may contribute to the stress-sensitive phenotypes of this mutant. Consistent with this is the observation that Pi is mobilized from polyP stores following exposure to either cationic or alkaline stress (5). Therefore, we asked whether preventing polyP mobilization impacts resistance to stresses known to require Pho4 for resistance. Spot test assays were performed on Pi-limiting medium with the expectation that polyP mobilization would be more important under such conditions. Figure 3 illustrates that ppn1Δ ppx1Δ cells do display some impaired resistance to alkaline, cationic, and superoxide stress when grown under Pi-limiting conditions, albeit not to the same levels as pho4Δ cells (Fig. 3A). However, stress-sensitive phenotypes were not evident on YPD medium containing Pi (Fig. 3A) or with the single ppn1Δ and ppx1Δ mutants (Fig. S4). This indicates that Ppn1 and Ppx1 contribute to stress resistance only under Pi-limiting conditions and, as seen before, that these polyphosphatases function redundantly to promote stress resistance. Consistent with this, the impaired stress resistance associated with Ppn1 and Ppx1 loss was rescued upon reintegration of either PPN1 or PPX1 (Fig. 3A). These results show that polyP mobilization does contribute to the cellular resistance to stresses that are also dependent on the Pho4 transcription factor.
FIG 3.
PolyP mobilization and stress responses. (A) PolyP mobilization promotes stress resistance under Pi-limiting conditions. Exponentially growing strains were spotted in serial dilutions onto YPD-LPi plates containing 1 M NaCl and 300 μM menadione or at pH 8 (top, −Pi) or in YPD plates containing the same stresses (bottom, +Pi). Plates were incubated for 24 h at 30°C. (B) PolyP mobilization in response to alkaline stress is dependent on Ppn1 and Ppx1. Neisser staining of the indicated strains grown in YPD or after 10 min growth in YPD (pH 8). Bar, 10 μm. (C) Toluidine blue staining of RNA/polyP extracts from indicated strains after electrophoresis on urea-polyacrylamide gels, before and after 30 min growth in YPD medium (pH 8). (D) WT and ppn1Δ ppx1Δ cells expressing Pho4-GFP were grown in YPD, left untreated or transferred to YPD medium (pH 8) for 30 min, and processed to examine Pho4-GFP localization using fluorescence microscopy. DAPI staining illustrates nuclear positioning.
Stress phenotypes of the single Ppn1 and Ppx1 mutants. Exponentially growing strains were spotted in serial dilutions onto YPD-LPi plates containing the indicated stresses. Download FIG S4, TIF file, 1 MB (1MB, tif) .
Copyright © 2022 Ahmed et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
It has been documented in S. cerevisiae that alkaline stress triggers a Pi starvation response (8) which is accompanied by a rapid mobilization of polyP (33). Consistent with this, alkaline stress in C. albicans triggers polyP mobilization and the subsequent nuclear accumulation of Pho4 (5). As ppx1Δ ppn1Δ cells display some sensitivity to alkaline stress (Fig. 3A), we examined whether polyP mobilization and Pho4 nuclear accumulation, following growth in pH 8 medium, were dependent on Ppn1 and Ppx1. Both Neisser staining of cells and visualization of polyP by urea-PAGE replicated previous findings that polyP is rapidly mobilized in wild-type cells following alkaline stress (Fig. 3B and C). However, this alkaline stress-stimulated Pi mobilization was significantly impaired in ppn1Δ ppx1Δ cells. In wild-type cells, mobilization of polyP was evident after 10 min after switching to pH 8 medium, but this was not seen in cells lacking Ppn1 and Ppx1. Mobilization of polyP is also dependent on Ppx1 and Ppn1 when cells are grown in medium at pH 7.4 (Fig. S5), which reflects the slightly alkaline pH of blood. As impaired polyP mobilization impacted the nuclear accumulation of Pho4 following Pi limitation (Fig. 2C), we next examined the cellular localization of Pho-GFP in wild-type and ppn1Δ ppx1Δ cells following alkaline pH stress. As shown in Fig. 3D, Pho4 clearly accumulated in the nucleus in wild-type cells following growth in pH 8 medium for 30 min. In contrast, no nuclear accumulation of Pho4 was evident in ppn1Δ ppx1Δ cells following the same treatment. Collectively, these results indicate that polyP mobilization, mediated by Ppn1 and Ppx1, plays an important role in the cellular response of C. albicans to alkaline stress.
PolyP mobilization in response to growth at pH 7.4 is dependent on Ppn1 and Ppx1. Neisser staining of the indicated strains grown in YPD and after 60 min growth in YPD (pH 7.4). Download FIG S5, TIF file, 2.3 MB (2.4MB, tif) .
Copyright © 2022 Ahmed et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Cells lacking both polyphosphatases display severe morphological defects.
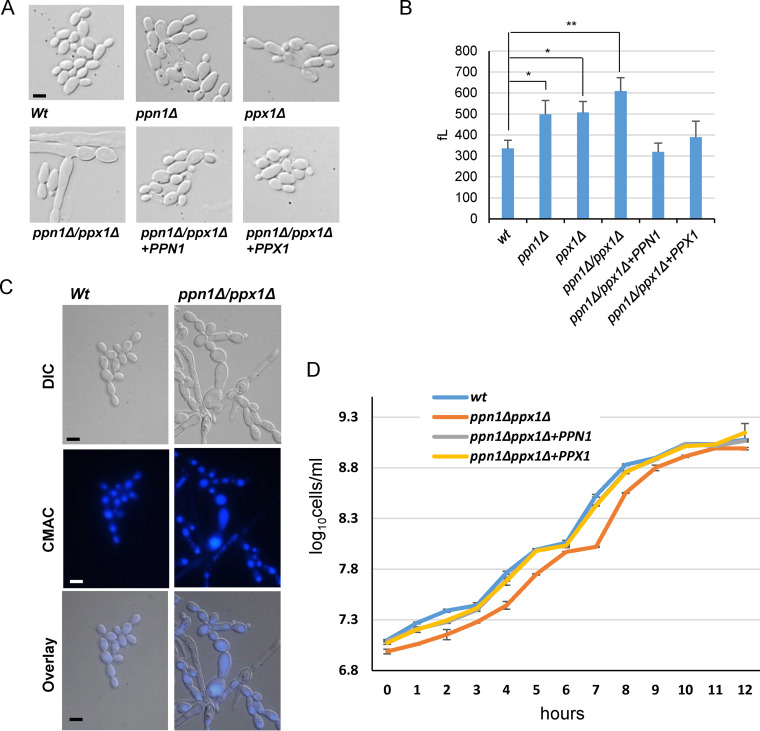
During our investigations into polyP mobilization in C. albicans, we observed morphological defects that were particularly evident in cells lacking both Ppn1 and Ppx1 (Fig. 4A). Cell volume analysis revealed that loss of PPN1 or PPX1 led to a significant increase in cell volume, which was exacerbated in the double ppn1Δ ppx1Δ mutant and reversed upon reintegration of PPN1 or PPX1 (Fig. 4B). The larger cell size can also be seen in the Neisser-stained cells in Fig. 1A and the fluorescence microscopy images in Fig. 2C and 3D. In addition to larger yeast cells, ppn1Δ ppx1Δ cells often formed swollen pseudohypha-like structures under non-filament-inducing conditions (Fig. 4A). As the majority of polyP is stored in the vacuole, we asked whether prevention of polyP mobilization would result in altered vacuolar morphology. Microscopy of cells stained with the vacuolar marker dye 7-amino-4-chloromethylcoumarin (CMAC) revealed that a proportion of ppn1Δ ppx1Δ cells have highly expanded vacuoles. Moreover, such expanded vacuoles tended to be restricted to cells displaying highly abnormal morphologies (Fig. 4C). Vacuole volume is implicated in cell cycle control in C. albicans (34), and thus, such expanded vacuoles seen in some ppn1Δ ppx1Δ cells may contribute to their morphological defects. Indeed, blocking polyP mobilization results in cells that grow slightly more slowly than wild-type cells (Fig. 4D); the doubling time for wild-type cells was 64.3 ± 0.6 min, and that for ppn1Δ ppx1Δ cells was 70.7 ± 1.2 min (P = 0.001).
FIG 4.
Morphological and growth characteristics of ppn1Δ ppx1Δ cells. (A) Cells lacking Ppn1 and Ppx1 have altered morphologies. DIC images of exponentially growing cells. (B) Cells lacking Ppn1 and Ppx1 are larger than wild-type cells. Cell volume analysis showing the cell volumes (means and SD). The data were analyzed statistically using Student’s two-sample t test. ns, not significant; *, P < 0.05; **, P < 0.01. (C) Some ppn1Δ ppx1Δ cells exhibit large vacuoles. Vacuolar morphology was captured by CMAC staining. (D) Cells lacking PPN1 and PPX1 have a slight slow-growth phenotype. Analysis of growth of the indicated strains in YPD. Cells numbers rather than OD were recorded due to the morphological defects seen in ppn1Δ ppx1Δ cells. Bars (A and C) represent 10 μm, and scale is the same across each row of images.
Recent work in S. cerevisiae has shown that polyP levels show a cyclical decrease during the S phase of the cell cycle. Furthermore, loss of Ppn1 and Ppx1 leads to a delay in progression through the G1/S phase of the cell cycle which correlates with impaired deoxyribonucleoside triphosphate (dNTP) production (25). These findings support a model whereby Pi release from polyP is important to support the synthesis of dNTPs necessary for DNA replication. Due to the morphological defects exhibited by C. albicans ppn1Δ ppx1Δ cells, we were unable to obtain a synchronous population (via centrifugal elutriation) to ask whether such cells also exhibited an S phase delay. Hence, instead, we asked whether such cells were sensitive to DNA replication stress elicited by hydroxyurea (HU), a potent inhibitor of ribonucleotide reductase (RNR) which synthesizes the dNTPs necessary for replication (35). Cells lacking both polyphosphatases were extremely sensitive to HU, and this sensitivity was rescued by reintegration of either PPN1 or PPX1 (Fig. 5A). Moreover, such sensitivity was restricted to HU-mediated replication stress and was not seen with other genotoxic stresses such as UV light or the DNA alkylating agent methyl methanesulfonate (MMS). Next, we explored whether cells lacking polyP would also show sensitivity to DNA stress, but as illustrated in Fig. 5A, cells lacking the polyP polymerase Vtc4 displayed wild-type levels of resistance to HU. This suggests that an inability to mobilize, rather than synthesize, polyP results in cells acutely sensitive to replication stress. Relevant to this, an interesting phenomenon has been reported in C. albicans, in which a range of genotoxic stresses, including replication stress, induce filamentous growth due to the significant elongation of the daughter bud (36, 37). Thus, we examined whether HU-induced hyperpolarized bud growth was impacted in ppn1Δ ppx1Δ cells. Consistent with previous results (36, 37), wild-type cells formed extensive filaments following HU exposure (Fig. 5B). However, ppn1Δ ppx1Δ cells demonstrated a heightened response in that longer filaments were generated following HU exposure than in wild-type cells (Fig. 5B). Quantification of filament length revealed statistically different differences between those formed in wild-type and ppn1Δ ppx1Δ cells and showed that reintegration of either PPN1 or PPX1 reversed this difference (Fig. 5C). The greater extent of filamentation seen in ppn1Δ ppx1Δ cells may be linked to the enhanced sensitivity of such cells to HU, due to delays in S phase progression.
FIG 5.
Ppn1 and Ppx1 are required for replication stress resistance and the formation of hyphae. (A) Exponentially growing strains were spotted in serial dilutions onto YPD plates that contained HU (40 mM) or MMS (0.02%) or that were exposed to UV (75 J/m2). Plates were incubated for 24 h at 30°C. (B) DIC images of cells grown in YPD and following treatment with 40 mM HU for 4 h. Bars represent 10 μm, and scale is the same across each row of images. (C) Quantification of hyperpolarized bud length was carried out using Zeiss imaging software on 200 cells for each strain. Data are means and SD. Statistical analysis was performed using Student’s two-sample t test. *, P < 0.05. (D) Stationary-phase cells were diluted 1:10 in YPD medium containing 10% fetal bovine serum and incubated at 37°C for 3 h (YPD + serum). Bars represent 10 μm, and scale is the same across each row of images.
Morphological switching is a key virulence determinant in C. albicans (38). Therefore, we examined whether the morphological defects presented by ppn1Δ ppx1Δ cells impacted the ability of this strain to undergo morphological switching to form true hyphae. As expected, wild-type cells rapidly formed true hyphae when grown under the filament-inducing conditions of medium supplemented with 10% serum at 37°C (Fig. 5D). Strikingly, serum-induced filamentation was drastically impaired in the ppn1Δ ppx1Δ mutant, with only very few cells forming true hypha-like structures (Fig. 5D). This defect was completely reversed upon reintegration of either PPN1 or PPX1, which indicates that preventing polyP mobilization inhibits morphological switching in C. albicans, a key virulence trait in this fungal pathogen.
PolyP mobilization is required for virulence in multiple infection models.
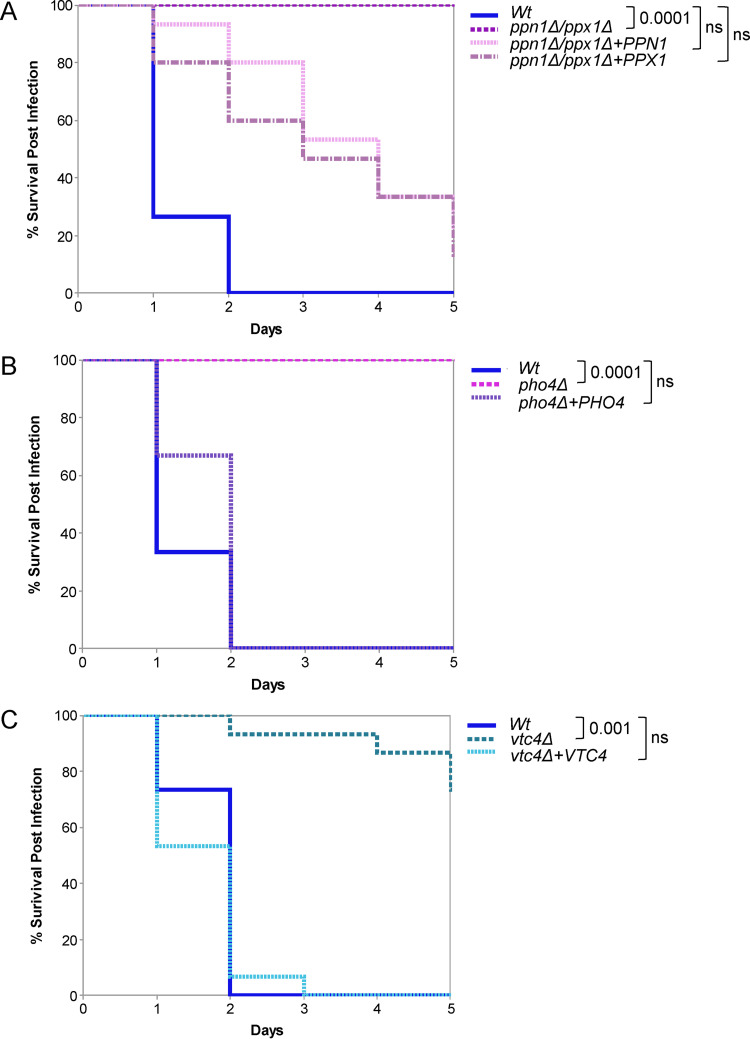
Given that preventing polyP mobilization impacts a number of virulence traits in C. albicans, including stress resistance and morphological switching, we investigated the importance of polyP mobilization in C. albicans virulence using the Galleria mellonella invertebrate model of systemic candidiasis (39). Cells lacking both PPX1 and PPN1 displayed significantly attenuated virulence compared to wild-type cells (P < 0.001) (Fig. 6A). Interestingly, despite the redundancy displayed by Ppn1 and Ppx1 in previous experiments, reconstitution with either PPN1 or PPX1 only partially restored infectivity to that of wild-type cells (Fig. 6A).
FIG 6.
Virulence analysis in the Galleria mellonella model of infection. (A to C) Comparison of virulence of the indicated strains in the Galleria model of systemic infection (15 larvae per fungal strain). The data were analyzed statistically using the log-rank (Mantel-Cox) test. ns, not significant.
As polyP mobilization is important for the activation of Pho4, we predicted that Pho4 would also be an important virulence determinant in the G. mellonella model. As shown in Fig. 6B, pho4Δ cells display significantly impaired virulence in this model of infection (P < 0.001), similar to that exhibited by ppn1Δ ppx1Δ cells. Thus, Pi mobilization and acquisition are important for the virulence of C. albicans in the G. mellonella model, which is consistent with our findings that polyP mobilization is an essential prerequisite for Pho4-mediated Pi acquisition.
To explore the importance of polyP presence in mediating C. albicans virulence, vtc4Δ cells were also tested in the G. mellonella model. Interestingly, deletion of VTC4 did impair C. albicans virulence (P < 0.01), albeit not to the same extent as that observed for pho4Δ and ppn1Δ ppx1Δ cells (Fig. 6C). Thus, although in vitro experiments have yet to reveal the precise roles of polyP in C. albicans biology (with the exception of manganese storage [5]), the presence of polyP does appear to contribute to the virulence of this important human fungal pathogen.
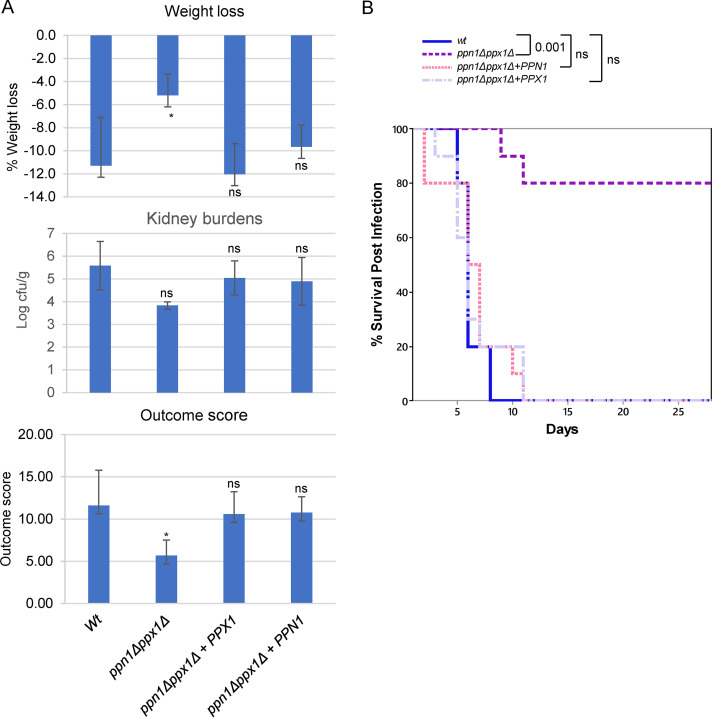
Following on from the observation that cells lacking Ppn1 and Ppx1 have a dramatic effect on C. albicans virulence in G. mellonella, the virulence of ppn1Δ ppx1Δ cells was examined in murine models of systemic candidiasis. The 3-day murine intravenous challenge model of C. albicans infection (10) combines weight loss and kidney fungal burden measurements after 72 h of infection to give an outcome score calculated as log(renal CFU/gram) − (0.5 × percent weight change), with a higher score indicating greater virulence. Mice infected with ppn1Δ ppx1Δ cells had a significantly lower kidney fungal burden and weight loss than those infected with wild-type cells, which culminated in a significantly lower outcome score (P < 0.01) (Fig. 7A). This virulence defect was restored by reintroduction of either PPN1 or PPX1, which resulted in kidney fungal burdens, weight loss, and thus outcome scores similar to those seen with wild-type cells (Fig. 7A). The same strains were examined in the 28-day survival model of systemic candidiasis (Fig. 7B). Mice injected with ppn1Δ ppx1Δ cells survived significantly longer than mice injected with wild-type cells or reconstituted strains expressing either PPN1 or PPX1. Mean survival times were 6.2 ± 0.3 days for wild-type (WT), 6.0 ± 0.7 for ppn1Δ ppx1Δ+PPX1, 7.4 ± 0.5 for ppn1Δ ppx1Δ+PPN1, and 19.8 ± 2.4 for ppn1Δ ppx1Δ cells (means ± standard errors of the means [SEM]). Therefore, cells lacking PPN1 and PPX1 display significantly attenuated virulence in both 3-day and 28-day murine models of systemic candidiasis which mirrors that seen in the G. mellonella model (Fig. 6). However, in both murine models of infection, reintroduction of either PPN1 or PPX1 restored wild-type levels of virulence to ppn1Δ ppx1Δ cells, thus indicating that Ppn1 and Ppx1 function redundantly to promote C. albicans virulence.
FIG 7.
Virulence analysis in murine models of infection. (A) Three-day infection model. Kidney burden, percentage weight loss, and outcome scores for mice (n = 6) infected with the indicated strains. Comparison of WT, ppn1Δ ppx1Δ+PPN1, and ppn1Δ ppx1Δ+PPX1 strain-infected mice with ppn1Δ ppx1Δ strain-infected mice by Kruskal-Wallis statistical analysis demonstrated a significant difference with ppn1Δ ppx1Δ strain-infected mice across all three parameters. ns, not significant; *, P < 0.05. (B) Twenty-eight-day survival model. Mice were injected with the same strains as for panel A, and survival was monitored daily. Survival curves were created using 10 mice per group except for the ppn1Δ ppx1Δ+PPX1 strain (n = 9). Comparing survival of each strain with WT cells, only ppn1Δ ppx1Δ cells were highly significantly different (Kruskal-Wallis nonparametric test).
DISCUSSION
Here, we show that two polyphosphatases, Ppn1 and Ppx1, function redundantly to mobilize polyP stores in C. albicans. Moreover, we find that blocking polyP mobilization impairs PHO pathway activation, stress resistance, and morphogenetic switching (Fig. 8). Consistent with these traits being important for the pathobiology of C. albicans, cells lacking Ppx1 and Ppn1 display significantly attenuated virulence in both G. mellonella and murine models of infection.
FIG 8.
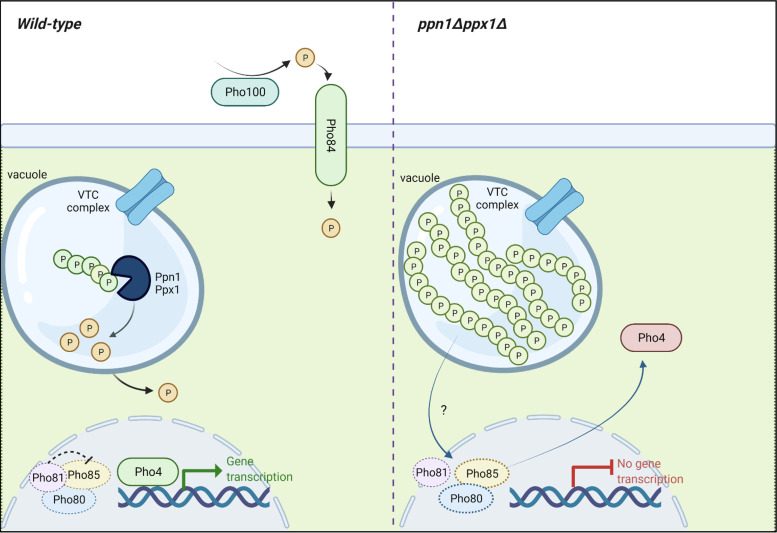
Activation of Pho4 is inhibited when polyP mobilization is hindered. Growth of wild-type C. albicans cells in Pi-limiting medium stimulates polyP mobilization, the nuclear accumulation of Pho4, and the induction of Pho4-dependent genes with roles in Pi acquisition. However, in cells lacking the Ppn1 and Ppx1 polyphosphatases, polyP mobilization is dramatically impaired following growth in Pi-limiting medium. Furthermore, Pho4 fails to accumulate in the nucleus and activate Pi acquisition genes. This suggests that an inability to mobilize polyP in C. albicans prevents the activation of Pho4. In S. cerevisiae, Pho4 is activated following Pi limitation via Pho81 inhibition of the Pho80-Pho85 cyclin-CDK complex, which negatively regulates Pho4. It is unknown if C. albicans Pho4 is regulated in the same way (as indicated by the dashed lines), but it is possible that polyP presence interferes with mechanism by which the cell senses Pi-limiting environments. The figure was created with BioRender.com.
The functional redundancy that exists between Ppn1 and Ppx1 in C. albicans was unanticipated, as in S. cerevisiae, deletion of Ppn1 alone results in the accumulation of longer-chain polyP molecules than in wild-type cells (22, 40). However, both Ppn1 and Ppx1 need to be deleted from the C. albicans genome before clear differences in polyP chain length and defects in polyP mobilization are seen. In S. cerevisiae, a total of four enzymes exhibiting polyphosphatase activity have been identified: Ppn1 (18), Ppn2 (41), Ppx1 (20), and Ddp1 (42). Only Ppn1 and Ppn2 are located in the vacuole, with Ppx1 and Dpd1 being predominantly cytoplasmic enzymes (40). Ppn1 and Ppn2 appear to be the major polyphosphatases in S. cerevisiae; deleting Ppn2 in ppn1Δ cells further increases the size of polyP chains (40). The C. albicans genome contains, in addition to Ppn1 and Ppx1 genes, homologues of Ppn2 (C7_03500W) and Ddp1 (C5_02220C) genes, which share 29% and 49% identity to their respective S. cerevisiae homologues. Thus, although we find that deletion of Ppn1 and Ppx1 in C. albicans largely prevents polyP mobilization, the small amount of mobilization seen in ppn1Δ ppx1Δ cells following Pi starvation may be due to Ppn2 or Ddp1 activity. Nonetheless, the functional redundancy that exists between Ppn1 and Ppx1 in C. albicans is clear; reintroduction of either PPX1 or PPN1 into ppx1Δ ppn1Δ cells rescues the myriad of phenotypes exhibited by the double mutant. This is particularly intriguing with regard to Ppx1; it is predicted to be a cytoplasmic enzyme, and yet the majority of polyP is stored in the vacuole.
In this study, we also found that blocking polyP mobilization impairs activation of the PHO pathway, and this, to the best of our knowledge, is the first such report (Fig. 8). Even following sustained growth under Pi starvation conditions (16 h), we saw no clear nuclear accumulation of Pho4 in ppn1Δ ppx1Δ cells and the induction of Pi acquisition genes was significantly impaired. This indicates that polyP presence (including polyP that cannot be mobilized) inhibits activation of the PHO pathway. The mechanism underlying this is unknown, although there have been recent breakthroughs in understanding how cells sense intracellular Pi levels (6). Many proteins involved in Pi homeostasis contain an SPX domain, which has been found to form a basic binding region for inositol pyrophosphate (PP-IP) ligands (43). As changes in Pi availability alter PP-IP levels (43), these molecules have been defined as “metabolic messengers” able to signal the Pi levels of the cell (44). In S. cerevisiae, the cyclin-dependent kinase (CDK) inhibitor Pho81 is regulated by PP-IP binding, which in turn regulates the activity of the Pho80-Pho85 cyclin-CDK complex; a negative regulator of the Pho4 transcription factor (45). Moreover, a recent study revealed that in the fungal pathogen Cryptococcus neoformans, PP-IP binding to Pho81 stabilizes the association of this CDK inhibitor with Pho80-Pho85 to promote PHO pathway activation and phosphate acquisition (46). In S. cerevisiae and C. albicans, polyP levels are also governed by PP-IPs, which bind to the SPX domain in Vtc4, stimulating its polyP-synthetase activity (43). Could the reverse also be true, i.e., polyP levels influence the levels of PP-IPs to inform the cell of Pi levels? In this scenario, the abundant yet unavailable polyP levels present in ppn1Δ ppx1Δ cells could signal to the cell that Pi levels are plentiful. This requires further investigation, but the observation that in C. albicans, as in S. cerevisiae (47, 48), the activation of Pho4-dependent genes occurs more rapidly in cells lacking polyP is consistent with the overall concept that polyP presence inhibits activation of Pi acquisition.
Consistent with Pho4 activation being compromised in cells that cannot mobilize polyP, ppn1Δ ppx1Δ cells display impaired growth on the same stresses that require Pho4 for resistance. However, cells lacking Pho4 are considerably more stress-sensitive than ppn1Δ ppx1Δ cells. This could be due to the fact that pho4Δ cells have no polyP and significantly lower Pi levels than wild-type cells (5), whereas there is residual polyphosphatase activity in ppn1Δ ppx1Δ cells.
Blocking polyP mobilization in C. albicans also results in significant morphological defects; both single ppx1Δ and ppn1Δ mutants are larger than wild-type cells, which is exacerbated in the double mutant, in which large swollen pseudohyphal cells are prevalent. Such morphological defects may be due in part to delays in cell cycle progression, as a recent study in S. cerevisiae revealed that Pi is mobilized from polyP to support dNTP synthesis and normal S-phase progression (25). Consistent with this, we found that C. albicans cells lacking both Ppn1 and Ppx1 are extremely sensitive to hydroxyurea, which blocks dNTP synthesis. Moreover, as Pho4 regulation is impaired in ppn1Δ ppx1Δ cells, it is noteworthy that “cell cycle” is one of the GO biological processes that are deregulated in pho4Δ cells compared with wild-type cells (5). A further morphological facet of ppn1Δ ppx1Δ cells is their impaired ability to form true hyphae following growth at 37°C in the presence of serum. PolyP mobilization does not appear to be stimulated during the yeast-to-hypha transition (Y. Ahmed and J. Quinn, unpublished data); thus, the inability of ppn1Δ ppx1Δ cells to form true hyphae may instead be linked to the cell cycle/morphological defects characteristic of this mutant. It is noteworthy, in this regard, that in a fungal pathogen of maize, Ustilago maydis, polyP is important for filamentous growth (49).
As we show that preventing polyP mobilization impacts the virulence of C. albicans, this study adds to a growing body of evidence that Pi homeostasis is important for the pathogenesis of this major human pathogen. Previous studies have shown that the Pho4 transcription factor is important for survival of C. albicans following phagocytosis and in systemic and commensal models of infection (5, 30), and deletion of the major phosphate transporter Pho84 also significantly impairs virulence (9), with a recent study revealing that Pho84 also promotes fungal commensalism (50). In this study, we show that preventing polyP mobilization impairs important pathogenesis traits, including PHO pathway activation and the yeast-to-hypha switch, which likely contribute to the virulence defect in ppn1Δ ppx1Δ cells. Indeed, the PHO pathway targets, PHO84 and PHO100, which are downregulated in ppn1Δ ppx1Δ cells, are both required for C. albicans virulence (9, 10). We also provide the first evidence that polyP presence contributes to the pathogenesis of C. albicans, as cells lacking the Vtc4 polyP synthase display impaired virulence in G. mellonella, albeit not to the same extent as in ppn1Δ ppx1Δ cells. Other studies have shown that Pi homeostasis is important for the virulence of a further major human fungal pathogen, Cryptococcus neoformans. Phosphate acquisition is essential, as cells lacking the Pho4 transcription factor were hypovirulent in both intranasal and intravenous models of cryptococcosis (51), and in a separate study, deletion of three phosphate transporters also resulted in reduced virulence (52). In the latter study, C. neoformans cells lacking homologues of the Ppn1 and Ppx1 polyphosphatases, Epp1 and Xpp1, were also created. While the double epp1Δ xpp1Δ mutant had higher levels of polyP than wild-type cells, this strain behaved like wild-type cells in a macrophage interaction assay (52). Hence, it would be interesting to explore if, as reported here for C. albicans, PHO pathway activation is likewise attenuated in C. neoformans cells lacking Epp1 and Xpp1.
In summary, we have demonstrated that polyP mobilization plays a multifaceted role in the pathobiology of C. albicans. It is intriguing that blocking polyP mobilization in C. albicans results in much stronger phenotypes than actually preventing the synthesis of this polymer. This may be due to the fact that blocking polyP mobilization impairs activation of the PHO pathway and therefore acquisition of extracellular Pi. Pi acquisition is an important virulence trait in several pathogens, and Pi homeostasis mechanisms differ significantly between fungal pathogen and host, making this an excellent target for new antifungal drug discovery.
MATERIALS AND METHODS
Media.
All strains were grown at 30°C in YPD medium (2% Bacto peptone, 1% Bacto yeast extract, 2% glucose) unless otherwise stated. For Pi-limiting conditions, strains were either grown in low-phosphate YPD medium (YPD-LPi) (2% Bacto peptone, 1% yeast extract base without phosphate [ForMedium], 2% glucose) or PNMC (peptone, 2.5 g/L; NaCl, 3 g/L; MgSO4, 1 mM; CaCl2, 1 mM) (53) supplemented with 20% glucose. As Bacto peptone contains 0.4% Pi, this equates to 0.8 mM Pi in YPD-LPi and 0.1 mM Pi in PNMC. Both YPD-LPi and PNMC were supplemented with 10 mM KH2PO4 to generate YPD+Pi and PNMC+Pi, respectively.
Strain construction.
All strains used in this study are listed in Table 1, and oligonucleotides are in Table S1.
TABLE 1.
Strains used in this study
| Strain | Name | Genotype | Reference or source |
|---|---|---|---|
| SN148 | arg4Δ/arg4Δ leu2Δ/leu2Δ his1Δ/his1Δ ura3Δ::imm434/ura3Δ::imm434 iro1Δ::imm434/iro1Δ::imm434 | 55 | |
| JC747 | WT SN148 + CIp30 | arg4 leu2/leu2 his1/his1 ura3::λimm434/ura3::λimm434 iro1::λimm434/iro1::λimm434 CIp10 | 59 |
| JC1936 | WT | SN152 arg4Δ/arg4Δ leu2Δ/leu2Δ his1Δ/his1Δ ura3/ura3Δ::imm434 IRO1/iro1Δ::imm434 CIp10 | 5 |
| JC1928 | pho4Δ | SN152 arg4Δ/arg4Δ leu2Δ/leu2Δ his1Δ/his1Δ ura3/ura3Δ::imm434 IRO1/iro1Δ::imm434 pho4Δ::HIS1/pho4Δ::LEU2 CIp10 | 5 |
| JC1917 | pho4Δ + CIp10-PHO4 | SN152 arg4Δ/arg4Δ leu2Δ/leu2Δ his1Δ/his1Δ ura3/ura3Δ::imm434 IRO1/iro1Δ::imm434 pho4Δ::HIS1/pho4Δ::LEU2 CIp10-PHO4 | 5 |
| JC1977 | PHO4-GFP | SN148 pACT-PHO4-GFP:URA3 | 5 |
| JC1984 | vtc4Δ + CIp10 | SN148 vtc4::loxP-ARG4-ura3-loxP/vtc4::loxP-HIS1-loxP CIp10 | 5 |
| JC2014 | vtc4Δ + CIp10-VTC4 | SN148 vtc4::loxP-ARG4-ura3-loxP/vtc4::loxP-HIS1-loxP CIp10-VTC4 | 5 |
| JC1991 | ppx1Δ + CIp10 | SN148 ppx1::loxP-ARG4-ura3-loxP/ppx1::loxP-HIS1-loxP CIp10 | This work |
| JC1985 | ppn1Δ + CIp10 | SN148 ppn1::loxP-ARG4-ura3-loxP/ppn1::loxP-HIS1-loxP CIp10 | This work |
| JC2016 | ppn1Δ + CIp10-PPN1 | SN148 ppn1::loxP-ARG4-ura3-loxP/ppn1::loxP-HIS1-loxP CIp10-PPN1 | This work |
| JC2283 | ppx1Δ + CIp10-PPX1 | SN148 ppx1::loxP-ARG4-ura3-loxP/ppx1::loxP-HIS1-loxP CIp10-PPX1 | This work |
| JC2210 | ppn1Δ ppx1Δ + CIp10 | SN148 ppn1::loxP-ARG4-ura3-loxP/ppn1::loxP-HIS1-loxP ppx1Δ::loxP/ppx1Δ::loxP CIp10 | This work |
| JC2257 | ppn1Δ ppx1Δ + CIp10-PPN1 | SN148 ppn1::loxP-ARG4-ura3-loxP/ppn1::loxP-HIS1-loxP ppx1Δ::loxP/ppx1Δ::loxP CIp10-PPN1 | This work |
| JC2267 | ppn1Δ ppx1Δ + CIp10-PPX1 | SN148 ppn1::loxP-ARG4-ura3-loxP/ppn1::loxP-HIS1-loxP ppx1Δ::loxP/ppx1Δ::loxP CIp10-PPX1 | This work |
| JC2303 | ppn1Δ ppx1Δ + PHO4-GFP | SN148 ppn1::loxP-ARG4-ura3-loxP/ppn1::loxP-HIS1-loxP ppx1Δ::loxP/ppx1Δ::loxP/pACT-PHO4-GFP:URA3 | This work |
Oligonucleotides used in this study. Download Table S1, DOCX file, 0.02 MB (26KB, docx) .
Copyright © 2022 Ahmed et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Deletion of PPN1 and PPX1.
To delete PPN1, disruption cassettes containing ARG4 or HIS1 nutritional marker genes, flanked by loxP sites and 91 bp 5′ and 3′ of the PPN1 open reading frame (ORF), were generated by PCR using oligonucleotide primers Ppn1delF and Ppn1delR and the plasmid template pLAL or pLHL (54). Following amplification, deletion cassettes were sequentially transformed into SN148 wild-type cells (55) to disrupt both alleles of PPN1. The same strategy was used to disrupt both alleles of PPX1, using the oligonucleotides Ppx1delF and Ppx1delR to create the ppx1Δ strain. PCR was used to confirm disruption of each allele. Uridine prototrophy was restored by integrating Clp10 at the RSP1 locus. This generated the ppn1Δ (JC1991) and ppx1Δ (JC1985) strains. To reintegrate PPN1 into ppn1Δ cells, the ORF and the promoter and terminator regions were amplified by PCR using the oligonucleotide pair PPN1CIFBamHI and PPN1CIRBamHI and ligated into the BamHI site of Clp10 to generate CIp10-PPN1. Clp10-PPN1 was linearized with StuI to integrate at the RPS1 locus of ppn1Δ cells, generating the ppn1Δ+PPN1 strain (JC2016). The same strategy but with the oligonucleotides PPX1CIfBamHI and PPX1CIRBamHI was used to create the ppx1Δ+PPX1 strain (JC2283).
To delete both PPX1 and PPN1, the Clox system with nourseothricin selection was used (56). A PPX1 disruption cassette containing the NAT1-Clox marker gene was PCR amplified using the oligonucleotides Ppx1natDelF and Ppx1natDelR. This was sequentially transformed into ppn1Δ cells to disrupt both alleles of PPX1, generating the ppn1Δ ppx1Δ mutant. CIp10 was integrated at the RSP1 locus to generate ppn1Δ ppx1Δ (JC2210). To reintegrate either PPN1 or PPX1, CIp10-PPN1 and CIp10-PPX1 were linearized with StuI to integrate at the RPS1 locus of ppn1Δ ppx1Δ cells, generating ppn1Δ ppx1Δ+PPN1 (JC2257) and ppn1Δ ppx1Δ+PPX1 (JC2267) cells, respectively. To facilitate localization of Pho4 in ppn1Δ ppx1Δ cells, the plasmid pACT-PHO4GFP (5) was linearized with StuI and transformed into ppn1Δ ppx1Δ cells, generating JC2303.
Polyphosphate analysis.
Intracellular polyP granules were examined by Neisser staining (31) and visualized by light microscopy. Cells grown in PNMC were fixed with paraformaldehyde (57), mounted on a slide, and stained with solution A (methylene blue, 0.1%; glacial acetic acid, 5%; ethanol, 5%) and solution B (crystal violet, 10%) for 15 s. Slides were stringently rinsed with water and allowed to dry, followed by staining with solution C (chrysoidin Y, 1%) for 45 s and stringent washing. Images were captured using a Zeiss Axioscope (differential inference contrast [DIC] setting) with a 63× oil immersion objective.
For urea-PAGE analysis, RNA and polyP were extracted as previously described (5). Twenty micrograms of RNA containing polyP was resolved on 12% polyacrylamide TBE-urea gels (Bio-Rad) in 1× Tris-borate-EDTA (TBE) buffer. Following electrophoresis, gels were fixed with glycerol and methanol, stained with toluidine blue, and destained as described previously (5).
ICP-MS.
Exponentially growing cells, grown in YPD at 30°C, were harvested by centrifugation, washed twice with 25 mL of Tris buffer (50 mM Tris, pH 7.5), incubated in the same buffer containing 10 mM EDTA for 5 min at room temperature to remove surface-bound metal, and then washed twice with 25 mL of the same buffer without EDTA. Washed pellets were digested in 1 mL of 65% (wt/vol) HNO3 (Merck) and incubated for >48 h at room temperature. The triplicate digested samples were centrifuged (13,000 × g, 20 min), and the supernatants were diluted 1:10 with 2% (wt/vol) HNO3 solution, which contained 20 μg/L Ag and Pt as internal standards, and analyzed by inductively coupled plasma mass spectrometry (ICP-MS) essentially as previously described (58). Differences were tested for statistical significance by one-way analysis of variance (ANOVA).
Yeast-hypha switch assay.
Hyphal formation was induced by diluting stationary-phase cells 1:10 in YPD medium containing 10% fetal calf serum (FCS) and incubated at 37°C for 3 h. DIC images were captured using a Zeiss Axioscope with a 63× oil immersion objective.
Spot tests.
Overnight C. albicans cultures grown in YPD were diluted back to an optical density at 660 nm (OD660) of 0.2 and then grown to mid-exponential phase (OD660 ≈ 0.7) before being diluted back to an OD660 of 0.2 in fresh YPD or YPD-LPi, and serial 10-fold dilutions were spotted onto YPD or YPD-LPi agar plates containing the specified stress-inducing compounds using a 48-well replica plater (Sigma). Plates were incubated for 24 to 48 h at 30°C.
Cell volume and growth analysis.
Cell volume was measured using a Beckman cell counter and analyzer system via pulse area analysis. Cells were grown to an equal OD660 of 0.7 before being sonicated (35 kHz, 15 s) to negate any artifacts cause by cells clumping together. From this, 200 μL of sample was added to 10 mL of filtered CASYton in sterile CASY cups. Samples were mixed immediately prior to measurement. The Beckman cell counter and analysis system was washed 3 times prior to sample analysis and checked for background. A wash step was performed between each sample read. Student’s two-sample t test was used to investigate whether the cell volume of specified strains differed from that of the wild type.
Growth analysis was performed as follows. Overnight cultures were grown in YPD and diluted back to a starting OD660 of 0.1. Samples were taken at time zero from the OD660 0.1 culture, which included an OD reading and a cell count using a hemocytometer. This was repeated hourly.
Pho4-GFP localization and Western blotting.
Wild-type and ppn1Δ ppx1Δ cells expressing Pho4-GFP (5) were grown in YPD, a sample was removed (t = 0), and then the remaining cells were washed 3 times, resuspended in YPD-LPi, and grown for the indicated times. Samples were processed as previously described (57). GFP and DAPI fluorescence was captured with a Zeiss AxioImager with a 63× oil immersion objective (Newcastle University Bioimaging Facility). Protein samples were subjected to electrophoresis on 8% SDS polyacrylamide gels and transferred to a nitrocellulose membrane. Membranes were blocked in 10% bovine albumin serum (BSA) in TBST (1 mM Tris-HCl [pH 8], 15 mM NaCl, 0.1% Tween 20 [vol/vol]) at room temperature for 30 min with gentle agitation. Following blocking, membranes were incubated with an anti-GFP antibody (Sigma, Dorset, UK) overnight at 4°C with gentle agitation. Membranes were subsequently washed 3 times in TBST before being incubated with a horseradish peroxidase (HRP)-conjugated anti-mouse secondary antibody (Sigma, Dorset, UK) for 1 h at room temperature. Development of membranes was carried out manually using an ECL Western blot detection system (Amersham Pharma Biotech) and Fuji Medical X-ray film. Tubulin was used as a loading control using an anti-tubulin monoclonal primary antibody (DSHB, University of Iowa) and the secondary antibody described above.
Hyperpolarized bud stimulation.
Hyperpolarized bud formation was induced by diluting stationary-phase cells grown overnight 1:10 in fresh YPD liquid medium containing 40 mM HU and incubating at 30°C for 6 h at 180 rpm. Cells were fixed with 3.7% paraformaldehyde, and images were captured using a Zeiss Axioscope with a 63× oil immersion objective. For each strain, 200 cells were measured using Zeiss imaging software. Analysis was carried out to determine the means and standard deviations (SD). Statistical analysis was performed using Student’s two-sample t test.
Vacuolar staining.
For vacuolar staining, prior to fixing, cells were washed 3 times in phosphate-buffered saline (PBS) to remove traces of YPD, as this can interfere with the dye. Cultures were incubated with 100 mM 7-amino-4-chloromethylcoumarin (CMAC) for 30 min in the dark followed by fixation with 3.7% paraformaldehyde. CAMC-stained images were captured using a Zeiss Axioscope by excitation at 460 nm.
RNA extraction.
Cells were grown to mid-log phase in at 30°C YPD prior to being harvested, washed twice in YPD-LPi, and then resuspended in YPD-LPi. Samples were collected following 0, 4, and 16 h growth in YPD-LPi, washed twice in ice-cold H2O, and then snap-frozen in liquid nitrogen.
For extraction, pellets were thawed on ice before being resuspended in 750 μL TES (10 mM Tris-HCl [pH 7.5], 5 mM EDTA [pH 7.5], 1% SDS [wt/vol]) and 750 μL of acidic phenol-chloroform. Following this, samples were incubated at 65°C for 1 h with vortexing every 10 min. Subsequently, samples were incubated on ice for 1 min and centrifuged at 6,000 rpm for 15 min 4°C to separate the aqueous layer. To the aqueous layer, 700 μL of acidic phenol-chloroform was added and mixed by inversion before undergoing centrifugation at 13,000 rpm for 5 min 4°C to separate the aqueous layer. Following this, 700 μL of phenol-chloroform was added to the aqueous layer, mixed by inversion, and centrifuged at 13,000 rpm for 5 min at 4°C. To the aqueous layer, 2 volumes of 100% ethanol and 1/10 volume 3 M sodium acetate (NaAc) (pH 7) were added, and samples were incubated overnight at −80°C to precipitate the RNA. Following incubation, samples were pelleted at 13,000 rpm for 15 min at 4°C. Pellets were washed in 400 μL 70% ethanol, centrifuged at 13,000 rpm for 5 min at 4°C, and then resuspended in 50 μL of sterile nano-H2O. Samples were stored at −80°C until use.
RT-qPCR.
For real-time qPCR, samples extracted were diluted according to their concentration to be used as a template for one-step reactions using the SuperScript III Platinum one-step qRT-PCR kit (Thermo Fisher) in 96-well plates run on an ABI machine. Threshold cycle (CT) values were determined using ABI software. For the tested targets, the single enrichment for each target was calculated using the comparative CT method. Actin was used for normalization using the primers ACT1F and ACT1R. RT-qPCR analysis was performed on transcripts of PHO pathway targets, PHO84 (PHO84F and PHO84R) and PHO100 (PHO100F and PHO100R), of 3 biological replicates in technical duplicate. Statistical significance was determined using ANOVA with Dunnett’s posttest for multiple comparisons.
G. mellonella virulence assay.
To investigate C. albicans virulence, cultures were grown to mid-exponential phase (OD660 ≈ 0.7) and washed 3 times in PBS. Following this, 5 × 105 cells were injected directly into the hemocoel via the last left proleg of 15 Galleria larvae. As a control, sterile PBS was injected into a further 15 larvae. Survival was assayed over a 5-day period at 37°C and is represented by Kaplan-Meier curves and analyzed by a log-rank test.
Murine virulence assays.
Sixty-four female BALB/c mice (6 to 8 weeks old) were purchased from Envigo Ltd. and were allowed to acclimatize for 1 week in the animal facility. Mice were randomly assigned to 12 cages, eight with five mice each and four with six mice each. Mice were allowed free access to food and water throughout the study. Procedures were carried out under UK Home Office project license 70/9027 awarded to Donna MacCallum and were carried out by a UK Home Office personal license holder.
C. albicans strains were grown for 16 to 24 h in 0.1% Neopeptone, 0.4% glucose, 0.1% yeast extract (NGT) medium at 30°C at 200 rpm. Cells were harvested, washed twice in saline, and enumerated using a hemocytometer. For each strain, 10 mice were injected in the tail vein with 3.5 × 104 CFU/g body weight in 100 μL sterile saline. Inoculum level was confirmed by viable-cell counting on Sabouraud dextrose agar. Inocula were randomly assigned to sets of cages, and strains were coded so that the researcher was blind to strain identity.
For the 3-day outcome experiment, the 6 mice in one cage were culled, weight change from day 0 to 3 was determined, and kidneys and brain were used to determine fungal burdens. Outcome score was determined using weight change and kidney burdens (10).
For the survival experiment, 10 mice (2 cages of 5 mice) were monitored over 28 days. Mice were weighed and monitored daily, with more frequent monitoring if mice became ill. Mice were culled if they lost 20% of their initial body weight, along with exhibiting ruffled fur and hunched posture, or if they developed a severe head tilt which affected their ability to reach food and water. Culled mice were recorded as having “died” on the following day. After 28 days, any surviving mice were culled. Kidneys and brains were removed for organ burden determination.
Results were compared in IBM SPSS version 25 using Kaplan-Meier log rank statistics to compare survival and the Kruskal-Wallis test (multiple groups) and Mann-Whitney U test (pairwise comparisons) for weight change, organ burdens, and outcome score comparisons. Surviving mice were considered censored data in survival curve statistics.
ACKNOWLEDGMENTS
We thank members of the Quinn lab and Tracy Palmer for useful comments on the manuscript.
This work was funded by BBSRC BB/P020119/1, BBSRC BB/P02050X/1, and a BBSRC-DTP studentship to Y.A. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Janet Quinn, Email: janet.quinn@ncl.ac.uk.
James W. Kronstad, University of British Columbia
REFERENCES
- 1.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Ene IV, Brunke S, Brown AJ, Hube B. 2014. Metabolism in fungal pathogenesis. Cold Spring Harb Perspect Med 4:a019695. doi: 10.1101/cshperspect.a019695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Citiulo F, Jacobsen ID, Miramón P, Schild L, Brunke S, Zipfel P, Brock M, Hube B, Wilson D. 2012. Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog 8:e1002777. doi: 10.1371/journal.ppat.1002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikeh M, Ahmed Y, Quinn J. 2017. Phosphate acquisition and virulence in human fungal pathogens. Microorganisms 5:48. doi: 10.3390/microorganisms5030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeh MAC, Kastora SL, Day AM, Herrero-de-Dios CM, Tarrant E, Waldron KJ, Banks AP, Bain JM, Lydall D, Veal EA, MacCallum DM, Erwig LP, Brown AJP, Quinn J. 2016. Pho4 mediates phosphate acquisition in Candida albicans and is vital for stress resistance and metal homeostasis. Mol Biol Cell 27:2784–2801. doi: 10.1091/mbc.e16-05-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azevedo C, Saiardi A. 2017. Eukaryotic phosphate homeostasis: the inositol pyrophosphate perspective. Trends Biochem Sci 42:219–231. doi: 10.1016/j.tibs.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Lenburg ME, O'Shea EK. 1996. Signaling phosphate starvation. Trends Biochem Sci 21:383–387. doi: 10.1016/0968-0004(96)10048-7. [DOI] [PubMed] [Google Scholar]
- 8.Serrano R, Ruiz A, Bernal D, Chambers JR, Ariño J. 2002. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol Microbiol 46:1319–1333. doi: 10.1046/j.1365-2958.2002.03246.x. [DOI] [PubMed] [Google Scholar]
- 9.Liu N-N, Uppuluri P, Broggi A, Besold A, Ryman K, Kambara H, Solis N, Lorenz V, Qi W, Acosta-Zaldívar M, Emami SN, Bao B, An D, Bonilla FA, Sola-Visner M, Filler SG, Luo HR, Engström Y, Ljungdahl PO, Culotta VC, Zanoni I, Lopez-Ribot JL, Köhler JR. 2018. Intersection of phosphate transport, oxidative stress and TOR signalling in Candida albicans virulence. PLoS Pathog 14:e1007076. doi: 10.1371/journal.ppat.1007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacCallum DM, Castillo L, Nather K, Munro CA, Brown AJP, Gow NAR, Odds FC. 2009. Property differences among the four major Candida albicans strain clades. Eukaryot Cell 8:373–387. doi: 10.1128/EC.00387-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Austin S, Mayer A. 2020. Phosphate homeostasis—a vital metabolic equilibrium maintained through the INPHORS signaling pathway. Front Microbiol 11:1367. doi: 10.3389/fmicb.2020.01367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerasimaitė R, Sharma S, Desfougères Y, Schmidt A, Mayer A. 2014. Coupled synthesis and translocation restrains polyphosphate to acidocalcisome-like vacuoles and prevents its toxicity. J Cell Sci 127:5093–5104. doi: 10.1242/jcs.159772. [DOI] [PubMed] [Google Scholar]
- 13.Saito K, Ohtomo R, Kuga-Uetake Y, Aono T, Saito M. 2005. Direct labeling of polyphosphate at the ultrastructural level in Saccharomyces cerevisiae by using the affinity of the polyphosphate binding domain of Escherichia coli exopolyphosphatase. Appl Environ Microbiol 71:5692–5701. doi: 10.1128/AEM.71.10.5692-5701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hothorn M, Neumann H, Lenherr ED, Wehner M, Rybin V, Hassa PO, Uttenweiler A, Reinhardt M, Schmidt A, Seiler J, Ladurner AG, Herrmann C, Scheffzek K, Mayer A. 2009. Catalytic core of a membrane-associated eukaryotic polyphosphate polymerase. Science 324:513–516. doi: 10.1126/science.1168120. [DOI] [PubMed] [Google Scholar]
- 15.Desfougères Y, Gerasimaitė RU, Jessen HJ, Mayer A. 2016. Vtc5, a novel subunit of the vacuolar transporter chaperone complex, regulates polyphosphate synthesis and phosphate homeostasis in yeast. J Biol Chem 291:22262–22275. doi: 10.1074/jbc.M116.746784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiménez J, Bru S, Ribeiro MP, Clotet J. 2017. Polyphosphate: popping up from oblivion. Curr Genet 63:15–18. doi: 10.1007/s00294-016-0611-5. [DOI] [PubMed] [Google Scholar]
- 17.Gerasimaitė R, Mayer A. 2016. Enzymes of yeast polyphosphate metabolism: structure, enzymology and biological roles. Biochem Soc Trans 44:234–239. doi: 10.1042/BST20150213. [DOI] [PubMed] [Google Scholar]
- 18.Sethuraman A, Rao NN, Kornberg A. 2001. The endopolyphosphatase gene: essential in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 98:8542–8547. doi: 10.1073/pnas.151269398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi X, Kornberg A. 2005. Endopolyphosphatase in Saccharomyces cerevisiae undergoes post-translational activations to produce short-chain polyphosphates. FEBS Lett 579:2014–2018. doi: 10.1016/j.febslet.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 20.Wurst H, Shiba T, Kornberg A. 1995. The gene for a major exopolyphosphatase of Saccharomyces cerevisiae. J Bacteriol 177:898–906. doi: 10.1128/jb.177.4.898-906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreeva N, Trilisenko L, Eldarov M, Kulakovskaya T. 2015. Polyphosphatase PPN1 of Saccharomyces cerevisiae: switching of exopolyphosphatase and endopolyphosphatase activities. PLoS One 10:e0119594. doi: 10.1371/journal.pone.0119594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa N, DeRisi J, Brown PO. 2000. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol Biol Cell 11:4309–4321. doi: 10.1091/mbc.11.12.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao NN, Gómez-García MR, Kornberg A. 2009. Inorganic polyphosphate: essential for growth and survival. Annu Rev Biochem 78:605–647. doi: 10.1146/annurev.biochem.77.083007.093039. [DOI] [PubMed] [Google Scholar]
- 24.Gray MJ, Wholey W-Y, Wagner NO, Cremers CM, Mueller-Schickert A, Hock NT, Krieger AG, Smith EM, Bender RA, Bardwell JCA, Jakob U. 2014. Polyphosphate is a primordial chaperone. Mol Cell 53:689–699. doi: 10.1016/j.molcel.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bru S, Martínez-Laínez JM, Hernández-Ortega S, Quandt E, Torres-Torronteras J, Martí R, Canadell D, Ariño J, Sharma S, Jiménez J, Clotet J. 2016. Polyphosphate is involved in cell cycle progression and genomic stability in Saccharomyces cerevisiae. Mol Microbiol 101:367–380. doi: 10.1111/mmi.13396. [DOI] [PubMed] [Google Scholar]
- 26.Docampo R, Ulrich P, Moreno SN. 2010. Evolution of acidocalcisomes and their role in polyphosphate storage and osmoregulation in eukaryotic microbes. Philos Trans R Soc Lond B Biol Sci 365:775–784. doi: 10.1098/rstb.2009.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrissey JH, Choi SH, Smith SA. 2012. Polyphosphate: an ancient molecule that links platelets, coagulation, and inflammation. Blood 119:5972–5979. doi: 10.1182/blood-2012-03-306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azevedo C, Livermore T, Saiardi A. 2015. Protein polyphosphorylation of lysine residues by inorganic polyphosphate. Mol Cell 58:71–82. doi: 10.1016/j.molcel.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Urrialde V, Prieto D, Pla J, Alonso-Monge R. 2015. The Pho4 transcription factor mediates the response to arsenate and arsenite in Candida albicans. Front Microbiol 6:118. doi: 10.3389/fmicb.2015.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urrialde V, Prieto D, Pla J, Alonso-Monge R. 2016. The Candida albicans Pho4 transcription factor mediates susceptibility to stress and influences fitness in a mouse commensalism model. Front Microbiol 7:1062. doi: 10.3389/fmicb.2016.01062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurr E. 1965. The rational use of dyes in biology and general staining methods. Leonard Hill, London, United Kingdom. [Google Scholar]
- 32.Smith SA, Morrissey JH. 2007. Sensitive fluorescence detection of polyphosphate in polyacrylamide gels using 4',6-diamidino-2-phenylindol. Electrophoresis 28:3461–3465. doi: 10.1002/elps.200700041. [DOI] [PubMed] [Google Scholar]
- 33.Castro CD, Meehan AJ, Koretsky AP, Domach MM. 1995. In situ 31P nuclear magnetic resonance for observation of polyphosphate and catabolite responses of chemostat-cultivated Saccharomyces cerevisiae after alkalinization. Appl Environ Microbiol 61:4448–4453. doi: 10.1128/aem.61.12.4448-4453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barelle CJ, Bohula EA, Kron SJ, Wessels D, Soll DR, Schäfer A, Brown AJP, Gow NAR. 2003. Asynchronous cell cycle and asymmetric vacuolar inheritance in true hyphae of Candida albicans. Eukaryot Cell 2:398–410. doi: 10.1128/EC.2.3.398-410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koç A, Wheeler LJ, Mathews CK, Merrill GF. 2004. Hydroxyurea arrests DNA replication by a mechanism that preserves basal dNTP pools. J Biol Chem 279:223–230. doi: 10.1074/jbc.M303952200. [DOI] [PubMed] [Google Scholar]
- 36.Bachewich C, Nantel A, Whiteway M. 2005. Cell cycle arrest during S or M phase generates polarized growth via distinct signals in Candida albicans. Mol Microbiol 57:942–959. doi: 10.1111/j.1365-2958.2005.04727.x. [DOI] [PubMed] [Google Scholar]
- 37.Shi QM, Wang YM, Zheng XD, Lee RT, Wang Y. 2007. Critical role of DNA checkpoints in mediating genotoxic-stress-induced filamentous growth in Candida albicans. Mol Biol Cell 18:815–826. doi: 10.1091/mbc.e06-05-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlisle PL, Banerjee M, Lazzell A, Monteagudo C, López-Ribot JL, Kadosh D. 2009. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc Natl Acad Sci USA 106:599–604. doi: 10.1073/pnas.0804061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobsen ID. 2014. Galleria mellonella as a model host to study virulence of Candida. Virulence 5:237–239. doi: 10.4161/viru.27434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azevedo C, Desfougères Y, Jiramongkol Y, Partington H, Trakansuebkul S, Singh J, Steck N, Jessen HJ, Saiardi A. 2020. Development of a yeast model to study the contribution of vacuolar polyphosphate metabolism to lysine polyphosphorylation. J Biol Chem 295:1439–1451. doi: 10.1074/jbc.RA119.011680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerasimaitė R, Mayer A. 2017. Ppn2, a novel Zn(2+)-dependent polyphosphatase in the acidocalcisome-like yeast vacuole. J Cell Sci 130:1625–1636. doi: 10.1242/jcs.201061. [DOI] [PubMed] [Google Scholar]
- 42.Lonetti A, Szijgyarto Z, Bosch D, Loss O, Azevedo C, Saiardi A. 2011. Identification of an evolutionarily conserved family of inorganic polyphosphate endopolyphosphatases. J Biol Chem 286:31966–31974. doi: 10.1074/jbc.M111.266320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wild R, Gerasimaite R, Jung J-Y, Truffault V, Pavlovic I, Schmidt A, Saiardi A, Jessen HJ, Poirier Y, Hothorn M, Mayer A. 2016. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 352:986–990. doi: 10.1126/science.aad9858. [DOI] [PubMed] [Google Scholar]
- 44.Shears SB. 2009. Diphosphoinositol polyphosphates: metabolic messengers? Mol Pharmacol 76:236–252. doi: 10.1124/mol.109.055897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee YS, Huang K, Quiocho FA, O'Shea EK. 2008. Molecular basis of cyclin-CDK-CKI regulation by reversible binding of an inositol pyrophosphate. Nat Chem Biol 4:25–32. doi: 10.1038/nchembio.2007.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desmarini D, Lev S, Furkert D, Crossett B, Saiardi A, Kaufman-Francis K, Li C, Sorrell TC, Wilkinson-White L, Matthews J, Fiedler D, Djordjevic JT. 2020. IP(7)-SPX domain interaction controls fungal virulence by stabilizing phosphate signaling machinery. mBio 11:e01920-20. doi: 10.1128/mBio.01920-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neef DW, Kladde MP. 2003. Polyphosphate loss promotes SNF/SWI- and Gcn5-dependent mitotic induction of PHO5. Mol Cell Biol 23:3788–3797. doi: 10.1128/MCB.23.11.3788-3797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas MR, O'Shea EK. 2005. An intracellular phosphate buffer filters transient fluctuations in extracellular phosphate levels. Proc Natl Acad Sci USA 102:9565–9570. doi: 10.1073/pnas.0501122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyce KJ, Kretschmer M, Kronstad JW. 2006. The vtc4 gene influences polyphosphate storage, morphogenesis, and virulence in the maize pathogen Ustilago maydis. Eukaryot Cell 5:1399–1409. doi: 10.1128/EC.00131-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Zhou J, Zou Y, Chen X, Liu L, Qi W, Huang X, Chen C, Liu N-N. 2022. Fungal commensalism modulated by a dual-action phosphate transceptor. Cell Rep 38:110293. doi: 10.1016/j.celrep.2021.110293. [DOI] [PubMed] [Google Scholar]
- 51.Lev S, Kaufman-Francis K, Desmarini D, Juillard PG, Li C, Stifter SA, Feng CG, Sorrell TC, Grau GER, Bahn Y-S, Djordjevic JT. 2017. Pho4 is essential for dissemination of Cryptococcus neoformans to the host brain by promoting phosphate uptake and growth at alkaline pH. mSphere 2:e00381-16. doi: 10.1128/mSphere.00381-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kretschmer M, Reiner E, Hu G, Tam N, Oliveira DL, Caza M, Yeon JH, Kim J, Kastrup CJ, Jung WH, Kronstad JW. 2014. Defects in phosphate acquisition and storage influence virulence of Cryptococcus neoformans. Infect Immun 82:2697–2712. doi: 10.1128/IAI.01607-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romanowski K, Zaborin A, Valuckaite V, Rolfes RJ, Babrowski T, Bethel C, Olivas A, Zaborina O, Alverdy JC. 2012. Candida albicans isolates from the gut of critically ill patients respond to phosphate limitation by expressing filaments and a lethal phenotype. PLoS One 7:e30119. doi: 10.1371/journal.pone.0030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dennison PM, Ramsdale M, Manson CL, Brown AJ. 2005. Gene disruption in Candida albicans using a synthetic, codon-optimised Cre-loxP system. Fungal Genet Biol 42:737–748. doi: 10.1016/j.fgb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Noble SM, Johnson AD. 2005. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot Cell 4:298–309. doi: 10.1128/EC.4.2.298-309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shahana S, Childers DS, Ballou ER, Bohovych I, Odds FC, Gow NAR, Brown AJP. 2014. New Clox systems for rapid and efficient gene disruption in Candida albicans. PLoS One 9:e100390. doi: 10.1371/journal.pone.0100390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Enjalbert B, Smith DA, Cornell MJ, Alam I, Nicholls S, Brown AJP, Quinn J. 2006. Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol Biol Cell 17:1018–1032. doi: 10.1091/mbc.e05-06-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tottey S, Waldron KJ, Firbank SJ, Reale B, Bessant C, Sato K, Cheek TR, Gray J, Banfield MJ, Dennison C, Robinson NJ. 2008. Protein-folding location can regulate manganese-binding versus copper- or zinc-binding. Nature 455:1138–1142. doi: 10.1038/nature07340. [DOI] [PubMed] [Google Scholar]
- 59.da Silva Dantas A, Patterson MJ, Smith DA, Maccallum DM, Erwig LP, Morgan BA, Quinn J. 2010. Thioredoxin regulates multiple hydrogen peroxide-induced signaling pathways in Candida albicans. Mol Cell Biol 30:4550–4563. doi: 10.1128/MCB.00313-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence alignment of Ppn1 and Ppx1 orthologues in S. cerevisiae and C. albicans. Sequences were aligned using ClustalW. Identical amino acids are annotated with asterisks, conserved amino acids with colons, and semiconserved amino acids with dots. Gaps introduced to maximize alignment are indicated with dashes. (A) Sequences of C. albicans (Ca) Ppn1 and S. cerevisiae (Sc) Ppn1. The endopolyphosphatase domain is highlighted in yellow. (B) Sequences of C. albicans (Ca) Ppx1 and S. cerevisiae (Sc) Ppx1. The Ppx1 superfamily domain is highlighted in red and the DHHA2 domain in yellow. Download FIG S1, TIF file, 1.9 MB (2MB, tif) .
Copyright © 2022 Ahmed et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Analysis of PHO pathway activation after extended growth in Pi-limiting medium. (A) WT and ppn1Δ ppx1Δ cells expressing Pho4-GFP were grown in YPD until mid-log phase (t = 0), moved to YPD-LPi medium for 16 h, and processed to examine Pho4-GFP localization using fluorescence microscopy. DAPI staining illustrates nuclear positioning. (B) WT and ppn1Δ ppx1Δ cells expressing Pho4-GFP were grown in YPD-LPi medium for 16 h, and Pho4-GFP expression was examined by Western blotting of whole-cell extracts using an anti-GFP antibody. An antitubulin antibody was used as a loading control. (C) RT-qPCR analysis showing fold induction of Pho4 target genes PHO84 and PHO100 after growth of the indicated strains for 16 h in YPD-LPi. Transcript levels were measured relative to the internal ACT1 mRNA control and normalized to the level of transcript in WT cells with Pi. Means and standard deviations for three biological replicates are shown. ns, not significant; *, P < 0.05; **, P < 0.01. Download FIG S2, TIF file, 2.7 MB (2.8MB, tif) .
Copyright © 2022 Ahmed et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) Analysis of growth of the indicated strains under the conditions used for cells in the RT-qPCR experiments. Cells numbers rather than OD were recorded due to morphological defects of ppn1Δ ppx1Δ cells. Means and standard deviations for three biological replicates are shown. (B) Reintegration of PPN1 or PPX1 restores Pho4-dependent gene expression. RT-qPCR analysis of Pho4 target genes PHO84 and PHO100 in the indicated strains. Means and standard deviations for three biological replicates are shown. ns, not significant; *, P < 0.05; **, P < 0.01. (C) Pho4-dependent gene expression occurs with faster kinetics in cells lacking polyP. RT-qPCR analysis of Pho4 target genes PHO84 and PHO100 in wild-type and vtc4Δ strains. Means and standard deviations for three biological replicates are shown. ns, not significant; *, P < 0.05; **, P < 0.01. Download FIG S3, TIF file, 1.6 MB (1.7MB, tif) .
Copyright © 2022 Ahmed et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Stress phenotypes of the single Ppn1 and Ppx1 mutants. Exponentially growing strains were spotted in serial dilutions onto YPD-LPi plates containing the indicated stresses. Download FIG S4, TIF file, 1 MB (1MB, tif) .
Copyright © 2022 Ahmed et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
PolyP mobilization in response to growth at pH 7.4 is dependent on Ppn1 and Ppx1. Neisser staining of the indicated strains grown in YPD and after 60 min growth in YPD (pH 7.4). Download FIG S5, TIF file, 2.3 MB (2.4MB, tif) .
Copyright © 2022 Ahmed et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Oligonucleotides used in this study. Download Table S1, DOCX file, 0.02 MB (26KB, docx) .
Copyright © 2022 Ahmed et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.