Abstract
We describe the first case of Wunderlich syndrome as a hemorrhagic complication in a patient with severe acute respiratory syndrome coronavirus 2 infection and acute respiratory distress syndrome. The possible underlying pathophysiological mechanisms have been extensively discussed. Emergency management included selective angioembolization of the upper polar arterial branches of the left kidney and discontinuation of thromboprophylaxis. The patient was discharged after 18 days. No other localizations or local recurrence of bleeding occurred during the hospitalization. Our report suggests a broad spectrum of clinical manifestations in patients with coronavirus disease 2019. As observed in our clinical case, in addition to thrombotic complications, bleeding is a significant cause of morbidity in coronavirus disease 2019 patients. Further studies should determine whether these urological bleeding sequelae are a direct manifestation of the infection or an indirect effect of thromboprophylaxis.
Keywords: Bleeding, Coagulopathy, Coronavirus disease 2019, Interventional radiology, Wunderlich syndrome
1. Introduction
Coronavirus disease 2019 (COVID-19) is a new disease that is responsible for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and is characterized by a wide spectrum of clinical manifestations, ranging from mild upper respiratory tract symptoms to life-threatening conditions, such as acute respiratory distress syndrome and multi-organ failure.[1] Moreover, SARS-CoV-2 infection has been associated with arterial and venous thrombotic complications including venous thromboembolism, pulmonary embolism, stroke, myocardial infarction, microvascular thrombosis, and acute arterial throm-bosis.[2,3] Several trials are ongoing to establish the ideal antithrombotic strategy. While awaiting more precise data, guidelines from the American College of Chest Physicians recommend prophylaxis with low-molecular-weight heparin or fondaparinux instead of unfractionated heparin or direct oral anticoagulants for all hospitalized patients with COVID-19 in the absence of contraindications, such as active bleeding.[2,3]
Wunderlich syndrome (WS) is a rare life-threatening emergency medical condition that refers to spontaneous, nontraumatic renal bleeding limited to the subcapsular and/or perirenal spaces.[4] WS may present with the classic Lenk's triad of acute flank pain, flank mass, and hypovolemic shock.[4] To the best of our knowledge, this is the first report of WS in a COVID-19 patient.
2. Case presentation
An 80-year-old man with a clinical history of hypertension and obesity presented with muscle pain, fever, and a dry cough. Three days later, he experienced acute shortness of breath and dyspnea, requiring admission to the emergency ward. Physical examination showed a respiratory rate of 32 breaths per minute, blood oxygen saturation of 92% in ambient air, rate of partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2:FiO2) of 280 mm Hg, and body temperature of 39.2°C. A complete blood workup showed mild thrombocytopenia (125 × 109/L), lymphopenia (0.9 × 109/L), and elevated levels of lactate dehydrogenase (310 U/L), C-reactive protein (14 mg/L), serum ferritin (695 ng/mL), and D-dimer (2850 ng/mL). However, other coagulation parameters, liver enzyme levels, creatinine levels, and blood urea nitrogen levels were normal.
Chest computed tomography (CT) and CT angiography showed diffuse and bilateral involvement of the pulmonary parenchyma with peripheral ground-glass opacities, thickening of the interlobular septa, and parenchymal condensation. No signs of proximal pulmonary embolism were identified (Fig. 1).
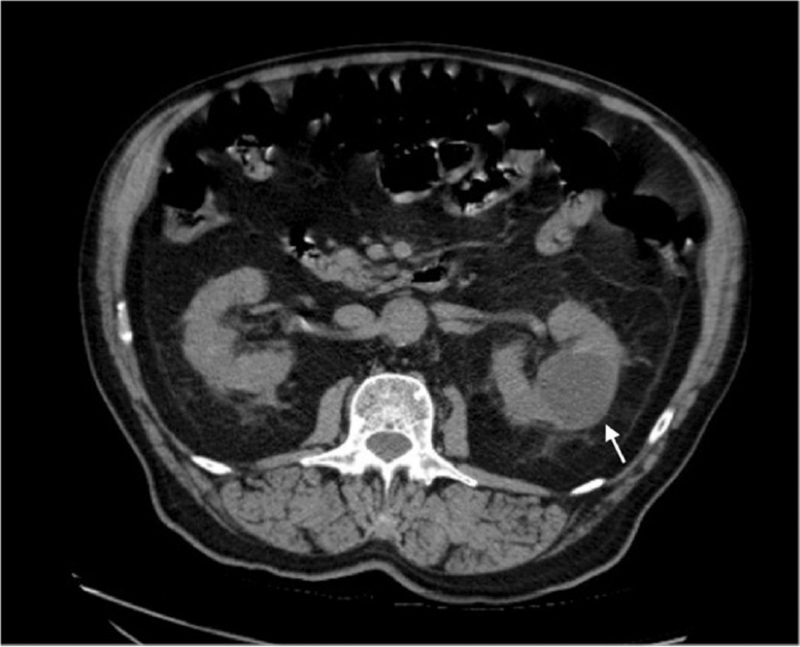
Figure 1.
Kidneys with normal appearance on CT scan performed upon arrival at the emergency ward. Simple renal cyst at the left mesorenal level (white arrow). CT = computed tomography.
Supportive care with supplemental oxygen, enoxaparin 40 mg twice a day, and dexamethasone 6 mg once daily was started, and rapid clinical improvement was observed. However, 2 days later, the patient experienced tachycardia and intense left flank pain. No history of abdominal trauma was reported. Physical examination revealed moderate tenderness across the entire abdomen, which was more evident on the left side. The patient had hypotension, 85 bpm, and a body temperature of 37.1 °C. Hemoglobin of 8.7 g/dL and moderate thrombocytopenia were reported in the complete blood count.
Abdominal CT revealed a large retroperitoneal hematoma with active bleeding from a branch of the renal artery (Fig. 2). Considering hemodynamic stability and the apparent absence of underlying renal pathologies, minimally invasive treatment was first proposed. Selective angioembolization of the upper polar arterial branches of the left kidney was performed effectively (Fig. 3), and thromboprophylaxis was discontinued. The patient was successfully discharged after 18 days. No other localizations or local recurrence of bleeding occurred during the hospitalization.
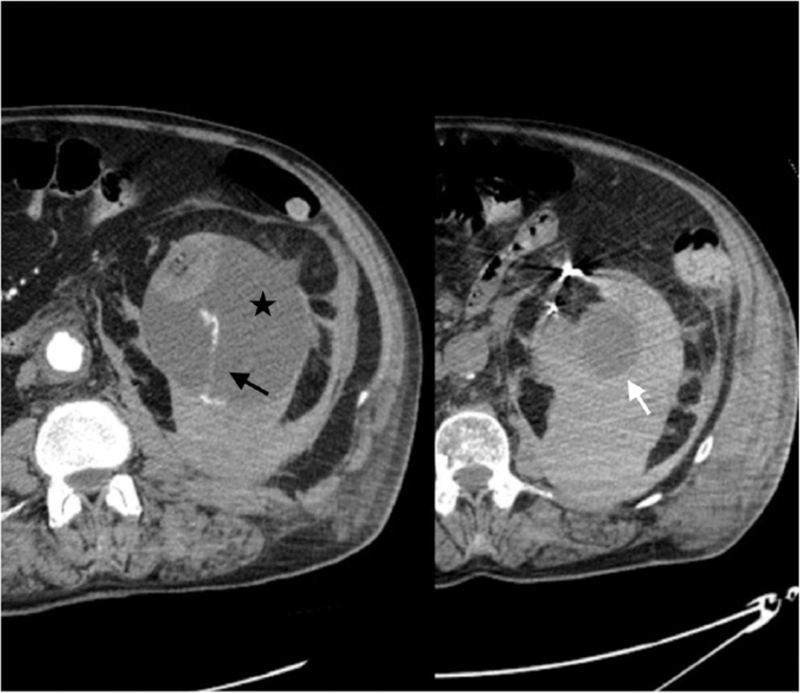
Figure 2.
Extensive left subcapsular renal hematoma (black star) caused by spontaneous rupture of the renal parenchyma with evidence of active arterial bleeding (black arrow). The simple renal cyst on the left mesorenal level, diagnosed at the initial CT scan, appears intact (white arrow). In this patient, WS would not appear to be associated with an underlying kidney disorder (ie, spontaneous rupture of renal cyst). Therefore, systemic coagulopathy is probably the underlying pathophysiological mechanism of WS in this case. CT = computed tomography; WS = Wunderlich syndrome.
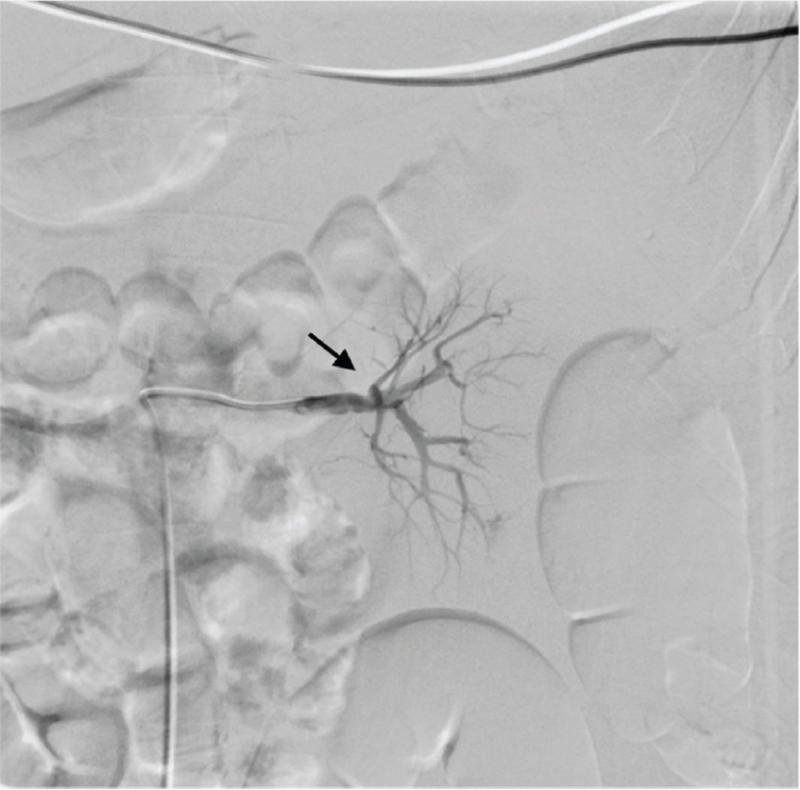
Figure 3.
Postembolization renal artery angiogram showing obliteration of the bleeding with microcoils (black arrow).
3. Discussion and conclusions
To the best of our knowledge, this is the first case of WS observed in a COVID-19 patient. WS is a potentially life-threatening condition, first described by Carl Wunderlich in 1856, consisting of spontaneous, nontraumatic renal bleeding limited to the subcapsular and/or perirenal spaces.[4] Clinically, WS is characterized by Lenk's triad, which includes acute flank pain, a palpable mass, and hypovolemic shock. The presence of all 3 symptoms is uncommon, occurring in only 20% of cases, although WS can also present with gross or microscopic hematuria.[4] Symptomatically 83% of the patients had acute flank or abdominal pain, 19% presented with hematuria, and 11% had hypovolemic shock.[5] The differential diagnosis includes rupture of an abdominal aortic aneurysm, visceral aneurysms, or other visceral bleeding such as in WS. Some cases may be misdiagnosed as renal colic; however, hemodynamic instability with hypotension requires urgent imaging.[4,5] Although initial imaging is usually abdominal ultrasound, abdominal CT scan is the exam of choice and provides information on the etiology, as well as the severity of the associated disease, revealing the presence of perirenal hematoma with a sensitivity of 92%–100%.[4]
The underlying pathological condition is often undetectable in the acute phase due to the presence of perinephric blood. In addition, when a mass that causes bleeding cannot be confirmed, arteriography can be used to diagnose or treat certain patients.[4,5] Renal neoplasms are the most common cause of WS, representing approximately 60%–65% of all cases. Their etiology can be further distinguished as benign or malignant. Angiomyolipoma is the most common benign cause of the condition, whereas renal cell carcinoma is the most common malignant etiology, causing up to 30%-35% of cases of WS.
Other neoplasms that cause spontaneous perinephric hemorrhage include metastases, sarcomas, adenomas, oncocytomas, and transitional cell carcinomas.[4,5]
Renal vascular diseases are the second most frequent cause of WS (20%–30% of all cases), with polyarteritis nodosa being the most common. They are further categorized into arterial and venous etiologies. Arterial causes include polyarteritis nodosa and renal artery aneurysms, while venous causes include renal vein thrombosis, renal arteriovenous malformations, fistulas, and portal hypertension.[4,5]
Complications of renal infections are a rare cause of WS. These include renal abscesses, acute pyelonephritis, emphysematous pyelonephritis, and xanthogranulomatous pyelonephritis, which account for 5%-10% of spontaneous perinephric hemor-rhages.[4,5] Other rare causes of WS include hematological disorders such as hemophilia and blood dyscrasias, anticoagulation therapy, and postpuerperal status.[4,5]
Definitive treatment for WS depends on the clinical condition and underlying causes; possible therapeutic options include conservative treatment, angioembolization, nephron-sparing surgery, or radical nephrectomy.[4,5]
In our case, WS did not appear to be associated with an underlying kidney alteration. The left kidney, which was partially visualized during the initial chest CT scan performed at admission to the hospital, appeared normal (Fig. 1). Therefore, systemic coagulopathy was probably the underlying pathophysiological mechanism of WS in this patient. It remains open to discussion whether renal bleeding is an indirect consequence of antithrombotic therapy or a primary coagulation disorder associated with COVID-19. Coagulation disorders are frequent in COVID-19 patients. In a case series, Al Samkari et al. reported an overall thrombotic complication rate of 9.5%. On the contrary, the overall bleeding rate was 4.8% (7.6% in critically ill patients), with a major bleeding rate of 2.3%.[6] Elevated D-dimer levels at admission were predictive of coagulation-associated complications during hospitalization (D-dimer >2500 ng/mL; adjusted odds ratio for thrombosis, 6.79 [95% CI = 2.39–19.30]; adjusted odds ratio for bleeding, 3.56 [95% CI = 1.01–12.66]).[6] Therefore, it is crucial to identify this characteristic in patients with COVID-19 admitted to the emergency department.
Finally, our report once again suggests the broad spectrum of clinical manifestations in patients with COVID-19. Consequently, optimal clinical management requires a high level of coordination in the emergency wards and close multidisciplinary collaboration. As observed in our clinical case, in addition to thrombotic complications, bleeding is a significant cause of morbidity in COVID-19 patients. Further studies should determine whether these bleeding sequelae are a direct manifestation of infection or an indirect effect of thromboprophylaxis.
Acknowledgments
None.
Statement of ethics
This study was approved by the Institutional Review Board (IRB) of Ospedale San Filippo Neri, and the protocols used in the study were approved by the Committee of Human Subjects Protection of Ospedale San Filippo Neri, Rome, Italy. Written informed consent was obtained from the patient for publication of details of the medical case and any accompanying images. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest statement
The authors declare no conflicts of interest.
Funding source
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
FP: Design and drafting of the article and final approval of the version to be submitted;
FM and CF: Data acquisition, drafting of the article, and final approval of the version to be submitted;
MM, CF, and FM: Data acquisition, critical revision of the article, and final approval of the version to be submitted;
AF: Critical revision of the article and final approval of the version to be submitted;
All authors have read and approved the final manuscript.
References
- [1].Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 2020;3248:782–793. [DOI] [PubMed] [Google Scholar]
- [2].Piazza G, Morrow DA. Diagnosis, management, and pathophysiology of arterial and venous thrombosis in COVID-19. JAMA 2020;32424:2548–2549. [DOI] [PubMed] [Google Scholar]
- [3].Albani F, Sepe L, Fusina F, et al. Thromboprophylaxis with enoxaparin is associated with a lower death rate in patients hospitalized with SARS-CoV-2 infection. A cohort study. E Clinical Medicine 2020;27:100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim JW, Kim JY, Ahn ST, et al. Spontaneous perirenal hemorrhage (Wunderlich syndrome): An analysis of 28 cases. Am J Emerg Med 2019;371:45–47. [DOI] [PubMed] [Google Scholar]
- [5].Dhanapal V, Ramachandran R, Radhan P, Vivekanandan B, Jeevanand-ham B, Jacob P. The many facets of Wunderlich syndrome: A multidetector computed tomography based review. Int J Contemp Med 2019;41:A88–A93. [Google Scholar]
- [6].Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020;1364:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]