Abstract
Ovarian tissue cryopreservation (OTC) is the only pre-treatment option currently available to preserve fertility for prepubescent girls and patients who cannot undergo ovarian stimulation. Currently, there is no standardized method of processing ovarian tissue for cryopreservation, despite evidence that fragmentation of ovaries may trigger primordial follicle activation. Because fragmentation may influence ovarian transplant function, the purpose of this systematic review was (1) to identify the processing sizes and dimensions of ovarian tissue within sites around the world, and (2) to examine the reported outcomes of ovarian tissue transplantation including, reported duration of hormone restoration, pregnancy, and live birth. A total of 2,252 abstracts were screened against the inclusion criteria. In this systematic review, 103 studies were included for analysis of tissue processing size and 21 studies were included for analysis of ovarian transplantation outcomes. Only studies where ovarian tissue was cryopreserved (via slow freezing or vitrification) and transplanted orthotopically were included in the review. The size of cryopreserved ovarian tissue was categorized based on dimensions into strips, squares, and fragments. Of the 103 studies, 58 fertility preservation sites were identified that processed ovarian tissue into strips (62%), squares (25.8%), or fragments (31%). Ovarian tissue transplantation was performed in 92 participants that had ovarian tissue cryopreserved into strips (n = 51), squares (n = 37), and fragments (n = 4). All participants had ovarian tissue cryopreserved by slow freezing. The pregnancy rate was 81.3%, 45.5%, 66.7% in the strips, squares, fragment groups, respectively. The live birth rate was 56.3%, 18.2%, 66.7% in the strips, squares, fragment groups, respectively. The mean time from ovarian tissue transplantation to ovarian hormone restoration was 3.88 months, 3.56 months, and 3 months in the strips, squares, and fragments groups, respectively. There was no significant difference between the time of ovarian function’ restoration and the size of ovarian tissue. Transplantation of ovarian tissue, regardless of its processing dimensions, restores ovarian hormone activity in the participants that were reported in the literature. More detailed information about the tissue processing size and outcomes post-transplant are required to identify a preferred or more successful processing method.
Systematic Review Registration
[https://www.crd.york.ac.uk], identifier [CRD42020189120].
Keywords: fertility preservation, ovarian tissue cryopreservation, ovarian tissue transplantation, ovarian tissue size, oncofertility
Introduction
According to the American Cancer Society, 927,910 women and 10,500 children were diagnosed with cancer in 2021 (1). Advancements in cancer treatments, such as chemotherapy and radiation, have led to an increased chance of survival in these patients. Specifically, the 5-year survival rate of women aged 15-39 and children under the age of 14 is 86.7% and 84%, respectively (2). Cancer survivors are interested in methods that would improve their quality of life after treatment (3).
Cancer treatments such as the alkylating agent cyclophosphamide are gonadotoxic and cause irreversible damage to the germ cells by triggering double-stranded DNA breaks leading to apoptosis (4). This extreme decline in germ cells directly impairs ovarian endocrine function, which has systemic effects in the body, such as the increased risk of osteoporosis, high blood pressure, cardiovascular disease, and decline in cognitive function (5). The American Society of Clinical Oncology has recommended that oncologists describe and offer fertility preservation to their patients (6). Fertility preservation methods include ovarian transposition, gonadal shielding during pelvic radiotherapy, egg cryopreservation, embryo cryopreservation, and ovarian tissue cryopreservation (OTC) (7). The latter of these methods is the only option currently available to preserve fertility for prepubescent since they do not produce mature gametes (7, 8). OTC preserves primordial follicles within ovarian cortical microenvironment (7). Four key components are involved in OTC: ovarian surgical procurement, ovarian tissue processing, tissue cryopreservation, and storage. A unique feature of OTC is that in the future, the patient can choose to reimplant the ovarian cortical tissue orthotopically or heterotopically for fertility purposes or to restore ovarian endocrine function (7). Not only has this fertility preservation method been used to benefit cancer patients, but also women who want to postpone fertility and menopause (7). In 1999, the first successful autotransplantation of frozen-thawed ovarian cortical tissue was performed, but it was not until 2006 when Meirow et al. reported the first live birth obtained from OTC (9, 10).
Reported in 2017, ovarian tissue transplantation (OTT) has resulted in over 130 births (11). A recent meta-analysis of three centers has stated a pregnancy rate of about 50% (12). Although a vast majority of participants (95%) have the return of endocrine function post-transplantation, the average duration of endocrine function of ovarian tissue after transplantation is approximately 2-5 years (13). It has been shown that the duration of the ovarian tissue function is correlated to its ovarian reserve (14). Additionally, in xenograft experiments there is a decline in primordial follicles via activation and apoptosis in human, bovine, and marmoset 3-days post-transplantation of ovarian tissue (14). A review from Roness et al. has outlined that every step of OTC/OTT from the participant’s initial reserve to transplantation site can impact the premature loss of the primordial follicles and impact ovarian tissue function (15). Studies done in mice and humans have shown that fragmentation of ovarian tissue stimulates follicle activation pathways, such as Hippo and PI3K-AKT (16, 17). Fragmenting the ovarian cortex during the tissue processing for OTC may activate primordial follicles and reduce the ovarian reserve. OTC has recently been designated as a nonexperimental procedure by the American Society for Reproductive Medicine (ASRM) (18). However, there is currently no standard method of processing ovarian tissue, which emphasizes the importance of examining if fragmentation impacts the function of transplanted ovarian tissue. In this systematic review, we sought (1) to identify the sizes and processing techniques used to cryopreserve ovarian tissue around the world, and (2) to examine the reported results of different sized ovarian cortical tissue in participants who have undergone OTT on functional longevity, hormone restoration, pregnancy, and live birth. This report examines different dimensions of cryopreserved ovarian cortical tissue on fertility and ovarian function post-transplantation.
Materials and Methods
Search Strategy
This systematic review followed the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines and statement. This review’s protocol is registered on PROSPERO and is available on Centre for Reviews and Dissemination (CRD) website at https://www.crd.york.ac.uk (registration number #: CRD42020189120). All literature searches were conducted on the National Institute of Health (NIH) PubMed database (https://www.ncbi.nlm.nih.gov/pubmed/). We performed all searches until February 2022. The searches had no date restrictions, the document types included were case studies, multi-center studies, and articles that focused on the processing size of ovarian cortical tissue and reproductive outcomes in human patients who have undergone OTT. We included the following keywords in the search: “ovarian tissue cryopreservation”, “hormone restoration”, “live birth”, “success”, “output”, “size”, “fragments”, “strips”, “cubes”, “squares”, “slivers”, “processing”, and “transplantation”. A total of 14 searches were conducted. The parameters for the searches were: ((ovarian tissue cryopreservation), (ovarian tissue cryopreservation) AND ((hormone restoration) OR (pregnancy) OR (live birth) OR (success) OR (output)), (((ovarian tissue cryopreservation) AND((hormone restoration) OR (pregnancy) OR (live birth) OR (success) OR (output)) AND ((fragments) OR (strips) OR (squares) OR (cubes) OR (slices) OR (pieces) OR (slivers))), (((fragments) OR (strips) OR (squares) OR (cubes) OR (slices) OR (pieces) OR (slivers)) AND (Ovarian tissue processing)), ((((fragments) OR (strips) OR (squares) OR (cubes) OR (slices) OR (pieces) OR (slivers)) AND (Ovarian tissue processing)) AND (((Live birth) or (pregnancy) or (hormone restoration)))) AND (ovarian tissue cryopreservation), ((fragments) OR (strips) OR (squares) OR (cubes) OR (slices) OR (pieces) OR (slivers)) AND (Ovarian tissue cryopreservation)), (((fragments) OR (strips) OR (squares) OR (cubes) OR (slices) OR (pieces) OR (slivers)) AND (Ovarian tissue cryopreservation))) AND (((Pregnancy) or (live birth) or (success))), ((ovarian tissue cryopreservation) AND ((squares) OR (strips) OR (cubes) OR (slivers) OR (fragments) OR (pieces)), (((ovarian tissue cryopreservation) AND ((squares) OR (strips) OR (cubes) OR (slivers) OR (fragments) OR (pieces))) AND ((hormone function) OR (ovarian activity)), ((ovarian tissue cryopreservation) AND ((squares) OR (strips) OR (cubes) OR (slivers) OR (fragments) OR (pieces))) AND (restoration), (ovarian tissue cryopreservation) AND ((size) OR (fragments) OR (cubes) OR (strips) OR (slivers) OR (processing)), (ovarian tissue cryopreservation) AND (processing), (ovarian tissue cryopreservation) AND (size), (ovarian tissue cryopreservation) AND ((fragments) OR (strips) OR (cubes) OR (squares) OR (slivers)), respectively. The results were compiled, and duplicated results were removed. Titles and abstracts were manually reviewed to determine articles that met the inclusion/exclusion criteria. We presented a PRISMA flow diagram to layout the identification, screening, eligibility, and included studies for this review.
Inclusion/Exclusion Criteria
In this systematic review, we had two subgroups: ovarian tissue processing for cryopreservation and transplanted frozen/thawed processed ovarian tissue. Studies focused on ovarian tissue processing for cryopreservation were gathered for the processing analysis, and studies that included outcomes of OTT were considered for the outcomes analysis. Only studies that contained participants who have undergone OTC regardless of age, diagnosis, and previous cancer treatment for fertility preservation and hormone restoration purposes were included for the outcomes analysis. Studies were excluded if ovarian tissue was used for other reasons than future fertility or hormone restoration purposes such as for experimental analysis. These included studies underwent further screening which focused on OTT outcomes. For the outcomes analysis, studies which contained participants who have undergone OTC and orthotopic auto transplantation regardless of age, diagnosis, and previous cancer treatment were included. Studies that contained participants who have only undergone OTC without a record of transplantation, non-human studies, ovarian tissue that was cultured/incubated in drugs before transplantation, heterotopic OTT, and OTT using donor ovaries were not included in this analysis.
Study Selection
Duplicated articles were removed from the lists that were generated from the search strategies above. The titles and abstracts of the remaining articles were manually examined by three independent reviewers (AAD, NH, MTH) for inclusion and exclusion criteria based on the criteria of this study, previously mentioned. Reasons for exclusion and inclusion for all articles were recorded in the screening process. To avoid disagreements and bias in the study selection process, a fourth reviewer (HK) screened the title, abstracts, and full text of all articles for inclusion criteria. Articles that met the inclusion criteria were independently examined for data extraction by three reviewers (AAD, NH, MTH).
Data Extraction
Three reviewers (AAD, NH, MTH) assessed the full-length articles that met the inclusion criteria to extract study measures. The reasons for exclusion were recorded for those articles that did not meet the inclusion criteria after full-article examination. Articles that met the inclusion criteria for OTC had the following data extracted: lab name, location of site, country of origin, number of participants, age of participant(s) at OTC, condition of participant(s), previous cancer treatment prior to OTC, date range of which tissue was processed, surgical technique of ovary removal, partial or whole ovary removal, methods used to process ovarian tissue during OTC, techniques used to process tissue, name of the ‘size’ of cortical ovarian tissue processed, dimensions of tissue pieces (length x width x thickness, mm), number of total tissue processed/cryopreserved, and cryopreservation technique (slow freeze, vitrification). Only articles that cryopreserved human ovarian tissue for future fertility or hormone purposes, explicitly stated methods of processing ovarian tissue, and had clear dimensions (length, width) of OTC tissue were assessed. Additional data were gathered for studies that met inclusion criteria for OTT outcomes analyses including: age of participant(s) at OTT, site of transplantation, number of total ovarian tissue pieces transplanted, beginning of ovarian hormone restoration (months), the longevity of ovarian function (months), the number of participants which underwent transplantation, number of participants that underwent assisted reproductive technology (ART) to conceive (yes/no, number of rounds, number of participants), number of participants that conceived spontaneously (yes/no, number of participants), the number of pregnancies, and the number of live births. Every participant in these studies were treated individually to avoid bias and to effectively assess the ovarian activity outcomes. The extracted data was verified by one reviewer (AAD) for validity and bias.
Characterization of Size
The size of cryopreserved ovarian tissue was characterized into three categories based on dimensions of tissue pieces (length, width): strips, squares, fragments. Processed ovarian tissue were categorized as strips if the length (≥ 5 mm) and width had different measurements. Squares were determined to be ovarian tissue which had the same measurements for length (≥ 5 mm) and width (≥ 5 mm). Fragments were determined to be tissue which had the length and width < 5 mm. Only studies that included clear dimensions of the length and width of the processed ovarian tissue were included in this review.
Outcome Measurements
For the outcomes analysis the following data were evaluated: dimensions of processed ovarian tissue (length, width, thickness), most common dimensions of ovarian tissue, area of tissue transplanted, pregnancy rate, live birth rate, time to resumption of ovarian function post-transplantation, and longevity of ovarian hormone function. Only articles that contain these data measurements were assessed. The most common dimension of ovarian tissue was defined by the greatest number of participants that have the length and width of the ovarian tissue pieces in common. The average area of tissue transplanted was determined by the average area of the size of ovarian tissue pieces and the average amount of total tissue transplanted. Fertility rate was calculated to be percentage of the number of participants that obtained pregnancy to the number of participants that attempted pregnancy. Live birth rate was calculated to be the percentage of the number of participants who obtained live birth to participants who attempted pregnancy. We defined ovarian function restoration as the time of decline of FSH levels to premenopausal state, an increase in estradiol levels, or to time of resumption of menses, if the study included all these factors the former was noted.
Statistical Analysis
The number of tissues transplanted, and area of tissue transplanted were expressed as mean and range. The following parameters: age at OTC, age at OTT, and time of ovarian restoration from OTT were expressed as mean with standard deviations. To determine the differences between size of ovarian tissue and age at OTC, OTT, and ovarian restoration a One-way ANOVA was performed, P<0.05 were considered significantly different. Statistical analyses were performed using GraphPad PRISM.
Risk of Bias
Two authors (AAD, HK) independently evaluated each article included in this systematic review for risk for bias. In this systematic review case reports, multi-center studies, and articles were assessed for quality assessment. For these platforms, the Joanna Briggs Institute Critical appraisal checklist was utilized to examine the clarity on the participant’s demographic characteristics, medical history, current clinical condition, intervention or treatment procedure, post-interventions, adverse/unanticipated events. Disagreements or conflicts on risk of bias assessment were resolved by a third reviewer (MML) ( Supplementary Tables 1, 2 ).
Results
Study Selection
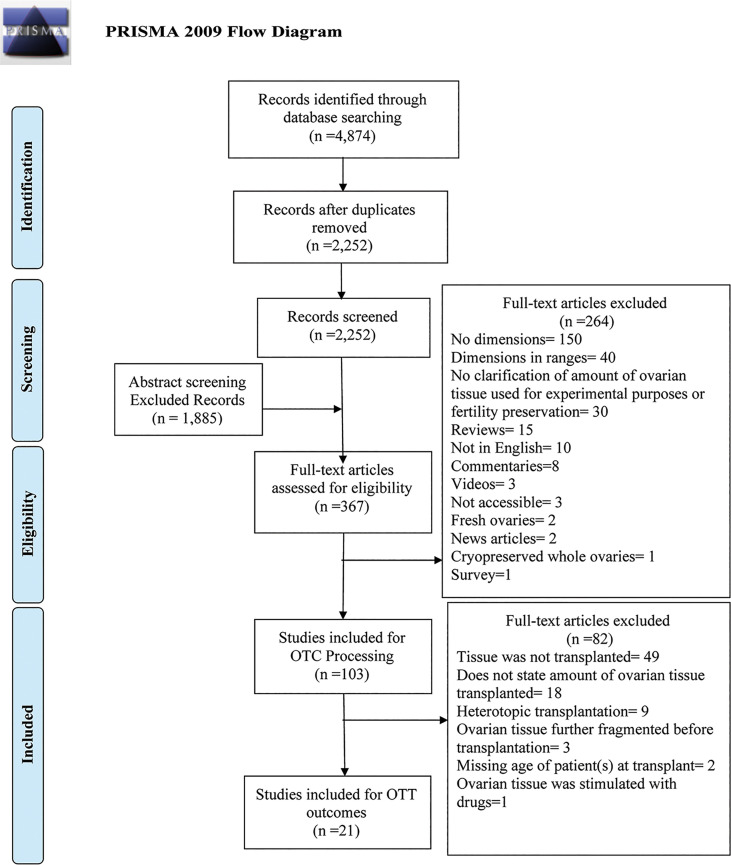
Figure 1 represents the PRISMA Flow diagram that was constructed for this systematic review. This diagram shows the screening, identification, eligibility, and inclusion steps conducted for OTC processing and OTT outcomes analyses. Studies that focused on OTC were gathered for OTC processing research; these articles underwent further screening for OTT and for OTT outcomes analysis. A total of 4,874 results were identified in PubMed; after manual removal of duplicate results, a total of 2,252 results were assessed for screening against the OTC inclusion criteria ( Figure 1 ).
Figure 1.
PRISMA Flow diagram for ovarian tissue cryopreservation and transplantation.
Of the abstract screened, 367 results met the inclusion criteria for the tissue processing analysis. Two-hundred and sixty-four results were excluded with reasons ( Figure 1 ). A total of 103 studies specifying the methods of processing ovarian tissue for cryopreservation, dimensions of cryopreserved ovarian tissue pieces, and location of the site were identified and included in this review for the outcomes analysis ( Figure 1 ). As previously mentioned, the articles included for processing analysis were further screened against the inclusion criteria for OTT. Twenty-one articles that were used for processing analysis were included for OTT outcomes analysis in this review. A total of 82 articles were excluded from outcomes analysis with reasons ( Figure 1 ). In this systematic review, 103 studies that contained participants that underwent OTC were included ( Supplementary Table 3 ). Additionally, 21 studies described 92 participants who underwent OTC and autologous transplantation ( Supplementary Table 4 ).
Ovarian Tissue Processing Sites
In this review, 58 unique sites published how ovarian tissue was processed for cryopreservation and tissue size categorized into three groups: strips, squares, and fragments ( Table 1 ). There were 15, 3, and 10 unique length and width dimensions for strips, squares, and fragments, respectively. A total of 36 sites (62%) from 18 countries processed the ovarian tissue into strips (19–71) ( Table 1 ). The most common dimension (length, width) for strips of 10 mm x 5 mm is processed in 19 different sites, with a range of 5-30 mm by 1-20 mm, (length, width). A total of 15 sites (25.8%) process ovarian tissue into squares, with the dimensions of 5 x 5 mm being the most common in 12 different sites and a range of 5-10 mm by 5-10 mm (length, width) ( Table 1 ). Ovarian tissue was processed into squares in 15 sites from 11 countries (20, 65, 72–98). In total, 17 different sites (29.3%) from 12 countries process ovarian tissue into dimensions that were categorized into fragments ( Table 1 ) (21, 62, 77, 99–122). Fragments with the dimensions of 2 x 2 mm (length, width) were the most common in 5 sites. The fragment dimensions ranged from 0.5-4 by 0.3-3 mm (length, width) ( Table 1 ). Reported thicknesses ranged from 1 – 2 mm, and one site reporting a thickness of 5 mm ( Table 1 ).
Table 1.
Ovarian tissue cryopreservation processing sizes across different sites around the world.
| Size | Dimensions length x width (mm2) thickness (site) | Sites of OTC processing |
|---|---|---|
| Strips |
|
|
| Squares | ||
| Fragments |
thickness no mentioned.
thickness of 1 mm.
thickness of 1-1.5 mm.
thickness of 1-2 mm.
thickness of 2 mm.
thickness of 5 mm.
Of the 58 unique OTC processing sites, 8 (13.8%) cut ovarian tissue into different dimensions within the same size categories, 6,1, and 2 sites for strips, squares, and fragments, respectively ( Table 1 ). Additionally, 9 sites (15.5%) processed ovarian tissue into different sizes ( Table 2 ).
Table 2.
Processing sites that cut ovarian tissue into different sizes.
| Size of processed ovarian tissue | Site(s) | Dimensions length x width (mm2) |
|---|---|---|
| All three sizes | The Juliane Marie Centre for Women, University Hospital of Copenhagen, University of Copenhagen, Copenhagen, Denmark. | Strips: 5 x 4, 5 x 1 Squares: 5 x 5 Fragments: 4 x 3, 3 x 3, 3 x 2, 2 x 2 |
| Royal Women’s Hospital, Parkville, Victoria, Australia. | Strips: 5 x 1 Squares: 5 x 5 Fragments: 2 x 2, 3 x 3, 4 x 3 |
|
| Strips and fragments | Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Brussels, Belgium. | Strips: 12 x 4, 10 x 5, 10 x 4, 10 x 3 Fragments: 2 x 2 |
| Lis Maternity Hospital, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel. | Strips: 10 x 5 Fragments: 0.5 x 0.3 |
|
| University Medical Center Utrecht, Utrecht, The Netherlands. | Strips: 10 x 3 Fragments: 2 x 2 |
|
| Medical University of Bonn, Bonn, Germany. | Strips: 8 x 4 Fragments: 2 x 1 |
|
| Strips and squares | St. Luke’s Hospital, St. Louis, Missouri, USA. | Strips: 20 x 10 Squares: 10 x 10 |
| Marianna University School of Medicine, Kawasaki City, Japan. | Strips: 5 x 3 Squares: 10 x 10 |
|
| Squares and fragments | Erasme Hospital, Université Libre de Bruxelles, Brussels, Belgium. | Squares: 5 x 5 Fragments: 3 x 2 |
To determine if there is a correlation between age at OTC, participant diagnosis or ovarian tissue procurement method and the chosen size for tissue processing, additional data was extracted from articles where sites reported using multiple tissue processing sizes. Two sites (3.4%) cryopreserved ovarian tissue into all three sizes ( Table 2 .) The Juliane Marie Centre for Women at University Hospital of Copenhagen in Copenhagen, Denmark reported cutting tissue into the following dimensions (1): strips: 5 x 4, 5 x 1 (2), squares: 5 x 5 (3), fragments: 4 x 3, 3 x 3, 3 x 2, 2 x 2 mm2 (length, width) ( Table 2 ). This site processed a 9-year-old Ewing’s sarcoma participant’s ovarian tissue into 5 x 4 mm2 (64). Six galactosemia participants at this site had one ovary removed for fertility preservation at the ages of 1.7, 0.9, 4.5, 0.3, 2.9, and 11.7 and ovarian tissue was cut into the dimensions: 5 x 5, 4 x 3, 3 x 3, 3 x 2, 2 x 2, and 2 x 2 mm2, respectively (77). Additionally at this site, 25 leukemia participants had 5 x 1 mm2 strips cryopreserved (71). The Royal Women’s Hospital in Victoria, Australia also reported processing ovarian tissue into multiple different sizes (1): strips: 5 x 1 (2), squares: 5 x 5 (3), fragments: 2 x 2, 3 x 3, 4 x 3 mm2 ( Table 2 ). Over 40 participants at this site had ovarian tissue processed into squares at the mean age of 26 (range 17.8 – 40.7), these participants had wide range of diagnoses (79). Also at this site 17 participants that had a mean age of 27.4 (range 17.8 – 35.9) at OTC had their ovarian tissue processed and cryopreserved into 5 x 1 mm2 strips (68). The Royal Women’s Hospital processed 9 participants (mean age at OTC= 20.6, range 18-31) ovarian tissue into 4 x 2 mm2 fragments, these participants had various from of diseases (120).
In total 4 sites (6.9%) cut ovarian tissue into strips and fragments. The Cliniques Universitaires Saint-Luc in Brussels, Belgium, reported processing ovarian tissue into strips and fragments with the following dimensions: 12 x 4, 10 x 5, 10 x 4, 10 x 3, and 2 x 2 mm2 ( Table 2 ). At this site, 6 participants with different diagnoses and a mean age of 23.8 (range 21-28) underwent partial or whole oophorectomy had 12 x 4 mm2 strips cryopreserved (22). A 23-year-old sickle cell anemia participant, at the Cliniques Universitaires Saint-Luc, that underwent a unilateral oophorectomy for fertility preservation had 10 x 5 mm2 ovarian tissue strips cryopreserved (29). Four participants, with the mean age of 23.5 (range 21-28) at OTC, had their ovarian tissue was cut into 10 x 4 mm2 strips for cryopreservation (123). A 22-year-old participant at this site had 10 x 3 mm2 ovarian strips cryopreserved. Additionally at this site, 2 participants (17 and 25 years old at OTC) had fragments cryopreserved in the dimensions of 2 x 2 mm2 (22). In the Lis Maternity Hospital at Tel Aviv Sourasky Medical Center in Tel Aviv, Israel, 93 participants (average at OTC 15.4, range 0–25) with a wide range of diagnosis at OTC, had 0.5 x 0.3 mm2 fragments cryopreserved ( Table 2 ) ( 116). Also at this site, an 18 year old participant with Hodgkin’s disease had ovarian tissue cut into 10 x 5 mm2 strips for OTC (43). At University Medical Center Utrecht in Utrecht, The Netherlands, 10 participants with different diagnoses underwent unilateral oophorectomy for fertility preservation and had 10 x 3 strips and 2 x 2 mm2 fragments cryopreserved (52) ( Table 2 ).
Two additional sites report processing tissue into strips and squares (3.4%). St. Luke’s Hospital in St. Louis, Missouri, USA cryopreserves participants’ ovarian tissue into 20 x 10 mm2 strips and 10 x 10 mm2 squares (20) ( Table 2 ). From 1997 to 2007, slow freezing was this site’s main method of cryopreservation and therefore cut ovarian tissue into 20 x 10 mm2 strips (20). This site changed its method of cryopreservation to vitrification in 2007. From 2007 to 2017, participants at this site had 10 x 10 mm2 squares cryopreserved for fertility preservation (20). In Japan, the Marianna University School of Medicine uses 5 x 3 mm2 strips and 10 x 10 mm2 squares for participants with various diagnosis at OTC (17, 73) ( Table 2 ).
Of the 58 processing sites 1 site uses both squares and fragments as its size for cryopreserving ovarian tissue. Erasme Hospital in Brussels, Belgium cryopreserves tissue as 5 x 5 mm2 squares, which has been used for over 200 participants (age range 0-27) with different diagnoses at OTC, and regardless of partial or whole ovary removal (89) ( Table 2 ). Additionally, a 13-year-old sickle cell anemia participant at this site had 3 x 2 mm2 fragments ovarian tissue cryopreserved for fertility preservation (100).
Characteristics of OTT Participants
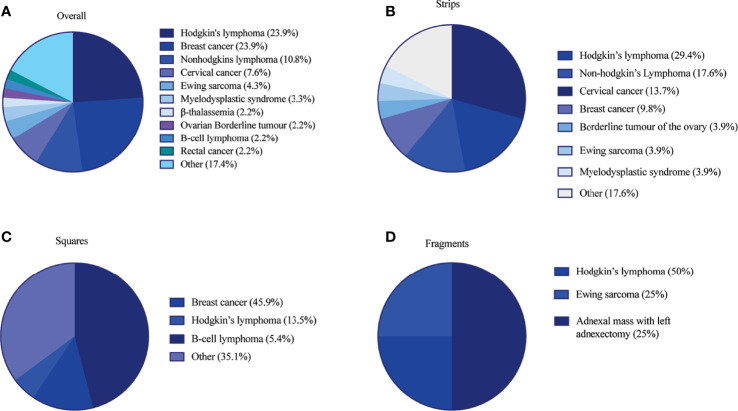
The clinical metadata for participants that were included in this systematic review is detailed in Supplementary Table 4 . A total of 92 unique participants who underwent OTT after OTC with the goal of having a biological child or restoring hormones were included in this analysis (21, 22, 25, 34, 37, 44, 57, 59, 61, 64, 66, 78, 84, 91–93, 95, 111, 115, 118, 124). Overall, 51, 37, and 4 participants had their ovarian tissue processed into strips, squares, and fragments, respectively ( Table 3 ). The three most predominant conditions that were present in the total participant population were Hodgkin’s lymphoma (23.9%), breast cancer (29.3%), and other conditions (17.4%) ( Figure 2A ). In the strips and fragment participant populations, Hodgkin’s lymphoma was the most prevalent diagnosis ( Figures 2B, D ). Additionally, the most prevalent condition for participants whose ovaries were processed into squares was breast cancer ( Figure 2C ). The mean age at OTC was 27.4, 29.8, and 22.8 years for participants whose ovaries were processed into strips, squares, and fragments, respectively ( Figure 3A ). The mean age at the time of OTT was 33.1, 33.8, and 30.3 for strips, squares, and fragments groups, respectively ( Figure 3B ). There was statistically significant difference in the age at the time of OTC (P-value= 0.0390) between all three sizes, but no significant difference between two sizes. Furthermore, there was no statistically significant difference between the age at the time OTT (P-value= 0.4130) in the three different size ovarian tissue pieces, respectively ( Figures 3A, B ). Although all groups had at least one participant with previous cancer treatment before OTC, the strips group had the greatest number of participants in this subgroup (n=21) ( Table 3 ). The ovarian tissue was most often processed into the dimensions of 6 x 4 mm2, 5 x 5 mm2, and 2 x 2 mm2 (length x width) in the strips, squares, and fragments groups, respectively prior to OTT ( Table 3 ). Eighteen participants underwent a second OTT 7, 10, and 1 strips, squares, and fragments groups, respectively and one participant underwent a third OTT ( Table 3 ). A total of 51 participants whose ovarian tissue was processed into strips, 9 into squares, and 3 into fragment were included for analysis because information on hormone restoration and longevity was reported. The average area of tissue per first OTT was 334.0 mm2, 271.8 mm2, and 123.8 mm2 for strips, squares, and fragments groups, respectively. Overall, the total average area of tissue transplanted was 394.58 mm2, 328.4 mm2, and 134.8 mm2 for strips, squares, and fragments groups, respectively ( Table 3 ). There was a statistically significant difference between the total and first average area of tissue per OTT in all three size ovarian tissue pieces. (P-value= 0.0432 and 0.0013, respectively) Additionally, there was not a statistically significant difference in total and first average area of tissue per OTT between two groups ( Table 3 ; Figures 4A, B ).
Table 3.
OTC/OTT Participant characteristics for fertility and hormone outcomes (21, 22, 25, 34, 37, 44, 57, 59, 61, 64, 66, 78, 84, 91–93, 95, 111, 115, 118, 124).
| Size of processed ovarian tissue | Strips | Squares | Fragments |
|---|---|---|---|
| Range Dimensions of processed ovarian tissue Length x Width (mm2 ) | 6-10 x 2-5 | 5 x 5 | 2-3 x 1-2 |
| Most common dimension Length x Width (mm2 ), (n=67) | 6 x 4 (n = 28) | 5 x 5 (n = 37) | 2 x 2 (n = 2) |
| No. of OTC/OTT participants | 51 | 37 | 4 |
|
Method of cryopreservation
(Slow freeze, Vitrification) |
Slow freeze: 50 Vitrification: 0 |
Slow freeze: 37 Vitrification: 0 |
Slow freeze: 4 Vitrification: 0 |
|
Mean Age at OTC
Years ± SD, Range |
27.4 ± 6.6, 9-40 | 29.8 ± 5.31, 15.4-38 | 22.8 ± 3.3, 18-25 |
|
Mean Age at OTT
Years ± SD, Range |
33.1 ± 5.3, 13.6-41.9 | 33.8± 4.95, 27-43 | 30.3 ± 1.7, 28-32 |
| No. of participants with previous treatment prior to OTC | 21 | 2 | 1 |
|
Average number of tissues transplanted, ± SD, Range
(1st, 2nd, 3rd) |
1st: 11.5 ± 7.8, 2-46 2nd: 11.7 ± 4, 8-16 (n=7) |
1st:10.9 ± 2.8, 6-17 2nd: 8.3 ± 4.3, 3-16 (n=10) |
1st: 21.8 ± 30.3, 4-67 2nd: 20 (n=1) 3rd: 49 (n=1) |
|
Av. Area of tissue transplanted (mm2) ± SD, Range, mm2
(Total, 1st, 2nd, 3rd) |
Total: 394.58 ± 262.7, 40 – 1152 1st: 334.0 ± 172.5, 40-920 2nd: 385.75 ± 100.7, 216-550 3rd: - |
Total: 328.4 ± 128, 150 -750 1st; 271.8 ± 69.9, 150-425 2nd: 207.5 ± 108, 75-400 mm2 3rd: - |
Total: 134.8 ± 139.8, 12-268 1st; 83 ± 123.8, 12-268 2nd: 60 (n=1) 3rd:147 (n=1) |
Figure 2.
Participant diagnosis at time of OTC in (A) overall patient population (B) strips (C) squares and (D) fragments group. Diagnoses in the other category included the following in (1) overall: acute lymphocytic leukemia, adnexal mass with left adnexectomy, aplastic anemia, autoimmune vasculitis, choriocarcinoma, colorectal cancer, endometrial cancer, granulomatosis with polyangiitis, leukemia, neuroendocrine tumor, ovarian cancer, Schwachman-diamond syndrome, sickle cell anemia, synovial sarcoma of the lung and pelvic sarcoma, systemic lupus erythematosus, T-cell lymphoma (2), strips: acute lymphocytic leukemia, aplastic anemia, β-thalassemia, colorectal cancer, endometrial cancer, rectal cancer, Schwachman-diamond syndrome, sickle cell anemia, systemic lupus erythematosus, and (3) squares: autoimmune vasculitis, β-thalassemia, choriocarcinoma, Ewing’s sarcoma, granulomatosis with polyangiitis, leukemia, myelodysplastic syndrome, neuroendocrine tumor, non-Hodgkin’s lymphoma, ovarian cancer, rectal cancer, synovial sarcoma of the lung and pelvic sarcoma, T-cell lymphoma (21, 22, 25, 34, 37, 44, 57, 59, 61, 64, 66, 78, 84, 91–93, 95, 111, 115, 118, 124).
Figure 3.
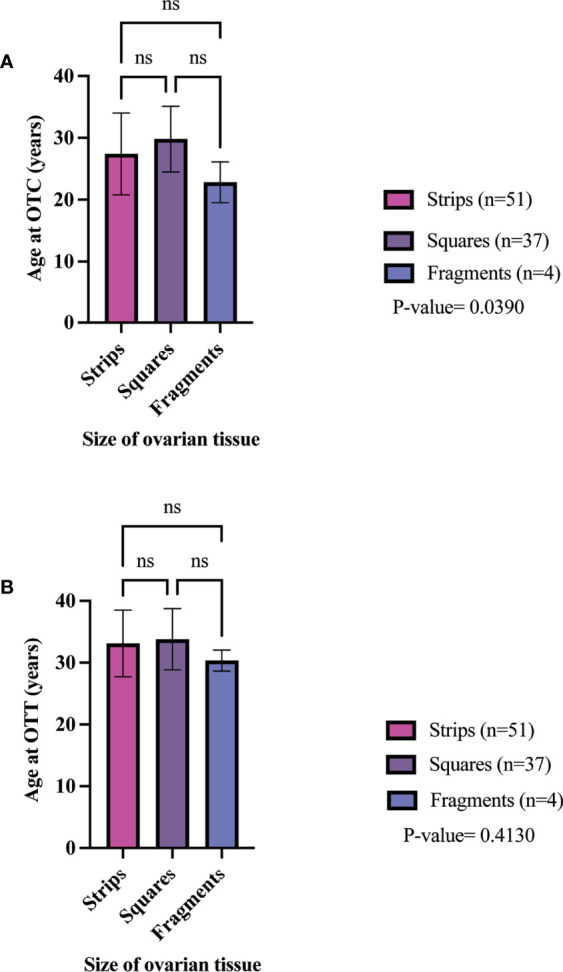
Age of participant at OTC (A) and OTT (B) in different size cryopreserved and transplanted ovarian tissue. P-values greater than 0.05 were considered not significantly different (ns) (21, 22, 25, 34, 37, 44, 57, 59, 61, 64, 66, 78, 84, 91–93, 95, 111, 115, 118, 124).
Figure 4.
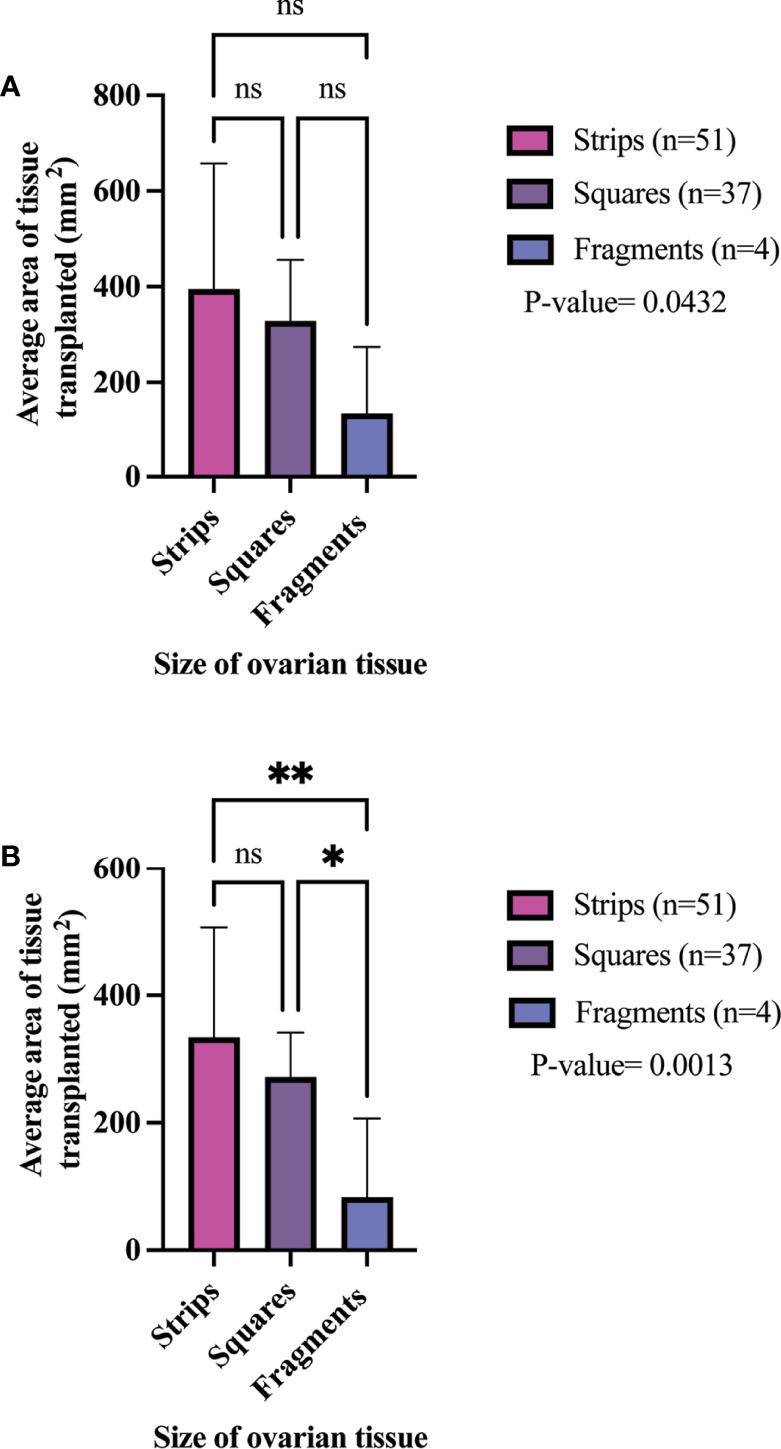
Total average area of tissue transplanted (A) and per 1st OTT (B) in different size cryopreserved and transplanted ovarian tissue. P-values greater than 0.05 were considered not significantly different (ns) P-values greater than 0.05 were considered not significantly different (ns).P-values less than 0.05 (*) and 0.005(**) were considered significantly different. (21, 22, 25, 34, 37, 44, 57, 59, 61, 64, 66, 78, 84, 91–93, 95, 111, 115, 118, 124).
Ovarian Tissue Processing Size and Pregnancy Outcomes
52.2% (48/92) of all participants who were reported to undergo OTT were also monitored for pregnancy attempts using assisted reproductive technologies (ART) or spontaneously (21, 22, 25, 34, 37, 44, 57, 59, 61, 64, 66, 78, 84, 91–93, 95, 111, 115, 118, 124). A total of 16, 11 and 3 participants with strip, square, and fragment ovarian tissue pieces attempted pregnancy ( Table 4 ). The pregnancy rates were 81.3, 45.5, and 66.7% for strip, square, and fragment ovarian tissue pieces (21, 22, 25, 34, 37, 44, 57, 59, 61, 64, 66, 78, 84, 91–93, 95, 111, 115, 118, 124).
Table 4.
Pregnancy and live birth outcome after transplantation per 1st transplant (21, 22, 25, 34, 37, 44, 57, 59, 61, 64, 66, 78, 84, 91–93, 95, 111, 115, 118, 124).
| Size of processed ovarian tissue (No. of OTT Participants) | Participants who attempted to become pregnant n, (%) | Pregnancy rate n, (%) | Number of Pregnancies | Live birth rate n, (%) | Number of Live Births |
|---|---|---|---|---|---|
|
Strips (n = 51) Total ART spontaneous |
16g/51, (31.4) 6 13 |
13h/16, (81.3) 4 11 |
14 4 10i |
9j/16, (56.3) 2 8 |
12k
2 10 |
|
Squares (n = 37) Total ART spontaneous |
11/37, (29.7) 6 5 |
5/11, (45.5) 2 3 |
5 2 3 |
2/11, (18.2) 1 1 |
2 1 1 |
|
Fragments (n = 4) Total ART spontaneous |
3/4, (75) 0 3 |
2/3, (66.7) 0 2 |
2 0 2 |
2/3, (66.7) 0 2 |
2 0 2 |
gThree participants in the strips category used both ART and spontaneous methods to attempt pregnancy.
hTwo participants in the strips category that used both ART and spontaneous methods to attempt pregnancy obtained pregnancy with both methods, one participant in the strips category that used both ART and spontaneous methods to attempt pregnancy obtained pregnancy only with spontaneous methods.
iOne participant in the strips category that used both ART and spontaneous methods to attempt pregnancy obtained pregnancy only with spontaneous methods, had an ongoing pregnancy.
jOne participant in the strips category obtained a live birth from both ART and spontaneous methods.
k Two participants in the strips category had twins.
In the strips group, 6 participants used ART methods, 13 participants used spontaneous methods, and 3 participants used both methods to attempt pregnancy (25, 34, 37, 44, 57, 59, 61, 63, 64). Overall, there were 14 pregnancies in this group; 4 pregnancies resulted from ART, and 10 were obtained spontaneously. Of the three strips participants that used both methods to attempt pregnancy, 2 participants achieved pregnancy with ART and spontaneous methods, and one participant was successful at achieving pregnancy spontaneously (34, 59). In total, 9 strips participants had pregnancies that resulted in a live birth. One participant has a total of two separate live births from both ART and spontaneous pregnancies. The live birth rate for the strips groups was 56.3% and there was a total of 12 live births (2 ART, 10 spontaneous) ( Table 4 ). Two strips participants birthed twins (34, 61). Two participants in the strips groups had miscarriages, one participant had a termination of pregnancy, and one participant had an ongoing pregnancy at the end of the study (59, 63). In total, four participants in the strips group who attempted pregnancy had a second transplant (34, 63). Two participants were unsuccessful in obtaining pregnancy with the first transplant, so to increase chances of pregnancy they underwent a second OTT ( Table 4 ) (34, 63). The first participant achieved three spontaneous pregnancies that resulted in two separate successful live births and one miscarriage (63). The second participant had a successful spontaneous pregnancy and subsequent live birth (34, 63). Of the two other participants in the strips group that had a second OTTs, one participant got pregnant with the first OTT but resulted in a miscarriage, and the other had a termination of pregnancy (63). These two participants had a successful spontaneous pregnancy and live birth with the second transplant (63).
The square group had a pregnancy rate of 45.5%. These participants obtained pregnancy with ART (n=2) or spontaneously (n=3) ( Table 4 ) ( 78, 84, 91–95). In addition, there were a total of five pregnancies in this group; two were achieved using ART, and three were achieved spontaneously. In total, two participants in the squares group had a termination of pregnancy, and one participant had an ongoing pregnancy at the end of the study (78, 92, 95). The live birth rate for the squares group was 18.2%. In this group, 10 participants had a second transplantation and 8 of these participants were not included for pregnancy outcomes due to unclear pregnancy attempts ( Table 4 ) (91, 93). A total of 3 spontaneous pregnancies were obtained from these excluded participants (93). Two participants had a total of two separate pregnancies, where in one participant both pregnancies resulted in termination and in the other participant both pregnancies resulted in live birth (93). The third participant that was excluded had a single spontaneous pregnancy which was terminated (93). Two participants were included that underwent a second OTT ( Table 4 ) (91, 93). One participant attempted pregnancy spontaneously with first transplantation to increase chances of pregnancy, and then requested a second transplant that resulted in a successful live birth (91). The other participant was unsuccessful at obtaining pregnancy with the first and second OTT (91).
In the fragments group, 3 participants attempted pregnancy spontaneously ( Table 4 ) (21, 111, 118). Two of these participants were successful at obtaining pregnancy and live birth (21, 111). In this group, one participant had a total of three separate OTTs (118). This participant attempted pregnancy with the first transplant, however, due to decline ovarian hormone levels the participant had multiple transplants that resulted in a single pregnancy and live birth (118).
OTT Hormone Restoration and Function Longevity
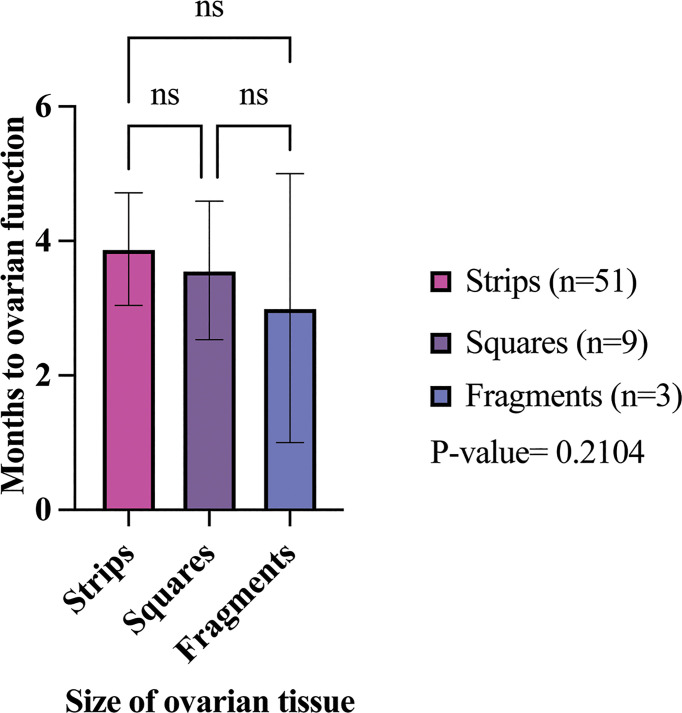
Decreased FSH levels or menstrual cycle resumption and the duration of ovarian hormone production post-transplantation determined ovarian function. Overall, 98% of participants had restoration of ovarian function with first OTT; only one participant in the strips group showed no reestablishment of ovarian activity ( Table 5 ) (34). The mean time from OTT to ovarian hormone restoration was 3.88 months (range: 1.6-7.5 months), 3.56 months (range: 1.8-4.6), and 3 months (range:1-5 months) in the strip, square, and fragment groups per first OTT, respectively ( Table 5 ; Figure 5 ) (21, 22, 25, 34, 37, 44, 57, 59, 61, 64, 66, 78, 84, 91, 92, 95, 111, 115, 124). There was no statistically significant difference of the mean time from OTT to ovarian restoration in all three sizes (P-value= 0.2104) ( Figure 5 ). In all three groups, over 88% of participants had ovarian activity lasting over six months ( Table 5 ). One participant who had OTT with strips and one with squares had continuous function at 2.4- and 2.8-months post-transplantation, respectively (34, 92). The strips group had the highest percentage of participants with hormone production lasting over one-year post-transplantation with their first OTT (86%) ( Table 5 ). In total, two participants from the strips group had ovarian activity lasting over five years with their first OTT (63). The strips, squares, and fragments groups had 84, 55.6, and 100% of participants with ongoing ovarian function with first OTT, respectively. In total, eight (16%) and four (44.4%) participants had cessation of ovarian function with first OTT in the strips and square groups, respectively (44, 63, 64).In total, 10 participants underwent a second OTT(8 strips and 2 squares) ( Table 5 ) ( 34, 63, 78, 91). In the strips group, 8 participants had an end of ovarian function with the first OTT at a mean of 1.29 years, (range 0.75-3.2 years) post transplantation (34, 63). Additionally, one participant in the strips group that had two OTT did not have restoration of ovarian function with second OTT (63). Participants with ovarian restoration from second OTT, six participants had ongoing function and one participant had an ovarian cessation at one year post second OTT (34, 63). In the squares group, 4 participants had the end of ovarain fuction, two participant had a second OTT ( Table 5 ) (84, 91). Of the two participants that did not have a second OTT, ovarian function ceased at 2 and 3.75 years, respectively (mean=2.9 years) (84, 91). Two participants from the squares group underwent a second OTT due to cessation of ovarian function at 1.25 years and 7 months post first OTT (78, 91).However, the second OTT in one participant did not restore ovarian activity (91). The other participant had ongoing ovarian activity lasting over 1.5 years with second OTT (91).
Table 5.
Ovarian function outcome after transplantation.
| Size of processed ovarian tissue (No. of OTT Participants) | Months from OTT HP mean ± SD, range | HP lasting ≥6 months n, (%) | HP lasting ≥1 year n, (%) | HP lasting ≥ 2 years n, (%) | HP ≥ 5 years n, (%) | Participants with ongoing ovarian function n, (%) | Participants with reported cessation of ovarianfunction n, (%) |
|---|---|---|---|---|---|---|---|
|
Strips
1st (n = 51) 2nd (n = 8) |
3.88 ± 0.84, 1.6-7.5 | 49/51, (96) 7/8, (87.5) |
45/51, (88.2) 7/8, (87.5) |
19/51, (37.3) 4/8, (50) |
2/51, (4) 1/8, (12.5) |
42/51, (84) 6/8, (75) |
9q/51, (17.6) 2q/8, (25) |
|
Squares
1st (n = 9) 2nd (n = 2) |
3.56 ± 1.03, 1.8-4.6 |
8/9, (88.9) 1/2, (50) |
4/9, (44.4) 1/2, (50) |
3/9, (33.3) 0/2, (0) |
0/9, (0) 0/2, (0) |
5/9, (55.6) 1/2, (50) |
4/9, (44.4) 1q/2, (50) |
|
Fragments
1st (n = 3) |
3 ± 2, 1-5 |
3/3, (100) | 1/3, (33.3) | 0/3, (0) | 0/3, (0) | 3/3, (100) | 0/3, (0) |
Figure 5.
Months to restoration of ovarian activity in different size transplanted ovarian tissues. The average time to ovarian restoration per first OTT was 3.88, 3.56, and 3 months in the strip, square, and fragment groups, respectively. P-values greater than 0.05 were considered not significantly different (ns) (21, 22, 25, 34, 37, 44, 57, 59, 61, 64, 66, 78, 84, 91–93, 95, 111, 115, 118, 124).
Discussion
There have been several recent systematic reviews of OTC and OTT that have identified the different cryoprotectant protocols OTC, fresh and frozen OTT for hormone restoration, ovarian tissue transport prior to OTC, age at OTC, and vitrification versus slow-freezing methods for OTC (13, 125–127).This is the first systematic review that considered the size of ovarian tissue pieces that were processed for OTC and the outcomes of OTT within those size categories. OTT is a technique emerging in the field of reproductive science and has been performed in over 318 patients (128). The ovarian tissue is processed for OTC by thinning the tissue, removing most of the ovarian medulla and isolating the ovarian cortical tissue. The cortex is then cut into pieces to allow for penetration of the cryoprotectant in preparation for cryopreservation (129). In this systematic review, 58 unique sites reported details on the dimensions used for OTC. This analysis identified that ovarian tissue is cut into multiple different sizes. Ten sites located in the U.S., Belgium, Australia, China, Japan, Israel, Germany, Denmark, and Netherlands processed the ovarian tissue in multiple sizes within the same clinical site. There were no correlations found between age at OTC or diagnosis and the size of processed ovarian tissue. There were no indications of a regional preference for ovarian tissue processing size and, furthermore, seven sites cut ovarian tissue into different dimensions within the same size category. These findings highlight that there is no standard size of cryopreserved ovarian tissue. Studies have shown that fragmentation of the ovarian cortex leads to follicle activation (17). This investigation revealed that 36 sites (62% of sites) process ovarian tissue into strips and is the most predominant cryopreserved size. These findings also demonstrate that most sites around the world process the ovarian tissue to have a thickness of 1-2 mm, which coincides with a thickness which has been shown to reduce ice crystal formation and injury, reduce ischemic time, and increase oxygen diffusion once transplanted (130, 131). Transplantation of ovarian tissue has been reported to restore ovarian activity in 95% of transplantations (12, 128). In the results described here, the ovarian function was restored in 98% of participants that underwent OTT.
Fertility clinics in Denmark and Japan utilize fragmentation of ovarian tissue for POI patients to activate dormant follicles and increase fertilization rates (132, 133). Based on this and other evidence from animal models that suggest that disruption of the microenvironment can initiate follicle activation, it was hypothesized that ovarian tissue used in OTT that had been processed into smaller pieces would result in fewer pregnancies and shorter duration of ovarian function, and a shorter time to initiate ovarian function after OTT (16). After transplantation there is up to an 80% loss in ovarian reserve due to factors such as ischemic time (14, 15). The graft size can influence ischemic time, such that smaller graft sizes can have shorter ischemic time than larger graft sizes, leading to earlier ovarian function (15, 134, 135). The resumption of ovarian activity on average did decrease with a smaller processing size (strips 3.88 months, squares 3.56 months, and fragments 3 months). Additionally, the squares group had the highest rate (44.4%) of participants that had a reported cessation of ovarian activity with the first OTT, followed by the strips group (17.6%). Even though more tissue on average was transplanted in the strips than squares group, the strips group had a shorter ovarian tissue lifespan (1.29 years) for the first OTT compared to squares group (2.9 years). While some of these results followed the expected trend, the longevity of ovarian tissue function in the strips group is lower than the reported literature range of 2-5 years due to the lack of follow-ups on participants with OTT in the reviewed reports (128). If further participant follow-up was performed for each study through to cessation of ovarian function, then the longevity of ovarian tissue function would be better understood. While the analysis accounted for the location of the transplant by excluding heterotopic sites, and the reported results only included tissue that was cryopreserved using a slow freezing and not vitrification technique, there are other confounding factors that may contribute to ovarian tissue function after OTT. We also note that there were studies with exciting findings that did not meet our inclusion criteria. For instance, Oktay et al. have implemented the use of extracellular matrix scaffolds, which show promise in extending graft longevity (136).
OTC followed by OTT has resulted in over 140 published live births worldwide and it has been reported to lead to multiple live births from the same OTT procedure (60, 128). This systematic review of reports that contained information on ovarian tissue processing size and OTT outcomes identified an overall pregnancy rate of 81.3%, 45.5%, 66.7% of participants in strips, squares, and fragments groups, respectively. In the strips and fragments groups, the pregnancy rates were higher than the 50% pregnancy rate from OTT reported in the literature (12). This vast difference in pregnancy rate is probably attributed to the fact that authors have not published or described the additional information required for this analysis. The live birth rate was higher in the strips groups (56.3%) compared to squares (18.2%). However, in these two groups, participants (2 squares, 1 strips) had a termination of pregnancy which could have impacted the live birth rate (63, 92, 93, 95). The fragments group had the highest birth rate (66.7%) in all three groups. The small sample size of participants in the fragments group (n=4) compared to the strips (n=51) and squares (n=37) was a contributing factor to these high rates. The participant data utilized for this systematic review was limited by the number of published case studies that contain participants who underwent autologous orthotopic OTT, follow-up time points in studies that discussed OTT outcomes, and contained data on the ovarian tissue sizes. This systematic review relies heavily on the information of case studies, therefore, can introduce an internal validity threat to our reported results. An additional caveat to this study is that authors publishing on OTT might not be reporting unsuccessful OTT cases and have not reported detailed follow-ups of past reported case studies. Evidence is needed to identify and understand factors that could contribute to follicle burnout within the first few months post-OTT, such as impact of tissue fragmentation on preventing ischemia-reperfusion injury and initiating angiogenesis, to develop a unified best practice for ovarian tissue processing (20).
Conclusion
This systematic review determined the processing sizes of cryopreserved ovarian tissue in sites across the world and examined ovarian function restoration in different sized ovarian tissue pieces in OTT participants. It is shown in this review that there is no standard for processing ovarian tissue and documentation of ovarian restoration outcomes in participants. Although the time of resumption of ovarian activity was not statistically significant between the different sizes, this could have been due to the small population size of the fragments and squares group. The participant population size used in this systematic review could have been more prominent by changing the rigorous inclusion/exclusion criteria to include studies that did not contain enough information about the participants. However, changing the inclusion/exclusion criteria of this systematic review to include studies that lacked important participant information would increase bias within the review. This systematic review has shown the importance of documenting information on the participant before OTC, explicit ovarian tissue processing and transplantation methods, and updating participants’ ovarian outcomes post-transplantation via follow-ups. This call to action of proper documentation and consistent OTT participant follow-ups will allow scientists and clinicians to drive research questions with the goals of improving OTC/OTT and maximizing fertility outcomes.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author Contributions
AD and ML contributed to the study concept, design, and data interpretation. AD contributed to data analysis. HK contributed to the study quality assessment and unbiased reviewer. AD, HK, and ML contributed to manuscript preparation. NH, MH, and AD contributed to data collection. All authors contributed to the article and approved the submitted version.
Funding
This works was supported in part by the Children’s Research Fund, the Ann & Robert H. Lurie Children’s Hospital Faculty Practice Plan award (ML), the Warren and Eloise Batts endowment (ML).
Conflict of Interest
ML is an Advisor for Dimension Inx, LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Drs. Francesca Duncan and Erin Rowell for guidance in study design and impact.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.918899/full#supplementary-material
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: A Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2. Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer Statistics for Adolescents and Young Adults, 2020. CA: A Cancer J Clin (2020) 70(6):443–59. doi: 10.3322/caac.21637 [DOI] [PubMed] [Google Scholar]
- 3. Shrestha A, Martin C, Burton M, Walters S, Collins K, Wyld L. Quality of Life Versus Length of Life Considerations in Cancer Patients: A Systematic Literature Review. Psycho-Oncol (2019) 28(7):1367–80. doi: 10.1002/pon.5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bedoschi G, Navarro PA, Oktay K. Chemotherapy-Induced Damage to Ovary: Mechanisms and Clinical Impact. Future Oncol (2016) 12(20):2333–44. doi: 10.2217/fon-2016-0176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chahal H, Drake W. The Endocrine System and Ageing. J Pathol (2007) 211(2):173–80. doi: 10.1002/path.2110 [DOI] [PubMed] [Google Scholar]
- 6. Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology Recommendations on Fertility Preservation in Cancer Patients. J Clin Oncol (2006) 24(18):2917–31. doi: 10.1200/JCO.2006.06.5888 [DOI] [PubMed] [Google Scholar]
- 7. Kim S, Lee Y, Lee S, Kim T. Ovarian Tissue Cryopreservation and Transplantation in Patients With Cancer. Obstetrics Gynecol Sci (2018) 61(4):431. doi: 10.5468/ogs.2018.61.4.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wallace WHB, Anderson RA, Irvine DS. Fertility Preservation for Young Patients With Cancer: Who is at Risk and What can be Offered? Lancet Oncol (2005) 6(4):209–18. doi: 10.1016/S1470-2045(05)70092-9 [DOI] [PubMed] [Google Scholar]
- 9. Marin L, Bedoschi G, Kawahara T, Oktay KH. History, Evolution and Current State of Ovarian Tissue Auto-Transplantation With Cryopreserved Tissue: A Successful Translational Research Journey From 1999 to 2020. Reprod Sci (2020) 27(4):955–62. doi: 10.1007/s43032-019-00066-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy After Transplantation of Cryopreserved Ovarian Tissue in a Patient With Ovarian Failure After Chemotherapy. N Engl J Med (2005) 353(3):318–21. doi: 10.1056/NEJMc055237 [DOI] [PubMed] [Google Scholar]
- 11. Donnez J, Dolmans MM. Fertility Preservation in Women. N Engl J Med (2017) 377(17):1657–65. doi: 10.1056/NEJMra1614676 [DOI] [PubMed] [Google Scholar]
- 12. Shapira M, Dolmans MM, Silber S, Meirow D. Evaluation of Ovarian Tissue Transplantation: Results From Three Clinical Centers. Fertil Steril (2020) 114(2):388–97. doi: 10.1016/j.fertnstert.2020.03.037 [DOI] [PubMed] [Google Scholar]
- 13. Corkum KS, Rhee DS, Wafford QE, Demeestere I, Dasgupta R, Baertschiger R, et al. Fertility and Hormone Preservation and Restoration for Female Children and Adolescents Receiving Gonadotoxic Cancer Treatments: A Systematic Review. J Pediatr Surg (2019) 54(11):2200–9. doi: 10.1016/j.jpedsurg.2018.12.021 [DOI] [PubMed] [Google Scholar]
- 14. Gavish Z, Spector I, Peer G, Schlatt S, Wistuba J, Roness H, et al. Follicle Activation is a Significant and Immediate Cause of Follicle Loss After Ovarian Tissue Transplantation. J Assisted Reprod Genet (2018) 35(1):61–9. doi: 10.1007/s10815-017-1079-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roness H, Meirow D. FERTILITY PRESERVATION: Follicle Reserve Loss in Ovarian Tissue Transplantation. Reproduction (2019) 158(5):F35–44. doi: 10.1530/REP-19-0097 [DOI] [PubMed] [Google Scholar]
- 16. Nagamatsu G, Shimamoto S, Hamazaki N, Nishimura Y, Hayashi K. Mechanical Stress Accompanied With Nuclear Rotation is Involved in the Dormant State of Mouse Oocytes. Sci Advances (2019) 5(6):eaav9960. doi: 10.1126/sciadv.aav9960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo Signaling Disruption and Akt Stimulation of Ovarian Follicles for Infertility Treatment. Proc Natl Acad Sci (2013) 110(43):17474–9. doi: 10.1073/pnas.1312830110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fertility Preservation in Patients Undergoing Gonadotoxic Therapy or Gonadectomy: A Committee Opinion. Fertility Sterility (2019) 112(6):1022–33. doi: 10.1016/j.fertnstert.2019.09.013 [DOI] [PubMed] [Google Scholar]
- 19. Meirow D, Ra’anani H, Shapira M, Brenghausen M, Derech Chaim S, Aviel-Ronen S, et al. Transplantations of Frozen-Thawed Ovarian Tissue Demonstrate High Reproductive Performance and the Need to Revise Restrictive Criteria. Fertil Steril (2016) 106(2):467–74. doi: 10.1016/j.fertnstert.2016.04.031 [DOI] [PubMed] [Google Scholar]
- 20. Silber SJ, DeRosa M, Goldsmith S, Fan Y, Castleman L, Melnick J. Cryopreservation and Transplantation of Ovarian Tissue: Results From One Center in the USA. J Assist Reprod Genet (2018) 35(12):2205–13. doi: 10.1007/s10815-018-1315-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth After Orthotopic Transplantation of Cryopreserved Ovarian Tissue. Lancet (2004) 364(9443):1405–10. doi: 10.1016/S0140-6736(04)17222-X [DOI] [PubMed] [Google Scholar]
- 22. Donnez J, Squifflet J, Van Eyck AS, Demylle D, Jadoul P, Van Langendonckt A, et al. Restoration of Ovarian Function in Orthotopically Transplanted Cryopreserved Ovarian Tissue: A Pilot Experience. Reprod BioMed Online (2008) 16(5):694–704. doi: 10.1016/S1472-6483(10)60484-1 [DOI] [PubMed] [Google Scholar]
- 23. Mayerhofer K, Ott J, Nouri K, Stoegbauer L, Fischer EM, Lipovac M, et al. Laparoscopic Ovarian Tissue Harvesting for Cryopreservation: An Effective and Safe Procedure for Fertility Preservation. Eur J Obstet Gynecol Reprod Biol (2010) 152(1):68–72. doi: 10.1016/j.ejogrb.2010.05.034 [DOI] [PubMed] [Google Scholar]
- 24. Ott J, Nouri K, Stögbauer L, Fischer EM, Lipovac M, Promberger R, et al. Ovarian Tissue Cryopreservation for non-Malignant Indications. Arch Gynecol Obstet. (2010) 281(4):735–9. doi: 10.1007/s00404-009-1224-8 [DOI] [PubMed] [Google Scholar]
- 25. Ruan X, Du J, Korell M, Kong W, Lu D, Jin F, et al. Case Report of the First Successful Cryopreserved Ovarian Tissue Retransplantation in China. Climacteric (2018) 21(6):613–6. doi: 10.1080/13697137.2018.1514005 [DOI] [PubMed] [Google Scholar]
- 26. González C, Devesa M, Boada M, Coroleu B, Veiga A, Barri PN. Combined Strategy for Fertility Preservation in an Oncologic Patient: Vitrification of In Vitro Matured Oocytes and Ovarian Tissue Freezing. J Assist Reprod Genet (2011) 28(12):1147–9. doi: 10.1007/s10815-011-9628-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Virant-Klun I, Vogler A. In Vitro Maturation of Oocytes From Excised Ovarian Tissue in a Patient With Autoimmune Ovarian Insufficiency Possibly Associated With Epstein-Barr Virus Infection. Reprod Biol Endocrinol (2018) 16(1):33. doi: 10.1186/s12958-018-0350-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Philippart C, Masciangelo R, Camboni A, Donnez J, Dolmans MM. Basal Lamina Characterization in Frozen-Thawed and Long-Term Grafted Human Prepubertal Ovarian Tissue. Reprod BioMed Online (2021) 42(5):859–69. doi: 10.1016/j.rbmo.2021.02.012 [DOI] [PubMed] [Google Scholar]
- 29. Dolmans MM, Jadoul P, Gilliaux S, Amorim CA, Luyckx V, Squifflet J, et al. A Review of 15 Years of Ovarian Tissue Bank Activities. J Assist Reprod Genet (2013) 30(3):305–14. doi: 10.1007/s10815-013-9952-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abir R, Ben-Aharon I, Garor R, Yaniv I, Ash S, Stemmer SM, et al. Cryopreservation of In Vitro Matured Oocytes in Addition to Ovarian Tissue Freezing for Fertility Preservation in Paediatric Female Cancer Patients Before and After Cancer Therapy. Hum Reprod (2016) 31(4):750–62. doi: 10.1093/humrep/dew007 [DOI] [PubMed] [Google Scholar]
- 31. Abtahi NS, Ebrahimi B, Fathi R, Khodaverdi S, Mehdizadeh Kashi A, Valojerdi MR. An Introduction to The Royan Human Ovarian Tissue Bank. Int J Fertil Steril (2016) 10(2):261–3. doi: 10.22074/ijfs.2016.4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jenninga E, Louwe LA, Peters AA, Nortier JW, Hilders CG. Timing of Fertility Preservation Procedures in a Cohort of Female Patients With Cancer. Eur J Obstet Gynecol Reprod Biol (2012) 160(2):170–3. doi: 10.1016/j.ejogrb.2011.11.011 [DOI] [PubMed] [Google Scholar]
- 33. Hoekman EJ, Smit VT, Fleming TP, Louwe LA, Fleuren GJ, Hilders CG. Searching for Metastases in Ovarian Tissue Before Autotransplantation: A Tailor-Made Approach. Fertil Steril (2015) 103(2):469–77. doi: 10.1016/j.fertnstert.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 34. Hoekman EJ, Louwe LA, Rooijers M, Westerlaken LAJ, Klijn NF, Pilgram GSK, et al. Ovarian Tissue Cryopreservation: Low Usage Rates and High Live-Birth Rate After Transplantation. Acta Obstetricia Gynecologica Scandinavica (2020) 99(2):213–21. doi: 10.1111/aogs.13735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jenninga E, Hilders CG, Louwe LA, Peters AA. Female Fertility Preservation: Practical and Ethical Considerations of an Underused Procedure. Cancer J (2008) 14(5):333–9. doi: 10.1097/PPO.0b013e31818860ac [DOI] [PubMed] [Google Scholar]
- 36. Bastings L, Liebenthron J, Westphal JR, Beerendonk CC, van der Ven H, Meinecke B, et al. Efficacy of Ovarian Tissue Cryopreservation in a Major European Center. J Assist Reprod Genet (2014) 31(8):1003–12. doi: 10.1007/s10815-014-0239-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sonmezer M, Ozkavukcu S, Sukur YE, Kankaya D, Arslan O. First Pregnancy and Live Birth in Turkey Following Frozen-Thawed Ovarian Tissue Transplantation in a Patient With Acute Lymphoblastic Leukemia Who Underwent Cord Blood Transplantation. J Assist Reprod Genet (2020) 37(8):2033–43. doi: 10.1007/s10815-020-01850-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Radford JA, Lieberman BA, Brison DR, Smith AR, Critchlow JD, Russell SA, et al. Orthotopic Reimplantation of Cryopreserved Ovarian Cortical Strips After High-Dose Chemotherapy for Hodgkin’s Lymphoma. Lancet (2001) 357(9263):1172–5. doi: 10.1016/S0140-6736(00)04335-X [DOI] [PubMed] [Google Scholar]
- 39. Gracia CR, Chang J, Kondapalli L, Prewitt M, Carlson CA, Mattei P, et al. Ovarian Tissue Cryopreservation for Fertility Preservation in Cancer Patients: Successful Establishment and Feasibility of a Multidisciplinary Collaboration. J Assist Reprod Genet (2012) 29(6):495–502. doi: 10.1007/s10815-012-9753-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hanfling SN, Parikh T, Mayhew A, Robinson E, Graham J, Gomez-Lobo V, et al. Case Report: Two Cases of Mature Oocytes Found in Prepubertal Girls During Ovarian Tissue Cryopreservation. F S Rep (2021) 2(3):296–9. doi: 10.1016/j.xfre.2021.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sermondade N, Sonigo C, Sifer C, Valtat S, Ziol M, Eustache F, et al. Serum Antimüllerian Hormone Is Associated With the Number of Oocytes Matured In Vitro and With Primordial Follicle Density in Candidates for Fertility Preservation. Fertil Steril (2019) 111(2):357–62. doi: 10.1016/j.fertnstert.2018.10.018 [DOI] [PubMed] [Google Scholar]
- 42. Shai D, Aviel-Ronen S, Spector I, Raanani H, Shapira M, Gat I, et al. Ovaries of Patients Recently Treated With Alkylating Agent Chemotherapy Indicate the Presence of Acute Follicle Activation, Elucidating its Role Among Other Proposed Mechanisms of Follicle Loss. Fertil Steril (2021) 115(5):1239–49. doi: 10.1016/j.fertnstert.2020.11.040 [DOI] [PubMed] [Google Scholar]
- 43. Azem F, Hasson J, Cohen T, Shwartz T, Mey-Raz N, Almog B, et al. Retrieval of Immature Oocytes After Chemotherapy for Hodgkin’s Disease and Prolonged Ovarian Down-Regulation With Gonadotropin-Releasing Hormone Agonist. Fertil Steril (2009) 92(2):828.e1–2. doi: 10.1016/j.fertnstert.2009.04.052 [DOI] [PubMed] [Google Scholar]
- 44. Fabbri R, Pasquinelli G, Magnani V, Macciocca M, Vicenti R, Parazza I, et al. Autotransplantation of Cryopreserved Ovarian Tissue in Oncological Patients: Recovery of Ovarian Function. Future Oncol (2014) 10(4):549–61. doi: 10.2217/fon.13.234 [DOI] [PubMed] [Google Scholar]
- 45. Meirow D, Baum M, Yaron R, Levron J, Hardan I, Schiff E, et al. Ovarian Tissue Cryopreservation in Hematologic Malignancy: Ten Years’ Experience. Leuk Lymphoma (2007) 48(8):1569–76. doi: 10.1080/10428190701471957 [DOI] [PubMed] [Google Scholar]
- 46. Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra’anani H, et al. Searching for Evidence of Disease and Malignant Cell Contamination in Ovarian Tissue Stored From Hematologic Cancer Patients. Hum Reprod (2008) 23(5):1007–13. doi: 10.1093/humrep/den055 [DOI] [PubMed] [Google Scholar]
- 47. Hickman LC, Uy-Kroh MJ, Chiesa-Vottero A, Desai N, Flyckt R. Ovarian Tissue Cryopreservation for Benign Gynecologic Disease: A Case of Ovarian Torsion and Review of the Literature. J Minim Invasive Gynecol (2016) 23(3):446–9. doi: 10.1016/j.jmig.2015.12.011 [DOI] [PubMed] [Google Scholar]
- 48. Oktay K, Oktem O. Ovarian Cryopreservation and Transplantation for Fertility Preservation for Medical Indications: Report of an Ongoing Experience. Fertil Steril (2010) 93(3):762–8. doi: 10.1016/j.fertnstert.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 49. Schermerhorn SMV, Rosen MP, Blevins EM, Byrd KA, Rabban JT, Marsh P, et al. Regional Air Transportation of Ovarian Tissue for Cryopreservation in a Prepubertal Female With Cancer. Pediatr Blood Cancer (2021) 68(9):e29107. doi: 10.1002/pbc.29107 [DOI] [PubMed] [Google Scholar]
- 50. Dolmans MM, Donnez J, Camboni A, Demylle D, Amorim C, Van Langendonckt A, et al. IVF Outcome in Patients With Orthotopically Transplanted Ovarian Tissue. Hum Reprod (2009) 24(11):2778–87. doi: 10.1093/humrep/dep289 [DOI] [PubMed] [Google Scholar]
- 51. Fabbri R, Vicenti R, Macciocca M, Pasquinelli G, Lima M, Parazza I, et al. Cryopreservation of Ovarian Tissue in Pediatric Patients. Obstet Gynecol Int (2012) 2012:910698. doi: 10.1155/2012/910698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Janse F, Donnez J, Anckaert E, De Jong FH, Fauser BCJM, Dolmans M-M. Limited Value of Ovarian Function Markers Following Orthotopic Transplantation of Ovarian Tissue After Gonadotoxic Treatment. (2011) 96(4):1136–44. doi: 10.1210/jc.2010-2188 [DOI] [PubMed] [Google Scholar]
- 53. Policiano C, Subirá J, Aguilar A, Monzó S, Iniesta I, Rubio Rubio JM. Impact of ABVD Chemotherapy on Ovarian Reserve After Fertility Preservation in Reproductive-Aged Women With Hodgkin Lymphoma. J Assist Reprod Genet (2020) 37(7):1755–61. doi: 10.1007/s10815-020-01844-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Delattre S, Segers I, Van Moer E, Drakopoulos P, Mateizel I, Enghels L, et al. Combining Fertility Preservation Procedures to Spread the Eggs Across Different Baskets: A Feasibility Study. Hum Reprod (2020) 35(11):2524–36. doi: 10.1093/humrep/deaa193 [DOI] [PubMed] [Google Scholar]
- 55. Peek R, Schleedoorn M, Smeets D, van de Zande G, Groenman F, Braat D, et al. Ovarian Follicles of Young Patients With Turner’s Syndrome Contain Normal Oocytes But Monosomic 45,X Granulosa Cells. Hum Reprod (2019) 34(9):1686–96. doi: 10.1093/humrep/dez135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li Y, Ruan X, Liebenthron J, Montag M, Zhou Q, Kong W, et al. Ovarian Tissue Cryopreservation for Patients With Premature Ovary Insufficiency Caused by Cancer Treatment: Optimal Protocol. Climacteric (2019) 22(4):383–9. doi: 10.1080/13697137.2018.1554644 [DOI] [PubMed] [Google Scholar]
- 57. Ruan X, Cheng J, Korell M, Du J, Kong W, Lu D, et al. Ovarian Tissue Cryopreservation and Transplantation Prevents Iatrogenic Premature Ovarian Insufficiency: First 10 Cases in China. Climacteric (2020) 23(6):574–80. doi: 10.1080/13697137.2020.1767569 [DOI] [PubMed] [Google Scholar]
- 58. Du J, Ruan X, Jin F, Li Y, Cheng J, Gu M, et al. Abnormalities of Early Folliculogenesis and Serum Anti-Müllerian Hormone in Chinese Patients With Polycystic Ovary Syndrome. J Ovarian Res (2021) 14(1):36. doi: 10.1186/s13048-021-00786-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ruan X, Du J, Lu D, Duan W, Jin F, Kong W, et al. First Pregnancy in China After Ovarian Tissue Transplantation to Prevent Premature Ovarian Insufficiency. Climacteric (2021) 24(6):624–8. doi: 10.1080/13697137.2021.1956453 [DOI] [PubMed] [Google Scholar]
- 60. Liebenthron J, Montag M, Reinsberg J, Köster M, Isachenko V, van der Ven K, et al. Overnight Ovarian Tissue Transportation for Centralized Cryobanking: A Feasible Option. Reprod BioMed Online (2019) 38(5):740–9. doi: 10.1016/j.rbmo.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 61. Chehab G, Krüssel J, Fehm T, Fischer-Betz R, Schneider M, Germeyer A, et al. Successful Conception in a 34-Year-Old Lupus Patient Following Spontaneous Pregnancy After Autotransplantation of Cryopreserved Ovarian Tissue. Lupus (2019) 28(5):675–80. doi: 10.1177/0961203319839482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Van der Ven H, Liebenthron J, Beckmann M, Toth B, Korell M, Krüssel J, et al. Ninety-Five Orthotopic Transplantations in 74 Women of Ovarian Tissue After Cytotoxic Treatment in a Fertility Preservation Network: Tissue Activity, Pregnancy and Delivery Rates. Hum Reprod (2016) 31(9):2031–41. doi: 10.1093/humrep/dew165 [DOI] [PubMed] [Google Scholar]
- 63. Poirot C, Fortin A, Lacorte JM, Akakpo JP, Genestie C, Vernant JP, et al. Impact of Cancer Chemotherapy Before Ovarian Cortex Cryopreservation on Ovarian Tissue Transplantation. Hum Reprod (2019) 34(6):1083–94. doi: 10.1093/humrep/dez047 [DOI] [PubMed] [Google Scholar]
- 64. Ernst E, Kjærsgaard M, Birkebæk NH, Clausen N, Andersen CY. Case Report: Stimulation of Puberty in a Girl With Chemo- and Radiation Therapy Induced Ovarian Failure by Transplantation of a Small Part of Her Frozen/Thawed Ovarian Tissue. Eur J Cancer (2013) 49(4):911–4. doi: 10.1016/j.ejca.2012.09.028 [DOI] [PubMed] [Google Scholar]
- 65. Takae S, Furuta S, Keino D, Shiraishi E, Iwahata Y, Oyama K, et al. Surgical Management of Unilateral Oophorectomy for Ovarian Tissue Cryopreservation in High-Risk Children and Adolescents With Varied Backgrounds. Pediatr Surg Int (2021) 37(8):1021–9. doi: 10.1007/s00383-021-04900-7 [DOI] [PubMed] [Google Scholar]
- 66. Poirot C, Brugieres L, Yakouben K, Prades-Borio M, Marzouk F, de Lambert G, et al. Ovarian Tissue Cryopreservation for Fertility Preservation in 418 Girls and Adolescents Up to 15 Years of Age Facing Highly Gonadotoxic Treatment. Twenty Years of Experience at a Single Center. Acta Obstet Gynecol Scand (2019) 98(5):630–7. doi: 10.1111/aogs.13616 [DOI] [PubMed] [Google Scholar]
- 67. Mohsenzadeh M, Khalili MA, Tabibnejad N, Yari N, Agha-Rahimi A, Karimi-Zarchi M. Embryo Cryopreservation Following In-Vitro Maturation for Fertility Preservation in a Woman With Mullerian Adenosarcoma: A Case Report. J Hum Reprod Sci (2017) 10:138–41. doi: 10.4103/jhrs.JHRS_93_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gook D, Hale L, Polyakov A, Manley T, Rozen G, Stern K. Experience With Transplantation of Human Cryopreserved Ovarian Tissue to a Sub-Peritoneal Abdominal Site. Hum Reprod (2021) 36(9):2473–83. doi: 10.1093/humrep/deab167 [DOI] [PubMed] [Google Scholar]
- 69. Karavani G, Schachter-Safrai N, Chill HH, Mordechai Daniel T, Bauman D, Revel A. Single-Incision Laparoscopic Surgery for Ovarian Tissue Cryopreservation. J Minim Invasive Gynecol (2018) 25(3):474–9. doi: 10.1016/j.jmig.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 70. Karavani G, Schachter-Safrai N, Revel A, Mordechai-Daniel T, Bauman D, Imbar T. In Vitro Maturation Rates in Young Premenarche Patients. Fertil Steril (2019) 112(2):315–22. doi: 10.1016/j.fertnstert.2019.03.026 [DOI] [PubMed] [Google Scholar]
- 71. Greve T, Clasen-Linde E, Andersen MT, Andersen MK, Sørensen SD, Rosendahl M, et al. Cryopreserved Ovarian Cortex From Patients With Leukemia in Complete Remission Contains No Apparent Viable Malignant Cells. Blood (2012) 120(22):4311–6. doi: 10.1182/blood-2012-01-403022 [DOI] [PubMed] [Google Scholar]
- 72. Kikuchi I, Kagawa N, Silber S, Kuwayama M, Takehara Y, Aono F, et al. Oophorectomy for Fertility Preservation via Reduced-Port Laparoscopic Surgery. Surg Innov (2013) 20(3):219–24. doi: 10.1177/1553350612449074 [DOI] [PubMed] [Google Scholar]
- 73. Takae S, Sugishita Y, Yoshioka N, Hoshina M, Horage Y, Sato Y, et al. The Role of Menstrual Cycle Phase and AMH Levels in Breast Cancer Patients Whose Ovarian Tissue Was Cryopreserved for Oncofertility Treatment. J Assist Reprod Genet (2015) 32(2):305–12. doi: 10.1007/s10815-014-0392-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kawamura K, Ishizuka B, Hsueh AJW. Drug-Free in-Vitro Activation of Follicles for Infertility Treatment in Poor Ovarian Response Patients With Decreased Ovarian Reserve. Reprod BioMed Online (2020) 40(2):245–53. doi: 10.1016/j.rbmo.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 75. Meng L, Kawamura K, Yoshioka N, Tamura M, Furuyama S, Nakajima M, et al. Learning Curve of Surgeons Performing Laparoscopic Ovarian Tissue Transplantation in Women With Premature Ovarian Insufficiency: A Statistical Process Control Analysis. J Minim Invasive Gynecol (2022) 29(4):559–66. doi: 10.1016/j.jmig.2021.12.014 [DOI] [PubMed] [Google Scholar]
- 76. Rosendahl M, Loft A, Byskov AG, Ziebe S, Schmidt KT, Andersen AN, et al. Biochemical Pregnancy After Fertilization of an Oocyte Aspirated From a Heterotopic Autotransplant of Cryopreserved Ovarian Tissue: Case Report. Hum Reprod (2006) 21(8):2006–9. doi: 10.1093/humrep/del140 [DOI] [PubMed] [Google Scholar]
- 77. Mamsen LS, Kelsey TW, Ernst E, Macklon KT, Lund AM, Andersen CY. Cryopreservation of Ovarian Tissue May Be Considered in Young Girls With Galactosemia. J Assist Reprod Genet (2018) 35(7):1209–17. doi: 10.1007/s10815-018-1209-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Burmeister L, Kovacs GT, Osianlis T. First Australian Pregnancy After Ovarian Tissue Cryopreservation and Subsequent Autotransplantation. Med J Aust (2013) 198(3):158–9. doi: 10.5694/mja12.11768 [DOI] [PubMed] [Google Scholar]
- 79. Rozen G, Avagliano S, Agresta F, Gook D, Polyakov A, Stern C. Ovarian Tissue Grafting: Lessons Learnt From Our Experience With 55 Grafts. Reprod Med Biol (2021) 20(3):277–88. doi: 10.1002/rmb2.12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. De Roo C, Lierman S, Tilleman K, De Sutter P. In-Vitro Fragmentation of Ovarian Tissue Activates Primordial Follicles Through the Hippo Pathway. Hum Reprod Open (2020) 2020(4):hoaa048. doi: 10.1093/hropen/hoaa048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. El Issaoui M, Giorgione V, Mamsen LS, Rechnitzer C, Birkebæk N, Clausen N, et al. Effect of First Line Cancer Treatment on the Ovarian Reserve and Follicular Density in Girls Under the Age of 18 Years. Fertil Steril (2016) 106(7):1757–62.e1. doi: 10.1016/j.fertnstert.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 82. Greve T, Schmidt KT, Kristensen SG, Ernst E, Andersen CY. Evaluation of the Ovarian Reserve in Women Transplanted With Frozen and Thawed Ovarian Cortical Tissue. Fertil Steril (2012) 97(6):1394–8.e1. doi: 10.1016/j.fertnstert.2012.02.036 [DOI] [PubMed] [Google Scholar]
- 83. Schmidt KL, Andersen CY, Loft A, Byskov AG, Ernst E, Andersen AN. Follow-Up of Ovarian Function Post-Chemotherapy Following Ovarian Cryopreservation and Transplantation. Hum Reprod (2005) 20(12):3539–46. doi: 10.1093/humrep/dei250 [DOI] [PubMed] [Google Scholar]
- 84. Revelli A, Marchino G, Dolfin E, Molinari E, Delle Piane L, Salvagno F, et al. Live Birth After Orthotopic Grafting of Autologous Cryopreserved Ovarian Tissue and Spontaneous Conception in Italy. Fertil Steril (2013) 99(1):227–30. doi: 10.1016/j.fertnstert.2012.09.029 [DOI] [PubMed] [Google Scholar]
- 85. Lee JR, Lee D, Park S, Paik EC, Kim SK, Jee BC, et al. Successful in Vitro Fertilization and Embryo Transfer After Transplantation of Cryopreserved Ovarian Tissue: Report of the First Korean Case. J Korean Med Sci (2018) 33(21):e156. doi: 10.3346/jkms.2018.33.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tanbo T, Greggains G, Storeng R, Busund B, Langebrekke A, Fedorcsak P. Autotransplantation of Cryopreserved Ovarian Tissue After Treatment for Malignant Disease - The First Norwegian Results. Acta Obstet Gynecol Scand (2015) 94(9):937–41. doi: 10.1111/aogs.12700 [DOI] [PubMed] [Google Scholar]
- 87. Bystrova O, Lapina E, Kalugina A, Lisyanskaya A, Tapilskaya N, Manikhas G. Heterotopic Transplantation of Cryopreserved Ovarian Tissue in Cancer Patients: A Case Series. Gynecol Endocrinol (2019) 35(12):1043–9. doi: 10.1080/09513590.2019.1648413 [DOI] [PubMed] [Google Scholar]
- 88. Kim SS. Assessment of Long Term Endocrine Function After Transplantation of Frozen-Thawed Human Ovarian Tissue to the Heterotopic Site: 10 Year Longitudinal Follow-Up Study. J Assist Reprod Genet (2012) 29(6):489–93. doi: 10.1007/s10815-012-9757-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Imbert R, Moffa F, Tsepelidis S, Simon P, Delbaere A, Devreker F, et al. Safety and Usefulness of Cryopreservation of Ovarian Tissue to Preserve Fertility: A 12-Year Retrospective Analysis. Hum Reprod (2014) 29(9):1931–40. doi: 10.1093/humrep/deu158 [DOI] [PubMed] [Google Scholar]
- 90. Fasano G, Dechène J, Antonacci R, Biramane J, Vannin AS, Van Langendonckt A, et al. Outcomes of Immature Oocytes Collected From Ovarian Tissue for Cryopreservation in Adult and Prepubertal Patients. Reprod BioMed Online (2017) 34(6):575–82. doi: 10.1016/j.rbmo.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 91. Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two Successful Pregnancies Following Autotransplantation of Frozen/Thawed Ovarian Tissue. Hum Reprod (2008) 23(10):2266–72. doi: 10.1093/humrep/den244 [DOI] [PubMed] [Google Scholar]
- 92. Greve T, Ernst E, Markholt S, Schmidt KT, Andersen CY. Legal Termination of a Pregnancy Resulting From Transplanted Cryopreserved Ovarian Tissue. Acta Obstet Gynecol Scand (2010) 89(12):1589–91. doi: 10.3109/00016349.2010.512074 [DOI] [PubMed] [Google Scholar]
- 93. Dueholm Hjorth IM, Kristensen SG, Dueholm M, Humaidan P. Reproductive Outcomes After In Vitro Fertilization Treatment in a Cohort of Danish Women Transplanted With Cryopreserved Ovarian Tissue. Fertil Steril (2020) 114(2):379–87. doi: 10.1016/j.fertnstert.2020.03.035 [DOI] [PubMed] [Google Scholar]
- 94. Tryde Schmidt KL, Yding Andersen C, Starup J, Loft A, Byskov AG, Nyboe Andersen A. Orthotopic Autotransplantation of Cryopreserved Ovarian Tissue to a Woman Cured of Cancer - Follicular Growth, Steroid Production and Oocyte Retrieval. Reprod BioMed Online (2004) 8(4):448–53. doi: 10.1016/S1472-6483(10)60929-7 [DOI] [PubMed] [Google Scholar]
- 95. Ernst EH, Offersen BV, Andersen CY, Ernst E. Legal Termination of a Pregnancy Resulting From Transplanted Cryopreserved Ovarian Tissue Due to Cancer Recurrence. J Assist Reprod Genet (2013) 30(7):975–8. doi: 10.1007/s10815-013-0026-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kim SS, Hwang IT, Lee HC. Heterotopic Autotransplantation of Cryobanked Human Ovarian Tissue as a Strategy to Restore Ovarian Function. Fertil Steril (2004) 82(4):930–2. doi: 10.1016/j.fertnstert.2004.02.137 [DOI] [PubMed] [Google Scholar]
- 97. Radwan P, Abramik A, Wilczyński J, Radwan M. Successful Autotransplantation of Cryopreserved Ovarian Tissue With Recovery of the Ovarian Function. Ginekol Pol (2016) 87(3):235–40. doi: 10.17772/gp/61981 [DOI] [PubMed] [Google Scholar]
- 98. Demeestere I, Simon P, Buxant F, Robin V, Fernandez SA, Centner J, et al. Ovarian Function and Spontaneous Pregnancy After Combined Heterotopic and Orthotopic Cryopreserved Ovarian Tissue Transplantation in a Patient Previously Treated With Bone Marrow Transplantation: Case Report. Hum Reprod (2006) 21(8):2010–4. doi: 10.1093/humrep/del092 [DOI] [PubMed] [Google Scholar]
- 99. Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Restoration of Ovarian Function After Orthotopic (Intraovarian and Periovarian) Transplantation of Cryopreserved Ovarian Tissue in a Woman Treated by Bone Marrow Transplantation for Sickle Cell Anaemia: Case Report. Hum Reprod (2006) 21(1):183–8. doi: 10.1093/humrep/dei268 [DOI] [PubMed] [Google Scholar]
- 100. Demeestere I, Simon P, Dedeken L, Moffa F, Tsépélidis S, Brachet C, et al. Live Birth After Autograft of Ovarian Tissue Cryopreserved During Childhood. Hum Reprod (2015) 30(9):2107–9. doi: 10.1093/humrep/dev128 [DOI] [PubMed] [Google Scholar]
- 101. Donnez J, Squifflet J, Jadoul P, Demylle D, Cheron AC, Van Langendonckt A, et al. Pregnancy and Live Birth After Autotransplantation of Frozen-Thawed Ovarian Tissue in a Patient With Metastatic Disease Undergoing Chemotherapy and Hematopoietic Stem Cell Transplantation. Fertil Steril (2011) 95(5):1787.e1–4. doi: 10.1016/j.fertnstert.2010.11.041 [DOI] [PubMed] [Google Scholar]
- 102. Huang JY, Tulandi T, Holzer H, Tan SL, Chian RC. Combining Ovarian Tissue Cryobanking With Retrieval of Immature Oocytes Followed by In Vitro Maturation and Vitrification: An Additional Strategy of Fertility Preservation. Fertil Steril (2008) 89(3):567–72. doi: 10.1016/j.fertnstert.2007.03.090 [DOI] [PubMed] [Google Scholar]
- 103. Huang JY, Tulandi T, Holzer H, Lau NM, Macdonald S, Tan SL, et al. Cryopreservation of Ovarian Tissue and In Vitro Matured Oocytes in a Female With Mosaic Turner Syndrome: Case Report. Hum Reprod (2008) 23(2):336–9. doi: 10.1093/humrep/dem307 [DOI] [PubMed] [Google Scholar]
- 104. Elizur SE, Tulandi T, Meterissian S, Huang JY, Levin D, Tan SL. Fertility Preservation for Young Women With Rectal Cancer–a Combined Approach From One Referral Center. J Gastrointest Surg (2009) 13(6):1111–5. doi: 10.1007/s11605-009-0829-3 [DOI] [PubMed] [Google Scholar]
- 105. Cheng J, Ruan X, Zhou Q, Li Y, Du J, Jin F, et al. Long-Time Low-Temperature Transportation of Human Ovarian Tissue Before Cryopreservation. Reprod BioMed Online (2021) 43(2):172–83. doi: 10.1016/j.rbmo.2021.05.006 [DOI] [PubMed] [Google Scholar]
- 106. Isachenko E, Rahimi G, Isachenko V, Nawroth F. In-Vitro Maturation of Germinal-Vesicle Oocytes and Cryopreservation in Metaphase I/II: A Possible Additional Option to Preserve Fertility During Ovarian Tissue Cryopreservation. Reprod BioMed Online (2004) 8(5):553–7. doi: 10.1016/S1472-6483(10)61102-9 [DOI] [PubMed] [Google Scholar]
- 107. Maltaris T, Koelbl H, Fischl F, Seufert R, Schmidt M, Kohl J, et al. Xenotransplantation of Human Ovarian Tissue Pieces in Gonadotropin-Stimulated SCID Mice: The Effect of Ovariectomy. Anticancer Res (2006) 26(6b):4171–6. [PubMed] [Google Scholar]
- 108. Maltaris T, Dragonas C, Hoffmann I, Mueller A, Beckmann MW, Dittrich R. Simple Prediction of the Survival of Follicles in Cryopreserved Human Ovarian Tissue. J Reprod Dev (2006) 52(4):577–82. doi: 10.1262/jrd.18012 [DOI] [PubMed] [Google Scholar]
- 109. Maltaris T, Beckmann MW, Binder H, Mueller A, Hoffmann I, Koelbl H, et al. The Effect of a GnRH Agonist on Cryopreserved Human Ovarian Grafts in Severe Combined Immunodeficient Mice. Reproduction (2007) 133(2):503–9. doi: 10.1530/REP-06-0061 [DOI] [PubMed] [Google Scholar]
- 110. Dittrich R, Mueller A, Binder H, Oppelt PG, Renner SP, Goecke T, et al. First Retransplantation of Cryopreserved Ovarian Tissue Following Cancer Therapy in Germany. Dtsch Arztebl Int (2008) 105(15):274–8. doi: 10.3238/arztebl.2008.0274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dittrich R, Lotz L, Keck G, Hoffmann I, Mueller A, Beckmann MW, et al. Live Birth After Ovarian Tissue Autotransplantation Following Overnight Transportation Before Cryopreservation. Fertil Steril (2012) 97(2):387–90. doi: 10.1016/j.fertnstert.2011.11.047 [DOI] [PubMed] [Google Scholar]
- 112. Lotz L, Liebenthron J, Nichols-Burns SM, Montag M, Hoffmann I, Beckmann MW, et al. Spontaneous Antral Follicle Formation and Metaphase II Oocyte From a non-Stimulated Prepubertal Ovarian Tissue Xenotransplant. Reprod Biol Endocrinol (2014) 12:41. doi: 10.1186/1477-7827-12-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dittrich R, Hackl J, Lotz L, Hoffmann I, Beckmann MW. Pregnancies and Live Births After 20 Transplantations of Cryopreserved Ovarian Tissue in a Single Center. Fertil Steril (2015) 103(2):462–8. doi: 10.1016/j.fertnstert.2014.10.045 [DOI] [PubMed] [Google Scholar]
- 114. Raffel N, Lotz L, Hoffmann I, Liebenthron J, Söder S, Beckmann MW, et al. Repetitive Maturation of Oocytes From Non-Stimulated Xenografted Ovarian Tissue From a Prepubertal Patient Indicating the Independence of Human Ovarian Tissue. Geburtshilfe Frauenheilkd (2017) 77(12):1304–11. doi: 10.1055/s-0043-122601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Póvoa A, Xavier P, Calejo L, Soares S, Sousa M, Silva J, et al. First Transplantation of Cryopreserved Ovarian Tissue in Portugal, Stored for 10 Years: An Unexpected Indication. Reprod BioMed Online (2016) 32(3):334–6. doi: 10.1016/j.rbmo.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 116. Fouks Y, Hamilton E, Cohen Y, Hasson J, Kalma Y, Azem F. In-Vitro Maturation of Oocytes Recovered During Cryopreservation of Pre-Pubertal Girls Undergoing Fertility Preservation. Reprod BioMed Online (2020) 41(5):869–73. doi: 10.1016/j.rbmo.2020.07.015 [DOI] [PubMed] [Google Scholar]
- 117. Walker CA, Bjarkadottir BD, Fatum M, Lane S, Williams SA. Variation in Follicle Health and Development in Cultured Cryopreserved Ovarian Cortical Tissue: A Study of Ovarian Tissue From Patients Undergoing Fertility Preservation. Hum Fertil (Camb). (2021) 24(3):188–98. doi: 10.1080/14647273.2019.1616118 [DOI] [PubMed] [Google Scholar]
- 118. Rodriguez-Wallberg KA, Karlström PO, Rezapour M, Castellanos E, Hreinsson J, Rasmussen C, et al. Full-Term Newborn After Repeated Ovarian Tissue Transplants in a Patient Treated for Ewing Sarcoma by Sterilizing Pelvic Irradiation and Chemotherapy. Acta Obstet Gynecol Scand (2015) 94(3):324–8. doi: 10.1111/aogs.12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G, et al. First Reported Clinical Pregnancy Following Heterotopic Grafting of Cryopreserved Ovarian Tissue in a Woman After a Bilateral Oophorectomy. Hum Reprod (2013) 28(11):2996–9. doi: 10.1093/humrep/det360 [DOI] [PubMed] [Google Scholar]
- 120. Gook DA, Edgar DH, Borg J, Archer J, McBain JC. Diagnostic Assessment of the Developmental Potential of Human Cryopreserved Ovarian Tissue From Multiple Patients Using Xenografting. Hum Reprod (2005) 20(1):72–8. doi: 10.1093/humrep/deh550 [DOI] [PubMed] [Google Scholar]
- 121. Seshadri T, Gook D, Lade S, Spencer A, Grigg A, Tiedemann K, et al. Lack of Evidence of Disease Contamination in Ovarian Tissue Harvested for Cryopreservation From Patients With Hodgkin Lymphoma and Analysis of Factors Predictive of Oocyte Yield. Br J Cancer (2006) 94(7):1007–10. doi: 10.1038/sj.bjc.6603050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Stern CJ, Toledo MG, Hale LG, Gook DA, Edgar DH. The First Australian Experience of Heterotopic Grafting of Cryopreserved Ovarian Tissue: Evidence of Establishment of Normal Ovarian Function. Aust N Z J Obstet Gynaecol (2011) 51(3):268–75. doi: 10.1111/j.1479-828X.2011.01289.x [DOI] [PubMed] [Google Scholar]
- 123. Dolmans MM, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of Cryopreserved Ovarian Tissue From Patients With Acute Lymphoblastic Leukemia is Potentially Unsafe. Blood (2010) 116(16):2908–14. doi: 10.1182/blood-2010-01-265751 [DOI] [PubMed] [Google Scholar]
- 124. Schmidt KL, Ernst E, Byskov AG, Nyboe Andersen A, Yding Andersen C. Survival of Primordial Follicles Following Prolonged Transportation of Ovarian Tissue Prior to Cryopreservation. Hum Reprod (2003) 18(12):2654–9. doi: 10.1093/humrep/deg500 [DOI] [PubMed] [Google Scholar]
- 125. Khattak H, Malhas R, Craciunas L, Afifi Y, Amorim CA, Fishel S, et al. Fresh and Cryopreserved Ovarian Tissue Transplantation for Preserving Reproductive and Endocrine Function: A Systematic Review and Individual Patient Data Meta-Analysis. Hum Reprod Update (2022) 28(3):400–16. doi: 10.1093/humupd/dmac015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shi Q, Xie Y, Wang Y, Li S. Vitrification Versus Slow Freezing for Human Ovarian Tissue Cryopreservation: A Systematic Review and Meta-Anlaysis. Sci Rep (2017) 7(1):8538. doi: 10.1038/s41598-017-09005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Vilela JMV, Dolmans MM, Amorim CA. Ovarian Tissue Transportation: A Systematic Review. Reprod BioMed Online (2021) 42(2):351–65. doi: 10.1016/j.rbmo.2020.11.001 [DOI] [PubMed] [Google Scholar]
- 128. Gellert SE, Pors SE, Kristensen SG, Bay-Bjørn AM, Ernst E, Yding Andersen C. Transplantation of Frozen-Thawed Ovarian Tissue: An Update on Worldwide Activity Published in Peer-Reviewed Papers and on the Danish Cohort. J Assisted Reprod Genet (2018) 35(4):561–70. doi: 10.1007/s10815-018-1144-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Newton H, Fisher J, Arnold JR, Pegg DE, Faddy MJ, Gosden RG. Permeation of Human Ovarian Tissue With Cryoprotective Agents in Preparation for Cryopreservation. Hum Reprod (1998) 13(2):376–80. doi: 10.1093/humrep/13.2.376 [DOI] [PubMed] [Google Scholar]
- 130. Gavish Z, Ben-Haim M, Arav A. Cryopreservation of Whole Murine and Porcine Livers. Rejuvenation Res (2008) 11(4):765–72. doi: 10.1089/rej.2008.0706 [DOI] [PubMed] [Google Scholar]
- 131. Oktay K, Karlikaya G, Aydin B. Ovarian Cryopreservation and Transplantation: Basic Aspects. Mol Cell Endocrinology (2000) 169(1-2):105–8. doi: 10.1016/S0303-7207(00)00361-0 [DOI] [PubMed] [Google Scholar]
- 132. Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful Fertility Preservation Following Ovarian Tissue Vitrification in Patients With Primary Ovarian Insufficiency. Hum Reprod (2015) 30(3):608–15. doi: 10.1093/humrep/deu353 [DOI] [PubMed] [Google Scholar]
- 133. Lunding SA, Pors SE, Kristensen SG, Landersoe SK, Jeppesen JV, Flachs EM, et al. Biopsying, Fragmentation and Autotransplantation of Fresh Ovarian Cortical Tissue in Infertile Women With Diminished Ovarian Reserve. Hum Reprod (2019) 34(10):1924–36. doi: 10.1093/humrep/dez152 [DOI] [PubMed] [Google Scholar]
- 134. Cleary M, Snow M, Paris M, Shaw J, Cox SL, Jenkin G. Cryopreservation of Mouse Ovarian Tissue Following Prolonged Exposure to an Ischemic Environment. Cryobiology (2001) 42(2):121–33. doi: 10.1006/cryo.2001.2315 [DOI] [PubMed] [Google Scholar]
- 135. Kim SS, Yang HW, Kang HG, Lee HH, Lee HC, Ko DS, et al. Quantitative Assessment of Ischemic Tissue Damage in Ovarian Cortical Tissue With or Without Antioxidant (Ascorbic Acid) Treatment. Fertility Sterility (2004) 82(3):679–85. doi: 10.1016/j.fertnstert.2004.05.022 [DOI] [PubMed] [Google Scholar]
- 136. Oktay K, Marin L, Bedoschi G, Pacheco F, Sugishita Y, Kawahara T, et al. Ovarian Transplantation With Robotic Surgery and a Neovascularizing Human Extracellular Matrix Scaffold: A Case Series in Comparison to Meta-Analytic Data. Fertil Steril (2022) 117(1):181–92. doi: 10.1016/j.fertnstert.2021.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.