Abstract
Cellular mechanophenotype is often a defining characteristic of conditions like cancer malignancy/metastasis, cardiovascular disease, lung and liver fibrosis, and stem cell differentiation. However, acquiring living cells based on mechanophenotype is challenging for conventional cell sorters due to a lack of biomarkers. In this study, we demonstrate a workflow for surface protein discovery associated with cellular mechanophenotype. We sorted heterogeneous adipose-derived stem/stromal cells (ASCs) into groups with low vs. high lamin A/C, an intracellular protein linked to whole-cell mechanophenotype. Proteomic data of enriched groups identified surface protein candidates as potential biochemical proxies for ASC mechanophenotype. Select surface biomarkers were used for live-cell enrichment, with subsequent single-cell mechanical testing and lineage-specific differentiation. Ultimately, we identified CD44 to have a strong inverse correlation with whole-cell elastic modulus, with CD44lo cells exhibiting moduli three times greater than that of CD44hi cells. Functionally, these stiff and soft ASCs showed enhanced osteogenic and adipogenic differentiation potential, respectively. The described workflow can be replicated for any phenotype with a known correlated intracellular protein, allowing for the acquisition of live cells for further characterization, diagnostics, or therapeutics.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04351-w.
Keywords: Lamin, CD44, Elastic modulus, Atomic force microscopy, Mechanobiology, Proteomics
Introduction
The mechanophenotype of a cell, though normally well-regulated for tissue-specific functions, becomes dysregulated during many disease states throughout the body, such as fibrosis, arthritis, cardiovascular disease, and cancer [1–4]. Stem cell differentiation preference, especially during remodeling of damaged tissues, has also been shown to be linked to inherent cell stiffness, potentially a consequence of their niche environment. Specifically, clonal populations of stiffer mesenchymal stem cells (MSCs) differentiate more robustly down the osteogenic lineage and softer MSCs down the adipogenic lineage [5–8]. Though there have been many studies of the effects of local mechanical environment and the extracellular matrix (ECM) on cell behavior, intracellular mechanical changes also arise on a single-cell level. For instance, internal traction forces are generated to aid in cell division and motility and bidirectional mechanotransductive signaling also occurs in and out of the cell [9]. As a desirable phenotype for diagnostic or enrichment purposes, it would be advantageous to identify and sort live cells based on their inherent stiffness. While custom, microfluidic devices exist that can separate living cells based on their physical properties (e.g., size and deformability combined), no large-scale infrastructure exists for broad adoption of these technologies [10–12]. To achieve live-cell sorting based on elastic modulus on a larger scale, the identification of a stiffness-correlated surface biomarker is needed to enable the use of widely established methods like fluorescence-activated cell sorting (FACS).
In the search for molecular mechanisms that influence cell stiffness, researchers have identified a correlation between the expression levels of the intracellular protein lamin A/C and the inherent elastic moduli of cell nuclei, tissues, and even whole cells [13, 14]. This connection has been shown to be influenced by a host of nuclear and cytoskeletal proteins including the LINC (Linker of Nucleoskeleton and Cytoskeleton) complex, collagen I, actin, actomyosin, emerins, and proteins in the Wnt/β-catenin signaling pathway [13, 15–20]. As a specific example, the relationship between stem cell stiffness and differentiation potential has been correlated with lamin A/C expression, where stem cells engineered to overexpress lamin A/C exhibited increased osteogenic differentiation and decreased adipogenic differentiation, potentially related to a vascular endothelial growth factor (VEGF) feedback loop with lamin A/C [20, 21]. However, all of the intracellular proteins associated with stiffness, including lamin A/C, are incompatible with use in live sorting of non-genetically modified cells as this would require fixation and permeabilization for internal labeling. Acquiring live cells is critical for many downstream applications, whether that be basic science experiments or clinical therapies. Additionally, having a surface marker proxy for nuclear lamin A/C levels could benefit cancer diagnostics and predictions, as lower levels have been shown to correlate with increased cell migration/metastasis as well as poor prognosis and decreased survival times in many cancers [22–24].
The objective of this study was to develop and implement a surface protein biomarker discovery process to identify candidate targets that correlated with stem cell stiffness. We hypothesized that adipose-derived stem/stromal cell (ASC) differentiation potential in heterogeneous, primary cell sources would correlate with whole-cell mechanophenotypes, with stiffer cells being more osteogenic and softer cells more adipogenic. To test this hypothesis, we used our recently developed technique for formaldehyde-fixed, intracellular target-sorted antigen retrieval (FITSAR) to enrich for high and low lamin A/C-expressing subpopulations of fixed ASCs [25–27]. These samples underwent proteomic analysis to screen for associated surface markers. Top candidates were then evaluated by enrichment and characterization of live ASCs for single-cell mechanical properties and osteogenic/adipogenic differentiation potential. All experiments were conducted using unmanipulated cells based on their expression of endogenous, natural protein levels and inherent single-cell mechanophenotype, in contrast to the transfected and knockdown cells used in much of the supporting literature for this study. More broadly, this work demonstrates a process to discover surface protein markers for cellular phenotypes defined by a characteristic intracellular profile suitable for live-cell purification using widely available sorting techniques and infrastructure.
Results
Lamin A/C sort and proteomics
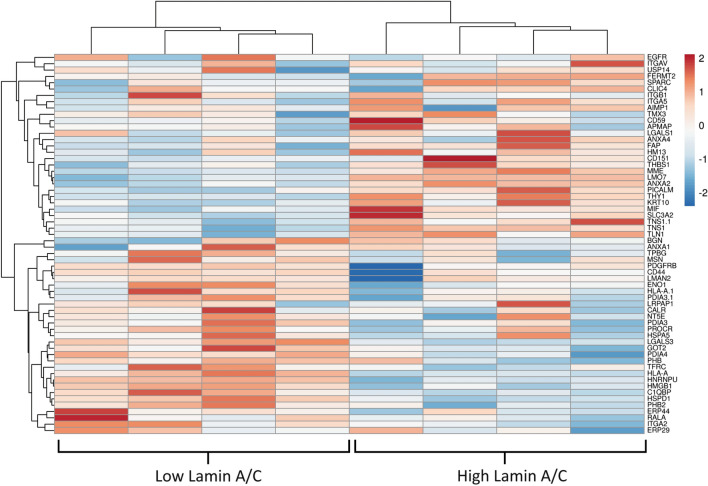
ASCs were fixed, permeabilized, and labeled with anti-lamin A/C antibody prior to sorting by FACS. Sorting gates included the top and bottom 20% of the population as “low” and “high” lamin A/C subsets, respectively (Fig. 1a). The relative levels of lamin A/C in these two groups was confirmed via western blot (Fig. 1b, c). While direct measurement of cell elastic moduli at this stage were not relevant due to the fixed nature of the samples, previously published work using overexpression models indicated a positive correlation between lamin A/C levels and cell stiffness [13]. We confirmed this relationship in our samples by siRNA knockdown of LMNA, which significantly decreased single-cell elastic moduli (Supplementary Fig. 1). Protein lysates for the low and high lamin A/C samples were processed and analyzed by mass spectrometry (n = 4), resulting in 1300 total identified proteins. We used the UniProt Gene Ontology groupings database to select for any surface proteins present based on their subcellular location classification and ranked them based on the absolute difference between the normalized protein amounts for “low” and “high” lamin A/C samples (Fig. 2). Approximately half of these proteins had a positive correlation with lamin A/C while the other half was negatively correlated. This pattern differed from that of cytoskeletal proteins, which as expected, skewed more towards a positive correlation (Supplementary Fig. 2). To select for surface marker candidates, we focused on proteins whose abundance differed the most between low and high lamin A/C (> 20%). Of those, only a subset has been characterized as being exclusively on the cell plasma membrane, such as CD44, TPBG, MME (CD10), and THY1 (CD90). We decided to focus on CD44 and CD90 as they are well-known stem cell markers [28].
Fig. 1.
Enrichment of fixed ASCs by whole-cell mechanophenotype using intracellular, lamin A/C protein levels. a ASCs labeled with AlexaFluor 488-conjugated anti-lamin A/C antibody were gated to collect the top and bottom 20% of cells based on fluorescence. b Relative lamin A/C levels were confirmed by western blot with bands at 74 and 67 kDa, with β-tubulin as a loading control at 55 kDa. c The lamin A and lamin C bands were quantified via densitometry, as well as combined for a total lamin A/C amount, and normalized to the loading control, which confirmed expected expression levels in the two sorted groups
Fig. 2.
Proteomic heat map of identified surface proteins for low and high lamin A/C ASC samples. The top half of listed proteins showed a positive correlation with lamin A/C levels while the bottom half were negatively correlated. Surface proteins were pulled from the whole proteome dataset (1300 proteins) based on UniProt subcellular location classification. Results show n = 4 technical replicates from singular low and high lamin A/C samples. Figure produced with ClustVis online tool
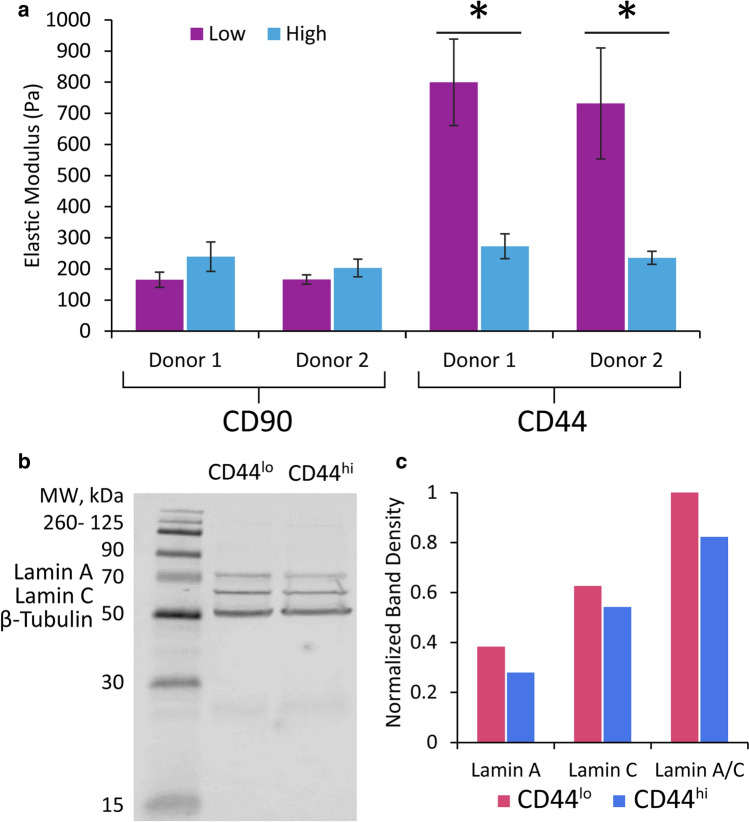
Atomic force microscopy (AFM) of CD90lo/hi and CD44lo/hi subpopulations
ASCs from two, distinct donors were labeled with antibodies for CD90 and CD44 and sorted into the top and bottom 20% of expressers by FACS. The elastic moduli of 20–30 cells in a spherical morphology on glass were measured via AFM (Fig. 3a). CD90lo/hi populations exhibited the expected correlations based on proteomic findings, but elastic modulus measurements were not statistically different at p < 0.05. CD44lo/hi populations also exhibited the expected correlations but with strong statistical significance (p < 0.0001). The average elastic modulus for CD44lo cells was about three times that of CD44hi cells (Fig. 3a). This trend was again confirmed via western blot for lamin A/C using cells from the CD44lo/hi samples (Fig. 3b, c). Both lamin A and C protein levels were consistently higher in the “stiffer” cell group (CD44lo) than the “softer” cell group (CD44hi).
Fig. 3.
Single-cell mechanical testing was performed by AFM on two different human donor ASC samples sorted by CD90 or CD44 surface protein levels. a Expected trends and statistically significant differences were observed between “stiff” CD90hi and CD44lo cells and “soft” CD90lo and CD44hi cells (n = 20–30). Statistical significance between low and high protein levels denoted by asterisks above bars (mean ± standard error). b, c The amount of lamin A/C in the CD44-sorted samples was quantified via western blot to confirm an inverse correlation existed between the two proteins
Spurred by our findings for CD44, a limited exploration into the universality of the CD44-mechanophenotype relationship was examined for two cancer cell lines, MG-63 osteosarcoma cells and U251 glioblastoma cells. MG-63 cells have important similarities to ASCs such as a fibroblastic morphology and strong expression of lamin A/C [14]. Comparatively, U251 cells are much smaller with a high nuclear-to-cytoplasmic ratio and exhibit low-to-no lamin A/C [13]. Both cell lines were labeled with anti-CD44 antibody, sorted into top and bottom 20% expressers by FACS, and mechanically tested by AFM following the same protocols as for ASCs. Results showed no significant difference in elastic modulus for CD44lo/hi groups in either cell type (Supplementary Fig. 3). Average modulus values were nearly the same for sorted U251 cells (p = 0.697); however, sorted MG-63 cells exhibited a similar trend to ASCs, with CD44lo cells having an average elastic modulus ~ 20% greater than CD44hi cells (p = 0.475).
Differentiation potential of CD44lo/hi ASCs
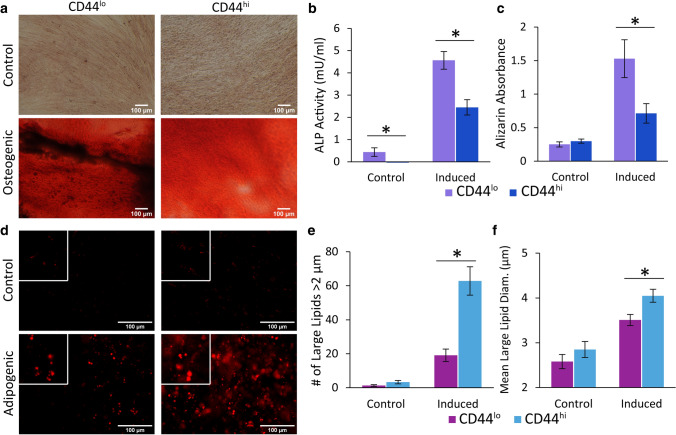
CD44lo/hi ASCs were cultured in control or lineage differentiation media for 21 days, progressing them down the osteogenic and adipogenic lineages. We measured significantly higher alkaline phosphatase (ALP) activity, which is associated with osteogenesis, on day 7 in CD44lo vs. CD44hi samples (p < 0.05). Interestingly, ALP could also be detected in control CD44lo samples which lacked chemical induction stimuli (Fig. 4b). Alizarin red S staining of deposited calcified matrix, when eluted, showed more than twice as much calcium secretion in CD44lo samples than CD44hi (Fig. 4c, p < 0.05). The opposite relationship was seen in adipogenically induced samples, with the softer, CD44hi cells exhibiting more robust differentiation as determined by the number of large lipids (> 2 µm in diameter), the mean diameter of these lipids, and overall oil red O lipid staining (Fig. 4d–f, p < 0.05). As observed previously, samples in control medium lacked the formation of large lipids but instead exhibited many small lipid droplets [29]. Considering the overall differentiation results, a strong case can be made for the predictive value of CD44 towards lineage-specific differentiation potential.
Fig. 4.
Lineage-specific differentiation potential differed for ASCs based on CD44 levels. a CD44lo/hi ASCs were chemically differentiated down the osteogenic lineage and compared to cells kept in control medium. b, c Osteogenesis samples were assessed for ALP activity after 7 days and stained with alizarin red S after 21 days to visualize calcium deposits. Induced CD44lo cells, the stiffer phenotype, showed more robust osteogenic differentiation based on these two assays (*p < 0.05). d CD44lo/hi were also chemically differentiated down the adipogenic lineage and lipids stained with oil red O. Insets are 2.5 × the original magnification. Scale bars are 100 µm in all images (a, d). e, f Adipogenesis samples were stained with oil red O to visualize intracellular lipids, and the number of large lipids/image (diameter > 2 µm) was quantified along with the mean large-lipid diameter (*p < 0.05). Error bars signify standard error
Discussion
In this study, we demonstrated an experimental workflow to discover surface protein markers that correlated with a functional, cell phenotype. We used a characteristic intracellular protein to enrich for “stiff” and “soft” ASC subpopulations that were then interrogated for their proteomic profiles. Ultimately, we were able to discover a biochemical proxy for ASC mechanophenotype that was compatible with live cell sorting. This methodological process was enabled by a technique previously developed in our lab, FITSAR, which allows for protein extraction from fixed cells, facilitating the proteomic analysis of cells based on intracellular protein sorting [25]. Of the proteins identified in our initial screen of high and low lamin A/C ASC proteomes, CD44 exhibited the largest difference between the two groups. When used for live cell sorting, CD44-expressing cells exhibited statistically significant differences in elastic moduli, with low expressers being over three times stiffer than high expressers. While CD44 has multiple biological functions, our results indicate it can be used as a surface biomarker associated with ASC stiffness level, a phenotype that correlates with lineage-specific differentiation potential. These relationships were elucidated without needing genetic or chemical modification of cell populations, so protein levels and mechanophenotypes reflect natural, unbiased variation within these human ASC samples. Greater in vitro osteogenic differentiation, particularly when indicated by higher ALP activity levels, has been associated with more mineralized bone formation and healing when those stem cells are implanted in vivo in mice [30–32]. This bolsters the impact of our in vitro findings, implying that CD44lo ASCs could be isolated and implanted for better bone healing, while CD44hi ASCs could benefit fat grafting.
As part of our workflow demonstrations, we prioritized assessment of surface proteins that showed the largest differences in lamin A/C levels and had known relevance to our cell type of interest (i.e., ASCs) rather than conducting an exhaustive screen. These criteria resulted in CD90 and CD44 as our most promising targets. Many of the proteins identified by the UniProt database as existing on the cell surface also have documented intracellular locations, which eliminated their utility for our purposes. CD44, the hyaluronan receptor, has previously been linked to softer cell phenotypes, particularly in cancer and aging stem cells [2, 33–35]. While the current work does not include a mechanistic study of how CD44 (or any of its various isoforms) and lamin A/C are connected, substantial literature exists pointing to their common interaction with the cytoskeleton. In turn, the cytoskeleton is a direct contributor to whole-cell mechanical properties [14, 36–39]. At least one physical linkage exists connecting CD44 to lamin A/C (i.e., CD44 to actin to nesprin-1/2 to lamin A/C), though each of these proteins are themselves components of many other pathways that could play a role in mechanophenotype regulation [15, 40, 41]. Actin is well documented as one of the largest influencers of cell structure and elastic modulus, with stiffer cell regions as well as whole cells containing more dense and organized actin filaments [36, 42–44]. The importance of actin in cell mechanics is generally demonstrated using pharmacologic disruptors, like cytochalasin D and latrunculin, to inhibit actin filament formation as well as downregulate actin-binding proteins to prevent proper crosslinking and branching [45]. In the current study, we observed no major differences in overall actin abundance between the CD44lo/hi populations (Supplementary Fig. 4). Cell morphologies were also similar, suggesting that the difference in mechanophenotype was likely not due to actin amount or organization. Since CD44 has no major effect on morphology, its mechanobiological role is likely downstream of actin filament polymerization. Similar results were previously shown with CD44-knockout mouse fibroblasts compared to wild type, indicating that CD44 influences cell motility but not focal adhesion creation or stress fiber formation in response to the mechanical environment [46]. Our exploratory experiments with MG-63 and U251 cell lines do suggest the critical nature of lamin A/C in the CD44-mechanophenotype relationship. U251 cells, which lack robust expression of lamin A/C, showed no difference between the elastic moduli of CD44lo/hi populations. Comparatively, MG-63 cells, which do have lamin A/C, showed a similar trend for this relationship as ASCs, although the magnitude of modulus difference was much less. A potential explanation for this muted difference is the greater homogeneity of cell lines compared to primary cell populations. Future work can investigate this by examining the CD44-mechanophenotype relationship in primary tumor cells.
Non-physical signaling linkages between CD44 and lamin A/C also exist, such as through the extra-to-intracellular Hippo communication pathway and Wnt/β-catenin signaling for cell adhesion, migration, differentiation, and epithelial-mesenchymal transition (EMT) in metastasis [20, 47]. The directionality of this relationship can vary, though. For example, LMNA-null cells have been shown to exhibit decreased RhoA activity, which is a key player in the Hippo pathway regulating the overall mechanobiology of cells [48]. Knockdown of CD44 has also been demonstrated to inhibit RhoA expression and reduce yes-associated protein (YAP) expression, a transcriptional co-activator of Hippo communication regulated by RhoA [49, 50]. Together, this positive correlation between CD44 and lamin A/C conflicts with our current findings. However, CD44 activity can mediate vascular endothelial growth factor (VEGF) production, which at high levels has been shown to repress lamin A/C, a feedback mechanism related to PPAR-γ and RUNX2 expression associated with adipocytic and osteoblastic stem cell differentiation, respectively [21, 51, 52]. This provides at least one pathway that could drive the inverse relationship we observed between CD44 and lamin A/C. EMT and Wnt signaling pathways are alternative possibilities. During osteogenic stem cell differentiation, lamin A/C can facilitate the nuclear translocation of β-catenin in the Wnt pathway, which induces greater expression of osteogenic genes and proteins for stiff bone formation [20]. Silencing of LMNA increases overall β-catenin levels and decreases E-cadherin, a protein that spans the cell membrane and regulates cell–cell binding [53]. When β-catenin is released from the nucleus, it forms a complex with E-cadherin in the cytoplasm that functions as an intercellular junction, and loss of E-cadherin leads to increased β-catenin concentration as it is no longer sequestered with the cadherins [54]. Nuclear β-catenin concentration is positively correlated with CD44 levels, with downregulation of CD44 in leukemia cells having been shown to cause the phosphorylation of β-catenin in the cytoplasm, leading to ubiquitin-driven degradation [55, 56]. Comparatively, E-cadherin is negatively correlated with CD44, acting to inhibit its binding to hyaluronan [57, 58]. Thus, the combination of β-catenin and E-cadherin provide another explanation for the negative correlation observed between lamin A/C and CD44 in our experiments, as high lamin A/C is correlated with high E-cadherin and low β-catenin while high E-cadherin and low β-catenin are also correlated with low CD44.
CD44 has a well-documented role in disease states and progression. Some of these conditions directly relate to underlying cell mechanical properties. Others may only manifest this as a characteristic. High levels of CD44 have been identified in cancer cells, especially those with greater capabilities for migration and metastasis as CD44 and its ligand, hyaluronan, contribute to mechanosensing and motility [59, 60]. Increased deformability and low elastic modulus are also characteristic of malignant cell types [2]. Loss of E-cadherin is a characteristic of the epithelial-mesenchymal transition that occurs when cancer cells metastasize, which corroborates the inverse correlation between CD44 and E-cadherin expression in spreading cancer cells [57]. These reports correspond well with our findings that CD44hi cells exhibit lower moduli, at least for cells that express lamin A/C. Cancer stem cells of the lung that persist after ionizing radiation treatments have been shown to express high CD44 and increased β-catenin in the nucleus, a positive correlation between the two proteins that we described above [61]. Fibrosis is another condition in which mechanical changes are a central feature. In a kidney injury rat model, ischemia and fibrosis were mitigated by preconditioning ASCs in hyaluronic acid, which upregulated CD44 levels [62]. If CD44 is eliminated or blocked by an antibody, pulmonary fibrosis is reduced and fibroblasts become less invasive as the cells cannot bind hyaluronan [63]. Though this would seem contradictory based on the relationships discovered in our study, as lower CD44 would result in the stiffer cell phenotypes normally associated with fibrosis, this study did not include any mechanical testing and made claims based on histology and disease outcomes alone [64]. In another tissue-stiffening disease, low CD44 levels in articular cartilage have been found to correspond with stiffer chondrocytes as well as increased severity of osteoarthritis [65, 66]. Outside of CD44-related diseases, the correlation we have found between CD44 and lamin A/C potentially allows for live-cell diagnosis and study of laminopathies correlated with high and low lamin A/C levels. Breast cancer cells from larger tumors and of worse histological grade have lower levels of lamin A/C [22]. Loss of normal levels of lamin A/C also correlates with more likely recurrence of stage II and III colon cancer [24]. Lamin A has even been called a biomarker of prostate cancer cells and severity of disease progression based on a proteomic screen of benign vs. malignant cells [67]. This indicates how useful a surface biomarker correlating with lamin A/C levels could be to facilitate diagnoses and additional tests using live cell samples, which is what we have made feasible with CD44. While we are only conjecturing on diseases involving dysregulated lamin A/C quantity, there are many other laminopathies involving genetic mutations, including muscular dystrophies, lipodystrophies, and accelerated aging disorders, whose connections to CD44 or other surface biomarkers have yet to be explored [68].
In this work, a proteomic screen of low vs. high lamin A/C-expressing cells led to the discovery of CD44 as a surface biomarker proxy of ASC stiffness. Cells sorted by CD44 levels, which inversely correlated with cellular elastic moduli, predicted lineage-specific, stem cell differentiation potential, with stiffer CD44lo cells exhibiting more robust osteogenic differentiation and softer CD44hi cells exhibiting more robust adipogenic differentiation. This demonstration in heterogeneous, primary ASC samples replicates and extends previous observations of the stem cell mechanophenotype-differentiation association observed with clonal populations [6]. The experimental workflow described herein is a powerful approach to biomarker discovery using characteristic intracellular molecules associated with a phenotype of interest that may be unique to a single cell type or cross many cell types. Ultimately, we identified a ubiquitously present extracellular marker on ASCs that can be used to generally identify the mechanophenotype of individual cells. This will facilitate live-cell sorting of stem cells based on their elastic modulus using easily accessible, widely adopted methods and infrastructure.
Materials and methods
Cells and culture reagents
Primary human adipose-derived stem cells contained in the stromal vascular fraction (SVF) were isolated from axillary lipoaspirate of two healthy 34 and 55-year-old female donors (IRB Registration #0000396, 00004624, CMTT/PROJ: 210312) according to published methods [29, 69]. Briefly, lipoaspirate samples were thoroughly washed with phosphate-buffered saline (PBS). The adipose tissue was then digested with an equal volume of PBS with 0.1% collagenase, 2 mM calcium chloride, and 1% bovine serum albumin (BSA) for 1 h shaking at 37 °C. After incubation, the digested tissue was centrifuged at 300×g for 5 min, and the top layer containing mature adipocytes and free lipids was removed. The remaining pellet was resuspended in complete medium of DMEM/F12 (HyClone, SH30023.01), 1× penicillin/streptomycin/Amphotericin B (HyClone, Cytiva, SV30079.01), and 10% fetal bovine serum (FBS) (Zen-Bio Inc., SER-500). Following centrifugation at 400×g for 5 min, cells were filtered sequentially through 100 µm and 70 µm cell strainers before incubation for 10 min in 160 mM ammonium chloride to lyse red blood cells. After an additional centrifugation and medium wash to remove the lysate, the pelleted cells, now identified as the ASCs, were frozen in medium containing 80% FBS, 10% complete medium, and 10% dimethyl sulfoxide (DMSO).
Cells were thawed and grown to confluence in T-182 flasks prior to the start of experiments. MG-63 cells were expanded and maintained in medium containing MEM (Hyclone, SH3002401), 1× penicillin/streptomycin (Hyclone, SV30010) and 10% fetal bovine serum (FBS) (Genesee Sci. 25–514). U251 cells were expanded and maintained in medium containing DMEM (Hyclone, SH3024301), 1× penicillin/streptomycin (Hyclone, SV30010), 10% FBS (Genesee Sci. 25-514), and 1% l-glutamine (Hyclone, SH3003401). ASCs were expanded and maintained in expansion medium containing DMEM/F12 (HyClone, SH30023.01), 1× penicillin/streptomycin/Amphotericin B (HyClone, Cytiva, SV30079.01), 10% FBS (Zen-Bio Inc., SER-500), 1 ng/mL fibroblast growth factor (R&D Systems, 233-FB), 5 ng/mL epidermal growth factor (R&D Systems, 236-EG), and 0.25 ng/mL transforming growth factor-β1 (R&D Systems, 240-B). Prior to sorting based on lamin A/C level for proteomic analysis, ASCs were switched to serum-free expansion media for 16 h to prevent an abundance of extraneous serum proteins from creating noise in the ASC proteome data [70, 71]. The effect of serum starvation before sorting was also later explored in surface marker-based FACS (Supplementary Fig. 5).
Lamin A/C-labeled ASC sorting and protein extraction
Four sets of three T-182 flasks containing confluent ASCs were uplifted with Accutase (Fisher Scientific) for 30 min at 37 °C, and triplicate flasks were pooled and counted. Cells were then fixed for 10 min at room temperature in 4% paraformaldehyde in PBS, permeabilized for 15 min at room temperature with 1% Triton X-100 (Fisher Scientific) in PBS and blocked with 3% bovine serum albumin (BSA) in PBS for 1 h. Cells were then incubated for 1 h at room temperature with anti-lamin A/C pre-conjugated to AlexaFluor 488 (Cell Signaling Technology, 8617S, 1:50 in 1% BSA/PBS), with a small portion of cells left unlabeled as a control. These four identical sets of lamin A/C-labeled cells were sorted using a BD FACSAria IIIu instrument (BD Biosciences) at 2500 cells/s. Gates were set to collect only ASCs in the top and bottom 20% of fluorescence intensities in separate tubes, with unlabeled ASCs used as a control for background fluorescence level.
Protein extraction was accomplished according to the formaldehyde-fixed, intracellular target-sorted antigen retrieval (FITSAR) method [25–27]. Briefly, fixed samples of high and low lamin A/C-sorted ASCs were lysed and incubated for 30 min at 100 °C, then for 2 h at 60 °C in 300 mM Tris HCl (pH 8.0) with 2% sodium dodecyl sulfate (SDS). Samples were centrifuged at 16,000×g for 10 min at 4 °C, and supernatants were retained and stored at −80 °C prior to protein analyses.
Western blot protein quantification
Protein content of the low and high lamin A/C lysates was quantified using a bicinchoninic acid (BCA) assay (Pierce, Thermo Fisher Scientific). For western blots, 5 µg of protein from high lamin A/C and low lamin A/C samples were loaded in a gel, run for 1 h at 120 V, and transferred to membrane paper for blotting. Antibodies used included anti-lamin A/C (Cell Signaling Technology, 2032S, 1:500), anti-beta tubulin (Developmental Studies Hybridoma Bank, E7-S, 1:1000), anti-rabbit secondary with IRDye 680RD (LI-COR, 925-68073, 1:5000), and anti-mouse secondary with IRDye 800CW (LI-COR, 925-32210, 1:5000). The western blot was imaged on a LI-COR Odyssey Clx Infrared Imaging System, and densitometry of the bands was determined using ImageJ gel analysis tool.
For CD44lo/hi samples, ~ 3 million cells were lysed using an SDS/urea buffer determined to be efficient for lamin A/C extraction [14]. Briefly, cells were resuspended in 100 µL of 2 M urea (Sigma Aldrich), 34 mM SDS (Thermo Fisher Scientific), and 50 mM Tris HCl pH 8.0 (Thermo Fisher Scientific) and incubated 30 min with shaking on ice. Samples were then vortexed and pulled through a 21-gauge needle to eliminate clumping due to released nucleic acid material prior to centrifugation at 14,000×g for 10 min at 4 °C. Supernatant was transferred to clean tubes and stored at −80 °C until use.
Following a BCA assay, 20 µg of total protein was loaded into a gel and run for 1 h at 120 V. The blot was probed with anti-lamin A/C (Cell Signaling Technology, 2032S, 1:500), anti-beta tubulin (Developmental Studies Hybridoma Bank, E7-S, 1:1000), anti-rabbit secondary with IRDye 680RD (LI-COR, 925-68073, 1:5000), and anti-mouse secondary with IRDye 800CW (LI-COR, 925-32210, 1:5000). The western blot was imaged and quantified in the same manner as before to confirm that lamin A/C levels maintained the same correlation with CD44 when sorted in the opposite manner as the initial proteomics sorts. The additional western blot for actin expression was run with the same lysates using anti-actin antibody (Thermo Fisher Scientific, MA5-11869).
Atomic force microscopy (AFM) of ASCs and cell lines
Single-cell mechanical measurements were made using AFM as described previously [72, 73]. For initial experiments, ASCs expanded to passage 5 were treated with either 50 nM LMNA siRNA (siLMNA, s8221, 4390824, sense: 5'-CCAAAAAGCGCAAACUGGATT-3′, antisense: 5′-UCCAGUUUGCGCUUUUUGGTG-3′, LMNA Silencer Select Validated siRNA, Ambion, Thermo Fisher Scientific) or 50 nM Scramble siRNA (siScramble, 4390843, Silencer Select Negative Control #1 siRNA, Ambion, Thermo Fisher Scientific) for 72 h prior to mechanical testing. Cells at a density of 40,000 cells/mL in 500 µL PBS were allowed to adhere to glass coverslips for 30 min at 37 °C, which facilitated a spherical morphology previously documented to enhance mechanical property differences among cell types [6, 29, 39, 72–74]. All mechanical testing experiments were carried out at room temperature in fluid environments, and the AFM was equilibrated before tests to minimize laser deflection and piezo drift. While still in a spherical morphology, the mechanical properties of single cells were measured for each condition (one untreated and two siRNA treated) using established AFM techniques for single-cell indentation tests (MFP-3D-BIO, Asylum Research) [73, 74]. Briefly, cantilevers with 5 µm diameter spherical tips (k ~ 0.03 N/m, Novascan Technologies, Inc.) were used for single indentation tests on the perinuclear region of individual cells (n = 20–45 cells per condition). Force–indentation data were sampled with an approach velocity of 10 μm/s. Trigger forces ranged from 1.5 to 2 nN with deflections of 50–70 nm for all samples. Indentation and force data were used to determine whole-cell elasticity measurements by fitting with a modified Hertz contact model [72, 75]. Whole-cell elasticity tests were repeated for all post-sorting ASCs and cell line samples (MG-63 and U251) to acquire the elastic modulus for CD90lo/hi (ASCs only) and CD44lo/hi sorted groups of spherical cells with n = 20–30 cells per sample.
Mass spectrometry and proteomic data analysis
Aliquots of high and low lamin A/C protein lysates of 30 µg each were sent to IQ Proteomics, LLC (Cambridge, MA) for mass spectrometry analysis. ASCs from a single, human donor were used to create four biological replicates, which were then processed using in-house protocols. Briefly, protein samples were reduced in 20 mM DTT for 1 h, cysteines alkylated in 60 mM iodoacetamide for 1 h, and methanol-chloroform extracted with 1 µg trypsin in 100 mM EPPS buffer, pH 8.0 for 4 h. Tandem Mass Tags (TMT, Pierce, 400 µg) were added for 2 h at room temperature to each sample. Labeling efficiency was confirmed to be > 98%. Samples were then quenched with 0.5% hydroxylamine and pH lowered with 2% trifluoroacetic acid before being pooled together and evaporated via SpeedVac. Peptide fractionation was achieved using the high pH reverse-phase kit (Pierce) into three samples and desalted with Empore-C18 (3 M) StageTips before mass spectrometry on an Orbitrap Fusion Lumos with easy nanoLC-1000 (Thermo Fisher Scientific). A 75 µm capillary column packed with Sepax GP-C18 resin (Sepax) with a 35 cm final length was loaded with 2 µg of peptides per sample. A 110 min linear gradient from 8 to 28% acetonitrile in 0.1% formic acid was applied to separate peptides. Following initial FTMS1 spectral acquisition, the top ten most intense precursor ions were subjected to MS2 analysis with collisional-induced dissociation and then the top eight MS2 products were selected via synchronous-precursor-selection for high energy collisional-induced dissociation analysis in the Orbitrap. Peptides were identified using the SEQUEST algorithm and a 2018 human UniProt database with common contaminants with search criteria including fully tryptic peptides with two missed cleavages, precursor mass tolerance of 50 ppm, and fragment ion tolerance of 1 Da [76] Matches were assessed using linear discriminant analysis and paired down to 1% peptide false discovery rate before further stringency of 1% false discovery rate at the protein level. Protein levels were quantified by the sum of the total reporter intensities for all peptide-spectrum matches. The resulting list of proteins and their normalized abundance levels were used to identify candidate surface markers for subsequent evaluation. A simple ranking approach was used to distinguish which proteins differed most between the high and low lamin A/C groups, which involved calculating the absolute value of the difference between average scaled amounts of proteins that existed in both groups. UniProt online database was used to classify the identified proteins into relevant groupings such as cytoskeletal proteins and surface proteins [77]. The ClustVis online tool was used to make clustered heat maps [78].
Live-cell sorting with CD90 and CD44
Ten T-182 flasks of ASCs were grown to confluence in expansion media for each stem cell experiment. Five T-182 flasks of the cell lines (MG-63 and U251) were grown to confluence for each cell line experiment. ASCs, MG-63, and U251 cells were uplifted, counted, and kept on ice during all labeling steps. Cells were blocked in 3% BSA in Hank’s Balanced Salt Solution (HBSS) for 30 min prior to antibody labeling. Primary antibodies included anti-CD90 (ASCs only, Thermo Fisher Scientific, MA5-16671, 1:2000) and anti-CD44 (ASCs, MG-63, and U251 cells, Thermo Fisher Scientific, MA5-13890, 1:800 for ASCs and 1:100 for cell lines), which were diluted in 1% BSA/HBSS and incubated for 40 min. Fluorescent secondary antibody, goat anti-mouse IgG (H&L) conjugated to AlexaFluor 647 (Thermo Fisher Scientific, A-21236, 1: 1000 for ASCs and 1:250 for cell lines) was then added in 1% BSA/HBSS for 40 min. Cells were filtered through a 35 µm cell strainer prior to FACS sorting with a BD Biosciences Influx cell sorter. Gates were established using unlabeled and secondary-only labeled cell populations to distinguish expressing cells from background fluorescence, of which the top and bottom 20% of expressers were retained in separate tubes (Supplementary Fig. 6). Experiments were performed using the same lipoaspirate donor as the proteomics sort as well as a second iteration using cells isolated from a different donor. Additional runs investigated the two cancer cell lines of interest (MG-63 and U251). A quality-control comparison was also performed whereby cultured cells were switched to serum-free expansion media 16 h prior to sorting, as was done for the proteomics experiments, to assess whether the targeted surface proteins were affected noticeably by serum starvation (Supplementary Fig. 5). No differences in mechanics were observed so all experiments used medium containing serum.
Differentiation of CD44-sorted cells
After sorting by CD44 abundance levels, ASCs were seeded in three 96-well plates at 8000 cells/well with 16 wells per CD44lo/hi group for ALP analysis with osteogenic induction, 16 wells per group for matrix deposition analysis with osteogenic induction, 16 wells per group for intracellular lipid analysis with adipogenic induction, and 24 wells for matched control medium conditions for both the CD44lo/hi populations. Cells were cultured in expansion medium for 5 days to subconfluent densities prior to switching to induction or control media to allow for natural removal of anti-CD44 antibodies as previously determined [79]. For adipogenic differentiation, induced wells were cultured in DMEM/F-12, 10% FBS, 10 µM insulin, 1 µM dexamethasone, 0.25 mM isobutyl-1-methylxanthine, 200 µM indomethacin, and 1% penicillin/streptomycin/Amphotericin B (HyClone, Cytiva, SV30079.01) [80]. For osteogenic differentiation, induced wells were cultured in DMEM/high glucose, 10% FBS, 10 mM β-glycerophosphate, 10 nM 1,25-(OH)2 vitamin D3, 0.15 mM ascorbate-2-phosphate, 10 nM dexamethasone, and 1% penicillin/streptomycin/Amphotericin B [81]. Control medium contained DMEM/F-12, 10% FBS, 1% penicillin/streptomycin/Amphotericin B. Every other day for 7 or 21 days, wells underwent a 75% media exchange with fresh media. At 7 days of osteogenic induction, samples were assessed for alkaline phosphatase activity levels (ALP, BioVision, K422-500), which was indicative of early bone formation. After 21 days of osteogenic or adipogenic induction, samples were fixed with 10% formalin and stained with alizarin red S (Sigma-Aldrich) or oil red O (Sigma-Aldrich), respectively, and imaged with brightfield and fluorescence microscopy for comparison with control medium samples. Alizarin red S was eluted using 10% cetylpyridinium chloride in 10 mM sodium phosphate for 1 h, and optical density was measured by spectrophotometer at 540 nm. Oil red O-stained samples were fluorescently imaged (ex 628 nm/em 685 nm) for quantification of lipid size and large lipid counts greater than 2 µm in diameter. Lipid size and quantity were calculated using a custom MATLAB program using intensity thresholding to eliminate background signal, masking, and particle diameter measurements.
Confocal imaging of actin in CD44-sorted cells
The abundance and organization of cytoskeletal actin was visualized for CD44hi/lo ASCs in both suspended and adhered states. Cells were fixed with 4% formaldehyde for 10 min at room temperature in suspension or after 30 min adherence to glass coverslips. Following fixation, cells were permeabilized with 0.1% Triton X-100 (Fisher Scientific) in PBS for 15 min at room temperature, stained with AlexaFluor 488 phalloidin (Thermo Fisher Scientific, A12379, 1:40) in 1% BSA/PBS for 20 min at room temperature, and fluorescently stained for nuclei visualization with DAPI (Fisher Scientific, 1:50,000) in PBS for 30 min at room temperature with PBS washes between all steps. Adhered cells were preserved on glass slides with 10% glycerol, and cells in suspension settled by gravity in dishes prior to imaging. Cells were imaged on Olympus FV3000 Confocal Microscope at 60× magnification over 25–100 z slices depending on degree of cell attachment with 0.39 µm separation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Kevin Carlson of the Brown University Flow Cytometry and Sorting Facility as well as Mark Dooner of the COBRE Flow Cytometry Core at Rhode Island Hospital for performing all cell sorts.
Author contributions
MED performed flow cytometry co-stain experiments, preparation of ASCs for FACS sorts, and differentiation experiments and assays. GRC prepared cell lines for FACS sorts. RDGC carried out initial pilot experiments as well as the fixed-cell sort and preparation of those samples for proteomic analysis. VCF performed AFM mechanical testing. EMD expanded on proteomics analysis and experimental design. MED and EMD wrote the manuscript with editing from other authors.
Funding
We acknowledge the financial support of the National Institutes of Health (R01 AR054673, P30 GM122732).
Availability of data and material
Proteomics available on MassIVE, UCSD: MSV000087538
Code availability
Not applicable
Declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Costa KD. Single-cell elastography: probing for disease with the atomic force microscope. Dis Mark. 2004;19:139–154. doi: 10.1155/2004/482680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alibert C, Goud B, Manneville JB. Are cancer cells really softer than normal cells? Biol Cell. 2017;109:167–189. doi: 10.1111/boc.201600078. [DOI] [PubMed] [Google Scholar]
- 3.Ingber D. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35:564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- 4.Handorf AM, Zhou Y, Halanski MA, Li WJ. Tissue stiffness dictates development, homeostasis, and disease progression. Organo. 2015;11:1–15. doi: 10.1080/15476278.2015.1019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 6.González-Cruz RD, Fonseca VC, Darling EM. Cellular mechanical properties reflect the differentiation potential of adipose-derived mesenchymal stem cells. Proc Natl Acad Sci. 2012;109:E1523–E1529. doi: 10.1073/pnas.1120349109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benayahu D, Wiesenfeld Y, Sapir-Koren R. How is mechanobiology involved in mesenchymal stem cell differentiation toward the osteoblastic or adipogenic fate? J Cell Phys. 2019;234:12133–12141. doi: 10.1002/jcp.28099. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Cruz RD, Darling EM. Adipose-derived stem cell fate is predicted by cellular mechanical properties. Adipocyte. 2013;2:87–91. doi: 10.4161/adip.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen KA, Donato DM, Balcioglu HE, Schmidt T, Danen EH, Koenderink GH. A guide to mechanobiology: where biology and physics meet. Biochim Biophys Acta Mol Cell Res. 1853;2015:3043–3052. doi: 10.1016/j.bbamcr.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Urbanska M, Muñoz HE, Bagnall JS, Otto O, Manalis SR, Di Carlo D, Guck J. A comparison of microfluidic methods for high-throughput cell deformability measurements. Nat Methods. 2020;17:587–593. doi: 10.1038/s41592-020-0818-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G, Mao W, Byler R, Patel K, Henegar C, Alexeev A, Sulchek T. Stiffness dependent separation of cells in a microfluidic device. PLoS ONE. 2013;8:e75901. doi: 10.1371/journal.pone.0075901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam M, Mezencev R, McFarland B, Brink H, Campbell B, Tasadduq B, Waller EK, Lam W, Alexeev A, Sulchek T. Microfluidic cell sorting by stiffness to examine heterogenic responses of cancer cells to chemotherapy. Cell Death Dis. 2018;9:1–12. doi: 10.1038/s41419-018-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PDP, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin—a scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104–1240101–1240115. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González-Cruz RD, Sadick JS, Fonseca VC, Darling EM. Nuclear lamin protein C is linked to lineage-specific, whole-cell mechanical properties. Cell Mol Bioeng. 2018;11:131–142. doi: 10.1007/s12195-018-0518-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon DN, Zastrow MS, Wilson KL. Direct actin binding to A and B-type lamin tails and actin filament bundling by the lamin-A tail. Nucleus. 2010;1:264–272. doi: 10.4161/nucl.11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buxboim A, Swift J, Irianto J, Spinler KR, Dingal PDP, Athirasala A, Kao YRC, Cho S, Harada T, Shin JW, Discher DE. Matrix elasticity regulates lamin A, C phosphorylation and turnover with feedback to actomyosin. Curr Biol. 2014;24:1909–1917. doi: 10.1016/j.cub.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, Garcia-Mata R, Burridge K. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol. 2014;16:376–381. doi: 10.1038/ncb2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI200419670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bermeo S, Vidal C, Zhou H, Duque G. Lamin A/C acts as an essential factor in mesenchymal stem cell differentiation through the regulation of the dynamics of the Wnt/β-catenin pathway. J Cell Biochem. 2015;116:2344–2353. doi: 10.1002/jcb.25185. [DOI] [PubMed] [Google Scholar]
- 21.Alcorta-Sevillano N, Macías I, Rodríguez CI, Infante A. Crucial role of Lamin A/C in the migration and differentiation of MSCs in bone. Cells. 2020;9:1330. doi: 10.3390/cells9061330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alhudiri IM, Nolan CC, Ellis IO, Elzagheid A, Rakha EA, Green AR. C J Chapman Expression of Lamin A/C in early-stage breast cancer and its prognostic value. Breast Cancer Res Treat. 2019;174:661–668. doi: 10.1007/s10549-018-05092-w. [DOI] [PubMed] [Google Scholar]
- 23.Kaspi E, Frankel D, Guinde J, Perrin S, Laroumagne S, Robaglia-Schlupp A, Ostacolo K, Harhouri K, Tazi-Mezalek R, Micallef J, Dutau H. Low lamin-A expression in lung adenocarcinoma cells from pleural effusions is a pejorative factor associated with high number of metastatic sites and poor performance status. PLoS ONE. 2017;12:e0183136. doi: 10.1371/journal.pone.0183136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belt ET, Fijneman RJA, Van Den Berg EG, Bril H, Delis-van Diemen PM, Tijssen M, Van Essen HF, De Lange-De Klerk ESM, Beliën JAM, Stockmann HBAC, Meijer S. Loss of lamin A/C expression in stage II and III colon cancer is associated with disease recurrence. Eur J Cancer. 2011;47:1837–1845. doi: 10.1016/j.ejca.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Sadick JS, Darling EM. Processing fixed and stored adipose-derived stem cells for quantitative protein array assays. Biotechniques. 2017;63:275–280. doi: 10.2144/000114620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadick JS, Boutin ME, Hoffman-Kim D, Darling EM. Protein characterization of intracellular target-sorted, formalin-fixed cell subpopulations. Sci Rep. 2016;6:33999. doi: 10.1038/srep33999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadick JS, Crawford LA, Cramer HC, 3rd, Franck C, Liddelow SA, Darling EM. generating cell type-specific protein signatures from non-symptomatic and diseased tissues. Ann Biomed Eng. 2020;48:2218–2232. doi: 10.1007/s10439-020-02507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alt E, Yan Y, Gehmert S, Song YH, Altman A, Gehmert S, Vykoukal D, Bai X. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell. 2011;103:197–208. doi: 10.1042/BC20100117. [DOI] [PubMed] [Google Scholar]
- 29.Kanthilal M, Darling EM. Characterization of mechanical and regenerative properties of human, adipose stromal cells. Cell Mol Bioeng. 2014;7:585–597. doi: 10.1007/s12195-014-0350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaquiéry C, Schaeren S, Farhadi J, Mainil-Varlet P, Kunz C, Zeilhofer HF, Heberer M, Martin I. In vitro osteogenic differentiation and in vivo bone-forming capacity of human isogenic jaw periosteal cells and bone marrow stromal cells. Ann Surg. 2005;242:859–868. doi: 10.1097/01.sla.0000189572.02554.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright VJ, Peng H, Usas A, Young B, Gearhart B, Cummins J, Huard J. BMP4-expressing muscle-derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Mol Ther. 2002;6:169–178. doi: 10.1006/mthe.2002.0654. [DOI] [PubMed] [Google Scholar]
- 32.Prins HJ, Braat AK, Gawlitta D, Dhert WJ, Egan DA, Tijssen-Slump E, Yuan H, Coffer PJ, Rozemuller H, Martens AC. In vitro induction of alkaline phosphatase levels predicts in vivo bone forming capacity of human bone marrow stromal cells. Stem Cell Res. 2014;12:428–440. doi: 10.1016/j.scr.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Maherally Z, Smith JR, Ghoneim MK, Dickson L, An Q, Fillmore HL, Pilkington GJ. Silencing of CD44 in glioma leads to changes in cytoskeletal protein expression and cellular biomechanical deformation properties as measured by AFM nanoindentation. Bionanoscience. 2016;6:54–64. doi: 10.1007/s12668-015-0189-2. [DOI] [Google Scholar]
- 34.Zhou Z, Akinbiyi T, Xu L, Ramcharan M, Leong DJ, Ros SJ, Colvin AC, Schaffler MB, Majeska RJ, Flatow EL, Sun HB. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell. 2010;9:911–915. doi: 10.1111/j.1474-9726.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohammadalipour A, Burdick MM, Tees DF. Deformability of breast cancer cells in correlation with surface markers and cell rolling. FASEB J. 2018;32:1806–1817. doi: 10.1096/fj.201700762R. [DOI] [PubMed] [Google Scholar]
- 36.Li QS, Lee GY, Ong CN, Lim CT. AFM indentation study of breast cancer cells. Biochem Biophys Res Commun. 2008;374:609–613. doi: 10.1016/j.bbrc.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Cruz RD, Dahl KN, Darling EM. The emerging role of lamin C as an important LMNA isoform in mechanophenotype. Front Cell Dev Biol. 2018;6:151. doi: 10.3389/fcell.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labriola NR, Darling EM. Temporal heterogeneity in single-cell gene expression and mechanical properties during adipogenic differentiation. J Biomech. 2015;48:1058–1066. doi: 10.1016/j.jbiomech.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah MK, Garcia-Pak IH, Darling EM. Influence of inherent mechanophenotype on competitive cellular adherence. Ann Biomed Eng. 2017;45:2036–2047. doi: 10.1007/s10439-017-1841-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 41.Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, Fry AM, Trembath RC, Shackleton S. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol Cell Biol. 2006;26:3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo Q, Kuang D, Zhang B, Song G. Cell stiffness determined by atomic force microscopy and its correlation with cell motility. Biochim Biophys Acta Gen Subj. 1860;2016:1953–1960. doi: 10.1016/j.bbagen.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Rotsch C, Radmacher M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophys J. 2000;78:520–535. doi: 10.1016/S0006-3495(00)76614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pogoda K, Jaczewska J, Wiltowska-Zuber J, Klymenko O, Zuber K, Fornal M, Lekka M. Depth-sensing analysis of cytoskeleton organization based on AFM data. Eur Biophys J. 2012;41:79–87. doi: 10.1007/s00249-011-0761-9. [DOI] [PubMed] [Google Scholar]
- 45.Schaefer A, Te Riet J, Ritz K, Hoogenboezem M, Anthony EC, Mul FP, de Vries CJ, Daemen MJ, Figdor CG, van Buul JD, Hordijk PL. Actin-binding proteins differentially regulate endothelial cell stiffness, ICAM-1 function and neutrophil transmigration. J Cell Sci. 2014;127:4470–4482. doi: 10.1242/jcs.164814. [DOI] [PubMed] [Google Scholar]
- 46.Razinia Z, Castagnino P, Xu T, Vázquez-Salgado A, Puré E, Assoian RK. Stiffness-dependent motility and proliferation uncoupled by deletion of CD44. Sci Rep. 2017;7:1–10. doi: 10.1038/s41598-017-16486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitt M, Metzger M, Gradl D, Davidson G, Orian-Rousseau V. CD44 functions in Wnt signaling by regulating LRP6 localization and activation. Cell Death Differ. 2015;22:677–689. doi: 10.1038/cdd.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hale CM, Shrestha AL, Khatau SB, Stewart-Hutchinson PJ, Hernandez L, Stewart CL, Hodzic D, Wirtz D. Dysfunctional connections between the nucleus and the actin and microtubule networks in laminopathic models. Biophys J. 2008;95:5462–5475. doi: 10.1529/biophysj.108.139428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Xia H, Ge X, Chen Q, Yuan D, Chen Q, Leng W, Chen L, Tang Q, Bi F. CD44 acts through RhoA to regulate YAP signaling. Cell Signal. 2014;26:2504–2513. doi: 10.1016/j.cellsig.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 50.Li S, Li C, Zhang Y, He X, Chen X, Zeng X, Liu F, Chen Y, Chen J. Targeting mechanics-induced fibroblast activation through CD44-RhoA-YAP pathway ameliorates crystalline silica-induced silicosis. Theranostics. 2019;9:4993. doi: 10.7150/thno.35665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, Olsen BR. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest. 2012;122:3101–3113. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy JF, Lennon F, Steele C, Kelleher D, Fitzgerald D, Long A. Engagement of CD44 modulates cyclooxygenase induction, VEGF generation, and cell proliferation in human vascular endothelial cells. FASEB J. 2005;19:1–17. doi: 10.1096/fj.03-1376fje. [DOI] [PubMed] [Google Scholar]
- 53.Zuo L, Zhao H, Yang R, Wang L, Ma H, Xu X, Zhou P, Kong L. Lamin A/C might be involved in the EMT signalling pathway. Gene. 2018;663:51–64. doi: 10.1016/j.gene.2018.04.040. [DOI] [PubMed] [Google Scholar]
- 54.Yu W, Yang L, Li T, Zhang Y. Cadherin signaling in cancer: its functions and role as a therapeutic target. Front Oncol. 2019;9:989. doi: 10.3389/fonc.2019.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schulenburg A, Cech P, Herbacek I, Marian B, Wrba F, Valent P, Ulrich-Pur H. CD44-positive colorectal adenoma cells express the potential stem cell markers musashi antigen (msi1) and ephrin B2 receptor (EphB2) J Pathol. 2007;213:152–160. doi: 10.1002/path.2220. [DOI] [PubMed] [Google Scholar]
- 56.Chang G, Zhang H, Wang J, Zhang Y, Xu H, Wang C, Zhang H, Ma L, Li Q, Pang T. CD44 targets Wnt/β-catenin pathway to mediate the proliferation of K562 cells. Cancer Cell Int. 2013;13:1–13. doi: 10.1186/1475-2867-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Bras GF, Allison GL, Richards NF, Ansari SS, Washington MK, Andl CD. CD44 upregulation in E-cadherin-negative esophageal cancers results in cell invasion. PLoS ONE. 2011;6:e27063. doi: 10.1371/journal.pone.0027063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Y, Yu Q. E-cadherin negatively regulates CD44-hyaluronan interaction and CD44-mediated tumor invasion and branching morphogenesis. J Biol Chem. 2003;278:8661–8668. doi: 10.1074/jbc.M208181200. [DOI] [PubMed] [Google Scholar]
- 59.Henry TK, Gossett DR, Moon YS, Masaeli M, Sohsman M, Ying Y, Mislick K, Adams RP, Rao J, Di Carlo D. Quantitative diagnosis of malignant pleural effusions by single-cell mechanophenotyping. Sci Transl Med. 2013;5:212ra163. doi: 10.1126/scitranslmed.3006559. [DOI] [PubMed] [Google Scholar]
- 60.Kim Y, Kumar S. CD44-mediated adhesion to hyaluronic acid contributes to mechanosensing and invasive motility. Mol Cancer Res. 2014;12:1416–1429. doi: 10.1158/1541-7786.MCR-13-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gomez-Casal R, Bhattacharya C, Ganesh N, Bailey L, Basse P, Gibson M, Epperly M, Levina V. Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial-mesenchymal transition phenotypes. Mol Cancer. 2013;12:1–13. doi: 10.1186/1476-4598-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Awadalla A, Hussein AM, Ali M, Barakat N, Hamam ET, Magar RW, Shokeir AA. Possible mechanisms for the renoprotective action of adipose-derived mesenchymal stem cells with CD44-targeted hyaluronic acid against renal ischemia. Life Sci. 2021;272:119221. doi: 10.1016/j.lfs.2021.119221. [DOI] [PubMed] [Google Scholar]
- 63.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208:1459–1471. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaffar J, Yang SH, Kim SY, Kim HW, Faiz A, Chrzanowski W, Burgess JK. Greater cellular stiffness in fibroblasts from patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2018;315:L59–L65. doi: 10.1152/ajplung.00030.2018. [DOI] [PubMed] [Google Scholar]
- 65.Trickey WR, Lee GM, Guilak F. Viscoelastic properties of chondrocytes from normal and osteoarthritic human cartilage. J Orthop Res. 2000;18:891–898. doi: 10.1002/jor.1100180607. [DOI] [PubMed] [Google Scholar]
- 66.Zhang FJ, Luo W, Gao SG, Su DZ, Li YS, Zeng C, Lei GH. Expression of CD44 in articular cartilage is associated with disease severity in knee osteoarthritis. Mod Rheumatol. 2013;23:1186–1191. doi: 10.3109/s10165-012-0818-3. [DOI] [PubMed] [Google Scholar]
- 67.Skvortsov S, Schäfer G, Stasyk T, Fuchsberger C, Bonn GK, Bartsch G, Klocker H, Huber LA. Proteomics profiling of microdissected low-and high-grade prostate tumors identifies Lamin A as a discriminatory biomarker. J Proteome Res. 2011;10:259–268. doi: 10.1021/pr100921j. [DOI] [PubMed] [Google Scholar]
- 68.Worman HJ, Bonne G. “Laminopathies”: a wide spectrum of human diseases. Exp Cell Res. 2007;313:2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5:1294–1311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zvonic S, Lefevre M, Kilroy G, Floyd ZE, DeLany JP, Kheterpal I, Gravois A, Dow R, White A, Wu X, Gimble JM. Secretome of primary cultures of human adipose-derived stem cells: modulation of serpins by adipogenesis. Mol Cell Proteom. 2007;6:18–28. doi: 10.1074/mcp.M600217-MCP200. [DOI] [PubMed] [Google Scholar]
- 71.Frazier TP, Gimble JM, Kheterpal I, Rowan BG. Impact of low oxygen on the secretome of human adipose-derived stromal/stem cell primary cultures. Biochimie. 2013;95:2286–2296. doi: 10.1016/j.biochi.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 72.Darling EM, Zauscher S, Block JA, Guilak F. A thin-layer model for viscoelastic, stress-relaxation testing of cells using atomic force microscopy: do cell properties reflect metastatic potential? Biophys J. 2007;92:1784–1791. doi: 10.1529/biophysj.106.083097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Darling EM, Zauscher S, Guilak F. Viscoelastic properties of zonal articular chondrocytes measured by atomic force microscopy. Osteoarthr Cartil. 2006;14:571–579. doi: 10.1016/j.joca.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 74.Darling EM, Topel M, Zauscher S, Vail TP, Guilak F. Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, and adipocytes. J Biomech. 2008;41:454–464. doi: 10.1016/j.jbiomech.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dimitriadis EK, Horkay F, Maresca J, Kachar B, Chadwick RS. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys J. 2002;82:2798–2810. doi: 10.1016/S0006-3495(02)75620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 77.T. U. Consortium UniProt: the universal protein knowledgebase in. Nucleic Acids Res. 2021;49(2021):D480–D489. doi: 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43:W566–W570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dempsey ME, Woodford-Berry O, Darling EM. Quantification of antibody persistence for cell surface protein labeling. Cell Mol Bioeng. 2021;14:1–11. doi: 10.1007/s12195-021-00670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng B, Cao B, Li G, Huard J. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Eng. 2006;12:1891–1901. doi: 10.1089/ten.2006.12.1891. [DOI] [PubMed] [Google Scholar]
- 81.Guilak F, Lott KE, Awad HA, Cao Q, Hicok KC, Fermor B, Gimble JM. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Phys. 2006;206:229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Proteomics available on MassIVE, UCSD: MSV000087538
Not applicable