Abstract
Objectives
To compare tumor best overall response (BOR) by RECIST 1.1 and iRECIST, to explore the incidence of pseudoprogression in melanoma treated with pembrolizumab, and to assess the impact of pseudoprogression on overall survival (OS).
Methods
221 patients with locally advanced/unresectable melanoma who received pembrolizumab as part of KEYNOTE-002 trial were included in this study. Radiological assessment of imaging was centrally reviewed to assess tumor response. Incidence of discordance in BOR between RECIST 1.1 and iRECIST as well as rate of pseudoprogression were measured. OS of patients with pseudoprogression was compared to those with uncontrolled disease.
Results
Of the 221 patients in this cohort, 136 patients developed PD as per RECIST v1.1 and 78 patients with PD continued treatment and imaging beyond initial RECIST 1.1 defined PD. Among the 78 patients who continued therapy and imaging post-progression, RECIST 1.1 and iRECIST were discordant in 10 patients (12.8%) and pseudoprogression was encountered in 14 patients (17.9%). OS of patients with pseudoprogression was longer than that of patients with uncontrolled disease/true progression (29.9 months versus 8.0 months, p-value <0.001).
Conclusions
Effectiveness of immunotherapy in clinical trials depends on the criterion used to assess tumor response (RECIST 1.1 vs iRECIST) with iRECIST being more appropriate to detect pseudoprogression and potentially prevent premature termination of effective therapy. Pseudoprogression was associated with improved OS in comparison to patient with uncontrolled disease.
Keywords: RECIST, Melanoma, Immunotherapy
INTRODUCTION:
Immune checkpoint blockers targeting CTLA-4, PD-1 and PD-L1 have revolutionized the management of these patients as well as a wide range of cancer types.1 First authorized in patients with advanced (metastatic or unresectable) melanoma who progressed after treatment with ipilimumab2, pembrolizumab rapidly became a standard of care in first line therapy for these patients and more recently as adjuvant immunotherapy in melanoma patients with lymph node involvement following complete resection.3 Clinical trials have demonstrated that 34 to 46% of melanoma patients achieve objective response with anti-PD-1 including patients achieving durable response.4,5 In fact, 90% of patients with melanoma who achieve complete response while on pembrolizumab will continue to have disease-free survival of at least 24 months, even after discontinuation of therapy.6
Pseudoprogression is defined as an atypical response of tumor lesions manifested on clinical imaging as increase in tumor burden enough to meet RECIST 1.1 criteria for progressive disease followed by stabilization or shrinkage of tumors. Melanoma was reported to have one of the highest rates of pseudoprogression, observed in up to 19% of melanoma patients receiving PD-1/PD-L1 inhibitors.7–11
In March 2017, the Response Evaluation Criteria in Solid Tumors (RECIST) working group introduced guidelines for assessment of treatment response among patients receiving immunotherapy (iRECIST) which allows detection of pseudoprogression.12 iRECIST was built on the principal metrics of RECIST 1.113 and is identical to RECIST 1.1 assessment at time points when there is complete response (CR), partial response (PR), and stable disease (SD). However, iRECIST allows for continuation of treatment upon first occurrence of progressive disease (PD) by considering it unconfirmed progression (iUPD, a new category introduced in iRECIST) that needs to be followed with imaging in 4–8 weeks as shown in table 1. iUPD may remain as iUPD (if the tumor burden does not increase but remains above the threshold for progression), revert back to a controlled disease (immune-related stable disease (iSD), immune-related partial response (iPR), or immune-related complete response (iCR) if the tumor burden falls below the threshold for progression), or change to confirmed progressive disease status (iCPD) if there is further worsening of any existing cause of progression, or appearance of a new cause of progression).12,14
Table 1.
Comparison between RECIST 1.1 and iRECIST
| RECIST 1.1 | iRECIST | |
|---|---|---|
| Measurements | ||
| Size measurement | Unidimensional | Unidimensional |
| Number of target lesions | 5 lesions total, 2 per organ | 5 lesions total, 2 per organ |
| Tumor burden | Sum of the diameters of target lesions | -Same at baseline -Sum of longest diameters of new target lesions are followed separately from baseline tumor burden |
| Definitions | ||
| Target lesion | Non-nodal lesion ≥ 10 mm in longest axis | Same |
| Nodal lesion ≥ 15 mm in shortest axis | ||
| Non-target lesion | Non-nodal lesion <10 mm in longest axis | Same |
| Nodal lesion ≥10 and <15 mm in shortest axis | ||
| Non-measurable lesions | ||
| New lesion | New measurable or non-measurable lesion | Same |
| Response categories | ||
| Complete Response (CR) | Complete disappearance of all lesions | Same |
| No new lesion | Can occur after iUPD but not after iCPD | |
| Partial Response (PR) | Decrease in target lesion burden by ≥30% from baseline | Same |
| Absence of new lesion or PD based on non-target lesion | Can occur after iUPD but not after iCPD | |
| Stable Disease (SD) | Residual/persistent tumor lesions | Same |
| Neither PD nor PR has been achieved | Can occur after iUPD but not after iCPD | |
| Progression of Disease (PD) | Increase in target lesion burden by ≥20% from nadir | First occurrence of PD will be considered iUPD. |
| New lesion or PD based on non-target lesion | iCPD occurs when there is further increase in tumor burden (target, non-target, or new lesions) 4–8 weeks from time of iUPD. | |
| Progression of Disease | ||
| Appearance of new lesion | Always means PD | Need to be confirmed in 4–8 weeks to be considered as iCPD |
| Measurement of new measurable lesion is not included in sum of diameters | Sum of diameters of new measurable lesions will be followed separately from initial tumor burden (sum of diameters at baseline) | |
| Confirmation of PD | Not required | Required after iUPD to differentiate pseudoprogression from iCPD |
| Consideration of clinical status | Not included in assessment | Considered to continue treatment in patients with iUPD |
Notes - iUPD: immune-unconfirmed PD; iCPD: Immune-confirmed PD
Since the introduction of iRECIST, there have been a few attempts to compare RECIST 1.1 and iRECIST in evaluation of patients receiving PD-1/PD-L1 inhibitors.15,16 However, these comparisons were only made in lung cancer patients, limited by retrospective approach, or confined to a single institution with a small sample size. Therefore, it remains unclear if pseudoprogression occur in the same frequency in patients with melanoma.11
In the immunotherapy arm of a multi-institutional phase II clinical trial, KEYNOTE-0022 (which allowed continuing therapy beyond RECIST 1.1 defined progression) we explore the differences in best overall response (BOR) between iRECIST and RECIST 1.1 assessments, report the incidence of pseudoprogression, and compare the overall survival (OS) of patients with pseudoprogression to patients with controlled disease (BOR=iSD, iPR, iCR) and patients with uncontrolled disease (BOR=iCPD).
METHODS:
Phase II clinical trial setting:
KEYNOTE-002 was a double-blind randomized controlled clinical trial (Phase II) that enrolled adult patients with advanced (histologically/cytologically proved stage III or IV) melanoma in 73 medical institutions across 12 countries from November 30th, 2012 through November 13th, 2013.2 The clinical trial was approved by each participating IRB and patients’ informed consent was obtained before enrollment. Key elements pertinent to this study of the imaging findings were that patients: 1) had to have lesions for which local therapy would not be amenable, 2) tumor should have progressed (within the last 24 weeks) on ipilimumab and BRAF/MEK inhibitor therapy, or both, prior to enrollment, and 3) had to have measurable disease burden as per RECIST 1.1 criteria.13 Exclusion criteria included intracranial metastatic disease, HIV infection, active autoimmune disease, and other immunological disorders. Further description of the clinical trial setting was reported previously.2
Participants Included in this study:
Imaging of 427 patients (79% of the 540 patients included in the randomization) was provided by the trial’s sponsor which included 286 patients who received pembrolizumab. Of those 286 patients, 65 patients were excluded due to either lack of RECIST-defined target lesions at baseline (n=30), baseline CT date beyond 4 weeks from randomization date (n=3), or due to lack of follow-up CT (n=32). The remaining 221 patients were included in the final analysis of this study. Overview of the study cohort is shown in figure 1.
Figure 1.
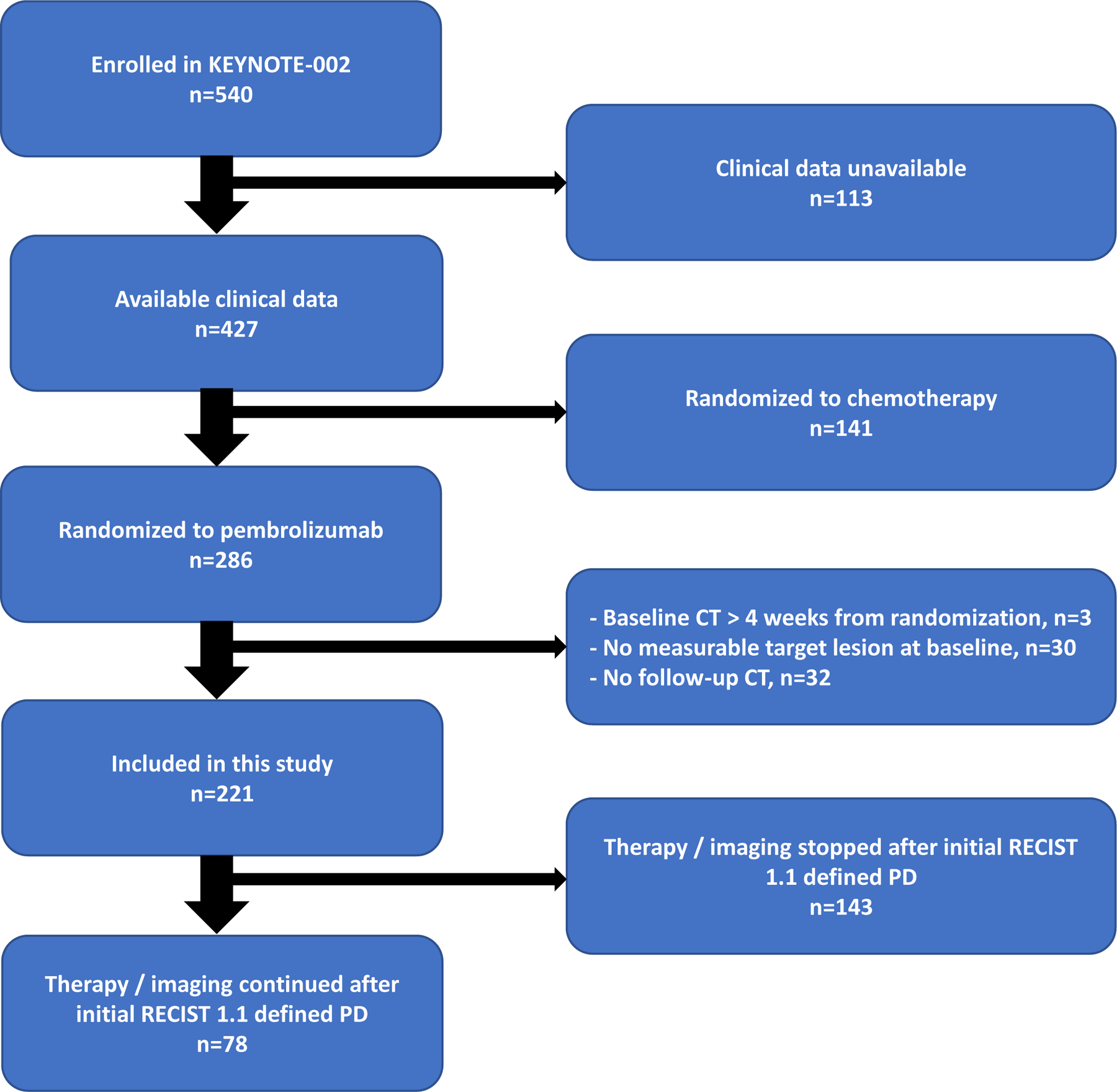
CONSORT diagram showing treatment all enrolled patients in KEYNOTE-002.
Central Imaging Review of Tumor Burden and Response to Therapy:
Retrospectively, an independent central review of all patients’ imaging was performed by two radiologists experienced in cancer imaging (FSA is an abdominal fellowship trained radiologist with 9-year experience in radiology and 4-year experience in assessing tumor response to therapy in clinical trials. LD is a nuclear medicine trained radiologist with PhD in oncology and 9-year experience in cancer imaging). As per RECIST 1.1, the radiologists reviewed the imaging to determine target tumor lesions, measure sum of diameters, assess non-target lesions, and identify/measure new lesions.13 In addition to the RECIST unidimensional approach, all measurable tumor lesions were segmented (had their margins marked by the radiologist using dedicated oncological imaging platform)17 on every slice of the CT-scan where it was seen. This method of segmentation allowed for estimation of tumor volume (three-dimensional estimation of target tumor size) which was used in a secondary analysis to corroborate response assessments made based on conventional RECIST unidimensional approach (Supplemental material and figure 5).18 After segmentation process, both radiologists simultaneously reviewed the recorded tumor measurements/non-target lesion comments and conducted consensus read/assessment of tumor response according to RECIST 1.1 and iRECIST at each time point. Further details of imaging review are included in the supplemental material.
Statistical Analysis:
The primary outcome was to explore the discordance rate between RECIST 1.1 and iRECIST in terms of best overall response (BOR) among all patients included in this study as well as in patient with post-progression imaging. The agreement between iRECIST and RECIST 1.1 was evaluated using the Cohen’s kappa agreement.
The secondary outcome was to estimate pseudoprogression rate and to compare OS of pseudoprogression patients with uncontrolled disease patients. OS was defined as the time from randomization in the clinical trial to death from any cause. To this end, patients were classified in three response categories: controlled disease (BOR = iCR, iPR or iSD), uncontrolled disease (BOR = iCPD), and pseudoprogression. The difference in OS between those groups was assessed using log-rank testing (Kaplan-Meier analysis). Landmark analysis was performed to control for immortal time bias in which landmark analysis divided the follow-up time at a pre-specified time point (in this study it is 6-month). Groups were then defined by response categories (controlled disease, uncontrolled disease, and pseudoprogression) having occurred before the landmark, and outcome events were only considered if they occurred after the landmark. Differences in survival were analyzed using the log-rank test and a p-value less than 0.05 was considered significant.
Analyses were conducted using MatLab (v9.5, 2018), Microsoft Excel (v2019), SPSS (v25.0, 2017), and R (Version 1.2.1335).
RESULTS
Participants
In the 221 patients of the KEYNOTE-002 trial included in this study, the first CT assessment (initially intended at 12 weeks from randomization) was performed at a median time of 13.0 weeks (interquartile range: 12.1, 14.4 weeks). According to the evaluation of all available time points, 136 (61.5%) developed PD using RECIST v1.1. In these patients with radiographic progression, 58 patients (42.6%) were not imaged after initial RECIST 1.1 defined PD time point while 78 patients (57.3%) were kept on treatment and imaged beyond initial RECIST 1.1 defined PD allowing real comparison of RECIST v1.1 to iRECIST.
I. Comparison of RECIST 1.1 and iRECIST
The agreement between iRECIST and RECIST 1.1 was high; the Kappa’s Cohen agreement was 0.98 (95CI: 0.96 – 1.00, P-value <0.001). Both RECIST 1.1 and iRECIST were in agreement in ranking the BOR in 211 patients; 87 patients (39.4%) had PD/iCPD, 53 patients (24.0%) had SD/iSD, 49 patients (22.2%) had PR/iPR, and 22 patients (10.0%) had CR/iCR as shown in table 2.
Table 2.
Comparison of best overall response RECIST 1.1 and iRECIST
| BOR iRECIST | |||||
|---|---|---|---|---|---|
| CR | PR | SD | PD | Total | |
| BOR RECIST v1.1 | |||||
| CR | 22 | 22 | |||
| PR | 2 | 49 | 51 | ||
| SD | 0 | 1 | 53 | 54 | |
| PD | 0 | 3 | 4 | 87 | 94 |
| Total | 24 | 53 | 57 | 87 | 221 |
However, RECIST 1.1 and iRECIST BOR was discordant in 10 patients. Thus, the rate of BOR discordance was 4.5% among the entire study cohort (n=221) while in the sub-cohort of patients with imaging beyond initial RECIST v1.1 defined PD (n=78), BOR discordance rate was 12.8%. Six patients were considered as having immune-response (2 additional CR, 4 additional PR) as per iRECIST (iPR, iCR) while their tumor was considered progressive (PD) as per RECIST v1.1. Similarly, four patients were considered as having immune-stable disease as per iRECIST (iSD) while the disease was considered progressive (PD) as per RECIST v1.1.
Secondary analysis focusing on the difference in progression free survival between the two assessment criteria (RECIST 1.1 and iRECIST) is described in details along with Kaplan-Meier’s graphs in the supplemental material.
II. Incidence of Pseudoprogression
Pseudoprogression was encountered in 14 patients representing 6.3% of the total study cohort (n=221) and 17.9% of the sub-cohort of patients with imaging beyond initial RECIST 1.1 defined PD (n=78) allowing investigators to detect pseudoprogression.
Early pseudoprogression was observed in 5 patients (36%) at the initial imaging time point of the first CT assessment 12 weeks from immunotherapy initiation. Late pseudoprogression was observed in the remaining 9 patients after 12 weeks from date of immunotherapy initiation (i.e. on the second or later response assessment imaging time point).
Patients with pseudoprogression and true progression were similar in terms of pembrolizumab dose received, organs involved, and the timing of true progression/pseudoprogression as shown in table 3. Of the 14 pseudoprogression patients, the increase in tumor burden that triggered the initial RECIST 1.1 defined PD was the appearance of new target lesions in 7 patients (50.0%), increase in target lesions sum of diameter by 20% or more in 9 patients (64.3%), and simultaneous observation of these phenomena (new lesions and increase in target lesions sum of diameter by >20%) at the time of pseudoprogression in 2 patients (14.3%) as shown in table 3. Pseudoprogression was predominantly observed in lymph nodes (57.1%) and visceral organs such as the lung (21.4%), and the liver (28.6%) but this pattern of organ involvement was not significantly different from its corresponding pattern in patients with true progression. Pseudoprogression occurred at an average time of 5.42 months (standard deviation = 3.52 months) which was not significantly different from the time of occurrence of true progression (average of 4.57 months and standard deviation of 3.94) as shown in table 3. Among the 14 patients with pseudoprogression, 71% (10/14) continued to have a controlled disease status (iSD, iPR, or iCR). The ultimate outcome and tumor assessment after initial RECIST 1.1 defined PD among patients with pseudoprogression is shown in details in the Supplemental Material - Table 3.
Table 3.
Characteristics of patients with true progression and pseudoprogression
| True Progression | Pseudo-Progression | p-value | ||
|---|---|---|---|---|
| n = 64 | n = 14 | |||
| Treatment group (%) | 10 mg Q3 (%) | 34 (55.7) | 7 ( 50.0) | 0.927 |
| 2 mg Q3 (%) | 27 (44.3) | 7 ( 50.0) | 0.927 | |
| BOR RECIST (%)* | PD | 45 (70.3) | 6 ( 42.9) | 0.016 |
| PR | 5 ( 7.8) | 5 ( 35.7) | ||
| SD | 14 (21.9) | 3 ( 21.4) | ||
| CR | 0 (0.0) | 0 (0.0) | ||
| Timing after immunotherapy initiation | Delay (months) (mean (SD) | 4.57 (3.94) | 5.42 (3.52) | 0.463 |
| Frequency of imaging beyond initial PD | mean (SD) | 2.39 (2.82) | 4.93 (4.07) | 0.006 |
| Number of CTs beyond initial PD | 0.013 | |||
| 1 (%) | 32 (50.0) | 1 ( 7.1) | ||
| 2 (%) | 15 (23.4) | 5 ( 35.7) | ||
| 3 (%) | 6 ( 9.4) | 1 ( 7.1) | ||
| 4 (%) | 2 ( 3.1) | 1 ( 7.1) | ||
| 5+ (%) | 9 ( 14.1) | 6 ( 42.9) | ||
| Cause of progression (%) | New lesion | 36 (57.1) | 7 ( 50.0) | 0.850 |
| Non-target | 33 (52.4) | 0 ( 0.0) | 0.001 | |
| Target | 47 (74.6) | 9 ( 64.3) | 0.651 | |
| New lesion & Non-target | 24 (37.5) | 0 ( 0.0) | 0.015 | |
| New lesion & Target | 23 (35.9) | 2 ( 14.3) | 0.209 | |
| Non target & Target | 25 (39.1) | 0 ( 0.0) | 0.012 | |
| New lesion & Non-target & Target | 17 (26.6) | 0 ( 0.0) | 0.068 | |
| Site of PD (%) | Lymph node | 40 (62.5) | 8 (57.1) | 0.944 |
| Lung | 18 (28.1) | 3 ( 21.4) | 0.858 | |
| Subcutaneous and soft tissue | 14 (21.9) | 2 ( 14.3) | 0.786 | |
| Liver | 10 (15.6) | 4 ( 28.6) | 0.448 | |
| Adrenal | 9 (14.1) | 0 ( 0.0) | 0.303 | |
| Spleen | 6 ( 9.4) | 1 ( 7.1) | 1.000 | |
| Bone | 2 ( 3.1) | 0 ( 0.0) | 1.000 |
BOR from baseline until the time point when true progression or pseudoprogression was encountered.
The only significant difference between patients with pseudoprogression and those with true progression was the rate of partial response to therapy before first RECIST 1.1 defined PD; approximately 36% (5/14) of patients with pseudoprogression experienced initial RECIST 1.1 defined PR prior to the time of pseudoprogression while only 8% (5/64) of patients with true PD experienced initial RECIST 1.1 defined PR before they progressed (p-value = 0.016).
III. OS of patients with pseudoprogression
In the study cohort, 135 patients died during the follow-up and the median (95% CI) OS was 16.5 months (95% CI: 14.8–23.1). We compared the OS of patients with pseudoprogression (n=14) to the OS of patients with controlled disease (n=119 patients with iCR, iPR or iSD as per BOR) and OS of patients with uncontrolled disease (n=87 patients with iCPD as BOR) as a benchmark. As shown in table 4, the median OS was 29.9 months (95CI: 29.2-NA) in patients with pseudoprogression and was comparable to the 31.2 months (95CI: 23.54-NA) OS among patients with controlled disease. However, the OS was only 8.0 months (95CI: 6.3–11.1) among patients with uncontrolled disease, significantly shorter than patients with controlled disease or pseudoprogression (p-value <0.0001), as shown in figure 2.
Table 4.
Overall survival (non-landmark analysis) months and estimates across patients with controlled disease (CR, PR, SD as BOR), patients with true progression (PD as BOR) and patients with pseudoprogression
| Median OS and 95%CI |
Estimation of OS probability at 6, 12 and 18 months |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strata | n | events | median | 95%CI | time (months) | Number at risk | Number of events | Overall Survival estimate | 95%CI | ||
| Overall | 221 | 135 | 16.5 | 14.8 | 23.1 | 6 | 183 | 35 | 0.84 | 0.79 | 0.89 |
| 12 | 136 | 44 | 0.64 | 0.58 | 0.70 | ||||||
| 18 | 14 | 8 | 0.38 | 0.30 | 0.49 | ||||||
| Controlled Disease | 119 | 52 | 31.2 | 23.54 | NA | 6 | 114 | 5 | 0.96 | 0.92 | 0.99 |
| 12 | 96 | 17 | 0.81 | 0.75 | 0.89 | ||||||
| 18 | 61 | 13 | 0.60 | 0.52 | 0.70 | ||||||
| True Progression | 88 | 78 | 8.0 | 6.26 | 11.1 | 6 | 55 | 30 | 0.65 | 0.56 | 0.76 |
| 12 | 27 | 27 | 0.33 | 0.24 | 0.45 | ||||||
| 18 | 9 | 1 | 0.18 | 0.11 | 0.32 | ||||||
| Pseudoprogression | 14 | 5 | 29.9 | 29.21 | NA | 6 | 14 | 0 | 1.00 | 1.00 | 1.00 |
| 12 | 13 | 0 | 1.00 | 1.00 | 1.00 | ||||||
| 18 | 7 | 1 | 0.70 | 0.47 | 1.00 | ||||||
Notes-The strata are defined by the best overall response. Disease control is defined by patients having CR, PR or SD as best overall response. Pseudoprogression is defined by patients having a pseudoprogression per iRECIST criteria at anytime during treatment.
Figure 2.
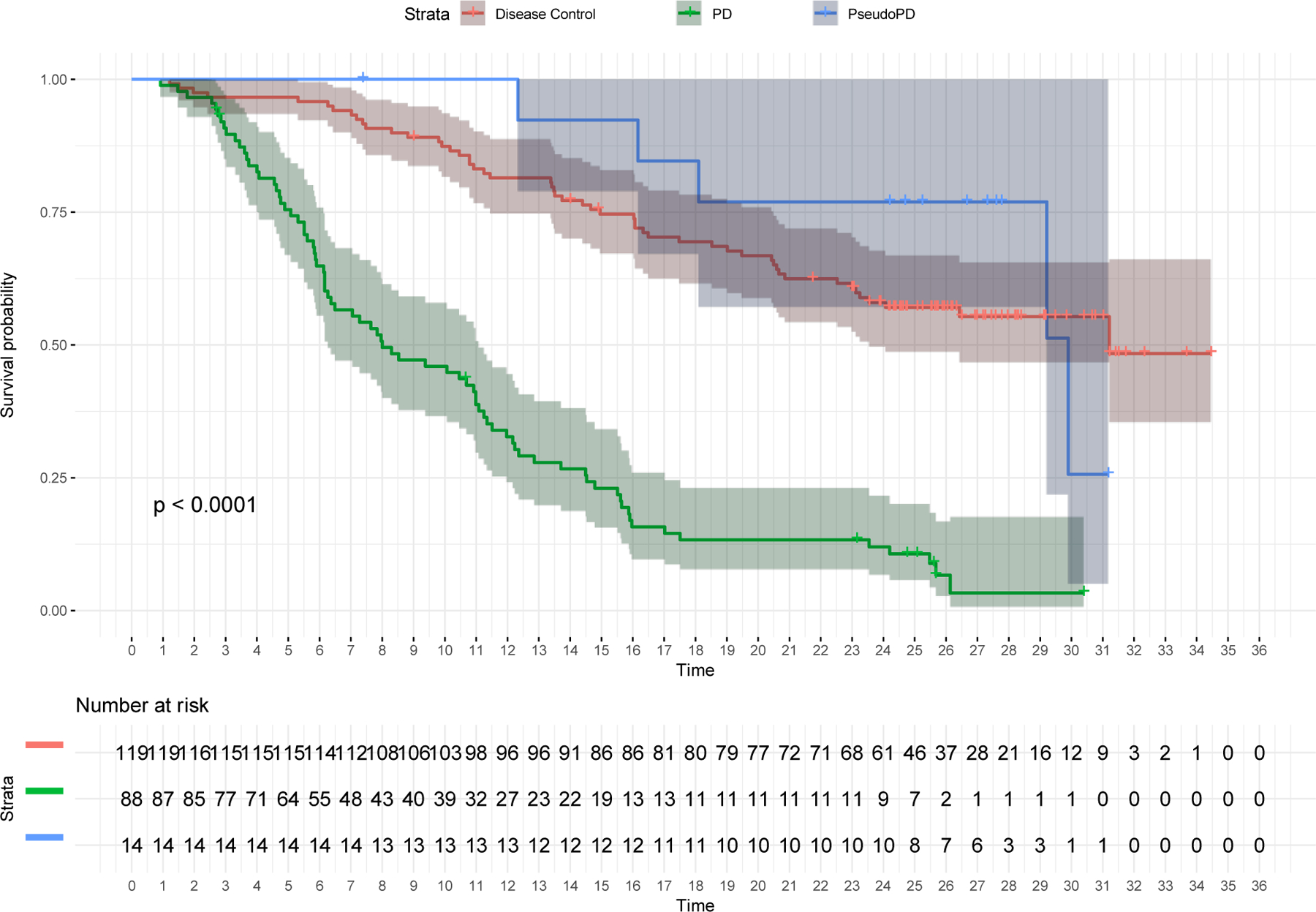
Kaplan-Meier curves depicting differences in overall survival between patients with pseudoprogression, uncontrolled disease (BOR=iCPD) and controlled disease (BOR=iCR, iPR, and iSD) among the entire cohort (n=221)
In the landmark analysis performed 6 months after randomization (n=180, supplemental table 2), the median OS time was not reached among patients with controlled disease (95%CI: 20.4-NA), and was 23.2 months after landmark (95%CI: 12.1-NA, n=11) in patients with pseudoprogression, significantly longer than the 6.2 months after landmark (95%CI: 5.0–9.5, n=55, p-value <0.0001) in patients with uncontrolled disease.
DISCUSSION:
In the strict setting of a double-blind randomized controlled clinical trial (Phase II) among patients with locally advanced/unresectable melanoma receiving pembrolizumab, we found: 1) The rate of discordance between RECIST 1.1 and iRECIST criteria in assessing BOR to therapy reached up to 12.8% among patients with imaging beyond initial RECIST 1.1 defined PD. 2) The rate of pseudoprogression in advanced/unresectable melanoma patients receiving immunotherapy reached up to 17.9%. 3) Patients with pseudoprogression and true progression were similar in terms of organ involvement and timing of progression but they were significantly different regarding the incidence of initial objective response (incidence of initial PR among patients with pseudoprogression was 36% versus 8% among patients with true progression). 4) Patients with pseudoprogression have similar OS time and estimates when compared patients with controlled disease (BOR = iCR, iPR, or iSD) and significantly better than OS time and estimates among patients with uncontrolled disease (BOR = iCPD).
A recently published study19 demonstrated that implementation of another immune-related RECIST assessment (irRECIST proposed in 201420) resulted in significantly longer median progression free survival defined by irRECIST in comparison to that defined by RECIST 1.1. Concordant with that study, we found that iRECIST defined progression free survival (PFS) is significantly longer than RECIST 1.1 defined PFS among patients with post-progression imaging (supplemental material). Furthermore, our study extends these findings to the most recently published iRECIST criterion12 now adopted by the cancer imaging community for immunotherapy trials.
Although treatment and imaging beyond initial RECIST 1.1 defined PD were allowed in this study, 42.6% of patients who had PD based on RECIST 1.1 did not receive such post-PD imaging, which could potentially underestimate the prevalence of pseudoprogression. However, the prevalence of pseudoprogression estimates from this study are very close to ones recently published in a pooled analysis of clinical trials of anti-PD-1 therapy in unresectable melanoma patients submitted to the Food and Drug Administration.10
An interesting finding was that a significant proportion of pseudoprogression occurred after an objective response (5 patients [36%] achieved PR before developing pseudoprogression (i.e. progression from nadir)) while true progression tends to occur from baseline in the majority of cases (45 patients [70%] developed PD from start of the trial without any prior objective response). This finding is similar to results from a recent study21 highlighting the paradoxical disadvantage for any cancer therapy that results in initial decrease in measurable tumor burden followed by mild increase in tumor size (high enough for RECIST 1.1 defined PD from nadir but not from baseline). This initial decrease in size of tumor burden will create a nadir from which PD will later be established even if the tumor burden did not exceed baseline measurement by 20% as per RECIST 1.1 criterion. Our results suggest that patients who achieve initial objective response to immunotherapy may herald a more durable response. An objective initial response followed by RECIST 1.1-defined PD from nadir may be a sign of pseudoprogression that is worth waiting to confirm on follow up imaging, as proposed by the iRECIST guidelines.12
The landmark analysis demonstrated that patients with pseudoprogression had a similar OS to patients with controlled disease (iCR, iPR, iSD). Therefore, iRECIST is clinically relevant since it will avoid premature cessation of treatment in patients with pseudoprogression. Based on these results, iRECIST appeared to be an enhanced assessment criterion among patients who are treated with immunotherapy by allowing continuation of treatment past initial progression in order to avoid premature cessation of effective therapy among patients who appeared to have pseudoprogression.
Tazdait et al. compared assessment of response to PD-1 or PDL-1 inhibitor therapy among patients with non-small cell lung cancer.15 They found the rate of pseudoprogression to be 5% in their single center retrospective cohort study with variable immunotherapeutic agents. Our results corroborate those of Tazdait et al., however, the rate of pseudoprogression in advanced melanoma patients in our cohort ranged between being 6.3% and 17.9% and is similar to pseudoprogression in melanoma patients published before.7,10,22 We also agree with Tazdait et al. in stressing the importance of using the more flexible solid tumor assessment criteria (iRECIST) in the era of immunotherapy to allow for capturing the delayed but potentially effective response to therapy without losing the window for salvage therapy in patients who prove to have true disease progression.14
A small but prospective study by Beer et al. among patients with advanced non-small cell lung cancer on PD1/PD-L1 inhibitors found that RECIST 1.1 and iRECIST were discordant in 5% (2/42), mainly due to pseudoprogression phenomenon. These reported discordance rate between RECIST 1.1 and iRECIST and pseudoprogression rate are smaller than what we found in our study, which could be related to the difference in type of cancer; higher incidence of pseudoprogression are generally reported in patients with melanoma.10
A pooled analysis of multiple clinical trials submitted to FDA focusing on use of PD-1/PD-L1 inhibitors among solid tumors patients was recently published.23 Mulkey et al. found that patients with objective iRECIST defined response (without matching RECIST 1.1 response), i.e. patients with pseudoprogression, have OS similar to patients with objective response based on RECIST 1.1. This finding is concordant with our results, despite the fact that this analysis included other solid tumors in addition to melanoma (non-small cell lung cancer, renal cell carcinoma, and head and neck squamous cell carcinoma) and the reliance on investigator-assessed tumor measurements rather than having a centralized radiological review of imaging.
Limitations of our study include incomplete utilization of the treatment/experimental arm (only 79% of the experimental arm patients were included in this study) as this is what was provided to the study team by the sponsor.24 However, the 79% of the experimental arm included in our study was randomly selected and we therefore propose that the rate of pseudoprogression in the remaining 21% of the data would not be significantly different. The controlled nature of a clinical trial with pre-specified imaging modalities and time intervals afforded an excellent environment for exploring differences in these response criteria.
In conclusion, assessment of solid tumor’s BOR to immunotherapy is better performed with iRECIST allowing to detect pseudoprogression in patients with advanced melanoma. Our findings also suggest that iRECIST may help to avoid premature termination of immunotherapy given the improved OS in patients with pseudoprogression. We also found that, unlike true progression, pseudoprogression tend to occur after initial objective response; future studies should attempt to verify this phenomenon which may potentially help earlier discrimination between patients with pseudoprogression and those with true progression.
Supplementary Material
Key Points:
Discordance between iRECIST and RECIST 1.1 was found in 12.8% of unresectable melanoma patients on pembrolizumab who continued therapy beyond initial RECIST 1.1-defined progression.
Pseudoprogression, captured with iRECIST, occurred in 17.9% and was significantly associated with improved overall survival in comparison with uncontrolled disease.
1. [Acknowledgements]
Authors would like to thank other members of the Vol-PACT team (especially Mithat Gonen, PhD and Chaya Moskowitz, PhD) for their feedback and suggestions during the preparation of the manuscript.
2. Funding
This study has (through multi-institutional collaboration as a project of the Foundation for the National Institute of Health (FNIH) Biomarkers Consortium, Advanced Metrics and Modeling with Volumetric CT for Precision Analysis of Clinical Trial Results (Vol-PACT)) received funding support provided to the FNIH by: Amgen, Inc.; Boehringer Ingelheim; Merck KGaA, Darmstadt, Germany; Genentech, Inc.; Merck Sharp & Dohme Corp.; Regeneron Pharmaceuticals, Inc., and Takeda Pharmaceuticals International, Inc.
Abbreviations:
- RECIST 1.1
Response Evaluation Criteria in Solid Tumors, updated in 2009
- iRECIST
immune-Response Evaluation Criteria in Solid Tumors, proposed in 2017
- CR
complete response
- iCR
immune-complete response
- PR
partial response
- iPR
immune-partial response
- SD
stable disease
- iSD
immune-stable disease
- PD
progressive disease
- iUPD
immune-unconfirmed progressive disease
- iCPD
immune-confirmed progressive disease
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death protein 1 ligand
- PFS
progression free survival
- OS
overall survival
- BOR
best overall response
Footnotes
Guarantor:
The scientific guarantor of this publication:
Lawrence H. Schwartz, MD
James Picker Professor of Radiology
Chair, Department of Radiology, Columbia University
Service Chief and Attending Physician, New York-Presbyterian Hospital-Columbia Campus
Email: lhs2120@cumc.columbia.edu
Conflict of Interest:
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and Biometry:
One of the authors, Laurent Dercle, MD, PhD, has significant statistical expertise.
No complex statistical methods were necessary for this paper.
Informed Consent:
Written informed consent was not required for this study because the study is a retrospective analysis of imaging that had already been collected during Keynote 002 clinical trial.
Ethical Approval:
Institutional Review Board approval was obtained for the keynote 002 clinical trial.
Institutional Review Board approval was not required for this retrospective study of the de-identified imaging data which was collected during keynote 002 clinical trial.
Study subjects or cohorts overlap:
Some study subjects or cohorts have been previously reported in the original publication of Keynote 002 clinical trial manuscript in Lancet Oncology: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(15)00083-2/fulltext
Methodology
Methodology:
• Retrospective
• Randomised controlled trial (only the immunotherapy arm)
• Multicenter study
References:
- 1.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nature reviews Immunology 2018;18:153–67. [DOI] [PubMed] [Google Scholar]
- 2.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. The Lancet Oncology 2015;16:908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med 2018;378:1789–801. [DOI] [PubMed] [Google Scholar]
- 4.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014;32:1020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019;20:1239–51. [DOI] [PubMed] [Google Scholar]
- 6.Robert C, Ribas A, Hamid O, et al. Durable Complete Response After Discontinuation of Pembrolizumab in Patients With Metastatic Melanoma. J Clin Oncol 2018;36:1668–74. [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients With Advanced Melanoma Treated With Pembrolizumab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34:1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Queirolo P, Spagnolo F. Atypical responses in patients with advanced melanoma, lung cancer, renal-cell carcinoma and other solid tumors treated with anti-PD-1 drugs: A systematic review. Cancer Treat Rev 2017;59:71–8. [DOI] [PubMed] [Google Scholar]
- 9.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412–20. [DOI] [PubMed] [Google Scholar]
- 10.Beaver JA, Hazarika M, Mulkey F, et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol 2018;19:229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33:3541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. The Lancet Oncology 2017;18:e143–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 14.Ferte C, Marabelle A. iRECIST: A clarification of tumour response assessment in the immunotherapy era. Eur J Cancer 2017;77:165–7. [DOI] [PubMed] [Google Scholar]
- 15.Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer 2018;88:38–47. [DOI] [PubMed] [Google Scholar]
- 16.Beer L, Hochmair M, Haug AR, et al. Comparison of RECIST, iRECIST, and PERCIST for the Evaluation of Response to PD-1/PD-L1 Blockade Therapy in Patients With Non-Small Cell Lung Cancer. Clinical nuclear medicine 2019;44:535–43. [DOI] [PubMed] [Google Scholar]
- 17.Yang H, Schwartz LH, Zhao B. A Response Assessment Platform for Development and Validation of Imaging Biomarkers in Oncology. Tomography (Ann Arbor, Mich) 2016;2:406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao B, Lee SM, Lee HJ, et al. Variability in assessing treatment response: metastatic colorectal cancer as a paradigm. Clin Cancer Res 2014;20:3560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pignon JC, Jegede O, Shukla SA, et al. irRECIST for the Evaluation of Candidate Biomarkers of Response to Nivolumab in Metastatic Clear Cell Renal Cell Carcinoma: Analysis of a Phase II Prospective Clinical Trial. Clin Cancer Res 2019;25:2174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohnsack O, Hoos A, Ludajic K. Adaptation and modification of the immune related response criteria (IRRC): IrRECIST. Journal of Clinical Oncology 2014;32. [Google Scholar]
- 21.Johnson K, Gomez A, Burton J, et al. Directional inconsistency between Response Evaluation Criteria in Solid Tumors (RECIST) time to progression and response speed and depth. Eur J Cancer 2019;109:196–203. [DOI] [PubMed] [Google Scholar]
- 22.Kurra V, Sullivan R, F. Gainor J, et al. Pseudoprogression in cancer immunotherapy: Rates, time course and patient outcomes. Journal of Clinical Oncology 2016;34:6580-. [Google Scholar]
- 23.Mulkey F, Theoret MR, Keegan P, Pazdur R, Sridhara R. Comparison of iRECIST versus RECIST V.1.1 in patients treated with an anti-PD-1 or PD-L1 antibody: pooled FDA analysis. Journal for immunotherapy of cancer 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dercle L, Connors DE, Tang Y, et al. Vol-PACT: A Foundation for the NIH Public-Private Partnership That Supports Sharing of Clinical Trial Data for the Development of Improved Imaging Biomarkers in Oncology. JCO Clin Cancer Inform 2018;2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


