Abstract
Research suggests that inflammation is an important mediator in the pathophysiology of anxiety disorders. In addition, women are more likely to develop an anxiety and depression disorder, in comorbidity with a wide spectrum of diseases related to the immune system. In recent years, hydrogen-rich water has emerged as a promising therapeutic strategy to prevent and intervene in stress-related disorders, due to its antioxidant and anti-inflammatory properties. The present study aims to analyze the effects of psychological treatment and a hydrogen-rich drink on the severity of anxiety and depression, pro-inflammatory cytokine levels, the cortisol awakening response, and general health state in a sample of women with panic disorder. This is a completely randomized, placebo-controlled study. The treatment group simultaneously received psychological treatment and 1.5 L of hydrogenated water for three months, compared to the control group that received psychological treatment and placebo. The results show that the treatment group was not significantly better than the control group. But there was a further reduction in measured pro-inflammatory cytokine scores, improving body pain and physical health. When between-group treatment effects were removed, psychological treatment significantly decreased measured variables, including cytokines and cortisol. The results support the presence of a maladaptive inflammatory process in women with panic disorder.
Keywords: pro-inflammatory cytokines, gastrointestinal symptoms, stress, anxiety, intestinal microbiota
INTRODUCTION
Anxiety disorders (AD) are characterized by experiencing excessive and irrational fear of potentially aversive stimuli.1 In addition, it is common for women to be almost twice as likely as men to suffer from these prevalent disorders. Increasing the risk of later developing another AD and major depression, in comorbidity with other medical pathologies.2,3 In recent years, the interrelationships emerged between the gut microbiota (GM), the gut, the immune system, the endocrine system, and the brain known as the Microbiome-Gut-Brain Axis (MGBA). As well as, its relationship with AD, depression, and gastrointestinal (GI) health; highlighting the fundamental role of inflammatory processes as a common mechanism of AD and chronic inflammatory diseases (CID).4–9
It is well known that chronic psychosocial stress, whether through psychological, emotional or physical threats, triggers harmful physiological and behavioral responses to mental health and GI, which are generally accompanied by dysregulated MGBA and a compromised immune system.5,9–11 The overactivation of the hypothalamic-pituitary-adrenal (HPA) axis and the consequent disproportionate release of glucocorticoids such as cortisol, could produce changes in GM generating a state of dysbiosis, which would increase the risk of bacterial translocation into the bloodstream. The immune system detects pathogenic bacteria and sends information to immune cells, triggering an inflammatory process that affects the intestine and the central nervous system (CNS). Consequently, there is an inflammatory response mediated by the production of pro-inflammatory cytokines and harmful reactive oxygen species (ROS), which change the neuronal activity of brain regions involved in AD. This maladaptive process results in the homeostatic imbalance of the MGBA, producing profound changes in anxiety-like behavior and a more depressed mood.5,6,9,12–18 Although research is limited, studies have found elevated concentrations of pro-inflammatory cytokines in AD.4,14
In recent years, hydrogen-rich water (HRW) has emerged as a promising therapeutic strategy to prevent and intervene in stress-related disorders, due to its antioxidant and anti-inflammatory properties; positively influencing the body without generating unwanted side effects.19–22 Molecular hydrogen (H2) is a natural element present in nature and in living beings, with the ability to mitigate the inflammatory response and excessive oxidative stress, generating neuroprotective effects in the brain.19,23 Although the exact mechanisms are unknown, HRW can spread rapidly through cells and tissues, protecting neuronal cells. It is capable of selectively deactivating harmful ROS, suppressing bacterial endotoxin, and restoring pro-inflammatory cytokines such as tumor necrosis factor Alpha (TNF-α), interleukin (IL) 6, and IL-1β.19,20,23,24 To our knowledge, there are no studies that have tested HRW in patients with AD. But if we start from the inflammatory hypothesis, preclinical research has discovered that H2 has anxiolytic and antidepressant effects, since it improves resistance to stress by reducing the activity of the HPA axis and the inflammatory response, through the inhibition of corticosterone, IL- 6, IL-1β, IL-12 and TNF-α, as well as anxiety and depression behaviors, improving social interaction.19,22,25,26 In a clinical study with healthy volunteers, they found that drinking 600ml of HRW improves mood and anxiety by reducing the activity of the sympathetic nervous system.23 In a recent study they found that drinking 1.5 liters of H2 water for four weeks reduces the inflammatory response and cell death, promoting antioxidant capacity in adults over 30 years of age.27 Likewise, H2 is beneficial to normalize the activity of the GI system, attenuating GI symptoms (such as diarrhea, constipation, abdominal distension, among others).28 This fact may be due to the fact that bacterial species of GM genetically have the ability to metabolize H2, affecting the state of GM.29,30 On the other hand, cognitive-behavioral treatment (CBT) is the treatment of choice due to its efficacy and safety in addressing AD and reducing stress.31 Through the restructuring of cognitive distortions associated with interpretations of events that initiate physiological responses related to stress, CBT could reduce the body’s inflammatory response and the reactivity of the HPA axis.4 Although studies in this field are scarce, CBT is known to help mitigate pro-inflammatory cytokines IL-6, IL-8, and TNF-α and diurnal cortisol levels; being able to become a beneficial strategy to attenuate the inflammatory levels of the organism and reestablish the communication of the MGBA.32–35
Starting from this context and given that people who suffer from panic attacks live with an intense state of anticipatory anxiety due to the extreme fear of suffering, again, another panic attack.36 It constitutes a significant psychological stressor to study the inflammatory hypothesis and its effects on MGBA in women suffering from panic disorder (PD). One might think that a comprehensive intervention directed at reestablishing the MGBA, can decrease the inflammatory response and normalization of the HPA axis; recovering more effectively the communication between both distal organs and, ultimately, the symptoms associated with anxiety and panic. The present study aims to: a) to study the therapeutic effects of CBT and an ARW drink, on the levels of pro-inflammatory cytokines and the cortisol awakening response (CAR), and other psychological variables; b) and, to verify the inflammatory hypothesis by means of indicators of the function of the HPA axis (CAR) and inflammation (pro-inflammatory cytokines). As far as we know, this is the first study to evaluate a comprehensive treatment aimed at MGBA in women with PD.
MATERIALS AND METHODS
Participants
The final sample consisted of 25 women (M: 42.92, SD: 9.84) with an age range between 19 and 64 years. The patients were selected from two centers of the Mental Health network of the Region of Murcia: 52% from the Mental Health Center (MHC) of Caravaca de la Cruz (area IV Murcia Northwest) (n = 13); and 48% of the Mula CSM (Area I Murcia Oeste) (n = 12). The diagnosis of PD [F41.0] was obtained based on the ICD-10 diagnostic criterio.37 Most of the sample presented comorbidity with another AD (72%; n = 18). 28% (n = 7) had the diagnosis of PD; 16% (n = 4) PD and a mixed anxiety-depressive disorder (MADD); 12% (n = 3) PD, agoraphobia and MADD; 8% (n = 2) PD and generalized anxiety disorder (GAD); 8% (n = 2) PD and specific phobia (SP); 4% (n = 1) PD, SP and MADD; 4% (n = 1) PD and agoraphobia; 4% (n = 1) PD, MADD and adjustment disorder; 4% (n = 1) PD and adjustment disorder; 4% (n = 1) PD, agoraphobia and social phobia; 4% (n = 1) PD, MADD and SP; and 4% (n = 1) PD, agoraphobia, MADD and adjustment disorder.
Instruments
A psychological interview was constructed and all participants were administered in the first and last session, the following battery of instruments: Perceived Stress Scale (PSS)38–40; State-Trait Anxiety Inventory (STAI)41,42; Beck Depression Inventory-II (BDI-II)43–45; Gastrointestinal Symptom Rating Scale (GSRS)46–48; and the Short Form 36 Health Survey (SF-36v2®).49–56
To determine cortisol levels and pro-inflammatory cytokine values, three saliva samples were collected with Deltalab brand sterile swabs, before and after treatment. In order to avoid errors in the collection of the samples, a record sheet was provided that included variables such as the time of awakening, the hours of sleep, spontaneous awakening or with an alarm clock, follicular phase, the exact time of taking the samples, as well as the instructions to follow:
Cortisol analysis salivary. To determine the reactivity of the HPA axis, the CAR was used as a measure.57,58 Participants were asked to take a sample upon awakening, at 30 minutes, and at 45 minutes after awakening. They were instructed to refrain from brushing their teeth, drinking, eating, smoking, or vigorous physical exercise for the duration of the test. We insisted on the correct fulfillment of the collection of the samples in time and form to avoid bias in the results. After collection, it was advised that the samples be kept refrigerated until they were taken to the corresponding MHC that same day. A member of the research staff duly collected them and sent them to the laboratory. Once delivered, they were frozen at -20 ° C until all the study samples were obtained. On the day of testing, samples were thawed, vortexed, and centrifuged at 2000-3000 xg for 10 minutes. Next, cortisol levels were quantified in µg / dl by ELISA enzyme immunoassay (Thermo Fisher Scientific®, Waltham, MA USA) and with the Merck Millipore reagent kit (Ref. HNCSMAG-35K), following the manufacturers instructions. The values of the area under the curve (AUC) were calculated with the three measurements collected as a measure of the CAR.58–60
Analysis of cytokines in saliva. The values of the cytokines IL-1β, IL-6, IL-8, IL-12, Interferon Alpha (IFN-γ) and TNF-α were analyzed through the multiplex immunoassay by means of the catch sandwich technique and with technology Luminex® xMAP (Chicago, IL, EEUU). The analyzer was used Luminex® 100/200TM and the software xPONENT®, with microsphere reagent kit MagPlex® 6.5 microns in diameter according to the manufacturer Luminex®.61–63 Cytokine concentrations were expressed in µg/ml.
Intervention
Psychological treatment. The CBT treatment was based on the treatment manual for the control of panic and anxiety of Barlow and Craske.36 It is an evidence-based program that was implemented at the group level, structured in 12 weekly sessions of 90 minutes duration. In general, the first session was devoted to the presentation, evaluation, consolidation of the group and a brief introduction to the cognitive model of panic was made. The other sessions were aimed at incorporating new strategies for observation and learning of the disorder through psychoeducation (sessions 2 and 3); physical control techniques through diaphragmatic breathing and relaxation (sessions 4, 5 and 6); cognitive restructuring (session 7); progressive interoceptive exposure and elimination of avoidance and escape behaviors (sessions 8, 9, 10 and 11); and, finally, there was a review of the knowledge acquired and its maintenance to avoid future relapses (session 12).36,64,65 In each session, skills were learned that were put into practice and reviewed, and homework assignments were delivered with self-registration and information documents.
Hydrogen water (HW). The model HW generator was used Jarra Hydrogen® from the range Osmostar (Osmostar Soriano, Elche, ALC, Spain), it uses the electrolysis method to break down water (H2O) into hydrogen (H2). The HRW was administered orally with a concentration of H2 diluted in water of 1.2 ppm and an ORP of -550 mV. For its transformation, weak mineralized water was used, with a dry residue of less than 100 mg/liter. The company’s technical staff was in charge of installing and starting the generator, providing information on its operation and maintenance. Aluminum bottles were supplied for transport and maintenance of the properties up to 8 hours.22 The participants had to drink a total of one and a half liters per day, distributed throughout the day (fasting, at noon, before lunch, in the middle of the afternoon, before dinner and before going to bed) in 250 ml. Machines designed with characteristics and anagrams similar to the originals, which provided normal water, were used as placebo.
Process
The clinical sample consisted of participants drawn from a waiting list, who derived only clinical psychologists and psychiatrists from each corresponding MHC. Patients were referred to the group if they met the diagnostic criteria for PD. The start of each group was managed by the nurses of each MHC who also acted as co-therapists. The CBT for the control of anxiety and panic was taught by the same clinical psychologist, specialized in AD and with more than 30 years of experience. The data were obtained from a total of five groups, carried out between September 14, 2018 and July 17, 2019. Three groups belonged to the Mula MHC and received sessions on Wednesdays, and two groups from the Caravaca MHC, with sessions on Fridays. At the beginning of each group (session 1) the possibility of participating in the research was offered, and those who agreed were registered in a list to be interviewed individually before the next session. During the individual interview, sociodemographic and clinical information was collected and the questionnaires were completed. Those who met the inclusion criteria, the interviewer continued to offer information related to HW. A sterile swab kit, a record sheet and instructions to follow for the correct collection of biological samples were delivered, which they had to record and deliver in session 2. At the end of all the interviews, the main researcher used a simple random sampling to assign the subjects, obtaining a control group (psychological treatment and placebo) and an experimental group (psychological treatment and HW). Before session 3, the machines were delivered to the homes of the subjects for the start of the session. During the three months that the CBT lasted, two telephone follow-ups were carried out in order to confirm the appropriate use and procedure. In session 11, another saliva collection kit and another record sheet were given again. Finally, in session 12, saliva samples were collected, the battery of selected questionnaires was administered in groups, and the samples were taken to the laboratory properly refrigerated. Repeating the protocol of action in each new group that started. Both the psychologist and the technician who delivered the machines to the participants remained blinded after assigning the interventions.
The sample was selected according to the following inclusion criteria: (1) the patients were selected if they met the diagnostic criteria according to the ICD-10 of PD [F41.0]; (2) it is essential to be over 18 years old and under 65 years old; (3) attend a minimum of eight therapy sessions and regularly to ensure the effectiveness of CBT; (4) not have cognitive difficulties to follow the group sessions; (5) have no other mental disorders (bipolar disorder, personality disorder, schizophrenia, substance abuse, hypochondriac disorder, etc.); (6) drink a minimum of one and a half liters of water daily; (7) do not consume glucocorticoids or other medications that affect the functioning of the immune and endocrine systems; (8) not having night work shifts that influenced the circadian rhythm; (9) not having or having had serious physical illnesses such as cancers, heart disease, viral infections or operations of the digestive system; (10) and not have oral diseases, injuries or inflammations that could cause bleeding.
Ethical considerations
The study was approved on 27/04/2018 by the ethics committee of the Universidad Católica San Antonio - UCAM with the code CE041807. Authorization was obtained from the coordinator of mental health areas I Murcia West and IV Northwest, and the written informed consent of the participants prior to participation in the study. This clinical trial was registered with the ISRCTN on 10/09/2021 retroactively (registration number ISRCTN95058526). All methods were performed in accordance with the relevant guidelines and regulations.
Statistic analysis
It was an experimental study with a completely randomized, design with pre- and post-treatment measures. Cronbach’s Alpha internal consistency coefficient was used in the battery of standardized questionnaires. The Kolmogorov-Smirnov normality test was used to assess the normal distribution of the data. The quantitative variables between the two treatment groups were compared using the Mann-Whitney U test for independent samples. The analysis of qualitative variables was carried out using the Chi-square test. A one-way ANOVA was calculated to study the effects of the variable “chronic inflammatory disease” on the clinical and biological variables. As well as “drug use” in GI symptoms. To check the variations and the results of the clinical variables after treatment between the two groups, it was compared using the ANOVA of one factor (type of group) of repeated measures. Eta squared (η2) was used as the effect size. For the analysis of the biological variables, an ANCOVA was performed using the covariate “tobacco” to eliminate the influence on the results. For cases that did not meet the assumption of homogeneity with Levene’s test, the Welsh and Brown-Forsythe test was used. No treatment was performed for missing values as there were none. All data were treated with a confidence level of 95% (p < .05). The data analysis was developed with the JASP statistical software version 0.9.0.1.
RESULTS
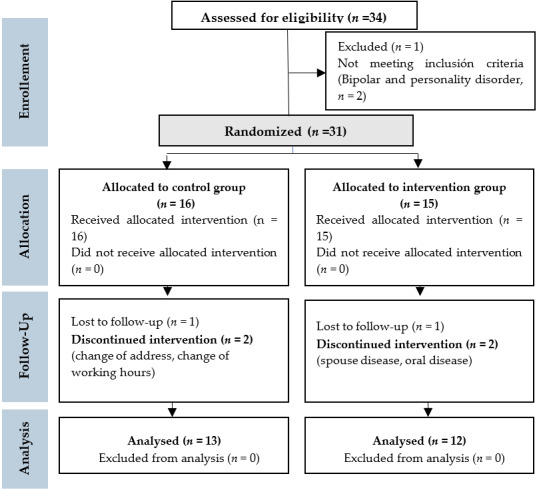
The final sample of the study consisted of 25 patients with PD: 13 subjects belonged to the control group (CG) and 12 to the treatment group (TG) (see Figure 1).
Figure 1. The CONSORT Flow Diagram.
In Table 1, you can see the sociodemographic characteristics of the sample of women with PD. Regarding the diseases reported, of the total sample, only 20% (n = 5) indicated that they did not have CID. 36% (n = 9) suffered from allergic diseases; 4% (n = 1) an autoimmune disease; 4% (n = 1) a metabolic disease; 20% (n = 5) an allergic disease in combination with an autoimmune disease; 8% (n = 2) an allergic and metabolic disease; 4% (n = 1) an allergic, autoimmune and metabolic disease; and 4% (n = 1) an autoimmune and metabolic disease. The rest of the reported diseases, 12% (n = 3) suffered from migraines, 12% (n = 3) herniated discs and 16% (n = 4) herniated discs and migraines.
Table 1. Clinical characteristics of the study sample.
| Variables | TG (n = 12) | CG (n = 13) | p-value |
| Age, mean years (SD) | 45 (12.31) | 41 (6.83) | 0.30a |
| Start anxiety crisis, n (%) | |||
| Adolescense | 4 (33.30) | 9 (69.20) | 0.07b |
| Adulthood | 8 (66.70) | 4 (30.80) | |
| Tobacco, n (%) | |||
| Yes | 4 (33.30) | 8 (61.50) | 0.16b |
| No | 8 (66.70) | 5 (38.50) | |
| Alcohol, n (%) | |||
| Never | 1 (8.30) | 3 (23.10) | 0.18b |
| Almost never | 11 (91.70) | 7 (72.00) | |
| Sometimes | 0 (0.00) | 1 (7.70) | |
| Almost always | 0 (0.00) | 2 (15.40) | |
| Physical exercise, n (%) | |||
| Never | 6 (50.00) | 4 (30.80) | 0.50b |
| Almost never | 0 (0.00) | 1 (7.70) | |
| Sometimes | 2 (16.70) | 2 (15.40) | |
| Almost asways | 3 (25.00) | 2 (15.40) | |
| Always | 1 (8.30) | 4 (30.80) | |
| Insomnia, n (%) | |||
| Yes | 5 (41.70) | 6 (46.20) | 0.82b |
| No | 7 (58.30) | 7 (53.80) | |
| CID, n (%) | |||
| Yes | 11 (91.70) | 9 (69.20) | 0.16b |
| No | 1 (8.30) | 4 (30.80) | |
| Psychotropic drugs, n (%) | |||
| Bz | 3 (25.00) | 2 (15.40) | 0.67b |
| AD | 1 (8.30) | 1 (7.70) | |
| Bz y AD | 6 (50.00) | 5 (38.50) | |
| No psychotropic drug | 2 (16.70) | 5 (38.50) | |
| Menstrual cycle, n (%) | |||
| Si | 8 (66.70) | 12 (92.30) | 0.11b |
| No | 4 (33.30) | 1 (7.70) | |
| PSS (mean ± SD) | 33.42 ± 6.51 | 34.69 ± 7.46 | 0.46a |
| STAI-State (mean ± SD) | 33.50 ± 8.45 | 33.46 ± 11.03 | 0.95a |
| STAI-Trait (mean ± SD) | 39.50 ± 6.58 | 39.46 ± 8.66 | 0.82a |
| BDI-II (mean ± SD) | 25.83 ± 11.04 | 27.62 ± 11.03 | 0.70a |
| GSRS (mean ± SD) | 43.33 ± 9.35 | 41.38 ± 13.17 | 0.60a |
| SF36-BP (mean ± SD) | 37.81 ± 7.65 | 39.51 ± 7.79 | 0.68a |
| SF36-PSC (mean ± SD) | 45.58 ± 8.20 | 50.65 ± 8.11 | 0.10a |
| SF36-MSC (mean ± SD) | 32.03 ± 5.32 | 30.05 ± 9.95 | 0.43a |
Note. aMann-Whitney U test for independent samples; bChi-cuadrado test. (CID) Chronic inflammatory disease; (PSS) Perceived Stress Scale; (STAI) State-Trait Anxiety Inventory; (BDI-II) Beck Depression Inventory; (GSRS) Gastrointestinal Symptom Rating Scale; (SF-36) Short Form 36 Health Survey (BP) Body pain, (PSC) Physical Summation Component y (MSC) Mental Summation Component. (Bz) Benzodiazepines; (AD) Antidepressants.
In order to control for GI symptoms as possible side effects caused by the consumption of psychotropic drugs, a one-way ANOVA was performed to check the relationships between means. The results do not show significant differences between the groups in the GSRS variable [(Bz M: 49.40, SD: 18.71), (AD M: 33.00, SD: 11.31), (Bz y AD M: 39.55, SD: 7.51), (No psichotropic drugs M: 44.29, SD: 8.55), F = 1.48; p < 0.25].
Table 2 shows the results of the repeated measures factorial ANOVA for the clinical variables, which studies the effects of treatment in women in each group (TG and CG). As can be seen, the effects of treatment with HRW and CBT (TG) are practically null. Only greater differences were observed in the scores in the TG of the variables SF36-BP and SF36-PSC.
Table 2. Descriptive data and mean differences of the clinical variables for independent groups before and after treatment.
| TG (n=12) | CG (n=13) | |||||
| Variables | pretest | postest | pretest | postest | F | p |
| PSS | 33.42 ± 6.51 | 26.25 ± 5.08 | 34.69 ± 7.46 | 24.69 ± 9.21 | 0.00 | 0.95 |
| STAI-State | 33.50 ± 8.45 | 25.08 ± 6.03 | 33.46 ± 11.03 | 25.15 ± 10.99 | 0.00 | 0.99 |
| STAI-Trait | 39.50 ± 6.58 | 31.83 ± 6.01 | 39.46 ± 8.66 | 27.69 ± 10.84 | 0.55 | 0.46 |
| BDI-II | 25.83 ± 11.04 | 13.50 ± 9.04 | 27.61 ± 11.03 | 14.77 ± 8.66 | 0.19 | 0.66 |
| GSRS | 43.33 ± 9.35 | 31.42 ± 11.20 | 41.38 ± 13.17 | 31.62 ± 11.02 | 0.04 | 0.84 |
| SF36-BP | 37.81 ± 7.65 | 46.88 ± 9.58 | 39.51 ± 7.79 | 43.20 ± 9.84 | 0.09 | 0.75 |
| SF36-PSC | 45.58 ± 8.20 | 49.40 ± 7.71 | 50.65 ± 8.11 | 50.93 ± 5.31 | 1.45 | 0.24 |
| SF36-MSC | 32.03 ± 5.31 | 38.49 ± 4.49 | 30.05 ± 9.95 | 40.31 ± 7.77 | 0.00 | 0.97 |
Note. The data shows the mean ± SD. (PSS) Perceived Stress Scale; (STAI) State-Trait Anxiety Inventory; (BDI-II) Beck Depression Inventory; (GSRS) Gastrointestinal Symptom Rating Scale; (SF-36) Short Form 36 Health Survey (BP) Body pain, (PSC) Physical Summation Component y (MSC) Mental Summation Component.
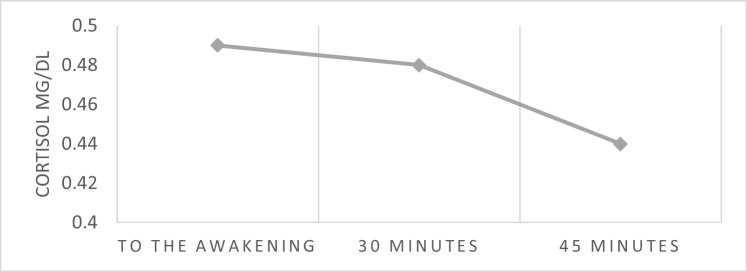
Figure 2 shows the mean values of CAR measured before treatment. The decline pattern is slightly constant, showing nearly flat cortisol levels between waking (M: 0,49, SD: 0,21) and the 30 minutes later (M: 0,48, SD: 0,20).
Figure 2. Mean values of the cortisol response on awakening.
Note. (CAR) Cortisol awakening response
Table 3 shows the results of the repeated measures factorial ANCOVA using the covariate “tobacco” to eliminate the effects on the results. Likewise, the significance of the TG does not turn out to be different from the GC. What is observed are greater changes in the means of the scores of each and every one of the proinflammatory cytokines in TG (IL-1β, IL-8, IL-12, IL-6, TNF-α, IFN). Possibly, statistical significance is not reached due to the small sample size. Regarding the AUCG variable, the effects of the interaction were significant, showing an effect size of small magnitude. The Levene test was higher than 0.05 (F = 1.33, p <0.25) fulfilling the assumption of homogeneity.
Table 3. Descriptive data and mean differences of the biological variables for independent groups before and after treatment.
| TG (n=12) | CG (n=13) | ||||||
| Variables | pretest | postest | pretest | postest | F | p | η2 |
| AUCG | 15.85 ± 7.88 | 9.83 ± 544 | 27.03 ± 7.44 | 12.22 ± 7.28 | 11.04 | 0.00 | 0.30 |
| IL-1β | 94.08 ± 11.32 | 65.50 ± 14.15 | 88.23 ± 10.74 | 67.69 ± 17.64 | 0.42 | 0.52 | 0.01 |
| IL-8 | 80.83 ± 16.19 | 59.83 ± 15.92 | 75.69 ± 12.52 | 62.00 ± 17.76 | 0.49 | 0.49 | 0.02 |
| IL-12 | 75.75 ± 6.87 | 54.50 ± 13.81 | 74.38 ± 13.19 | 63.62 ± 15.95 | 0.01 | 0.92 | 0.00 |
| IL-6 | 72.33 ± 13.26 | 51.67 ± 12.20 | 78.69 ± 17.05 | 61.15 ± 21.51 | 1.07 | 0.31 | 0.05 |
| FNT-α | 74.50 ± 11.07 | 49.25 ± 18.22 | 77.00 ± 12.10 | 60.69 ± 18.24 | 1.11 | 0.30 | 0.05 |
| IFN-γ | 71.00 ± 13.71 | 50.25 ± 8.50 | 66.00 ± 11.91 | 51.38 ± 14.78 | 0.43 | 0.51 | 0.02 |
Note. The data shows the mean ± SD. (AUCG) Area under the curve with respect to the groud; (IL) Interleukin; (TNF-α) Tumor Neutrotrophic Factor; (IFN-γ) Interferón gamma. The effect of the tobacco covariate on the results was controlled.
Now, if we eliminate the differences between groups, the intra-subject contrast test shows a significant decrease in all the variables measured, both for the clinical and biological variables. Except for the variable SF36-PSC, staying close to significance (F = 3,30, p <0.08). The rest of the variables, including those that measure GI symptoms and body pain, show a large effect size, reaching an η2 of 0.68 in GSRS (see Table 4).
Table 4. Intra-subject contrast test between clinical and biological variables.
| variables | F | p | η2 |
| PSS | 25.70 | 0.001 | 0.52 |
| STAI-E | 13.40 | 0.001 | 0.37 |
| STAI-R | 28.85 | 0.001 | 0.54 |
| BDI-II | 37.37 | 0.001 | 0.62 |
| GSRS-T | 49.80 | 0.001 | 0.68 |
| SF36-DC | 15.78 | 0.001 | 0.38 |
| SF36-PSC | 3.30 | 0.082 | 0.11 |
| SF36-MSC | 29.19 | 0.001 | 0.54 |
| AUCG | 55.96 | 0.001 | 0.65 |
| IL-1β | 58.64 | 0.001 | 0.66 |
| IL-8 | 53.31 | 0.001 | 0.68 |
| IL-12 | 45.84 | 0.001 | 0.62 |
| IL-6 | 45.32 | 0.001 | 0.66 |
| FNT-α | 33.95 | 0.001 | 0.58 |
| IFN-γ | 27.03 | 0.001 | 0.53 |
Note. (PSS) Perceived Stress Scale; (STAI) State-Trait Anxiety Inventory; (BDI-II) Beck Depression Inventory; (GSRS) Gastrointestinal Symptom Rating Scale; (SF-36) Short Form 36 Health Survey (BP) Body pain, (PSC) Physical Summation Component y (MSC) Mental Summation Component. (AUCG) Area under the curve with respect to the ground; (IL) Interleukin; (TNF-α) Tumor Neurotrophic Factor; (IFN-γ) Interferon gamma. The effect of the tobacco covariate on the results of the biological variables was controlled.
DISCUSSION
The aim of the present study was to analyze the clinical characteristics of women who come to mental health with a PD; and evaluate the effects of CBT and an HRW drink on the levels of pro-inflammatory cytokines (IL-1β, IL-8, IL-12, IL-6, TNF-α, IFN-γ), the cortisol response on awakening (AUCG) and the values of the subjective scales (PSS, STAI, BDI-II, GSRS, SF-36) from the perspective of the MGBA.
In the first place, and after analyzing the descriptive results of the clinical sample, it is striking that the women, in addition to having high symptoms of perceived stress, anxiety and depression, presented high scores of GI symptoms and a more deteriorated physical health, with significant levels of body pain. In fact, most of the patients had CID, whether it was allergic, autoimmune, hormonal and / or metabolic or in combination with each other. This would indicate that PD in women are frequently comorbid with CID, so typical of developed countries.8,14,66 There are many reports that show that dysregulation of the inflammatory response, as a consequence of an aberrant microbial structure, contributes to the development of a large number of CIDs.67,68 And now, inflammation is beginning to be associated with ATs, directly modulating affective behavior.14 At the same time, these medical conditions presented with migraines and back pain, which are usually common characteristics in the Spanish population with mood and anxiety disorders.69,70 And that, likewise, have been related to maladaptive inflammatory processes.71 About half of the patients showed harmful behavior patterns that could possibly be contributing to increased levels of inflammation and anxiety symptoms. It has been discovered that certain factors related to lifestyle have a negative impact on the communication of the MGBA with negative consequences on the GI system and on the immune response with manifestations in the CNS, such as the maintenance of anxiety and depression.18,72–75 Tobacco and alcohol consumption, sedentary lifestyle, poor sleep quality and the intake of psychoactive drugs could generate an inflammatory response in the intestine, affecting microbial quality and modifying GI function, influencing anxiety behavior.16,76–80 In addition, the participants exhibited an elevated CAR corresponding to a reactive HPA axis, with an increased cortisol concentration on awakening, relative to the second and third saliva samples. It seems that the patients have a clinical profile with characteristics related to a possible deregulated MGBA, where PDs and CIDs could be closely related to each other.4,5,8
In relation to the clinical variables, according to our results, the intervention with CBT and HRW did not show better results in the subjective variables, compared to the CG that only received CBT. What can be seen in the TG is a greater increase in the body pain score (SF36-BP) and in the global mean of physical health (SF36-PSC), indicating an improvement in symptoms, although without statistical significance. Regarding the biological variables, likewise, the intervention with CBT and with HRW did not report statistically significant results. However, in this group there was a greater reduction in the levels of pro-inflammatory cytokines, showing a continuous pattern in all the parameters measured. Although the results were not statistically significant, possibly as a consequence of the small sample size, it is interesting to note that both the variables related to physical health and the pro-inflammatory cytokines obtained better scores in the results. It would be possible that CBT and HRW can generate more beneficial effects on the health of participants with PD, helping to restore the immune response. In fact, H2 has the ability to mitigate the inflammatory response through the reduction of IL-6, IL-1β, IL-12 and TNF-α.19,26,30 This inhibition of pro-inflammatory cytokines could be linked to decreased levels of body pain and increased physical health in patients with PD. In recent years, evidence has shown that certain pro-inflammatory cytokines are involved in the initiation and maintenance of pathological pain, specifically IL-1β, IL-6 and TNF-α.71 ARH has anti-inflammatory and antioxidant properties that help improve people’s quality of life, without causing adverse effects on the body.22,25,26 In addition, GM has the genetic ability to assimilate and transform H2,29,30 facilitating intestinal fermentation and the increase of substances beneficial to health.81 However, the psychological variables were not better than the CG, probably because the treatment with HRW was not implemented long enough to influence the symptoms of anxiety and depression. Nevertheless, the understanding of the mechanisms of action of H2 is limited, although it can become an attractive strategy in disorders that share inflammatory mechanisms, due to its biological properties. But, it is necessary to define the appropriate mode, frequency and time of administration to ensure the therapeutic effects of treatment with HRW.29
Now, if we eliminate the different types of treatment, according to the within-subjects analysis, the results show statistically significant effects in all the variables measured, both clinical and biological. It is clear that CBT is effective in improving the psychological processes involved in reducing stress, anxiety and depression, being the treatment of choice for its efficacy and safety.1,31 But the most striking thing is that it is also capable of normalizing the reactivity of the HPA axis and the inflammatory response, through the reduction of pro-inflammatory cytokines; also contributing to improve symptoms related to body pain, GI symptoms and physical health; which could help reestablish MGBA communication. Research has shown the efficacy of CBT in reducing inflammatory processes and normalizing the HPA axis.4 It seems that the chronic inflammatory process is involved in the onset and maintenance of symptoms of anxiety and depression and, furthermore, it is common in CID.14,33,67,75 Based on our results, the inflammatory response could be modulated through cognitive restructuring, modifying irrational thoughts that activate physiological response mechanisms associated with stress. This would decrease pro-inflammatory cytokines and CAR, resulting in anti-inflammatory effects and less activation of the HPA axis; improving clinical psychological symptoms of anxiety and depression, and symptoms related to physical health.4,14,32,33,35
However, this study had some limitations such as low statistical power, which may be influencing the fact that some of the results were not statistically significant. Furthermore, the effects of HRW could not be explained separately and the intervention was administered for a short period of time, so the results cannot assess the effect of HRW in the long term. Finally, the sample may not be representative since only two MHCs were recruited from the Region of Murcia. Also, there are other inflammatory factors not considered in this research that are important for future research, and that may be maintaining these disorders that are so frequent and comorbid with diseases related to the immune system (type of diet, sex, exposures to trauma, consumption of antibiotics, etc.).
In conclusion, our results show evidence of an associated inflammatory response in women with PD in comorbidity with CID, possibly due to a compromised immune system as a consequence of prolonged exposure to stressful stimulus. In addition to anxiety and depression, the sample presented GI symptoms, body pain and a more deteriorated physical health, together with unhealthy behavior patterns that may be contributing to the alteration of the MGBA and the increase in inflammation. Likewise, after the intervention, and although the effect of HRW was not significant, there was a greater reduction in all the pro-inflammatory cytokines measured and, when the effect between groups was eliminated, CBT significantly decreased both the psychological and biological variables, helping to restore the communication of the MGBA. To date, we did not have results on the effects of HRW and CBT in a clinical sample of women with PD and with a completely randomized design. The results would support the presence of a maladaptive inflammatory process in women suffering from PD. These data would be contributing, on the one hand, to broadening the etiopathogenic perspective of PD and, on the other hand, to promoting healthy lifestyles and implementing new therapeutic approaches in order to reduce inflammatory processes and reestablish MGBA communication.
AUTHORS’ CONTRIBUTIONS
The authors’ contributions were as follows: FJMF, CAGA, JCFR, and ABFS designed the research; MTM, JFSG, and ABFS conducted the research; JFSG and ABFS collected and analyzed the data; ABFS prepared the manuscript; FJMF, CAGA, JCFR, and ABFS reviewed and edited the manuscript.
CONFLICT OF INTERESTS
The authors declare no competing interests.
Acknowledgments
ACKNOWLEDGMENTS
Thank the company Hydrogen for supporting this project from the beginning and betting on resources that promote health. To my directors of the Doctoral Thesis for their involvement and commitment. To the administrative and nursing staff of the Mental Health Center of Mula and Caravaca for the great help and service provided. And, especially, to Martín del Toro Mellado, Clinical Psychologist, for his human and professional work.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This work was supported by Health Sciences PhD program, Universidad Católica de Murcia UCAM. And the data that support the findings of this study are available from the corresponding author.
References
- 1. Barlow DH. Anxiety and Its Disorders: The Nature and Treatment of Anxiety and Panic. 2nd ed. The Guilford Press; 2002.
- 2. Audet MC. Stress-induced disturbances along the gut microbiota-immune-brain axis and implications for mental health: does sex matter? Front Neuroendocrinol. 2019;54:100772. doi:10.1016/j.yfrne.2019.100772 [DOI] [PubMed]
- 3. Jalnapurkar I, Allen M, Pigott AT. Sex Differences in Anxiety Disorders: A Review. Psychiatry, Depression Anxiety. 2018;4:1-9. doi:10.24966/PDA-0150/100011
- 4. Alessi MG, Bennett JM. Mental health is the health of the whole body: How psychoneuroimmunology health psychology can inform improve treatment. J Eval Clin Pract. Published online March 14, 2020:jep.13386. doi:10.1111/jep.13386 [DOI] [PubMed]
- 5. Amini-Khoei H, Haghani-Samani E, Beigi M, et al. On the role of corticosterone in behavioral disorders, microbiota composition alteration and neuroimmune response in adult male mice subjected to maternal separation stress. Int Immunopharmacol. 2019;66:242-250. doi:10.1016/j.intimp.2018.11.037 [DOI] [PubMed]
- 6. Dinan TG, Cryan JF. Brain-gut-microbiota axis and mental health. Psychosom Med. 2017;79(8):920-926. doi:10.1097/PSY.0000000000000519 [DOI] [PubMed]
- 7. Foster JA, Rinaman L, Cryan JF. Stress the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017;7:124-136. doi:10.1016/j.ynstr.2017.03.001 [DOI] [PMC free article] [PubMed]
- 8. Paiva IHR, Duarte-Silva E, Peixoto CA. The role of prebiotics in cognition, anxiety, and depression. Eur Neuropsychopharmacol. 2020;34:1-18. doi:10.1016/j.euroneuro.2020.03.006 [DOI] [PubMed]
- 9. Sarkar A, Harty S, Lehto SM, et al. The Microbiome in Psychology and Cognitive Neuroscience. Trends Cogn Sci. 2018;22(7):611-636. doi:10.1016/j.tics.2018.04.006 [DOI] [PubMed]
- 10. Kuti D, Winkler Z, Horváth K, et al. Gastrointestinal (non-systemic) antibiotic rifaximin differentially affects chronic stress-induced changes in colon microbiome and gut permeability without effect on behavior. Brain Behav Immun. 2020;84:218-228. doi:10.1016/j.bbi.2019.12.004 [DOI] [PubMed]
- 11. Mörkl S, Butler MI, Holl A, Cyran JF, Dinan TG. Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry. Curr Nutr Rep. Published online May 13, 2020. doi:10.1007/s13668-020-00313-5 [DOI] [PMC free article] [PubMed]
- 12. Burokas A, Arboleya S, Moloney RD, et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. 2017;82(7):472-487. doi:10.1016/j.biopsych.2016.12.031 [DOI] [PubMed]
- 13. Furtado M, Katzman MA. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res. 2015;229(1-2):37-48. doi:10.1016/j.psychres.2015.05.036 [DOI] [PubMed]
- 14. Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T. Inflammation in fear-and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology. 2017;42(1):254. [DOI] [PMC free article] [PubMed]
- 15. Quagliato LA, Nardi AE. Cytokine alterations in panic disorder: a systematic review. J Affect Disord. 2018;228:91-96. doi:10.1016/j.jad.2017.11.094 [DOI] [PubMed]
- 16. Rea K, Dinan TG, Cryan JF. Gut Microbiota: A Perspective for Psychiatrists. Neuropsychobiology. 2020;79(1):50-62. doi:10.1159/000504495 [DOI] [PubMed]
- 17. Salim S, Chugh G, Asghar M. Inflammation in anxiety. In: Advances in Protein Chemistry and Structural Biology. Vol 88. Elsevier; 2012:1-25. [DOI] [PubMed]
- 18. Tao H, Wang CR, Guo JC, Guo M. Research Progress on the Relationship between Intestinal Flora and Mental and Psychological Diseases. Advances in Microbiology. 2020;10(06):295-305. doi:10.4236/aim.2020.106021
- 19. Gao Q, Song H, Wang X ting, et al. Molecular hydrogen increases resilience to stress in mice. Sci Rep. 2017;7(1):9625. doi:10.1038/s41598-017-10362-6 [DOI] [PMC free article] [PubMed]
- 20. Iketani M, Ohsawa I. Molecular Hydrogen as a Neuroprotective Agent. Curr Neuropharmacol. 2017;15(2):324-331. doi:10.2174/1570159X14666160607205417 [DOI] [PMC free article] [PubMed]
- 21. Mizuno K, Sasaki A, Ebisu K, et al. Hydrogen-rich water for improvements of mood, anxiety, and autonomic nerve function in daily life. Med Gas Res. 2017;7(4):247. doi:10.4103/2045-9912.222448 [DOI] [PMC free article] [PubMed]
- 22. Yang Y, Zhu Y, Xi X. Anti‑inflammatory and antitumor action of hydrogen via reactive oxygen species (Review). Oncol Lett. Published online June 26, 2018. doi:10.3892/ol.2018.9023 [DOI] [PMC free article] [PubMed]
- 23. Mikami T, Tano K, Lee H, et al. Drinking hydrogen water enhances endurance and relieves psychometric fatigue: a randomized, double-blind, placebo-controlled study. Can J Physiol Pharmacol. 2019;97(9):857-862. doi:10.1139/cjpp-2019-0059 [DOI] [PubMed]
- 24. Spulber S, Edoff K, Hong L, Morisawa S, Shirahata S, Ceccatelli S. Molecular Hydrogen Reduces LPS-Induced Neuroinflammation and Promotes Recovery from Sickness Behaviour in Mice. PLoS One. 2012;7(7). doi:10.1371/journal.pone.0042078 [DOI] [PMC free article] [PubMed]
- 25. Guo Q, Yin X, Qiao M, et al. Hydrogen-Rich Water Ameliorates Autistic-Like Behavioral Abnormalities in Valproic Acid-Treated Adolescent Mice Offspring. Front Behav Neurosci. 2018;12. doi:10.3389/fnbeh.2018.00170 [DOI] [PMC free article] [PubMed]
- 26. Niu Y, Nie Q, Dong L, et al. Hydrogen Attenuates Allergic Inflammation by Reversing Energy Metabolic Pathway Switch. Sci Rep. 2020;10. doi:10.1038/s41598-020-58999-0 [DOI] [PMC free article] [PubMed]
- 27. Sim M, Kim CS, Shon WJ, Lee YK, Choi EY, Shin DM. Hydrogen-rich water reduces inflammatory responses and prevents apoptosis of peripheral blood cells in healthy adults: a randomized, double-blind, controlled trial. Sci Rep. 2020;10. doi:10.1038/s41598-020-68930-2 [DOI] [PMC free article] [PubMed]
- 28. Tanaka Y, Kanazawa M, Kano M, et al. Differential activation in amygdala and plasma noradrenaline during colorectal distention by administration of corticotropin-releasing hormone between healthy individuals and patients with irritable bowel syndrome. PLoS ONE. 2016;11(7). http://search.ebscohost.com/login.aspx?direct=true=psyh=2016-39996-001=es=ehost-live [DOI] [PMC free article] [PubMed]
- 29. Ghanizadeh A, Berk M. Molecular hydrogen: an overview of its neurobiological effects and therapeutic potential for bipolar disorder and schizophrenia. Med Gas Res. 2013;3(1):11. doi:10.1186/2045-9912-3-11 [DOI] [PMC free article] [PubMed]
- 30. Zhang Y, Xu J, Yang H. Hydrogen: An Endogenous Regulator of Liver Homeostasis. Front Pharmacol. 2020;11. doi:10.3389/fphar.2020.00877 [DOI] [PMC free article] [PubMed]
- 31. Cordero-Andrés P, González-Blanch C, Umaran-Alfageme O, et al. Tratamiento psicológico de los trastornos emocionales en atención primaria: fundamentos teóricos y empíricos del estudio PsicAP. Ansiedad Y Estrés. 2017;23(2-3):91-98.
- 32. Lelli L, Castellini G, Cassioli E, Monteleone AM, Ricca V. Cortisol levels before and after cognitive behavioural therapy in patients with eating disorders reporting childhood abuse: A follow-up study. Psychiatry Res. 2019;275:269-275. doi:10.1016/j.psychres.2019.03.046 [DOI] [PubMed]
- 33. Lopresti AL. Cognitive behaviour therapy and inflammation: A systematic review of its relationship and the potential implications for the treatment of depression. Aust N Z J Psychiatry. 2017;51(6):565-582. doi:10.1177/0004867417701996 [DOI] [PubMed]
- 34. Manigault AW, Shorey RC, Hamilton K, et al. Cognitive behavioral therapy, mindfulness, and cortisol habituation: a randomized controlled trial. Psychoneuroendocrinology. 2019;104:276-285. doi:10.1016/j.psyneuen.2019.03.009 [DOI] [PubMed]
- 35. Zabihiyeganeh M, Vafaee Afshar S, Amini Kadijani A, et al. The effect of cognitive behavioral therapy on the circulating proinflammatory cytokines of fibromyalgia patients: a pilot controlled clinical trial. Gen Hosp Psychiatry. 2019;57:23-28. doi:10.1016/j.genhosppsych.2019.01.003 [DOI] [PubMed]
- 36. Barlow DH, Craske MG. Mastery of Your Anxiety and Panic. Graywind Publications; 1989.
- 37. World Health Organization. Guia de bolsillo de la clasificación CIE-10: clasificación de los trastornos mentales y del comportamiento. Editorial Médica Panamericana; 2000. https://apps.who.int/iris/handle/10665/42326
- 38. Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav. 1983;24(4):385. doi:10.2307/2136404 [PubMed]
- 39. Remor E. Psychometric Properties of a European Spanish Version of the Perceived Stress Scale (PSS). Span J Psychol. 2006;9(1):86-93. doi:10.1017/S1138741600006004 [DOI] [PubMed]
- 40. Remor E, Carrobles JA. Versión Española de la Escala de Estrés Percibido (PSS-14): Estudio psicométrico en una muestra VIH+. [Spanish version of the Perceived Stress Scale (PSS-14): Psychometric study in a HIV+ sample.]. Ansiedad y Estrés. 2001;7(2-3):195-201.
- 41. Buela-Casal G, Guillen-Riquelme A, Seisdedos N. Cuestionario de Ansiedad Estado-Rasgo. 9th ed. TEA Ediciones; 2015.
- 42. Spielberger CD, Gorsuch RL, Lushene RE. STAI. Manual for the State-Trait Anxiety Inventory (Self-Evaluation Questionnaire). Consulting Psychologist Press; 1970.
- 43. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571. [DOI] [PubMed]
- 44. Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and-II in Psychiatric Outpatients. J Pers Asses. 1996;67(3):588-597. doi:10.1207/s15327752jpa6703_13 [DOI] [PubMed]
- 45. Sanz J, García-Vera MP, Espinosa R, Fortún M, Vázquez C. Adaptación española del Inventario para la Depresión de Beck-II (BDI-II): 3. Propiedades psicométricas en pacientes con trastornos psicológicos. Clínica y Salud. 2005;16:23.
- 46. Dimenäs E, Glise H, Hallerbäck B, Hernqvist H, Svedlund J, Wiklund I. Well-being and gastrointestinal symptoms among patients referred to endoscopy owing to suspected duodenal ulcer. Scand J Gastroenterol. 1995;30(11):1046-1052. [DOI] [PubMed]
- 47. Kulich KR, Piqué JM, Vegazo O, et al. Validación psicométrica de la traducción al español de la escala de evaluación de síntomas gastrointestinales (GSRS) y del cuestionario de calidad de vida de reflujo y dispepsia (QOLRAD) en los pacientes con enfermedad por reflujo gastroesofágico. Revista clinica espanola. 2005;205(12):588-594. [DOI] [PubMed]
- 48. Revicki DA, Wood M, Wiklund I, Crawley J. Reliability and validity of the Gastrointestinal Symptom Rating Scale in patients with gastroesophageal reflux disease. Qual Life Res. 1997;7(1):75-83. [DOI] [PubMed]
- 49. Alonso J. La versión española del SF-36 Health Survey (Cuestionario de Salud SF-36): un instrumento para la medida de los resultados clínicos. Medicina Clínica. 1995;104:771-776. [PubMed]
- 50. Alonso J, Prieto L, Ferrer M, et al. Testing the measurement properties of the Spanish version of the SF-36 Health Survey among male patients with chronic obstructive pulmonary disease. J Clin Epidemiol. 1998;51(11):1087-1094. [DOI] [PubMed]
- 51. López-García E, Banegas JR, Pérez-Regadera AG, Gutiérrez-Fisac JL, Alonso J, Rodríguez-Artalejo F. Valores de referencia de la versión española del Cuestionario de Salud SF-36 en población adulta de más de 60 años. Medicina Clínica. 2003;120(15):568-573. doi:10.1016/S0025-7753(03)73775-0 [DOI] [PubMed]
- 52. Maruish ME. User’s Manual for the Sf-36v2 Health Survey. 3rd ed. QualityMetric Incorporated; 2009.
- 53. Vilagut G, María Valderas J, Ferrer M, Garin O, López-García E, Alonso J. Interpretación de los cuestionarios de salud SF-36 y SF-12 en España: componentes físico y mental. Medicina Clínica. 2008;130(19):726-735. doi:10.1157/13121076 [DOI] [PubMed]
- 54. Ware JE. How to Score the Revised MOS Short-Form Health Scale (SF-36®). The Health Institute, New England Medical Center Hospitals; 1988:10, 17-18.
- 55. Ware JE, Kosinski M, Bjorner JB, Turner‐Bowker DM, Gandek B, Maruish ME. User’s Manual for the SF-36v2 Health Survey. 2nd ed. QualityMetric Incorporated; 2007.
- 56. Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. The Health Institute, New England Medical Center Hospitals; 1993.
- 57. Cohen A, Colodner R, Masalha R, Haimov I. The Relationship Between Tobacco Smoking, Cortisol Secretion, and Sleep Continuity. Subst Use Misuse. 2019;54(10):1705-1714. doi:10.1080/10826084.2019.1608250 [DOI] [PubMed]
- 58. Powell DJ, Schlotz W. Daily Life Stress and the Cortisol Awakening Response: Testing the Anticipation Hypothesis. PLoS ONE. 2012;7(12):e52067. doi:10.1371/journal.pone.0052067 [DOI] [PMC free article] [PubMed]
- 59. Fekedulegn DB, Andrew ME, Burchfiel CM, et al. Area Under the Curve and Other Summary Indicators of Repeated Waking Cortisol Measurements: Psychosom Med. 2007;69(7):651-659. doi:10.1097/PSY.0b013e31814c405c [DOI] [PubMed]
- 60. Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916-931. doi:10.1016/S0306-4530(02)00108-7 [DOI] [PubMed]
- 61. Angeloni S, Cordes R, Dunbar S, et al. xMAP® Cookbook. A collection of methods and protocols for developing multiplex assays with xMAP Technology. Luminex xMAP Technology; 2013. http://www.luminexcorp.com
- 62. Arellano-Garcia ME, Hu S, Wang J, et al. Multiplexed immunobead-based assay for detection of oral cancer protein biomarkers in saliva. Oral Dis. 2008;14(8):705-712. doi:10.1111/j.1601-0825.2008.01488.x [DOI] [PMC free article] [PubMed]
- 63. Bjerre M, Hansen TK, Flyvbjerg A, Tønnesen E. Simultaneous detection of porcine cytokines by multiplex analysis: development of magnetic bioplex assay. Vet Immunol Immunopathol. 2009;130(1-2):53-58. doi:10.1016/j.vetimm.2009.01.007 [DOI] [PubMed]
- 64. Craske MG, Lewin MR. Trastorno por pánico. In: Manual para el tratamiento cognitivo-conductual de los trastornos psicológicos. Trastornos por ansiedad, sexuales, afectivos y psicóticos. Vol 1. Siglo XXI; 2007:777.
- 65. Moreno P, Martín JC. Tratamiento psicológico del trastorno de pánico y la agorafobia: manual para terapeutas. 2nd ed. Desclée de Brouwer; 2011.
- 66. Ouabbou S, He Y, Butler K, Tsuang M. Inflammation in Mental Disorders: Is the Microbiota the Missing Link? Neurosci Bull. Published online June 27, 2020. doi:10.1007/s12264-020-00535-1 [DOI] [PMC free article] [PubMed]
- 67. Rizzetto L, Fava F, Tuohy KM, Selmi C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: the role of sex. J Autoimmun. 2018;92:12-34. doi:10.1016/j.jaut.2018.05.008 [DOI] [PubMed]
- 68. Zhong J, Shi G. Editorial: Regulation of Inflammation in Chronic Disease. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.00737 [DOI] [PMC free article] [PubMed]
- 69. Cano-Vindel A. Los desórdenes emocionales en atención primaria. Ansiedad y Estrés. 2011;17(1):75-97.
- 70. Haro JM, Palacín C, Vilagut G, et al. Prevalencia de los trastornos mentales y factores asociados: resultados del estudio ESEMeD-España. Medicina Clínica. 2006;126(12):445-451. [DOI] [PubMed]
- 71. Zhang JM, An J. Cytokines, Inflammation and Pain. Int Anesthesiol Clin. 2007;45(2):27-37. doi:10.1097/AIA.0b013e318034194e [DOI] [PMC free article] [PubMed]
- 72. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018 [DOI] [PubMed]
- 73. Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci. 2011;12(8):453. [DOI] [PMC free article] [PubMed]
- 74. Misiak B, Łoniewski I, Marlicz W, et al. The HPA axis dysregulation in severe mental illness: can we shift the blame to gut microbiota? Prog Neuropsychopharmacol Biol Psychiatry. 2020;102:109951. doi:10.1016/j.pnpbp.2020.109951 [DOI] [PubMed]
- 75. Schnorr SL, Bachner HA. Integrative Therapies in Anxiety Treatment with Special Emphasis on the Gut Microbiome. Yale J Biol Med. 2016;89(3):397-422. [PMC free article] [PubMed]
- 76. Cook MD, Allen JM, Pence BD, et al. Exercise and gut immune function: evidence of alterations in colon immune cell homeostasis and microbiome characteristics with exercise training. Immunol Cell Biol. 2016;94(2):158-163. doi:10.1038/icb.2015.108 [DOI] [PubMed]
- 77. Cussotto S, Strain CR, Fouhy F, et al. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology. 2019;236(5):1671-1685. doi:10.1007/s00213-018-5006-5 [DOI] [PubMed]
- 78. Gao T, Wang Z, Dong Y, et al. Role of melatonin in sleep deprivation‐induced intestinal barrier dysfunction in mice. J Pineal Res. Published online April 12, 2019:e12574. doi:10.1111/jpi.12574 [DOI] [PubMed]
- 79. Hillemacher T, Bachmann O, Kahl KG, Frieling H. Alcohol, microbiome, and their effect on psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;85:105-115. doi:10.1016/j.pnpbp.2018.04.015 [DOI] [PubMed]
- 80. Leprun PMB, Clarke G. The gut microbiome and pharmacology: a prescription for therapeutic targeting of the gut–brain axis. Curr Opin Pharmacol. 2019;49:17-23. doi:10.1016/j.coph.2019.04.007 [DOI] [PubMed]
- 81. Higashimura Y, Baba Y, Inoue R, et al. Effects of molecular hydrogen-dissolved alkaline electrolyzed water on intestinal environment in mice. Med Gas Res. 2018;8(1):6-11. doi:10.4103/2045-9912.229597 [DOI] [PMC free article] [PubMed]