Abstract
Background
Numerous strategies are used to decrease the risk of the need for [allogeneic blood transfusion (ABT)], including [tranexamic acid (TXA)].
Objective
In a single-center retrospective observational study, we have assessed the impact of TXA on the need and average volume of blood used during transfusion.
Methods
We have reviewed medical records of a total of 491 patients undergoing arthroplasty in our hospital from Dec 2016 to Dec 2019.
Results
226 patients were administered TXA IV, and 265 did not receive an additional intervention. In the TXA group, 7/226 patients required ABT vs. 41/265 in the non-TXA group (p<0,001). The Non-TXA group required a significantly higher blood transfusion volume than the TXA group (mean 82,42 mL vs. 12,74 mL; p<0,001).
Conclusion
We conclude that two doses of 1g TXA administered [intravenously (IV)] before incision and during skin suturing reduce the need for blood transfusion in patients undergoing JRS.
Keywords: tranexamic acid, arthroplasty, blood loss reduction, observational study
Introduction
Orthopedic surgery accounts for up to 10% of all blood transfusions.1 Intraoperative and postoperative bleeding has been a major concern in [joint replacement surgery (JRS)]. It might lead to several complications, such as hematomas and infections, translating to a lengthened hospital stay and healthcare costs.2 Furthermore, up to 45% of patients undergoing either [total knee arthroplasty (TKA)] or [total hip replacement (THR)] will require [allogeneic blood transfusion (ABT)].1 ABT might contribute to an increased risk of infection and other complications related to immunosuppressive and immunomodulating consequences.1,3
Several methods have been proposed to reduce blood loss and the need for subsequent ABT related to arthroplasty, which might be divided into pharmacological and non-pharmacological interventions, mainly blood-saving strategies.3,4 Among the pharmacological methods to reduce the need for ABT are preoperative erythropoietin use to boost hematopoiesis, and desmopressin, which affects platelet aggregation and is used in platelet dysfunctions.3–5 Another pharmacological agent is tranexamic acid (TXA), an antifibrinolytic synthetic lysine analog.6 It was introduced more than 40 years ago and is indicated in various hemorrhagic conditions.7,8 TXA acts by inhibiting fibrinolysis by preventing the binding of fibrin and plasminogen reversibly.3,7 The use of TXA for blood loss reduction was studied extensively in many surgical areas, including cardiac surgery, obstetrics, traumatology, gastrointestinal, hepatic, and joint replacement surgeries.4,7,9
Objective
To date, numerous studies have investigated the impact of TXA on blood loss and the need for ABT in arthroplasty. However, most of these include a small number of patients in the TXA arm.10 Thus, the purpose of this study was to assess the efficacy of TXA in a larger group of patients with grade IV osteoarthritis according to the Kellegren-Lawrence classification.
Material and methods
It was a single-center, retrospective observational study performed in the Orthopedic Surgery Unit in Ciechanów Regional Hospital. The Warsaw’s Medical University Ethics Committee approval was obtained (KB/180/2016).
We retrospectively reviewed medical records of adult patients (≥18 years) scheduled for elective primary arthroplasty between December 2016 and December 2019. All patients had grade IV of osteoarthritis according to the Kellegren-Lawrence scale.
Patients undergoing surgery in the first 1,5 years of the observation period (from December 2016 until the end of May 2018) did not receive TXA (non-TXA group). The use of TXA was introduced in our Clinic in June 2018. All patients undergoing arthroplasty from the beginning of June 2018 until the end of December 2019 received a single dose of 1 g of undiluted TXA [intravenously (IV)] approximately 10 minutes before skin incision and another dose of 1g during sin suturing (the TXA group). All patients who underwent surgery during the observation period have signed informed consent for the proposed treatment.
Surgical technique and perioperative management
For all patients, the same medical staff conducted surgery and was responsible for the postoperative management. Two experienced surgeons performed arthroplasties. Spinal cord anesthesia was used in all cases. The tourniquet during total knee arthroplasty was placed before the procedure and released after skin sutures were placed in the TXA and the non-TXA group. The limb was elevated during the postoperative period to minimize passive congestion. In the early period after surgery (after the first 24h), the verticalization of patients and the rehabilitation process were initiated. Patients were discharged on the 4th day postoperatively.
Each patient underwent a physical examination before surgery, and routine blood tests were performed. Patients’ demographic data, including age, gender, and BMI, were also collected. The blood tests were then repeated up to 2 days after surgery. All patients participating in the study received the standard antibiotic prophylaxis consisting of 1g of cefazolin (for patients weighting <80 kg) or 2g (for patients weighing ≥80 kg) and 1g at 6 and 12h after surgery, nadroparin 3800 IU (0.4 mL) - for patients weighting <70 kg, and 5700 IU - for patients weighing ≥70 kg before and 12h after surgery. Patients received pain medication according to the anaesthesiologist’s recommendation.
Patients received ABT if the criteria defined by the Polish Ministry of Health and National Blood Center from 2014 were fulfilled:
[Hemoglobin (HGB)] level ≤6.0 g/dL – in all cases
HGB between 6.0-8.0 g/dL and symptoms suggestive of anemia such as hypotension, acidosis, changes in [electrocardiogram (ECG)] suggestive of cardiac ischemia, or presence of risk factors such as cardiac disease (cardiac insufficiency, coronary disease, cerebrovascular failure)
HGB >8-10 g/dL accompanied by symptoms suggestive of anemia such as hypotension, acidosis, ECG changes suggestive of cardiac ischemia.
During hospitalization, routine screening for [deep vein thrombosis (DVT)] consisted of the Wells prediction score, D-dimer, ultrasound examination. Patients were observed for [pulmonary embolism (PE)] symptoms as well. The screening for TEE was also done during the patient interview at follow-up.
Patients were assessed at three follow-up visits after surgery, scheduled at two weeks, 12-14 weeks, nine months after surgery, which is a standard in our practice. The last follow-up visit was scheduled after 12 months. On each visit, patients were examined and interviewed to screen for any adverse events, including symptoms of thromboembolic events. An X-ray was taken on the second follow-up visit and later visits as screening for loosening and evaluation of the placement of the prosthetic material.
Outcomes
The primary outcomes of interest were ABT rates and mean volume of blood used in the TXA and non-TXA group during the hospital stay. In patients requiring ABT, another blood test was performed the next day. The other outcomes we analyzed were postoperative HGB and [red blood count (RBC)] in both TXA and non-TXA groups.
For subgroup analysis, the mean blood volume was calculated for patients undergoing either THR or TKA. Analysis of preoperative and postoperative changes in blood parameters (HGB, RBC, and erythrocytes) were also analyzed for THR and TKA groups - in both groups (TXA and non-TXA group) combined, and a comparison between the non-TXA group and the group receiving TXA for each type of surgery (TKA or THR) separately.
Statistical analysis
The difference in average values between two independent groups was tested using non-parametric tests Mann-Whitney U and student t-Test without the Welch correction. The decision whether to use non-parametric or parametric analysis was made based on the results of the Kolmogorov-Smirnov test, and the decision whether to use the correction was made by observing the results of Levene’s test of equality of variances. Tests for the independence of two categorical variables was performed using Pearson’s chi-squared test. The strength of the relationship between the variables is provided by Yule phi. The relationship strength is provided as Cohen d (for parametric tests) and r (for non-parametric tests).
Results
Between Dec 2016 and Dec 2019, a total of 491 patients underwent arthroplasty (either TKA or THR). Two hundred twenty-six patients (90 male, 136 female) were administered TXA IV, and 265 (101 male and 164 female) did not receive an additional intervention. In the TXA group, 139 patients underwent THR and 87 – TKA, whereas in the group without intervention, 147 patients underwent THR and 118 – TKA. There was no significant difference between the two groups regarding BMI (p = 0,854) and age (p = 0,166) between the TXA and the non-TXA group. There were no discrepancy in frequencies between TXA, non-TXA groups and THR and TKA χ2(1) = 1,83; p = 0,177; φ = 0,06 (Table 1).
Table 1. Number of THR and TKA in the TXA and non-TXA groups.
| THR | TKA | χ2 | p | Φ | |
| TXA | 139 | 87 | 1,83 | 0,177 | 0,06 |
| No TXA | 147 | 118 |
Notes: TXA – tranexamic acid group; T HR - total hip replacement; TKA – total knee arthroplasty
Primary outcomes
For analysis of blood transfusion rates, Chi-squared analysis was performed and showed that in the non-TXA group (41/265), compared to the TXA (7/226), significantly more blood transfusions were needed (p < 0,001). On the other hand there were no significant difference between patients with knee arthroplasty and hip replacement in the need for blood transfusion χ2(1) = 1,55; p = 0,213; φ = -0,06. The results are presented in Table 2.
Table 2. Number of patients requiring blood transfusion in each group.
| Blood transfusion | χ2 | p | Φ | ||
| Required | Not required | ||||
| TXA | 7 | 219 | 21,18 | <0,001 | 0,21 |
| No TXA | 41 | 224 | |||
| THR | 32 | 254 | 1,55 | 0,213 | -0,06 |
| TKA | 16 | 189 | |||
Notes: THR - total hip replacement; TKA – total knee arthroplasty, TXA – tranexamic acid
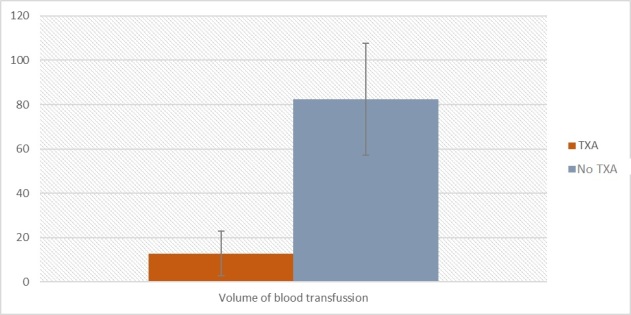
In the next analysis, a difference between the non-TXA and TXA groups in blood transfusion volume was checked. Kolmogorov-Smirnov test showed that the assumption of normal distribution of residuals was not fulfilled, a non-parametric test was performed. Analysis of Mann-Whitney’s U showed a significant difference between non-TXA and TXA groups Z = -4,65; p < 0,001; r = -0,21. Participants in the non-TXA group (M = 82,42; Me = 0,00; SD = 209,81) required significantly higher volume of blood transfusion compared to the TXA group (M = 12,74; Me = 0,00; SD = 77,34). Results are presented in Table 3 and Figure 1. Effect size is small.
Table 3. Volume of blood transfusion between TXA and non-TXA group.
| TXA | No TXA | ||||||||
| M | Mdn | SD | M | Mdn | SD | Z | P | r | |
| Volume of blood transfusions [mL] | 12,74 | 0,00 | 77,34 | 82,42 | 0,00 | 209,81 | -4,65 | <0,001 | -0,21 |
Notes: M – mean; Me – median; SD –standard deviation; Min and Maks. – minimum and maximum values; p-value; Gr – group; All – all participants; TXA – tranexamic acid
Figure 1. The volume of blood transfusion between TXA and non-TXA group in mL.
Secondary outcomes
Based on results of Kolmogorov-Smirnov tests, pre-operative HGB (D = 0,06; p = 0,002) and HGB after a surgical intervention (D = 0,05; p = 0,022) have not fulfilled the assumption of normality of residuals distribution, required for parametric tests. To analyze these variables, non-parametric tests were used. An effect size for non-parametric tests, was provided as r parameter (Rosenthal, 1994). Analysis of Wilcoxon signed-rank test showed a significant difference between pre-operative and post-operative measurements Z = -16,99; p < 0,001; r = -0,85. Participants had higher level of HGB before the intervention (M = 13,88 g/dL; Mdn = 13,90; SD = 1,45) compared to measurement after the intervention (M = 11,39 g/dL; Mdn = 11,50; SD = 1,59). Effect size analysis showed that this effect could be considered large. The results are presented on Figure 2 and in Table 4.
Figure 2a. The differences in HGB level before and after intervention between the TXA and non-TXA group.
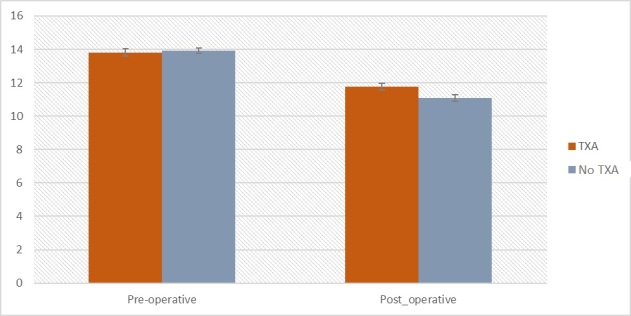
Table 4. Secondary outcomes - HGB and erythrocytes level before and after intervention between TXA and non-TXA groups.
| TXA | No TXA | ||||||||
| M | Me | SD | M | Me | SD | Z | P | r | |
| Pre-operative [g/dL] | 13,82 | 13,90 | 1,56 | 13,92 | 13,90 | 1,37 | -0,47 | 0,636 | 0,02 |
| Post-operative [g/dL] | 11,76 | 11,80 | 1,41 | 11,07 | 11,10 | 1,66 | -4,85 | <0,001 | 0,22 |
| Pre-operative [*106/mm3] | 4,46 | 4,50 | 0,47 | 4,51 | 4,52 | 0,44 | -0,72 | 0,470 | 0,04 |
| Post-operative [*106/mm3] | 3,80 | 3,80 | 0,44 | 3,60 | 3,60 | 0,55 | -4,67 | <0,001 | 0,21 |
Notes: M – mean; Me – median; SD –standard deviation; Min and Maks. – minimum and maximum values; p-value; Gr – group; All – all participants; THR – total hip replacement; TKA – total knee arthroplasty
Figure 2b. The differences in erythrocytes level before and after intervention between the TXA and non-TXA groups.
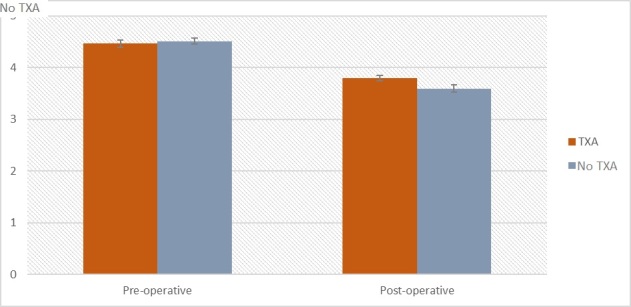
For analysis perioperative changes in erythrocytes level, non-parametric test was conducted Both measurements, erythrocytes before intervention (D = 0,06; p = 0,003) and after intervention (D = 0,07; p < 0,001) did not meet assumptions for residuals distribution normality and Wilcoxon signed-rank test is preferred. Based on results of Wilcoxon signed-rank there is visible difference between measurements Z = -16,94; p < 0,001; r = -0,85. Participants had higher level of erythrocytes before the surgical intervention (M = 4,49 *106/mm3; Mdn = 4,50; SD = 0,45) compared to a measurement after intervention (M = 3,69 *106/mm3; Mdn = 3,70; SD = 0,51). Effect size analysis (r = -0,85) showed that this effect is large.
Subgroup analysis
For subgroup analysis, the differences between the non-TXA and TXA groups in the mean volume of blood transfusions for hip replacement and knee arthroplasty separately were checked. Based Kolmogorov-Smirnov test, which showed that the assumption of normal distribution of residuals was not fulfilled by a variable of the volume of blood transfusion, non-parametric tests were performed, i.e., Mann-Whitney’s U analysis which showed significant differences between non-TXA and TXA groups for hip replacement and knee arthroplasty. Participants in non-TXA groups have a significantly higher volume of blood transfusion compared to participants in TXA groups. The effect size for both groups is small.
Moreover, there was no significant difference between hip replacement and knee arthroplasty groups exposed to TXA in terms of volume of blood transfusion (p<0,001). The results are presented in Table 5 and Figure 3.
Table 5. Volume of blood transfused for the TXA and non-TXA groups for hip replacement and knee arthroplasty separately [mL].
| TXA | No TXA | |||||||||
| Gr | M | Mdn | SD | M | Mdn | SD | Z | P | r | |
| The volume of blood transfused [mL] | THR | 13,82 | 0,00 | 75,18 | 94,64 | 0,00 | 216,78 | -4,01 | <0,001 | -0,24 |
| Volume of blood transfusion [mL] | TKA | 11,04 | 0,00 | 81,08 | 67,12 | 0,00 | 200,64 | -2,53 | 0,011 | -0,18 |
Notes: M – mean; Me – median; SD –standard deviation; Min and Maks. – minimum and maximum values; p-value; Gr – group; All – all participants; THR – total hip replacement; TKA – total knee arthroplasty
Figure 3. The volume of blood transfused for TXA and non-TXA groups after fr hip replacement and knee arthroplasty [mL].
![Figure 3. The volume of blood transfused for TXA and non-TXA groups after fr hip replacement and knee arthroplasty [mL].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/0e10/9239398/572de8900f97/orthopedicreviews_2022_14_2_33875_86310.jpg)
In the next step, we conducted comparative analyses between THR and TKA groups (TXA and non-TXA groups combined) in terms of differences of in HGB level and erythrocytes in patients undergoing knee arthroplasty and hip replacement to investigate if type of surgery has an influence on these two parameters. There were no differences between THR (M = 13,97 g/dL; Mdn = 14,05; SD = 1,50) and TKA (M = 13,75 g/dL; Mdn = 13,80; SD = 1,37) groups preoperatively (Z = -1,78; p = 0,076; r = 0,09). In post-operative there is no difference between THR (M = 11,38 g/dL; Mdn = 11,50; SD = 1,63) and TKA (M = 11,41 g/dL; Mdn = 11,40; SD = 1,54) groups as well Z = -0,11; p = 0,914; r < 0,01. To investigate differences between THA and TKA groups (TXA and non-TXA group combined) in pre-operative and post-operative levels of erythrocytes, two non-parametric Mann-Whitney’s U tests were performed. For a pre-operative measurement there was no statistically significant difference between THR (M = 4,50 [*106/mm3]; Mdn = 4,53; SD = 0,48) and TKA (M = 4,48 [*106/mm3]; Mdn = 4,50; SD = 0,41) groups Z = -0,54; p = 0,590; r = 0,03. In post-operative analysis no difference was found as well Z = -1,01; p = 0,311; r = 0,05 (THR (M = 3,66 [*106/mm3]; Mdn = 3,70; SD = 0,52); TKA (M = 3,72 [*106/mm3]; Mdn = 3,70; SD = 0,49)). Results are presented in Table 6 and on Figure 4.
Table 6. Pre- and postoperative levels of HGB, erythrocytes, and volume of blood transfused in all patients and THR and TKA patients.
| Gr | M | Me | SD | Min. | Max. | p | |
| Age | All | 68,00 | 69,00 | 9,53 | 32,00 | 90,00 | <0,001 |
| Pre-operative HGB [g/dL] | All | 13,88 | 13,90 | 1,45 | 9,00 | 17,70 | 0,002 |
| Pre-operative HGB [g/dL] | THR | 13,97 | 14,00 | 1,49 | 9,00 | 17,70 | >0,200 |
| Pre-operative HGB [g/dL] | TKA | 13,76 | 13,80 | 1,37 | 9,00 | 16,80 | 0,004 |
| Post-operative HGB [g/dL] | All | 11,39 | 11,50 | 1,59 | 6,40 | 16,60 | 0,022 |
| Post-operative HGB [g/dL] | THR | 11,24 | 11,40 | 1,65 | 6,40 | 16,60 | 0,001 |
| Post-operative HGB [g/dL] | TKA | 11,35 | 11,20 | 1,56 | 7,40 | 14,70 | >0,200 |
| Pre-operative erythrocytes [*106/mm3] | All | 4,49 | 4,50 | 0,45 | 2,90 | 5,80 | 0,003 |
| Pre-operative erythrocytes [*106/mm3] | THR | 4,50 | 4,53 | 0,48 | 2,90 | 5,80 | 0,033 |
| Pre-operative erythrocytes [*106/mm3] | TKA | 4,49 | 4,50 | 0,41 | 3,20 | 5,40 | >0,200 |
| Post-operative erythrocytes [*106/mm3] | All | 3,69 | 3,70 | 0,51 | 2,30 | 5,30 | <0,001 |
| Post-operative erythrocytes [*106/mm3] | THR | 3,62 | 3,70 | 0,53 | 2,30 | 5,30 | 0,001 |
| Post-operative erythrocytes [*106/mm3] | TKA | 3,70 | 3,70 | 0,49 | 2,50 | 4,93 | >0,200 |
| Volume of blood transfusions [mL] | All | 50,35 | 0,00 | 166,35 | 0,00 | 960,00 | <0,001 |
| Volume of blood transfusions [mL] | THR | 55,38 | 0,00 | 168,67 | 0,00 | 960,00 | <0,001 |
| Volume of blood transfusions [mL] | TKA | 43,32 | 0,00 | 163,19 | 0,00 | 960,00 | <0,001 |
Notes: M – mean; Me – median; SD –standard deviation; Min and Maks. – minimum and maximum values; p-value; Gr – group; All – all participants; THR – total hip replacement; TKA – total knee arthroplasty
Figure 4. The differences in HGB level before and after intervention between THR and TKA groups (TXA and non-TXA group combined) [g/dL].
![Figure 4. The differences in HGB level before and after intervention between THR and TKA groups (TXA and non-TXA group combined) [g/dL].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/0e10/9239398/4d21d1318efe/orthopedicreviews_2022_14_2_33875_86312.jpg)
In the next two analyses, differences between non-TXA and TXA groups in preoperative HGB were checked for hip replacement and knee arthroplasty separately. Based on statistics of the Kolmogorov-Smirnov test, a residual distribution of preoperative HGB in the knee arthroplasty group is significantly different from the normal distribution, a decision about conducting non-parametric tests was made. Analysis of Mann-Whitney’s U showed a no difference between non-TXA and TXA groups for hip replacement Z = -0,21; p = 0,831; r = -0,01 and knee arthroplasty, Z = -0,73; p = 0,465; r = -0,06.
In the last two analyses, differences between non-TXA and TXA group in post-operative HGB, for hip replacement and knee arthroplasty separately, were checked. Based on statistics of the Kolmogorov-Smirnov test, which showed that the assumption of normal distribution of residuals was not fulfilled by a variable of number of blood transfusions, nonparametric tests were performed. Analysis of Mann-Whitney’s U showed a significant difference between non-TXA and TXA groups for hip replacement Z = -3,74; p < 0,001; r = -0,22. Participants in non-TXA group (M = 11,05; Me = 11,30; SD = 1,73) have significantly less post-operative HGB level compared to participants in TXA group (M = 11,73; Me = 11,90; SD = 1,43). For knee arthroplasty, there was a difference between groups as well Z = -3,10; p = 0,002; r = -0,22. Participants in non-TXA group (M = 11,10; Me = 11,05; SD = 1,58) have significantly more blood transfusions compared to participants in TXA group (M = 11,82; Me = 11,80; SD = 1,39). Effect size for both groups are small. Results are presented in Table 6 and on Figure 5.
Figure 5a. HGB level before arthroplasty in both TXA and non-TXA group [g/dL].
![Figure 5a. HGB level before arthroplasty in both TXA and non-TXA group [g/dL].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/0e10/9239398/e104f4697a7f/orthopedicreviews_2022_14_2_33875_86313.jpg)
Figure 5b. HGB level after arthroplasty in both TXA and non-TXA group [g/dL].
![Figure 5b. HGB level after arthroplasty in both TXA and non-TXA group [g/dL].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/0e10/9239398/9da3b510e463/orthopedicreviews_2022_14_2_33875_86314.jpg)
Discussion
Our study has found that TXA effectively reduces transfusion rates following both knee and hip replacement surgery. We found that the number of patients requiring blood transfusion in the TXA group was significantly lower than in the non-TXA group (7 vs. 41 patients; p<0,001) as well as the average volume of blood transfused (mean 12,74 ± 77,34 mL vs. 82,42 ±209,81 mL; p<0.001).
ABT is a known risk factor for surgical site infection and other prolonged hospital stay and mortality complications.2,3,11 Numerous studies have proven that in JRS, as well as in other types of surgery, the use of TXA is not only an effective method to reduce total blood loss but also healthcare costs without compromising patient’s safety.3,12–14
Our results are in line with the results of other studies and meta-analyses.1–3,6,11–20 It is worth noting that the transfusion rates in the TXA group were even lower than in some of the previous reports. According to the meta-analysis by Kuo et al., in revision JRS, the transfusion rates can be as high as 15% with TXA and 38,7% in patients not receiving TXA.14 In a study by Chin et al., where the same dosing regimen was used, 19% in the TXA group received ABT, compared with the 20% in the placebo group, and the difference did not reach the threshold of statistical significance (p=0.87).15 Also, another retrospective study reported higher transfusion rates in patients receiving TXA (33.9% vs. 48% in patients without this intervention). However, the dose of TXA in this study was variable (250-1000 mg).16 A Cochrane review from 2011 suggested that TXA use is associated with a 38% reduction in the probability of transfusion, but not mortality.17
We decided to use two doses of TXA IV of 1g, one during skin incision and the second one during wound closure. Most of the studies on the use of TXA in arthroplasty used doses between 1-2g IV.18 According to the Clinical Practice Guidelines by Fillingham et al., it has not been proven that the benefits of TXA would increase with higher doses or multiple administrations, including combinations of various routes (IV, intra-articular, oral route).11 Timing of the administration and the dose administered before the incision has more influence on the outcome, similar to our study, and it is associated with lower blood loss and transfusion rates. However, one must always take into account an increased risk of complications with an increased dose.11 Some authors speculate that the topical route is safer, especially in higher-risk populations such as patients with renal impairment due to lower systemic exposure.13 However, this route also has some disadvantages, as a preclinical study has found that TXA administered to the joint cavity might have potentially toxic effects on the cartilage, resulting in atypical morphology and cellular death if its concentration exceeds 20mg/ml, which would have implications for patients undergoing partial arthroplasty.21 In summary, we found that the dosing regimen we used is justified and easy to implement into routine clinical practice.
One of the significant concerns regarding the use of TXA is the risk of TEE.1,2,11 Although many studies conducted to date investigated the effectiveness of TXA in blood loss reduction in orthopedic surgery, there are still essential areas left for investigation, such as safety in patients with risk factors for thromboembolic complications. Age is one of the significant risk factors for both adverse outcomes, and in most of the studies and analyses we have reviewed, the mean age of patients included exceeded 65 years.2,10,14,19,22,23 On average, almost 65% of patients in this age group have an average of 2.6 concomitant diseases, which may also contribute to an increased risk of TEE.24 Other risk factors, such as obesity, hypertension, hypercholesterolemia, and diabetes, are frequent in the general adult population.25,26 Arthroplasty itself is also a significant risk factor for VTE, and the three most common risk factors for such complications: age ≥40 years, obesity, VTE in the medical history, which are very frequent in the population most often subjected to JRS.27 As a result, in most studies regarding TXA use in JRS, risk factors for TEEs were exclusion criteria, which could explain why the sample sizes rarely exceed 100 patients in the treatment group.7,13,14,22,23,28 As a consequence, although many authors have described TXA use in JRS, there are no guidelines to standardize the method of administration, dosing, intervals between dosing of TXA in patients with an increased risk of arterial thromboembolic events (ATE) and venous thromboembolic events (VTE).11 For example, Tsukada et al. have reported that TEE’s were recorded in 12% of patients in the IV TXA group.28 In the study comparing IV and IA TXA with and without epinephrine added a total of 3 out of 204 patients suffered thromboembolic complications.20 These numbers are very high, taking into account that one of the recent analyses concerning over 5,700 subjects has determined that the frequency of symptomatic TEE events is low after JRS; both for TKA (DVT – 0.05%; PE – 0.27%) and THR ( DVT – 0.77% and PE – 0.46%) in the first 90 days after surgery.29 In our study group, there was one case of TEE complications in a 68-year old patient undergoing TKA who did not receive TXA. At six months postoperatively, she was diagnosed with deep vein thrombosis in the popliteal and posterior tibial veins, with minor symptoms of pain in the popliteal fossa, without edema in this localization.
Our study has certain limitations. As this was a single-center study with a non-randomized and retrospective design, there is a risk of bias, including reporting bias. On the other hand, we were able to eliminate many confounding factors, e.g., standards of perioperative care remained unchanged during the study period. Safety analysis, which is still an important area of investigation in the TXA use, was not a part of our primary study design. Therefore, we believe that there is still a need to conduct more studies on TXA use in risk groups.
Conclusion
We have shown that 2g of TXA administered IV in two doses effectively reduces the need for blood transfusion in patients undergoing both knee or hip replacement surgery. However, we believe that there is still a need for a detailed assessment of its effect in patients with comorbidities related to increased TEEs in a larger population.
Authors contributions
Conceptualization, TP, and WK.; methodology, TP.; formal analysis, TP, WK and KS.; investigation, TP.; data curation, TP, WK and KS.; writing—original draft preparation, TP and WK.; writing—review and editing, KS.; visualization, TP.; supervision, TP. All authors have read and agreed to the published version of the manuscript.
Disclosures
The authors have no conflicts of interest to declare.
Funding Statement
No funding was received for this research, as well as writing this manuscript.
References
- 1. Tille E, Mysliwietz J, Beyer F, Postler A, Lützner J. Intraarticular use of tranexamic acid reduces blood loss and transfusion rate after primary total knee arthroplasty. BMC Musculoskelet Disord. 2019;20(1):341. doi:10.1186/s12891-019-2715-9 [DOI] [PMC free article] [PubMed]
- 2. Adravanti P, Di Salvo E, Calafiore G, Vasta S, Ampollini A, Rosa MA. A prospective, randomized, comparative study of intravenous alone and combined intravenous and intraarticular administration of tranexamic acid in primary total knee replacement. Arthroplast Today. 2017;4(1):85-88. doi:10.1016/j.artd.2017.08.004 [DOI] [PMC free article] [PubMed]
- 3. Goldstein M, Feldmann C, Wulf H, Wiesmann T. Tranexamic acid prophylaxis in hip and knee joint replacement. Dtsch Arztebl Int. 2017;114:824-830. [DOI] [PMC free article] [PubMed]
- 4. Sambandam B, Batra S, Gupta R, Agrawal N. Blood conservation strategies in orthopedic surgeries: A review. J Clin Orthop Trauma. 2013;4(4):164-170. doi:10.1016/j.jcot.2013.11.002 [DOI] [PMC free article] [PubMed]
- 5. Horstman LL, Valle-Riestra BJ, Jy W, Wang F, Mao W, Ahn YS. Desmopressin (DDAVP) acts on platelets to generate platelet microparticles and enhanced procoagulant activity. Thromb Res. 1995;79(2):163-174. doi:10.1016/0049-3848(95)00102-w [DOI] [PubMed]
- 6. Xiong H, Liu Y, Zeng Y, Wu Y, Shen B. The efficacy and safety of combined administration of intravenous and topical tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2018;19(1). doi:10.1186/s12891-018-2181-9 [DOI] [PMC free article] [PubMed]
- 7. Lin ZX, Woolf SK. Safety, Efficacy, and Cost-effectiveness of Tranexamic Acid in Orthopedic Surgery. Orthopedics. 2016;39(2):119-130. doi:10.3928/01477447-20160301-05 [DOI] [PubMed]
- 8. Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57(6):1005-1032. doi:10.2165/00003495-199957060-00017 [DOI] [PubMed]
- 9. Ng WCK, Jerath A, Wąsowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther. 2015;47(4):339-350. doi:10.5603/ait.a2015.0011 [DOI] [PubMed]
- 10. Fillingham YA, Ramkumar DB, Jevsevar DS, et al. Tranexamic Acid Use in Total Joint Arthroplasty: The Clinical Practice Guidelines Endorsed by the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. J Arthroplasty. 2018;33(10):3065-3069. doi:10.1016/j.arth.2018.08.002 [DOI] [PubMed]
- 11. Klasan A, Dworschak P, Heyse TJ, et al. Transfusions increase complications and infections after hip and knee arthroplasty: An analysis of 2760 cases. Technol Health Care. 2018;26(5):825-832. doi:10.3233/thc-181324 [DOI] [PubMed]
- 12. Riaz O, Aqil A, Asmar S, et al. Epsilon-aminocaproic acid versus tranexamic acid in total knee arthroplasty: a meta-analysis study. J Orthop Traumatol. 2019;20(1):28. doi:10.1186/s10195-019-0534-2 [DOI] [PMC free article] [PubMed]
- 13. Sun Q, Li J, Chen J, Zheng C, Liu C, Jia Y. Comparison of intravenous, topical or combined routes of tranexamic acid administration in patients undergoing total knee and hip arthroplasty: a meta-analysis of randomised controlled trials. BMJ Open. 2019;9(1):e024350. doi:10.1136/bmjopen-2018-024350 [DOI] [PMC free article] [PubMed]
- 14. Guo P, He Z, Wang Y, et al. efficacy and safety of oral tranexamic acid in total knee arthroplasty: a systematic review and meta-analysis. Medicine. 2018;97(18):e0587. doi:10.1097/md.0000000000010587 [DOI] [PMC free article] [PubMed]
- 15. Chin J, Blackett J, Kieser DC, Frampton C, Hooper G. The Value of Routine Intravenous Tranexamic Acid in Total Hip Arthroplasty: A Preliminary Study. Adv Orthop. 2020;2020(2943827):1-5. doi:10.1155/2020/2943827 [DOI] [PMC free article] [PubMed]
- 16. Liu KL, Chen IH, Wen SH. Low dose tranexamic acid reduces blood transfusion rate after total knee arthroplasty: a population-based study in Taiwan. J Formos Med Assoc. 2017;116(1):24-31. doi:10.1016/j.jfma.2015.12.012 [DOI] [PubMed]
- 17. Henry DA, Carless PA, Moxey AJ, et al. Antifibrinolytic use for minimizing perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;3:001886. doi:10.1002/14651858.cd001886.pub3 [DOI] [PMC free article] [PubMed]
- 18. Melvin JS, Stryker LS, Sierra RJ. Tranexamic Acid in Hip and Knee Arthroplasty. J Am Acad Orthop Surg. 2015;23(12):732-740. doi:10.5435/jaaos-d-14-00223 [DOI] [PubMed]
- 19. Yu Y, Lin H, Wu Z, Xu P, Lei Z. Perioperative combined administration of tranexamic acid and dexamethasone in total knee arthroplasty—benefit versus harm? Medicine. 2019;98(34):e15852. doi:10.1097/md.0000000000015852 [DOI] [PMC free article] [PubMed]
- 20. Stamatopoulos A, Stamatopoulos T, Kenanidis E, et al. Intravenous and intraarticular tranexamic acid plus epinephrine for the management of blood loss after cemented total knee arthroplasty: a case-control study. Hippokratia. 2018;22:86-90. [PMC free article] [PubMed]
- 21. Parker JD, Lim KS, Kieser DC, Woodfield TBF, Hooper GJ. Is tranexamic acid toxic to articular cartilage when administered topically? What is the safe dose? Bone Joint J. 2018;100-B(3):404-412. doi:10.1302/0301-620x.100b3.bjj-2017-1135.r1 [DOI] [PubMed]
- 22. Xu Y, Sun S, Feng Q, et al. The efficiency and safety of oral tranexamic acid in total hip arthroplasty: a meta-analysis. Medicine. 2019;98(46):e17796. doi:10.1097/md.0000000000017796 [DOI] [PMC free article] [PubMed]
- 23. Zhang LK, Ma JX, Kuang MJ, et al. The efficacy of tranexamic acid using oral administration in total knee arthroplasty: a systematic review and meta-analysis. J Orthop Surg Res. 2017;12(1). doi:10.1186/s13018-017-0660-6 [DOI] [PMC free article] [PubMed]
- 24. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37-43. doi:10.1016/s0140-6736(12)60240-2 [DOI] [PubMed]
- 25. Zeymer U, Senges J, Parhofer KG, Röther J. Risk Factors and Event Rates in Patients With Atherothrombotic Disease in Germany: Results of the REACH Registry. Dtsch Arztebl Int. 2008;105:769-775. [DOI] [PMC free article] [PubMed]
- 26. The health of citizens of Poland. Chief Statistical Office (GUS). Published online 2014. Accessed May 2020. https://stat.gov.pl/obszary-tematyczne/zdrowie/zdrowie/stan-zdrowia-ludnosci-polski-w-2014-r-,6,6.html
- 27. Anderson FAJr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:I9-I16. doi:10.1161/01.cir.0000078469.07362.e6 [DOI] [PubMed]
- 28. Tsukada S, Kurosaka K, Nishino M, Maeda T, Yonekawa Y, Hirasawa N. Intra-articular tranexamic acid as an adjunct to intravenous tranexamic acid for simultaneous bilateral total knee arthroplasty: a randomized double-blind, placebo-controlled trial. BMC Musculoskelet Disord. 2019;20(1). doi:10.1186/s12891-019-2890-8 [DOI] [PMC free article] [PubMed]
- 29. Mula V, Parikh S, Suresh S, Bottle A, Loeffler M, Alam M. Venous thromboembolism rates after hip and knee arthroplasty and hip fractures. BMC Musculoskelet Disord. 2020;21(1):95. doi:10.1186/s12891-020-3100-4 [DOI] [PMC free article] [PubMed]


