Abstract
Background
Periodontitis is a highly prevalent, chronic inflammation that causes damage to the soft tissues and bones supporting the teeth. Conventional treatment is quadrant scaling and root planing (the second step of periodontal therapy), which comprises scaling and root planing of teeth in one quadrant of the mouth at a time, with the four different sessions separated by at least one week. Alternative protocols for anti‐infective periodontal therapy have been introduced to help enhance treatment outcomes: full‐mouth scaling (subgingival instrumentation of all quadrants within 24 hours), or full‐mouth disinfection (subgingival instrumentation of all quadrants in 24 hours plus adjunctive antiseptic). We use the older term 'scaling and root planing' (SRP) interchangeably with the newer term 'subgingival instrumentation' in this iteration of the review, which updates one originally published in 2008 and first updated in 2015.
Objectives
To evaluate the clinical effects of full‐mouth scaling or full‐mouth disinfection (within 24 hours) for the treatment of periodontitis compared to conventional quadrant subgingival instrumentation (over a series of visits at least one week apart) and to evaluate whether there was a difference in clinical effects between full‐mouth disinfection and full‐mouth scaling.
Search methods
An information specialist searched five databases up to 17 June 2021 and used additional search methods to identify published, unpublished and ongoing studies.
Selection criteria
We included randomised controlled trials (RCTs) lasting at least three months that evaluated full‐mouth scaling and root planing within 24 hours, with or without adjunctive use of an antiseptic, compared to conventional quadrant SRP (control). Participants had a clinical diagnosis of (chronic) periodontitis according to the International Classification of Periodontal Diseases from 1999. A new periodontitis classification was launched in 2018; however, we used the 1999 classification for inclusion or exclusion of studies, as most studies used it. We excluded studies of people with systemic disorders, taking antibiotics or with the older diagnosis of 'aggressive periodontitis'.
Data collection and analysis
Several review authors independently conducted data extraction and risk of bias assessment (based on randomisation method, allocation concealment, examiner blinding and completeness of follow‐up). Our primary outcomes were tooth loss and change in probing pocket depth (PPD); secondary outcomes were change in probing attachment (i.e. clinical attachment level (CAL)), bleeding on probing (BOP), adverse events and pocket closure (the number/proportion of sites with PPD of 4 mm or less after treatment). We followed Cochrane's methodological guidelines for data extraction and analysis.
Main results
We included 20 RCTs, with 944 participants, in this updated review. No studies assessed the primary outcome tooth loss. Thirteen trials compared full‐mouth scaling and root planing within 24 hours without the use of antiseptic (FMS) versus control, 13 trials compared full‐mouth scaling and root planing within 24 hours with adjunctive use of an antiseptic (FMD) versus control, and six trials compared FMS with FMD.
Of the 13 trials comparing FMS versus control, we assessed three at high risk of bias, six at low risk of bias and four at unclear risk of bias. We assessed our certainty about the evidence as low or very low for the outcomes in this comparison. There was no evidence for a benefit for FMS over control for change in PPD, gain in CAL or reduction in BOP at six to eight months (PPD: mean difference (MD) 0.03 mm, 95% confidence interval (CI) –0.14 to 0.20; 5 trials, 148 participants; CAL: MD 0.10 mm, 95% CI –0.05 to 0.26; 5 trials, 148 participants; BOP: MD 2.64%, 95% CI –8.81 to 14.09; 3 trials, 80 participants). There was evidence of heterogeneity for BOP (I² = 50%), but none for PPD and CAL.
Of the 13 trials comparing FMD versus control, we judged four at high risk of bias, one at low risk of bias and eight at unclear risk of bias. At six to eight months, there was no evidence for a benefit for FMD over control for change in PPD or CAL (PPD: MD 0.11 mm, 95% CI –0.04 to 0.27; 6 trials, 224 participants; low‐certainty evidence; CAL: 0.07 mm, 95% CI –0.11 to 0.24; 6 trials, 224 participants; low‐certainty evidence). The analyses found no evidence of a benefit for FMD over control for BOP (very low‐certainty evidence). There was no evidence of heterogeneity for PPD or CAL, but considerable evidence of heterogeneity for BOP, attributed to one study. There were no consistent differences in these outcomes between intervention and control (low‐ to very low‐certainty evidence).
Of the six trials comparing FMS and FMD, we judged two trials at high risk of bias, one at low risk of bias and three as unclear. At six to eight months, there was no evidence of a benefit of FMD over FMS for change in PPD or gain in CAL (PPD: MD –0.11 mm, 95% CI –0.30 to 0.07; P = 0.22; 4 trials, 112 participants; low‐certainty evidence; CAL: MD –0.05 mm, 95% CI –0.23 to –0.13; P = 0.58; 4 trials, 112 participants; low‐certainty evidence). There was no evidence of a difference between FMS and FMD for BOP at any time point (P = 0.98; 2 trials, 22 participants; low‐ to very low‐certainty evidence). There was evidence of heterogeneity for BOP (I² = 52%), but not for PPD or CAL.
Thirteen studies predefined adverse events as an outcome; three reported an event after FMD or FMS. The most important harm identified was an increase in body temperature.
We assessed the certainty of the evidence for most comparisons and outcomes as low because of design limitations leading to risk of bias, and the small number of trials and participants, leading to imprecision in the effect estimates.
Authors' conclusions
The inclusion of nine new RCTs in this updated review has not changed the conclusions of the previous version of the review. There is still no clear evidence that FMS or FMD approaches provide additional clinical benefit compared to conventional mechanical treatment for adult periodontitis. In practice, the decision to select one approach to non‐surgical periodontal therapy over another should include patient preference and the convenience of the treatment schedule.
Keywords: Adult; Humans; Anti-Infective Agents, Local; Anti-Infective Agents, Local/therapeutic use; Chronic Periodontitis; Chronic Periodontitis/drug therapy; Tooth Loss
Plain language summary
Treating all teeth (full mouth) within 24 hours for gum disease (periodontitis) in adults
Background
Long‐lasting gum disease (periodontitis) is a common chronic inflammatory disease that causes damage to soft tissues (gums) and bone around teeth, and can result in tooth loss. Non‐surgical treatments are used to stop and control the disease. These are based on 'subgingival instrumentation', that is, the mechanical removal of bacteria below the gums from the infected root surfaces of the teeth.
Conventional treatment is carried out in two to four sessions over several weeks, scaling a different section (or 'quadrant') of the mouth each time. This has traditionally been known as 'scaling and root planing' (SRP). An alternative approach is to treat the whole mouth within 24 hours in one or two sessions (known as full‐mouth scaling (FMS)). When an antiseptic agent (like chlorhexidine) is added to FMS, the intervention is called full‐mouth disinfection (FMD). The rationale for using these full‐mouth approaches is to reduce the likelihood of re‐infection in already treated sites.
Review question
This review, produced within Cochrane Oral Health, is the second update of one we originally published in 2008. It evaluates the effectiveness of full‐mouth treatments within 24 hours (FMS and FMD) compared to conventional treatment over a number of weeks, and whether there is a difference between FMS and FMD. The evidence is current up to June 2021.
Study characteristics
The included studies were randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) that evaluated a full‐mouth approach to subgingival instrumentation, with at least three months of monitoring (follow‐up). Both FMS and FMD were compared to conventional quadrant SRP (control). Participants had a clinical diagnosis of chronic periodontitis and we excluded studies of people with aggressive periodontitis, systemic disorders (affecting other part of the body) or who were taking antibiotics.
We included nine new studies in this update and we excluded one trial that had been included in the previous version of the review. In total, the review now includes 20 studies that involved 944 participants.
Key results
Treatment effects of FMS and FMD are modest and there are no clear implications for periodontal care. Neither treatment was superior to the usual treatment of scaling and root planing a quarter of the mouth at a time.
The most important harm identified was an increased body temperature after FMS or FMD treatments, reported in three out of 13 studies.
In practice, the decision to select one approach over another will be based on preference and convenience for patient and dentist.
Certainty of the evidence
Our confidence in the results is low for most comparisons and outcomes, due to the small number of studies and participants involved, and limitations in study designs. The addition of nine studies has not changed the findings of our previous version of this review.
Summary of findings
Summary of findings 1. Full‐mouth scaling compared to control for periodontitis in adults.
| FMS compared to control for periodontitis in adults | ||||||
| Population: adults with periodontitis Setting: university dental departments Intervention: FMS Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with FMS | |||||
| Tooth loss | None of the studies comparing FMS vs control reported tooth loss. | |||||
| Change in PPD: whole mouth, single‐ and multi‐rooted teeth Follow‐up: 6–8 months | The mean change in PPD was 0.27 mm to 1.80 mm | MD 0.03 mm higher (0.14 lower to 0.20 higher) | — | 148 (5 RCTs) | ⊕⊕⊝⊝ Lowa | Similar results at 3–4 months. Subgroup analyses of 6‐ to 8‐month data were undertaken for:
See Table 2; Table 3; Table 4. There was no consistent evidence of a benefit for FMS. |
| Change in CAL: whole mouth, single‐ and multi‐rooted teeth Follow‐up: 6–8 months | The mean change in CAL was 0.19 mm to 1.10 mm | MD 0.1 mm higher (0.05 lower to 0.26 higher) | — | 148 (5 RCTs) | ⊕⊕⊝⊝ Lowa | |
| Change in BOP: whole mouth, single‐ and multi‐rooted teeth Follow‐up: 6–8 months | The mean change in BOP was 23% to 58% | MD 2.64% higher (8.81 lower to 14.09 higher) | — | 80 (3 RCTs) | ⊕⊝⊝⊝ Very lowb,c,d | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BOP: bleeding on probing; CAL: clinical attachment level; CI: confidence interval; FMS: full‐mouth scaling; MD: mean difference; mm: millimetres; PPD: probing pocket depth; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for risk of bias (three trials at high risk of detection bias and one at unknown risk of bias). bDowngraded one level for inconsistency ‐ some concern about unexplained heterogeneity. cDowngraded two levels for risk of bias (two trials at high risk of detection bias). dDowngraded one level for design limitations and imprecision.
1. Full‐mouth scaling versus control: change in probing pocket depth.
| Tooth type: single‐ or multi‐rooted, or both | Baseline pocket depth (mm) | Time (months) | Number of studies (participants) | Mean difference (95% CI) (random‐effects meta‐analysis) | Heterogeneity |
| Both | 5–6 | 3/4 | 6 (177) | –0.05 (–0.19, 0.09); P = 0.47 | P = 0.97; I² = 0% |
| Both | > 6 | 3/4 | 7 (193) | –0.04 (–0.29, 0.21); P = 0.77 | P = 0.66; I² = 0% |
| Both | 5–6 | 6/8 | 3 (88) | –0.14 (–0.45, 0.18); P = 0.39 | P = 0.85; I² = 0% |
| Both | > 6 | 6/8 | 4 (104) | –0.16 (–0.60, 0.28); P = 0.48 | P = 0.41; I² = 0% |
| Single‐rooted | 5–6 | 3/4 | 1 (16) | 0.63 (0.29, 0.97); P = 0.0002 | Not applicable |
| Single‐rooted | > 6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Single‐rooted | 5–6 | 6/8 | 3 (69) | 0.16 (–0.01, 0.32); P = 0.06 | P = 0.89; I² = 0% |
| Single‐rooted | > 6 | 6/8 | 2 (53) | 0.26 (–0.21, 0.73); P = 0.27 | P = 0.64; I² = 0% |
| Multi‐rooted | 5–6 | 3/4 | 1 (16) | 1.00 (0.41, 1.59); P = 0.0008 | Not applicable |
| Multi‐rooted | > 6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Multi‐rooted | 5–6 | 6/8 | 3 (69) | 0.21 (–0.14, 0.55); P = 0.24 | P = 0.06; I² = 64% |
| Multi‐rooted | > 6 | 6/8 | 2 (53) | 0.18 (–0.26, 0.62); P = 0.42 | P = 0.42; I² = 0% |
CI: confidence interval.
2. Full‐mouth scaling versus control: change in clinical attachment level.
| Tooth type: single‐ or multi‐rooted, or both | Baseline pocket depth (mm) | Time (months) | Number of studies (participants) | Mean difference (95% CI) (random‐effects meta‐analysis) | Heterogeneity |
| Both | 5–6 | 3/4 | 4 (111) | 0.02 (–0.19, 0.23); P = 0.85 | P = 0.90; I² = 0% |
| Both | > 6 | 3/4 | 5 (127) | 0.09 (–0.22, 0.41); P = 0.57 | P = 1.00; I² = 0% |
| Both | 5–6 | 6/8 | 3 (89) | 0.22 (–0.05, 0.49); P = 0.11 | P = 0.87; I² = 0% |
| Both | > 6 | 6/8 | 4 (105) | 0.05 (–0.64, 0.74); P = 0.89 | P = 0.005; I² = 77% |
| Single‐rooted | 5–6 | 3/4 | 1 (16) | 0.41 (–0.00, 0.82); P = 0.05 | Not applicable |
| Single‐rooted | > 6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Single‐rooted | 5–6 | 6/8 | 2 (40) | 0.04 (–0.19, 0.27); P = 0.71 | P = 0.50; I² = 0% |
| Single‐rooted | > 6 | 6/8 | 1 (24) | 0.47 (–0.37, 1.31); P = 0.27 | Not applicable |
| Multi‐rooted | 5–6 | 3/4 | 1 (16) | 1.11 (0.45, 1.77); P = 0.0009 | Not applicable |
| Multi‐rooted | > 6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Multi‐rooted | 5–6 | 6/8 | 2 (40) | 0.00 (–0.34, 0.34); P = 1.00 | P = 0.19; I² = 41% |
| Multi‐rooted | > 6 | 6/8 | 1 (24) | 0.38 (–0.28, 1.04); P = 0.26 | Not applicable |
CI: confidence interval.
3. Full‐mouth scaling versus control: change in bleeding on probing.
| Tooth type: single‐ or multi‐rooted, or both | Baseline pocket depth (mm) | Time (months) | Number of studies (participants) | Mean difference (95% CI) (random‐effects meta‐analysis) | Heterogeneity |
| Both | 5–6 | 3/4 | 3 (61) | –8.05 (–30.25, 14.16); P = 0.48 | P = 0.02; I² = 80% |
| Both | > 6 | 3/4 | 4 (77) | –0.33 (–7.70, 7.04); P = 0.93 | P = 0.51; I² = 0% |
| Both | 5–6 | 6/8 | 1 (20) | –6.10 (–24.12, 11.92); P = 0.51 | Not applicable |
| Both | > 6 | 6/8 | 2 (36) | 10.22 (–0.59, 21.03); P = 0.06 | P = 0.92; I² = 0% |
| Single‐rooted | 5–6 | 3/4 | 1 (16) | 3.00 (–2.43, 8.43); P = 0.28 | Not applicable |
| Single‐rooted | > 6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Single‐rooted | 5–6 | 6/8 | 2 (45) | –3.06 (–10.47, 4.35); P = 0.42 | P = 0.27; I² = 18% |
| Single‐rooted | > 6 | 6/8 | 1 (29) | –4.00 (–20.17, 12.17); P = 0.63 | Not applicable |
| Multi‐rooted | 5–6 | 3/4 | 1 (16) | 7.00 (4.54, 9.46); P < 0.00001 | Not applicable |
| Multi‐rooted | > 6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Multi‐rooted | 5–6 | 6/8 | 2 (45) | 2.38 (–2.95, 7.71); P = 0.38 | P = 0.50; I² = 0% |
| Multi‐rooted | > 6 | 6/8 | 1 (29) | –4.00 (–23.29, 15.29); P = 0.68 | Not applicable |
CI: confidence interval.
Summary of findings 2. Full‐mouth disinfection compared to control for periodontitis in adults.
| FMD compared to control for periodontitis in adults | ||||||
| Population: adults with periodontitis Setting: university dental departments Intervention: FMD Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with FMD | |||||
| Tooth loss | None of the studies comparing FMD vs control reported tooth loss. | |||||
| Change in PPD: whole mouth, single‐ and multi‐rooted teeth Follow‐up: 6–8 months | The mean change in PPD was 0.26 mm to 1.54 mm | MD 0.11 mm higher (0.04 lower to 0.27 higher) | — | 214 (6 RCTs) | ⊕⊕⊝⊝ Lowa,b | Similar results were found at 3–4 months. Subgroup analyses were undertaken for:
See Table 6; Table 7; Table 8. There was no consistent evidence of a benefit for FMD. |
| Change in CAL: whole mouth, single‐ and multi‐rooted teeth Follow‐up: 6–8 months | The mean change in CAL was 0.16 mm to 1.05 mm | MD 0.07 mm higher (0.11 lower to 0.24 higher) | — | 214 (6 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| Change in BOP: whole mouth, single‐ and multi‐rooted teeth Follow‐up: 6–8 months | The mean change in BOP was 3.11% to 49.18% | MD 9.54% higher (2.24 lower to 21.32 higher) | — | 92 (4 RCTs) | ⊕⊝⊝⊝ Very lowb,c,d | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BOP: bleeding on probing; CAL: clinical attachment level; CI: confidence interval; FMD: full‐mouth disinfection; MD: mean difference; mm: millimetres; PPD: probing pocket depth; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for risk of bias (two trials at high risk of detection bias and three trials at unknown risk of bias). bDowngraded one level for design limitations and imprecision. cDowngraded one level for inconsistency – some concern with unexplained heterogeneity. dDowngraded one level for risk of bias (one trial at high risk of detection bias and two at unknown risk of bias).
4. Full‐mouth disinfection versus control: change in probing pocket depth.
| Tooth type: single‐ or multi‐rooted, or both | Baseline pocket depth (mm) | Time (months) | Number of studies (participants) | Mean difference (95% CI) (random‐effects meta‐analysis) | Heterogeneity |
| Both | 5–6 | 3/4 | 2 (56) | 0.50 (–0.33, 1.33); P = 0.24 | P = 0.02; I² = 83% |
| Both | > 6 | 3/4 | 3 (72) | 0.32 (–1.22, 1.85); P = 0.69 | P < 0.0001; I² = 91% |
| Both | 5–6 | 6/8 | 1 (28) | 0.88 (0.20, 1.56); P = 0.01 | Not applicable |
| Both | > 6 | 6/8 | 2 (44) | –0.10 (–0.47, 0.26); P = 0.58 | P = 0.46; I² = 0% |
| Single‐rooted | 5–6 | 3/4 | 3 (50) | 0.28 (–0.59, 1.15); P = 0.52 | P = 0.0005; I² = 87% |
| Single‐rooted | > 6 | 3/4 | 2 (34) | 1.28 (–0.48, 3.04); P = 0.15 | P = 0.03; I² = 78% |
| Single‐rooted | 5–6 | 6/8 | 5 (103) | 0.41 (0.11, 0.70); P = 0.006 | P = 0.01; I² = 70% |
| Single‐rooted | > 6 | 6/8 | 4 (87) | 0.78 (–0.01, 1.57); P = 0.05 | P = 0.03; I² = 67% |
| Multi‐rooted | 5–6 | 3/4 | 3 (50) | 0.18 (–0.79, 1.15); P = 0.72 | P = 0.003; I² = 83% |
| Multi‐rooted | > 6 | 3/4 | 2 (34) | 1.28 (0.44, 2.11); P = 0.003 | P = 0.92; I² = 0% |
| Multi‐rooted | 5–6 | 6/8 | 5 (103) | 0.21 (–0.12, 0.53); P = 0.21 | P = 0.03; I² = 62% |
| Multi‐rooted | > 6 | 6/8 | 4 (87) | 0.56 (–0.23, 1.34); P = 0.16 | P = 0.04; I² = 65% |
CI: confidence interval.
5. Full‐mouth disinfection versus control: change in clinical attachment level.
| Tooth type: single‐ or multi‐rooted, or both | Baseline pocket depth (mm) | Time (months) | Number of studies (participants) | Mean difference (95% CI) (random‐effects meta‐analysis) | Heterogeneity |
| Both | 5–6 | 3/4 | 1 (28) | 0.18 (–0.21, 0.57); P = 0.37 | Not applicable |
| Both | > 6 | 3/4 | 2 (44) | –0.39 (–1.32, 0.54); P = 0.42 | (P = 0.06); I² = 71% |
| Both | 5–6 | 6/8 | 0 (0) | Not estimable | Not applicable |
| Both | > 6 | 6/8 | 1 (16) | –0.16 (–0.41, 0.09); P = 0.20 | Not applicable |
| Single‐rooted | 5–6 | 3/4 | 2 (40) | 0.08 (–0.87, 1.04); P = 0.86 | (P = 0.04); I² = 75% |
| Single‐rooted | > 6 | 3/4 | 1 (24) | 1.90 (0.73, 3.07); P = 0.001 | Not applicable |
| Single‐rooted | 5–6 | 6/8 | 3 (64) | 0.14 (0.00, 0.28); P = 0.05 | (P = 0.48); I² = 0% |
| Single‐rooted | > 6 | 6/8 | 2 (48) | 0.72 (–0.94, 2.37); P = 0.40 | (P = 0.03); I² = 79% |
| Multi‐rooted | 5–6 | 3/4 | 2 (40) | 0.27 (–1.21, 1.75); P = 0.72 | (P = 0.001); I² = 90% |
| Multi‐rooted | > 6 | 3/4 | 1 (24) | 1.30 (0.20, 2.40); P = 0.02 | Not applicable |
| Multi‐rooted | 5–6 | 6/8 | 3 (64) | 0.12 (–0.17, 0.41); P = 0.43 | (P = 0.07); I² = 62% |
| Multi‐rooted | > 6 | 6/8 | 2 (48) | 0.52 (–1.30, 2.34); P = 0.57 | (P = 0.005); I² = 87% |
CI: confidence interval.
6. Full‐mouth disinfection versus control: change in bleeding on probing.
| Tooth type: single‐ or multi‐rooted, or both | Baseline pocket depth (mm) | Time (months) | Number of studies (participants) | Mean difference (95% CI) (random‐effects meta‐analysis) | Heterogeneity |
| Both | 5–6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Both | > 6 | 3/4 | 1 (16) | –5.00 (–11.70, 1.70); P = 0.14 | Not applicable |
| Both | 5–6 | 6/8 | 0 (0) | Not estimable | Not applicable |
| Both | > 6 | 6/8 | 1 (16) | 2.00 (–7.83, 11.83); P = 0.69 | Not applicable |
| Single‐rooted | 5–6 | 3/4 | 1 (16) | 5.00 (1.97, 8.03); P = 0.001 | Not applicable |
| Single‐rooted | > 6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Single‐rooted | 5–6 | 6/8 | 2 (45) | 4.83 (1.86, 7.80); P = 0.001 | P = 0.60; I² = 0% |
| Single‐rooted | > 6 | 6/8 | 1 (29) | 14.00 (–2.17, 30.17); P = 0.09 | Not applicable |
| Multi‐rooted | 5–6 | 3/4 | 1 (16) | 2.00 (0.38, 3.62); P = 0.02 | Not applicable |
| Multi‐rooted | > 6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Multi‐rooted | 5–6 | 6/8 | 2 (45) | 8.72 (–2.61, 20.06); P = 0.13 | P = 0.22; I² = 34% |
| Multi‐rooted | > 6 | 6/8 | 1 (29) | –8.00 (–25.00, 9.00); P = 0.36 | Not applicable |
CI: confidence interval.
Summary of findings 3. Full‐mouth scaling compared to full‐mouth disinfection for periodontitis in adults.
| FMS compared to FMD for periodontitis in adults | ||||||
| Population: adults with periodontitis Setting: university dental departments Intervention: FMS Comparison: FMD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with FMD | Risk with FMS | |||||
| Tooth loss | None of the studies comparing FMS vs FMD reported tooth loss. | |||||
| Change in PPD: whole mouth, single‐ and multi‐rooted teeth Follow‐up: 6–8 months | The mean change in PPD was 0.57 mm to 1.73 mm | MD 0.11 mm lower (0.3 lower to 0.07 higher) | — | 112 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | Similar results were found at 3–4 months. Subgroup analyses were undertaken for:
See Table 10; Table 11; Table 12. There was no consistent evidence of a benefit for either intervention. |
| Change in CAL: whole mouth, single‐ and multi‐rooted teeth Follow‐up: 6–8 months | The mean change in CAL was 0.43 mm to 1.07 mm | MD 0.05 mm lower (0.23 lower to 0.13 higher) | — | 112 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| Change in BOP: whole mouth, single‐ and multi‐rooted teeth Follow‐up: 6–8 months | The mean change in BOP was 23% to 56.4% | MD 0.2% lower (13.27 lower to 12.87 higher) | — | 42 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,c,d | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BOP: bleeding on probing; CAL: clinical attachment level; CI: confidence interval; FMD: full mouth disinfection; FMS: full mouth scaling; MD: mean difference; mm: millimetres; PPD: probing pocket depth; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for design limitations and imprecision. bDowngraded one level for risk of bias (two trials at high risk of detection bias and one trial at unknown risk of bias). cDowngraded one level for moderate imprecision. dDowngraded one level for risk of bias (one trial at unknown risk of detection bias).
7. Full‐mouth scaling versus full‐mouth disinfection: change in probing pocket depth.
| Tooth type: single‐ or multi‐rooted, or both | Baseline pocket depth (mm) | Time (months) | Number of studies (participants) | Mean difference (95% CI) (random‐effects meta‐analysis) | Heterogeneity |
| Both | 5–6 | 3/4 | 2 (57) | –0.52 (–1.34, 0.30); P = 0.22 | P = 0.01; I² = 84% |
| Both | > 6 | 3/4 | 3 (75) | –0.05 (–1.84, 1.73); P = 0.95 | P < 0.00001; I² = 94% |
| Both | 5–6 | 6/8 | 1 (30) | –0.88 (–1.53, –0.23); P = 0.008 | Not applicable |
| Both | > 6 | 6/8 | 2 (48) | –0.50 (–2.00, 0.99); P = 0.51 | P = 0.03; I² = 80% |
| Single‐rooted | 5–6 | 3/4 | 1 (18) | 0.95 (0.65, 1.25); P < 0.00001 | Not applicable |
| Single‐rooted | > 6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Single‐rooted | 5–6 | 6/8 | 3 (70) | –0.10 (–0.40, 0.20); P = 0.52 | P = 0.02; I² = 76% |
| Single‐rooted | > 6 | 6/8 | 2 (52) | –0.03 (–0.48, 0.41); P = 0.88 | P = 0.55; I² = 0% |
| Multi‐rooted | 5–6 | 3/4 | 1 (18) | 1.37 (0.81, 1.93); P < 0.00001 | Not applicable |
| Multi‐rooted | > 6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Multi‐rooted | 5–6 | 6/8 | 3 (70) | 0.04 (–0.16, 0.25); P = 0.68 | P = 0.63; I² = 0% |
| Multi‐rooted | > 6 | 6/8 | 2 (52) | 0.05 (–0.38, 0.47); P = 0.83 | P = 0.29; I² = 9% |
CI: confidence interval.
8. Full‐mouth scaling versus full‐mouth disinfection: change in clinical attachment level.
| Tooth type: single‐ or multi‐rooted, or both | Baseline pocket depth (mm) | Time (months) | Number of studies (participants) | Mean difference (95% CI) (random‐effects meta‐analysis) | Heterogeneity |
| Both | 5–6 | 3/4 | 1 (27) | –0.05 (–0.50, 0.40); P = 0.83 | Not applicable |
| Both | > 6 | 3/4 | 2 (45) | 0.41 (–0.45, 1.27); P = 0.35 | P = 0.17; I² = 47% |
| Both | 5–6 | 6/8 | 0 (0) | Not estimable | Not applicable |
| Both | > 6 | 6/8 | 1 (18) | –0.51 (–1.24, 0.22); P = 0.17 | Not applicable |
| Single‐rooted | 5–6 | 3/4 | 1 (18) | 0.71 (0.31, 1.11); P = 0.0005 | Not applicable |
| Single‐rooted | > 6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Single‐rooted | 5–6 | 6/8 | 2 (42) | –0.09 (–0.30, 0.11); P = 0.38 | P = 0.44; I² = 0% |
| Single‐rooted | > 6 | 6/8 | 1 (24) | 0.56 (–0.37, 1.49); P = 0.24 | Not applicable |
| Multi‐rooted | 5–6 | 3/4 | 1 (18) | 1.53 (0.89, 2.17); P < 0.00001 | Not applicable |
| Multi‐rooted | > 6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Multi‐rooted | 5–6 | 6/8 | 2 (42) | –0.02 (–0.53, 0.49); P = 0.93 | P = 0.06; I² = 73% |
| Multi‐rooted | > 6 | 6/8 | 1 (24) | 0.74 (0.17, 1.31); P = 0.01 | Not applicable |
CI: confidence interval.
9. Full‐mouth scaling versus full‐mouth disinfection: change in bleeding on probing.
| Tooth type: single‐ or multi‐rooted, or both | Baseline pocket depth (mm) | Time (months) | Number of studies (participants) | Mean difference (95% CI) (random‐effects meta‐analysis) | Heterogeneity |
| Both | 5–6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Both | > 6 | 3/4 | 1 (18) | 7.00 (0.43, 13.57); P = 0.04 | Not applicable |
| Both | 5–6 | 6/8 | 0 (0) | Not estimable | Not applicable |
| Both | > 6 | 6/8 | 1 (18) | 8.00 (1.18, 14.82); P = 0.02 | Not applicable |
| Single‐rooted | 5–6 | 3/4 | 1 (18) | 2.00 (–3.27, 7.27); P = 0.46 | Not applicable |
| Single‐rooted | > 6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Single‐rooted | 5–6 | 6/8 | 2 (46) | –6.69 (–12.18, –1.19); P = 0.02 | P = 0.45; I² = 0% |
| Single‐rooted | > 6 | 6/8 | 1 (28) | –18.00 (–30.83, –5.17); P = 0.006 | Not applicable |
| Multi‐rooted | 5–6 | 3/4 | 1 (18) | 5.00 (2.93, 7.07); P < 0.00001 | Not applicable |
| Multi‐rooted | > 6 | 3/4 | 0 (0) | Not estimable | Not applicable |
| Multi‐rooted | 5–6 | 6/8 | 2 (46) | –4.16 (–8.72, 0.39); P = 0.07 | P = 0.68; I² = 0% |
| Multi‐rooted | > 6 | 6/8 | 1 (28) | 4.00 (–13.37, 21.37); P = 0.65 | Not applicable |
CI: confidence interval.
Background
Description of the condition
Periodontitis is understood as a chronic multi‐factorial inflammatory disease associated with dysbiotic dental plaque biofilm, affecting the tissues surrounding the teeth characterised by clinical attachment loss (CAL) and radiographically assessed alveolar bone loss, presence of periodontal pocketing and gingival bleeding (Papapanou 2018; Sanz 2020a). Some 10% to 12% of the population have severe periodontitis (Kassebaum 2017), though mild to moderate periodontitis affects the majority of adults (AAP 2005; Billings 2018; Oliver 1991).
Periodontitis is seen as resulting from a complex interplay of bacterial infection and host response, modified by behavioural and systemic risk factors (Papapanou 2018). In people with periodontitis, key pathogens such as Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia have been found to colonise nearly all niches in the oral cavity, such as the tongue, mucosa, saliva and tonsils (Beikler 2004). Translocation of these pathogens may occur rapidly and a recently instrumented deep pocket might be re‐colonised from remaining untreated pockets or from other intraoral niches before a less pathogenic ecosystem can be established.
Description of the intervention
Conventional treatment involves subgingival instrumentation, formerly known as scaling and root planing (SRP), which is performed at several appointments over a period of weeks. This is now understood as second step of periodontal therapy following step one (Sanz 2020a). The first step of therapy targets adequate self‐performed oral hygiene practices as well as a professional mechanical removal of supragingival plaque and calculus and elimination of local retentive factors (Sanz 2020a). There is considerable evidence to support SRP as an effective procedure for the treatment of infectious periodontal diseases (Heitz‐Mayfield 2002; Sanz 2020a; van der Weijden 2002), provided that the procedures included in the first step of therapy have been successfully implemented (Sanz 2020a). However, based on the risk of the recolonisation hypothesis, a full‐mouth disinfection (FMD) approach, which consists of SRP of all pockets in two visits within 24 hours, in combination with adjunctive chlorhexidine treatments of all oral niches, has been proposed (Quirynen 2006). This was first evaluated in a series of studies by the same research group (Bollen 1998; Mongardini 1999; Vandekerckhove 1996). A later report indicated that this full‐mouth treatment approach resulted in superior clinical outcomes and microbiological effects than conventional quadrant SRP (control), regardless of the adjunctive use of chlorhexidine (Quirynen 2000). However, more‐recent studies from other research centres have not been able to demonstrate an advantage of full‐mouth scaling (FMS) within 24 hours over the control regimen (Afacan 2020; Apatzidou 2004; Babaloo 2018; Del Peloso 2008; Fonseca 2015; Graziani 2015; Jervøe‐Storm 2006; Koshy 2005; Loggner Graff 2009; Pontillo 2018; Predin 2014; Roman‐Torres 2018; Santuchi 2015; Soares 2015; Swierkot 2009; Wennström 2005; Zanatta 2006; Zijnge 2010).
How the intervention might work
It is thought that the comprehensive reduction of bacteria from several oral niches by application of antiseptics within 24 hours will reduce the recolonisation of already treated sites leading to reductions of probing pocket depth (PPD) and bleeding on probing (BOP), and gains in clinical attachment.
Why it is important to do this review
This is the second update of a Cochrane Review first published in 2008 (Eberhard 2008a). Three systematic reviews were conducted to assess the evidence for full‐mouth treatment modalities (Eberhard 2008b; Farman 2008; Lang 2008). A review article was published by the advocates of the full‐mouth treatment concept (Teughels 2009), who disagreed with the results of these systematic reviews. Since then, more reviews have been published (Eberhard 2015; Fang 2016; Pockpa 2018; Suvan 2020; Zhao 2020). Our present systematic review is an update from 2015 (Eberhard 2015), and includes the most recent studies on this topic to ensure the evidence base for this important clinical question is up to date. The reason for the second update is the increased number of publications since 2015, more than doubling the number of included participants.
Objectives
To evaluate the clinical effects of full‐mouth scaling or full‐mouth disinfection (within 24 hours) for the treatment of periodontitis compared to conventional quadrant subgingival instrumentation (over a series of visits at least one week apart) and to evaluate whether there was a difference in clinical effects between full‐mouth disinfection and full‐mouth scaling.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) with at least three months of follow‐up. We excluded trials with a split‐mouth or cross‐over design due to potential carryover effects.
Types of participants
Although the new classification of periodontal diseases no longer distinguishes between chronic or aggressive forms of periodontitis (Papapanou 2018), this will take some time to filter through to research studies, and so we retained our inclusion criteria of people with a clinical diagnosis of 'chronic periodontitis' based on the International Classification of Periodontal Diseases (Armitage 1999), and excluded studies of people with 'aggressive periodontitis'. We also excluded studies of participants with systemic disorders, and people taking antibiotics.
Types of interventions
Full‐mouth scaling (FMS), comprising subgingival instrumentation of all quadrants within 24 hours.
Full‐mouth disinfection (FMD), comprising subgingival instrumentation of all quadrants within 24 hours along with adjunctive antiseptic treatments (such as chlorhexidine), which could include rinsing, pocket irrigation, spraying of the tonsils and tongue brushing.
Quadrant subgingival instrumentation (SRP) (control), comprising SRP of each quadrant at a separate session, each session separated by an interval of at least one week.
The comparisons were: FMS versus control, FMD versus control and FMS versus FMD.
Types of outcome measures
Primary outcomes
Tooth loss.
Change in probing pocket depth (PPD) after three to four months and six to eight months.
Secondary outcomes
Change in clinical attachment level (CAL) after three to four months and six to eight months.
Change in bleeding on probing (BOP) after three to four months and six to eight months.
Adverse events.
Pocket closure (number/proportion of sites with PPD of 4 mm or less after treatment).
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for RCTs and controlled clinical trials. There were no language, publication year or publication status restrictions:
Cochrane Oral Health's Trials Register (searched 17 June 2021) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 5) in the Cochrane Library (searched 17 June 2021) (Appendix 2);
MEDLINE Ovid (1946 to 17 June 2021) (Appendix 3);
Embase Ovid (1980 to 17 June 2021) (Appendix 4);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 17 June 2021) (Appendix 5).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. A filter to limit the search to RCTs was not used as the yield was low.
Searching other resources
We searched the following trial registries for ongoing studies (see Appendix 6 for the search strategy):
US National Institutes of Health Trials Register ClinicalTrials.gov (clinicaltrials.gov) (to 17 June 2021);
World Health Organization Clinical Trials Registry Platform (apps.who.int/trialsearch/default.aspx) (to 17 June 2021).
Incomplete information and ambiguous data were researched further by contacting the author or researcher (or both) responsible for the study directly. For unpublished material, we searched the conference proceedings of the International Association for Dental Research (IADR), American Academy of Periodontology (AAP) and European Federation of Periodontology (EFP) up to June 2021. We sought relevant 'in press' manuscripts from the Journal of Clinical Periodontology, Journal of Periodontology, Journal of Dental Research and Journal of Periodontal Research and by contact with the journal editors.
We handsearched the following journals:
Journal of Periodontology (1980 to 17 June 2021);
Journal of Clinical Periodontology (1980 to 17 June 2021);
Journal of Periodontal Research (1980 to 17 June 2021).
We searched the reference lists of included studies and relevant systematic reviews for further studies.
We checked that none of the included studies in this review were retracted due to error or fraud.
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Titles and abstracts were downloaded to EndNote 9 software. The search was designed to be sensitive and include controlled clinical trials; these were filtered out early in the selection process if they were not randomised. For this update, three review authors (PS, JE and SJ), independently and in duplicate, carried out the selection of papers and made decisions about eligibility. They resolved any disagreements by discussion. We recorded reasons for studies that were rejected at full‐text stage in the Characteristics of excluded studies table.
Data extraction and management
Four review authors (PS, HW, JE and SJ) extracted and entered data into a computer. Review authors who were authors on an included study did not extract data from that study. We extracted the following data.
General study characteristics: year of the study, country of origin, authors, funding, university/private practice based.
Specific trial characteristics: population, diagnosis of chronic periodontitis, gender, age, severity of periodontal disease, inclusion and exclusion criteria not already stated.
Primary outcomes: tooth loss, PPD (after three and six months if available, otherwise the nearest assessment time point evaluation).
Secondary outcomes: CAL and BOP before and after different treatment modalities (after three and six months if available, otherwise the nearest assessment time point evaluation), and adverse events.
Assessment of risk of bias in included studies
Three review authors (PS, HW and SJ) assessed the methodological quality of included studies mainly using the risk of bias components shown to affect study outcomes, including method of randomisation, allocation concealment and blinding of examiners. We also examined completeness of outcome reporting, selective outcome reporting and other potential threats to validity. Risk of bias was used in sensitivity analyses to test the robustness of the conclusions but was not used to exclude studies qualifying for the review. We used the definitions of risk of bias (RoB 1 tool) categories from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (see Appendix 7). Review authors who were authors on an included study did not assess the risk of bias for that study.
To examine overall risk of bias for each study, we used all the domains of risk of bias. If all domains were at low risk, the study was deemed at low risk of bias. If any domains had an unclear risk, then the study was classed as having an unclear risk of bias; however, if one or more domains was assessed at a high risk of bias, then so was the study. We did not score performance bias of the operator as it is impossible to blind the therapist as they perform quadrant‐wise or full‐mouth instrumentation.
Measures of treatment effect
We used change scores for the secondary outcomes as this is how the data were generally presented in these trials. If studies presented only post‐scores or covariance adjusted means, we included these and conducted a subgroup analysis for the different outcome measures. For continuous outcomes, we used mean differences (MD) and 95% confidence intervals (CI) to summarise the data for each group. For dichotomous outcomes, we expressed the estimates of effect of an intervention as risk ratios with 95% CI.
Unit of analysis issues
Whole‐mouth, single‐rooted teeth and multi‐rooted teeth outcomes were the basis for data analysis, and we calculated means for all the primary and secondary outcomes.
Dealing with missing data
We calculated missing standard deviations using the methods in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
Prior to each meta‐analysis, we assessed heterogeneity by inspection of a graphical display of the estimated treatment effects from trials, along with Cochran's test for heterogeneity, and I² statistics.
Assessment of reporting biases
We considered the different types of reporting bias that might have been present in this review. If there had been more than 10 studies included in a meta‐analysis, we would have created a funnel plot to detect possible publication bias, although an asymmetrical funnel plot may be due to other factors. However, no single comparison of the present review included more than 10 studies.
Data synthesis
Where there were studies of similar comparisons reporting the same outcome measures, we performed a meta‐analysis. We combined risk ratios for dichotomous data, and MDs for continuous data, using the random‐effects model.
We categorised teeth into the following groups for the meta‐analysis, as these categories are thought to have clinical relevance: whole mouth (all teeth), teeth that had moderate pocket depth at baseline and teeth that had deep pocket depth at baseline. These analyses were repeated for single‐rooted and multi‐rooted teeth separately for all outcomes, and for two outcome assessment times: three to four months and six to eight months after treatment. Based on current treatment concepts, we categorised the pocket depth of 4 mm to 6 mm as moderate and 7 mm or more as deep. This is described in more detail for each study in the Results section.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses for different outcome measures (post‐treatment, change, covariance adjusted). The following factors were recorded to assess the clinical heterogeneity of outcomes across studies.
Plaque levels.
Time allowed for treatment.
Age of participants.
Initial probing depth.
Smoking status.
Risk of bias.
There were insufficient studies in any one comparison to use subgroup analyses to investigate any clinical heterogeneity.
Sensitivity analysis
We conducted sensitivity analyses by analysing only studies assessed at low risk of bias. We had also planned, if appropriate, to conduct sensitivity analyses by excluding unpublished literature.
Summary of findings and assessment of the certainty of the evidence
We summarised the findings of the main comparisons and outcomes in summary of findings tables. These included:
Tooth loss.
Change in PPD.
Change in CAL.
Change in BOP.
We assessed the certainty of the evidence, using GRADE criteria, as high, moderate, low or very low, explaining rationale for our judgements in footnotes. We assessed the GRADE domains using GRADEpro GDT (Schünemann 2020). We assessed the following domains.
Study design: any limitations in design or execution of the study.
Inconsistency: unexplained heterogeneity of the results.
Indirectness: indirect comparisons or differences in participants, interventions or outcomes of interest.
Imprecision: studies including small numbers of participants with wide CIs.
Publication bias: we had planned to assess this using funnel plots.
To make our decisions about the certainty of the evidence, we followed the same guideline as in the first update of this review in 2015 (Schünemann 2011), but used data from six to eight months of follow‐up for the summary of findings tables rather than data from three to four months (see Differences between protocol and review).
Results
Description of studies
Our original review, Eberhard 2008a, included seven studies (Apatzidou 2004; Jervøe‐Storm 2006; Koshy 2005; Mongardini 1999; Quirynen 2006; Wennström 2005; Zanatta 2006). In our first update, which was published in 2015 (Eberhard 2015), we added another five studies (Del Peloso 2008; Knöfler 2007; Swierkot 2009; Vandekerckhove 1996; Zijnge 2010). In the present (second) update, we reconsidered these studies and determined that one should be excluded (Knöfler 2007). We included nine new studies from the updated searches (Afacan 2020; Babaloo 2018; Fonseca 2015; Graziani 2015; Pontillo 2018; Predin 2014; Roman‐Torres 2018; Santuchi 2015; Soares 2015).
Results of the search
For this review update, we screened 391 titles and abstracts and rejected 370. We obtained the full text for 21 potentially eligible articles. Of these, we excluded 11 articles (10 studies). In addition, we excluded one article formerly awaiting assessment (Zhao 2005) and one formerly included RCT (Knöfler 2007); see information above under Description of studies. Therefore, in total, the review includes 20 trials reported in 23 articles (Mongardini 1999 was reported in three articles and Babaloo 2018 was reported in two). The 20 included trials are: Afacan 2020; Apatzidou 2004; Babaloo 2018; Del Peloso 2008; Fonseca 2015; Graziani 2015; Jervøe‐Storm 2006; Koshy 2005; Mongardini 1999; Pontillo 2018; Predin 2014; Quirynen 2006; Roman‐Torres 2018; Santuchi 2015; Soares 2015; Swierkot 2009; Vandekerckhove 1996; Wennström 2005; Zanatta 2006; Zijnge 2010. See Figure 1 for a diagrammatic representation of the selection of included studies.
1.
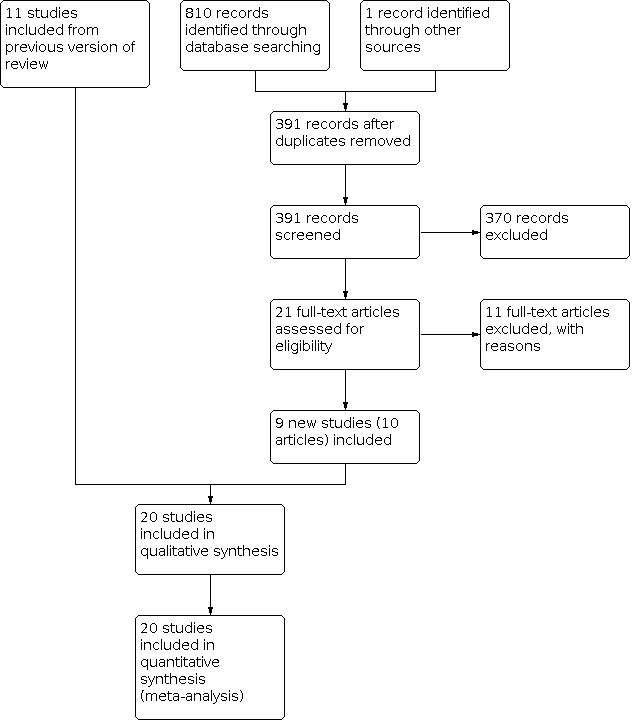
Study flow chart.
Included studies
Design
The 20 included studies were all parallel‐group RCTs of between three and 12 months' duration. Most studies had two or three arms. One paper relating to Mongardini 1999 referred to an FMS group ('FRp group') that was not randomised and so was not part of the review. In addition, four studies involved randomised arms that we did not include: Fonseca 2015 (one FMD arm using an alternative protocol and two SRP arms that included antiseptics); Pontillo 2018 (one control arm that consisted of people without periodontitis); Quirynen 2000 (two FMD arms using alternative antiseptic protocols); and Soares 2015 (one FMD arm and one SRP arm that included tongue scraping as part of the intervention).
Setting
Ten studies were conducted in Europe; seven in Brazil; and one each in Japan (Koshy 2005), Iran (Babaloo 2018), and Turkey (Afacan 2020).
Participants
In total, the 20 studies included 944 adults with periodontitis. Participants were aged 23 to 77 years (one study did not specify the age range (Graziani 2015)). Seven studies involved only non‐smokers (Afacan 2020; Del Peloso 2008; Koshy 2005; Pontillo 2018; Roman‐Torres 2018; Soares 2015; Zijnge 2010); 10 studies involved a mix of smokers and non‐smokers, and three studies were unclear about smoking status (Babaloo 2018; Santuchi 2015; Zanatta 2006). The number of participants enrolled in the included studies ranged from 10 to 230. Eleven trials had no dropouts and the other trials had dropouts ranging from 2% to 17%.
Interventions
Six studies included more than one comparison. The comparisons included in the trials were:
FMS versus control (13 trials): Afacan 2020; Apatzidou 2004; Del Peloso 2008; Fonseca 2015; Graziani 2015; Jervøe‐Storm 2006; Koshy 2005; Predin 2014; Quirynen 2006; Swierkot 2009; Wennström 2005; Zanatta 2006; Zijnge 2010;
FMD versus control (13 trials): Afacan 2020; Babaloo 2018; Fonseca 2015; Koshy 2005; Mongardini 1999; Pontillo 2018; Quirynen 2006; Roman‐Torres 2018; Santuchi 2015; Soares 2015; Swierkot 2009; Vandekerckhove 1996; Zanatta 2006;
FMS versus FMD (six trials): Afacan 2020; Fonseca 2015; Koshy 2005; Quirynen 2006; Swierkot 2009; Zanatta 2006.
Outcomes
Nineteen studies provided whole‐mouth data, with one study only providing partial‐mouth scores (Quirynen 2006).
None of the studies provided information on the primary outcome 'tooth loss'.
Thirteen studies provided full information on the primary outcome 'change in PPD', as well as on the secondary outcomes 'change in attachment loss' (CAL) and 'change in BOP'. Five studies reported only PPD and CAL (Afacan 2020; Fonseca 2015; Predin 2014; Roman‐Torres 2018; Santuchi 2015). One study reported only PPD and BOP (Zijnge 2010); and one study reported only PPD (Vandekerckhove 1996). All studies provided change scores and we were able to use these in all analyses.
Eleven studies provided data for the comparison of single‐ and multi‐rooted teeth between FMS and control three or four (in the following, designated as 3/4) months after baseline (Afacan 2020; Del Peloso 2008; Fonseca 2015; Graziani 2015; Jervøe‐Storm 2006; Predin 2014; Quirynen 2006; Swierkot 2009; Wennström 2005; Zanatta 2006; Zijnge 2010). Seven studies provided these data after six or eight (in the following, designated as 6/8) months (Afacan 2020; Apatzidou 2004; Fonseca 2015; Jervøe‐Storm 2006; Koshy 2005; Quirynen 2006; Swierkot 2009). Two studies performed retreatment after three months (Del Peloso 2008; Wennström 2005). These two studies were included in the meta‐analysis, but only data measured before retreatment were used for the comparisons.
Ten studies provided data for the comparison between FMD and control 3/4 months after baseline (Afacan 2020; Babaloo 2018; Fonseca 2015; Mongardini 1999; Quirynen 2006; Roman‐Torres 2018; Soares 2015; Swierkot 2009; Vandekerckhove 1996; Zanatta 2006); nine studies showed such data after 6/8 months (Afacan 2020; Fonseca 2015; Koshy 2005; Mongardini 1999; Pontillo 2018; Quirynen 2006; Santuchi 2015; Swierkot 2009; Vandekerckhove 1996). Six studies compared the three different treatment modalities after 3/4 and 6/8 months (Afacan 2020; Fonseca 2015; Koshy 2005; Quirynen 2006; Swierkot 2009; Zanatta 2006). Five studies separated the data into the subcategories 'single‐rooted' or 'multi‐rooted' teeth in terms of PPD (Koshy 2005; Mongardini 1999; Quirynen 2006; Swierkot 2009; Vandekerckhove 1996).
With regard to 'moderate' pocket depth, two studies defined this as 4 mm to 5.5 mm (Fonseca 2015; Quirynen 2006); two studies defined it as 4 mm to 6 mm (Swierkot 2009; Zijnge 2010); one study defined it as 6 mm or less (Del Peloso 2008); while seven studies classified pocket depths of 5 mm to 6 mm as moderate (Apatzidou 2004; Jervøe‐Storm 2006; Koshy 2005; Mongardini 1999; Vandekerckhove 1996; Wennström 2005; Zanatta 2006). Ten studies defined 'deep' pockets as being 7 mm or more (Apatzidou 2004; Del Peloso 2008; Jervøe‐Storm 2006; Koshy 2005; Mongardini 1999; Swierkot 2009; Vandekerckhove 1996; Wennström 2005; Zanatta 2006; Zijnge 2010), and two studies defined deep pockets as 6 mm or deeper (Fonseca 2015; Quirynen 2006). Three studies provided data from the first quadrant only (Mongardini 1999; Quirynen 2006; Vandekerckhove 1996); the other studies generated the data from the whole mouth (Afacan 2020; Apatzidou 2004; Babaloo 2018; Del Peloso 2008; Fonseca 2015; Graziani 2015; Jervøe‐Storm 2006; Koshy 2005; Pontillo 2018; Predin 2014; Roman‐Torres 2018; Santuchi 2015; Soares 2015; Swierkot 2009; Wennström 2005; Zanatta 2006; Zijnge 2010).
Excluded studies
We excluded 22 studies for the reasons below (see Characteristics of excluded studies table).
Type of disease (aggressive periodontitis, data not split regarding classification of periodontitis) (Bollen 1998).
Results of subgroups for QRP (quadrant‐wise subgingival SRP) (with and without use of chlorhexidine gluconate (CHX)) and FMS (with and without use of CHX) were not split into subgroups. The clinical data of QRP and FMS were presented as two groups only (Cortelli 2015).
Intervention after 24 hours (Eren 2002).
No control group (Jothi 2009).
No randomisation (Lee 2009).
Retreatment of participants prior to outcome assessment at six months (Loggner Graff 2009).
Data only available as figures; no reply from authors to request for supplemental data (Meulman 2013).
Participants in all arms received azithromycin (Oliveira 2019).
Participants in all arms received chlorhexidine rinse (Knöfler 2007; Preus 2013; Preus 2015a; Preus 2015b; Preus 2017a; Preus 2017b) or chlorhexidine gel (Silveira 2017).
Commentary on Preus 2013; Preus 2015a; Preus 2017a; Preus 2017b (Devji 2017).
Length of follow‐up was less than three months (Quirynen 1995; Serrano 2011).
Same group as presented in Santuchi 2015, but outcomes presented insufficiently (Santuchi 2016).
Several retreatments prior to outcome assessment at 18 months (Tomasi 2006).
Immunological study, lack of clinical data (Ushida 2008).
Still preliminary results only; was awaiting classification in 2015 version of this review (Zhao 2005).
Risk of bias in included studies
Overall risk of bias
Based on all domains, we assessed five studies at high risk of bias (Afacan 2020; Apatzidou 2004; Mongardini 1999; Swierkot 2009; Vandekerckhove 1996), and six at low risk of bias (Del Peloso 2008; Graziani 2015; Jervøe‐Storm 2006; Koshy 2005; Wennström 2005; Zijnge 2010), with the remaining nine being at unclear risk of bias. The risk of bias for each domain for each study is summarised in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Overall, we assessed 11 studies at low risk of selection bias (Del Peloso 2008; Fonseca 2015; Graziani 2015; Jervøe‐Storm 2006; Koshy 2005; Predin 2014; Quirynen 2006; Santuchi 2015; Swierkot 2009; Wennström 2005; Zijnge 2010).
Random sequence generation (selection bias)
Eleven trials described the method of randomisation, which was performed using a computer (Afacan 2020; Del Peloso 2008; Graziani 2015; Jervøe‐Storm 2006; Koshy 2005; Predin 2014; Quirynen 2006; Wennström 2005; Zijnge 2010) or sealed numbered envelopes (Fonseca 2015; Santuchi 2015). In three trials, the method of randomisation was a coin toss (Babaloo 2018; Mongardini 1999; Swierkot 2009). In six trials, the method of randomisation was uncertain or not stated (Apatzidou 2004; Pontillo 2018; Roman‐Torres 2018; Soares 2015; Vandekerckhove 1996; Zanatta 2006).
Allocation concealment (selection bias)
Five trials performed concealment using sealed opaque envelopes (Fonseca 2015; Graziani 2015; Koshy 2005; Santuchi 2015; Wennström 2005). Six trials provided adequate information about allocation concealment (Del Peloso 2008; Jervøe‐Storm 2006; Predin 2014; Quirynen 2006; Swierkot 2009; Zijnge 2010). In four studies, the concealment was unclear (Roman‐Torres 2018; Soares 2015; Vandekerckhove 1996; Zanatta 2006). The remaining five trials gave no comments about concealment (Afacan 2020; Apatzidou 2004; Babaloo 2018; Mongardini 1999; Pontillo 2018).
Blinding
We did not score performance bias of the operator as it was impossible to blind the therapist as they perform quadrant‐wise or full‐mouth instrumentation.
Thirteen trials blinded the outcome assessor to the treatment groups. Two trials gave no information about blinding and were at unclear risk of detection bias (Roman‐Torres 2018; Soares 2015), and five trials did not blind the outcome assessor (Afacan 2020; Apatzidou 2004; Mongardini 1999; Swierkot 2009; Vandekerckhove 1996).
Incomplete outcome data
Fourteen studies adequately described the completeness of follow‐up (the number of participants who entered the study and subsequently finished it) (Afacan 2020; Apatzidou 2004; Del Peloso 2008; Graziani 2015; Jervøe‐Storm 2006; Koshy 2005; Mongardini 1999; Pontillo 2018; Roman‐Torres 2018; Santuchi 2015; Swierkot 2009; Vandekerckhove 1996; Wennström 2005; Zijnge 2010). Five studies did not describe timing or reason for dropout, which we judged at unclear risk of attrition bias (Fonseca 2015; Predin 2014; Quirynen 2006; Soares 2015; Zanatta 2006). We also judged Babaloo 2018 as unclear because, although the study implied all participants were included in follow‐up assessments, it did not explicitly state this.
Selective reporting
Outcome reporting bias
All studies reported their data on all primary and secondary outcomes.
Publication bias
We did not assess publication bias using funnel plots as the comparisons included fewer than 10 studies with data at 6/8 months. Due to the small number of studies in each comparison, publication bias was difficult to assess.
Other potential sources of bias
We judged 15 studies at low risk of any other potential biases. Five studies were unclear: the baseline balance for smoking was unclear in four studies (Babaloo 2018; Fonseca 2015; Santuchi 2015; Zanatta 2006), and there was insufficient information to make a judgement about other potential risks of bias in one study (Roman‐Torres 2018).
Effects of interventions
See: Table 1; Table 5; Table 9
It must be stated that studies defined whole‐mouth evaluation differently. Fourteen studies carried out evaluation on all pockets (Afacan 2020; Apatzidou 2004; Babaloo 2018; Fonseca 2015; Graziani 2015; Koshy 2005; Pontillo 2018; Predin 2014; Roman‐Torres 2018; Santuchi 2015; Soares 2015; Swierkot 2009; Wennström 2005; Zanatta 2006); one study evaluated only pockets initially greater than 3 mm (Zijnge 2010); one study evaluated only pockets initially greater than 5 mm (Jervøe‐Storm 2006); one study presented only results in the subcategories initially moderate or deep pockets (Del Peloso 2008); one study only reported results in the subcategories single‐ or multi‐rooted teeth (Quirynen 2006); and two studies evaluated only pockets of the upper right quadrant (Mongardini 1999; Vandekerckhove 1996).
Full‐mouth scaling versus control
The results for evaluations at 6/8 months are reported below and summarised in Table 1. Results for evaluations at 3/4 months are found in Analysis 1.1; Analysis 1.2; and Analysis 1.3.
1.1. Analysis.

Comparison 1: Full‐mouth scaling (FMS) versus control, Outcome 1: Change in probing pocket depth: whole mouth, single‐ and multi‐rooted teeth
1.2. Analysis.

Comparison 1: Full‐mouth scaling (FMS) versus control, Outcome 2: Change in clinical attachment level: whole mouth, single‐ and multi‐rooted teeth
1.3. Analysis.

Comparison 1: Full‐mouth scaling (FMS) versus control, Outcome 3: Change in bleeding on probing: whole mouth, single‐ and multi‐rooted teeth
Tooth loss
None of the studies comparing FMS versus control reported tooth loss.
Change in probing pocket depth
Whole‐mouth data on PPD are shown in a forest plot (Analysis 1.1).
Five studies (three at high, one at unclear and one at low risk of bias) compared whole‐mouth scores in single‐ and multi‐rooted teeth after 6/8 months (Afacan 2020; Apatzidou 2004; Fonseca 2015; Koshy 2005; Swierkot 2009). The evidence suggests that FMS, when compared with control, results in little to no difference in change in PPD for whole‐mouth scores (MD 0.03 mm, 95% CI –0.14 to 0.20; P = 0.70; Chi² = 2.56, 4 degrees of freedom (df), P for heterogeneity (Phet) = 0.63, I² = 0%; low‐certainty evidence).
Sensitivity analysis for probing pocket depth
A sensitivity analysis was undertaken for low risk trials only for PPD at 6/8 months; the MD was 0.24 mm (95% CI –0.09 to 0.57; heterogeneity not applicable, Koshy 2005), which is consistent with the overall finding of no evidence of a difference.
Change in clinical attachment level
Whole‐mouth data on CAL are shown in a forest plot (Analysis 1.2).
We included five studies (three at high, one at unclear and one at low risk of bias) in the meta‐analysis for whole‐mouth scores in single‐ and multi‐rooted teeth after 6/8 months (Afacan 2020; Apatzidou 2004; Fonseca 2015; Koshy 2005; Swierkot 2009). The evidence suggests that FMS results in little to no difference in change in CAL when compared with control for whole‐mouth data comparisons (MD 0.10 mm, 95% CI –0.05 to 0.26; P = 0.18; Chi² = 3.78, 4 df, Phet = 0.44, I² = 0%; low‐certainty evidence). There was no evidence of heterogeneity for whole‐mouth recordings.
Change in bleeding on probing
Whole‐mouth data on BOP are shown in a forest plot (Analysis 1.3).
We included three studies (two at high and one at low risk of bias) in the meta‐analysis after 6/8 months for single‐ and multi‐rooted teeth combined (Apatzidou 2004; Koshy 2005; Swierkot 2009). The evidence suggests that FMS results in little to no difference in change in BOP when compared with control for the whole‐mouth evaluation (MD 2.64%, 95% CI –8.81 to 14.09; P = 0.65; Chi² = 3.97, 2 df, Phet = 0.14, I² = 50%; very‐low‐certainty evidence). There was some evidence of heterogeneity for whole‐mouth recording.
Subgroup analyses full‐mouth scaling versus control
Results for 3/4‐months subgroup analyses are found in Table 2; Table 3; and Table 4.
Change in probing pocket depth
Single‐ and multi‐rooted teeth
We included four studies (two at high, one at unclear and one at low risk of bias) in the meta‐analysis for moderate (Apatzidou 2004; Fonseca 2015; Jervøe‐Storm 2006) and deep pockets (Apatzidou 2004; Fonseca 2015; Jervøe‐Storm 2006; Swierkot 2009) in single‐ and multi‐rooted teeth after 6/8 months (Table 2). The evidence suggests that FMS results in little to no difference in change in PPD when compared with control for moderate pockets (5 mm to 6 mm) (MD –0.14 mm, 95% CI –0.45 to 0.18; P = 0.39; Chi² = 0.33, 2 df, Phet = 0.85, I² = 0%). The same applied for the deep pockets (greater than 6 mm) (MD –0.16 mm, 95% CI –0.60 to 0.28; P = 0.48; Chi² = 2.91, 3 df, Phet = 0.41, I² = 0%).
Single‐ or multi‐rooted teeth
We included three studies (one at high, one at unclear and one at low risk of bias) in the meta‐analysis for single‐rooted teeth alone after 6/8 months. The evidence suggests that FMS results in little to no difference in change in PPD when compared with control for moderate (MD 0.16 mm, 95% CI –0.01 to 0.32; P = 0.06; Chi² = 0.24, 2 df, Phet = 0.89, I² = 0%; Koshy 2005; Quirynen 2006; Swierkot 2009) or deep pockets in single‐rooted teeth (MD 0.26 mm, 95% CI –0.21 to 0.73; P = 0.27; Chi² = 0.21, 1 df, Phet = 0.64, I² = 0%; Koshy 2005; Quirynen 2006).
The same three studies (one at high, one at unclear and one at low risk of bias) were included in the meta‐analysis for multi‐rooted teeth alone after 6/8 months. The evidence suggests that FMS results in little to no difference in change in PPD when compared with control for moderate (MD 0.21 mm, 95% CI –0.14 to 0.55; P = 0.24; Chi² = 5.60, 2 df, Phet = 0.06, I² = 64%; Koshy 2005; Quirynen 2006; Swierkot 2009) or deep pockets in multi‐rooted teeth (MD 0.18 mm, 95% CI –0.26 mm to 0.62 mm; P = 0.42; Chi² = 0.65, 1 df, Phet = 0.42, I² = 0%; Koshy 2005; Quirynen 2006).
Change in clinical attachment level
Single‐ and multi‐rooted teeth
We included four studies in the meta‐analysis for moderate and deep pockets in single‐ and multi‐rooted teeth (Apatzidou 2004; Jervøe‐Storm 2006; Quirynen 2006; Swierkot 2009) after 6/8 months. The evidence suggests that FMS results in little to no difference in change in CAL when compared with control for moderate (MD 0.22 mm, 95% CI –0.05 to 0.49; P = 0.11; Chi² = 0.29, 2 df, Phet = 0.87, I² = 0%; Apatzidou 2004; Jervøe‐Storm 2006; Quirynen 2006) or deep pockets (MD 0.05 mm, 95% CI –0.64 to 0.74; P = 0.89; Chi² = 12.89, 3 df, Phet = 0.005, I² = 77%; Apatzidou 2004; Jervøe‐Storm 2006; Quirynen 2006; Swierkot 2009). There was no evidence of heterogeneity for moderate pockets, but evidence of substantial heterogeneity for deep pockets (Table 3).
Single‐ or multi‐rooted teeth
Only two studies (one at high and one at low risk of bias) provided data after 6/8 months for single‐rooted or multi‐rooted teeth alone (Koshy 2005; Swierkot 2009). The evidence suggests that FMS results in little to no difference in change in CAL when compared with control for moderate pockets in single‐rooted (MD 0.04 mm, 95% CI –0.19 to 0.27; P = 0.71; Chi² = 0.46, 1 df, Phet = 0.50, I² = 0%) and multi‐rooted teeth (MD 0.00 mm, 95% CI –0.34 to 0.34; P = 1.00; Chi² = 1.71, 1 df, Phet = 0.19, I² = 41%) as for deep pockets in single‐ (MD 0.47 mm, 95% CI –0.37 to 1.31; P = 0.27; heterogeneity not applicable) and multi‐rooted teeth (MD 0.38 mm, 95% CI –0.28 to 1.04; P = 0.26; heterogeneity not applicable) (Table 3).
Change in bleeding on probing
Single‐ and multi‐rooted teeth
The evidence suggests that at 6/8 months FMS results in little to no difference in change in BOP when compared with control for moderate (MD –6.10%, 95% CI –24.12 to 11.92; P = 0.51; heterogeneity not applicable; Jervøe‐Storm 2006) or for deep pockets (MD 10.22%, 95% CI –0.59 to 21.03; P = 0.06; Chi² = 0.01, 1 df, Phet = 0.92, I² = 0%; Jervøe‐Storm 2006; Swierkot 2009). There was no evidence of heterogeneity (Table 4).
Single‐ or multi‐rooted teeth
Only two studies (with high and unclear risk of bias) provided data at 6/8 months for single‐rooted alone and multi‐rooted teeth alone (Quirynen 2006; Swierkot 2009). The evidence suggests that FMS results in little to no difference in change in BOP after 6/8 months when compared with control for moderate pockets (single‐rooted: MD –3.06%, 95% CI –10.47 to 4.35; P = 0.42; Chi² = 1.22, Phet = 0.27, I² = 18%; multi‐rooted: MD 2.38%, 95% CI –2.95 to 7.71; P = 0.38; Chi² = 0.45, 1 df, Phet = 0.50, I² = 0%; Quirynen 2006; Swierkot 2009), or deep pockets (single‐rooted: MD ‐4.00%, 95% CI –20.17 to 12.17; P = 0.63; heterogeneity not applicable; multi‐rooted: MD ‐4.00%, 95% CI –23.29 to 15.29; P = 0.68; heterogeneity not applicable; Quirynen 2006) (Table 4).
Full‐mouth disinfection versus control
The results are summarised in Table 5. Results for 3/4‐month evaluation are found in Analysis 2.1; Analysis 2.2; and Analysis 2.3.
2.1. Analysis.

Comparison 2: Full‐mouth disinfection (FMD) versus control, Outcome 1: Change in probing pocket depth: whole mouth, single‐ and multi‐rooted teeth
2.2. Analysis.

Comparison 2: Full‐mouth disinfection (FMD) versus control, Outcome 2: Change in clinical attachment level: whole mouth, single‐ and multi‐rooted teeth
2.3. Analysis.

Comparison 2: Full‐mouth disinfection (FMD) versus control, Outcome 3: Change in bleeding on probing: whole mouth, single‐ and multi‐rooted teeth
Tooth loss
None of the studies comparing FMD versus control reported tooth loss.
Change in probing pocket depth
Whole‐mouth data are shown in a forest plot (Analysis 2.1).
Six studies (two at high, three at unclear and one at low risk of bias) compared whole‐mouth scores in single‐ and multi‐rooted teeth after 6/8 months (Afacan 2020; Fonseca 2015; Koshy 2005; Pontillo 2018; Santuchi 2015; Swierkot 2009). The evidence suggests that FMD when compared with control results in little to no difference in change in PPD for whole‐mouth scores (MD 0.11 mm, 95% CI –0.04 to 0.27; P = 0.14; Chi² = 2.68, 5 df, Phet = 0.75, I² = 0%).
Sensitivity analysis
We undertook a sensitivity analysis for low risk of bias trials only for change in PPD at 6/8 months. The MD was 0.23 mm (95% CI –0.15 to 0.61; heterogeneity not applicable, Koshy 2005), which is consistent with the overall finding of no evidence of a difference.
Change in clinical attachment level
Whole‐mouth for 6/8‐month comparisons are shown in a forest plot (Analysis 2.2).
We included six studies (two at high, three at unclear and one at low risk of bias) in the meta‐analysis for whole‐mouth scores in single‐ and multi‐rooted teeth after 6/8 months (Afacan 2020; Fonseca 2015; Koshy 2005; Pontillo 2018; Santuchi 2015; Swierkot 2009). The evidence suggests that FMD when compared with control results in little to no difference in change in CAL for whole‐mouth scores (MD 0.07 mm 95% CI –0.11 to 0.24; P = 0.47; Chi² = 1.14, 5 df, Phet = 0.95, I² = 0%). There was no evidence of heterogeneity.
Change in bleeding on probing
Whole‐mouth data for 6/8‐month comparisons are shown in a forest plot (Analysis 2.3).
We included four studies (two at high, one at unclear and one at low risk of bias) in the meta‐analysis after 6/8 months for single‐ and multi‐rooted teeth combined (Koshy 2005; Mongardini 1999; Pontillo 2018; Swierkot 2009). The evidence suggests that FMD results in little to no difference in change in BOP for whole‐mouth scores when compared with control (MD 9.54%, 95% CI –2.24 to 21.32; P = 0.11; Chi² = 14.68, 3 df, Phet = 0.002, I² = 80%). There was evidence of considerable heterogeneity for the whole‐mouth findings.
Subgroup analyses full‐mouth disinfection versus control
Results for 3/4‐month subgroup analyses are found in Table 6; Table 7; and Table 8.
Change in probing pocket depth
Single‐ and multi‐rooted teeth
Only two studies at unclear and high risk of bias were included for moderate and deep pockets in single‐ and multi‐rooted teeth after 6/8 months (Fonseca 2015; Swierkot 2009; Table 6). The evidence suggests FMD reduces PPD more than control for moderate pockets (MD 0.88 mm, 95% CI 0.20 to 1.56; P = 0.01; heterogeneity not applicable; Fonseca 2015). The evidence suggests that FMD results in little to no difference in change in PPD when compared with control for deep pockets (MD –0.10 mm, 95% CI –0.47 to 0.26; P = 0.58; Chi² = 0.56, 1 df, Phet = 0.46, I² = 0%; Fonseca 2015; Swierkot 2009). The outcome for moderate pockets was based on one trial with unclear risk of bias and the numbers of participants were low (18).
Single‐ or multi‐rooted teeth
We included five studies (three at high, one at unclear and one at low risk of bias) in the meta‐analysis after 6/8 months for single‐rooted teeth alone (Koshy 2005; Mongardini 1999; Quirynen 2006; Swierkot 2009; Vandekerckhove 1996). The evidence suggests FMD reduces PPD more than control for moderate (MD 0.41 mm, 95% CI 0.11 to 0.70; P = 0.006; Chi² = 13.13, 4 df, Phet = 0.01, I² = 70%; Koshy 2005; Mongardini 1999; Quirynen 2006; Swierkot 2009; Vandekerckhove 1996) and deep pockets (MD 0.78 mm, 95% CI –0.01 to 1.57; P = 0.05; Chi² = 9.41, 3 df, Phet = 0.03, I² = 67%; Koshy 2005; Mongardini 1999; Quirynen 2006; Vandekerckhove 1996). However, there was substantial heterogeneity for both analyses. Three studies for these analyses had a high risk of detection bias. In all three studies, the same person performed treatment and assessment. One study had unclear risk of bias because the rate of dropouts was 15.7%.
We included five studies (three at high, one at unclear and one at low risk of bias) in the meta‐analysis for multi‐rooted teeth alone after 6/8 months (Koshy 2005; Mongardini 1999; Quirynen 2006; Swierkot 2009; Vandekerckhove 1996). The evidence suggests that FMD results in little to no difference in change in PPD when compared with control for moderate (MD 0.21 mm, 95% CI –0.12 to 0.53; P = 0.21; Chi² = 10.56, 4 df, Phet = 0.03, I² = 62%) or deep pockets (MD 0.56 mm, 95% CI –0.23 to 1.34; P = 0.16; Chi² = 8.52, 3 df, Phet = 0.04, I² = 65%). There was evidence of substantial heterogeneity for both comparisons.
Change in clinical attachment level
Single‐ and multi‐rooted teeth
No studies provided data for moderate pockets after 6/8 months. Only one study reported data for deep pockets in single‐ and multi‐rooted teeth (Swierkot 2009). The evidence suggests that FMD results in little to no difference in change in CAL when compared with control for deep pockets without evidence of heterogeneity (MD –0.16 mm, 95% CI –0.41 to 0.09; P = 0.20; heterogeneity not applicable; Table 7). However, this outcome was generated from one study at high risk of bias. The concern was about blinding of assessment of data, as the same person performed treatment and assessment. Additionally, data were generated in 16 participants (FMD: nine; control: seven), reaching a difference of 0.16 mm, which is unlikely to be clinically relevant.
Single‐ or multi‐rooted teeth
Three studies (two at high and one at low risk of bias) provided data after 6/8 months for single‐rooted teeth alone (Koshy 2005; Mongardini 1999; Swierkot 2009). The evidence suggests FMD may result in a small difference in CAL compared with control for moderate pockets (MD 0.14 mm; 95% CI 0.00 to 0.28; P = 0.05; Chi² = 1.45, 2 df, Phet = 0.48, I² = 0%). For deep pockets, the MD was 0.72 mm (95% CI –0.94 to 2.37; P = 0.40; Chi² = 4.66, 1 df, Phet = 0.03, I² = 79%) with considerable heterogeneity. Outcomes were based on three trials, two with high risk of bias. The concerns for both high‐risk studies were blinding of assessment of data, as the same person performed treatment and assessment. Additionally, the difference, based on the comparison of 55 participants versus 57 participants, between the two treatment modalities was 0.14 mm, which is unlikely to be clinically relevant.
Three studies (two at high and one at low risk of bias) provided data after 6/8 months for multi‐rooted teeth (Koshy 2005; Mongardini 1999; Swierkot 2009). The evidence suggests that FMD results in little to no difference in change in CAL when compared with control for moderate (MD 0.12 mm, 95% CI –0.17 to 0.41; P = 0.43; Chi² = 5.32, 2 df, Phet 0.07, I² = 62%; Koshy 2005; Mongardini 1999; Swierkot 2009) or deep pockets (MD 0.52 mm, 95% CI –1.30 to 2.34; P = 0.57; Chi² = 7.79, 1 df, Phet = 0.005, I² = 87%; Koshy 2005; Mongardini 1999), both with evidence of considerable heterogeneity.
Change in bleeding on probing
Single‐ and multi‐rooted teeth
There were no data for moderate pockets. The evidence is very uncertain about the effect of FMD at 6/8 months for deep pockets (MD 2.00%, 95% CI –7.83 to 11.83; P = 0.69; heterogeneity not applicable). However, this outcome was generated from one study at high risk of bias (Swierkot 2009). The concern was about blinding of assessment of data, as the same person performed treatment and assessment. Additionally, the difference of 2% is not clinically relevant and the level of evidence for BOP was very low.
Single‐ or multi‐rooted teeth
Two studies (one at high and one at unclear risk of bias) provided data after 6/8 months for single‐rooted teeth (Quirynen 2006; Swierkot 2009). FMD reduced BOP slightly compared with control (MD 4.83%, 95% CI 1.86 to 7.80; P = 0.001; Chi² = 0.28, 1 df, Phet = 0.60, I² = 0%). The evidence is very uncertain about the effect of FMD in deep pockets at 6/8 months (MD 14.00%, 95% CI –2.17 to 30.17; P = 0.09; heterogeneity not applicable; Quirynen 2006). A difference in BOP of 4.83% or 14% is probably not clinically relevant. The results must be seen in the context of the risk of bias assessed for both studies. One study had concerns with detection blinding; the other with the rate of dropouts (15.7%). Reasons and time point for dropouts were unclear and not declared. The level of evidence for BOP was very low certainty.
Two studies (one at high and one at unclear risk of bias) provided data after 6/8 months for multi‐rooted teeth (Quirynen 2006; Swierkot 2009). The evidence is very uncertain about the effect of FMD at 6/8 months for moderate pockets (MD 8.72%, 95% CI –2.61 to 20.06; P = 0.13; Chi² = 1.52, 1 df, Phet = 0.22, I² = 34%) or deep pockets (MD –8.00%, 95% CI –25.00 to 9.00; P = 0.36; heterogeneity not applicable; Quirynen 2006). There was evidence of moderate heterogeneity for moderate pockets.
Full‐mouth scaling versus full‐mouth disinfection
The results are summarised in Table 9. Results for evaluation at 3/4 months are found in Analysis 3.1; Analysis 3.2; and Analysis 3.3.
3.1. Analysis.

Comparison 3: Full‐mouth scaling (FMS) versus full‐mouth disinfection (FMD), Outcome 1: Change in probing pocket depth: whole mouth, single‐ and multi‐rooted teeth
3.2. Analysis.

Comparison 3: Full‐mouth scaling (FMS) versus full‐mouth disinfection (FMD), Outcome 2: Change in clinical attachment level: whole mouth, single‐ and multi‐rooted teeth
3.3. Analysis.

Comparison 3: Full‐mouth scaling (FMS) versus full‐mouth disinfection (FMD), Outcome 3: Change in bleeding on probing: whole mouth, single‐ and multi‐rooted teeth
Tooth loss
None of the studies comparing FMS versus FMD reported tooth loss.
Change in probing pocket depth
Whole‐mouth results for 6/8‐month comparisons are shown in a forest plot (Analysis 3.1).
Four studies (two at high and two at unclear risk of bias) compared FMS with FMD after 6/8 months (Afacan 2020; Fonseca 2015; Swierkot 2009; Zanatta 2006). The evidence suggests that FMS results in little to no difference in change in PPD when compared with FMD for whole‐mouth scores (MD –0.11 mm, 95% CI –0.30 to 0.07; P = 0.22; Chi² = 2.79, 3 df, Phet = 0.43, I² = 0%).
Sensitivity analysis
We undertook a sensitivity analysis for low‐risk trials only for PPD at 6/8 months. The MD was 0.01 mm (–0.43 to 0.45; heterogeneity not applicable; Koshy 2005), which is consistent with the overall finding of no evidence of a difference.
Change in clinical attachment level
Whole‐mouth data are shown in a forest plot (Analysis 3.2).
We included four studies (two at high risk of bias, one at low risk and one unclear) in the meta‐analysis for whole‐mouth scores in single‐ and multi‐rooted teeth after 6/8 months (Afacan 2020; Fonseca 2015; Koshy 2005; Swierkot 2009). The evidence suggests that FMS results in little to no difference in change in CAL when compared with FMD for whole‐mouth at 6/8‐month evaluation (MD –0.05 mm, 95% CI –0.23 to 0.13; P = 0.58; Chi² = 2.69, 3 df, Phet = 0.44, I² = 0%).
Change in bleeding on probing
Whole‐mouth results for 6/8‐month comparisons are shown in a forest plot (Analysis 3.3).
We included two studies (one at high and one at low risk of bias) in the meta‐analysis after 6/8 months for single‐ and multi‐rooted teeth combined (Koshy 2005; Swierkot 2009). The evidence suggests that FMS results in little to no difference in change in BOP when compared with FMD for the whole‐mouth evaluation (MD –0.20%, 95% CI –13.27 to 12.87; P = 0.98; Chi² = 2.09, 1 df, Phet = 0.15, I² = 52%). There was evidence of heterogeneity for the whole‐mouth findings.
Subgroup analyses full‐mouth scaling versus full‐mouth disinfection
Results for 3/4‐months subgroup analyses are found in Table 10; Table 11; and Table 12.
Change in probing pocket depth
Single‐ and multi‐rooted teeth
Two studies (one at high and one at unclear risk of bias) were included in the analysis for moderate and deep pockets in single‐ and multi‐rooted teeth after 6/8 months (Fonseca 2015; Swierkot 2009). The evidence suggests FMD results in a reduction in PPD in comparison with FMS in moderate pockets (MD –0.88 mm, 95% CI –1.53 to –0.23; P = 0.008; heterogeneity not applicable), but may not result in a difference for reduction in PPD for deep pockets (MD –0.50 mm, 95% CI –2.00 to 0.99 mm; P = 0.51; Chi² = 4.89, 1 df, Phet = 0.03, I² = 80%). The evidence for the moderate pockets based on one study with unclear risk of bias in the domain 'attrition bias', due to unclear dropouts. Even if there was a difference of 0.88 mm, results were generated from one study with low numbers of participants in each group. There was considerable heterogeneity for deep pockets.
Single‐ or multi‐rooted teeth
We included three studies (one at high, one at unclear, and one at low risk of bias) in the meta‐analysis after 6/8 months for single‐rooted teeth (Koshy 2005; Quirynen 2006; Swierkot 2009). The evidence suggests that FMS results in little to no difference in change in PPD when compared with FMD for moderate (MD –0.10 mm, 95% CI –0.40 to 0.20; P = 0.52; Chi² = 8.24, 2 df, Phet = 0.02, I² = 76%; Koshy 2005; Quirynen 2006; Swierkot 2009) or deep pockets (MD –0.03 mm, 95% CI –0.48 to 0.41; P = 0.88; Chi² = 0.35, 1 df, Phet = 0.55, I² = 0%; Koshy 2005; Quirynen 2006), with a high degree of heterogeneity for moderate pockets.
We included three studies (one at high, one at unclear and one at low risk of bias) in the meta‐analysis for multi‐rooted teeth alone after 6/8 months (Koshy 2005; Quirynen 2006; Swierkot 2009). The evidence suggests that FMS results in little to no difference in change in PPD when compared with FMD for moderate (MD 0.04 mm, 95% CI –0.16 to 0.25; P = 0.68; Chi² = 0.94, 2 df, Phet = 0.63, I² = 0%; Koshy 2005; Quirynen 2006; Swierkot 2009) or deep pockets (MD 0.05 mm, 95% CI –0.38 to 0.47; P = 0.83; Chi² = 1.10, 1 df, Phet = 0.29, I² = 9%; Koshy 2005; Quirynen 2006).
Change in clinical attachment level
Single‐ and multi‐rooted teeth
No studies provided data at 6/8 months for moderate pockets and only one study was included in the analysis for deep pockets in single‐ and multi‐rooted teeth (Swierkot 2009). The evidence suggests that FMS results in little to no difference in change in CAL when compared with FMD for deep pockets (MD –0.51 mm, 95% CI –1.24 to 0.22; P = 0.17; heterogeneity not applicable). However, this outcome was generated from one study at high risk of bias. The concern was about blinding of assessment of data, as the same person performed treatment and assessment.
Single‐ or multi‐rooted teeth
Two studies (one at high and one at low risk of bias) provided data after 6/8 months for single‐ or multi‐rooted teeth (Koshy 2005; Swierkot 2009). The evidence suggests that FMS results in little to no difference in change in CAL when compared with FMD for moderate pockets (single‐rooted: MD –0.09 mm, 95% CI –0.30 to 0.11; P = 0.38; Chi² = 0.60, 1 df, Phet = 0.44, I² = 0%; multi‐rooted: MD –0.02 mm, 95% CI –0.53 to 0.49; P = 0.93; Chi² = 3.68, 1 df, Phet = 0.06, I² = 73%), with substantial risk of heterogeneity for multi‐rooted teeth (Koshy 2005; Swierkot 2009). Regarding deep pockets in multi‐rooted teeth only, FMS may result in an increase in CAL when compared with FMD (single‐rooted: MD 0.56 mm, 95% CI –0.37 to 1.49; P = 0.24; heterogeneity not applicable; multi‐rooted: MD 0.74 mm; 95% CI 0.17 to 1.31; P = 0.01; heterogeneity not applicable; Koshy 2005). This outcome was generated from one study with low risk of bias. However, data were reported from the treatment of only 24 participants, 12 in each group.
Change in bleeding on probing
Single‐ and multi‐rooted teeth
There were no data for moderate pockets independent of pocket depth. The evidence is very uncertain about the effect of FMS when compared with FMD in deep pockets at 6/8 months. The difference was based on one study reporting only deep pockets (MD 8.00%, 95% CI 1.18 to 14.82; P = 0.02; heterogeneity not applicable; Swierkot 2009).
Single‐ or multi‐rooted teeth
Two studies (one at high and one at unclear risk of bias) provided data after 6/8 months for single‐ or multi‐rooted teeth (Quirynen 2006; Swierkot 2009). FMD may have little effect on reduction in BOP when compared with FMS, but the evidence is very uncertain. Values were calculated for single‐rooted teeth in moderate pockets (MD –6.69%, 95% CI –12.18 to –1.19; P = 0.02; Chi² = 0.38, 1 df, Phet = 0.54, I² = 0%; Quirynen 2006; Swierkot 2009) and in deep pockets (MD –18.00%, 95% CI –30.83 to –5.17; P = 0.006; heterogeneity not applicable; Quirynen 2006). For multi‐rooted teeth, the evidence is very uncertain about the effect of FMS compared with FMD for moderate pockets (MD –4.16%, 95% CI –8.72 to 0.39; P = 0.07; Chi² = 0.18, 1 df, Phet = 0.68, I² = 0%; Quirynen 2006; Swierkot 2009) or deep pockets (MD 4.00%, 95% CI –13.37 to 21.3; P = 0.65; heterogeneity not applicable; Quirynen 2006).
Adverse events
A summary of the findings for this outcome is presented in Table 13.
10. Adverse events and participant‐reported outcomes.
| Study | Comparison | Outcome |
| Afacan 2020 | FMS vs FMD vs control | Adverse events or side effects: no harm reported or observed. |
| Apatzidou 2004 | FMS vs control | VAS (0–10) of pain, percentage of participants taking analgesics, number of analgesics, body temperature (axilla) all recorded after 24 and 48 hours. Occurrence of labial herpes or oral ulcers recorded after 2 weeks. Higher pain rating with FMS; no difference in body temperature. |
| Babaloo 2018 | FMD vs control | Adverse events or side effects were not planned outcomes. |
| Del Peloso 2008 | FMS vs control | Body temperature (axilla), VAS (0–10) of pain, reports of analgesics, reports of oral ulcerations or other adverse effects. No difference between groups. |
| Fonseca 2015 | FMS vs FMD vs control | The use of CHX in the FMD group was associated with adverse events such as tooth staining, taste changing, and difficulties in participants' adherence and side effects over the course of 60 days. |
| Graziani 2015 | FMS vs control | Body temperature, acute‐phase responses in terms of CRP more elevated in the FMS group. |
| Jervøe‐Storm 2006 | FMS vs control | Adverse events or side effects were not planned outcomes. |
| Koshy 2005 | FMS vs FMD vs control | VAS of pain (1–10), number of analgesics, body temperature (axilla) all recorded after treatment same day and next day. No significant differences between groups. |
| Mongardini 1999 | FMD vs control | VAS of pain (10‐cm scale), number of analgesics, body temperature (axilla) all recorded same and next day. Occurrence of labial herpes or oral ulcers recorded during the first week: no differences between groups. |
| Pontillo 2018 | FMD vs control | Adverse events or side effects were not planned outcomes. |
| Predin 2014 | FMS vs control | Adverse events or side effects were not planned outcomes. |
| Quirynen 2006 | FMS vs FMD vs control | Adverse events or side effects were not planned outcomes. |
| Roman‐Torres 2018 | FMD vs control | Participants were asked for their use of analgesics and their satisfaction with the treatments. However, no standardised measurements were used or presented. No differences between groups reported. |
| Santuchi 2015 | FMD vs control | VAS of pain. Fear (DFS) and anxiety (DAS) were monitored with questionnaires. No increase in body temperature and occurrence of abscess. No differences between groups. |
| Soares 2015 | FMD vs control | Adverse events or side effects were not planned outcomes. |
| Swierkot 2009 | FMS vs FMD vs control | Adverse events or side effects: no harm reported or observed. |
| Vandekerckhove 1996 | FMD vs control | Questionnaire of pain, number of analgesics, body temperature all recorded after first session of treatment. Occurrence of labial herpes. All complaints occurred only in the FMD group. |
| Wennström 2005 | FMS vs control | Overall degree of treatment discomfort on a 100‐mm VAS. No differences between groups. |
| Zanatta 2006 | FMS vs FMD vs control | Adverse events or side effects were not planned outcomes. |
| Zijnge 2010 | FMS vs control | Adverse events or side effects: no harm reported or observed. |
CHX: chlorhexidine gluconate; CRP: C‐reactive protein; DAS: Dental Anxiety Scale; DFS: Dental Fear Scale; FMD: full‐mouth disinfection; FMS: full‐mouth scaling; VAS: visual analogue scale.
In seven studies, adverse events, side effects or participant‐related outcomes were not part of the investigation plan and they presented no results (Babaloo 2018; Jervøe‐Storm 2006; Pontillo 2018; Predin 2014; Quirynen 2006; Soares 2015; Zanatta 2006).
Thirteen studies provided information about adverse events or participant‐reported outcomes (Afacan 2020; Apatzidou 2004; Del Peloso 2008; Fonseca 2015; Graziani 2015; Koshy 2005; Mongardini 1999; Roman‐Torres 2018; Santuchi 2015; Swierkot 2009; Vandekerckhove 1996; Wennström 2005; Zijnge 2010).
Five studies did not look for adverse events or side effects of the various treatments as a specific outcome (Afacan 2020; Fonseca 2015; Roman‐Torres 2018; Swierkot 2009; Zijnge 2010); they only reported that "no harmful effect was observed/there were no reports of adverse events or side effects". However, one of these trials reported tooth staining and taste changing in the FMD group, which led to difficulties in participants' adherence to the study during the 60 days (Fonseca 2015).
Five trials found no differences between groups in terms of adverse events (Del Peloso 2008; Koshy 2005; Mongardini 1999; Santuchi 2015; Wennström 2005). Participants were asked to fill out questionnaires or visual analogue scales about post‐treatment pain, use of analgesics and other adverse events. Four of the studies found no differences between groups. One study found a small, but significant, elevation in body temperature after the second treatment in the FMD group (Mongardini 1999, as explained in Quirynen 1999); this observation was made in a combined group of participants with aggressive and chronic periodontitis. The authors explained this comprehensibly with the prolonged treatment or repeated transient bacteraemia (or both) during the second subgingival instrumentation. The diagnosis was not clinically relevant. There were no differences in other parameters.
Only three trials reported any signs of any type of reaction after treatment (Apatzidou 2004; Graziani 2015; Vandekerckhove 1996). There were increased use of analgesics, higher body temperature and occurrence of herpes in the full‐mouth treatment groups in comparison with the quadrant treatment (Apatzidou 2004; Vandekerckhove 1996). In Apatzidou 2004, the difference between groups was significant; Vandekerckhove 1996 was a pilot study and there were no comments about significance. One study investigated levels of C‐reactive protein (CRP), interleukin (IL)‐6, and tumour necrosis factor (TNF)α in addition to the clinical parameters (Graziani 2015). At day one, there was a marked significant increase in the serum levels of CRP, IL‐6 and TNFα in the FMS group compared to the control group, which was no longer evident after seven days.
Pocket closure
One method of evaluation is to compare the number/proportion of shallow ('closed') pockets before and after therapy. Pocket closure was originally described by Wennström 2005 as the number/proportion of sites with PPD of 4 mm or less after treatment. Five studies provided information on this outcome (Afacan 2020; Graziani 2015; Jervøe‐Storm 2006; Koshy 2005; Wennström 2005), whereas another four studies employed slightly modified definitions for their evaluations (Apatzidou 2004; Fonseca 2015; Santuchi 2015; Zijnge 2010). All nine studies reported an increase in the number/proportion of 'closed' pockets after treatment, and eight studies found no differences between groups. In one study, there was a greater change in both full‐mouth treatment groups compared with the control treatment (Koshy 2005). The results are presented in Table 14.
11. Pocket closure.
| a Study | Outcome | Category of PD included for evaluation | Results |
| Afacan 2020 | Control, FMS, FMD | Percentage of PPD ≥ 5 mm; before and after treatment | No difference between groups, significant reductions in all groups. |
| Apatzidou 2004 | Control, FMS | Number of sites with PPD > 5 mm; before and after treatment | No difference between groups, significant reductions in both groups. |
| Babaloo 2018 | Pocket closure was not a planned outcome. | ||
| Del Peloso 2008 | Pocket closure was not a planned outcome. | ||
| Fonseca 2015 | Control, FMS, FMD | Percentage of periodontal diseased sites ('PDS': sites with PD ≥ 4 mm and CAL ≥ 3 mm); before and after treatment | No difference between groups, significant reductions in all groups. |
| Graziani 2015 | Control, FMS | Number of pockets with PPD > 4 mm before and after treatment | No differences between groups, there was a reduction in number and percentage of pockets with PPD ≤ 4 mm after therapy in both groups. |
| Jervøe‐Storm 2006 | Control, FMS | Proportions of sites with PPD ≤ 4 mm before and after treatment | No differences between groups, there was an increase of sites with PPD ≤ 4 mm after therapy in both groups, with a slightly higher proportion in the FMS group. |
| Koshy 2005 | Control, FMS, FMD | Reduction in number of sites ≥ 5 mm; before and after treatment | The number of pocket sites (≥ 5 mm) reduced significantly in all 3 groups 6 months after treatment, with greater reduction observed in both full‐mouth groups compared with the control group. |
| Mongardini 1999 | Pocket closure was not a planned outcome. | ||
| Pontillo 2018 | Pocket closure was not a planned outcome. | ||
| Predin 2014 | Pocket closure was not a planned outcome. | ||
| Quirynen 2006 | Pocket closure was not a planned outcome. | ||
| Roman‐Torres 2018 | Pocket closure was not a planned outcome. | ||
| Santuchi 2015 | Control, FMD | Percentage of sites with PD 5–6 mm; before and after treatment | No difference between groups, significant reductions in both groups. |
| Soares 2015 | Pocket closure was not a planned outcome. | ||
| Swierkot 2009 | Pocket closure was not a planned outcome. | ||
| Vandekerckhove 1996 | Pocket closure was not a planned outcome. | ||
| Wennström 2005 | Control, FMS | Proportions of sites with PPD ≤ 4 mm before and after treatment | No differences between both groups, proportion of pockets with PPD ≤ 4 increased in both groups. QRP showed a tendency to have a more favourable outcome in sites with PPD ≥ 7 mm. |
| Zanatta 2006 | Pocket closure was not a planned outcome. | ||
| Zijnge 2010 | Control, FMS | Percentage of pockets initially measuring ≥ 5 mm and which were reduced to ≤ 3 mm and considered healthy or remained ≥ 5 mm after 3 months | No differences between both groups, significant reductions in both groups. |
FMS: full‐mouth scaling: FMD: full‐mouth scaling and disinfection; PD: pocket depth; PPD: probing pocket depth; QRP: quadrant‐wise subgingival scaling and root planing.
Discussion
Summary of main results
In this review, we included 20 trials that assessed the effects of full‐mouth treatment modalities within 24 hours, with or without adjunctive antiseptics, compared to the conventional quadrant approach. We assessed the certainty of the evidence using GRADE (Schünemann 2020), and our assessment for the key comparisons and outcomes is presented in Table 1; Table 5; and Table 9.
None of the trials reported data on one of our primary outcomes, tooth loss.
For our other primary outcome, PPD, there is low‐certainty evidence from the analyses for all teeth at two time points (3/4 and 6/8 months) that neither FMS nor FMD were more beneficial than conventional SRP. In terms of secondary outcomes of CAL and BOP at 6/8 months, the analyses also found no evidence of superior benefit between any of the treatment groups (low‐ to very low‐certainty evidence).
We conducted various meta‐analyses for single‐ and multi‐rooted teeth, and teeth at different initial levels of PPD, with some inconsistent findings.
Thirteen studies provided information about adverse events or participant‐reported outcomes, four of which reported the occurrence of harms and adverse events. The most frequent adverse effect was an increased body temperature after FMS or FMD treatments.
In addition, we considered the effect of the different treatment modalities on pocket closure. In recent years, there has been consensus that the use of 'no bleeding following probing' and 'a PPD of ≤ 4 mm' (pocket closure) can be considered a meaningful clinical endpoint of treatment success (Chapple 2018; Loos 2020; Sanz 2020a). Most of the 20 studies included in this review were conducted prior to this consensus; however, nine had included the number/proportion of shallow (closed) pockets below a certain threshold of probing depth after therapy in their analysis. Interestingly, only one study found differences between the treatment modalities for this outcome, thereby supporting the overall findings of the present review.
Overall completeness and applicability of evidence
The objectives of this review were to assess the effects of three treatment modalities of periodontitis for the clinical outcomes tooth loss, and change in PPD, CAL, and BOP. We excluded what was formerly known as 'aggressive periodontitis' due to its low incidence and the application of systemic antibiotics during therapy. The new classification of periodontal and peri‐implant diseases does not use the terms 'aggressive' or 'chronic' to describe the progression or severity of attachment loss (Papapanou 2018). Instead, the terms 'staging' and 'grading' are used to define a case, taking into account severity, complexity and extent, as well as direct and indirect evidence of progression rate (Tonetti 2018). We continued to use the former terms 'aggressive' and 'chronic' periodontitis to determine inclusion and exclusion of studies in the present review since most papers were conducted and published before the implementation of the new classification.
Overall, there was insufficient evidence to claim or refute a benefit for one of the three investigated treatment modalities for the treatment of adult periodontitis. None of the trials reported the primary outcome of tooth loss. This is not surprising as they were conducted over relatively short time periods from three to 12 months. Longer studies would be needed to monitor tooth loss. Furthermore, tooth loss can be problematic since the reason for extraction is often unclear and might not be due to progression of the periodontitis (Loos 2020).
Study participants were aged between 23 and 78 years, and there were overall equal numbers of men and women (44.9% men) took part in the studies. The setting of the trials was predominantly university clinics. Only one study was performed in a dental practice (Zijnge 2010); a second trial was performed as a multi‐centre (two) study in a university and a dental practice (Wennström 2005). For daily practice, it is important to know that results might have been generated without time pressure. However, in one study the duration of treatment was measured (Koshy 2005), and it was shown that the treatment time could be shortened when performing full‐mouth treatment. This could be an secondary effect as participants were only placed in the dentist's chair once. With a quadrant‐wise treatment, the participants had to visit the practice four times. There was no investigation on operator fatigue. The concentration of the dental professional and effectiveness of treatment may decrease after several hours of subgingival treatment on one person.
Although economic costs and patient burdens may be important for any treatment comparison, they could not be addressed in this review because of lack of data. There is a paucity of studies of long duration because supportive periodontal care begins six to 12 weeks after treatment. Therefore, effects due to different treatment modalities may be lost after longer observation periods.
Readers of this review are likely to be interested in the safety of treatment modalities; however, it was not possible to assess this in the long term, as RCTs are not appropriate study designs to assess the possible systemic effects related to safety. In the short term, three of 13 studies reported adverse systemic effects. Most were increased body temperature after the full‐mouth treatment modalities. Moreover, signs of an acute systemic reaction are associated with full‐mouth protocols. There was a greater acute‐phase response 24 hours after full‐mouth treatment (a three‐fold increase in CRP, two‐fold increase in IL‐6 and a slight increase in TNFα) in comparison with a conventional quadrant instrumentation (Graziani 2015). This evidence for systemic implications/adverse systemic effects of full‐mouth protocols led to the recommendation that this choice of treatment approach should always include careful consideration of the general health status of the patient (Sanz 2020a). According to a consensus report from an expert conference of the EFP and the World Heart Federation (WHF), in people with cardiovascular disease (CVD), irrespective of the level of CVD or specific medication, non‐surgical periodontal therapy should be provided, preferably in several 30‐ to 45‐minute sessions to minimise a spike of acute systemic inflammation (Sanz 2020b).
Quality of the evidence
The body of evidence for FMS versus control at both three to four (3/4) and six to eight (6/8) months was of very‐low to low certainty for PPD, CAL and BOP. This was downgraded two levels from 'high' due to some studies being at high or unclear risk of bias and there being a small number of trials and participants for some of the comparisons. We made a change to a risk of bias judgement in this comparison: we changed the judgement for Zijnge 2010 from unclear to low risk of reporting bias when updating the present review after considering up‐to‐date recommendations for assessing risk of bias. This made the study at low risk of bias overall. There was no evidence of heterogeneity in this comparison.
The body of evidence comparing FMD with control for both time periods was also of very‐low to low certainty for the same reasons, plus inconsistency in findings. We changed our judgement of the risk of detection bias in the largest study, Mongardini 1999, as we found some further information through revisiting the text in the original manuscript (see Characteristics of included studies table). The change from low to high risk of detection bias put the study at high risk of bias overall. There was some concern with unexplained heterogeneity in this comparison. There was considerable heterogeneity in the meta‐analyses for PPD reduction, CAL gain and BOP reduction, possibly due to differences in the time point of probing in relation to subgingival instrumentation and the type of probe used, as well as differences in the quality of instrumentation. Differences also existed for the use of chlorhexidine (or other antiseptic) and the time schedule for full‐mouth approaches, which ranged from 12 to 24 hours. More discrepancies might have resulted from the fact that not every group included oral hygiene instructions before baseline. Furthermore, even though all studies included minimal observation periods of three months, re‐evaluation was conducted at varying time points three to 12 months after treatment.
The body of evidence comparing FMS with FMD was of low certainty due to the small number of trials and participants in each comparison and the risk of bias in the studies. Only one of the five studies included in this comparison had a low risk of bias. Two of the other four studies were at high risk of bias.
The nine new studies added to the studies in the last update in 2015 increased the number of participants considerably (notwithstanding 37 participants were removed due to the exclusion of one previously included study after reconsideration (Knöfler 2007)). There are now 944 participants in contrast to the 389 in the 2015 update (Eberhard 2015).
Potential biases in the review process
We conducted a sensitive search of multiple databases to identify suitable studies for this review, with no restrictions on language or publication status. We attempted to contact some study authors for missing information, but we were unable to include all missing data. We recognise that some deviations from protocol may have introduced bias in the review process. However, we clearly reported the reasoning behind our judgements and we tried to be consistent.
Agreements and disagreements with other studies or reviews
Following completion and publication of the first version of this Cochrane Review (Eberhard 2008a); another systematic review originating from the sixth European Workshops on Periodontology was published with confirmatory data and conclusions (Lang 2008). Additionally, the authors attempted to perform a meta‐analysis of the microbiological results of the included studies; however, no conclusions could be drawn, mainly due to the differences in the microbiological techniques utilised. Another review published in the British Dental Journal suggested that both the traditional quadrant approach and the newer full‐mouth debridement could be equally effective as treatments for chronic periodontitis (Farman 2008). A review focusing on treatment time and oral hygiene in combination with different treatment modalities found no differences between full‐ and partial‐mouth treatment modalities (Tomasi 2009). It was concluded that long‐term treatment success mainly depends on the quality of patients' oral hygiene and instrumentation and less on the choice of treatment protocol or time spent on subgingival instrumentation. At the same time, another review article was published by the advocates of the full‐mouth treatment concept (Teughels 2009). Conclusions drawn by Teughels and colleagues were merely based on a literature overview without statistical evaluation. In their opinion, the one‐stage, full‐mouth disinfection concept results in significant additional clinical and microbiological improvements in non‐surgical periodontal therapy, which is in contrast to the findings of our systematic review and those of other groups (Eberhard 2008a; Eberhard 2015; Farman 2008; Lang 2008; Tomasi 2009). One systematic review with meta‐analysis that included 13 RCTs reported modest additional benefits for FMD versus Q‐SRP only for sites with moderate initial depth with regard to PPD reduction (based on four studies) and CAL gain (based on three studies), but no differences between treatment approaches with regard to all other analyses (Fang 2016).
Another review including 21 RCTs compared various full‐mouth treatments with SRP (Pockpa 2018). However, the review included several antimicrobial protocols, including the use of systemic antibiotics, probiotics and photodynamic therapy. A further systematic review with meta‐analysis also had a different focus and studied the effect of subgingival application of chlorhexidine gel in non‐surgical treatment (Zhao 2020).
As a background paper for the EFP S3 Level Clinical Practice Guideline (Sanz 2020a), one systematic review including meta‐analyses compared quadrant‐wise and full‐mouth approaches for subgingival instrumentation in the second step of therapy (Suvan 2020). There were no significant differences between treatment groups irrespective of examination time point or initial PPD (Suvan 2020).
Authors' conclusions
Implications for practice.
The inclusion of nine additional randomised controlled trials (RCT) in this updated review comparing the clinical effects of conventional mechanical treatment with full‐mouth scaling (FMS) and full‐mouth disinfection (FMD) approaches for the treatment of periodontitis has not changed the conclusions of the previous version of the review. From the 20 included trials, there is still no clear evidence that FMS or FMD is more beneficial than conventional subgingival instrumentation (scaling and root planing). There is insufficient evidence of a benefit for either FMS or FMD. In practice, the decision to select one approach to non‐surgical periodontal therapy over another can focus on patient preference and the convenience of the treatment schedule.
Implications for research.
To increase the quality of the evidence base, studies with low risk of bias are warranted, with attention paid to allocation concealment, complete outcome data reporting and blinding of outcome assessments. In this context, RCTs with higher numbers of participants would be useful. However, outcome assessment blinding can be compromised by participant awareness of differences between interventions and visible signs of differences in intervention groups, if, for example, not all debridement has been completed in comparison groups to FMS or FMD. Objective outcomes such as tooth loss might be less amenable to bias, although their value is limited by the duration of follow‐up needed (likely three to five years).
Future studies should address economic costs and patient burden, follow the recommendations of the CONSORT statement (www.consort-statement.org/), and ensure means and standard deviations are reported for all continuous outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 1 February 2022 | New citation required but conclusions have not changed | The addition of nine new RCTs has not changed the review's previous conclusions. |
| 17 June 2021 | New search has been performed | Search updated. Nine new RCTs included and seven new RCTs excluded. One RCT (from update 2015) excluded due to violation of inclusion criteria. Title changed from 'Full‐mouth treatment modalities (within 24 hours) for chronic periodontitis in adults' to 'Full‐mouth treatment modalities (within 24 hours) for periodontitis in adults'. Order of authors changed. Pocket closure added as secondary outcome. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 26 March 2015 | New citation required but conclusions have not changed | Five new trials included, one new trial excluded and one study awaiting classification. Title changed from 'Full‐mouth disinfection for the treatment of adult chronic periodontitis' to 'Full‐mouth treatment modalities (within 24 hours) for chronic periodontitis in adults'. |
| 26 March 2015 | New search has been performed | Search updated. |
| 6 March 2012 | Amended | Additional tables linked to text. |
| 30 July 2008 | Amended | Converted to new review format. |
Notes
This is an update of a published Cochrane Review (Eberhard 2004; Eberhard 2008a; Eberhard 2015).
Acknowledgements
For the 2022 update, we appreciate the excellent support from Cochrane Oral Health, specifically Laura MacDonald (Managing Editor), Anne Littlewood (Information Specialist) and Anne‐Marie Glenny (Co‐ordinating Editor). We want to acknowledge the valuable comments from the editors Thomas Lamont and Marco Esposito, and peer reviewers Akira Hasuike and Mark A Reynolds. We thank Anne Lawson (Copy Editor) for her valuable help in finalising the update. For previous versions, we thank Luisa Fernandez Mauleffinch, Anne‐Marie Glenny, Anne Littlewood, Philip Riley, Helen Wakeford, Janet Lear, Laura MacDonald, Esmonde Corbet, Bruce Pihlstrom and Carolyn Wayne.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
Cochrane Oral Health's Trials Register is available via the Cochrane Register of Studies. For information on how the register is compiled, see oralhealth.cochrane.org/trials.
From January 2014, updated searches of Cochrane Oral Health's Trials Register were undertaken using the Cochrane Register of Studies and the search strategy below:
1 ((periodont* or "furcation defect" or "intra‐bony defect*" or "intra bony defect*"or "infra‐bony defect*" or "infra bony defect*")) AND (INREGISTER) 2 ((scaling or scale or prophylaxis or "root plane*" OR "root planing" or debridem* or curett* or "pocket irrigat*" or chlorhexidine or eludril or chlorohex or corsodyl)) AND (INREGISTER) 3 (("full‐mouth" OR "full mouth")) AND (INREGISTER) 4 (#1 AND #2 AND #3) AND (INREGISTER)
Previous searches of the trials register were undertaken using the Procite software and the following search strategy:
((periodont* or "furcation defect" or "intra‐bony defect*" or "intra bony defect*"or "infra‐bony defect*" or "infra bony defect*") AND (scaling or scale or prophylaxis or "root plane*" OR "root planing" or debridem* or curett* or "pocket irrigat*" or chlorhexidine or eludril or chlorohex or corsodyl) AND ("full‐mouth" OR "full mouth"))
Appendix 2. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
#1 Exp PERIODONTAL DISEASES #2 periodont* #3 ((dental near scaling) or (tooth near scaling) or (tooth near scale*) or (teeth near scaling) or (teeth near scaled) or (supragingival next scaling) or (subgingival next scaling)) #4 Exp DENTAL PROPHYLAXIS #5 ((dental near prophylaxis) or (oral next prophylaxis) #6 ((root near plane*) or (root near planning)) #7 ((mechanical* near debride*) or (periodontal next debridement)) #8 (subgingival near curettage) #9 Exp SUBGINGIVAL CURRETTAGE #10 (pocket near irrigat*) #11 CHLORHEXIDINE #12 chlorhexidine #13 (eludril or chlorohex or corsodyl) #14 #1 or #2 #15 (#3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13) #16 ((full‐mouth near disinfection) or ((full next mouth) near disinfection) or ((full next mouth) near scaling) or (full‐mouth near scaling) or (full‐mouth near root‐planing) or ((full next mouth) near (root next planing)) or (full‐mouth near debridement) or ((full next mouth) near debridement)) #17 #14 AND #15 AND #16
Appendix 3. MEDLINE Ovid search strategy
1.exp Periodontal Diseases/ 2. periodont$.mp. 3. ((dental adj6 scaling) or (tooth adj6 scaling) or (tooth adj6 scale$) or (teeth adj6 scaling) or (teeth adj6 scale$) or (supragingival$ adj (scaling or scale$)) or (subgingival$ adj (scaling or scale$))).mp. 4. exp Dental Prophylaxis/ 5. (dental prophylaxis or oral prophylaxis).mp. 6. ((root adj plane$) or (root adj6 planing)).mp. 7. ((mechanical$ adj6 debride$) or periodontal adj debridem$).mp. 8. (subgingival adj curettage).mp. 9. exp Subgingival Curettage/ 10. (pocket adj6 irrigat$).mp. 11. CHLORHEXIDINE/ 12. chlorhexidine.mp. 13. (Eludril or Chlorohex or corsodyl).mp. 14. or/1‐2 15. or/3‐13 16. ((full‐mouth adj6 disinfection) or (full mouth adj6 disinfection) or (full mouth adj6 debridement) or (full mouth adj6 debridement) or full mouth scaling or full‐mouth scaling).mp. 17. 14 and 15 and 16
Appendix 4. Embase Ovid search strategy
1. exp Periodontal Diseases/ 2. periodont$.mp. 3. ((dental adj6 scaling) or (tooth adj6 scaling) or (tooth adj6 scale$) or (teeth adj6 scaling) or (teeth adj6 scale$) or (supragingival$ adj (scaling or scale$)) or (subgingival$ adj (scaling or scale$))).mp. 4. exp Dental Prophylaxis/ 5. (dental prophylaxis or oral prophylaxis).mp. 6. ((root adj plane$) or (root adj6 planing)).mp. 7. ((mechanical$ adj6 debride$) or periodontal adj debridem$).mp. 8. (subgingival adj curettage).mp. 9. exp Subgingival Curettage/ 10. (pocket adj6 irrigat$).mp. 11. CHLORHEXIDINE/ 12. chlorhexidine.mp. 13. (Eludril or Chlorohex or corsodyl).mp. 14. or/1‐2 15. or/3‐13 16. ((full‐mouth adj6 disinfection) or (full mouth adj6 disinfection) or (full mouth adj6 debridement) or (full mouth adj6 debridement) or full mouth scaling or full‐mouth scaling).mp. 17. 14 and 15 and 16
Appendix 5. CINAHL EBSCO search strategy
S1 MH "Periodontal Diseases+" S2 periodont* S3 ((dental N5 scaling) or (tooth N5 scaling) or (tooth N5 scale*) or (teeth N5 scaling) or (teeth N5 scale*) or (supragingival N5 scaling) or (subgingival N5 scaling)) S4 MH "Dental Prophylaxis+" S5 ((dental N5 prophylaxis) or (oral N5 prophylaxis)) S6 ((root N5 plane*) or (root N5 planing)) S7 ((mechanical* N5 debride*) or (periodontal N5 debridement)) S8 (subgingival N5 curettage) S9 (pocket N5 irrigat*) S10 MH Chlorhexidine S11 chlorhexidine S12 (eludril or chlorohex or corsodyl) S13 S1 or S2 S14 S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 S15 ((full‐mouth N5 disinfection) or ("full mouth" N5 disinfection) or ("full mouth" N5 scaling) or (full‐mouth N5 scaling) or (full‐mouth N5 root‐planing) or ("full mouth" N5 "root planing") or (full‐mouth N5 debridement) or ("full mouth" N5 debridement)) S16 S13 and S14 and S15
Appendix 6. US National Institutes of Health Trials Register and WHO International Clinical Trials Registry Platform search strategy
periodontitis AND full mouth
Appendix 7. Assessment of risk of bias in included studies
Method of randomisation sequence generation was classified as:
low risk of bias when random number generation was used such as computer generated schemes;
high risk of bias when other methods of randomisation were used (such as alternate assignment, hospital number);
unclear when method of randomisation was not reported or explained.
Allocation concealment (i.e. how the randomisation sequence was hidden from the examiners) was classified as:
low risk of bias when examiners were kept unaware of randomisation sequence (e.g. by means of central randomisation, sequentially numbered, opaque envelopes);
high risk of bias when other methods of allocation concealment were used (such as alternate assignment, hospital number);
unclear when method of allocation concealment was not reported or explained.
Blinding of examiners was classified as:
low risk of bias when the outcome assessors were blinded to the intervention;
high risk of bias when the outcome assessors knew which intervention a participant had received;
unclear when there was insufficient information to determine if the outcome assessors were blinded or not.
Completeness of outcome data was assessed as:
low risk of bias if there was no missing data, or missing data was balanced across the groups with similar reasons unlikely to be due to the intervention, or missing data were imputed using appropriate methods;
high risk of bias if reason for missing data was likely to be related to outcomes, or if there was a large proportion of missing data;
unclear when there is insufficient reporting of attrition/exclusions.
Selective outcome reporting was assessed as:
low risk of bias if all primary and secondary outcomes were reported;
high risk of bias if not all of the study's prespecified outcomes (protocol/abstract) were reported;
unclear if there was insufficient information on prespecified outcomes.
Other potential threats to validity were assessed as:
high risk of bias if a potential source of bias was related to a specific study design issue not already covered (high baseline imbalance for periodontal severity and smoking);
low risk of bias if there was no evidence of any other biases;
unclear if there was insufficient information provided to make decision.
Data and analyses
Comparison 1. Full‐mouth scaling (FMS) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Change in probing pocket depth: whole mouth, single‐ and multi‐rooted teeth | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 At 3/4 months | 7 | 228 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.10, 0.16] |
| 1.1.2 At 6/8 months | 5 | 148 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.14, 0.20] |
| 1.2 Change in clinical attachment level: whole mouth, single‐ and multi‐rooted teeth | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 At 3/4 months | 7 | 228 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.20, 0.15] |
| 1.2.2 At 6/8 months | 5 | 148 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.05, 0.26] |
| 1.3 Change in bleeding on probing: whole mouth, single‐ and multi‐rooted teeth | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 At 3/4 months | 5 | 158 | Mean Difference (IV, Random, 95% CI) | ‐2.30 [‐6.73, 2.13] |
| 1.3.2 At 6/8 months | 3 | 80 | Mean Difference (IV, Random, 95% CI) | 2.64 [‐8.81, 14.09] |
Comparison 2. Full‐mouth disinfection (FMD) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Change in probing pocket depth: whole mouth, single‐ and multi‐rooted teeth | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 At 3/4 months | 7 | 427 | Mean Difference (IV, Random, 95% CI) | 0.07 [0.06, 0.08] |
| 2.1.2 At 6/8 months | 6 | 214 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.04, 0.27] |
| 2.2 Change in clinical attachment level: whole mouth, single‐ and multi‐rooted teeth | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 At 3/4 months | 7 | 427 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.09, ‐0.07] |
| 2.2.2 At 6/8 months | 6 | 214 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.11, 0.24] |
| 2.3 Change in bleeding on probing: whole mouth, single‐ and multi‐rooted teeth | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.3.1 At 3/4 months | 5 | 153 | Mean Difference (IV, Random, 95% CI) | 6.37 [‐7.32, 20.06] |
| 2.3.2 At 6/8 months | 4 | 92 | Mean Difference (IV, Random, 95% CI) | 9.54 [‐2.24, 21.32] |
Comparison 3. Full‐mouth scaling (FMS) versus full‐mouth disinfection (FMD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Change in probing pocket depth: whole mouth, single‐ and multi‐rooted teeth | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1.1 At 3/4 months | 4 | 115 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.34, ‐0.00] |
| 3.1.2 At 6/8 months | 4 | 112 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.30, 0.07] |
| 3.2 Change in clinical attachment level: whole mouth, single‐ and multi‐rooted teeth | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.2.1 At 3/4 months | 4 | 115 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.37, ‐0.05] |
| 3.2.2 At 6/8 months | 4 | 112 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.23, 0.13] |
| 3.3 Change in bleeding on probing: whole mouth, single‐ and multi‐rooted teeth | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.3.1 At 3/4 months | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐1.59 [‐9.97, 6.80] |
| 3.3.2 At 6/8 months | 2 | 42 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐13.27, 12.87] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Afacan 2020.
| Study characteristics | ||
| Methods |
Study design: RCT with 3‐arm parallel design Recruitment period: October 2006–May 2008 Setting: University Dental Hospital, Turkey Number of centres: 1 Funding source: The Scientific and Technological Research Council of Turkey, grant/award number 106S212 |
|
| Participants |
Inclusion criteria: diagnosis of chronic periodontitis with PPD ≥ 6 mm Exclusion criteria: systemic disease or on antibiotics from 6 months before or during study Age: 35–60 years Sex: 24 F (FMD: 8; FMS: 7; control: 9), 36 M (FMD: 12; FMS: 13; control: 11) Smokers: 0 Number randomised: 68 (FMD: 23; FMS: 22; control: 23) Number evaluated: 60 (FMD: 20; FMS: 20; control: 20) |
|
| Interventions |
Comparison: FMD vs control; FMS vs control; FMD vs FMS FMD group: FMD US instrumentation in 2 sessions within 24 hours, after instrumentation: tongue brushing: CHX 1%, 1 minute; rinse: CHX 0.2%, 2 × 1 minute; spray pharynx: CHX 0.2%; subgingival: CHX 1%, 3 × within 10 minutes, repeated at day 8. Home: rinse CHX 0.2%, 1 minute, 2 × day; spray tonsils: CHX 0.2%, 2 × day, 2 weeks FMS group: (FMUD) US instrumentation in 2 sessions within 24 hours Control group: (QRP) 4 sessions – 1‐weekly intervals, start 1 Q, hand instruments OHI before study start: no Instruments used: hand and US instruments Time per Q: unknown Maintenance: at 1, 3 and 6 months from last instrumentation Retreatment: none Duration of study: 6 months |
|
| Outcomes |
Primary outcome: change in GCF biomarker levels Secondary outcomes: PPD, CAL (6 sites per tooth), changes of percentage of pockets with initial PD ≥ 5 mm, changes periodontal pathogen levels Teeth: whole‐mouth recordings with manual probe Pocket depth at baseline: sampling sites (GCF) ≥ 6 mm for selected sites Outcome time reported: 3‐ and 6‐month data used. Baseline, 1, 3 and 6 months measured Other outcomes: PI, PBI, number of single‐rooted teeth, tooth loss, any harm after treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study co‐ordinator randomly allocated participants into 3 groups using a computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | Unknown; operator and examiner was the same person. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Clinical measurements, GCF, plaque sampling and all treatments were performed by the same trained investigator in a standardised way. Comment: the same person undertook the interventions and the outcome assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed study. |
| Selective reporting (reporting bias) | Low risk | Data reported on all primary and secondary outcomes. |
| Other bias | Low risk | Baseline balance good for pocket depth. No apparent other biases. |
Apatzidou 2004.
| Study characteristics | ||
| Methods |
Study design: RCT with 2‐arm parallel design Recruitment period: unclear Setting: University Dental Hospital, Scotland Number of centres: 1 Funding source: unclear |
|
| Participants |
Inclusion criteria: diagnosis of chronic periodontitis with PD ≥ 5 mm Exclusion criteria: systemic disease or on antibiotics from 3 months before or during study Age: 31–70 years Sex: 17 F and 23 M Smokers: 15 Number randomised: 40 (20 per group), 2 Asian (1 per group), 38 Caucasian (assumed to be white people) Number evaluated: 40 (20 per group) |
|
| Interventions |
Comparison: FMS vs control FMS group: (FM‐SRP) 2 sessions same day Control group: (Q‐SRP) QRP 4 sessions at 2‐weekly intervals OHI before study start: unknown Instruments used: hand and US instruments Time per Q: 1 hour Maintenance: at 7 weeks (FMS) or 13 weeks (QRP) and 6 months from baseline (both groups) Retreatment: none Duration of study: 6 months |
|
| Outcomes |
Primary outcome: PPD (6 sites per tooth) Secondary outcomes: CAL/RAL, BOP (6 sites per tooth) Teeth: whole‐mouth recordings with manual probe, moderate and deep PD at baseline Pocket depth at baseline: moderate (≥ 5 and < 7 mm), deep (≥ 7 mm), for selected sites (deepest site per Q) Outcome time reported: 6‐month data used. Baseline, 6‐week re‐assessment after last instrumentation (FM‐SRP: 7 weeks; Q‐SRP: 13 weeks from baseline), 25 weeks. Computer‐assisted disk probe for selected sites Other outcomes: MGI, PI, SUP (selected site clinical analysis = 1 deepest pocket per Q). Mean pain VAS score (0–10), body temperature, number of analgesics, cold sores or oral ulcers |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomised into two groups". |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Clinical measurements were collected by a calibrated single examiner (D. A. A.) and unbiased data collection was assured by having no access to recordings of previous visits". Comment: blinding at high risk of bias as the same person undertook the interventions and the outcome assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed study. |
| Selective reporting (reporting bias) | Low risk | Data reported on all primary and secondary outcomes. |
| Other bias | Low risk | Baseline balance good for smokers and pocket depth. No apparent other biases. |
Babaloo 2018.
| Study characteristics | ||
| Methods |
Study design: RCT with 2‐arm parallel design Recruitment period: unclear Setting: University Dental Hospital, Iran Number of centres: 1 Funding source: none |
|
| Participants |
Inclusion criteria: diagnosis: chronic periodontitis with ≥ 12 teeth Exclusion criteria: systemic disease or on antibiotics from 2 months before or during study Age: 25–70 years (mean: FMD: 43 (SD 12.47) years; control: 47.7 (SD 9.4))* Sex: 19 F (FMD: 11; control: 8) and 21 M (FMD: 9; control 12)* Smokers: unknown Number randomised: 40 (20 per group)* Number evaluated: 40 (20 per group)* (implied but not explicitly stated) |
|
| Interventions |
Comparison: FMD vs control FMD group: US instrumentation in 2 sessions within 24 hours, subgingival: CHX 0.2%; tongue brushing: CHX 0.2%; rinse: CHX 0.2% Control group: (Q‐SRP) QRP 4 sessions – 2‐weekly intervals, type of instruments unknown OHI before study start: unknown Instruments used: US instruments, unknown if hand instruments were used Time per Q: unknown Maintenance: at week 2, 4, 8, 12 and 16 after treatment (both groups), PI recorded, OHI given Retreatment: none Duration of study: 4 months |
|
| Outcomes |
Primary outcome: unknown Secondary outcomes: PPD, CAL, BOP (4 sites per tooth), serum IL‐27 (see Shirmohammadi 2013 under Babaloo 2018; also serum IL‐17 And IL‐1β were evaluated) Teeth: whole‐mouth recordings with manual probe Pocket depth at baseline: data not split for pocket depth categories Outcome time reported: 4‐month data used. Baseline, 2 and 4 months reported Other outcomes: PI, MGI* |
|
| Notes | *additional information found in Shirmohammadi 2013 under Babaloo 2018. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to two groups using toss of a coin". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information for judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All the measurements were performed by one calibrated periodontist who was blind to the treatment groups" (additional information from Shirmohammadi 2013 under Babaloo 2018). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information for judgement. It is implied that all participants completed the study but not explicitly stated. |
| Selective reporting (reporting bias) | Low risk | Data reported on secondary outcomes; primary outcome not defined. |
| Other bias | Unclear risk | Baseline balance good for pocket depth; however smoking unclear. No apparent other biases. |
Del Peloso 2008.
| Study characteristics | ||
| Methods |
Study design: RCT with 2‐arm parallel design Recruitment period: July 2005–June 2006 Setting: University Dental Hospital, Brazil Number of centres: 1 Funding source: unclear |
|
| Participants |
Inclusion criteria: diagnosis of severe chronic periodontitis with PD ≥ 5 mm and BOP positive Exclusion criteria: medical disorders, SRP in past 6 months or on antibiotics from 6 months before or during study, smokers, pregnancy Age: 30–66 years Sex: 18 F (9 per group) and 7 M (FMS: 4; control: 3) Smokers: 0 Number randomised: 25 (FMS: 13; control: 12) Number evaluated: 25 (FMS 13; control: 12) |
|
| Interventions |
Comparison: FMS vs control FMS group: 1 session within 45 minutes Control group: (SRP) QRP 4 sessions at 1‐week intervals OHI before study start: yes Instruments used: hand and US instruments Time per Q: 45 minutes for test group Maintenance: every month Retreatment: after 3 months (PPD ≥ 5 mm) Duration of study: 6 months |
|
| Outcomes |
Primary outcome: PPD (6 sites per tooth) Secondary outcomes: RAL, BOP (6 sites per tooth) Teeth: whole‐mouth recordings with manual probe, moderate and severe PD at baseline Pocket depth at baseline: moderate (5 mm and 6 mm), deep (≥ 7 mm) (authors' information). Manual probe with stent Outcome time reported: 3 months used, 6 months also reported* Other outcomes: plaque score, GBI, recession (6 sites per tooth), body temperature, VAS for patient, VPI after initial prophylaxis, 30% in FMS and 40% in control group |
|
| Notes | Starting Q of SRP unclear. On request only BOP for sites > 4 mm; not for subgroups. * 6 months data not used because of retreatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised into two groups according to a computer‐generated list". |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation concealment was secured by having a person not involved in the study performing the randomisation. This person was different from the one responsible for the treatment (S. B.) and different from the examiner (E. D. P. R.). The randomisation code was not broken until all data had been collected. Thus, the treatment group was not revealed to the clinical examiner or to the statistician". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treatment and examination by 2 independent people. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed study. |
| Selective reporting (reporting bias) | Low risk | Data reported on all primary and secondary outcomes. |
| Other bias | Low risk | Baseline balance good for pocket depth. No apparent other biases. |
Fonseca 2015.
| Study characteristics | ||
| Methods |
Study design: RCT with 6‐arm parallel design (3 arms included) Recruitment period: 2011–2012 Setting: University Dental Hospital, Brazil Number of centres: 1 Funding source: Productivity Research fellows (PQ) and the National Program of Academic Cooperation (PROCAD) grant 552264/2011‐3 from National Council of Scientific and Technological Development (CNPq), Brasilia, Brazil (to FOC) |
|
| Participants |
Inclusion criteria: diagnosis of chronic periodontitis Exclusion criteria: systemic disease or on antibiotics from 3 months before or during study Age: 35–60 years Sex: 52 F (grouping unknown), 33 M (grouping unknown) Smokers: 72 non‐smokers, 13 smokers, grouping unknown Number randomised: 90 (15 in each of 6 groups) Number evaluated: 85 (groups included in review – FMD: 15; FMS: 15; control: 13) |
|
| Interventions |
Comparison: FMD vs control; FMS vs control; FMD vs FMS FMD group: (FC) hand instrumentation in 2 sessions within 24 hours, 60 minutes per session, for 2 consecutive days, after instrumentation: subgingival CHX 1%; tongue brushing: CHX 1%, 1 minute; rinse: CHX 0.12%, 30 seconds, last 10 seconds involving gargling, 2 × per session. Home: rinse: CHX 0.12%, 2 × day, 2 weeks FMS group: (FNC): hand instrumentation within 24 hours in 2 sessions, 60 minutes each session, for 2 consecutive days Control group: (QSNC) QRP 4 sessions, 1‐weekly intervals, hand instruments, 30 minutes for each Q Groups not used in the review: FMD using AZ; SRP plus AZ; SRP plus CHX OHI before study start: unknown Instruments used: hand instruments Time per Q: 30 minutes Maintenance: none reported Retreatment: none Duration of study: 6 months |
|
| Outcomes |
Primary outcome: PPD (6 sites per tooth). PPD used for whole mouth as well as for pocket categories Secondary outcomes: CAL (6 sites per tooth), changes total bacterial counts. CAL used for whole‐mouth recordings* Teeth: whole‐mouth recordings with manual probe Pocket depth at baseline: 4 mm and 5 mm, ≥ 6 mm Outcome time reported: 3‐ and 6‐month data used. Baseline, 3 and 6 months measured Other outcomes: *changes in baseline CAL 3–4 mm and ≥ 5 mm (not used, does not correspond the PPD sites) |
|
| Notes | Quote: "The groups were homogeneous in regard to sex, age, and smoking status (P >0.05)". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised into groups according to opaque envelopes. |
| Allocation concealment (selection bias) | Low risk | Quote: "Ninety opaque envelopes containing the groups' therapy identifications were sealed, mixed, and numbered sequentially". Envelopes "were opened by a masked researcher (LOMC)". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Examiners (FOC and JRC) were masked to the intervention group", "Treatment procedures were performed by four experienced periodontists (DCF, SCC, LCMC, and MVMC) masked to the adjuvant groups." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout of 2 participants in the control group before 3‐month examination for unclear reasons. |
| Selective reporting (reporting bias) | Low risk | Data reported on all primary and secondary outcomes. |
| Other bias | Unclear risk | Baseline balance good for pocket depth, however smoking unclear. No apparent other biases. |
Graziani 2015.
| Study characteristics | ||
| Methods |
Study design: RCT with 2‐arm parallel design Recruitment period: July 2012–July 2013 Setting: university dental hospital, Pisa, Italy Number of centres: 1 Funding source: Unit of Dentistry and Oral Surgery of the University of Pisa and by the Italian Ministry Health and the Tuscan Region (Grant # GR‐2009‐1592229). FD holds a Clinical Senior Lectureship Award supported by the UK Clinical Research Collaboration. MO holds a UCL Impact Award partially supported with a fellowship grant by Johnson and Johnson Consumer Services EAME Limited. FD and MO work at UCL, which received a proportion of funding from the Department of Health's National Institute of Health Research (NIHR) Biomedical Research Centres funding scheme. |
|
| Participants |
Inclusion criteria: diagnosis of chronic periodontitis* Exclusion criteria: medical disorders, SRP in past 6 months or on antibiotics from 3 months before or during study, smokers, pregnancy Age: FMS: 46 (SD 12) years; control: 48 (SD 9) years Sex: 19 F (FMS: 9; control: 10) and 19 M (FMS: 10; control: 9) Smokers: FMS: 7; control: 6 Number randomised: 38 (19 per group), all 'Caucasian' Number evaluated: 38 (19 per group) |
|
| Interventions |
Comparison: FMS vs control FMS group: 2 session within 24 hours Control group: (SRP) QRP 4 sessions at 1‐week intervals OHI before study start: yes Instruments used: hand and US instruments Time per Q: unclear Maintenance: none Retreatment: none Duration of study: 3 months |
|
| Outcomes |
Primary outcome: CRP increase Secondary outcomes: changes in a broad array of inflammatory and endothelial injury markers Teeth: whole‐mouth recordings with UNC‐15 manual probe Pocket depth at baseline: PPD > 4 mm Outcome time reported: 3 months used Other outcomes: PPD, CAL, BOP, PI (6 sites per tooth), body temperature |
|
| Notes | *Diagnosis provided by corresponding author on request. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table. |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation to treatment was concealed to the clinical examiner and statistician with opaque envelopes which were opened by the clinician on the day of treatment". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Examination by a blinded calibrated examiner, treatment by a single periodontist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed study. |
| Selective reporting (reporting bias) | Low risk | Data reported on all primary and secondary outcomes. |
| Other bias | Low risk | Baseline balance good for pocket depth. No apparent other biases. |
Jervøe‐Storm 2006.
| Study characteristics | ||
| Methods |
Study design: RCT with 2‐arm parallel design Recruitment period: 2003–2004* Setting: University Dental Hospital, Bonn, Germany Number of centres: 1 Funding source: unclear |
|
| Participants |
Inclusion criteria: diagnosis of chronic periodontitis with PD ≥ 5 mm and BOP positive. All participants in good general health Exclusion criteria: SRP in past 6 months or on antibiotics from 6 months before or during study, pregnancy Age: 53.1 (SD 10.2); range 37–77 years* Sex: 11 F (FMS: 5; control: 6) and 9 M (FMS: 5; control: 4) Smokers: 2 (1 in each group) (smoking ≥ 10 cigarettes per day) Number randomised: 20 Number evaluated: 20 (10 per group), all Caucasian (assumed to be white people) |
|
| Interventions |
Comparison: FMS vs control FMS group: (FM‐RP) FMS 2 sessions within 24 hours on 2 consecutive days Control group: QRP 4 sessions at 1‐week intervals OHI before study start: yes Instruments used: hand and US instruments Time per Q: 1 hour Maintenance: every month after 3 months Retreatment: none Duration of study: 6 months |
|
| Outcomes |
Primary outcome: PPD (6 sites per tooth) Secondary outcomes: RAL, BOP (only for PPD > 4 mm) (6 sites per tooth) Teeth: whole‐mouth recordings with computer‐assisted probe with stent for all measurements, moderate and severe PD at baseline Pocket depth at baseline: moderate (5 to < 7 mm), deep (≥ 7 mm) Outcome time reported: 3‐ and 6‐month data used Other outcomes: data from first Q |
|
| Notes | * on request, clarified by authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised into two groups according to a computer generated list provided by an external agent". |
| Allocation concealment (selection bias) | Low risk | Not mentioned in report of trial but author stated "treatment was concealed for all participants until first intervention. The randomisation was first made, when the patient was sitting in the office and treatment began. An independent person gave the treatment‐mode to the therapist". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All measurements were performed by one blinded examiner". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed study. |
| Selective reporting (reporting bias) | Low risk | Data reported on all primary and secondary outcomes. |
| Other bias | Low risk | Baseline balance good for pocket depth. No apparent other biases. |
Koshy 2005.
| Study characteristics | ||
| Methods |
Study design: RCT with 3‐arm parallel design Recruitment period: unclear Setting: university dental clinic, Japan Number of centres: 1 Funding source: grant from Scientific Society |
|
| Participants |
Inclusion criteria: diagnosis of chronic periodontitis with PD ≥ 5 mm. All participants in good general health Exclusion criteria: SRP in past 6 months or on antibiotics from 6 months before or during study, smokers, pregnancy, allergic to iodine Age: 34–66 years Sex: 23 F (FMD: 8; FMS: 7; control: 8) and 13 M (FMD: 4; FMS: 5; control: 4) Smokers: 0 Number randomised: 36 (12 per group); all Japanese Number evaluated: 36 (12 per group) |
|
| Interventions |
Comparison: FMS vs control; FMD vs control; FMS vs FMD FMS group: (FMS + water): FMS 1 session US scaling with water (duration 2–2.5 hours) FMD group: (FMS + povidone): FMS 1 session US scaling with 1% povidone iodine (duration 2–2.5 hours), participants rinsing with CHX 0.05% twice a day for 1 month, tongue brushing Control group: (QMD) QRP 4 sessions US scaling with water at 1‐week intervals (duration 40–50 minutes each) OHI before study start: yes Instruments used: US instruments Time per Q: unclear Maintenance: every month Retreatment: none Duration of study: 6 months |
|
| Outcomes |
Primary outcome: PPD (6 sites per tooth) Secondary outcomes: PAL, BOP (6 sites per tooth). Manual probe with stent for all measurements Teeth: whole‐mouth recordings (baseline, 1, 3 and 6 months). Data split in single‐/multi‐rooted teeth and initial moderate (PPD 5–6 mm) and deep pockets (PPD > 6 mm) Pocket depth at baseline: moderate (5 to < 7 mm), deep (≥ 7 mm) Outcome time reported: 6 months used Other outcomes: PI, mean pain VAS score (0–10), body temperature, number of analgesics, microbiology |
|
| Notes | PAL is equal to RAL. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random sequence was computer generated, with no stratification or balancing of factors". |
| Allocation concealment (selection bias) | Low risk | Quote: "The subjects chose a sequentially numbered opaque, sealed envelope, which enclosed the code for the treatment protocol they were to receive. The number of envelopes was same as the number of subjects". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The treatment groups were coded so that only the operator was aware of the protocol and the examiner remained blinded throughout the study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed study. |
| Selective reporting (reporting bias) | Low risk | Data reported on all primary and secondary outcomes. |
| Other bias | Low risk | Baseline balance good for pocket depth. No apparent other biases. |
Mongardini 1999.
| Study characteristics | ||
| Methods |
Study design: RCT with 2‐arm parallel design Recruitment period: unclear Setting: university dental hospital, Belgium Number of centres: 1 Funding source: supported by university |
|
| Participants |
Inclusion criteria: diagnosis of chronic periodontitis with PD ≥ 7 mm and BOP (people with aggressive periodontitis also included). All participants in good general health Exclusion criteria: antibiotics from 4 months before or during study, smokers Age: 23–69 years (based on all 40) Sex: 9 F (FMD: 7; control: 2), 15 M (FMD: 5; control 10) Smokers: 8 (FMD: 3; control: 5) (smoking ≥ 10 cigarettes per day) Number randomised: 24 (40 including aggressive periodontitis) Number evaluated: 24 (12 per group) |
|
| Interventions |
Comparison: FMD vs control* FMD group: 2 sessions within 24 hours, after instrumentation; tongue brushing: CHX 1%, 1 minute; rinse: CHX 0.2%, 1 minute; spray pharynx: CHX 0.2%; subgingival: CHX 1%, 3 times within 10 minutes, repeat subgingival after 8 days. Home: rinse CHX 0.2%, 1 minute, 2 × day, 2 months; spray: CHX 0.2%, 2 × day, 2 months Control group: SRP 4 sessions 2‐weekly intervals OHI before study start: no Instruments used: hand instruments Time per Q: unclear Maintenance: after 1, 2 and 4 months Retreatment: none Duration of study: 8 months |
|
| Outcomes |
Primary outcome: PPD (4 sites per tooth) Secondary outcomes: CAL, BOP (4 sites per tooth). Manual probe for all measurements Teeth: only recording of first Q. Data split in single‐/multi‐rooted teeth and initial moderate (PPD 4.5–6.5 mm) and deep pockets (PPD ≥ 7 mm) Pocket depth at baseline: moderate (PPD 4.5–6.5 mm) and deep pockets (PPD ≥ 7 mm) Outcome time reported: 4 and 8 months used, 1, 2, 4 and 8 months measured Other outcomes: SBI, plaque extent, pain and swelling on VAS, number of analgesics, occurrence of herpes labialis or oral ulcers |
|
| Notes | Only data from participants with chronic periodontitis were included in the meta‐analysis. *The follow‐up paper Quirynen 2000 involved a third group that was not randomised. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…the participants signed an informed consent form and were randomly distributed between test and control groups by coin toss…". |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The same person (CM) performed treatment and examination. Quote: "The sessions of scaling and root planing (SRP) were performed under local anaesthesia by the same investigator (CM)…" "…clinical parameters…were recorded by the same periodontist (CM)". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed study. |
| Selective reporting (reporting bias) | Low risk | Data reported on all primary and secondary outcomes. |
| Other bias | Low risk | Baseline balance good for pocket depth and smoking. No apparent other biases. |
Pontillo 2018.
| Study characteristics | ||
| Methods |
Study design: RCT with 3‐arm parallel design (2 arms included) Recruitment period: September 2015–October 2016 Setting: university dental hospital, Brazil Number of centres: 1 Funding source: unclear |
|
| Participants |
Inclusion criteria: diagnosis of moderate–severe chronic periodontitis with PD ≥ 5 mm. All participants in good general health Exclusion criteria: SRP in past 6 months or antibiotics from 6 months before or during study, compromised medical condition, pregnancy Age: 25–62 years Sex: 11 F (FMD: 6; control: 5) and 17 M (FMD: 8; control: 9) Smokers: 0 Number randomised: 28 in the 2 relevant arms (14 in each group) Number evaluated: 28 in the 2 relevant arms (14 in each group) |
|
| Interventions |
Comparison: FMD vs control FMD group: (FM‐SRP): 2 sessions scaling within 24 hours, subgingival: CHX 0.12%. Home: rinse: CHX 0.1 %, 15 days Control group: QRP (Q‐SRP): 4 sessions scaling, 1‐week intervals, no antiseptics Groups not used in the review: a third group involving periodontally healthy participants was labelled "control" in the paper; we used only the intervention groups (Q‐SRP, which we named "control" and FM‐SRP, which we named "FMD". OHI before study start: yes Instruments used: hand and US instruments Time per Q: "unrestricted" Maintenance: none Retreatment: none Duration of study: 6 months |
|
| Outcomes |
Primary outcome: PPD, CAL, BOP (6 sites per tooth), manual probe for all measurements Secondary outcomes: GCF and prostaglandin E2 Teeth: whole‐mouth recordings. PPD > 5 mm Pocket depth at baseline: PPD > 5 mm Outcome time reported: 6 months data used; 1, 3 and 6 months measured Other outcomes: GI, PI |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear. Quote: "Patients were randomly separated". |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The initial clinical examination was performed by a single previously trained examiner" and "the whole treatment was performed by a single operator". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | Data reported on all outcomes. |
| Other bias | Low risk | Baseline balance good for pocket depth and smoking. No apparent other biases. |
Predin 2014.
| Study characteristics | ||
| Methods |
Study design: RCT with 2‐arm parallel design Recruitment period: unknown Setting: university dental hospital, Serbia Number of centres: 1 Funding source: research grant No. 175075 from the Ministry of Education and Science of Serbia |
|
| Participants |
Inclusion criteria: diagnosis with chronic periodontitis Exclusion criteria: medical disorders, SRP in past 6 months or on antibiotics from 3 months before or during study, pregnancy Age: 32–75 years Sex: 31 F (FMS: 16; control: 15) and 9 M (FMS: 4; control: 5) Smokers: FMS: 4; control: 3 Number randomised: 48 (24 per group) Number evaluated: 40 (FMS: 21; control: 19), 8 dropouts (FMS: 3; control: 5) (Quote: "Subsequently, eight more patients were excluded from the study for various reasons") |
|
| Interventions |
Comparison: FMS vs control FMS group: (FMRP) 2 sessions within 24 hours Control group: QRP 4 sessions at 1‐week intervals OHI before study start: yes Instruments used: hand and US instruments Time per Q: unclear Maintenance: none Retreatment: none Duration of study: 3 months |
|
| Outcomes |
Primary outcome: unclear* Secondary outcomes: unclear* Teeth: whole‐mouth recordings (baseline, 1 and 3 months). Data split in initial moderate (PPD 5–7 mm) and deep pockets (PPD ≥ 7 mm). Williams manual probe Pocket depth at baseline: moderate (5 to < 7 mm), deep (≥ 7 mm) Outcome time reported: 3 months data used; 1 and 3 months measured Other outcomes: *PPD, CAL, BOP, PI, GI, PBI (4 sites per tooth), body temperature |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed with a computer‐generated list. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomised into two groups according to a computer‐generated list provided by a person not involved in the study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All the measurements have been conducted by the same investigator blind to the therapeutic protocol applied". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Time point of dropout for each of the 8 participants unclear. |
| Selective reporting (reporting bias) | Low risk | All parameters have been reported. |
| Other bias | Low risk | Baseline balance good. |
Quirynen 2006.
| Study characteristics | ||
| Methods |
Study design: RCT with 5‐arm parallel design (3 arms included) Recruitment period: unclear Setting: university dental hospital, Belgium Number of centres: 1 Funding source: supported by University |
|
| Participants |
Inclusion criteria: diagnosis of chronic periodontitis with PD ≥ 6 mm. All participants in good general health Exclusion criteria: SRP in past 12 months or antibiotics from 4 months before or during study, compromised medical condition, pregnancy Age: 31–75 years. All Caucasian (assumed to be white people) Sex: 19 F (FMS: 10; FMD: 4; control: 5) and 24 M (FMS: 4; FMD: 10; control: 10) Smokers: 11 (FMS: 3; FMD: 3; control: 5) Number randomised: 85 in 5 arms Number evaluated: 43 in 3 arms (FMS: 14; FMD: 14; control: 15) (71 in 5 arms) |
|
| Interventions |
Comparison: FMS vs control; FMD vs control; FMS vs FMD FMS group: (FRp): FMS 2 sessions over within 24 hours FMD group: (FM‐CHX): 2 sessions within 24 hours, after instrumentation: tongue brushing: CHX 1%, 1 minute; rinse: CHX 0.2%, 2 × 1 minute; spray pharynx: CHX 0.2%; subgingival: CHX 1%, 3 × within 10 minutes. Home: rinse CHX 0.2%, 1 minute, 2 × day, 2 months Control group: (NC): QRP 4 sessions scaling – 2‐week intervals, no antiseptics Groups not used in the review: 2 arms that were variations of the FMD intervention, i.e. amine fluoride/stannous fluoride for 2 months after full‐mouth scaling or chlorhexidine for the first 2 months followed by amine fluoride/stannous fluoride for another 6 months. OHI before study start: no Instruments used: hand instruments Time per Q: unclear Maintenance: 1, 2 and 4 months Retreatment: none Duration of study: 8 months |
|
| Outcomes |
Primary outcome: PPD (6 sites per tooth) Secondary outcomes: CAL (as sum of PPD and GR), BOP (6 sites per tooth). Manual probe for all measurements Teeth: first Q recordings (baseline, 2, 4 and 8 months). Data split in single‐/multi‐rooted teeth and initial medium (PPD 4–5.5 mm) and deep pockets (PPD > 5 mm) Pocket depth at baseline: moderate (PPD 4.5–6.5 mm) and deep pockets (PPD ≥ 7 mm) Outcome time reported: 8 months used, 1, 2, 4* and 8 months measured Other outcomes: SBI, PI, GR (6 sites per tooth) |
|
| Notes | Dropouts: 85 enrolled, 71 completed the study. Time point for dropouts unclear. Only 3 arms of trial included. *Authors could not provide data for 4 months evaluation on request. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A clinician who was informed about the baseline clinical data (but not about the content of the treatment strategies) randomly allocated (via a random‐number table) the consecutive participants (if fulfilling criteria) to one of the following groups". |
| Allocation concealment (selection bias) | Low risk | Quote: "A clinician who was informed about the baseline clinical data (but not about the content of the treatment strategies) randomly allocated (via a random‐number table) the consecutive participants (if fulfilling criteria) to one of the following groups". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treatment and examination by 2 independent people. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts 14/85; unclear reasons or timing of dropouts. |
| Selective reporting (reporting bias) | Low risk | Data reported on all primary and secondary outcomes. |
| Other bias | Low risk | Baseline balance good for pocket depth and smoking. No apparent other biases. |
Roman‐Torres 2018.
| Study characteristics | ||
| Methods |
Study design: RCT with 2‐arm parallel design Recruitment period: 2010–2014 Setting: university dental hospital, Brazil Number of centres: 1 Funding source: unknown |
|
| Participants |
Inclusion criteria: diagnosis of chronic periodontitis. All participants in good general health Exclusion criteria: SRP in past 12 months or antibiotics 6 months before or during study, pregnancy, smokers, orthodontic therapy Age: 41–60 years Sex: 138 F (FMD: 63; control: 75) and 92 M (FMD 52; control: 40) Smokers: none Number randomised: 230 Number evaluated: 230 (115 per group) |
|
| Interventions |
Comparison: FMD vs control FMD group: FMS 2 sessions scaling within 24 hours. Home: rinse CHX 0.12%, 1 minute, 1 × day, 1 week Control group: SRP 4 sessions 1‐week interval OHI before study start: yes Instruments used: hand instruments Time per Q: unknown Maintenance: FMD: OHI in both sessions, QRP: OHI in 1st and last session. OHI in both groups at 3 months examination Retreatment: none Duration of study: 3 months |
|
| Outcomes |
Primary outcome: PPD (6 sites per tooth) Secondary outcomes: CAL (6 sites per tooth). Manual probe for all measurements Teeth: whole‐mouth recordings Pocket depth at baseline: data not split for pocket depth categories Outcome time reported: 3‐month data Other outcomes: GI, PI (4 sites per tooth), microbiological changes in deep pockets (depth unknown) (Prevotella intermedia, Porphyromonas gingivalis) by cultivation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information for judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information for judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included participants completed study. |
| Selective reporting (reporting bias) | Low risk | All data reported. |
| Other bias | Unclear risk | Insufficient information for judgement. |
Santuchi 2015.
| Study characteristics | ||
| Methods |
Study design: RCT with 2‐arm parallel design Recruitment period: 2011–2012 Setting: university dental hospital, Brazil Number of centres: 1 Funding source: unknown |
|
| Participants |
Inclusion criteria: diagnosis of chronic periodontitis. All participants in good general health Exclusion criteria: SRP in past 12 months or antibiotics 3 months before or during study, orthodontic therapy Age: 35–60 years; mean 44.6 years Sex: 54 F (groups not reported) and 24 M (groups not reported) Smokers: unclear Number randomised: 90 Number evaluated: 78 (FMD: 41; control: 37). Dropouts happened before instrumentation |
|
| Interventions |
Comparison: FMD vs control FMD group: 2 sessions scaling within 24 hours, after instrumentation: tongue brushing: CHX 1%, 1 minute; rinse: CHX 0.12%, 2 × 1 minute + gargle the last 10 seconds; subgingival: CHX 1%. Home: rinse CHX 0.12%, 2 × day, 2 weeks Control group: (SRP‐Q): SRP 4 sessions weekly intervals OHI before study start: yes (PI < 30%) Instruments used: hand instruments Time per Q: 30 minutes Maintenance: monthly Retreatment: none Duration of study: 6 months after last instrumentation |
|
| Outcomes |
Primary outcome: impact of periodontal treatment on pain (VAS), fear (DFS; as proposed by Kleinknecht 1973), and anxiety (DAS; Corah's DAS) scores Secondary outcomes: PPD, CAL (6 sites per tooth). Manual probe (UNC‐15) for all measurements Teeth: whole‐mouth recordings Pocket depth at baseline: percentages of PPD ≥ 4 mm and PPD 5–6 mm Outcome time reported: 6 months used. 6 months after last instrumentation measured Other outcomes: GI, PI |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation with opaque envelopes. |
| Allocation concealment (selection bias) | Low risk | Quote: "Opaque envelopes containing identifications for treatment were mixed and then numbered. Each participant took a single envelope and was assigned to a specific group by a researcher (LOMC)". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Examinations were performed by two blinded examiners who were trained and calibrated (FOC and JRC)". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | Low risk | Data reported on all primary and secondary outcomes. |
| Other bias | Unclear risk | Baseline balance good for pocket depth; however, smoking unclear. No apparent other biases. |
Soares 2015.
| Study characteristics | ||
| Methods |
Study design: RCT with 4‐arm parallel design (2 arms included) Recruitment period: May 2010–September 2011 Setting: university dental hospital, Rio de Janeiro, Brazil Number of centres: 1 Funding source: unknown |
|
| Participants |
Inclusion criteria: diagnosis of chronic periodontitis with PPD ≥ 5 mm and halitosis. All participants in good general health Exclusion criteria: SRP in past 12 months or antibiotics 6 months before or during study, smoking Age: 38–66 years Sex: 44 F (groups unknown) and 46 M (groups unknown) Smokers: none Number randomised: 90 in 4 arms; 49 used for FMD (PTSS + CHX: 23)/QRP (PTQ‐TS: 26)* Number evaluated: 45 in 2 arms (FMD: 21; control: 24)* |
|
| Interventions |
Comparison: FMD vs control FMD group: (PTSS + CHX): 1 session scaling. Home: rinse CHX 0.2%, 2 × day, 60 seconds, 90 days Control group: (PTQ‐TS): SRP 4 sessions in weekly intervals Groups not used in review: 2 groups (1 FMD, 1 SRP) that included tongue scraping as part of the intervention OHI before study start: yes Instruments used: hand and US instruments Time per Q: unknown Maintenance: unknown Retreatment: none Duration of study: 3 months |
|
| Outcomes |
Primary outcome: VSC concentrations with Halimeter and organoleptic scores Secondary outcomes: PPD, CAL, BOP (4 sites per tooth). Manual probe (UNC‐15) for all measurements Teeth: whole‐mouth recordings Pocket depth at baseline: PPD ≥ 5 mm Outcome time reported: 3 months used. 1, 2 and 3 months measured Other outcomes: GBI, VPI, WTCI |
|
| Notes | * Information from author on request | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear. Quote: "Consecutive patients with periodontal disease were randomly divided into four groups". |
| Allocation concealment (selection bias) | Unclear risk | Unclear who randomised participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | 1 calibrated examiner for clinical assessments; unclear if blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 dropouts in each group used; time point of dropout unclear. |
| Selective reporting (reporting bias) | Low risk | Data reported on all primary and secondary outcomes. |
| Other bias | Low risk | Baseline balance good. |
Swierkot 2009.
| Study characteristics | ||
| Methods |
Study design: RCT with 3‐arm parallel design Recruitment period: unclear Setting: university dental department, Marburg, Germany Number of centres: 1 Funding source: supported by university |
|
| Participants |
Inclusion criteria: diagnosis of chronic periodontitis with PD ≥ 5 mm and BOP. All participants in good general health Exclusion criteria: antibiotics from 6 months before or during study, history of systemic disease, people attending for orthodontic treatment, pregnancy Age: 28–63 years Sex: 20 F (FMS: 7; FMD: 7; control: 6) and 5 M (FMS: 2; FMD: 2; control: 1) Smokers: 5 (FMS: 3; FMD: 1; control: 1) (smoking ≥ 10 cigarettes per day) Number randomised: 25 (FMS: 9 FMD: 9; control: 7) Number evaluated: 25 (FMS: 9 FMD: 9; control: 7) |
|
| Interventions |
Comparison: FMS vs control; FMD vs control; FMS vs FMD FMS group: (FM‐SRP): 2 sessions within 24 hours FMD group: (FMD) 2 sessions scaling within 24 hours; after instrumentation: tongue brushing: CHX 1%, 1 minute; rinse: CHX 0.2%, twice for 1 minute; spray pharynx: CHX 0.2% 4 × each, subgingival: CHX 1%. Home: rinse CHX 0.2%, 1 minute, 2 × day, 14 days; spray tonsils: CHX 0.2%, 1 × day, 14 days Control group: (Q‐SRP) 4 sessions Q wise, 1‐week interval starting first Q, hand and US instruments OHI before study start: yes Instruments used: hand and US instruments Time per Q: unclear Maintenance: 1, 2, 4 and 8 months Retreatment: none Duration of study: 8 months |
|
| Outcomes |
Primary outcome: PPD (4 sites per tooth) Secondary outcomes: CAL, BOP (4 sites per tooth). Manual probe for all measurements Teeth: whole‐mouth recordings (baseline, 1, 2, 4 and 8 months). Data split in single‐ and multi‐rooted teeth for moderate (4–6 mm) pockets and whole‐mouth recordings for deep (≥ 7 mm) pockets Pocket depth at baseline: moderate (PPD 4–6 mm) and deep pockets (PPD ≥ 7 mm) Outcome time reported: 4 and 8 months used. 1, 2, 4 and 8 months measured Other outcomes: PLI, API, microbiology |
|
| Notes | Blinding unclear. Exclusion of third molars, as well as teeth with furcation degree II and III | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation was performed with a combination of coin toss and drawing of lots by a second person not involved in the study to assign the patients into the following groups: full mouth disinfection (FMD), FM‐SRP (FMS) and Q‐SRP (control)". |
| Allocation concealment (selection bias) | Low risk | Quote: "The sequence was concealed until interventions were assigned". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The treatment and reassessment were performed by one periodontist who had been trained and tested previously for his reproducibility". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient in every group was excluded from the study due to prescribed antibiotics because of sinusitis maxillaris. The patient of the FM‐SRP group dropped out 2 months after treatment and the two patients of the other two groups dropped out 4 months after treatment. Their data were not included into the statistical analysis". |
| Selective reporting (reporting bias) | Low risk | Data reported on all primary and secondary outcomes. |
| Other bias | Low risk | Baseline balance good for pocket depth and smoking. No apparent other biases. |
Vandekerckhove 1996.
| Study characteristics | ||
| Methods |
Study design: RCT with 2‐arm parallel design Recruitment period: unclear Setting: university dental hospital, Leuven, Belgium Number of centres: 1 Funding source: supported by university |
|
| Participants |
Inclusion criteria: diagnosis of chronic periodontitis with PD ≥ 7 mm and BOP. All participants in good general health Exclusion criteria: no antibiotics from 4 months before or during study Age: 39–62 years Sex: 8 F (4 per group) and 2 M (1 per group) Smokers: 3 (FMD: 1; control: 2) (smoking ≥ 10 cigarettes per day) Number randomised: 10 (5 per group) Number evaluated: 10 (5 per group) |
|
| Interventions |
Comparison: FMD vs control FMD group: 2 sessions scaling within 24 hours, after instrumentation: tongue brushing: CHX 1%, 1 minute; rinse: CHX 0.2%, 2 × 1 minute + gargle the last 10 seconds; subgingival: CHX 1%, 3 × within 10 minutes. Home: rinse CHX 0.2%, 1 minute, 2 × day, 2 weeks Control group: SRP 4 sessions 2‐weekly intervals OHI before study start: no Instruments used: hand instruments Time per Q: 1 hour Maintenance: none Retreatment: none Duration of study: 8 months |
|
| Outcomes |
Primary outcome: PPD (6 sites per tooth) (data in graph) Secondary outcomes: CAL, BOP (6 sites per tooth). Manual probe for all measurements Teeth: only recording of first Q. Data split in single‐/multi‐rooted teeth and initial moderate (PPD 5–6 mm) and deep pockets (PPD ≥ 7 mm) Pocket depth at baseline: moderate (PPD 5–6 mm) and deep pockets (PPD ≥ 7 mm) Outcome time reported: 4 and 8 months used. 1, 2, 4 and 8 months measured Other outcomes: recession, GI, PI |
|
| Notes | Data extracted from graphs. No supplementary data (CAL, BOP) available on request | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects randomly distributed between the two treatment groups". |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The clinical parameters were recorded by the same periodontist…" Although blinded at 8 months, 4 months assessment was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | Data reported on all primary and secondary outcomes. |
| Other bias | Low risk | Baseline balance good for pocket depth and smoking. No apparent other biases. |
Wennström 2005.
| Study characteristics | ||
| Methods |
Study design: RCT with 2‐arm parallel design Recruitment period: during 2002 Setting: university dental hospital (Göteborg, Sweden), private dental clinic (Trento, Italy) Number of centres: 2 Funding source: industry funding (Electro Medical Systems, Nyon, Switzerland) |
|
| Participants |
Inclusion criteria: diagnosis of chronic periodontitis with PD ≥ 5 mm and BOP. All participants in good general health Exclusion criteria: SRP over last 12 months, antibiotics from 3 months before or during study, pregnant Age: 25–75 years Sex: 19 F (FMS: 8; control: 11) and 22 M (FMS: 12; control: 10) Smokers: 20 (FMS: 9; control: 11) Number randomised: 42 Number evaluated: 41 (FMS: 20; control: 21) |
|
| Interventions |
Comparison: FMS vs control FMS group: (FM‐UD): FMS 1‐hour session US scaling with water, re‐instrumentation after 3 months in PPD > 4 mm Control group: (Q‐SRP): QRP 4 sessions hand instrumentation, 1‐week intervals (time recorded, no time restriction), re‐instrumentation after 3 months in PPD > 4 mm OHI before study start: yes Instruments used: hand and US instruments Time per Q: 1 hour Maintenance: 1 month following completion of instrumentation (both groups) Retreatment: at 3 months Duration of study: 6 months |
|
| Outcomes |
Primary outcome: PPD (6 sites per tooth) Secondary outcomes: CAL, BOP (6 sites per tooth). Manual probe for all measurements Teeth: whole‐mouth recordings (baseline, 3 and 6 months). Data split in initial moderate (PPD 5–6 mm) and deep pockets (PPD > 6 mm) Pocket depth at baseline: moderate (PPD 5–6 mm) and deep pockets (PPD ≥ 7 mm) Outcome time reported: 3 months used. 3 and 6 months measured Other outcomes: PI, mean VAS pain score (100‐mm scale) |
|
| Notes | For BOP: data supplemented by authors. 6 months data not used because of retreatment at 3 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Within each of these subgroups, a random assignment to the two treatment protocols (Fig. 1) was subsequently performed by the use of computer‐generated tables". |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was secured by (i) having a person not otherwise involved in the study performing the randomisation and (ii) providing the centres (the dental hygienists) with sealed envelopes containing only the assignment for the individual subject". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "One examiner (a periodontist), who was masked with respect to the treatment assignments, performed all examinations". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 42 enrolled, 41 randomised, and 41 present at 6 months. |
| Selective reporting (reporting bias) | Low risk | Data reported on all primary and secondary outcomes. |
| Other bias | Low risk | Baseline balance good for pocket depth and smoking. No apparent other biases. |
Zanatta 2006.
| Study characteristics | ||
| Methods |
Study design: RCT with 3‐arm parallel design Recruitment period: March 2004–July 2004 Setting: university dental clinic, San Paulo, Brazil Number of centres: 1 Funding source: unclear |
|
| Participants |
Inclusion criteria: diagnosis: chronic periodontitis with PD ≥ 5 mm and BOP. All participants in good general health Exclusion criteria: SRP in past 6 months or on antibiotics from 6 months before or during study, pregnancy, allergic to iodine Age: 27–72 years Sex: 18 F and 27 M Smokers: unclear Number randomised: 45 (15 per group) Number evaluated: 40 (FMS: 12; FMD: 15; control: 13) |
|
| Interventions |
Comparison: FMS vs control; FMD vs control; FMS vs FMD FMS group: (PDG) FMS 1 session US scaling with 0.9% slaine (duration 45 minutes) FMD group: (PD‐PIG): FMS 1 session US scaling with povidone iodine 0.5% (duration 45 minutes) Control group: QRP 4 sessions US scaling with water, 1‐week intervals (duration unclear) OHI before study start: yes Instruments used: US instruments Time per Q: unclear Maintenance: twice weekly from baseline Retreatment: none Duration of study: 3 months |
|
| Outcomes |
Primary outcome: PPD (6 sites per tooth) Secondary outcomes: CAL, BOP (6 sites per tooth). Computerised probe with stent for all measurements Teeth: whole‐mouth recordings (baseline, 1 and 3 months). Data split initial moderate (PPD 5–6 mm) and deep pockets (PPD > 6 mm) Pocket depth at baseline: moderate (5–6 mm), deep (> 6 mm) Outcome time reported: 3 months used Other outcomes: PI, GR |
|
| Notes | Dropouts: 45 enrolled, 40 completed the study. Time point for dropouts unclear For BOP: data extracted from graphs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to one of the following treatment groups…". |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A previously calibrated examiner, masked to the type of treatment, performed all clinical assessments". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low dropout (5/45) and numbers by group given but reasons not given. |
| Selective reporting (reporting bias) | Low risk | Data reported on all primary and secondary outcomes. |
| Other bias | Unclear risk | Baseline balance good for pocket depth but smoking is unclear. No apparent other biases. |
Zijnge 2010.
| Study characteristics | ||
| Methods |
Study design: RCT with 2‐arm parallel design Recruitment period: September 2007–December 2008 Setting: private clinic, Groningen, The Netherlands Number of centres: 1 Funding source: university funding |
|
| Participants |
Inclusion criteria: diagnosis of chronic periodontitis with PD ≥ 6 mm at > 10% sites. All participants in good general health Exclusion criteria: SRP over last 5 years, antibiotics from 3 months before or during study, pregnant, smokers, removable denture Age: 25–75 years Gender: 16 F (8 per group) and 22 M (FMS: 10; control: 12) Smokers: 0 Number randomised: 39 (FMS: 19; control: 20) Number evaluated: 38 (FMS 18; control: 20) |
|
| Interventions |
Comparison: FMS vs control FMS group: (FM‐SRP) 1 × 3‐hour session Control group: (MS‐SRP): 3 sessions Q‐wise, 1‐hour duration per session, 10‐week interval starting first Q, hand instruments OHI before study start: no Instruments used: hand instruments Time per Q: 1 hour Maintenance: 1 and 2 weeks Retreatment: none Duration of study: 3 months |
|
| Outcomes |
Primary outcome: PPD (6 sites per tooth) Secondary outcomes: BOP (6 sites per tooth). Manual probe for all measurements Teeth: whole‐mouth recordings as well as test‐Q (1st Q). Data split in moderate (4–6 mm) and deep (≥ 7 mm) pockets Pocket depth at baseline: moderate (PPD 4–6 mm) and deep pockets (PPD ≥ 7 mm) Outcome time reported: 3 months Other outcomes: PI, microbiology |
|
| Notes | PI at baseline unclear Pockets < 3 mm were not recorded. No data for CAL on request. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a second independent person informed them whether they had to continue the treatment in the other quadrants (FM‐SRP) or continue treatment in another session (MS‐SRP), based on a computer‐generated randomisation table". |
| Allocation concealment (selection bias) | Low risk | Quote: "All study personnel was blinded to treatment assignment for the duration of the study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "After 3 months the patients were examined by a periodontist. All study personnel was blinded to treatment assignment for the duration of the study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 44 attended baseline examination but unclear if they were randomised. 1 participant dropped out of FMS group. Probably low risk of bias. |
| Selective reporting (reporting bias) | Low risk | Data reported on all outcomes. |
| Other bias | Low risk | Baseline balance good for pocket depth. No apparent other biases. |
API: Approximal Plaque Index; AZ: azithromycin; BOP: bleeding on probing; CAL: clinical attachment level; CHX: chlorhexidine gluconate; DAS: Dental Anxiety Scale; DFS: Dental Fear Scale; F: female; FC: FMD with CHX; FM‐CHX: FMD with CHX; FM‐RP: see FMS; FM‐UD: FMS with ultrasonic instrumentation; FMD: full‐mouth disinfection (full‐mouth subgingival scaling and root planing with use of antiseptics); FM‐SRP: full‐mouth scaling and root planing; FMRP: FMS; FMS: full‐mouth scaling (full‐mouth subgingival scaling and root planing); FNC: FMD without CHX; FRp: FMS; GBI: Gingival Bleeding Index; GCF: gingival crevicular fluid; GI: Gingival Index (Löe 1963); GR: gingival recession; IL: interleukin; M: male; MGI: modified Gingival Index; MS‐SRP: multiple session scaling and root planing; NC: see QRP; OHI: oral hygiene instruction; PAL: probing attachment level; PBI: Papilla Bleeding Index; PD: probing depth; PD‐PIG: see FMD; PDG: see FMS; PI: Plaque Index (O'Leary 1972); PLI: Plaque Index (Silness 1964); PPD: probing pocket depth; PTQ‐TS: see QRP; PTSS: full‐mouth periodontal therapy; Q: quadrant; Q‐SRP: see QRP; QRP: quadrant‐wise subgingival scaling and root planing, clockwise in 4 sessions; RAL: relative attachment level; RCT: randomised controlled trial; SBI: Sulcus Bleeding Index; SD: standard deviation; SI: Staining Index; SRP: scaling and root planing; SRP‐Q: see QRP; SUP: suppuration; UNC: University of North Carolina; US: ultrasonic; VAS: visual analogue scale; VPI: Visible Plaque Index; VSC: volatile sulphuric compound; WTCI: Winkel Tongue Coating Index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bollen 1998 | 6/16 participants with aggressive periodontitis. Data not shown separately for aggressive and chronic periodontitis. |
| Cortelli 2015 | Same 6 groups of participants as presented in Fonseca 2015; no results of subgroups provided. Results of 3 various FMD or QRP treatment‐modalities presented as 1. |
| Devji 2017 | Commentary of Preus 2013; Preus 2015a; Preus 2017a; Preus 2017b. |
| Eren 2002 | Participants in the intervention arm received FMS for 4 consecutive days (over 24 hours). |
| Jothi 2009 | No QRP control group. |
| Knöfler 2007 | Participants in both arms received a chlorhexidine rinse. |
| Lee 2009 | No randomisation. Quote: "The treatment group was determined according to the patients' preferences". |
| Loggner Graff 2009 | Retreatment of participants after 3 months in study prior to outcome assessment at 6 months. |
| Meulman 2013 | Data only available as figures. No reply from authors to request for supplemental data. |
| Oliveira 2019 | Participants in both arms received azithromycin. |
| Preus 2013 | Participants in all arms received a chlorhexidine rinse. |
| Preus 2015a | Participants in all arms received a chlorhexidine rinse. |
| Preus 2015b | All participants also in placebo arms received a chlorhexidine rinse. |
| Preus 2017a | Participants in all arms received a chlorhexidine rinse. |
| Preus 2017b | Participants in all arms received a chlorhexidine rinse. |
| Quirynen 1995 | 2‐month data only, data for 4 and 8 months later presented in Vandekerckhove 1996 (4‐month data used). |
| Santuchi 2016 | Same group as presented in Santuchi 2015, but outcomes presented insufficiently. |
| Serrano 2011 | 4‐ to 6‐week data only. |
| Silveira 2017 | Participants in both arms received a chlorhexidine rinse and a gel. |
| Tomasi 2006 | 18‐month evaluation after baseline but all participants had several retreatment sessions. These were the same participants as Wennström 2005, but it was an observational follow‐up of the trial. |
| Ushida 2008 | Immunology study with no clinical data. |
| Zhao 2005 | Only preliminary results. In 2020, still no publication on final results. |
FMS: full‐mouth scaling; QRP: quadrant‐wise scaling.
Differences between protocol and review
Differences introduced in 2022 update.
We modified the title according to the new Classification of Periodontal and Peri‐implant diseases launched in 2018 (Papapanou 2018).
We changed the term "scaling and root planing" to the now accepted term for the same treatment "subgingival instrumentation". For ease of reading, we have kept the abbreviation SRP for this treatment because the cited studies all use the term SRP for subgingival instrumentation/scaling and root planing. Likewise, the terms FMD and FMS have been kept as in the updated review from 2015 (Eberhard 2015), to facilitate reading of included studies.
We added a secondary outcome, 'pocket closure'.
We focused on 6/8 month data instead of 3/4 month data. In the previous version of 2015 (Eberhard 2015), three to four months of follow‐up was the basis for summary of findings tables. We switched to using six‐ to eight‐month follow‐up data in the summary of findings tables in this update based on the European Federation of Periodontology S3 Guidelines for the treatment of stage I to III periodontitis (Sanz 2020a), and the general consensus that six‐month data are a meaningful endpoint for step 2 of periodontal therapy (Loos 2020; Suvan 2020).
We changed two judgements of risk of bias (Mongardini 1999; Zijnge 2010), as we found further information through revisiting the text in the original manuscripts.
We excluded one study of 37 participants that we had included in the 2015 review (Knöfler 2007). Through revisiting all earlier included studies, we determined that this study did not provide an appropriate control condition as a disinfection agent had been used in both arms of the trial.
Differences introduced in 2015 update (Eberhard 2015).
We changed the title to include all treatment modalities, not just FMD.
We added an objective to compare FMS with FMD.
We restructured the presentation of the results by tooth type and justified this in the background.
We added three‐ to four‐month data to the six‐ to eight‐month data.
We changed the sensitivity analysis in the methods section to reduce the number of analyses. This now reads: "We conducted sensitivity analyses by analysing only studies assessed as having low risk of bias, and by excluding unpublished literature".
Contributions of authors
PS: abstract screening, review of full‐text articles, data extraction, data input, risk of bias assessment, creating summary of findings table using GRADEpro GDT, composition of the update and writing of the update.
JE: literature search, abstract screening and composition of the previous update.
IN: protocol development, consultant during the review process and composition of the update.
HW: abstract screening, data input, statistical analysis, risk of bias assessment and composition of the previous update.
SJ: literature search, abstract screening, review of full‐text articles, data extraction, risk of bias assessment and writing of the update.
Sources of support
Internal sources
-
School of Dentistry, The University of Manchester, UK
Support to Cochrane Oral Health and to Helen V Worthington
-
University Hospital Schleswig‐Holstein, Campus Kiel, Germany
Support to Jörg Eberhard for previous version of review
-
University College London, UK
Support to Ian Needleman
-
University Hospital Bonn, Germany
Support to Pia‐Merete Jervøe‐Storm and Søren Jepsen
External sources
-
National Institute of Health Research (NIHR), UK
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or Department of Health.
-
Cochrane Oral Health Group Global Alliance, Other
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (ohg.cochrane.org/partnerships-alliances). Contributors over recent years have been: British Association for the Study of Community Dentistry, UK; British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; Centre for Dental Education and Research at All India Institute of Medical Sciences, India; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; Swiss Society for Endodontology, Switzerland.
Declarations of interest
PS: I am an author of one of the included trials, but I did not select the trial, assess its risk of bias or extract data from it.
JE: none.
IN: I have received funding for lectures and research from industry related to oral hygiene products and prevention of ventilator‐associated pneumonia.
HW: none. I am an Editor with Cochrane Oral Health and was previously Co‐ordinating Editor, but I was not involved in the editorial processing of this review.
SJ: I am an author of one of the included trials, but I did not select the trial, assess its risk of bias or extract data from it.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Afacan 2020 {published data only}
- Afacan B, Çınarcık S, Gürkan A, Özdemir G, İlhan HA, Vural C, et al. Full-mouth disinfection effects on gingival fluid calprotectin, osteocalcin, and N-telopeptide of type I collagen in severe periodontitis. Journal of Periodontology 2020;91:638-50. [DOI] [PubMed] [Google Scholar]
Apatzidou 2004 {published data only}
- Apatzidou DA, Kinane DF. Quadrant root planing versus same-day full-mouth root planing. I. Clinical findings. Journal of Clinical Periodontology 2004;31(2):132-40. [DOI] [PubMed] [Google Scholar]
Babaloo 2018 {published data only}
- Babaloo A, Rahbar M, Babaloo Z, Ghasemi S, Amini A. Evaluation of clinical periodontal indices and serum interleukin-27 by one-stage full-mouth disinfection and quadrant scaling and root planing in periodontitis. Journal of Contemporary Dental Practice 2018;19:997-1004. [PubMed] [Google Scholar]
- Shirmohammadi A, Babaloo Z, Eskandari A, Purabbas R, Babaloo A. The effects of one-stage full-mouth disinfection and quadrant-wise scaling and root planing on serum levels of IL-17 and IL-1beta and clinical parameters (a randomized controlled trial study). Journal of Dentistry (Tehran) 2013;10:248-55. [PMC free article] [PubMed] [Google Scholar]
Del Peloso 2008 {published data only}
- Del Peloso RE, Bittencourt S, Sallum ER, Nociti FH Jr, Goncalves RB, Casati MZ. Periodontal debridement as a therapeutic approach for severe chronic periodontitis: a clinical, microbiological and immunological study. Journal of Clinical Periodontology 2008;35:789-98. [DOI] [PubMed] [Google Scholar]
Fonseca 2015 {published data only}
- Fonseca DC, Cortelli JR, Cortelli SC, Miranda Cota LO, Machado Costa LC, Moreira Castro MV, et al. Clinical and microbiologic evaluation of scaling and root planing per quadrant and one-stage full-mouth disinfection associated with azithromycin or chlorhexidine: a clinical randomized controlled trial. Journal of Periodontology 2015;86:1340-51. [DOI] [PubMed] [Google Scholar]
Graziani 2015 {published data only}
- Graziani F, Cei S, Orlandi M, Gennai S, Gabriele M, Filice N, et al. Acute-phase response following full-mouth versus quadrant non-surgical periodontal treatment: a randomized clinical trial. Journal of Clinical Periodontology 2015;42:843-52. [DOI] [PubMed] [Google Scholar]
Jervøe‐Storm 2006 {published data only}
- Jervøe-Storm PM, Semaan E, AlAhdab H, Engel S, Fimmers R, Jepsen S. Clinical outcomes of quadrant root planing versus full-mouth root planing. Journal of Clinical Periodontology 2006;33(3):209-15. [DOI] [PubMed] [Google Scholar]
Koshy 2005 {published data only}
- Koshy G, Kawashima Y, Kiji M, Nitta H, Umeda M, Nagasawa T, et al. Effects of single-visit full-mouth ultrasonic debridement versus quadrant-wise ultrasonic debridement. Journal of Clinical Periodontology 2005;32(7):734-43. [DOI] [PubMed] [Google Scholar]
Mongardini 1999 {published data only}
- Mongardini C, Steenberghe D, Dekeyser C, Quirynen M. One stage full- versus partial-mouth disinfection in the treatment of chronic adult or generalized early-onset periodontitis. I. Long-term clinical observations. Journal of Periodontology 1999;70(6):632-45. [DOI] [PubMed] [Google Scholar]
- Quirynen M, Mongardini C, Soete M, Pauwels M, Coucke W, Eldere J, et al. The role of chlorhexidine in the one-stage full-mouth disinfection treatment of patients with advanced adult periodontitis. Long-term clinical and microbiological observations. Journal of Clinical Periodontology 2000;27(8):578-89. [DOI] [PubMed] [Google Scholar]
- Quirynen M, Mongardini C, Pauwels M, Bollen CM, Eldere J, Steenberghe D. One stage full- versus partial-mouth disinfection in the treatment of chronic adult or generalized early-onset periodontitis. II. Long-term impact on microbial load. Journal of Periodontology 1999;70:646-56. [DOI] [PubMed] [Google Scholar]
Pontillo 2018 {published data only}
- Pontillo V, Miziak DB, Maller AC, Nassar PO, Nassar CA. Comparative clinical evaluation between conventional periodontal treatment and full mouth disinfection. Journal of the International Academy of Periodontology 2018;20:123-30. [PubMed] [Google Scholar]
Predin 2014 {published data only}
- Predin T, Djuric M, Nikolic N, Mirnic J, Gusic I, Petrovic D, et al. Clinical and microbiological effects of quadrant versus full-mouth root planing: a randomized study. Journal of Dental Sciences 2014;9:400-6. [Google Scholar]
Quirynen 2006 {published data only}
- Quirynen M, De Soete M, Boschmans G, Pauwels M, Coucke W, Teughels W, et al. Benefit of "one-stage full-mouth disinfection" is explained by disinfection and root planing within 24 hours: a randomized controlled trial. Journal of Clinical Periodontology 2006;33(9):639-47. [DOI] [PubMed] [Google Scholar]
Roman‐Torres 2018 {published data only}
- Roman-Torres CV, Bryington MS, Kussaba ST, Pimentel AC, Jimbo R, Cortelli JR, et al. Comparison of full-mouth scaling and quadrant-wise scaling in the treatment of adult chronic periodontitis. Brazilian Dental Journal 2018;29:296-300. [DOI] [PubMed] [Google Scholar]
Santuchi 2015 {published data only}
- Santuchi CC, Cortelli SC, Cortelli JR, Cota LO, Alencar CO, Costa FO. Pre- and post-treatment experiences of fear, anxiety, and pain among chronic periodontitis patients treated by scaling and root planing per quadrant versus one-stage full-mouth disinfection: a 6-month randomized controlled clinical trial. Journal of Clinical Periodontology 2015;42:1024-31. [DOI] [PubMed] [Google Scholar]
Soares 2015 {published data only}
- Soares LG, Castagna L, Weyne SC, Silva DG, Falabella ME, Tinoco EM. Effectiveness of full- and partial-mouth disinfection on halitosis in periodontal patients. Journal of Oral Science 2015;57:1-6. [DOI] [PubMed] [Google Scholar]
Swierkot 2009 {published data only}
- Swierkot K, Nonnenmacher CL, Mutters R, Flores-de-Jacoby L, Mengel R. One-stage full-mouth disinfection versus quadrant and full-mouth root planing. Journal of Clinical Periodontology 2009;36:240-9. [DOI] [PubMed] [Google Scholar]
Vandekerckhove 1996 {published data only}
- Vandekerckhove BN, Bollen CM, Dekeyser C, Darius P, Quirynen M. Full- versus partial-mouth disinfection in the treatment of periodontal infections. Long-term clinical observations of a pilot study. Journal of Periodontology 1996;67(12):1251-9. [DOI] [PubMed] [Google Scholar]
Wennström 2005 {published data only}
- Wennström JL, Tomasi C, Bertelle A, Dellasega E. Full-mouth ultrasonic debridement versus quadrant scaling and root planing as an initial approach in the treatment of chronic periodontitis. Journal of Clinical Periodontology 2005;32(8):851-9. [DOI] [PubMed] [Google Scholar]
Zanatta 2006 {published data only}
- Zanatta GM, Bittencourt S, Nociti FH Jr, Sallum EA, Sallum AW, Casati MZ. Periodontal debridement with povidone-iodine in periodontal treatment: short-term clinical and biochemical observations. Journal of Periodontology 2006;77(3):498-505. [DOI] [PubMed] [Google Scholar]
Zijnge 2010 {published data only}
- Zijnge V, Meijer HF, Lie MA, Tromp JA, Degener JE, Harmsen HJ, et al. The recolonization hypothesis in a full-mouth or multiple-session treatment protocol: a blinded, randomized clinical trial. Journal of Clinical Periodontology 2010;37:518-25. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bollen 1998 {published data only}
- Bollen CM, Mongardini C, Papaioannou W, Steenbergh D, Quirynen M. The effect of a one-stage full-mouth disinfection on different intra-oral niches. Clinical and microbiological observations. Journal of Clinical Periodontology 1998;25(1):56-66. [DOI] [PubMed] [Google Scholar]
Cortelli 2015 {published data only}
- Cortelli SC, Costa FO, Rodrigues E, Cota LO, Cortelli JR. Periodontal therapy effects on nitrite related to oral bacteria: a 6-month randomized clinical trial. Journal of Periodontology 2015;86:984-94. [DOI] [PubMed] [Google Scholar]
Devji 2017 {published data only}
- Devji T. Mechanical and antibiotic periodontal therapies may be no different in terms of tooth loss in patients with chronic periodontitis. Journal of the American Dental Association 2017;148:e124. [DOI] [PubMed] [Google Scholar]
Eren 2002 {published data only}
- Eren KS, Gurgan CA, Bostanci HS. Evaluation of non-surgical periodontal treatment using 2 time intervals. Journal of Periodontology 2002;73(9):1015-9. [DOI] [PubMed] [Google Scholar]
Jothi 2009 {published data only}
- Jothi MV, Bhat KM, Prathiba PK, Bhat GS. The evaluation of a biodegradable dental chip containing chlorhexidine in chitosan base as a targeted drug delivery in the management of chronic periodontitis in patients. Drug Development Research 2009;70:395-401. [Google Scholar]
Knöfler 2007 {published data only}
- Knöfler GU, Purschwtz RE, Jentsch HF. Clinical evaluation of partial- and full-mouth scaling in the treatment of chronic periodontitis. Journal of Periodontology 2007;78:2135-42. [DOI] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee S-H, Kim Y-J, Chung H-J, Kim O-S. The clinical effects of modified full-mouth disinfection in the treatment of moderate to severe chronic periodontitis patients. Journal of Korean Academy of Periodontology 2009;39(Suppl):239-51. [Google Scholar]
Loggner Graff 2009 {published data only}
- Loggner Graff I, Asklöw B, Thorstensson H. Full-mouth versus quadrant-wise scaling – clinical outcome efficiency and treatment discomfort. Swedish Dental Journal 2009;33:105-13. [PubMed] [Google Scholar]
Meulman 2013 {published data only}
- Meulman T, Oliveira Giorgetti AP, Gimenes J, Casarin RC, Peruzzo DC, Nociti FH Jr. One stage, full-mouth ultrasonic debridement in the treatment of severe chronic periodontitis in smokers: a preliminary blind and randomized clinical trial. Journal of the International Academy of Periodontology 2013;15:83-90. [PubMed] [Google Scholar]
Oliveira 2019 {published data only}
- Oliveira AM, Costa FO, Nogueira LM, Cortelli SC, Oliveira PA, Aquino DR, et al. Azithromycin and full-mouth scaling for the treatment of generalized stage III and IV periodontitis: a 6-month randomized comparative clinical trial. Brazilian Dental Journal 2019;30:429-36. [DOI] [PubMed] [Google Scholar]
Preus 2013 {published data only}
- Faveri M, Figueiredo LC, Feres M. Treatment of chronic periodontitis may be improved by the adjunctive use of systemic metronidazole. Journal of Evidence-based Dental Practice 2014;14(2):70-2. [DOI] [PubMed] [Google Scholar]
- Preus HR, Gunleiksrud TM, Sandvik L, Gjermo P, Baelum V. A randomized, double-masked clinical trial comparing four periodontitis treatment strategies: 1-year clinical results. Journal of Periodontology 2013;84(8):1075-86. [DOI] [PubMed] [Google Scholar]
Preus 2015a {published data only}
- Preus HR, Dahlen G, Gjermo P, Baelum V. Microbiologic observations after four treatment strategies among patients with periodontitis maintaining a high standard of oral hygiene: secondary analysis of a randomized controlled clinical trial. Journal of Periodontology 2015;86:856-65. [DOI] [PubMed] [Google Scholar]
Preus 2015b {published data only}
- Preus HR, Gjermo P, Scheie AA, Baelum V. The effect of metronidazole on the presence of P. gingivalis and T. forsythia at 3 and 12 months after different periodontal treatment strategies evaluated in a randomized, clinical trial. Acta Odontologica Scandinavica 2015;73:258-66. [DOI] [PubMed] [Google Scholar]
Preus 2017a {published data only}
- Preus HR, Gjermo P, Baelum V. A double-masked randomized clinical trial (RCT) comparing four periodontitis treatment strategies: 5-year clinical results. Journal of Clinical Periodontology 2017;44:1029-38. [DOI] [PubMed] [Google Scholar]
Preus 2017b {published data only}
- Preus HR, Gjermo P, Baelum V. A randomized double-masked clinical trial comparing four periodontitis treatment strategies: 5-year tooth loss results. Journal of Periodontology 2017;88:144-52. [DOI] [PubMed] [Google Scholar]
Quirynen 1995 {published data only}
- Quirynen M, Bollen CM, Vandekerckhove BN, Dekeyser C, Papaioannou W, Eyssen H. Full- vs. partial-mouth disinfection in the treatment of periodontal infections: short-term clinical and microbiological observations. Journal of Dental Research 1995;74(8):1459-67. [DOI] [PubMed] [Google Scholar]
Santuchi 2016 {published data only}
- Santuchi CC, Cortelli JR, Cortelli SC, Cota LO, Fonseca DC, Alencar CO, et al. Scaling and root planing per quadrant versus one-stage full-mouth disinfection: assessment of the impact of chronic periodontitis treatment on quality of life – a clinical randomized, controlled trial. Journal of Periodontology 2016;87:114-23. [DOI] [PubMed] [Google Scholar]
Serrano 2011 {published data only}
- Serrano C, Torres N, Bejarano A, Caviedes M, Castellanos ME. Clinical and microbiological comparison of three non-surgical protocols for the initial treatment of chronic periodontitis. Journal of the International Academy of Periodontology 2011;13:17-26. [PubMed] [Google Scholar]
Silveira 2017 {published data only}
- Silveira JO, Costa FO, Oliveira PA, Dutra BC, Cortelli SC, Cortelli JR, et al. Effect of non-surgical periodontal treatment by full-mouth disinfection or scaling and root planing per quadrant in halitosis – a randomized controlled clinical trial. Clinical Oral Investigation 2017;21:1545-52. [DOI] [PubMed] [Google Scholar]
Tomasi 2006 {published data only}
- Tomasi C, Bertelle A, Dellasega E, Wennström JL. Full-mouth ultrasonic debridement and risk of disease recurrence: a 1-year follow-up. Journal of Clinical Periodontology 2006;33:626-31. [DOI] [PubMed] [Google Scholar]
Ushida 2008 {published data only}
- Ushida Y, Koshy G, Kawashima Y, Kiji M, Umeda M, Nitta H, et al. Changes in serum interleukin-6, C-reactive protein and thrombomodulin levels under periodontal ultrasonic debridement. Journal of Clinical Periodontology 2008;35:969-75. [DOI] [PubMed] [Google Scholar]
Zhao 2005 {published data only}
- Zhao N, Ge SH, Yang PS. The clinical effect of full-mouth scaling and root planning on chronic periodontitis: a preliminary report. Shanghai Kou Qiang Yi Xue 2005;14(4):341-4. [PubMed] [Google Scholar]
Additional references
AAP 2005
- American Academy of Periodontology. Epidemiology of the periodontal diseases. Journal of Periodontology 2005;76:1406-19. [DOI] [PubMed] [Google Scholar]
Armitage 1999
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Annals of Periodontology 1999;4(1):1-6. [DOI] [PubMed] [Google Scholar]
Beikler 2004
- Beikler T, Abdeen G, Schnitzer S, Sälzer S, Ehmke B, Heinecke A, et al. Microbiological shifts in intra- and extraoral habitats following mechanical periodontal therapy. Journal of Clinical Periodontology 2004;31(9):777-83. [DOI] [PubMed] [Google Scholar]
Billings 2018
- Billings M, Holtfreter B, Papapanou PN, Mitnik GL, Kocher T, Dye BA. Age-dependent distribution of periodontitis in two countries: findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. Journal of Clinical Periodontology 2018;45(Suppl 20):S130-48. [DOI] [PubMed] [Google Scholar]
Chapple 2018
- Chapple IL, Mealey BL, Dyke TE, Bartold PM, Dommisch H, Eickholz P, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. Journal of Clinical Periodontology 2018;89(Suppl 1):S74-84. [DOI] [PubMed] [Google Scholar]
Eberhard 2008b
- Eberhard J, Jervøe-Storm P-M, Needleman I, Worthington H, Jepsen S. Full-mouth treatment concepts for chronic periodontitis: a systematic review. Journal of Clinical Periodontology 2008;35:591-604. [DOI] [PubMed] [Google Scholar]
Fang 2016
- Fang H, Han M, Li Q-L, Cao CY, Xia R, Zhang Z-H. Comparison of full-mouth disinfection and quadrant-wise scaling in the treatment of adult chronic periodontitis: a systematic review and meta-analysis. Journal of Periodontal Research 2016;51:417-30. [DOI] [PubMed] [Google Scholar]
Farman 2008
- Farman M, Joshi RI. Full-mouth treatment versus quadrant root surface debridement in the treatment of chronic periodontitis: a systematic review. British Dental Journal 2008;205:496-7. [DOI] [PubMed] [Google Scholar]
Heitz‐Mayfield 2002
- Heitz-Mayfield LJ, Trombelli L, Heitz F, Needleman I, Moles D. A systematic review of the effect of surgical debridement vs non-surgical debridement for the treatment of chronic periodontitis. Journal of Clinical Periodontology 2002;29(Suppl 3):92-102. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Kassebaum 2017
- Kassebaum NJ, Smith AG, Bernabé E, Fleming TD, Reynolds AE, Vos T, et al, GBD 2015 Oral Health Collaborators. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. Journal of Dental Research 2017;96:380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kleinknecht 1973
- Kleinknecht RA, Klepac RK, Alexander LD. Origins and characteristics of fear of dentistry. Journal of the American Dental Association 1973;86(4):842-8. [DOI] [PubMed] [Google Scholar]
Lang 2008
- Lang NP, Tan WC, Krähenmann MA, Zwahlen M. A systematic review of the effects of full-mouth debridement with and without antiseptics in patients with chronic periodontitis. Journal of Clinical Periodontology 2008;38(Suppl 8):8-21. [DOI] [PubMed] [Google Scholar]
Löe 1963
- Löe H, Silness J. Periodontal disease in pregnancy. Acta Odontologica Scandinavica 1963;21:533-51. [DOI] [PubMed] [Google Scholar]
Loos 2020
- Loos BG, Needleman I. Endpoints of active periodontal therapy. Journal of Clinical Periodontology 2020;47(Suppl 22):61-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Leary 1972
- O'Leary TJ, Drake RB, Naylor JE. The plaque control record. Journal of Periodontology 1972;43(1):38. [DOI] [PubMed] [Google Scholar]
Oliver 1991
- Oliver RC, Brown LJ, Loe H. Variations in the prevalence and extent of periodontitis. Journal of the American Dental Association 1991;122(6):43-8. [DOI] [PubMed] [Google Scholar]
Papapanou 2018
- Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. Journal of Clinical Periodontology 2018;45(Suppl 20):S162-70. [DOI] [PubMed] [Google Scholar]
Pockpa 2018
- Pockpa AD, Soueidan A, Louis P, Coulibaly NT, Badran Z, Struillou X. Twenty years of full-mouth disinfection: the past, the present and the future. Open Dentistry Journal 2018;12:435-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quirynen 1999
- Quirynen M, Mongardini C, Pauwels M, Bollen CM, Eldere J, Steenberghe D. One stage full- versus partial-mouth disinfection in the treatment of chronic adult or generalized early-onset periodontitis. II. Long-term impact on microbial load. Journal of Periodontology 1999;70:646-56. [DOI] [PubMed] [Google Scholar]
Quirynen 2000
- Quirynen M, Mongardini C, Soete M, Pauwels M, Coucke W, Eldere J, et al. The role of chlorhexidine in the one-stage full-mouth disinfection treatment of patients with advanced adult periodontitis. Long-term clinical and microbiological observations. Journal of Clinical Periodontology 2000;27(8):578-89. [DOI] [PubMed] [Google Scholar]
Sanz 2020a
- Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, et al. Treatment of stage I–III periodontitis – the EFP S3 level clinical practice guideline. Journal of Clinical Periodontitis 2020;47(Suppl 22):40-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanz 2020b
- Sanz M, Marco del Castillo A, Jepsen S, Gonzalez-Juanatey JR, D'Aiuto F, Bouchard P, et al. Periodontitis and cardiovascular diseases. Consensus report. Global Heart 2020;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.1.
Silness 1964
- Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontologica Scandinavica 1964;22:121-35. [DOI] [PubMed] [Google Scholar]
Suvan 2020
- Suvan J, Leira Y, Moreno Sancho FM, Graziani F, Derks J, Tomasi C. Subgingival instrumentation for treatment of periodontitis. A systematic review. Journal of Clinical Periodontology 2020;47(Suppl 22):155-175. [DOI] [PubMed] [Google Scholar]
Teughels 2009
- Teughels W, DeKeyser C, Essche M, Quirynen M. One-stage, full-mouth disinfection: fiction or reality? Periodontology 2000 2009;50:39-51. [DOI] [PubMed] [Google Scholar]
Tomasi 2009
- Tomasi C, Wennström JL. Full-mouth treatment vs. the conventional staged approach for periodontal infection control. Periodontology 2000 2009;51:45-62. [DOI] [PubMed] [Google Scholar]
Tonetti 2018
- Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. Journal of Clinical Periodontology 2018;45:S149-61. [DOI] [PubMed] [Google Scholar]
van der Weijden 2002
- Weijden U, Timmerman MF. A systematic review on the clinical efficacy of subgingival debridement in the treatment of chronic periodontitis. Journal of Clinical Periodontology 2002;29(Suppl 3):55-71. [DOI] [PubMed] [Google Scholar]
Zhao 2020
- Zhao H, Hu J, Zhao L. Adjunctive subgingival application of Chlorhexidine gel in nonsurgical periodontal treatment for chronic periodontitis: a systematic review and meta-analysis. BMC Oral Health 2020;20:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Eberhard 2004
- Eberhard J, Jepsen S, Needleman I, Worthington HV. Full-mouth disinfection for the treatment of adult periodontitis. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No: CD004622. [DOI: 10.1002/14651858.CD004622] [DOI] [PubMed] [Google Scholar]
Eberhard 2008a
- Eberhard J, Jepsen S, Jervøe-Storm P-M, Needleman I, Worthington HV. Full-mouth disinfection for the treatment of adult chronic periodontitis. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD004622. [DOI: 10.1002/14651858.CD004622.pub2] [DOI] [PubMed] [Google Scholar]
Eberhard 2015
- Eberhard J, Jepsen S, Jervøe-Storm P-M, Needleman I, Worthington HV. Full-mouth treatment modalities (within 24 hours) for chronic periodontitis in adults. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD004622. [DOI: 10.1002/14651858.CD004622.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


